ABSTRACT
Objective:
Obstructive sleep apnea (OSA) is associated with an increased risk of mortality and cardiometabolic diseases. The STOP-Bang questionnaire is a tool to screen populations at risk of OSA and prioritize complementary studies. Our objective was to evaluate the clinical utility of this questionnaire in identifying patients at an increased risk of mortality after discharge in a cohort of hospitalized patients.
Methods:
This was a prospective cohort study involving consecutive patients admitted to an internal medicine unit between May and June of 2017 who were reevaluated three years after discharge. At baseline, we collected data on comorbidities (hypertension, obesity, diabetes, and fasting lipid profile) and calculated STOP-Bang scores, defining the risk of OSA (0-2 score, no risk; ≥ 3 score, risk of OSA; and ≥ 5 score, risk of moderate-to-severe OSA), which determined the study groups. We also recorded data regarding all-cause and cardiovascular mortality at the end of the follow-up period.
Results:
The sample comprised 435 patients. Of those, 352 (80.9%) and 182 (41.8%) had STOP-Bang scores ≥ 3 and ≥ 5, respectively. When compared with the group with STOP-Bang scores of 0-2, the two groups showed higher prevalences of obesity, hypertension, diabetes, and dyslipidemia. Multivariate analysis showed an independent association between cardiovascular mortality and STOP-Bang score ≥ 5 (adjusted hazard ratio = 3.12 [95% CI, 1.39-7.03]; p = 0.01). Additionally, previous coronary heart disease was also associated with cardiovascular mortality.
Conclusions:
In this cohort of hospitalized patients, STOP-Bang scores ≥ 5 were able to identify patients at an increased risk of cardiovascular mortality three years after discharge.
Keywords: Sleep Apnea, Obstructive; Risk assessment; Surveys and questionnaires; Cardiovascular diseases/mortality
RESUMO
Objetivo:
A apneia obstrutiva do sono (AOS) está associada a um risco maior de mortalidade e doenças cardiometabólicas. O questionário STOP-Bang é uma ferramenta para rastrear populações em risco de AOS e assim priorizar estudos complementares. Nosso objetivo foi avaliar a utilidade clínica desse questionário na identificação de pacientes com risco aumentado de mortalidade após a alta em uma coorte de pacientes hospitalizados.
Métodos:
Estudo de coorte prospectivo com pacientes consecutivos internados em uma unidade de medicina interna entre maio e junho de 2017 que foram reavaliados três anos após a alta. No momento basal, coletamos dados sobre comorbidades (hipertensão, obesidade, diabetes e perfil lipídico em jejum) e calculamos as pontuações no STOP-Bang, definindo o risco de OSA (pontuação 0-2, sem risco; pontuação ≥ 3, risco de AOS; e pontuação ≥ 5, risco de AOS moderada a grave), que determinou os grupos de estudo. Também registramos dados sobre mortalidade por todas as causas e mortalidade cardiovascular ao final do período de acompanhamento.
Resultados:
Foram incluídos 435 pacientes. Desses, 352 (80,9%) e 182 (41,8%) apresentaram pontuações no STOP-Bang ≥ 3 e ≥ 5, respectivamente. Quando comparados com o grupo com pontuação no STOP-Bang de 0-2, os outros dois grupos apresentaram prevalências mais elevadas de obesidade, hipertensão, diabetes e dislipidemia. A análise multivariada mostrou uma associação independente entre mortalidade cardiovascular e pontuação no STOP-Bang ≥ 5 (razão de risco ajustada = 3,12 [IC95%, 1,39-7,03]; p = 0,01). Além disso, doença coronariana prévia também foi associada à mortalidade cardiovascular.
Conclusões:
Nesta coorte de pacientes hospitalizados, pontuações no STOP-Bang ≥ 5 foram capazes de identificar pacientes com risco aumentado de mortalidade cardiovascular três anos após a alta.
