Abstract
Introduction
COVID-19 large scale immunization in the US has been associated with breakthrough positive molecular testing. In this study, we investigated whether a positive test is associated with a high anti-viral IgG, specific viral variant, recovery of infectious virus, or symptomatic infection during an early phase after vaccination rollout.
Methods
We identified 133 SARS-CoV-2 positive patients who had received two doses of either Pfizer-BioNTech (BNT162b2) or Moderna (mRNA-1273) vaccines, the 2nd of which was received between January and April of 2021. The positive samples were collected between January and May of 2021. Samples were sequenced to characterize the whole genome and Spike protein changes and cycle thresholds that reflect viral loads were determined using a single molecular assay. Respiratory SARS-CoV-2 IgG antibodies were examined using ELISA and specimens were grown on cell culture to assess the recovery of infectious virus as compared to a control unvaccinated cohort.
Results
Of 133 specimens, 24 failed sequencing and yielded a negative or very low viral load on the repeat PCR. Of 109 specimens that were used for further genome analysis, 68 (62.4%) were from symptomatic infections, 11 (10.1%) were admitted for COVID-19, and 2 (1.8%) required ICU admission with no associated mortality. The predominant virus variant was the Alpha (B.1.1.7), however a significant association between lineage B.1.526 and amino acid change S: E484K with positives after vaccination was noted. A significant reduction of the recovery of infectious virus on cell culture was accompanied by an increase in localized IgG levels in respiratory samples of vaccinated individuals.
Conclusions
Vaccination reduces the recovery of infectious virus in breakthrough infections caused primarily by the Alpha variant accompanied by an increase in upper respiratory tract IgG levels.
Keywords: SARS-CoV-2, COVID-19, Cell culture, Vaccination
1. Introduction
SARS-CoV-2 has caused a devastating pandemic. Millions of global deaths have been recorded with thousands of new cases diagnosed daily, a trend that significantly changed with the large-scale vaccination in certain countries including the US (https://coronavirus.jhu.edu/map.html). Even though vaccines have high efficacy [1, 2] and undoubtedly, have reduced COVID-19 mortality and severe disease in countries that accelerated mass immunization [3], breakthrough infections have been reported.
With the appearance of SARS-CoV-2 variants that are more transmissible or capable of evading vaccine induced immune responses, surveillance has become of utmost importance and genome characterization of positives after vaccination is essential. Currently, data support that vaccines approved for use in the US are effective against most of the currently circulating variants [4, 5]. With the general increase in the circulation of variants of concern (VOC), it is expected to see a high percentage of breakthrough infections caused by these variants.
In this manuscript, we provide a comprehensive analysis of the first 133 positives after vaccination diagnosed by Johns Hopkins Clinical Virology laboratory during the early phase after vaccination rollout. Samples were enrolled in our whole genome sequencing for surveillance pipeline and were retested by the PerkinElmer PCR assay to obtain comparable cycle threshold values (Cts). The recovery of infectious virus from positives after vaccination was determined as well as local SARS-CoV-2 IgG levels in the respiratory samples using ELISA and compared to a control unvaccinated cohort.
2. Methods
2.1. Ethical considerations and data availability
The research was performed with a waiver of consent under IRB00221396. Whole genomes were made publicly available at GISAID.
2.2. Specimens and patient data
Remnant nasopharyngeal (symptomatic patients) or lateral mid-turbinate nasal swabs (asymptomatic screening) after standard of care diagnostic or screening testing were collected and used for genome sequencing. At Johns Hopkins Medical System, SARS-CoV-2 clinical testing is performed for inpatients and outpatients as well as standard of care asymptomatic presurgical screening and the laboratory serves a large geographic area in the National Capital Region (Baltimore, Virginia, and D.C.) [6]. Molecular methods used for screening and diagnosis include the NeuMoDx SARS-CoV-2 (Qiagen), Cobas SARS-CoV-2 (Roche), Xpert Xpress SARS-CoV-2/Flu/RSV (Cepheid), in addition to the RealStar® SARS-CoV-2 RT-PCR (Altona Diagnostics), ePlex Respiratory Pathogen Panel 2 (Roche), Aptima SARS-CoV-2 (Hologic), and Accula SARS-CoV-2 assays (ThermoFisher Scientific) [7], [8], [9], [10].
Breakthrough infections selected for the study were the first 133 positive samples from vaccinated patients who: 1) received two doses of either fizer/BioNTech BNT162b2 or Moderna mRNA-1273, and 2) were identified by our group through our SARS-CoV-2 genomic surveillance. Positive samples from vaccinated patients had collection times that ranged from 2 to 99 days from the second dose (Table S1). Samples had collection dates from January to May 2021 (during this time frame, the laboratory diagnosed 7396 positive samples of which, our group sequenced 2765). A Control sample group (N = 124) were selected randomly (convenience sample based on availability) from unvaccinated, SARS-CoV-2 positive individuals. The control group sample collection dates were from December 2020 to March 2021 and were used for cell culture, ELISA, and cycle thresholds studies. Lineages and clades of both groups are detailed in Tables S1 and S2 and Table 1 summarizes the clinical and metadata of the vaccinated and control cohorts.
