Abstract
Coronaviruses have been responsible for major epidemic crises in 2003 with SARS-CoV-1, in 2012 with MERS-CoV and in 2019 with SARS-CoV-2 (COVID-19), causing serious atypical pneumonia in humans. We intend, with this systematic analysis and meta-analysis, to clarify the prevalence of the various strains of coronavirus in different animal species. For this purpose, we carried out an electronic survey using Pubmed's Veterinary Science search tool to conduct a systematic assessment of published studies reporting the prevalence of different strains of coronavirus in different animal species between 2015 and 2020. We conducted different analysis to assess sensitivity, publication bias, and heterogeneity, using random effect. The final meta-analysis included 42 studies for systematic review and 29 in the meta-analysis. For the geographic regions with a prevalence greater than or equal to 0.20 (Forest plot overall; prevalence = 0.20, p < 0.01, Q = 10,476.22 and I2 = 100%), the most commonly detected viruses were: enteric coronavirus (ECoV), pigeon-dominant coronavirus, (PdCoV), Avian coronavirus M41, Avian coronavirus C46, Avian coronavirus A99, Avian coronavirus JMK, MERS-CoV, Bovine coronavirus, Ro-BatCoV GCCDC1, Alphacoronavirus, Betacoronavirus, Deltacoronavirus, Gamacoronavirus and human coronaviruses (HCoVs). The wide presence of different strains of coronavirus in different animal species on all continents demonstrates the great biodiversity and ubiquity of these viruses.
The most recent epidemiological crises caused by coronavirus demonstrates our unpreparedness to anticipate and mitigate emerging risks, as well as the need to implement new epidemiological surveillance programs for viruses. Combined with the need to create advanced training courses in One Health, this is paramount in order to ensure greater effectiveness in fighting the next pandemics.
Keywords: Coronavirus, One health, Zoonosis
1. Introduction
Coronavirus disease 2019 (COVID-19) is the most recent viral pandemic event in recent years associated with the Coronoviridae family, with SARS-CoV-2 being its seventh member [1]. The Coronaviridae comprise two subfamilies, including Coronavirinae, whose members are commonly referred to as coronaviruses (CoVs).
The outbreak was thought to have originated in Wuhan, spread rapidly to neighbouring provinces and, within three months, a pandemic was declared. Cases have been reported in every region of the world, with a high number of infections and deaths. New origin hypotheses, however, have been advanced in more recent studies [2].
Research studies indicate that 72% of events arising from zoonotic diseases originate from wildlife. Many of these diseases pose serious risks to human health, as demonstrated by the 2014 Ebola virus in West Africa, MERS-CoV in the Middle East in 2012 [3], SARS-CoV detected in 2002 in China and H5N1 in 2004. The existence of markets for trade of live animals brings wildlife closer to humans and domestic animals. These places of commerce have a potential role as an interface for the transmission of pathogens. This interface can contribute to the emergence, and the spread of a range of diseases, including pathogenic avian influenza H5N1, and Severe Acute Respiratory Syndrome (SARS) [4,5]. According to Leroy [2], since genetic recombination events within human CoVs are well documented, the known high prevalence of dog infections with canine coronaviruses in Europe might foster recombination with SARS-CoV-2 if an animal would be infected with both viruses. Such an event, if it happens, could lead to the emergence of a new coronavirus with unpredictable phenotypic characteristics (transmissibility and virulence). Unfortunately, the likelihood of such a scenario is difficult to assess. Hopefully, although genetic recombination documented for animal CoVs, for instance as feline and canine coronaviruses do not spread from cats. Investigations into homologous recombination of CoVs may help to clarify the mechanisms responsible for changes in host range [10].
The coronavirus is well known in the world of veterinary medicine. A translation of this experience can be very beneficial for human health [6,7]. For this purpose, One Health appears as an important concept. It is a new approach that is based on the relationship between humans, animals and the environment, and recognizes that the health and well-being of human beings is strongly related to the health of animals and their environment [3]. The risk of SARS-CoV-2 transmission to pets and other wild and domestic animals and SARS-CoV-2 potential for unknown animal reservoir hosts and public health implications strongly mandates a one-health strategy to control the COVID-19 and prevent future pandemics [42].
Belonging to the Coronaviridae family, in the order Nidovirales, the SARS-CoV-2 exhibits a genome of positive-sense, single-stranded, polyadenylated, nonsegmented RNA [8]. In order to anticipate the importance and evolution of the coronavirus, a broader point of view is needed to understand the behaviour of Coronaviridae. To date, from the seven coronaviruses reported in humans, four of them are ubiquitous with seasonal circulation and mostly causing relatively mild colds (HKU1, NL63, OC43 and 229E). The other three of more recent zoonotic origin, are associated with severe acute respiratory syndromes, namely SARS-CoV, MERS-CoV and now SARS-CoV-2. Of these seven human coronaviruses, NL63 and 229E belong to the alpha-CoV genus, while the other five are included within the beta-CoV genus. Coronaviruses detected in dogs and cats also belong to these two viral genera [9,10]. Like SARS-CoV-2 and the other respiratory syndrome viruses, the canine respiratory coronavirus (CRCoV), responsible for a respiratory condition in dogs, belongs to the beta-CoV genus. Canine coronavirus (CCoV) and Feline coronavirus (FCoV), both responsible for digestive diseases, belong to Alphacoronavirus.
Therefore, the study of the prevalence of different strains of coronaviruses in different animal species in the world provides important information for the implementation of surveillance strategies, as well as epidemiological and preventive public health policies [11,12].
2. Materials and methods
A systematic assessment of published studies reporting coronaviruses prevalence among different animal species and human, was performed based on PRISMA recommendation [13]. For this purpose, we used the Veterinary Science search tool at PubMed to retrieves published studies, combining different subject search terms, with filtered temporal delimitation in years, between 2015 and 2020.
2.1. Search strategy
The search strategy used was: veterinary[sb] AND ((“coronavirus”[MeSH Terms] OR “coronavirus”[All Fields]) AND (“one health”[MeSH Terms] OR (“one”[All Fields] AND “health”[All Fields]) OR “one health”[All Fields])) AND (“2015/04/18”[PDat]: “2020/04/15”[PDat]).
2.2. Article selection
The initial selection by title and abstract was conducted independently by two researchers according to the inclusion/exclusion criteria. In the selection of the titles, we included all those that presented one or more terms with a coronavirus and one health relationship. In a second phase, an exhaustive reading of the articles was carried out to confirm the presence of relevant data to include them in the systematic review and prevalence values for the meta-analysis. Discrepancies in the final decision to include or not an article were discussed with a third investigator to reach a consensus. The PRISMA model was used to organize the information resulting from the article selection process.
2.3. Inclusion and exclusion criteria
Only articles dealing with issues related to the coronavirus, both in the scope of human medicine and veterinary medicine, were considered eligible for a One Health approach. The main exclusion criteria were observed: articles published before 2015; studies unrelated to coronavirus; meta-analyses with data from sources that were not previously published articles with peer review and articles whose full text was not found. Studies without virus prevalence values. Studies related to drug development or optimization of new laboratory techniques were also excluded.
Due to the urgency of the situation and the state of the pandemic, studies with indications of public health policies were maintained, as well as other scientific data considered relevant to the discussion.
2.4. Data extraction
Quantitative and qualitative data extraction from the included studies was performed into four word table and an Excel spreadsheet, containing the following information: author name, year of publication, PubMed article link, article title, animal species, materials and methods, study location, and important note. During the data extraction process, information was extracted by one author and validated by a second author. Disagreements were resolved by discussion and consultation with a third author, whenever necessary.