Descritores: Apneia obstrutiva do sono, Medição de risco, Inquéritos e questionários, Doenças cardiovasculares/mortalidade
INTRODUCTION
Obstructive sleep apnea (OSA) is a prevalent condition, with an increased risk of cardiovascular complications. This condition is prevalent and underdiagnosed in some countries, mainly due to a lack of sleep study testing. Previous studies report that 1 billion people are at risk of OSA worldwide. 1 , 2 In Chile, the population at risk of OSA is 22%, and the risk of moderate-to-severe OSA is about 9%. 3 According to the American Academy of Sleep Medicine, OSA can be diagnosed through polysomnography or type III channel. The diagnosis requires the presence of an abnormal apnea-hypopnea index (AHI ≥ 5 events/h), and the presence of at least one of the following symptoms: sleepiness/fatigue, sleep disturbed by gasping or choking, snoring or apnea witnessed by a third party, or comorbidities such as hypertension, coronary artery disease/stroke, heart failure, diabetes, and mood changes. The diagnosis can be directly made with an AHI ≥ 15 events/h. Severity ranges from mild OSA (AHI between 5-15 events/h), moderate OSA (AHI between 15-29 events/h), and severe OSA (AHI ≥ 30 events/h). 4 , 5 Discrimination between mild OSA and moderate-to-severe OSA is relevant to clinical practice. Moderate-to-severe OSA is associated with an increased risk of hypertension, diabetes mellitus (DM), dyslipidemia, obesity, and major cardiovascular events-acute myocardial infarction (AMI), coronary heart disease (CHD), stroke, atrial fibrillation (AF), and cardiovascular mortality. 6
Although sleep studies are scant in some countries, different clinical questionnaires are available to identify populations at risk of OSA. This approach is also useful to define populations with an increased risk of cardiovascular comorbidities, such as hospitalized patients. Moreover, by means of these clinical predictor rules, clinicians can also identify populations with a high pre-test probability of OSA and, therefore, define the best cost-benefit diagnostic study after discharge.
The STOP-Bang questionnaire is a useful clinical screening tool for patients at risk of OSA (threshold score, ≥ 3), and of moderate-to-severe OSA (threshold score, ≥ 5). The sensitivity and specificity of this questionnaire are 90% and 49%, respectively, using a cut-off score ≥ 3 to identify the risk of OSA. However, using a cut-off score of ≥ 5, the sensitivity and specificity to identify the risk of moderate-to-severe OSA are 96% and 25%, respectively. Given the excellent sensitivity, the STOP-Bang questionnaire has been proposed as a screening tool in different epidemiological studies. 7 , 8
The combination of OSA and other significant cardiovascular comorbidities included in the STOP-Bang questionnaire suggests that it could help identify the population with an increased risk of mortality in the medium term, especially in hospitalized patients. The objective of the present study was to evaluate the association between the risk of OSA measured by the STOP-Bang questionnaire and the risk of all-cause and cardiovascular mortality in hospitalized patients.
METHODS
This was an observational, prospective cohort study following current recommendations from the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. 9 Between May and June of 2017, we consecutively included patients admitted to the internal medicine unit in one single university health care center located in the city of Los Ángeles, Chile.
The included cohort was prospectively followed up by June of 2020. Patients admitted for any medical reason were screened for potential inclusion. We included patients > 18 years of age who gave a written informed consent. We excluded patients who were unable to complete the questionnaire, those submitted to any type of surgery during hospitalization, transferred to an ICU, considered to be at the end of life due to any medical comorbidity, or lost to follow-up. The study protocol was approved by the Ethics Research Committee of the IRB Servicio de Salud Bio-Bio (Protocol #25, August of 2017). The study was conducted in accordance with the guidelines set forth in the Declaration of Helsinki and good clinical practice.
Demographic data (gender, age [being elderly was defined as being ≥ 65 years of age]), as well as smoking history, alcohol consumption, comorbidities at baseline (arterial hypertension, DM, CHD, and stroke), and current medications (lipid-lowering, antidiabetic, antihypertensive, and anticoagulant medications), were collected. Data about comorbidities and reason for admission were retrieved by both self-report and medical records.
Exposure was defined as the risk of OSA measured by the STOP-Bang score at admission. The questionnaire was evaluated according to a prior validation in the population in Chile. 7 , 8 Sleep-related symptoms (snoring, tiredness, and observed apnea) were recorded by either self-report or partner response. Blood pressure was measured with a standard mercury sphygmomanometer on the left arm after 10 min of rest in accordance with the current guidelines of the American Heart Association. 10 Weight and height were measured after an overnight fast with patients wearing only underwear. BMI was calculated as weight (kg)/height2(m2), and neck circumference was measured using a plastic tape meter at the cricoid level (Adam’s apple).
A venous blood sample was collected from all of the patients within the first 48 h after admission. The sample was obtained in the morning after an overnight fast. We included a fasting lipid profile. Patients with an abnormal lipid profile were classified according to the type of dyslipidemia: LDL dyslipidemia; hypertriglyceridemia; mixed dyslipidemia; and HDL dyslipidemia, in accordance with current recommendations. 10 Using the clinical and laboratory data, we calculated the cardiovascular risk using the American College of Cardiology/American Heart Association Atherosclerotic Cardiovascular Disease (ASCVD) risk score 10 at baseline in the population between 34 to 79 years of age.
