Abstract
Objective
The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to the most rapid response and scale-up in vaccine and therapeutic development in history. We highlight the history of these amazing achievements with a focus on the description of the classification and mechanisms of allergic reactions and adverse events relevant to the allergist and immunologist that have been associated with the SARS-CoV-2 vaccines. Finally, we offer a detailed management approach in the context of a possible allergic reaction.
Data Sources
Using defined search strategy, we identified peer-reviewed articles within PubMed that were published between January 1, 2019, and December 4, 2021.
Study Selections
All recent articles on COVID-19 published in English were reviewed with focus on the immunogenicity and allergenicity of the current existing COVID-19 vaccines.
Results
Following a detailed literature review, we discuss the evolution and development of the new vaccines for SARS-CoV-2. Furthermore, we provide evidence regarding the significance and mechanisms of allergic reactions associated with the vaccines and offer a management approach for those with an increased risk of presenting an allergic or other relevant vaccine reaction.
Conclusion
The international rollout of COVID-19 vaccination started with reports of immediate allergic reactions. Although we still need to understand the mechanisms of these reactions, we can be reassured that patients with underlying allergic disease will not need to avoid SARS-CoV-2 vaccination. In addition, the vast majority of those with a first-dose reaction will tolerate subsequent doses.
Key Messages.
• The current vaccines for severe acute respiratory syndrome coronavirus 2 currently approved for use by different international jurisdictions and in clinical phase I to III development are whole virus, protein subunit, nucleic acid (RNA and DNA), and viral vector vaccines.
• The adverse reactions associated with the coronavirus disease 2019 (COVID-19) vaccines can be broadly classified as reactogenic or allergic. They are further classified as local or systemic, immediate or non-immediate, and immune or non–immune-mediated reactions.
• Initial reports of allergic reactions led to a risk management strategy that triaged patients based on their prior history of a potential reaction to a vaccine or a component of the existing (messenger [m]RNA) vaccines.
• Now with a mature understanding of risk, and the reassurance of the very low risk of a preexisting allergy history affecting the safety of COVID-19 vaccination, we have pivoted to a different approach. This risk management approach focuses on those who have had an allergic or other serious reaction to a COVID-19 vaccine and provides support for the completion of primary and booster vaccinations.
• Anaphylaxis to excipients used in the manufacturing process of mRNA vaccines, such as polyethylene glycol used to stabilize the lipid nanoparticle, appears to occur rarely. This means that most individuals known to have allergy to polyethylene glycol (PEG) will usually tolerate mRNA vaccines. However, the flip side of this is that tolerance of mRNA vaccines does not confirm tolerance of PEG. Those that tolerate mRNA vaccines can still be susceptible to severe reactions to PEG, and caution should be applied to hidden ingredients in other products, such as bowel preparations and injectable corticosteroids.
Alt-text: Unlabelled box
Introduction
Since the first described case of coronavirus (COVID-19) disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) described in December 2019, the scientific community has united in a common battle against its associated global threats, morbidity, and the astounding 5 million deaths1 (Fig 1 ). Supportive care, corticosteroids, monoclonal antibodies, and other immunosuppressive and antiviral medications are being trialed and used since the beginning of this pandemic.2 Although patients who have recovered from the COVID-19 disease produce robust humoral and cellular responses, the appropriate memory CD4 cell response is most effectively attained with an optimal vaccination strategy.3 Currently, there are 139 SARS-CoV-2 vaccines in clinical development, and different types of vaccines have been developed and rolled out in the previous year using various strategies to generate an immune system response1 (Table 1 ). We aim to describe the evolution and the development of the new vaccines for SARS-CoV-2. Furthermore, we will give the context of what is known about the background of vaccine allergy and propose and provide an understanding of the classification and mechanisms of allergic reactions associated with the COVID-19 vaccines. We will synthesize the known information to provide a risk-based management approach for those with immediate and delayed hypersensitivity reactions associated with vaccination and other vaccine-related adverse events.
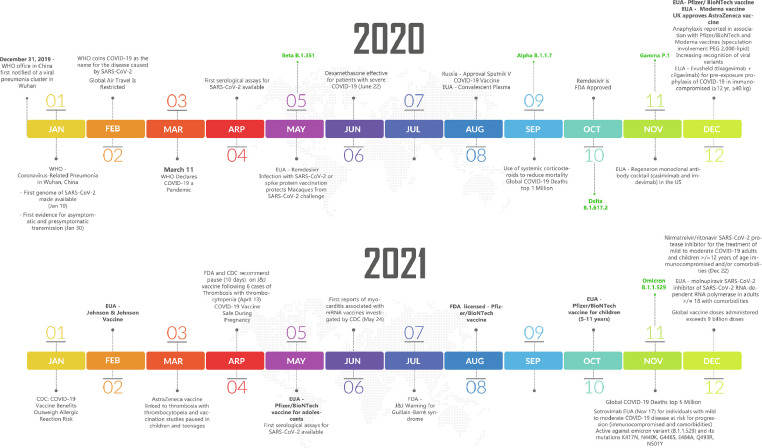
Figure 1.
Timeline of COVID-19 vaccines and therapeutics. COVID-19, coronavirus disease 2019; EUA, emergency use authorization; FDA, Food and Drug Administration; PEG, polyethylene glycol; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization.
Table 1.
Approved Vaccines for SARS-CoV-2
| Research name | Commercial name | Developer | Vaccine type | Active ingredient | Relevant details of excipients and formulationa | Dose | Numberof doses | Interval doses | Booster doseb | Efficacyc | Age indication | Storage |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BNT162b2 Tozinameran |
Pfizer Comirnaty >17 y |
Pfizer-BioNTech (and Fosun) |
RNA based | Nucleoside-modRNA encoding viral spike GP SARS-CoV-2 | 2-[PEG-2000]-N,N-ditetradecylacetamide | 0.3 mL IM (30 μg) |
2 | 21 d | ≥ 5 mo | 95%12 | 18 y + | −80°C to −60°C (−112°F to −76°F) |
| BNT162b2 Tozinameran | Pfizer Comirnaty 12-17 y |
Pfizer-BioNTech (and Fosun) |
RNA based | Nucleoside-modRNA encoding viral spike GP SARS-CoV-2 | 2-[PEG-2000]-N,N-ditetradecylacetamide | 0.3 mL IM (30 μg) |
2 | 21 d | ≥5 mo | 100%77 | 12-17 y | −80°C to −60°C (−112°F to −76°F) |
| BNT162b2 Tozinameran |
Pfizer Comirnaty 5-11 y |
Pfizer-BioNTech (and Fosun) | RNA based | Nucleoside-modRNA encoding viral spike GP SARS-CoV-2 | 2-[PEG-2000]-N,N-ditetradecylaacetamide Tromethamine |
0.2 mL IM (10 μg) |
2 | 21 d | n/ab | 90.7%78 | 5-11 y | −80°C to −60°C (−112°F to −76°F) |
| mRNA-1273 | Moderna Spikevax >17 y |
Moderna and NIAID | RNA based | mRNA encoding the pre-fusion stabilized spike GP (S) SARS-CoV-2 | PEG-2000-DMG Tromethamine |
0.5 mL IM (100 μg) |
2 | 28 d | 50 mcg ≥6 mo |
94.1%79 | 18 y + | −20°C (−4°F) |
| mRNA-1273 | Moderna Spikevax 12-17 y |
Moderna and NIAID | RNA based | mRNA encoding the pre-fusion stabilized spike GP (S) SARS-CoV-2 | PEG-2000-DMG Tromethamine |
0.5 mL IM (100 μg) |
2 | 28 d | n/a | 100%80 | 12-17 y | −20°C (−4°F) |
| AZD1222 ChAdOx1-S Vaxzevria |
AstraZeneca vaccine COVISHIELD Vaxzevria |
AstraZeneca and the University of Oxford | NR viral vector | Recombinant, replication-deficient chimpanzee adenovirus vector encoding SARS-CoV-2 spike GP | Polysorbate-80 | 0.5 mL IM (5 × 1010) |
2 | 4-12 wk | n/a | 62%81 | 18 y + | 2°C to 8°C (35.6°F to 46.4°F) |
| JNJ-78436735 Ad26.COV2.S | Johnson & Johnson | Janssen Pharmaceutical companies (Johnson & Johnson) | NR viral vector | Recombinant, replication-incompetent adenovirus type 26 expressing SARS-CoV-2 spike protein | Polysorbate-80 | 0.5 mL IM | 1 | n/a | ≥2 mo | 66% (overall) 72% (United States) 85% (severe disease)82 |
18 y + | −20°C (−4°F) |
| NVX-CoV2373 | Nuvaxovid | Novavax | Protein | SARS-CoV-2 recombinant spike protein | Polysorbate-80 | 0.5 mL IM | 2 | 21 d | n/a | 89.7%83 | 18 y + | ≤−60°C |
| BBIBP-CorV | Sinopharm | Sinopharm (Beijing) | Inactivated virus | SARS-CoV-2 virus (cultivated in Vero cell line) | n/a | 0.5 mL IM | 2 | 21-28 d | n/a | 78.1%84 | 18 y + | 2°C to 8°C |
| CoronaVac PiCoVacc |
CoronaVac PiCoVacc |
Sinovac | Inactivated virus | SARS-CoV-2 virus | n/a | 0.5 mL IM | 2 | 14-28 d | n/a | 50%-91%85, 86, 87 | 18 y + | Room temp. |
| BBV152 A, B, C | Covaxin | Bharat Biotech | Inactivated virus | SARS-CoV-2 virus | n/a | 0.5 mL IM | 2 | 28 d | n/a | 77.8%88 | 18 y + | 2°C to 8°C |
Abbreviations: COVID-19, coronavirus disease 2019; GP, glycoprotein; IM, intramuscular; mRNA, messenger RNA; modRNA, modified messenger RNA; NIAID, National Institute of Allergy and Infectious Diseases; PEG, polyethylene glycol; PEG-2000-DMG, 1,2-dimyristoyl-rac-glycero3-methoxypolyethylene glycol-2000; RNA, ribonucleic acid; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; NR, non-replicating; Temp, temperature.