Table 1.
Clinical and metadata of breakthrough infection cases and control groups.
| Positives after full vaccination | Positives after full vaccination (viral load below the limit of detection or false positives) | Control group 1 (ELISA, Ct, and cell culture) | Control group 2 (whole genome sequencing) | |
|---|---|---|---|---|
| Total number of patients | 109 | 24 | 124 | 335 |
| Median age in years (range) | 51 (23 - >90) | 52 (23 - 79) | 36 (0 - >90) | 40 (0 - >90) |
| >65 | 30 | 4 | 10 | 47 |
| 50–64 | 26 | 12 | 27 | 74 |
| 18–49 | 53 | 8 | 61 | 147 |
| <18 | 0 | 0 | 26 | 67 |
| Collection months | ||||
| Dec-20 | 0 | 0 | 2 (1.6) | 0 |
| Jan-21 | 2 (1.8) | 2 (8.3) | 17 (13.7) | 5 (1.5) |
| Feb-21 | 7 (6.4) | 3 (12.5) | 79 (63.7) | 5 (1.5) |
| Mar-21 | 16 (14.7) | 7 (29.2) | 26 (21) | 50 (14.9) |
| Apr-21 | 73 (67) | 9 (37.5) | 0 | 245 (73.1) |
| May-21 | 12 (11) | 2 (8.3) | 0 | 30 (9) |
| No. (%) of | ||||
| Male | 40 (36.7) | 6 (25) | 52 (41.9) | 151 (45.1) |
| Female | 69 (63.3) | 18 (75) | 72 (58.1) | 184 (54.9) |
| Symptomatic | 68 (62.4) | 8 (28.6) | 111 (89.5) | 291 (86.9) |
| Asymptomatic | 41 (37.6) | 16 (66.6) | 13 (10.5) | 44 (13.1) |
| Severity (%) | ||||
| Outpatient | 98 (89.9) | 24 (100) | 118 (95.2) | 269 (80.3) |
| Hospitalized for COVID-19 | 11 (10.1) | 0 | 6 (4.8) | 66 (19.7) |
| ICU admission | 2 (1.8) | 0 | 3 (2.4) | 23 (6.9) |
| Comorbidities (%) | ||||
| Hypertension | 49 (44.9) | 10 (41.7) | 42 (33.8) | 112 (33.4) |
| Respiratory Failure | 16 (14.7) | 0 | 9 (7.3) | 56 (16.7) |
| Pregnancy | 8 (7.3) | 2 (8.3) | 9 (7.3) | 18 (5.4) |
| Lung Disease | 29 (26.6) | 5 (20.8) | 31 (25) | 61 (18.2) |
| Kidney Disease | 26 (23.8) | 3 (12.5) | 17 (13.7) | 52 (15.5) |
| Immunosuppression | 29 (26.6) | 5 (20.8) | 27 (21.8) | 58 (17.3) |
| Diabetes | 26 (23.8) | 3 (12.5) | 14 (11.3) | 67 (20) |
| Heart Failure | 23 (21.1) | 3 (12.5) | 9 (7.3) | 31 (9.3) |
| Atrial Fibrillation | 12 (11) | 0 | 5 (4) | 22 (6.6) |
| Smoker | 9 (8.3) | 3 (12.5) | 21 (16.9) | 48 (14.3) |
| Cerebrovascular Disease | 6 (5.5) | 1 (4.2) | 7 (5.6) | 18 (5.4) |
| Cancer | 54 (49.5) | 10 (41.7) | 32 (25.8) | 56 (16.7) |
| Coronary Artery Disease | 29 (26.6) | 6 (25) | 20 (16.1) | 67 (20) |
| Patients with no/ unknown comorbidities | 28 (25.7) | 7 (29.2) | 42 (33.9) | 132 (39.4) |
| Patients with one comorbidity | 19 (17.4) | 6 (25) | 29 (23.4) | 66 (19.7) |
| Patients with two or more comorbidities | 62 (56.9) | 11 (45.8) | 53 (42.7) | 137 (40.9) |
| Median days after COVID-19 vaccine (range) | 52 (2 - 99) | 19.5 (3 - 100) | ||
The control group used for whole genome comparison were randomly selected from samples sequenced by our group (Table S3 includes GISAID submission information), using MatchIt in R (method= 'optimal', ratio=5) based on collection date (to control for differences that might be related to the frequency of the circulating variants at a given time frame). This control group (N = 335) was independent of the control samples used for cell culture, ELISA, and cycle thresholds comparisons (Table S3 and metadata shown in Table 1).
2.3. Clinical data analysis
Patient data was extracted by manual chart reviews of electronic health records. Symptomatic versus asymptomatic status was determined by the ordering clinician and included as a questionnaire in the patients’ charts. Vaccination status was determined through documented vaccinations given and self-reports documented in the system. Notably, Johns Hopkins has an agreement with the State of Maryland and Chesapeake Regional Information System for our Patients (CRISP) to provide vaccination status for all Johns Hopkins Patients.