The primary outcome of the present study was the risk of all-cause mortality after discharge in patients with a STOP-Bang score ≥ 3 and in those with a score ≥ 5. As a reference, both groups were compared with patients with a STOP-Bang score between 0-2 after follow-up. The secondary outcome was the risk of cardiovascular mortality in the same groups. Mortality data was obtained by the Servicio de Registro Civil e Identificación (www.registrocivil.cl) and categorized as either all-cause mortality or cardiovascular mortality in accordance with the International Classification of Diseases, version 9 (ICD-9). Details regarding cardiovascular mortality as defined in the present study are available in Chart S1 (124.9KB, pdf) .
Individual data were included in a case report form and transferred to an Excel spreadsheet. Continuous data were reported as means and standard deviations; categorical data were reported as frequencies. Intergroup differences were evaluated using the Student’s t-test for continuous data and using the chi-square test or Fisher’s exact test for categorical data. Odds ratio and respective 95% CIs were also reported.
The association between the groups and the primary outcome was evaluated using Kaplan-Meier survival analysis and the log-rank test (Mantel-Cox test). The incidence ratio of mortality was evaluated using an adjusted hazard ratio (HR) with Cox proportional hazards regression. As covariables, we included confounder variables related to an increased risk of cardiovascular mortality not included in the STOP-Bang questionnaire (dyslipidemia, smoking history, alcohol consumption, previous CHD, ASCVD risk score, and use of lipid-lowering, antidiabetic, antihypertensive, and anticoagulant medications at baseline). The level of significance was set at p < 0.05. Statistical analysis was performed with the SPSS Statistics software package, version 25.0 (IBM Corp., Armonk, NY, USA).
RESULTS
A flow chart of the patient selection process is shown in Figure 1. A total of 510 patients were screened, and 435 were included in the study for further analysis. The mean age of the cohort was 60.98 ± 17.10 years, 199 (45.7%) of the patients were considered elderly, 233 (53.6%) were male, 352 (80.9%) had a STOP-Bang score ≥ 3, and 192 (44.1%) had a STOP-Bang score ≥ 5. The ASCVD risk scores regarding patients with a STOP-Bang score of 0-2, ≥ 3, and ≥ 5, respectively, were 10.1 ± 11.2%, 22.6 ± 16.3%, and 24.6 ± 15.8%. A summary of baseline characteristics and reasons for admission is shown in Table S1 (124.9KB, pdf) .
Figure 1. Flow chart of the patient selection process during the study period.
A summary of clinical characteristics and differences among the groups are shown in Table 1. The mean age in the group with a STOP-Bang score ≥ 3 (n = 352) was 62.0 ± 16.5 years, 196 (55.7%) were male, and the mean BMI was 33.9 ± 6.5 kg/m2. The prevalences of hypertension, DM, and dyslipidemia were 73.3%, 43.8%, and 59.4%, respectively. Of the 209 patients with dyslipidemia, 71 (34.0%) had hypertriglyceridemia, 60 (28.7%) had LDL dyslipidemia, and 78 (37.3%) had mixed dyslipidemia. Cardiovascular reasons for admission were heart failure, new-onset AF, unstable angina, and AMI.
Table 1. Clinical characteristics of the patients included in the study according to STOP-Bang scores (N = 435).a .