2.5. Quality assessment
In the evaluation of quality, an instrument adapted from the 22 criteria proposed by the STROBE Statement was used, in compliance with the principles of epidemiological investigation. This assessment aimed to classify the relevance of the articles. The One Health/ERISA evaluation scale, consisting of 15 items to evaluate the articles with regards to the existence of relevant information for the definition of novel One Health recommendations and policies.
2.6. Data analysis
For the statistical analysis, data were stored in a predefined spread sheet file, including the authors and year of publication, number of animals and the number of infected animals.
Data were analysed using MetaXL version 5.3 software, an add-in for meta-analysis in Microsoft Excel for Windows (https://www.epigear.com/index_files/metaxl.html). The calculated results were represented in table and graphical formats. The heterogeneity across studies was evaluated by Cochrane's Q test and I2 statistics. The calculated value of I2 allows measuring the percentage of variability due to heterogeneity, rather than chance difference or sampling error. If the value of I2 was greater than 50% and the Q test yields P < 0.10, heterogeneity was considered statistically significant. The random effects model, based on DerSimonian-Laird method, which calculates the variability within and between studies, was applied to estimate the pooled prevalence and 95% CIs. The transformed double arcsine method was used for situations where the confidence limits and variance instability could appear due to any single studies with larger or small prevalence rates. The Luis Furuya-Kanamori asymmetry index (LFK index) and the Doi plot were calculated to estimate the publication bias. The presence of symmetry indicates no publication bias. The publication bias was determinate by LFK index, which can take the following assessments depending on the value obtained: no asymmetry if the LFK index is within ±1, minor asymmetry when out of the ±1 interval, but within ±2, and major asymmetry if the LFK index is beyond the ±2 interval. The LFK index for the general metanalysis is 3.71 (major asymmetry), for the mammals class it is 4.23 (major asymmetry), and for the birds class it is 0.34 (no asymmetry).
A sensitivity test was calculated to provide an indication of which study is the prime determinant of the pooled result, and which is the main source of heterogeneity. The test rejects each study, one by one, in the analysis performed, so that it is possible to indicate the combined effect sizes as well as the associated heterogeneity.
3. Results
3.1. Study selection
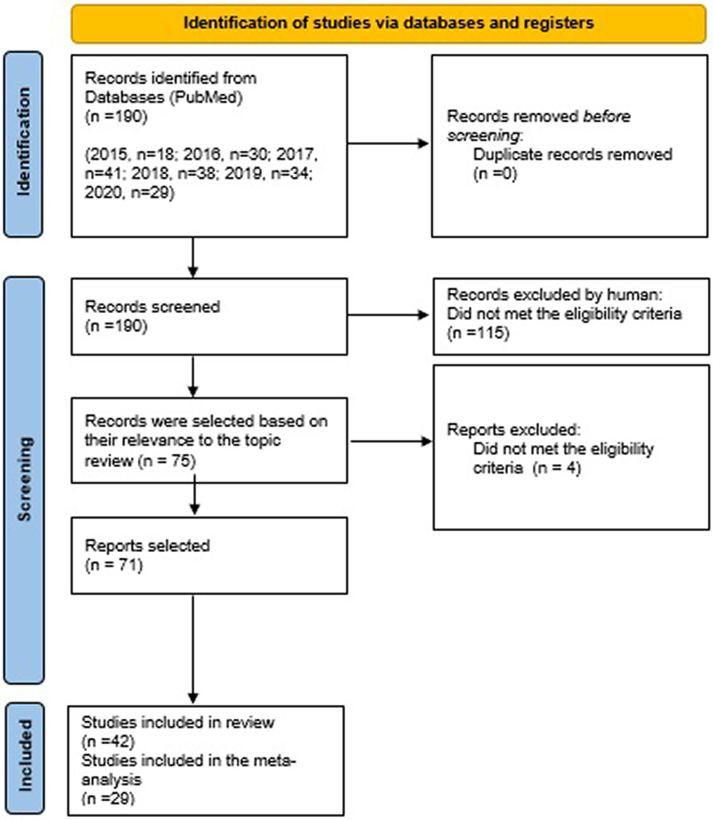
A total of 115 studies based on the title and abstract were excluded, the remaining 75 studies were selected for the continuation of our study, and 4 studies were subsequently excluded after full reading (2 studies on bibliometrics, 1 study with lack of data and 1 study did not fit the objectives of our study). Of the 71 studies considered eligible, 42 studies were considered for systematic review and 29 studies were included in the meta-analysis (Fig. 1).
Fig. 1.
Flow chart of systematic review process.
3.2. Quality assessment
From the one health evaluation scale, we obtained an average score of 9.33 points (62%), the scores of the articles evaluated varied between a minimum of 6 points (40%) and a maximum of 12 points (80%) in a total of 15 points (100%) possible. The following distribution of studies in relation to the average score values, obtained for each of them were: 2 studies had a score of 12 points (80%), 8 studies had a score of 11 points (73.33%), 11 studies had a score of 10 points (66.67%), 8 studies had a score of 9 points (60%), 4 studies had a score of 8 points (50.33%), 7 studies had a score of 7 points (46,67%) and one study had a score of 6 points (40%). Results are shown in Table 1.
Table 1.
Description and quality assessment of studies with information for the definition of new One Health (OH) measures and policies.
| Recommended measures |
||||||
|---|---|---|---|---|---|---|
| Studies | Local | Tipology | Epidemiology | Health policies | Species | OH Score (max 15) |
| Goodrich EL, et al., 2020 | USA | Case report | yes | No | Horse/Donkeys | 12 |
| Yadav PD et al., 2020 | India | Original | no | No | Bat | 10 |
| Leroy EM et al., 2020 | n.a | Editorial commentary | yes | No | Dogs/cats | 11 |
| Sun J et al., 2020 | China | Review | yes | No | Human | 11 |
| Foddai A et al., 2020 | world | Editorial Commentary | Yes | Yes | Human/animal | 10 |
| Foddai A et al. 2020 | world | methodology | Yes | Yes | Human/animal | 7 |
| ValituttoI et al., 2020 | Myanmar | original | Yes | No | Bat | 9 |
| Qingye Zhuang et al., 2020 | China | Original | Yes | No | Pigeons | 6 |
| Roya Mohammadpour et al., 2020 | Iran | Review | Yes | Yes | Camels | 8 |
| Masashi YAMADA et al., 2020 | Japan | Original | Yes | Yes | Calves | 7 |
| Nziza J, Goldstein T, Cranfield M, et al., 2020 | Rwanda | Original | Yes | Yes | Bat | 11 |
| Canuti M et al., 2019 | Canada | Original | Yes | No | Gull | 7 |
| Markotter W et al., 2019 | Rwanda | Original | Yes | Yes | Bat | 9 |