2.4. Nucleic acid extraction, PCR, and whole genome sequencing
Automated nucleic acid extraction was performed using the chemagic 360 (PerkinElmer) following the manufacturer's protocol, with an RNA elution volume of 60µL. Real-time reverse transcriptase PCR (rRT-PCR) was performed using the PerkinElmer SARS-CoV-2 Real-time RT-PCR Assay following the package insert (https://www.fda.gov/media/136410/download). Whole genome sequencing and analysis were performed using the ARTIC protocol as previously described [6, 11]. Thresholds were set to a minimum of 90% coverage and 100 mean depth. Lineages were assigned by Pangolin (COG-UK; cog-uk.io) and ambiguous mutations were visually confirmed with Integrated Genomics viewer (IGV) (Version 2.8.10).
2.5. ELISA
The EUROIMMUN Anti-SARS-CoV-2 ELISA (IgG) was run using undiluted respiratory samples and following the package insert (https://www.fda.gov/media/137609/download). This assay detects antibodies to the S1 domain of the spike protein of SARS-CoV-2. The assay has a cut-off < 0.8 for negative results and ≥ 0.8 to < 1.1 as borderline. The value 1.1 was used as a cut off for positivity for nasopharyngeal/ NMT swab specimen types even though these sources were not tested by the manufacturer.
2.6. Cell culture
Vero-TMPRSS2 cells were cultured and infected with aliquots of swab specimens as previously described for VeroE6 cells [12]. The presence of SARS-CoV-2 was confirmed by reverse transcriptase PCR (qPCR).
2.7. Statistical analyses
Statistics were performed using GraphPad Prism. Chi-square and Fisher Exact tests were used for categorical variable comparisons and t-test was used for comparing continuous independent variables. For Lineage and spike analyses (Fig. 1 C and D), samples with ≥ 90% genome coverage were selected from both vaccinated and control groups (N = 67 for the vaccinated group, and 335 for the control group, Tables S1 and S3). Control and vaccinated samples were plotted over time to verify a good match. Percentage of samples that matched to lineages were plotted. The full set of mutations present within the spike protein of vaccinated patients was determined, and a heatmap of percentage of samples from vaccinated and control groups were plotted. Chi-square analysis of lineages with at least 5 samples showed a correlation between lineage and vaccine status (p = 0.006). Chi-square analysis was performed for lineages P.1, B.1.1.7, B.1.351, B.1.526, and B.1.526.1.
Fig. 1.
SARS-CoV-2 genomes of positives after full vaccination. A) Lineages and B) Clades of genomes with more than 50% coverage and average depth of 50 (n = 88). C) A comparison between lineages from fully vaccinated (n = 67) and control (n = 335) genomes with coverage ≥ 90%. D) Spike amino acid changes in vaccinated and control groups. Chi-square test * p < 0.05, *** p < 0.001.
3. Results
3.1. SARS-CoV-2 genomes of positives after full vaccination
As a part of whole genome sequencing of SARS-CoV-2 for surveillance at Johns Hopkins Clinical Virology laboratory [6, 11], we identified 133 positives after the completion of either Moderna or Pfizer vaccination that were collected in the time frame of January 2021- May 2021. Of the 133 samples, 24 had failed sequencing with 0% coverage which we believe was related to very low viral loads (Table S1). Twenty-one sequences had coverage of less than 50% and average depth of less than 50 and a lineage was not called. Of the genomes that had more than 50% coverage and more than an average depth of 50 (a total of 88), the majority (61%) belonged to the B.1.1.7 lineage (Alpha variant) (20I/501Y.V1 clade), consistent with its predominance in this time frame [11, 13], followed by the 20C lineages (Iota variants) B.1.526 (9%) and B.1.526.1 (4.5%) (Fig. 1A and B). When a cohort of our characterized genomes for surveillance were randomly selected as a control group based on the sample collection dates, with only genomes that have ≥ 90% coverage used for the analysis (vaccinated: N = 67, and control: N = 335), a significantly higher proportion of individuals infected with lineage B.1.526 were vaccinated (Chi-square with Bonferroni correction p = 0.022, Fig. 1C). Spike substitution analysis showed that the S: E484K was associated with genomes of the vaccinated group (p = 0.0032, Fig. 1D and Tables S1 and S3).
3.2. Recovery of infectious virus from fully vaccinated individuals and the correlation with localized SARS-CoV-2 antibodies and relative viral loads
To assess the recovery of infectious virus from positive samples of fully vaccinated individuals, first, a cohort of control samples was selected. A total of 124 positive samples from unvaccinated individuals collected in the time frame between the end of December 2020 to the first week of March 2021 were used for comparison (Table S2). The cohort was selected randomly to include the Alpha variant lineage as well as prior predominant lineages. To compare the recovery of infectious virus between positives from fully vaccinated (N = 114 with sufficient volume) and control (N = 124) groups, samples were cultured on Vero-TMPRSS2 cells. As expected, samples that had failed sequencing and were thought of as very low viral load did not yield infectious virus and were excluded from further analysis (Table S1). Of the fully vaccinated group, 17 of 92 samples (18.5%) showed CPE on cell culture compared to 80 out of 124 (64.5%) of the control group (Fisher Exact test, p < 0.00001). Notably, the control group recovery on cell culture was faster than the vaccinated group with 44 out of 80 samples (55%) positive 2 days after culture compared to no samples showing CPE for the vaccinated group in the same day (Fig. 2 A and B).