| Characteristic | STOP-Bang score | p* | ||
|---|---|---|---|---|
| 0-2 | ≥ 3 | ≥ 5 | ||
| (n = 83) | (n = 352) | (n = 182) | ||
| Age, years | 56.61 ± 18.74 | 62.01 ± 16.57 | 62.55 ± 15.04 | 0.01 |
| Age ≥ 65 years | 38.5% | 47.4% | 65.1% | 0.12 |
| Male | 44.57% | 55.68% | 60.43% | 0.01 |
| BMI, kg/m2 | 30.72 ± 5.73 | 33.93 ± 6.58 | 35.78 ± 6.96 | < 0.01 |
| BMI ≥ 35 kg/m2 | 9.4% | 12.5% | 14.1% | < 0.01 |
| ASCVD, % | 10.1 ± 11.2 | 22.6 ± 16.3 | 24.6 ± 15.8 | < 0.01 |
| Former/current smoking | 38.4% | 52.3% | 59.1% | < 0.01 |
| Hypertension | 19.2% | 73.2% | 84.0% | < 0.01 |
| Diabetes mellitus | 14.4% | 43.7% | 50.0% | < 0.01 |
| Dyslipidemia | 37.3% | 59.3% | 59.3% | 0.01 |
| Hypertriglyceridemia | 41.9% | 33.9% | 34.3% | |
| High LDL | 38.7% | 28.7% | 24.0% | |
| Mixed dyslipidemia | 19.3% | 37.3% | 41.6% | |
| Medication | ||||
| Lipid-lowering | 31.6% | 48.3% | 43.5% | < 0.01 |
| Antidiabetic | 9.2% | 39.4% | 48.1% | < 0.01 |
| Anticoagulant | 16.2% | 52.0% | 71.5% | < 0.01 |
| Antihypertensive | 15.2% | 68.7% | 75.4% | < 0.01 |
| Reason for admission | ||||
| Decompensated HF | 12.0% | 13.0% | 10.9% | 0.47 |
| AF | 4.6% | 34.0% | 1.0% | 0.17 |
| CHD | 15.6% | 15.9% | 13.7% | 0.40 |
| AMI | 1.2% | 2.5% | 37.9% | 0.29 |
ASCVD: American College of Cardiology/American Heart Association Atherosclerotic Cardiovascular Disease risk score; HF: heart failure; AF: atrial fibrillation; CHD: coronary heart disease; and AMI: acute myocardial infarction; aValues expressed as mean ± SD, except where otherwise indicated. *In comparison with the STOP-Bang score 0-2 group.
The mean age in the group with a STOP-Bang score ≥ 5 (n = 182) was 62.55 ± 15.00, and the mean BMI was 35.78 ± 6.9 kg/m2. Of the 182 patients in this group, the prevalence of hypertension, DM, and dyslipidemia were 84.0%, 50.0%, and 59.3%, respectively. Of the 108 patients with dyslipidemia, 37 (34.3%) had hypertriglyceridemia, 26 (24.1%) had LDL dyslipidemia, and 45 (41.7%) had mixed dyslipidemia. The reasons for hospitalization in this group were heart failure, in 10.9%; new-onset AF, in 1.1%; unstable angina, in 13.7%; and AMI, in 37.9%.
In comparison with the control group (STOP-Bang score of 0-2), those with a score of ≥ 3 and ≥ 5 presented with a similar clinical profile, with no statistically significant differences. A greater number of patients in both study groups were elderly (p = 0.09) and male (p = 0.01). In addition, the two study groups showed greater prevalences of comorbidities (BMI ≥ 35 kg/m2, hypertension, DM, and dyslipidemia; p < 0.01 for all). Although no differences were found regarding cardiovascular events at admission between the control and the study groups (Table 1), there was a significant increased risk of both all-cause and cardiovascular mortality (p = 0.04 and p = 0.01, respectively) in the group with a STOP-Bang score ≥ 5 (Table 2).
Table 2. Summary of the associations of STOP-Bang scores with all-cause and cardiovascular mortality.
| Unadjusted HR (95% CI) | p | Adjusted HR* (95% CI) | p | |
|---|---|---|---|---|
| All-cause mortality | ||||
| STOP-Bang ≥ 3 | 1.36 (0.77-2.36) | 0.28 | 1.52 (0.83-2.79) | 0.17 |
| STOP-Bang ≥ 5 | 1.45 (0.96-2.20) | 0.07 | 1.58 (1.01-2.48) | 0.04 |
| Cardiovascular mortality | ||||
| STOP-Bang ≥ 3 | 2.94 (0.88-9.78) | 0.07 | 4.67 (1.27-17.08) | 0.02 |
| STOP-Bang ≥ 5 | 2.31 (1.13-4.70) | 0.04 | 3.12 (1.39-7.03) | 0.01 |
HR: hazard ratio. *Cox proportional hazards model adjusted by dyslipidemia, former or current smoking, alcohol consumption, previous cardiovascular heart disease, and American College of Cardiology/American Heart Association Atherosclerotic Cardiovascular Disease risk score, as well as lipid-lowering, antidiabetic, antihypertensive, and anticoagulant medications at baseline.