This column only contains the inactive lipids that are considered potential culprit for hypersensitivity reactions associated with these vaccines.
The recommendation for immunocompromised hosts for the mRNA vaccines is to administer a third dose 28 d after the second dose and a fourth booster dose 5 mo after the third dose. For immunocompromised children, a third dose is also recommended 28 d after the second dose but only the Pfizer-BioNTech vaccine has an EUA for 5- to 11-year-old children should this be used. Booster dosing 5 mo following primary vaccination has not yet received a EUA. The American College of Rheumatology has recommended adjusting the timing of immunosuppression where possible (eg, rituximab initiated 4 wk before primary series or delaying rituximab until 2-4 wk after completion of the primary vaccination series).89
Revaccination with the original series 3 mo following the intervention is recommended after hematopoietic cell transplant or CAR-T therapy.90
Refers to efficacy in phase III clinical trials against symptomatic COVID-19 illness. All vaccines have reduced efficacy against the SARS-CoV-2 viral variants although the effectiveness against severe COVID-19 disease, hospitalization, and mortality has remained for the delta and omicron variants particularly in adults who have received a booster dose with mRNA vaccines. Measurement of SARS-CoV-2 antibody titer or neutralizing antibody should not be measured because there is poor correlation and cellular immunity is likely playing a key role in protection against severe disease associated with newer variants.
Search Strategy and Selection Criteria
We searched PubMed for peer-reviewed articles published between January 1, 2019, and December 4, 2021 (date of previous search), with the following key terms: (“SARS-CoV-2” or “severe acute respiratory syndrome coronavirus 2” or “COVID-19”) and (“vaccine” or “mRNA” or “Pfizer-BioNTech” or “BNT162b2” or “Moderna” or “mRNA-1273” or “PEG” or “polyethylene glycol” or “AstraZeneca” or “AZD1222” or “Johnson & Johnson” or “Ad26.COV2.S” or “JNJ-78436735”) and (“allergy” or “anaphylaxis” or “allergenicity” or “adverse reaction” or “immune response” or “immunogenicity”). Articles published in English were selected and reviewed. We focused on articles classified as “clinical trials” and “meta-analysis.” This search provided 116 articles. The first screening was based on titles and abstracts followed by a second round of screening performed by reviewing the full-text articles for selected studies. This was performed by the first and last authors. We also identified several new references from the ones listed in the reviewed articles. Finally, we researched the ClinicalTrials.gov website to identify current trials on the allergenicity of the SARS-CoV-2 vaccines. The aim of this study was to provide a narrative review, and future systematic reviews are required to establish the allergenicity and adverse reactions associated with SARS-CoV-2 vaccines.
History of Allergic Reactions and Adverse Reactions Associated With Vaccines
In the last century of vaccine development, allergic reactions to vaccines have been an infrequent but measurable effect that may in some circumstances have led to exclusion from future vaccination.4, 5, 6 Most information gained about vaccine adverse events in the United States has been through the Vaccine Adverse Event Reporting System (VAERS) that was established in 1990 and is managed by both the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration. VAERS is a passive surveillance system and relies on individual reports of adverse experiences. A recent publication highlighted that from 1990 to 2016 and from 467,960 reports, 828 met Brighton collaboration case definition or a physician's diagnosis of anaphylaxis. Of the 42% of reports from adults, 80% were women, 41% had no history of hypersensitivity, and most were associated with influenza vaccination.7 One of the largest studies to date used health care data from vaccine safety datalink.8 The vaccine safety datalink is a collaborative effort between the CDC immunization safety organization and 9 health care organizations that is updated weekly since 1990 specifically to answer important vaccine safety questions in larger populations. This study confirmed 33 cases of anaphylaxis after 25,173,965 vaccine doses to give the vaccine rate of anaphylaxis that we most commonly quote today of 1.31 per million vaccine doses. Interestingly, this study also sets the context for events to follow with COVID-19 messenger RNA (mRNA) vaccines as it suggested that most (85%) of those who developed vaccine-associated anaphylaxis had preexisting atopic disease. Although there has been much interest in excipients being the culprits for vaccine-associated immediate reactions and anaphylaxis, there have only been a few case reports to support immunologic cross-reactivity between vaccines and drugs on the basis of shared common excipients, such as the polysorbates.4 , 9 For other vaccines, the rates of anaphylaxis have been modified by reduction or elimination of factors, such as gelatin and latex.4
Allergic Reactions Associated With Severe Acute Respiratory Syndrome Coronavirus 2 Vaccines
Shortly after the first administered Pfizer-BioNTech COVID-19 mRNA vaccine in the United Kingdom, anaphylaxis and other allergic reactions were reported on December 8, 2020. This led to the initial recommendation of the Medicines and Healthcare Products Regulatory Agency in the United Kingdom to exclude from vaccination any person with a history of food, drug, or vaccine anaphylaxis and initiating the threat of a vaccine hesitancy movement for patients with known allergies worldwide. This recommendation was later lifted after an additional review on December 30, 2020, that recommended continued mRNA vaccine avoidance in anyone with a history of an allergic reaction to components of the vaccine but allowed those with unrelated allergies (eg, food allergy) to receive COVID-19 mRNA vaccines. In the United States, the first cases were reported in health care workers in December 16, 2020. This led the US Food and Drug Administration and CDC to reinforce the recommendation against vaccination in individuals with a history of allergic reactions to any of the vaccine components, a recommendation instigated by the Medicines and Healthcare Products Regulatory Agency and all the other allergy associations worldwide10 , 11 (Fig 1). Of note, the clinical trials performed for the mRNA vaccines had excluded patients with a possible allergic reaction to any vaccine component and a past medical history of a severe adverse reaction with any type of vaccine.12 , 13 In the initial report from the VAERS monitoring database of the Pfizer-BioNTech (BNT162b2) mRNA COVID-19 vaccine between December 14, 2020, and December 23, 2020, 1,893,360 doses of the vaccine were administered and 21 cases of anaphylaxis (Brighton criteria) were reported giving a rate of 11.1 per million.14 As a more diverse group of individuals was vaccinated, the reported rates of anaphylaxis decreased to 2.5 to 5.1 events per million.11 , 15 One must also consider that there are various definitions of anaphylaxis and that the Brighton collaboration case definition has recently been reported to overestimate the prevalence of anaphylaxis compared with other criteria, such as the National Institute of Allergy and Infectious Disease 2005 and World Allergy Organization 2020.16 Various ongoing trials are aspiring to understand the mechanisms of these reactions focusing on possible risk factors, such as patients with multiple allergies or mast cell disorders (ClinicalTrials.gov Identifiers NCT04761822, NCT04977479). Furthermore, recent evidence reveals that patients who presented an allergic reaction to their first dose can safely tolerate their second dose.17
Epidemiology of Allergic Events and Vaccine Rollout of Severe Acute Respiratory Syndrome Coronavirus 2 Vaccines
The initial anaphylaxis reactions reported occurred within 15 minutes of vaccination and primarily in women with a previous history of self-reported allergic reactions.14 Further insights came from individual health care systems that had full ascertainment of vaccine allergic reactions, such as the Mass General Brigham.18 Mass General Brigham employees receiving their first dose from December 16, 2020, to February 12, 2021, were studied through follow-up on February 18, 2021. Overall acute allergic reactions occurred within 15 minutes of vaccine administration in 2.10%; more typically with the Moderna vaccine and in women with prior histories of allergic reactions. Approximately one-third (31%) had a history of prior anaphylaxis. No individual who experienced anaphylaxis required resuscitation or endotracheal intubation. At Vanderbilt University Medical Center, 23,094 health care workers were screened for history in the vaccine hall before vaccination with Pfizer-BioNTech COVID-19 mRNA vaccine.19 Among the 31 identified with higher risk histories, 28 went onto safe vaccination based on tolerance of polyethylene glycol (PEG) or polysorbate-containing medications or vaccines. Shavit et al20 conducted an 8-week prospective cohort study where they risk stratified patients with potential allergy before vaccination. Those considered highly to have allergy were monitored for 2 hours after vaccination in a specialized setting and those at low risk for 30 minutes in a routine setting.20 , 21 They did not vaccinate patients with a history of allergy to two or more injectable drugs or PEG. Of the 429 patients deemed highly to have allergy and observed for 2 hours, 9 patients, all women (2.1%), had immediate reactions, of which most were mild, and 3 (0.7% overall) experienced anaphylaxis, which resolved with epinephrine and without hospitalization.20 A more recent study reviewed 391,123 members at the Kaiser Permanente Southern California who received at least 1 dose of a COVID-19 mRNA vaccine between December 15, 2020, and March 11, 2021.22 Overall, 104 (0.028%) with 85% women had a first-dose reaction and only 2 of these (0.00033%) were consistent with anaphylaxis. Less than 10% of those with first-dose reactions had any reaction to the second dose. Similar to other studies, those who received treatment for a first dose or a second dose reaction were more likely to be younger women with a preexisting history of another allergy. To date, no clear risk factors for the COVID-19 vaccines have been definitively proven considering that the group that has safely tolerated the vaccine has not been described in most of the studies and that the atopic comorbidity data derive from self-reports.