Fig. 2.
Recovery of infectious SARS-CoV-2 on Vero-TMPRSS2 cells for A) fully vaccinated (samples with Ct values, N = 89) and B) control (N = 124) groups. C) SARS-CoV-2 IgG in upper respiratory swab samples from fully vaccinated (N = 114) and control (N = 124) groups. Dashed line demarcates the limit of borderline and negative ELISA results as specified per assay's package insert (1.1). CPE, cytopathic effect. t-test * p < 0.05, *** p < 0.0001.
To study the localized SARS-CoV-2 antibodies in the respiratory samples and their correlation to the observed CPE phenotype, respiratory samples were tested by ELISA for SARS-CoV-2 IgG. A significant increase in SARS-CoV-2 respiratory IgG levels were noted in the respiratory samples from vaccinated individuals when compared to the control group (Fig. 2C, t-test, P < 0.0001) with 66.7% positive samples from the vaccinated group versus 5.9% from the control group (Fig. 2C).
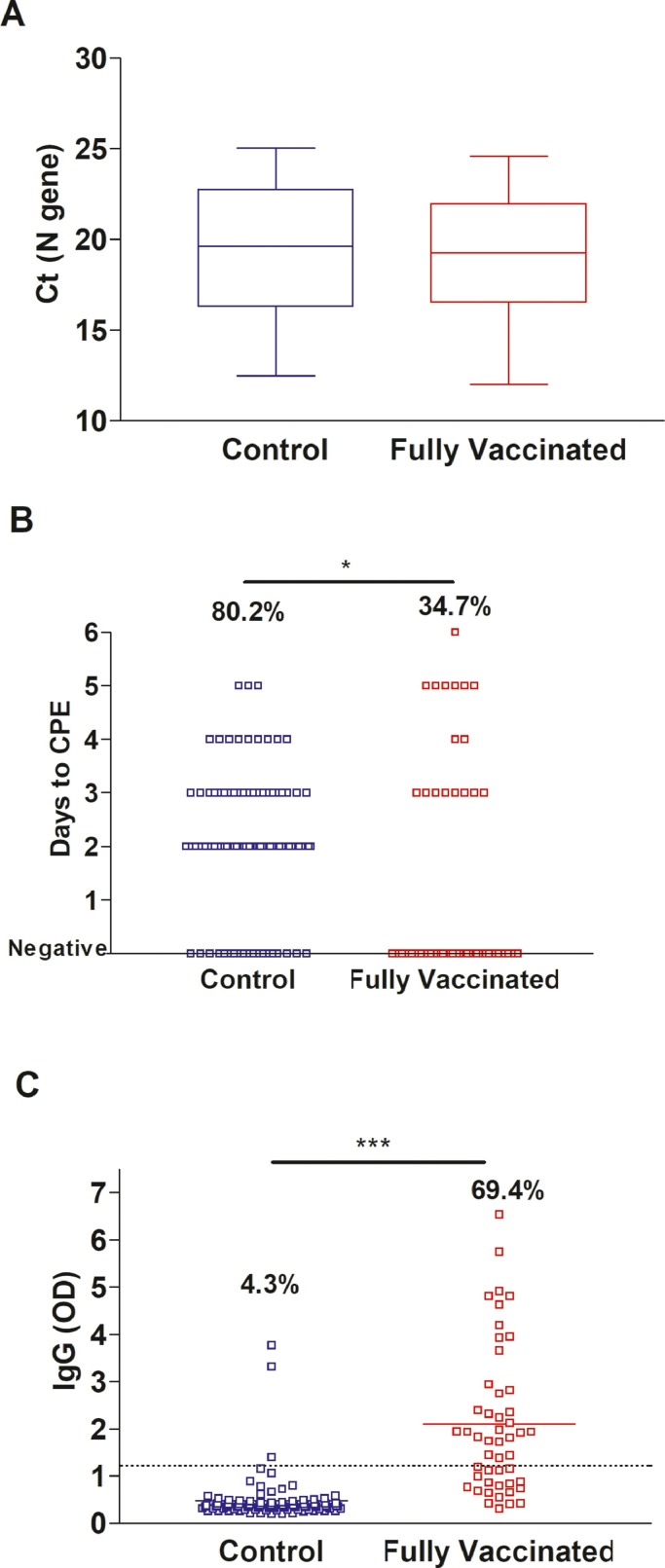
To examine if the discrepant virus recovery on cell culture is secondary to differences in the viral loads in vaccinated versus unvaccinated groups [12], we focused our analysis on samples with Ct values below 25. This constituted 49 samples in the vaccinated group versus 96 samples in the control group (Tables S1 and S2). Notably the distribution of the Ct values between the two groups for samples with Ct values lower than 25 was similar (Fig. 3 A). The majority of the control group samples were positive on cell culture (77, 80.2%), in contrast to 17 (34.7%) of the vaccinated group (Fig. 3B). Consistent with data from the whole cohort, higher nasal/ nasopharyngeal IgG levels (Fig. 3C, P < 0.0001) was noted for the fully vaccinated group.