After 36 months of follow-up, 95 patients died, cardiovascular events being the reason in 34. The clinical characteristics of those who died or survived are shown in Tables 3 and 4. In the unadjusted analysis, a STOP-Bang score ≥ 3 showed no significant associations with all-cause mortality (HR = 1.36 [95% CI, 0.77-2.36]; p = 0.28) or cardiovascular mortality (HR = 2.94 [95% CI, 0.88-9.78]; p = 0.07). However, the adjusted analysis showed a significant association with cardiovascular mortality (HR = 4.67 [95% CI, 1.27-17.08]; p = 0.02).
Table 3. Comparison between all-cause mortality and survival groups after follow-up.a .
| Baseline characteristic | Group | p | |
|---|---|---|---|
| All-cause mortality | Survival | ||
| (n = 95) | (n = 340) | ||
| Age, years | 68.1 ± 14.3 | 59.3 ± 17.3 | < 0.01 |
| Age ≥ 65 years | 61.7 | 42.0 | 0.01 |
| Male | 61.7 | 51.6 | 0.06 |
| STOP-Bang score | 4.2 ± 1.4 | 3.9 ± 1.8 | 0.19 |
| ≥ 3 | 87.6 | 79.3 | 0.05 |
| ≥ 5 | 48.1 | 40.3 | 0.12 |
| Hypertension | 77.7 | 59.6 | 0.01 |
| Diabetes mellitus | 44.4 | 36.7 | 0.12 |
| Dyslipidemia | 54.3 | 55.3 | 0.48 |
| BMI ≥ 35 kg/m2 | 9.8 | 10.7 | 0.50 |
| Reason for admission | |||
| Decompensated HF | 13.5 | 12.7 | 0.47 |
| Unstable angina | 49.3 | 18.3 | 0.01 |
| AMI | 1.2 | 25.4 | 0.41 |
| AF | 3.7 | 33.8 | 0.55 |
HF: heart failure; AMI: acute myocardial infarction; and AF: atrial fibrillation. aValues expressed as mean ± SD or %.
Table 4. Comparison between cardiovascular mortality and survival groups after follow-up.a .
| Baseline characteristic | Group | p | |
|---|---|---|---|
| CV mortality | Survival | ||
| (n = 34) | (n = 340) | ||
| Age, years | 73.8 ± 11.8 | 60.4 ± 17.1 | 0.02 |
| Age ≥ 65 years | 75.0 | 43.7 | 0.01 |
| Male | 67.8 | 52.5 | 0.08 |
| STOP-Bang score | 4.6 ± 1.1 | 3.9 ± 1.7 | 0.04 |
| ≥ 3 | 86.4 | 79.8 | 0.06 |
| ≥ 5 | 67.1 | 40.7 | 0.01 |
| Hypertension | 67.8 | 62.6 | 0.36 |
| Diabetes mellitus | 50.0 | 37.3 | 0.12 |
| Dyslipidemia | 46.4 | 55.7 | 0.08 |
| BMI ≥ 35 kg/m2 | 13.5 | 11.0 | 0.48 |
| Reason for admission | |||
| Decompensated HF | 25.0 | 12.0 | 0.05 |
| Unstable angina | 10.7 | 16.2 | 0.32 |
| AMI | 3.5 | 2.2 | 0.49 |
| AF | 10.7 | 2.9 | 0.06 |
HF: heart failure; AMI: acute myocardial infarction; and AF: atrial fibrillation. aValues expressed as mean ± SD or %.
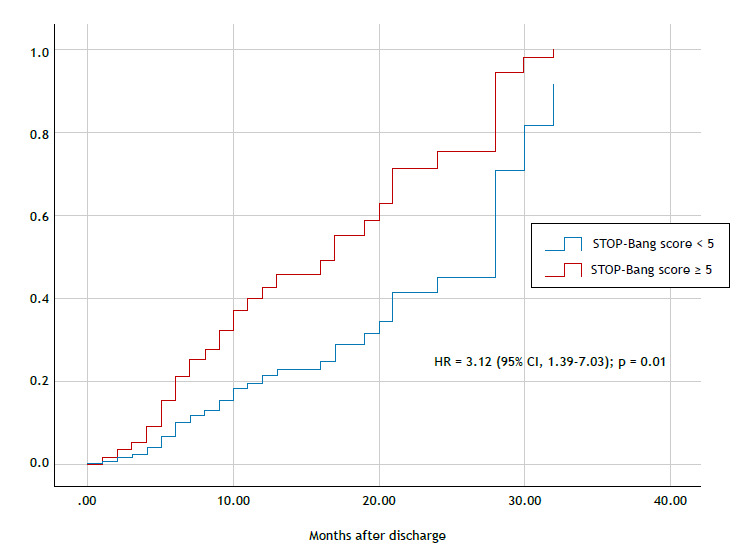
In the unadjusted analysis, a STOP-Bang score ≥ 5 had no association with all-cause mortality (HR = 1.45 [95% CI, 0.96-2.20]; p = 0.17). However, the adjusted analysis showed a significant association (HR = 1.58 [95% CI, 1.01-2.48]; p = 0.04). Regarding cardiovascular mortality, both unadjusted and adjusted analyses showed significant associations with a STOP-Bang score ≥ 5: HR = 2.31 (95% CI, 1.13-4.70); p = 0.02; and HR = 3.12 (95% CI, 1.39-7.03); p = 0.01, respectively (Table 2 and Figure 2). The summary of the unadjusted and adjusted analyses is shown in Table 2. In addition, previous CHD at baseline was also associated with cardiovascular mortality in the group with a STOP-Bang score ≥ 5 (HR = 2.34 [95% CI, 1.04-5.26]; p = 0.04).