| Uhart M et al., 2019 | Argentina | Original | Yes | No | Magellanic Penguins | 9 |
| Maboni G et al., 2019 | USA | Research | Yes | Yes | Dog | 10 |
| Farag E et al., 2019 | Quatar | – | Yes | Yes | Human and camels | 11 |
| Skariyachan S et al., 2019 | World | Review | Yes | Yes | More than one species | 10 |
| Farag EAB et al., 2019 | Gulf Cooperation Council countries | Online report | Yes | Yes | Human and camels | 7 |
| Ommeh S et al., 2018 | Kenya | Research | Yes | No | Camels Human |
11 |
| Dortmans JCFM et al., 2018 | Netherlands | Original | Yes | Yes | Dutch pigs Wild boar |
12 |
| David D et al., 2018 | Israel | Original | Yes | No | Alpacas Llamas |
10 |
| de Mira Fernandes A et al., 2018 | Brazil | Original | Yes | No | Calves | 9 |
| Bailey ES et al. 2018 | World | Review | Yes | No | More than one species | 7 |
| Obameso JO et al., 2017 | China | Research | Yes | No | Bat | 9 |
| Rizzo F et al., 2017 | Italy | Research | Yes | Yes | Bat | 10 |
| Lee S et al., 2017 | South Korea | Research | Yes | Yes | Bat | 7 |
| Hemida MG et al., 2017 | Saudi Arabia | Original | Yes | Yes | dromedary camel | 11 |
| Reperant LA et al., 2017 | World | Review | Yes | Yes | Human | 8 |
| Falzarano D et al., 2017 | Mali | Original | Yes | No | Dromedary camels | 8 |
| Lu S et al., 2017 | China | Original | Yes | No | Dog | 7 |
| Fish EJ et al., 2017 | USA | Original | Yes | No | Cat | 8 |
| Domańska-Blicharz K et al., 2017 | Poland | Research | Yes | No | Turkey | 9 |
| Saqib M et al., 2017 | Pakistan | Research letters | Yes | Yes | Dromedary Camels | 11 |
| Lacroix A et al., 2017 | Lao PDR Cambodia | Research | Yes | Yes | Bat | 10 |
| Torres CA et al., 2016 | Brasil | Original | Yes | Yes | Quail Chicken |
9 |
| Corman VM et al., 2016 | Middle East: KSA, UAE Africa: Kenya, Somalia, Sudan,Egypt |
Original | Yes | Yes | dromedary camels | 10 |
| Asano KM et al., 2016 | Brazil | Short report | Yes | Yes | Bat | 10 |
| Wille M et al., 2016 | Sweden | Research | Yes | Yes | Scandinavian Waterfowl | 10 |
| Liu L et al., 2016 | World | Perspective | Yes | Yes | Human | 11 |
| Ge XY et al., 2016 | China | Research | Yes | Yes | Bat | 10 |
| Crameri G et al., 2015 | Australia | Original | Yes | Yes | feral camels | 11 |
| Müller MA et al. 2015 | Saudi Arabia | Research | Yes | Yes | Human | 9 |
The origin of the studies carried out was as follows: three in the USA, five in China, three in Brazil, two in Saudi Arabia, one in Myanmar, one in Iran, one in Japan, one in Rwanda, one in Canada, one in Argentina, one in Qatar, one in Gulf Cooperation Council countries, one in Kenya, one in Netherlands, one in Israel, one in Italy, one in South Korea, one in Mali, one in Poland, one in Pakistan, one in Lao PDR, one in Cambodia, a joint study in Middle East (KSA, UAE, Africa: Kenya, Somalia, Sudan, Egypt), one in Sweden, one in Australia and 6 global studies (“world”) (see Table 1).
Of the 42 studies selected for the systematic review, thirty-eight (35) contained information on epidemiological data (one study without epidemiological data), twenty-five studies (25) had relevant data for the implementation of One Health policies.
3.3. Animal species, coronavirus strains and laboratory tests
The infected animal species described in the studies on the prevalence of different strains of coronavirus in the world were as follows: horses, donkeys, bats (various species), dogs, cats, human, bat, pigeons, camels (more than one species) calves, gull, Magellanic penguins, Dutch pigs, wild boar, alpacas, Llamas, dromedary camel, turkey, quail, chicken, Scandinavian waterfowl and feral camels. The respective identified strains of coronavirus can be found in Table 1, Table 2.
Table 2.
Characterization of studies according to the infected animal species and coronavirus.
| Population |
||||||
|---|---|---|---|---|---|---|
| Studies | Species | Coronavirus | n | n positive | Lab Technique | Prevalence |
| Yadav PD et al., 2020 | Bat (Pteropus) | BtCoV | 508 | 21 | RT-PCR | 4.13 |
| Bat (Rousettus) | BtCoV | 78 | 4 | RT-PCR | 5.13 | |
| Goodrich EL, et al., 2020 | AMH* and donkeys | BCoV/ECov | 30 | 25 | ||
| ValituttoI et al., 2020 | Bat** | PREDICT_CoV-35,47,82,92,93,96 | 464 | 7 | PCR | 1.5 |
| Qingye Zhuang et al., 2020 | Pigeons*** | CdCoV | 687 | 19 | RT-PCR | 2.77 |
| DdCoV | 687 | 6 | RT-PCR | 0.87 | ||
| PdCoV | 687 | 159 | RT-PCR | 23.14 | ||
| Roya Mohammadpour et al., 2020 | Camels | MERS-CoV*4 | 18 | 3 | Serology | 16.66 |
| 186 | 8 | Serology | 4.30 | |||
| 98 | 7 | RT-PCR | 7.14 | |||
| Masashi YAMADA et al., 2020 | Calves | BCV | 88 | 1 | RT-PCR | 1.14 |
| Nziza J, Goldstein T, Cranfield M, et al., 2020 | Bat | CoV*5 | 503 | 27 | c-PCR | 5.4 |
| Canuti M et al., 2019 | great black-backed | GuCoV B29*6 | 26 | 3 | PCR | 11.5 |
| American herring gulls | 24 | 2 | PCR | 8.3 | ||
| Markotter W et al., 2019 | Bat | Rh-BtCoV/441/Rwanda/08 Rh- BtCoV/445/Rwanda/08 (Betacoronavirus) |
101 | 2 | RT-PCR | 1.9 |
| Uhart M et al., 2019 | Magellanic Penguins | Avian coronavirus M41 Avian coronavirus C46 Avian coronavirus A99 Avian coronavirus JMK |
393 | 171 235 147 158 |
serological test (hemagglutination inhibition) | 43.5 59.8 37.4 40.2 |