Fig. 3.
Recovery of infectious SARS-CoV-2 on Vero-TMPRSS2 cells for samples with Ct values less than 25 (N gene). A) comparison of Ct values distribution between control (N = 96) and fully vaccinated groups (N = 49). B) Days to cytopathic effect (CPE) in control (N = 96) and fully vaccinated groups (N = 49). C) IgG in vaccinated (N = 49) and control (N = 96) groups’ respiratory samples. Dashed line demarcates the limit of borderline and negative ELISA results as specified per assay's package insert (1.1). t-test * p < 0.05, *** p < 0.0001.
3.3. Characterization of localized SARS-CoV-2 antibody responses
A correlation of SARS-CoV-2 local IgG with the days of sample collection after receiving the second dose of the COVID-19 vaccine showed a trend of reduction with the progress of time (Fig. 4 A, linear regression, p < 0.0001). No significant correlations between the IgG levels and Ct values were noticed (Fig. 4B). Negative recovery of infectious virus on cell culture correlated with higher levels of IgG in respiratory specimens, with no infectious virus isolated from samples with an IgG OD reading of >3.0 (Fig. 4C, t-test, P = 0.004). The absence of symptoms did not correlate with higher IgG levels when we compared samples from symptomatic vaccinated to asymptomatic vaccinated individuals (Fig. 5 A). Notably, a significant increase in the mean Ct value for the asymptomatic group was noted (27.6 versus 23.2, t-test, P = 0.0048, Fig. 5B) as well as a reduction in the mean genome coverage (70.8% versus 84.9%, t-test, P = 0.0284, Fig. 5C). Infectious virus was recovered from only 2 samples from asymptomatic patients (6.5%) in contrast to 15 from symptomatic patients (24.6%) (Table S1).
Fig. 4.
Localized SARS-CoV-2 antibodies in upper respiratory samples of vaccinated individuals. A) IgG levels by ELISA in the upper respiratory samples collected from patients positive after full vaccination (N = 114) and association with the days after receiving the second dose of the COVID-19 vaccine. B) SARS-CoV-2 IgG correlation to cycle thresholds of the N gene using the PerkinElmer SARS-CoV-2 assay. C) SARS-CoV-2 IgG correlation to days to the first appearance of cytopathic effect (CPE) on Vero-TMPRSS2 cells. Dashed line demarcates the limit of borderline and negative ELISA results as specified per assay's package insert.
Fig. 5.
Localized antibodies, viral loads, and recovery of whole genomes in symptomatic (N = 56) versus asymptomatic (N = 29) vaccinated individuals. A) IgG levels by ELISA in the upper respiratory samples. B) Comparison of Ct values distribution between symptomatic and asymptomatic groups. C) Percent genome coverage in symptomatic and asymptomatic groups. Dashed line demarcates the limit of borderline and negative ELISA results as specified per assay's package insert. t-test * p < 0.05, ** p < 0.001.
4. Discussion
In this study, we provide an analysis of a cohort of 133 SARS-CoV-2 positive specimens collected after the completion of COVID-19 vaccination during an early phase after vaccination rollout. Genomic analysis revealed a statistically significant increased representation of lineage B.1.526 as well as the Spike amino acid change S: E484K in genomes from the vaccinated group. Strikingly, when samples with Ct values less than 25 were compared to a control cohort of similar Ct values, the recovery of infectious virus from cases post-vaccination was significantly impaired, evident as both a delay in the first appearance of cytopathic effect as well as a significant decrease in the total number of positive samples on cell culture. This data indicates that infection in vaccinated individuals results in reduced infectious virus load compared to unvaccinated individuals. This may further reduce the likelihood that infected; vaccinated individuals can transmit SARS-CoV-2 to others. These observations were based on a cohort infected primarily by the Alpha variant.
Interestingly, the lower infectious virus load in vaccinated individuals was associated with an increase in localized SARS-CoV-2 specific IgG levels. The reduction in infectious virus in samples with Ct values of less than 25 could be explained if the nasal SARS-CoV-2 IgGs are neutralizing. The recovery of infectious virus was higher in symptomatic when compared to asymptomatic vaccinated individuals, but localized SARS-CoV-2 IgG levels were comparable between symptomatic and asymptomatic cases, indicating that localized antiviral IgG levels do not drive higher asymptomatic infection rates.
Data from the CDC showed that the widely used mRNA vaccines in the US reduce the infection risk by 91% and data from different groups confirm that breakthrough infections after full vaccination were scarce prior to the surge of the Delta variant [14], [15], [16]. Data also show that vaccines reduce symptomatic and asymptomatic infections and RNA loads [17], [18], [19], [20] and reduce viral replication in the respiratory tracts of animals [21], [22], [23].