Figure 2. Cumulative incidence of cardiovascular mortality in the groups of patients with a STOP-Bang score < 5 (blue line) and ≥ 5 (red line). HR: hazard ratio.
DISCUSSION
The main findings of the present study were that the STOP-Bang questionnaire was able to identify cardiovascular risk in hospitalized patients up to 36 months of follow-up; that, after adjusted analyses, the independent variables associated with a higher risk of cardiovascular mortality were a STOP-Bang score ≥ 3 and CHD at baseline; and that a STOP-Bang score ≥ 5 was associated with a higher risk of all-cause and cardiovascular mortality.
Hospitalized patients present with different levels of risk than does the general population. In our study, we hypothesized that the STOP-Bang questionnaire could identify populations with an increased risk of cardiovascular mortality to prioritize additional sleep studies. Moreover, we found an increased prevalence of patients at risk of OSA when we compared results from previous hospital-based studies. 11 - 13 Sharma et al. 11 reported a prevalence of OSA in 84% of hospitalized obese patients (BMI ≥ 30 kg/m2). Goring & Collop 12 reported a prevalence of OSA diagnosed by polysomnography of 77% in hospitalized patients. Identifying hospitalized patients at risk of OSA is an important issue, because they have response events more rapidly. Therefore, the use of validated questionnaires that indicate a higher risk of OSA is a useful intervention to reduce complications. 13 The present study found an increased risk for OSA (80%) in our sample, and 40% of patients had a STOP-Bang score ≥ 5. This high prevalence is due to older age, obesity, and hypertension, which are common in hospitalized patients. Moreover, after a three-year follow-up period, this increased prevalence was associated with an increased risk of cardiovascular mortality.
The STOP-Bang questionnaire was initially developed by researchers in the field of anesthesiology. 14 Previous studies evaluated its accuracy in different clinical practices, such as in sleep study centers or in perioperative settings. 15 , 16 This questionnaire is an easy tool to screen populations at risk of OSA. Additionally, the STOP-Bang questionnaire has been shown to be a good screening tool to identify individuals with hypertension or DM at risk of OSA. 17 - 19
Data regarding the use of the STOP-Bang questionnaire in hospitalized patients are scarce, and the clinical utility of this questionnaire in this population is unclear. Previously, we analyzed the cross-sectional relationships of STOP-Bang questionnaire scores with cardiovascular events (composite outcomes included major adverse cardiovascular events, cardiovascular mortality, acute coronary syndrome, and decompensated heart failure) during the first 30 days of hospitalization and with cardiovascular risk using the ASCVD risk score calculator. According to the ASCVD risk score, we found that the cardiovascular risk in patients with a STOP-Bang score ≥ 3 was 24.3%, whereas that in those with a STOP-Bang score of 0-2 was 10.9%. 20
In the present study, we included a single cohort of an understudied population (hospitalized individuals admitted to an internal medicine unit) followed for 36 months to determine the clinical utility of the STOP-Bang questionnaire in the identification of the risk for all-cause and cardiovascular mortality. We hypothesized that populations at risk of OSA would have an increased risk of cardiovascular mortality, mainly due to cardiovascular events defined in the ICD-9. We predefined to include hospitalized patients with mild-to-moderate disease in order to rule out those with severe acute disease, who might present with an increased risk of morbidity and sequelae. Our results provide new data about hospitalized patients. We studied the clinical utility of the STOP-Bang questionnaire in a specific population at risk of general or moderate-to-severe OSA. Our results demonstrated clinical differences between patients at risk of OSA and those without that risk. Additionally, after confounder analysis, previous CHD at baseline was also associated with cardiovascular mortality.