| Maboni G et al., 2019 | Dog | CoV | 559 | 26 | PCR | 4.6 |
| Pusterla N et al., 2019 | Horse | ECoV | 277 | 20 | qPCR | 7.2 |
| Ommeh S et al., 2018 | Turkana Rendille/Gabbra Somali (1) Improved/Pakistani Somali (2) Human |
MERS-CoV | 156 293 611 84 19 486 |
76 234 460 14 8 20 |
ELISA | 48.72 79.86 75.29 16.67 42.11 4.12 |
| Dortmans JCFM et al., 2018 | Dutch Pigs Wild boar |
Porcine epidemic diarrhea virus (PEDV) | 838 101 |
9 0 |
1.07 0 |
|
| David D et al., 2018 | Alpacas Llamas |
MERS-CoV | 102 19 |
35 7 |
ELISA | 34.3 36.8 |
| de Mira Fernandes A et al., 2018 | Calves | BCoV (Bovine coronaviru) | 44 70 |
10 7 |
22.72 10 |
|
| Obameso JO et al., 2017 | Bat | Ro-BatCoV GCCDC1 | 118 270 180 |
47 70 64 |
PCR | 39.8 38.8 35.6 |
| Rizzo F et al., 2017 | Bat | CoV | 302 | 36 | PCR | 12 |
| Lee S et al., 2017 | Bat | Bat-CoV-JTMC15 Bat-CoV-HKU5 Bat-CoV-SC2013 |
672 | 18 | RT-PCR | 2.7 |
| Falzarano D et al., 2017 | Dromedary camels | MERS-CoV | 570 | 502 | ELISA | 88 |
| Lu S et al., 2017 | Dog | CRCoV-BJ232*7 | 246 | 16 | RT-PCR | 6.5 |
| Fish EJ et al., 2017 | Cat | feline coronavirus (FCoV) | 205 | 9 | qRT-PCR | 4.4 |
| Domańska-Blicharz K et al., 2017 | Turkey | TCoV | 207 | 20 | RT-PCR | 9.8 |
| Domańska-Blicharz K et al., 2017 | Dromedary Camels | MERS-CoV | 565 | 315 | ELISA | 55.8 |
| Lacroix A et al., 2017 | Bat |
alpha-coronavirus (αCoV) strain HKU10, PREDICT-CoV-53 strains BatCoV Ratcha-67 BatCoV-25 PA201 beta-CoV(βCoV) PREDICT-CoV-22 strains PREDICT-CoV22, R91, R77, R74, R58 PREDICT-CoV-24 strains, R96, R75, R72, R65, R59, R71 PREDICT-CoV-34 strain, MERS-CoV JPDB144 BatCoV512_SL2-9_Pisp |
1965 | 93 | RT-PCR | 4.7 |
| Torres CA et al., 2016 | Quail Chicken |
Gammacoronavirus Deltacoronavirus | 60 30 |
28 6 |
RT-PCR | 46.6 20 |
| Corman VM et al., 2016 | dromedary camels | HCoV-229E | 364 | 150 | IFA | 41.2 |
| Asano KM et al., 2016 | Bat | Alphacoronavirus | 29 | 9 | RT-PCR | 31 |
| Wille M et al., 2016 | Scandinavian Waterfowl | Gammacoronavirus | 764 | 143 | QRT-PCR | 18.7 |
| Ge XY et al., 2016 | China | Alphacoronavirus Betacoronaviruse |
276 | 138 | RT-PCR | 50 |
| Müller MA et al. 2015 | Saudi Arabia | MERS-CoV | 10,009 | 15 | ELISA | 0.15 |
Note: *American Miniature Horse (AMH) of one farm in upstate New York. ECoV – enteric coronavirus.** 11 species across eight genera from six familie. PREDICT_CoV: unclassified Coronavirinae (Three novel alphacoronaviruses, three novel betacoronaviruses, and one known alphacoronavirus previously identified in the southeast Asian, were detected for the first time in bats in Myanmar).*** Viruses were organized into lineages based on phylogenetic analysis, and the CoVs dominant in chickens, ducks pigeons, and geese were named as pigeon-dominant coronavirus (PdCoV), chicken-dominant coronavirus (CdCoV), duck-dominant coronavirus (DdCoV) and geese-dominant coronavirus (GdCoV), respectively.*4: Khalaj, 2014a; Khalaj, 2014b and Khalili Bagaloy et al., 2017, respectively.*5 Known Coronaviruses Detected in Bats: 1) Strain of Kenya bat coronavirus/BtKY56/BtKY55, 2) Strain of Chaerephon bat/coronavirus/Kenya/KY22/2006, 3) Strain of Eidolon bat coronavirus/Kenya/KY24/2006, 4) Strain of Bat coronavirus HKU9; Novel Coronaviruses Detected in Bats: 1) PREDICT_CoV-42, 2) PREDICT_CoV-43, 3) PREDICT_CoV-44, 4) PREDICT_CoV-66.*6 The phylogenetic analyses of GuCoV B29 performed suggest that this virus could represent a novel species within the genus Gammacoronavirus.*7 the isolation of CRCoV-BJ232 failed on cell culture.
3.4. General analysis, Statistics, heterogeneity and publication bias
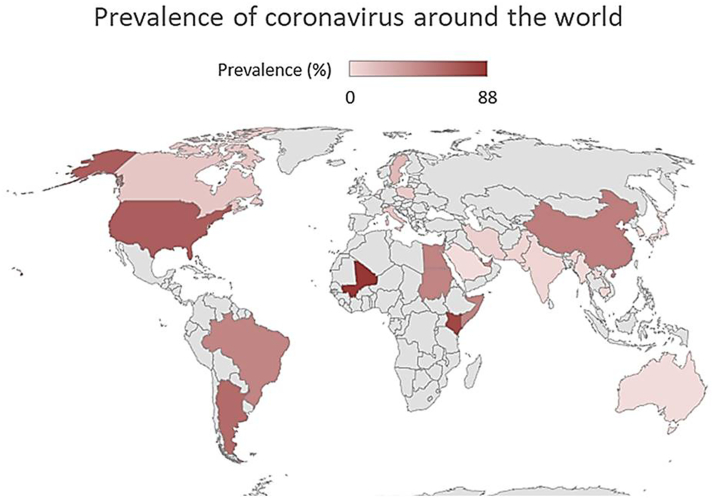
The prevalence values of the different strains of coronavirus identified in the infected animals and described in the studies selected for the meta-analysis were projected on a world map, where the red colour indicates the maximum prevalence value and the pink signifies the lowest value, see the Fig. 2.
Fig. 2.
Maximum prevalence of the different coronavirus strains for each country considered in this systematic review and metanalysis.
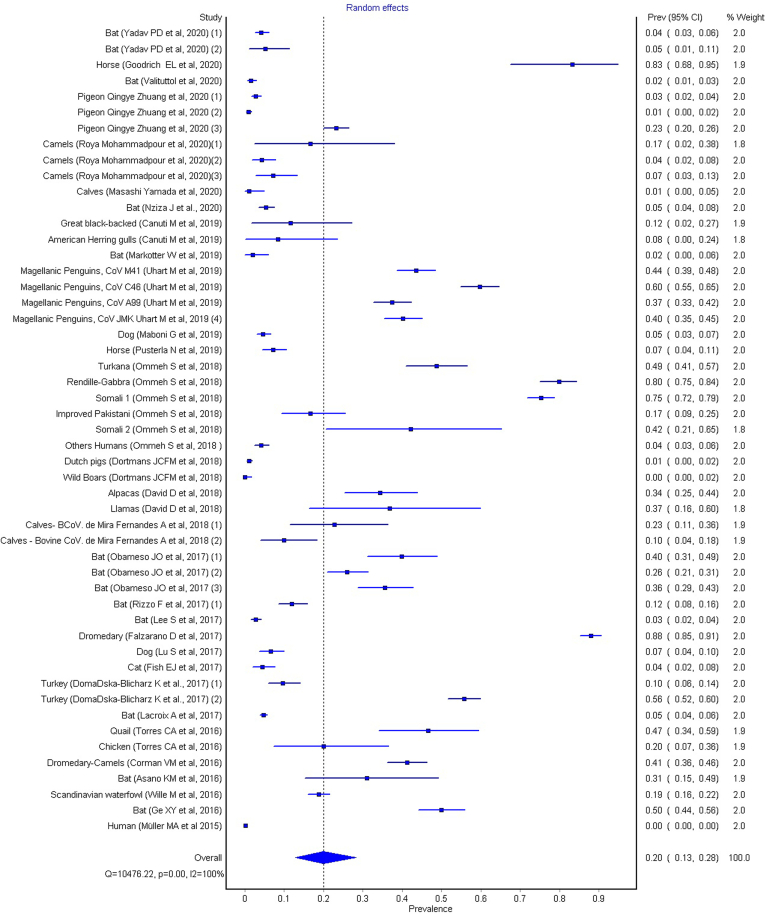
To calculate the meta-analysis of the general group, the random effect was calculated, and the summary measure (overall) of the meta-analysis, have a prevalence value of 0.20 (0.13–0.28, 95% CI), (see Fig. 3).
Fig. 3.
Global Results of metanalysis, fixed effects, heterogeneity, Q = 10,476.22, p = 0.00 and I2 = 100%.
The studies considered were assessed for heterogeneity through the Cochran Q test, calculated as the weighted sum of squared differences between individual study effects and the pooled effect across studies, with the weights being those used in the pooling method, and the results (Q = 10,476.22, p < 0.001) showed that there are variation in study outcomes between studies and variations are due to heterogeneity and not caused by chance. This was confirmed by the value of the I2 test (99.5%), suggesting a statistically significant heterogeneity. Unlike Q, I2 test does not inherently depend upon the number of studies considered. The value of τ2(0.473), which measures the estimated variation (heterogeneity) between the effects observed in different studies also supports the fact that effect sizes vary across studies.