Classification of Allergic Reactions to Severe Acute Respiratory Syndrome Coronavirus 2 Vaccines
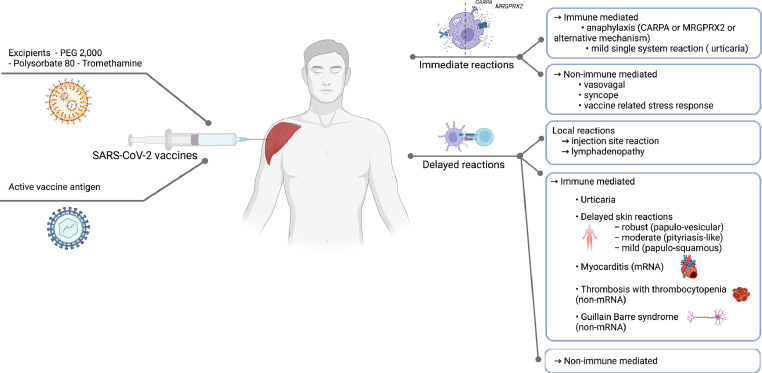
The adverse reactions associated with the COVID-19 vaccines can be classified as immediate or non-immediate, local or systemic, and immune or non–immune-mediated reactions (Fig 2 ).5
Figure 2.
Classification of COVID-19 vaccine reactions. CARPA, complement activation-related pseudoallergy; COVID-19, coronavirus disease 2019; MRGPRX2, Mas-related G protein-coupled receptor-X2; mRNA, messenger ribonucleic acid; PEG, polyethylene glycol; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Immediate Reactions
Systemic Reactions—Immune Mediated
Various described cases fall into the category of anaphylaxis with multisystem involvement. This raised concern that these reactions could be immunoglobulin (Ig)E mediated with allergen crosslinking on mast cells and rapid improvement following epinephrine administration.5 If this was the case, reactions would be expected to repeat and intensify on subsequent reactions, which fortunately was not the case. However, at the time these reactions were initially reported, distinguishing between an IgE-mediated reaction and a non–IgE-mediated reaction on history alone was a complex task in the acute clinical setting vaccination rollout in the health care setting where allergist and immunologist were not in close proximity. Indeed, in the case of a mast cell activation not triggered by IgE linking, it is considered that the reactions are caused by direct and indirect mast cell and basophil degranulation by activation of Mas-related G protein-coupled receptor-X223 or complement activation-related pseudoallergy.24 Mortality has not been reported following an allergic reaction associated with one of the SARS-CoV-2 vaccines,25 with higher anaphylaxis rates for mRNA vaccines compared with adenoviral vector and inactivated virus vaccines.11 As we will discuss in the allergenicity and management section, second-dose reactions in those with first-dose reactions are uncommon. This is evidence against an IgE-mediated mechanism.21
Systemic Reactions—Non-immune mediated
Vasovagal reactions are relatively common following vaccine administration and unless fastidiously documented can be confusing retrospectively. It is thus essential for a clinician to be able to recognize these reactions and to distinguish them from anaphylaxis. Important considerations for vasovagal reactions are (1) timing, with reactions occurring in the first seconds to minutes; (2) blood pressure that can transiently drop and pulse that is slow and weak; (3) slow breathing and possible apnea; and (4) marked pallor and diaphoresis that can be observed in the patient's skin.26 The differential diagnosis for immediate reactions also includes the anxiety-related symptoms, such as vocal cord dysfunction and panic attacks, that can manifest by stridor and dyspnea and globus sensation.27 In this context, the World Health Organization has defined a condition called “immunization stress-related response” and has defined this as a stress-related response that can include a variety of symptoms, such as fainting, hyperventilation, or a dissociative neurologic symptom reaction.28 This is also confirmed by looking at reports of adverse reactions to placebo where more than 35% of the participants in the placebo arms have reported systemic adverse reactions.29 Furthermore, reports have indicated that among previously reported severe allergic reactions, up to 50% were non-anaphylactic in nature.27 These can be challenging for allergists to recognize if the history is not well documented because allergists are typically not present to observe the initial vaccine reaction.
Delayed Reactions
Local Reactions
Local non-immediate reactions, which are not allergenic but possibly a consequence of an innate immune system activation, are common and may include swelling, soreness, and erythema at the injection site.10 , 30 , 31 Furthermore, delayed indurated-erythematous reactions have been reported following the administration of RNA vaccines.32 , 33 The manifestations known as “COVID-arm,” which have been more commonly associated with the Moderna mRNA-1273 SARS-CoV-2 vaccine, are considered based on supportive histopathology from the site of the reaction, possible T-cell–mediated cutaneous hypersensitivity reactions.34 , 35 These marked local reactions occurred in 0.8% after dose 1 of an mRNA vaccine and 0.2% after dose 232 (Table 2 ).
Table 2.