We also found an increased prevalence of cardiometabolic disease in patients at risk of OSA. First, the prevalence of hypertension ranged between 73% and 84%, which is higher than the prevalence reported in other studies. 21 However, most of those studies were performed in sleep study centers, not with hospitalized patients. Second, the prevalence of DM ranged between 44% and 50%. Pataka et al. 17 reported a prevalence of DM of 57% in patients with severe OSA; the sensitivity of the STOP-Bang questionnaire in the patients with DM was 81% for mild OSA and 95% for severe OSA. In that study, 17 the prevalence of dyslipidemia was 14%, whereas that was 59% in our study. An increased risk of dyslipidemia was previously reported by Chou et al. 22 in patients with OSA (61.1%). In another study, 23 the prevalence of dyslipidemia was 26% in the general population. In accordance with data from the European Sleep Apnea Database, 24 the prevalence of hyperlipidemia was 26% and 15% in patients with and without OSA, respectively, and this increase was independent after confounder analysis. However, those studies were performed in outpatient scenarios, either as population-based studies or at sleep study centers. Finally, regarding cardiovascular reasons for admission, the discharge of patients with heart failure and OSA was independently associated with an increased risk of mortality after 6-12 months of follow-up (RR = 1.53). 25
The strengths of the present study are its prospective design, including consecutive Latin American patients admitted for any medical reason to an internal medicine service and the fact that the completion of the STOP-Bang questionnaire and the initial evaluation were performed within the first 48 h after admission.
The study also has limitations. The patients included were not submitted to sleep studies to confirm or rule out OSA. However, this study was designed to evaluate the risk of mortality using the STOP-Bang questionnaire, and these data can be used to improve the identification of hospitalized patients with an increased risk of OSA and, therefore, of worse outcomes, which might allow the incorporation of various interventions to reduce that risk.
In conclusion, in this cohort of hospitalized patients in an internal medicine unit, the STOP-Bang questionnaire was able to identify those with an increased risk of mortality. A STOP-Bang score ≥ 5 is an easy and useful tool to identify patients at risk of all-cause and cardiovascular mortality within a three-year follow-up period.
Footnotes
Financial support: None.
Study carried out at the IRB Servicio de Salud Bio-Bio, Los Ángeles, Chile.
REFERENCES
- 1.Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of Obstructive Sleep Apnea a Population-based Perspective. Expert Rev Respir Med. 2008;2(3):349–364. doi: 10.1586/17476348.2.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ. Estimation of the global prevalence and burden of obstructive sleep apnoea a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrillo J, Vargas C, Cisternas A, Olivares-Tirado P. Obstructive sleep apnea findings from the Chilean National Health Survey 2010 Eur Resp. 2017;50:PA1190–PA1190. doi: 10.1183/1393003.congress-2017.PA1190. [DOI] [Google Scholar]
- 4.Qaseem A, Dallas P, Owens DK, Starkey M, Holty JE, Shekelle P. Diagnosis of obstructive sleep apnea in adults a clinical practice guideline from theAmerican College of Physicians. Ann Intern Med. 2014;161(3):210–220. doi: 10.7326/M12-3187. [DOI] [PubMed] [Google Scholar]
- 5.Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(3):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labarca G, Cruz NR, Descalzi F. Multisystemic involvement in obstructive sleep apnea [Article in Spanish] Rev Med. Chil. 2014;142(6):748–757. doi: 10.4067/S0034-98872014000600009. [DOI] [PubMed] [Google Scholar]
- 7.Saldías Peñafiel F, Gassmann Poniachik J, Canelo López A, Uribe Monasterio J, Díaz Patiño O. Accuracy of sleep questionnaires for obstructive sleep apnea syndrome screening [Article in Spanish] Rev Med. Chil. 2018;146(10):1123–1134. doi: 10.4067/S0034-98872018001001123. [DOI] [PubMed] [Google Scholar]