3.5. Sensitivity analysis
A sensitivity analysis of the fifty-one studies was performed to evaluate the effect of each individual study on the pooled result. The results showed that the studies of Falzarano and Müller [14,15], were the prime determinants of the pooled result.
3.6. Analysis of subgroups – Taxonomic classes
Taking into account the great diversity of species found in this study, two groups were organized according to the taxonomic class: the Aves class and mammals. For both cases, the random effect and heterogeneity were calculated.
3.7. Subgroup analysis – Aves class
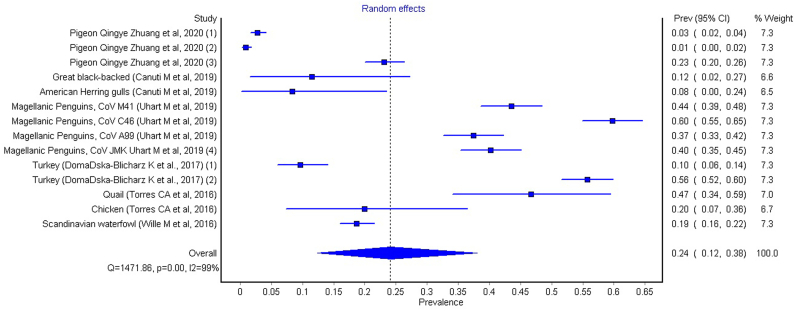
The species that constitute this subgroup are: Pigeon, Great Black-Backed, American Herring Gulls, Magellanic Penguins, Turkey, Quail, Chicken and Scandivian waterfowl. To calculate the meta-analysis of the Aves class of he random effect was calculated, having obtained an I2 of 99.117 (LCI95%: 98.934; HCI95%: 99.268), Cochran's Q test = 1471.863 and Chi2, p < 0.001.
The overall was calculated and the prevalence value is 0.24, with LCI95% of 0.12 and HCI95% of 0.38 (see Fig. 4).
Fig. 4.
Results of metanalysis for Aves Class, fixed effects, heterogeneity, Q = 1471,863, p = 0.00, I2 = 99.117.
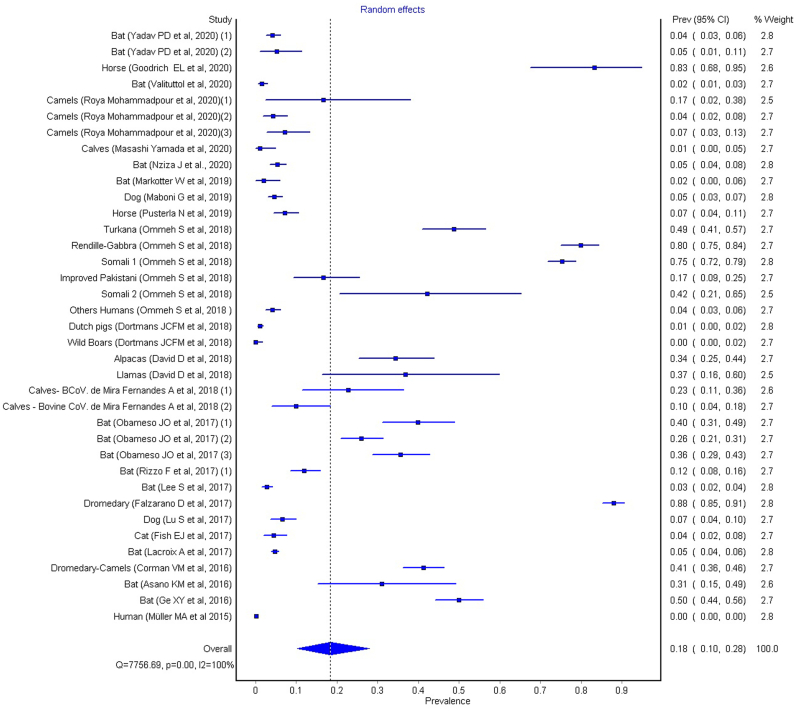
3.8. Subgroup analysis – Mammals class
The species that constitute this subgroup are: Bat, Dog, Horse, Dutch Pigs, Wild Boars, Alpacas, Llamas, Calves, Dromedary, Cat and Humans. To determine the meta-analysis of the Mammals class, the random effect was calculated, having obtained an I2 of 99.536 (LCI95%: 99.492; HCI95%: 99.576), Cochran's Q test = 7756.688 and Chi2, p < 0.001.
The overall was calculated and the prevalence value is 0.18, with LCI95% of 0.10 and HCI95% of 0.28 (see Fig. 5).
Fig. 5.
Results of metanalysis for Mammals Class, fixed effects, heterogeneity, Q = 7756.688, p = 0.00, I2 = 99.53.
The overall effects for both subgroups, aves and mammals, as well as for the whole sample, were calculated using the random effects model. These values are similar, with a prevalence value of 0.20 (0.13–0.28 for 95% CI) for all studies, and with prevalence values of 0.24 (0.12–0.38) for the aves subroup and a value of 0.18 (0.10–0.28) for the mammals subgroup. We can see that all effects can be considered significant, since none of the 95% confidence intervals shows a lower limit for the prevalence values below zero, therefore none of these intervals crosses the dividing line.
4. Discussion
4.1. Ubiquity of coronavirus
The results obtained in this systematic review and meta-analysis demonstrate a wide variety of coronaviruses capable of infecting a very wide range of animal species, namely vertebrates. It is possible over the last few decades to see coronavirus infections in several countries on all continents, with a globally endemic virus: Sweden, Italy, Holland, Poland, USA, Canada, Argentina, Brazil, Saudi Arabia, Myanmar, Iran, Rwanda, Middle East (KSA, United Arab Emirates, Africa: Kenya, Somalia, Sudan), Egypt, Qatar, Gulf Cooperation Council countries, Kenya, Israel, Mali, Pakistan, Japan, China, South Korea Lao PDR, Cambodia and Australia (see Table 1, Table 2).
4.2. Virological diversity
If we consider the geographic regions with a prevalence greater than or equal to 0.20 (Full metanalysis: Forest plot overall; prevalence = 0.20 (0.13–0.28 for 95% CI), p < 0.001, Q = 10,476.22 and I2 = 100%), we can highlight the following strains of coronavirus with the highest prevalence and confidence intervals (CI95%) (see Fig. 3): enteric coronavirus, ECoV, prevalence value of 0.83 (0.68–0.95 for 95% CI) (American Miniature Horse / USA) [16]; pigeon-dominant coronavirus, PdCoV, 0.23 (0.20–0.26) (pigeons / China) [17]; Avian coronavirus M41, 0.44 (0.39–0.48), Avian coronavirus C46, 0.37 (0.33–0.42), Avian coronavirus A99, 0.40 (0.35–0.45), Avian coronavirus JMK, 0.60 (0.55–0.65) (Magellanic Penguins / Argentina) [18]; MERS-CoV, 0.34 (0.25–0.44)/ 0.37 (0.16–0.60), (camels/ alpacas/ Israel), 0.88 (0.85–0.91) (llamas; dromedary camels/Mali); 0.80 (0.75–0.84) (llamas; dromedary camels Kenya) [19,20]; bovine coronavirus, 0.23 (0.11–0.36) (Calves / Brazil) [21]; Ro-BatCoV GCCDC1, 0.40 (0.31–0.49) (Bat / China) [22]; Gamacoronavirus, Deltacoronavirus, 0.47 (0.34–0.59) (Quail, Chiken / Brazil) [23]; Alphacoronavirus, 0.31 (0.15–0.49) (Bat / Brazil) [24]; Alphacoronavirus and Betacoronavirus, 0.50 (0.44–0.56) (Bat / China) [25]. These prevalence data should be investigated for their potential influence in evolutionary terms, in order to verify links with the four human coronaviruses (HCoVs) that have become endemic and global respiratory pathogens.