Immediate and Delayed Adverse Reactions to SARS-CoV-2 Vaccines
| Clinical phenotype | Specific type of vaccine | Risk group | Prevalence | Acute management | Advice for future vaccination |
|---|---|---|---|---|---|
| Immediate reactions | |||||
| Anaphylaxis | mRNA vaccines | Women > men History of previous anaphylaxis is prevalent |
2.5-5.1 events per million11 | Intramuscular epinephrine Serum tryptase |
Refer to allergy and immunology Increasing reports of tolerance of second and subsequent doses in the setting of anaphylaxis to the first dose suggests that a non–IgE-mediated mechanism may be prevalent.61,62 |
| Mild single-system reaction | mRNA vaccines | Women > men | 2.1%20 | Symptomatic management with antihistamines61,62 | Antihistamine premedication before subsequent dosing of mRNA vaccine.61,62 |
| Delayed reactions | |||||
| Mild-to-moderate urticaria | mRNA vaccines | Individuals with underlying urticaria Women > men |
Local reactions 0.8% (dose 1) 0.2% (dose 2)32 | Symptomatic management with ice (for local reactions) topical steroids and antihistamines | No contraindication for second and subsequent doses of vaccine Consider antihistamine premedication |
| Injection site | Women < 65 y | ||||
| Lymphadenopathy | Third dose booster > others | Can be >50% lasting up to 10 wk when sensitive imaging is performed48 | Symptomatic management | No contraindication for second and subsequent doses of vaccine (on side contralateral to tumor or other disease process if patient has known pathology for which they are being staged or followed) | |
| Myocarditis | mRNA vaccines | Men < 30 y | More common on second dose (vs first dose or booster) Estimated risk 12- to 29-y-old males—41 cases/million91 Moderna > Pfizer in Denmark (4.2/100,000 vs 1.4/100,000 vaccinated)51 |
Symptomatic management | Consider subsequent dose with mRNA vaccine on full recovery of all signs and symptoms of myocarditis particularly if patient has comorbidities or is immunosuppressed. Benefit on resolution of all symptoms and signs associated with myopericarditis |
| Guillain-Barré or transverse myelitis | Adenoviral vector vaccines | - | 8/million doses | Symptomatic management | Vaccinate with an alternative vaccine if the conditions is self-limited and resolved |
| Thrombosis with thrombocytopenia (multiple thrombotic episodes venous and arterial) |
Adenoviral vector vaccines (AstraZeneca (ChAdOx1) > Johnson and Johnson (Ad26.COV2.S)) |
Women > men (69% for J&J; median age < 50 y for 48% with J&J vaccine)92 Time course—median 9 d |
3.8/million doses for J&J92 | Avoid heparin Administer thrombin inhibitors IvIg may be of benefit |
Administer mRNA vaccine following occurrence. Since December 16, 2021, CDC recommends mRNA vaccines and the Johnson & Johnson vaccine for initial and booster dosing. |
Abbreviations: IgE, immunoglobulin E; IvIg, intravenous immunoglobulins; J&J, Johnson & Johnson; mRNA, messenger ribonucleic acid; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Systemic Reactions—Immune Mediated
Various maculopapular and morbilliform benign skin eruptions have been reported both with mRNA and adenoviral vector vaccines.11 , 36 Although most of these are benign rashes (morbilliform and urticarial reactions), acute generalized exanthematous pustulosis, erythema multiforme, and other blistering rashes have been described. Furthermore, a full spectrum of delayed phenotypes described includes bullous-pemphigoid like, dermal hypersensitivity reactions, lichen planus, pernio, neutrophilic dermatosis, leukocytoclastic vasculitis, granuloma annulare, and tatoo and sarcoidal reactions.37 Currently, it has been proposed that delayed reactions be divided into robust (papulovesicular), moderate (pityriasis-like), and mild (papulosquamous subphenotypes). In addition, a flare of chronic spontaneous urticaria, including new-onset acute urticaria, has been described in the days or weeks following the COVID-19 vaccine.31 , 36 Several case reports of reactivation of Varicella-Zoster virus (VZV) after the mRNA and adenoviral vector vaccines, including Herpes simplex virus reaction, have been described.38, 39, 40, 41, 42 Another article found no evidence of oropharyngeal shedding of human Herpes viruses before or after vaccination with the Pfizer-BioNTech COVID-19 mRNA vaccine in Israel.43 The potential mechanism of human herpesvirus (HHV) reactivation is not known. Furthermore, given that this HHV reactivation occurs soon after the first dose and infrequently after the second dose raises the question of whether cross-reactive HHV responses triggered with the initial vaccine dose cause a compensatory regulatory response leading to reactivation. A more recent study which was a retrospective cohort study using aggregated health records from 63 health care organizations that included more than 70 million patients and a control population without COVID-19 vaccination did not show a difference in VZV reactivation between those who had received a COVID-19 mRNA vaccine within 28 days and the historical control and contemporary cohort.44 , 45 Given that this may be affecting specific risk groups, the jury is still out as to whether this is a true association. In addition, clouding the picture is that COVID-19 infection itself has been associated with VZV reactivation in case reports.45
Other Systemic Delayed Reactions
Other delayed reactions may range from those that interestingly mimic the symptoms and signs of COVID-19 disease, such as anosmia, fever, fatigue, headache, and musculoskeletal pain, 24 to 48 hours following a vaccine dose.12 , 46 Lymphadenopathy is now a prevalent reactogenic symptom that is more common following the third-dose booster and may last for up to 3 months. For patients with cancer, it is recommended that vaccination occurs contralateral to any known adenopathy or tumor pathology.47 , 48 Studies have also reported neuropsychiatric symptoms with important morbidity and mortality, such as depression, anxiety, and altered mental status.46 In very rare instances (approximately 1%), ischemic and hemorrhagic strokes and seizures have been reported.46 Guillain-Barré Syndrome (GBS) has been more prevalently linked to the viral vector vaccines such as the J&J (Janssen) COVID-19 vaccination at a rate of 305 preliminary reports of GBS identified in VAERS after more than 18.1 million doses.25 Of note, GBS may be associated with other vaccines such as influenza and past GBS with influenza is not a contraindication to vaccination with a SARS-CoV-2 vaccine.4 Thrombosis with thrombocytopenia has been associated with the viral vector vaccines with AstraZeneca ChAdOx COVID-19 vaccine appearing to have a higher risk than the Janssen Ad26.COV2S (Johnson & Johnson). This appears to have an immunopathogenesis similar to heparin-induced thrombocytopenia in the absence of heparin. The case definition includes a COVID-19 adenoviral vector vaccine 4 to 42 days before symptom onset, any venous or arterial thrombosis (cerebral and abdominal prevalent), thrombocytopenia (platelets < 150 × 109/L), positive platelet factor 4 ELISA (HIT assay), and elevated D-dimer (>4 × upper limit normal). This should be suspected when there are severe headaches, visual changes, abdominal pain, back pain, shortness of breath, leg swelling or pain, easy bruising, or petechiae occurring 4 to 42 days following vaccination.49 A small case series of 20 patients with a prior history of heparin-induced thrombocytopenia tolerated the AstraZeneca ChAdOx COVID-19 adenoviral vector vaccine.50 Myocarditis has been reported primarily after the second dose of the mRNA vaccines and more prevalently in men less than 30 years of age.51, 52, 53 One study of interest suggested that this might be more common with the traditional 3- or 4-week interval between mRNA vaccines vs the extended 12- to 16-week interval in the rollout in Canada. In addition, heterologous vaccination (eg, Moderna mRNA-1273 vaccine following another vaccine) may also be associated with a heightened risk. Myocarditis has also been a prevalent feature of COVID-19 natural infection.41 The incidence of COVID-19–associated myocarditis and cardiac injury is of 1000 to 4000/100,000 people vs 0.3 to 5.0/100,000 after COVID-19 mRNA vaccination.55
Allergenicity of Vaccine Components
There are multiple vaccine components that could be responsible for allergic reactions, such as the active vaccine antigen, the residual nonhuman proteins, the excipients such as preservatives or stabilizers in the vaccine formulation, and other inactive products such as gelatin or latex5 , 54 (Fig 2). Contrary to drug hypersensitivity reactions, most of the reactions to vaccines have traditionally been thought to be associated with excipients contained in the formulation and not by the active ingredient.4 For the mRNA vaccines, concern was initially raised about the PEG-2000 lipid component of the mRNA BNT162b2 Pfizer-BioNTech and mRNA-1273 Moderna vaccines that stabilizes the lipid nanoparticle that carries the mRNA spike protein construct.5 This concern led to special care being taken during the rollout to risk stratify individuals who historically may have had a history of reaction to a vaccine (many of which contain a PEG-like sorbitan polysorbate 80 but not PEG) or PEG itself.31 The PEGs are synthetic agents used as excipients in various medicinal and cosmetic products.27 , 55 Although this agent is typically associated with a good safety profile and pharmacokinetic studies of PEG-3350 following laxative administration reveal a minimal systemic absorption,56 cases of anaphylaxis and severe immediate hypersensitivity reactions have been described.11 , 57 The mRNA-1273 Moderna mRNA vaccine and the newly formulated BNT162b2-Pfizer COVID-19 vaccine for more than 12 years of age (gray top vial) and that for children (5-11 years) (orange top vial) are tris rather than PBS buffered (contains tromethamine). The original adult BNT162b2-Pfizer COVID-19 mRNA equivalent vaccine is not PBS buffered (purple top vial) and did not contain tromethamine.54 The allergenic potential of this excipient is not known at this time and has been reported as a possible cause of anaphylaxis in the following 2 patients: (1) a patient receiving a gadolinium-based contrast agent58 and (2) a case report of a 45-year-old woman who presented an urticarial reaction following the Moderna mRNA-1273 vaccine and was positive on intradermal testing to an MRI contrast agent gadobutrol which contains tromethamine and negative to gadoteric acid which does not.59 The viral vector vaccines contain polysorbate 80,27 and this typically used excipient has also been linked to allergic reactions.54 Despite polysorbate 80 being an excipient in many vaccines and monoclonal antibodies, including other injectable drugs, it causes surprisingly few adverse events. Because excipients are not strictly regulated, the specific concentration present in drugs and vaccinations is often not precisely stated. Polysorbate 80 contains a lower molecular form of PEG (880 g/mL), and the evidence of cross-reactivity is through skin testing only.57