The emergence of SARS-CoV-2 variants of concern and interest were associated with changes in the spike protein within regions that could affect the receptor binding domain or impact the neutralization of the virus by natural or vaccine induced immune responses [24], [25], [26], [27]. Those variants were associated with an increase in transmissibility and in particular the S: E484K substitution was associated with a compromise in the neutralization by monoclonal antibodies rendering this change “of therapeutic concern”. The S: E484K independently emerged in multiple lineages in distant geographical locations including the Gamma and the Beta and those lineages showed some reduction in neutralization by sera collected from immunized individuals as well as decreased susceptibility to certain therapeutic monoclonal antibodies. Additionally, the Gamma and Beta were associated with reductions in the vaccine efficacy data in locations of their predominance [28, 29]. The S: E484K is also present in some strains of lineage B.1.526, a lineage which was significantly associated with positives after vaccination in our cohort, even though in a previous study, it was not reported to associate with positives after vaccination [30]. Our study shows that the S: E484K is significantly associated with breakthrough cases after vaccination in a well-controlled analysis that used a large cohort of controls from a matched time frame of sample collection.
In conclusion, our study combined genomic analysis, cell culture, and serology to correlate reduced recovery of infectious virus from positives after vaccination with increased localized IgG levels during the first few months after the COVID-19 vaccination rollout. Our data showed a significant association of S: E484K with positives after full vaccination using a well-controlled analysis and a relatively large sample size in a time when the Alpha variant predominated.
Data sharing
Whole genome data were made available publicly and raw genomic data requests could be directed to HHM.
Funding
NIH/NIAID Center of Excellence in Influenza Research and Surveillance contract HHS N2772201400007C, Johns Hopkins University, Maryland department of health, Centers for Disease Control and Prevention contract 75D30121C11061.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was only possible with the unique efforts of the Johns Hopkins Clinical Microbiology Laboratory faculty and staff. We also thank the Johns Hopkins Immunology Laboratory and Kim Jeemin for technical assistance. HHM is supported by the HIV Prevention Trials Network (HPTN) sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), National Institute on Drug Abuse, National Institute of Mental Health, and Office of AIDS Research, of the NIH, DHHS (UM1 AI068613), the NIH RADx-Tech program (3U54HL143541–02S2), National Institute of Health RADx-UP initiative (Grant R01 DA045556–04S1), National Institute of Allergy and Infectious Diseases (Johns Hopkins Center of Excellence in Influenza Research and Surveillance HHSN272201400007C), Johns Hopkins University President's Fund Research Response, the Johns Hopkins Department of Pathology, the Maryland Department of Health, and the CDC. This research was supported in part by the intramural research program of the National Institutes of Health. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Institute of Biomedical Imaging and Bioengineering; the National Heart, Lung, and Blood Institute; the National Institutes of Health, or the U.S. Department of Health and Human Services.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcv.2022.105151.
Appendix. Supplementary materials
References
- 1.Frenck R.W., Jr., Klein N.P., Kitchin N., Gurtman A., Absalon J., Lockhart S., Perez J.L., Walter E.B., Senders S., Bailey R., Swanson K.A., Ma H., Xu X., Koury K., Kalina W.V., Cooper D., Jennings T., Brandon D.M., Thomas S.J., Tureci O., Tresnan D.B., Mather S., Dormitzer P.R., Sahin U., Jansen K.U., Gruber W.C., Group CCT Safety, immunogenicity, and efficacy of the BNT162b2 covid-19 vaccine in adolescents. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T., Group C.S. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., Hernan M.A., Lipsitch M., Reis B., Balicer R.D. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu-Raddad L.J., Chemaitelly H., Butt A.A., National Study Group for C-V. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N. Engl. J. Med. 2021 doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woldemeskel B.A., Garliss C.C., Blankson J.N. SARS-CoV-2 mRNA vaccines induce broad CD4+ T cell responses that recognize SARS-CoV-2 variants and HCoV-NL63. J. Clin. Invest. 2021:131. doi: 10.1172/JCI149335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thielen P.M., Wohl S., Mehoke T., Ramakrishnan S., Kirsche M., Falade-Nwulia O., Trovao N.S., Ernlund A., Howser C., Sadowski N., Morris C.P., Hopkins M., Schwartz M., Fan Y., Gniazdowski V., Lessler J., Sauer L., Schatz M.C., Evans J.D., Ray S.C., Timp W., Mostafa H.H. Genomic diversity of SARS-CoV-2 during early introduction into the Baltimore-Washington metropolitan area. JCI Insight. 2021;6 doi: 10.1172/jci.insight.144350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarrett J., Uhteg K., Forman M.S., Hanlon A., Vargas C., Carroll K.C., Valsamakis A., Mostafa H.H. Clinical performance of the GenMark Dx ePlex respiratory pathogen panels for upper and lower respiratory tract infections. J. Clin. Virol. 2021;135 doi: 10.1016/j.jcv.2021.104737. [DOI] [PubMed] [Google Scholar]