The study by Corman [26] on HCoV-229E (dromedary camels / MiddleEast: KSA and UAE; Africa: Kenya, Somalia, Sudan and Egypt) present in dromedaries infected with MERS-CoV shows that 5.6% of these animals (n = 1033) tested positive for HCoV-229E. This study was important, because MERS-CoV is an emerging strain with a zoonotic reservoir in dromedary camels and allowed us to define a hypothesis about the origin of human coronaviruses (HCoVs). The study allowed to advance on knowledge that both viruses are monophyletics, with possible ecological isolation, being a descendant of camelid-associated viruses. Although HCoV-229E does not currently prove to be a risk for a global epidemic, its evolutionary history appears as a hypothesis for MERS-CoV emergence [26].
Since the appearance of the first global SARS-CoV crisis in 2003, which occurred in Guangdong province, China, with 305 cases of atypical pneumonia, the 2012 MERS-CoV and SARS-CoV-2 (COVID-19), the search for different species of animals that can be considered as a reservoir of the disease has been constant and in the case of SARS-CoV-2 inconclusive, although new evidence points to the bat [11].
4.3. Interactions between genera of coronaviruses – subgroup metanalysis
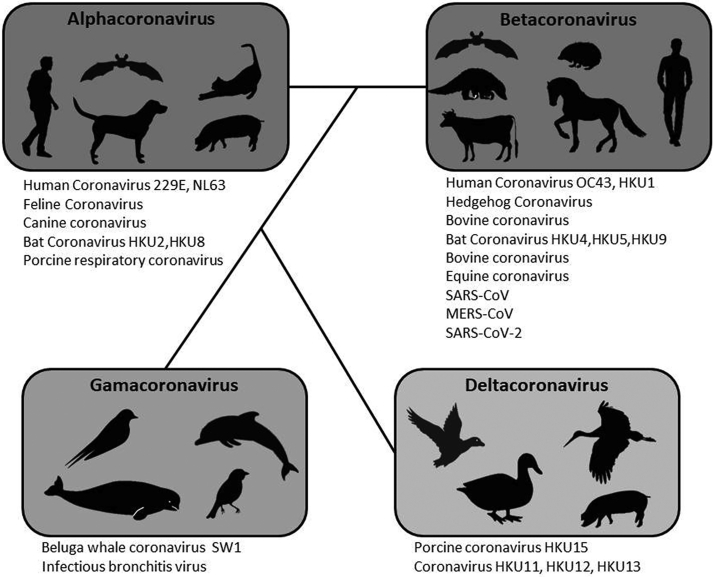
The meta-analysis of the subgroups shows a higher prevalence in the Aves class 0.24 (0.12–0.38, 95% CI) compared to the mammal class 0.18 (0.10–0.28, 95% CI), based on genetic and serological studies, the coronaviruses were divided into 4 genera, Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus (see Fig. 6), capable of infecting different species. It is known that Alphacoronaviruses and Betacoronaviruses mainly infect mammals, whereas Gammacoronaviruses and Deltacoronaviruses are mostly found in birds, although cases of infection in cetaceans have been reported [[38], [39], [40]].
Fig. 6.
Phylogenetic relationship between alphacoronaviruses, betacoronaviruses, deltacoronaviruses and gamacoronaviruses, and examples of strains capable of infecting several animal species.
The study of interactions between genera of coronaviruses, as well as a deepening of their ecology, is essential to understand to what extent each of them contributes to the emergence of new strains capable of breaking the species barrier, thus bringing to light the importance of a one health approach.
The coronaviruses that have caused recent epidemic and pandemic disease outbreaks, including SARS, MERS (well-represented in the subgroup meta-analysis – mammal class) and SARS-CoV-2, in human populations, belong to a subgroup of Betacoronaviruses known as Sarbecoviruses. Members of this group of coronaviruses are abundant in bats and other mammals, and these animal species end up having a higher prevalence of subgroup meta-analysis corresponding to the class of mammals.
If animals carrying different coronaviruses come into close contact and exchange viruses, then recombination can occur between the different strains, leading to diversification [40].
The presence of multiple different phylogenetic patterns indicates that co-infection and genetic recombination of distantly related mammalian coronaviruses has occurred in the recent evolutionary history of SARS-CoV-2 [40].
The current pandemic should be faced as an opportunity to study SARS-CoV-2 in order to create an evolutionary model that can be useful to prevent new coronaviruses with the capacity to infect humans. Monitoring SARS-CoV-2, its evolutionary biological process of genetic recombination, and studying interactions between major coronavirus genera, integrating human, veterinary medicine and ecology, may allow anticipating emerging risks from emerging variants.
4.4. Beyond the systematic revision – What are the lesson learned and where are we with regards to the One Health Approach?
Monitoring SARS-CoV-2 biological evolution and relating its emerging impacts to human medicine, veterinary medicine and ecology is a Herculean but unavoidable challenge. Emerging risks in agriculture and animal husbandry must be addressed through specific preventive detection and intervention tools. That implies a close cooperation and interaction between veterinarians, occupational health physicians and public health operators, so as to establish a worldwide strategy to expand interdisciplinary projects and better emerging risk communication policies in all aspects of health care for humans and animals, within a healthy environment. This is what the One Health Approach intends to be.
As stated by Wielinga in 2013, Food Safety and Public Health operators are at the center of One Health. Many, if not most, of all important zoonoses relate in some way to animals in the food production chain. Therefore, the food becomes an important vehicle for many, but not all, of these zoonotic pathogens, lacking plans for systematic collaboration between authorities and stakeholders in the animal health, food control and human health sectors [43].
.A pioneering review in 2018, discusses food safety aspects of importance from a One Health perspective, focusing on Europe [44]. Using examples of food pathogen/food commodity combinations, spread of antimicrobial resistance in the food web and the risk of transmission of zoonotic pathogens in a circular system, it demonstrates how different perspectives are interconnected.
Regarding food, the first incident related to COVID-19 occurred on June 12, 2020 at the Xinfadi agricultural products market in Beijing, where SARS-CoV-2 was detected on a cutting board used to process imported salmon [27]. Although subsequent investigations have not been conclusive as to its origin, this particular incident raised, before authorities and consumers, some questions about frozen foods as possible carriers of SARS-CoV-2. Since the beginning of July 2020, at least nine food contamination incidents have been reported in China, where SARS-CoV-2 has been detected in imported foods, mainly packaging materials, from shrimp imported from Ecuador, and in Shenzhen, in Guangdong province on August 12, 2020, on the surface of frozen chicken originating in Brazil, which became the first known case in which the new coronavirus was detected in real samples of imported foods [27].
It is worth considering the possibility that the food cold chain may promote contamination, because laboratory studies [28] have shown that SARS-CoV-2 remained highly stable under refrigeration, at 4 °C, and in freezing conditions, from −10 to −80 °C in fish, meat, poultry, and pig skin for 14–21 days. In a controlled laboratory study [29], the persistence of SARS-CoV-2 in chilled salmon, frozen chicken and pork for 21 days was examined. The study showed that SARS-CoV-2 titers remained virtually constant, and the inoculated viruses maintained their infectivity both in the refrigerated product (4 °C) and in the frozen samples (−20 °C and − 80 °C). In a previous study, researchers presented evidence that SARS-CoV-2 can remain quite stable in pig skin for 14 days, at 4 °C [30].
The most recent investigation, in an experimental context, points to the new coronavirus remaining up to 72 h in plastic and stainless steel with temperatures of around 20° and humidity of 40% [31,32]. Other multiple investigations have already reported that SARS-CoV-2 and other coronaviruses are able to remain on surfaces such as metal, glass, PVC, Teflon, and other materials, for several days [[33], [34], [35], [36]].