Lack of Evidence to Support Excipients as the Culprits of Allergic Reactions to Coronavirus Disease 2019 Messenger RNA Vaccines
Currently, there is a general lack of evidence to support that the prevalent immediate reactions associated with mRNA COVID-19 vaccines are related to an antigen-specific IgE-mediated reaction to an excipient component, such as PEG.60 Most reactions were occurring on the first dose of the mRNA vaccines in primarily women with a history of other allergic reactions. The reactions have rarely recurred on the second or subsequent dose and most patients were tolerant.61 , 62 The vast majority of people who experience these reactions have mild-to-moderate reactions that resolve without epinephrine. Tryptase levels, when done, have been normal compared with baseline.62 Those who have been rechallenged with a second or subsequent vaccine dose were largely without symptoms, and antihistamine administration pre- and post-dosing seems to help.61 , 62 This tolerance of the second and subsequent doses is very reassuring and is strongly suggestive against PEG or another excipient being the culprit of an antigen-specific IgE-mediated reaction. However, there may be rare patients who warrant risk mitigation and specific workup. Several studies now suggest that specific testing for excipients, such as PEG and PEG derivatives, is not helpful to risk stratify patients before first or second dosing.60 Furthermore, several studies have suggested that patients with immediate reactions to specific drugs containing PEG or PEG derivatives, such as pegaspargase of taxanes, are tolerant of the mRNA vaccines.63, 64, 65, 66 In addition, patients with known PEG anaphylaxis have tolerated the Janssen and AstraZeneca vaccines containing the PEG derivative polysorbate 80, despite being intradermal skin test positive to polysorbate 80 in 3 of 10 cases.67 , 68 Patients known to have PEG anaphylaxis have also tolerated the mRNA vaccines despite being skin test positive to polysorbate 80 or the mRNA vaccine.69 Importantly, tolerance of an mRNA vaccine in those with histories of PEG anaphylaxis does not suggest tolerance of PEG reagents. It appears that following tolerance of an mRNA vaccine, patients can still have anaphylaxis to PEG 3350 and higher molecular weight PEG products.69 Therefore, the role of detailed excipient testing in an individual who has a history of anaphylaxis to a non–COVID-19 vaccine-related PEG product is really to test the safety of administering PEG and other PEG derivatives and products unrelated to the COVID-19 vaccines in the future.55 , 57 It may be that the PEG 2000 in the vaccines is too low a molecular weight to be antigenic or that through the intramuscular injection of the vaccine, it is rapidly taken up by the reticuloendothelial system and not bioavailable to induce an immune response. Because mRNA technologies are now of great promise for vaccines and other therapeutics, this has great practical implications for the future. It is of interest that the epidemiology of PEG anaphylaxis, which occurs equally in men and women without prior allergic histories, is associated with PEG IgE and positive prick tests to PEG.57 , 70 PEG anaphylaxis intensifies over time and is often severe and initially requires repeat dosing of epinephrine. This is quite different from the COVID-19 vaccine allergy reactions that occur primarily in women with previous allergic histories and reactions typically remit with no treatment or antihistamines alone and do not intensify over time.57
Summary of Management of Allergic Reactions Associated With Severe Acute Respiratory Syndrome Coronavirus 2 Vaccines
Following the potentially life-threatening allergic reactions reported, allergists have responded rapidly during this period of high need to help risk stratify and manage patients based on the reported history of allergic reactions.26 An abundance of caution was taken during the first few weeks to months of the rollout of the COVID-19 vaccines and, after the billions of doses now given globally, these have now proven to be safe. Months after the beginning of the vaccination campaign, we are now reassured that any allergic potential of the mRNA vaccines is largely benign and that the vast majority of patients can be safely redosed without fear of a repeat reaction. Despite the fear that a component of the vaccines could be an allergen, there is clear evidence that even for the rare individuals confirmed to have allergy to PEG, these vaccines can be safely administered.69 In this context, investigations for possible PEG or polysorbate allergy before the vaccination are now not routinely suggested. Furthermore, we can be reassured that for those who tolerated the first and second doses of the vaccine, the chance of any subsequent reactions appears minimal and the longer observation periods initially recommended for those with an underlying allergy can now be relaxed for those with proven tolerance.
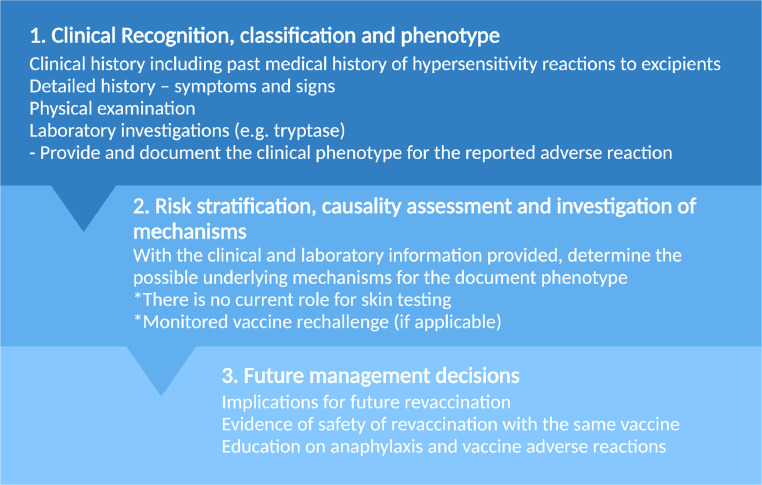
Overall, the confirmation of an allergic mechanism, such as an IgE-mediated reaction, should this occur rarely is suggested by in vivo investigations such as prick and intradermal testing or the detection of antigen-specific IgE antibodies.57 In the acute setting, it is recommended to test for tryptase, a biomarker that is elevated in anaphylaxis, and repeat this at minimum 24 hours later or at baseline. A suggested management strategy is described in Figure 3 .
Figure 3.
. Management approach.
Skin Testing
The evolving literature on skin testing in the previous year has allowed us to progress from the recommendation of performing PEG skin testing,27 despite absent information on sensitivity and specificity to avoiding routine skin testing with the vaccine or its excipients.11 Some study protocols have involved skin testing with 1:1 mRNA vaccine dilution with negative results in all patients confirmed by a negative mRNA vaccine challenge result.71, 72, 73 The previous guidelines for vaccine allergy testing have recommended performing skin prick test (SPT) and the undiluted vaccine followed by intradermal with a 1:100 dilution if the initial SPT result was negative.74 Despite the lack of international guidelines, non-irritating concentrations for vaccine excipients and standardization across different centers based on trialed concentrations, including for PEG testing, polysorbate 80, and tromethamine, are available.57 , 71 Currently, there is little evidence to support using excipient skin tests to risk stratify patients before vaccination, and it appears that most of the patients can go straight to first or subsequent mRNA vaccine dosing without testing. Indeed, the initial recommendation formulated for skin testing was based on a conservative expert recommendation derived from the 2012 vaccine anaphylaxis parameter guidance.6 Even if the positive predictive value of PEG SPT is not well described, this provides useful information in those with a high pretest probability of a PEG allergy in the context of a history of anaphylaxis to PEG.27
The PEG derivatives should be tested in individuals who have underlying suspected PEG anaphylaxis despite having tolerated an mRNA vaccine because tolerance of an mRNA vaccine does not reassure that the patient will tolerate the wide array of PEG products.
Vaccine Challenge
For patients with a history of anaphylaxis to any allergen, the recommendation is to offer a 30-minute observation period following the administration of the COVID-19 vaccine in a monitored area. The vaccination sites should have the equipment necessary to treat possible anaphylactic reactions. A shared decision-making approach is favored, and, in most cases, it appears that a single-dose vaccine rechallenge can be carried out in the setting of an immediate reaction on the first COVID-19 vaccine dose.11 Following previous cohort studies, it is now suggested that patients who are known for previous severe allergic reaction to a non-vaccine component can safely tolerate the COVID-19 vaccine.69 , 71 Various graded dosing75 or desensitization76 protocols have been suggested. As mentioned previously, oncology patients who presented immediate reactions to paclitaxel that contains polyoxyl-35 castor oil and pegaspargase, a multidrug chemotherapy regimen containing PEG 5000, have safely received the mRNA COVID-19 vaccines.64, 65, 66 Furthermore, evidence is emerging that patients who reported an immediate reaction and even anaphylaxis to the first mRNA vaccine dose can safely tolerate their second dose,17 , 61 , 62 supporting non–IgE-mediated mechanisms.
Follow-up and Long-term Advice
Currently, we have an incomplete understanding of the immune correlates of protection for SARS-CoV-2, and this affects our ability to understand the response to vaccines. It is clear that SARS-CoV-2 vaccines have the largest impact on reduction of illness severity, hospitalization, and death, and the very high effectiveness in preventing these primary outcomes in clinical trials has meant that it has not been easy to define the immune correlates of failure.