- 8.Mostafa H.H., Carroll K.C., Hicken R., Berry G.J., Manji R., Smith E., Rakeman J.L., Fowler R.C., Leelawong M., Butler-Wu S.M., Quintero D., Umali-Wilcox M., Kwiatkowski R.W., Persing D.H., Weir F., Loeffelholz M.J. Multi-center evaluation of the cepheid Xpert(R) Xpress SARS-CoV-2/Flu/RSV Test. J. Clin. Microbiol. 2020 doi: 10.1128/JCM.02955-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mostafa H.H., Hardick J., Morehead E., Miller J.A., Gaydos C.A., Manabe Y.C. Comparison of the analytical sensitivity of seven commonly used commercial SARS-CoV-2 automated molecular assays. J. Clin. Virol. 2020;130 doi: 10.1016/j.jcv.2020.104578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uhteg K., Jarrett J., Richards M., Howard C., Morehead E., Geahr M., Gluck L., Hanlon A., Ellis B., Kaur H., Simner P., Carroll K.C., Mostafa H.H. Comparing the analytical performance of three SARS-CoV-2 molecular diagnostic assays. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris C.P., Luo C.H., Amadi A., Schwartz M., Gallagher N., Ray S.C., Pekosz A., Mostafa H.H. An Update on SARS-CoV-2 Diversity in the United States national capital region: evolution of novel and variants of concern. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gniazdowski V., Morris C.P., Wohl S., Mehoke T., Ramakrishnan S., Thielen P., Powell H., Smith B., Armstrong D.T., Herrera M., Reifsnyder C., Sevdali M., Carroll K.C., Pekosz A., Mostafa H.H. Repeat COVID-19 molecular testing: correlation of SARS-CoV-2 culture with molecular assays and cycle thresholds. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo C.H., Morris C.P., Sachithanandham J., Amadi A., Gaston D.C., Li M., Swanson N.J., Schwartz M., Klein E.Y., Pekosz A., Mostafa H.H. Infection with the SARS-CoV-2 delta variant is associated with higher recovery of infectious virus compared to the alpha variant in both unvaccinated and vaccinated individuals. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobson K.B., Pinsky B.A., Rath M.E.M., Wang H., Miller J.A., Skhiri M., Shepard J., Mathew R., Lee G., Bohman B., Parsonnet J., Holubar M. Post-vaccination SARS-CoV-2 infections and incidence of the B.1.427/B.1.429 variant among healthcare personnel at a northern California academic medical center. medRxiv. 2021 doi: 10.1101/2021.04.14.21255431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haas E.J., Angulo F.J., McLaughlin J.M., Anis E., Singer S.R., Khan F., Brooks N., Smaja M., Mircus G., Pan K., Southern J., Swerdlow D.L., Jodar L., Levy Y., Alroy-Preis S. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angel Y., Spitzer A., Henig O., Saiag E., Sprecher E., Padova H., Ben-Ami R. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA. 2021 doi: 10.1001/jama.2021.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swift M.D., Breeher L.E., Tande A.J., Tommaso C.P., Hainy C.M., Chu H., Murad M.H., Berbari E.F., Virk A. Effectiveness of mRNA COVID-19 vaccines against SARS-CoV-2 infection in a cohort of healthcare personnel. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson M.G., Burgess J.L., Naleway A.L., Tyner H.L., Yoon S.K., Meece J., Olsho L.E.W., Caban-Martinez A.J., Fowlkes A., Lutrick K., Kuntz J.L., Dunnigan K., Odean M.J., Hegmann K.T., Stefanski E., Edwards L.J., Schaefer-Solle N., Grant L., Ellingson K., Groom H.C., Zunie T., Thiese M.S., Ivacic L., Wesley M.G., Lamberte J.M., Sun X., Smith M.E., Phillips A.L., Groover K.D., Yoo Y.M., Gerald J., Brown R.T., Herring M.K., Joseph G., Beitel S., Morrill T.C., Mak J., Rivers P., Harris K.M., Hunt D.R., Arvay M.L., Kutty P., Fry A.M., Gaglani M. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers - eight U.S. locations, December 2020-March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang L., Hijano D.R., Gaur A.H., Geiger T.L., Neufeld E.J., Hoffman J.M., Hayden R.T. Asymptomatic and symptomatic SARS-CoV-2 infections after BNT162b2 vaccination in a routinelyscreened workforce. JAMA. 2021 doi: 10.1001/jama.2021.6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine-Tiefenbrun M., Yelin I., Katz R., Herzel E., Golan Z., Schreiber L., Wolf T., Nadler V., Ben-Tov A., Kuint J., Gazit S., Patalon T., Chodick G., Kishony R. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat. Med. 2021;27:790–792. doi: 10.1038/s41591-021-01316-7. [DOI] [PubMed] [Google Scholar]