At the interface between the health of humans, animals and the ecosystem, host receptor recognition is a determinant for virus infection. Li [37] conducted sequence and structural analyses of angiotensin-converting enzyme 2 (ACE2) from different species, which sheds some light on cross-species receptor usage of SARS-CoV-2. Citing the authors, all these analyses raise an alert on a potential interspecies transmission of the virus and propose further surveillance in the diverse animal populations.
5. Conclusions
5.1. A true One Health approach is urgent
This systematic review and meta-analysis of the prevalence of the coronaviruses worldwide highlights the great biological diversity of these agents, as well as their ability to infect a wide variety of species. The latest most important epidemiological crises, which occurred in 2003 (SARS-CoV-1), 2012 (MERS-CoV) and 2019 (SARS-CoV-2, COVID-19) alert to the potential epidemic risk of this infection, which causes severe atypical pneumonia and several other systemic dysfunctions.
The wide variety of infected animal species or natural sources of the coronaviruses require a crosscutting and multidisciplinary approach. It is essential to put in place a One Health approach to this public health problem and to take advantage of the experience from the collaboration between Human and Veterinary Medicine. The concept of One Health has become increasingly important. However, there is much to do. It is essential to enhance the structuring of new One Health policies that allow the creation of epidemiological surveillance programs and the creation of advanced training courses in this new area of intervention that brings together human, animal and environment health, in order to prepare human resources to fight the next pandemic. EU Statement on the WHO-led COVID-19 origins established an agreement for scientific and collaborative field missions through WHO's close cooperation with the World Organization for Animal Health (OIE), the Food and Agriculture Organization of the United Nations (FAO) and countries, in line with a One Health Approach, which will enable targeted interventions and an international research agenda to reduce the risk of similar events occurring [accessed on 07/07/2021: https://eeas.europa.eu/delegations/un-geneva_en/95960/EU%20Statement%20on%20the%20WHO-led%20COVID-19%20origins%20study]. However, for now, the SARS-CoV-2 (COVID-19) pandemic only showed our lack of preparedness and responsiveness at the global level. Gathering data for collaborative, multisectoral, and trans-disciplinary approach — working at the local, regional, national, and global levels is the only way to be better prepared for the next One Health threat.
CRediT authorship contribution statement
Ricardo Faustino: Conceptualization, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing, Supervision. Miguel Faria: Investigation, Methodology, Validation, Writing – review & editing. Mónica Teixeira: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing. Filipe Palavra: Conceptualization, Investigation, Methodology, Validation. Paulo Sargento: Investigation, Methodology, Validation, Writing – review & editing. Maria do Céu Costa: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
The present study was supported by the Research Group in Health Sciences and Technologies - NICiTeS, Ribeiro Sanches Higher School of Health, Polytechnic Institute of Lusophony (IPLuso) Lisbon, Portugal, and by the Portuguese Red Cross Higher Health School (ESSCVP), Lisbon, Portugal.
References
- 1.Sun J., He W.T., Wang L., Lai A., Ji X., Zhai X., Li G., Suchard M.A., Tian J., Zhou J., Veit M., Su S. COVID-19: epidemiology, evolution, and cross-disciplinary perspectives. Trends Mol. Med. 2020;26(5):483–495. doi: 10.1016/j.molmed.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leroy E.M., Ar Gouilh M., Brugère-Picoux J. The risk of SARS-CoV-2 transmission to pets and other wild and domestic animals strongly mandates a one-health strategy to control the COVID-19 pandemic. One Health. 2020;10 doi: 10.1016/j.onehlt.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daly J.M. Middle East respiratory syndrome (MERS) coronavirus: putting one health principles into practice? Vet. J. 2017;222:52–53. doi: 10.1016/j.tvjl.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greatorex Z.F., Olson S.H., Singhalath S., Silithammavong S., Khammavong K., Fine A.E., Weisman W., Douangngeun B., Theppangna W., Keatts L., Gilbert M., Karesh W.B., Hansel T., Zimicki S., O’Rourke K., Joly D.O., Mazet J.A. Wildlife trade and human health in Lao PDR: an assessment of the zoonotic disease risk in markets. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zumla A., Dar O., Kock R., Muturi M., Ntoumi F., Kaleebu P., Eusebio M., Mfinanga S., Bates M., Mwaba P., Ansumana R., Khan M., Alagaili A.N., Cotten M., Azhar E.I., Maeurer M., Ippolito G., Petersen E. Taking forward a ‘One Health’ approach for turning the tide against the Middle East respiratory syndrome coronavirus and other zoonotic pathogens with epidemic potential. Int. J. Infect. Dis. 2016;47:5–9. doi: 10.1016/j.ijid.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foddai A., Lubroth J., Ellis-Iversen J. Base protocol for real time active random surveillance of coronavirus disease (COVID-19) - adapting veterinary methodology to public health. One Health. 2020;9 doi: 10.1016/j.onehlt.2020.100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foddai A., Lindberg A., Lubroth J., Ellis-Iversen J. Surveillance to improve evidence for community control decisions during the COVID-19 pandemic - opening the animal epidemic toolbox for public health. One Health. 2020;9 doi: 10.1016/j.onehlt.2020.100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su S., Wong G., Shi W., Liu J., Lai A., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erles K., Toomey C., Brooks H.W., Brownlie J. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology. 2003;310(2):216–223. doi: 10.1016/s0042-6822(03)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terada Y., Matsui N., Noguchi K., Kuwata R., Shimoda H., Soma T., Mochizuki M., Maeda K. Emergence of pathogenic coronaviruses in cats by homologous recombination between feline and canine coronaviruses. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0106534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey E.S., Fieldhouse J.K., Choi J.Y., Gray G.C. A Mini review of the zoonotic threat potential of influenza viruses, coronaviruses, adenoviruses, and enteroviruses. Front. Public Health. 2018;6:104. doi: 10.3389/fpubh.2018.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farag E., Nour M., El Idrissi A., Berrada J., Moustafa A., Mehmood M., Mahmoud M.H., El-Sayed A.M., Alhajri F., Al-Hajri M., Hassan O.A., Al-Romaihi H., Al-Thani M., Al-Marri S.A., Koopmans M., Ismail M.H. Survey on implementation of one health approach for MERS-CoV preparedness and control in gulf cooperation council and Middle East countries. Emerg. Infect. Dis. 2019;25(3) doi: 10.3201/eid2503.171702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donato H., Donato M. Stages for undertaking a systematic review. Acta Medica Port. 2019;32(3):227–235. doi: 10.20344/amp.11923. [DOI] [PubMed] [Google Scholar]