Newer therapies, such as tixagevimab co-packaged with cilgavimab (Evusheld), have been approved for preexposure prophylaxis with administration every 6 months. This agent is a SARS-CoV-2 monoclonal antibody combination reserved for the moderately to severely immune-compromised patients who may not mount an adequate response to vaccines. However, this drug should not replace vaccination event in the immunocompromised individuals. Another use for this antibody cocktail is listed as being those who have had a severe adverse reaction to a SARS-CoV-2 vaccine where future vaccination is precluded. A severe allergic reaction is listed as an example; however, we now know that it would be an extremely rare event for an allergic reaction to preclude future vaccination. Ideally, all patients with suspected allergic reactions associated with SARS-CoV-2 vaccines, such as the mRNA vaccines, should be assessed by an allergist with workup and observed vaccination as indicated. Interventions such as prophylactic monoclonal antibodies, a precious resource, should ideally be reserved for immunocompromised patients, such as those with primary immunodeficiency or transplantation. Following vaccine assessment, the patient needs to be provided with a clear management plan regarding future same-type vaccine administration and the likelihood of safely receiving other vaccines. One year following vaccine rollout, it is clear that allergy consultations continue to represent important guidance for risk assessment and vaccine guidance. We would suggest that the role of the allergist and immunologist is not to provide vaccine exemptions but to provide evidence-based confidence and reassurance to the patient who may be hesitant because of a reaction to a previous vaccine, drug, or a COVID-19 vaccine. Most immediate and delayed reactions seem to not recur on the second and subsequent doses. Over the next year, we will learn more about mechanisms; however, to date, the chance of tolerance with subsequent vaccination is excellent and the chance of harm is minimal.
Conclusion
Further research is needed to identify vaccine components that are responsible for immune-mediated hypersensitivity reactions and to understand the underlying mechanisms of vaccine reactions. This will shed light on the immunogenicity, reactogenicity, and allergenicity of current and future SARS-CoV-2 vaccine constructs. The use of effective vaccines is part of the long-time management strategy for SARS-CoV-2 and may continue to be important as the virus moves from a pandemic to an endemic infection. The risk of a reaction compared with the benefit of protection from severe illness and hospitalization is extremely small, and we can be reassured that although there are still many unanswered questions and controversies, there is already a very sound approach to ensure safe COVID-19 vaccination even in those with anaphylactic first-dose reactions (Table 3 ). As the pandemic and the number of SARS-CoV-2 viral variants evolve in the near future, new vaccines are built on an adapted mRNA construct, but the same delivery method and platform will rollout. The Allergy and Immunology community plays an enormous role in the education, clinical care, and research outputs that will result in optimized individual and public health.
Table 3.
Questions on How to Manage Those With a History of Anaphylaxis to a Vaccine Component or Anaphylaxis Following an mRNA Vaccine
| Question | Pro | Con | General consensus |
|---|---|---|---|
| Is there a role of skin testing to PEG or PEG derivatives to guide initial or future doses of COVID-19 vaccines? |
|
|
|
| Is there a role of skin testing to COVID-19 vaccines to guide initial or future doses of COVID-19 vaccines? |
|
|
|
| Is there a role for administering a future dose with a different COVID-19 vaccine platform |
|
|
|
| Is there a role for graded dosing of a COVID-19 vaccine |
|
|
|
| Is there a role to check SARS-CoV-2 antibodies to guide future dosing? |
|
|
|
| Should COVID-19 vaccination be deferred in someone who has had a history of a component reaction or first or second dose reaction? |
|
|
|
Abbreviations: COVID-19, coronavirus disease 2019; mRNA, messenger ribonucleic acid; PEG, polyethylene glycol; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Footnotes
Disclosures: Dr Phillips reports receiving grants from the National Institutes of Health (P50GM115305, R01HG010863, R01AI152183, U01AI154659, R13AR078623, UAI109565) and from the National Health and Medical Research Council of Australia; receiving royalties from UpToDate; receiving consulting fees from Janssen, Vertex, Biocryst, and Regeneron; serving as co-director of IIID Pty Ltd that holds a patent for HLA-B*57:01 testing for abacavir hypersensitivity and has a patent pending for Detection of Human Leukocyte Antigen-A*32:01 in connection with Diagnosing Drug Reaction with Eosinophilia and Systemic Symptoms without any financial remuneration and not directly related to the submitted work. The funders played no role in any aspect of this review.
Dr Copaescu reports receives support from the Montreal General Hospital Foundation and Research Institute of the McGill University Health Centre and was awarded The Anna Maria Solinas Laroche Career Award in Immunology and the Anita Garbarino Girard/Anna Maria Solinas/Dr Phil Gold Award of Distinction. Dr Rosa Duque has no conflicts of interest to report.
Funding: The authors have no funding sources to report.
References
- 1.World Health Organization. WHO coronavirus (COVID-19). 2021. Available at: https://covid19.who.int/2021. Accessed January 15, 2022.
- 2.Copaescu A, Smibert O, Gibson A, Phillips EJ, Trubiano JA. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J Allergy Clin Immunol. 2020;146(3):518–534. doi: 10.1016/j.jaci.2020.07.001. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou H, Zhang Y, Tang G, Luo Y, Liu W, Cheng C, et al. Immunologic memory to SARS-CoV-2 in convalescent COVID-19 patients at 1 year postinfection. J Allergy Clin Immunol. 2021;148(6):1481–1492. doi: 10.1016/j.jaci.2021.09.008. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone CA, Jr, Rukasin CRF, Beachkofsky TM, Phillips EJ. Immune-mediated adverse reactions to vaccines. Br J Clin Pharmacol. 2019;85(12):2694–2706. doi: 10.1111/bcp.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384(7):643–649. doi: 10.1056/NEJMra2035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelso JM, Greenhawt MJ, Li JT, Nicklas RA, Bernstein DI, Blessing-Moore J, et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130(1):25–43. doi: 10.1016/j.jaci.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Su JR, Moro PL, Ng CS, Lewis PW, Said MA, Cano MV. Anaphylaxis after vaccination reported to the Vaccine Adverse Event Reporting System, 1990-2016. J Allergy Clin Immunol. 2019;143(4):1465–1473. doi: 10.1016/j.jaci.2018.12.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNeil MM, Weintraub ES, Duffy J, Sukumaran L, Jacobsen SJ, Klein NP, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137(3):868–878. doi: 10.1016/j.jaci.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badiu I, Geuna M, Heffler E, Rolla G. Hypersensitivity reaction to human papillomavirus vaccine due to polysorbate 80. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr.02.2012.5797. bcr0220125797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampath V, Rabinowitz G, Shah M, Jain S, Diamant Z, Jesenak M, et al. Vaccines and allergic reactions: the past, the current COVID-19 pandemic, and future perspectives. Allergy. 2021;76(6):1640–1660. doi: 10.1111/all.14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenhawt M, Abrams EM, Shaker M, Chu DK, Khan D, Akin C, et al. The risk of allergic reaction to SARS-CoV-2 vaccines and recommended evaluation and management: a systematic review, meta-analysis, GRADE assessment, and international consensus approach. J Allergy Clin Immunol Pract. 2021;9(10):3546–3567. doi: 10.1016/j.jaip.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CDC COVID-19 Response Team, Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine - United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(2):46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US-December 14, 2020-January 18, 2021. JAMA. 2021;325(11):1101–1102. doi: 10.1001/jama.2021.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hourihane JO, Byrne AM, Blumchen K, Turner PJ, Greenhawt M. Ascertainment bias in anaphylaxis safety data of COVID-19 vaccines. J Allergy Clin Immunol Pract. 2021;9(7):2562–2566. doi: 10.1016/j.jaip.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu DK, Abrams EM, Golden DBK, Blumenthal KG, Wolfson AR, Jr Stone CA, et al. Risk of second allergic reaction to SARS-CoV-2 vaccines: a systematic review and meta-analysis. JAMA Intern Med. 2022;182(4):376–385. doi: 10.1001/jamainternmed.2021.8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blumenthal KG, Robinson LB, Camargo CA, Jr, Shenoy ES, Banerji A, Landman AB, et al. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325(15):1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krantz MS, Stone CA, Jr, Rolando LA, Nobis AE, Koo G, Corey KB, et al. An academic hospital experience screening mRNA COVID-19 vaccine risk using patient allergy history. J Allergy Clin Immunol Pract. 2021;9(10):3807–3810. doi: 10.1016/j.jaip.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shavit R, Maoz-Segal R, Iancovici-Kidon M, Offengenden I, Yahia SH, Maayan DM, et al. Prevalence of allergic reactions after Pfizer-BioNTech COVID-19 vaccination among adults with high allergy risk. JAMA Netw Open. 2021;4(8) doi: 10.1001/jamanetworkopen.2021.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips EJ. Allergic reactions after COVID-19 vaccination-putting risk into perspective. JAMA Netw Open. 2021;4(8) doi: 10.1001/jamanetworkopen.2021.22326. [DOI] [PubMed] [Google Scholar]
- 22.Macy E, Pandya S, Sheikh J, Bumette A, Shi JM, Chung J, et al. Population-based incidence, severity, and risk factors associated with treated acute-onset COVID-19 mRNA vaccination-associated hypersensitivity reactions. J Allergy Clin Immunol Pract. 2022;10(3):827–836. doi: 10.1016/j.jaip.2021.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porebski G, Kwiecien K, Pawica M, Kwitniewski M. Mas-related G protein-coupled receptor-X2 (MRGPRX2) in drug hypersensitivity reactions. Front Immunol. 2018;9:3027. doi: 10.3389/fimmu.2018.03027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang B, Li Q, Shi C, Zhang X. Drug-induced pseudoallergy: a review of the causes and mechanisms. Pharmacology. 2018;101(1-2):104–110. doi: 10.1159/000479878. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Selected adverse events reported after COVID-19 vaccination. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html. Accessed January 2, 2022.