- 21.Corbett K.S., Flynn B., Foulds K.E., Francica J.R., Boyoglu-Barnum S., Werner A.P., Flach B., O'Connell S., Bock K.W., Minai M., Nagata B.M., Andersen H., Martinez D.R., Noe A.T., Douek N., Donaldson M.M., Nji N.N., Alvarado G.S., Edwards D.K., Flebbe D.R., Lamb E., Doria-Rose N.A., Lin B.C., Louder M.K., O'Dell S., Schmidt S.D., Phung E., Chang L.A., Yap C., Todd J.M., Pessaint L., Van Ry A., Browne S., Greenhouse J., Putman-Taylor T., Strasbaugh A., Campbell T.A., Cook A., Dodson A., Steingrebe K., Shi W., Zhang Y., Abiona O.M., Wang L., Pegu A., Yang E.S., Leung K., Zhou T., Teng I.T., Widge A., et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N. Engl. J. Med. 2020;383:1544–1555. doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mercado N.B., Zahn R., Wegmann F., Loos C., Chandrashekar A., Yu J., Liu J., Peter L., McMahan K., Tostanoski L.H., He X., Martinez D.R., Rutten L., Bos R., van Manen D., Vellinga J., Custers J., Langedijk J.P., Kwaks T., Bakkers M.J.G., Zuijdgeest D., Rosendahl Huber S.K., Atyeo C., Fischinger S., Burke J.S., Feldman J., Hauser B.M., Caradonna T.M., Bondzie E.A., Dagotto G., Gebre M.S., Hoffman E., Jacob-Dolan C., Kirilova M., Li Z., Lin Z., Mahrokhian S.H., Maxfield L.F., Nampanya F., Nityanandam R., Nkolola J.P., Patel S., Ventura J.D., Verrington K., Wan H., Pessaint L., Van Ry A., Blade K., Strasbaugh A., Cabus M., et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586:583–588. doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogel A.B., Kanevsky I., Che Y., Swanson K.A., Muik A., Vormehr M., Kranz L.M., Walzer K.C., Hein S., Guler A., Loschko J., Maddur M.S., Ota-Setlik A., Tompkins K., Cole J., Lui B.G., Ziegenhals T., Plaschke A., Eisel D., Dany S.C., Fesser S., Erbar S., Bates F., Schneider D., Jesionek B., Sanger B., Wallisch A.K., Feuchter Y., Junginger H., Krumm S.A., Heinen A.P., Adams-Quack P., Schlereth J., Schille S., Kroner C., de la Caridad Guimil, Garcia R., Hiller T., Fischer L., Sellers R.S., Choudhary S., Gonzalez O., Vascotto F., Gutman M.R., Fontenot J.A., Hall-Ursone S., Brasky K., Griffor M.C., Han S., Su A.A.H., Lees J.A., et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592:283–289. doi: 10.1038/s41586-021-03275-y. [DOI] [PubMed] [Google Scholar]
- 24.Galloway S.E., Paul P., MacCannell D.R., Johansson M.A., Brooks J.T., MacNeil A., Slayton R.B., Tong S., Silk B.J., Armstrong G.L., Biggerstaff M., Dugan V.G. Emergence of SARS-CoV-2 B1.1.7 Lineage - United States, December 29, 2020-January 12, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:95–99. doi: 10.15585/mmwr.mm7003e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., Pearson C.A.B., Russell T.W., Tully D.C., Washburne A.D., Wenseleers T., Gimma A., Waites W., Wong K.L.M., van Zandvoort K., Silverman J.D., Group C.C.-.W., Consortium C.-.G.U., Diaz-Ordaz K., Keogh R., Eggo R.M., Funk S., Jit M., Atkins K.E., Edmunds W.J. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372 doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., Wang M., Yu J., Zhang B., Kwong P.D., Graham B.S., Mascola J.R., Chang J.Y., Yin M.T., Sobieszczyk M., Kyratsous C.A., Shapiro L., Sheng Z., Huang Y., Ho D.D. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 27.Wang P., Casner R.G., Nair M.S., Wang M., Yu J., Cerutti G., Liu L., Kwong P.D., Huang Y., Shapiro L., Ho D.D. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. Cell Host Microbe. 2021;29:747–751. doi: 10.1016/j.chom.2021.04.007. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadoff J., Gray G., Vandebosch A., Cardenas V., Shukarev G., Grinsztejn B., Goepfert P.A., Truyers C., Fennema H., Spiessens B., Offergeld K., Scheper G., Taylor K.L., Robb M.L., Treanor J., Barouch D.H., Stoddard J., Ryser M.F., Marovich M.A., Neuzil K.M., Corey L., Cauwenberghs N., Tanner T., Hardt K., Ruiz-Guinazu J., Le Gars M., Schuitemaker H., Van Hoof J., Struyf F., Douoguih M., Group E.S. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N. Engl. J. Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S., Briner C., Cicconi P., Collins A.M., Colin-Jones R., Cutland C.L., Darton T.C., Dheda K., Duncan C.J.A., Emary K.R.W., Ewer K.J., Fairlie L., Faust S.N., Feng S., Ferreira D.M., Finn A., Goodman A.L., Green C.M., Green C.A., Heath P.T., Hill C., Hill H., Hirsch I., Hodgson S.H.C., Izu A., Jackson S., Jenkin D., Joe C.C.D., Kerridge S., Koen A., Kwatra G., Lazarus R., Lawrie A.M., Lelliott A., Libri V., Lillie P.J., Mallory R., Mendes A.V.A., Milan E.P., Minassian A.M., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson C.N., Hughes S., Ngai S., Baumgartner J., Wang J.C., McGibbon E., Devinney K., Luoma E., Bertolino D., Hwang C., Kepler K., Del Castillo C., Hopkins M., Lee H., DeVito A.K., Rakeman J.L., PhD, Fine A.D. Rapid emergence and epidemiologic characteristics of the SARS-CoV-2 B1.526 Variant - New York City, New York, January 1-April 5, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:712–716. doi: 10.15585/mmwr.mm7019e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.