- 14.Falzarano D., Kamissoko B., de Wit E., Maïga O., Cronin J., Samaké K., Traoré A., Milne-Price S., Munster V.J., Sogoba N., Niang M., Safronetz D., Feldmann H. Dromedary camels in northern Mali have high seropositivity to MERS-CoV. One Health. 2017;3:41–43. doi: 10.1016/j.onehlt.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller M.A., Meyer B., Corman V.M., Al-Masri M., Turkestani A., Ritz D., Sieberg A., Aldabbagh S., Bosch B.J., Lattwein E., Alhakeem R.F., Assiri A.M., Albarrak A.M., Al-Shangiti A.M., Al-Tawfiq J.A., Wikramaratna P., Alrabeeah A.A., Drosten C., Memish Z.A. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. The lancet. Infect. Dis. Ther. 2015;15(5):559–564. doi: 10.1016/S1473-3099(15)70090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodrich E.L., Mittel L.D., Glaser A., Ness S.L., Radcliffe R.M., Divers T.J. Novel findings from a beta coronavirus outbreak on an American miniature horse breeding farm in upstate New York. Equine Vet. Educ. 2020;32(3):150–154. doi: 10.1111/eve.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhuang Q., Liu S., Zhang X., Jiang W., Wang K., Wang S., Peng C., Hou G., Li J., Yu X., Yuan L., Wang J., Li Y., Liu H., Chen J. Surveillance and taxonomic analysis of the coronavirus dominant in pigeons in China. Transbound. Emerg. Dis. 2020 doi: 10.1111/tbed.13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uhart M., Thijl Vanstreels R.E., Gallo L., Cook R.A., Karesh W.B. Serological survey for select infectious agents in wild Magellanic penguins (Spheniscus magellanicus) in argentina, 1994-2008. J. Wildl. Dis. 2020;56(1):66–81. [PubMed] [Google Scholar]
- 19.David D., Rotenberg D., Khinich E., Erster O., Bardenstein S., van Straten M., Okba N., Raj S.V., Haagmans B.L., Miculitzki M., Davidson I. Middle East respiratory syndrome coronavirus specific antibodies in naturally exposed Israeli llamas, alpacas and camels. One Health. 2018;5:65–68. doi: 10.1016/j.onehlt.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ommeh S., Zhang W., Zohaib A., Chen J., Zhang H., Hu B., Ge X.Y., Yang X.L., Masika M., Obanda V., Luo Y., Li S., Waruhiu C., Li B., Zhu Y., Ouma D., Odendo V., Wang L.F., Anderson D.E., Lichoti J., et al. Genetic evidence of Middle East respiratory syndrome coronavirus (MERS-Cov) and widespread Seroprevalence among camels in Kenya. Virol. Sin. 2018;33(6):484–492. doi: 10.1007/s12250-018-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Mira Fernandes A., Brandão P.E., Dos Santos Lima M., De Souza Nunes Martins M., Da Silva T.G., Da Silva Cardoso Pinto V., De Paula L.T., Vicente M., Okuda L.H., Pituco E.M. Genetic diversity of BCoV in Brazilian cattle herds. Vet. Med. scie. 2018;4(3):183–189. doi: 10.1002/vms3.102. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obameso J.O., Li H., Jia H., Han M., Zhu S., Huang C., Zhao Y., Zhao M., Bai Y., Yuan F., Zhao H., Peng X., Xu W., Tan W., Zhao Y., Yuen K.Y., Liu W.J., Lu L., Gao G.F. The persistent prevalence and evolution of cross-family recombinant coronavirus GCCDC1 among a bat population: a two-year follow-up. Science China. Life Sci. 2017;60(12):1357–1363. doi: 10.1007/s11427-017-9263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres C.A., Hora A.S., Tonietti P.O., Taniwaki S.A., Cecchinato M., Villarreal L.Y., Brandão P.E. Gammacoronavirus and Deltacoronavirus in quail. Avian Dis. 2016;60(3):656–661. doi: 10.1637/11412-032316-Reg.1. [DOI] [PubMed] [Google Scholar]
- 24.Asano K.M., Hora A.S., Scheffer K.C., Fahl W.O., Iamamoto K., Mori E., Brandão P.E. Alphacoronavirus in urban Molossidae and Phyllostomidae bats, Brazil. Virol. J. 2016;13:110. doi: 10.1186/s12985-016-0569-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge X.Y., Wang N., Zhang W., Hu B., Li B., Zhang Y.Z., Zhou J.H., Luo C.M., Yang X.L., Wu L.J., Wang B., Zhang Y., Li Z.X., Shi Z.L. Coexistence of multiple coronaviruses in several bat colonies in an abandoned mineshaft. Virol. Sin. 2016;31(1):31–40. doi: 10.1007/s12250-016-3713-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corman V.M., Eckerle I., Memish Z.A., Liljander A.M., Dijkman R., Jonsdottir H., Juma Ngeiywa K.J., Kamau E., Younan M., Al Masri M., Assiri A., Gluecks I., Musa B.E., Meyer B., Müller M.A., Hilali M., Bornstein S., Wernery U., Thiel V., Jores J., et al. Link of a ubiquitous human coronavirus to dromedary camels. Proc. Natl. Acad. Sci. U. S. A. 2016;113(35):9864–9869. doi: 10.1073/pnas.1604472113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han J., Zhang X., He S., et al. Can the coronavirus disease be transmitted from food? A review of evidence, risks, policies and knowledge gaps. Environ. Chem. Lett. 2021;19:5–16. doi: 10.1007/s10311-020-01101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher D., Reilly A., Zheng A.K.E., Cook A.R., Anderson D.E. Seeding of outbreaks of COVID-19 by contaminated fresh and frozen food. BioRxiv. 2020 [Google Scholar]
- 29.Harbourt D., Haddow A., Piper A., Bloomfield H., Kearney B., Gibson K., Minogue T. Modeling the stability of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on skin, currency, and clothing. medRxiv. 2020 doi: 10.1101/2020.07.01.20144253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H., Chan M.C.W., Peiris M., Poon L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1(1):e10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warnes S.L., Little Z.R., Keevil C.W. Human coronavirus 229E remains infectious on common touch surface materials. MBio. 2015;6 doi: 10.1128/mBio.01697-15. e01691-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Food and Drug Administration (FDA) 2020. Food safety and the coronavirus disease 2019 (COVID-19)https://www.fda.gov/food/food-safety-during-emergencis/food-safety-and-coronavirus-disease-2019-covid-19 (Accessed 2 Sept 2020) [PubMed] [Google Scholar]
- 34.Anderson E.L., Turnham P., Griffin J.R., Clarke C.C. Consideration of the aerosol transmission for COVID-19 and public health. Risk Anal. 2020;40(5):902–907. doi: 10.1111/risa.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morawska L., Milton D.K. It is time to address airborne transmission of COVID-19. Clin. Infect. Dis. 2020:ciaa939. doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anelich L., Lues R., Farber J., Parreira V. SARS-CoV-2 and risk for food safety. Fron. Nutr. 2020 doi: 10.3389/fnut.2020.580551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li R., Qiao S., Zhang G. Analysis of angiotensin-converting enzyme 2 (ACE2) from different species sheds some light on cross-species receptor usage of a novel coronavirus 2019-nCoV. J. Infect. 2020;80(4):469–496. doi: 10.1016/j.jinf.2020.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wertheim J.O., Chu D.K.W., Peiris J.S.M., Kosakovsky Pond S.L., Poon L.L.M. A case for the ancient origin of coronaviruses. J. Virol. 2013;87:7039. doi: 10.1128/JVI.03273-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forni D., Cagliani R., Clerici M., Sironi M. Molecular evolution of human coronavirus genomes. Trends Microbiol. 2017;25:35–48. doi: 10.1016/j.tim.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh D., Yi S.V. On the origin and evolution of SARS-CoV-2. Exp. Mol. Med. 2021;53(4):537–547. doi: 10.1038/s12276-021-00604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharun K., Dhama K., Pawde A.M., Gortázar C., Tiwari R., Bonilla-Aldana D.K., Rodriguez-Morales A.J., de la Fuente J., Michalak I., Attia Y.A. SARS-CoV-2 in animals: potential for unknown reservoir hosts and public health implications. Vet Q. 2021;41(1):181–201. doi: 10.1080/01652176.2021.1921311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wielinga P.R., Schlundt J. Food safety: at the center of a one health approach for combating zoonoses. Curr. Top. Microbiol. Immunol. 2013;366:3–17. doi: 10.1007/82_2012_238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boqvist S., Söderqvist K., Vågsholm I. Food safety challenges and one health within Europe. Acta Vet. Scand. 2018;60(1):1. doi: 10.1186/s13028-017-0355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]