- 26.Banerji A, Wickner PG, Saff R, Stone CA, Jr, Robinson LB, Long AA, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9(4):1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gold MS, MacDonald NE, McMurtry CM, Balakrishnan MR, Heininger U, Menning L, et al. Immunization stress-related response - redefining immunization anxiety-related reaction as an adverse event following immunization. Vaccine. 2020;38(14):3015–3020. doi: 10.1016/j.vaccine.2020.02.046. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Immunization stress-related response (ISRR)—a synopsis. 2019. Available at: https://www.who.int/immunization/sage/meetings/2019/april/2_A_synopsis_of_ISRR_Draft_SAGE.PDF. Accessed February 15, 2022.
- 29.Haas JW, Bender FL, Ballou S, Kelley JM, Wilhelm M, Miller FG, et al. Frequency of adverse events in the placebo arms of COVID-19 vaccine trials: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(1) doi: 10.1001/jamanetworkopen.2021.43955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catala A, Munoz-Santos C, Galvan-Casas C, Roncero Riesco M, revilla Nebreda D, Solá-Truyols A, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186(1):142–152. doi: 10.1111/bjd.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blumenthal KG, Freeman EE, Saff RR, Robinson LB, Wolfson AR, Foreman RK, et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384(13):1273–1277. doi: 10.1056/NEJMc2102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoff NP, Freise NF, Schmidt AG, Firouzi-Memarpuri P, Reifenberger J, Luedde T, et al. Delayed skin reaction after mRNA-1273 vaccine against SARS-CoV-2: a rare clinical reaction. Eur J Med Res. 2021;26(1):98. doi: 10.1186/s40001-021-00557-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Askenase PW. Rare skin reactions after mRNA vaccination, similar to Jones-Mote basophil responses. N Engl J Med. 2021;385(18):1720–1721. doi: 10.1056/NEJMc2111452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnston MS, Galan A, Watsky KL, Little AJ. Delayed localized hypersensitivity reactions to the moderna COVID-19 vaccine: a case series. JAMA Dermatol. 2021;157(6):716–720. doi: 10.1001/jamadermatol.2021.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niebel D, Novak N, Wilhelmi J, Ziob J, Wilsmann-Theis D, Bieber T, et al. Cutaneous adverse reactions to COVID-19 vaccines: insights from an immuno-dermatological perspective. Vaccines (Basel) 2021;9(9):944. doi: 10.3390/vaccines9090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freeman EE, Sun Q, McMahon DE, Singh R, Fathy R, Tyagi A, et al. Skin reactions to COVID-19 vaccines: an American Academy of Dermatology/International League of Dermatological Societies registry update on reaction location and COVID vaccine type. J Am Acad Dermatol. 2022;86(4):e165–e167. doi: 10.1016/j.jaad.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papasavvas I, de Courten C, Herbort CP., Jr. Varicella-zoster virus reactivation causing herpes zoster ophthalmicus (HZO) after SARS-CoV-2 vaccination - report of three cases. J Ophthalmic Inflamm Infect. 2021;11(1):28. doi: 10.1186/s12348-021-00260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furer V, Zisman D, Kibari A, Rimar D, Paran Y, Elkayam O. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford) 2021;60(SI):SI90–SI95. doi: 10.1093/rheumatology/keab345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katsikas Triantafyllidis K, Giannos P, Mian IT, Kyrtsonis G, Kechagias KS. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel) 2021;9(9):1013. doi: 10.3390/vaccines9091013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alkhalifah MI, Alsobki HE, Alwael HM, Al Fawaz AM, Al-Mezaine HS. Herpes simplex virus keratitis reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: a report of two cases. Ocul Immunol Inflamm. 2021;29(6):1238–1240. doi: 10.1080/09273948.2021.1986548. [DOI] [PubMed] [Google Scholar]
- 43.Brosh-Nissimov T, Sorek N, Yeshayahu M, Zherebovich I, Elmaliach M, Cahan A, et al. Oropharyngeal shedding of herpesviruses before and after BNT162b2 mRNA vaccination against COVID-19. Vaccine. 2021;39(40):5729–5731. doi: 10.1016/j.vaccine.2021.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Birabaharan M, Kaelber DC, Karris MY. Risk of herpes zoster reactivation after messenger RNA COVID-19 vaccination: a cohort study [e-pub ahead of print]. J Am Acad Dermatol. doi: 10.1016/j.jaad.2021.11.025. Accessed February 15, 2022. [DOI] [PMC free article] [PubMed]
- 45.Algaadi SA. Herpes zoster and COVID-19 infection: a coincidence or a causal relationship? Infection. 2022;50(2):289–293. doi: 10.1007/s15010-021-01714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers JP, Watson CJ, Badenoch J, Cross B, Butler M, Song J, et al. Neurology and neuropsychiatry of COVID-19: a systematic review and meta-analysis of the early literature reveals frequent CNS manifestations and key emerging narratives. J Neurol Neurosurg Psychiatry. 2021;92(9):932–941. doi: 10.1136/jnnp-2021-326405. [DOI] [PubMed] [Google Scholar]
- 47.Keshavarz P, Yazdanpanah F, Rafiee F, Mizandari M. Lymphadenopathy following COVID-19 vaccination: imaging findings review. Acad Radiol. 2021;28(8):1058–1071. doi: 10.1016/j.acra.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lam DL, Flanagan MR. Axillary lymphadenopathy after COVID-19 vaccination in a woman with breast cancer. JAMA. 2022;327(2):175–176. doi: 10.1001/jama.2021.20010. [DOI] [PubMed] [Google Scholar]
- 49.Pavord S, Scully M, Hunt BJ, Lester W, Bagot C, Craven B, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385(18):1680–1689. doi: 10.1056/NEJMoa2109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faruqi U, White K, Murray N, Cutler J, Breen K. The impact of COVID-19 vaccination on patients with a history of heparin induced thrombocytopenia [e-pub ahead of print]. Br J Haematol. doi: 10.1111/bjh.18048. Accessed February 15, 2022. [DOI] [PubMed]
- 51.Husby A, Hansen JV, Fosbol E, Thiesson EM, Madsen M, Thomsen RW, et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ. 2021;375 doi: 10.1136/bmj-2021-068665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patone M, Mei XW, Handunnetthi L, Dixon S, Zaccardi F, Shankar-Hari M, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28(2):410–422. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75–77. doi: 10.1038/s41569-021-00662-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caballero ML, Quirce S. Excipients as potential agents of anaphylaxis in vaccines: analyzing the formulations of currently authorized COVID-19 vaccines. J Investig Allergol Clin Immunol. 2021;31(1):92–93. doi: 10.18176/jiaci.0667. [DOI] [PubMed] [Google Scholar]
- 55.Caballero ML, Krantz MS, Quirce S, Phillips EJ, Stone CA., Jr. Hidden dangers: recognizing excipients as potential causes of drug and vaccine hypersensitivity reactions. J Allergy Clin Immunol Pract. 2021;9(8):2968–2982. doi: 10.1016/j.jaip.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pelham RW, Nix LC, Chavira RE, Cleveland MV, Stetson P. Clinical trial: single- and multiple-dose pharmacokinetics of polyethylene glycol (PEG-3350) in healthy young and elderly subjects. Aliment Pharmacol Ther. 2008;28(2):256–265. doi: 10.1111/j.1365-2036.2008.03727.x. [DOI] [PubMed] [Google Scholar]
- 57.Stone CA, Jr, Liu Y, Relling MV, Krantz MS, Pratt AL, Abreo A, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7(5):1533–1540. doi: 10.1016/j.jaip.2018.12.003. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lukawska J, Mandaliya D, Chan AWE, Foggitt A, Bidder T, Harvey J, et al. Anaphylaxis to trometamol excipient in gadolinium-based contrast agents for clinical imaging. J Allergy Clin Immunol Pract. 2019;7(3):1086–1087. doi: 10.1016/j.jaip.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 59.Rama TA, Coutinho RM, Mota D, Moreira A, Cernada J. Hypersensitivity to the Moderna COVID-19 vaccine caused by tromethamine: PEG is not always the culprit excipient [e-pub ahead of print]. J Investig Allergol Clin Immunol. doi: 10.18176/jiaci.0773. Accessed February 15, 2022. [DOI] [PubMed]