- 8.Labarca G, Dreyse J, Salas C, Gaete MI, Jorquera J. Performance of instruments aimed at detecting obstructive sleep apnea syndrome among individuals in Chile. J Bras Pneumol. 2019;46(1):e20190015. doi: 10.1590/1806-3713/e20190015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in J Am Coll. Pt BCardiol. 2014;63(25):3024–3025. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Sharma S, Mather PJ, Efird JT, Kahn D, Shiue KY, Cheema M. Obstructive Sleep Apnea in Obese Hospitalized Patients A Single Center Experience. J Clin Sleep Med. 2015;11(7):717–723. doi: 10.5664/jcsm.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goring K, Collop N. Sleep disordered breathing in hospitalized patients. J Clin Sleep Med. 2008;4(2):105–110. doi: 10.5664/jcsm.27125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma S, Chowdhury A, Tang L, Willes L, Glynn B, Quan SF. Hospitalized Patients at High Risk for Obstructive Sleep Apnea Have More Rapid Response System Events and Intervention Is Associated with Reduced Events. PLoS One. 2016;11(5):e0153790. doi: 10.1371/journal.pone.0153790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagappa M, Liao P, Wong J, Auckley D, Ramachandran SK, Memtsoudis S. Validation of the STOP-Bang Questionnaire as a Screening Tool for Obstructive Sleep Apnea among Different Populations A Systematic Review and Meta-Analysis. PLoS One. 2015;10(12):e0143697. doi: 10.1371/journal.pone.0143697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagappa M, Patra J, Wong J, Subramani Y, Singh M, Ho G. Association of STOP-Bang Questionnaire as a Screening Tool for Sleep Apnea and Postoperative Complications A Systematic Review and Bayesian Meta-analysis of Prospective and Retrospective Cohort Studies. Anesth Analg. 2017;125(4):1301–1308. doi: 10.1213/ANE.0000000000002344. [DOI] [PubMed] [Google Scholar]
- 16.Boynton G, Vahabzadeh A, Hammoud S, Ruzicka DL, Chervin RD. Validation of the STOP-BANG Questionnaire among Patients Referred for Suspected Obstructive Sleep Apnea. J Sleep Disord Treat Care. 2013;2(4) doi: 10.4172/2325-9639.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pataka A, Kalamaras G, Daskalopoulou E, Argyropoulou P. Sleep questionnaires for the screening of obstructive sleep apnea in patients with type 2 diabetes mellitus compared with non-diabetic patients. J Diabetes. 2019;11(3):214–222. doi: 10.1111/1753-0407.12835. [DOI] [PubMed] [Google Scholar]
- 18.Schiavone M, Ernst G, Blanco M, Avaca H, Acosta AL, Nosetto D. Performance of questionnaires aimed at detecting sleep disorders in patients attending a hypertension center. Clin Exp Hypertens. 2019;41(7):687–691. doi: 10.1080/10641963.2018.1539095. [DOI] [PubMed] [Google Scholar]
- 19.Westlake K, Plihalova A, Pretl M, Lattova Z, Polak J. Screening for obstructive sleep apnea syndrome in patients with type 2 diabetes mellitus a prospective study on sensitivity of Berlin and STOP-Bang questionnaires. Sleep Med. 2016;26:71–76. doi: 10.1016/j.sleep.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Labarca G, Valdivia G, Oñate A, Navarrete C, Araya J, Fernandez-Bussy I. Prevalence of STOP BANG questionnaire and association with major cardiovascular events in hospitalized population is it enough with currently used cardiovascular risk measurements?. Sleep. Med. 2019;61:82–87. doi: 10.1016/j.sleep.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 21.Salas C, Dreyse J, Contreras A, Nazar G, Astorquiza C, Cabezon R. Differences in patients derived from otolaryngology and other specialties with sleep apnea. J Otolaryngol Head Neck Surg. 2019;48(1):53–53. doi: 10.1186/s40463-019-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chou YT, Chuang LP, Li HY, Fu JY, Lin SW, Yang CT. Hyperlipidaemia in patients with sleep-related breathing disorders prevalence & risk factors. Indian J Med Res. 2010;131:121–125. [PubMed] [Google Scholar]
- 23.Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N. Prevalence of sleep-disordered breathing in the general population the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunduz C, Basoglu OK, Hedner J, Bonsignore MR, Hein H, Staats R. Hyperlipidaemia prevalence and cholesterol control in obstructive sleep apnoea Data from the European sleep apnea database (ESADA) J Intern Med. 2019;286(6):676–688. doi: 10.1111/joim.12952. [DOI] [PubMed] [Google Scholar]
- 25.Khayat R, Jarjoura D, Porter K, Sow A, Wannemacher J, Dohar R. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J. 2015;36(23):1463–1469. doi: 10.1093/eurheartj/ehu522. [DOI] [PMC free article] [PubMed] [Google Scholar]