- 60.Wolfson AR, Robinson LB, Li L, McMahon AE, Cogan AS, Fu X, et al. First-dose mRNA COVID-19 vaccine allergic reactions: limited role for excipient skin testing. J Allergy Clin Immunol Pract. 2021;9(9):3308–3320. doi: 10.1016/j.jaip.2021.06.010. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krantz MS, Kwah JH, Stone CA, Jr, Phillips EJ, Ortega G, Banerji A, et al. Safety evaluation of the second dose of messenger RNA COVID-19 vaccines in patients with immediate reactions to the first dose. JAMA Intern Med. 2021;181(11):1530–1533. doi: 10.1001/jamainternmed.2021.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krantz MS, Bruusgaard-Mouritsen MA, Koo G, Phillips EJ, Jr Stone CA, Garvey LH. Anaphylaxis to the first dose of mRNA SARS-CoV-2 vaccines: don't give up on the second dose! Allergy. 2021;76(9):2916–2920. doi: 10.1111/all.14958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rush C, Faulk KE, Bradley ZK, Turner A, Krumins M, Greenhawt M. The safety of SARS-CoV-2 vaccines in persons with a known history of pegaspargase allergy: a single institution experience. J Allergy Clin Immunol Pract. 2022;10(2):630–632. doi: 10.1016/j.jaip.2021.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koo G, Anvari S, Friedman DL, Zarnegar-Lumley S, Szafron V, Kahwash BM, et al. mRNA COVID-19 vaccine safety in patients with previous immediate hypersensitivity to pegaspargase. J Allergy Clin Immunol Pract. 2022;10(1):322–325. doi: 10.1016/j.jaip.2021.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mark C, Gupta S, Punnett A, Upton J, Orkin J, Atkinson A, et al. Safety of administration of BNT162b2 mRNA (Pfizer-BioNTech) COVID-19 vaccine in youths and young adults with a history of acute lymphoblastic leukemia and allergy to PEG-asparaginase. Pediatr Blood Cancer. 2021;68(11):e29295. doi: 10.1002/pbc.29295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banerji A, Wolfson AR, Robinson LB, McMahon AE, Cogan AS, Saff RR, et al. COVID-19 vaccines tolerated in patients with paclitaxel and docetaxel allergy. Allergy. 2022;77(3):1048–1051. doi: 10.1111/all.15178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sellaturay P, Gurugama P, Harper V, Dymond T, Ewan P, Nasser S. The polysorbate containing AstraZeneca COVID-19 vaccine is tolerated by polyethylene glycol (PEG) allergic patients. Clin Exp Allergy. 2022;52(1):12–17. doi: 10.1111/cea.14064. [DOI] [PubMed] [Google Scholar]
- 68.Bruusgaard-Mouritsen MA, Koo G, Heinrichsen AS, Melchiors BB, Krantz MS, Plager JH, et al. Janssen COVID-19 vaccine tolerated in 10 patients with confirmed polyethylene glycol allergy. J Allergy Clin Immunol Pract. 2022;10(3):859–862. doi: 10.1016/j.jaip.2021.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Picard M, Drolet JP, Masse MS, Filion CA, AlMuhizi F, Fein M, et al. Safety of COVID-19 vaccination in patients with polyethylene glycol allergy: a case series. J Allergy Clin Immunol Pract. 2022;10(2):620–625. doi: 10.1016/j.jaip.2021.11.021. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou ZH, Stone CA, Jr, Jakubovic B, Phillips EJ, Sussman G, Park J, et al. Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J Allergy Clin Immunol Pract. 2021;9(4):1731–1733. doi: 10.1016/j.jaip.2020.11.011. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rojas-Perez-Ezquerra P, Crespo Quiros J, Tornero Molina P, Baeza Ochoa de Ocariz ML, Zubeldia Ortuno JM. Safety of new mRNA vaccines against COVID-19 in severely allergic patients. J Investig Allergol Clin Immunol. 2021;31(2):180–181. doi: 10.18176/jiaci.0683. [DOI] [PubMed] [Google Scholar]
- 72.Marcelino J, Farinha S, Silva R, Didenko I, Proenca M, Tomaz E. Nonirritant concentrations for skin testing with SARS-CoV-2 mRNA vaccine. J Allergy Clin Immunol Pract. 2021;9(6):2476–2477. doi: 10.1016/j.jaip.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pienkowski MM, Pienkowski SM. Evaluation of anaphylaxis risk by skin testing with coronavirus disease 2019 messenger RNA vaccines on patients with anaphylaxis. Ann Allergy Asthma Immunol. 2022;128(1):101–103. doi: 10.1016/j.anai.2021.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Broyles AD, Banerji A, Barmettler S, Biggs CM, Blumenthal K, Brennan PJ, et al. Practical guidance for the evaluation and management of drug hypersensitivity: specific drugs. J Allergy Clin Immunol Pract. 2020;8(9S):S16–S116. doi: 10.1016/j.jaip.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 75.Tuong L, Capucilli P, Staicu M, Ramsey A, Walsh E, Mustafa S. Graded administration of second dose of moderna and Pfizer-BioNTech COVID-19 mRNA vaccines in patients with hypersensitivity to first dose. Open Forum Infect Dis. 2021;8(12):ofab507. doi: 10.1093/ofid/ofab507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.AlMuhizi F, Ton-Leclerc S, Fein M, Tsoukas C, Garvey LH, Lee D, et al. Successful desensitization to mRNA COVID-19 vaccine in a case series of patients with a history of anaphylaxis to the first vaccine dose. Front Allergy. 2022;3:1–8. doi: 10.3389/falgy.2022.825164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lutrick K, Rivers P, Yoo YM, Grant L, Hollister J, Jovel K, et al. Interim estimate of vaccine effectiveness of BNT162b2 (Pfizer-BioNTech) vaccine in preventing SARS-CoV-2 infection among adolescents aged 12-17 years - Arizona, July-December 2021. MMWR Morb Mortal Wkly Rep. 2021;70(5152):1761–1765. doi: 10.15585/mmwr.mm705152a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walter EB, Talaat KR, Sabharwal C, Gurtman A, Lockhart S, Paulsen GC, et al. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 years of age. N Engl J Med. 2022;386(1):35–46. doi: 10.1056/NEJMoa2116298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ali K, Berman G, Zhou H, Deng W, Faughnan V, Coronado-Voges M, et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N Engl J Med. 2021;385(24):2241–2251. doi: 10.1056/NEJMoa2109522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385(13):1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326(1):35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jara A, Undurraga EA, Gonzalez C, Paredes F, Fontecilla T, Jara G, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385(10):875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tanriover MD, Doganay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fadlyana E, Rusmil K, Tarigan R, Rahmadi AR, Prodjosoewojo S, Sofiatin Y, et al. A phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-CoV-2 inactivated vaccine in healthy adults aged 18-59 years: an interim analysis in Indonesia. Vaccine. 2021;39(44):6520–6528. doi: 10.1016/j.vaccine.2021.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ella R, Reddy S, Blackwelder W, Potdar V, Yadav P, Sarangi V, et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet. 2021;398(10317):2173–2184. doi: 10.1016/S0140-6736(21)02000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.American College of Rheumatology. COVID-19 vaccine clinical guidance summary for patients with rheumatic and musculoskeletal diseases. 2021. Available at: https://www.rheumatology.org/Portals/0/Files/COVID-19-Vaccine-Clinical-Guidance-Rheumatic-Diseases-Summary.pdf. Accessed January 11, 2022.
- 90.Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. 2021. Available at: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html. Accessed January 17, 2022.
- 91.Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977–982. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Centers for Disease Control and Prevention. Updates on thrombosis with thrombocytopenia syndrome (TTS). 2021. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-12-16/02-COVID-See-508.pdf2022. Accessed January 16, 2022.
- 93.Broyles AD, Banerji A, Castells M. Practical guidance for the evaluation and management of drug hypersensitivity: general concepts. J Allergy Clin Immunol Pract. 2020;8(9S):S3–S15. doi: 10.1016/j.jaip.2020.08.002. [DOI] [PubMed] [Google Scholar]