Abstract
The plasma pharmacokinetics of multilamellar liposomal nystatin were studied in normal, catheterized rabbits after single and multiple daily intravenous administration of dosages of 2, 4, and 6 mg/kg of body weight, and drug levels in tissues were assessed after multiple dosing. Concentrations of liposomal nystatin were measured as those of nystatin by a validated high-performance liquid chromatography method, and plasma concentration data were fitted into a two-compartment open model. Across the investigated dosage range, liposomal nystatin demonstrated nonlinear kinetics with more than proportional increases in the AUC0–24 and decreasing clearance, consistent with dose-dependent tissue distribution and/or a dose-dependent elimination process. After single-dose administration, the mean Cmax increased from 13.07 μg/ml at 2 mg/kg to 41.91 μg/ml at 6 mg/kg (P < 0.001); the AUC0–24 changed from 11.65 to 67.44 μg · h/ml (P < 0.001), the Vd changed from 0.205 to 0.184 liters/kg (not significant), the CLt from 0.173 to 0.101 liters/kg · h (P < 0.05), and terminal half-life from 0.96 to 1.51 h (P < 0.05). There were no significant changes in pharmacokinetic parameters after multiple dosing over 14 days. Assessment of tissue concentrations of nystatin near peak plasma levels after multiple dosing over 15 days revealed preferential distribution to the lungs, liver, and spleen at that time point. Substantial levels were also found in the urine, raising the possibility that renal excretion may play a significant role in drug elimination. Liposomal nystatin administered to rabbits was well tolerated and displayed nonlinear pharmacokinetics, potentially therapeutic peak plasma concentrations, and substantial penetration into tissues. Pharmacokinetic parameters were very similar to those observed in patients, thus validating results derived from infection models in the rabbit and allowing inferences to be made about the treatment of invasive fungal infections in humans.
Nystatin, a tetraene diene macrolide, was discovered as the first antifungal polyene in the early 1950s (11). Nystatin possesses potent and broad-spectrum antifungal activity in vitro (10); like amphotericin B, it binds to ergosterol, the main sterol in the cell membranes of fungi, leading to channel formation and cell death (14). While the compound has been used widely in topical formulations, problems with solubilization and toxicity after parenteral administration have curtailed its utility for systemic treatment (10; D. Andes, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother. abstr. 1002, p. 28, 1999).
In the late 1980s, however, laboratory investigators incorporated nystatin into a multilamellar liposome preparation consisting of dimyristoyl phosphatidylcholine (DMPC) and dimyristoyl phosphatidylglycerol (DMPG) in a 7:3 molar ratio (17). This novel formulation preserved antifungal activity in vitro but was considerably less toxic to mammalian cells than the free drug (17) and prolonged survival in a murine model of disseminated candidiasis (18). More recently, a multilamellar liposome formulation of nystatin with the same constituents but a defined particle size of 0.1 to 3 μm (25) was launched for clinical development. This liposomal formulation has demonstrated in vivo activity in stringent neutropenic animal models of invasive opportunistic fungal infections (5, 6, 8, 21). It was tolerated without reaching dose-limiting toxicities at dosages of up to 8 mg/kg of body weight/day in a phase I clinical trial in patients with hematological malignancies and refractory febrile neutropenia (E. Boutati, H. C. Maltezou, G. Lopez-Berestein, S. E. Vartivarian, and E. Anaissie, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. LM22, p. 330, 1995), and it is currently undergoing phase II and III clinical trials in patients with proven or suspected invasive fungal infections (R. Powles, S. Mawhorter, and A. H. Williams, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. LB-4, p. 14, 1999; A. H. Williams and J. E. Moore, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1420, p. 567, 1999).
Little is known, however, about the disposition of liposomal nystatin in the bloodstream and tissues. The purpose of this experimental study was therefore to assess the compartmental plasma pharmacokinetics and tissue distribution of liposomal nystatin in order to provide a foundation for understanding the pharmacokinetic and pharmacodynamic relationships of this novel liposomal antifungal compound.
MATERIALS AND METHODS
Study drug.
Liposomal nystatin (Nyotran; 50-mg vials; 50 mg of 95% pure nystatin A1 USP incorporated into a mixture of 350 mg of DMPC and 150 mg of DMPG; DMPC/DMPG ratio, 7:3 [wt/wt]; drug/lipids ratio, 1:10 [wt/wt]; particle size after reconstitution, 0.1 to 3.0 μm; Aronex Pharmaceuticals, The Woodlands, Tex.) was provided as a lyophilized powder and maintained at 4°C. The drug was freshly reconstituted prior to use with 50 ml of sterile normal saline to a 1-mg/ml solution. According to the recommendations of the manufacturer at the time of the study, following the addition of normal saline, vials were vigorously shaken for 1 min, placed in a 40°C water bath for 15 min, removed from the water bath, and vigorously shaken for another 60 s. The reconstituted drug was administered at ambient temperature as a slow intravenous infusion over 10 min.
Animals.
Healthy female New Zealand White rabbits (Hazleton, Denver, Pa.) weighing 2.8 to 3.5 kg were used in all experiments. They were individually housed and maintained with water and standard rabbit feed ad libitum according to the National Institutes of Health guidelines for laboratory animal care (3) and in fulfillment of American Association for Accreditation of Laboratory Animal Care criteria. Vascular access was established in each rabbit prior to experimentation by the surgical placement of a subcutaneous silastic central venous catheter as previously described (22).
Single-dose studies.
Three groups of four rabbits each were studied. Animals received liposomal nystatin at either 2, 4, or 6 mg/kg of body weight as a single, steady intravenous bolus over 10 min. Plasma samples (1.5 ml of blood) were drawn immediately before administration of the compound, immediately after administration, and then at 5, 10, 20, 40, and 60 min and 2, 3, 4, 5, 6, 7, 8, 10, 12, 18, and 24 h after administration.
Multiple-dose studies.
After completion of single-dose pharmacokinetics studies, the three cohorts continued to receive liposomal nystatin at either 2, 4, or 6 mg/kg of body weight as a single, steady intravenous bolus once a day for a total of 15 days. Animals were reweighed on day 7 and on day 14 prior to dosing, and dosages was adjusted to the new weight. On day 14, plasma samples were drawn immediately before dosing, immediately after dosing, and then at 5, 10, 20, 40, and 60 min and 2, 3, 4, 5, 6, 7, 8, 10, 12, 18, and 24 h postdosing. On day 15, following another blood draw, rabbits were euthanized with intravenous (i.v.) pentobarbital 30 min after administration of liposomal nystatin, and brain tissue, cerebrospinal fluid (CSF), choroid, vitreous and aqueous humors, lung, liver, spleen, kidney, psoas muscle, and fat (perirenal) tissues, bone marrow (femur), urine (expressed from the urinary bladder), and bile (aspirated from the gallbladder) were obtained at postmortem for determination of drug concentrations at near-peak levels in plasma.
Hepatic and renal toxicities were monitored during the multiple-dose pharmacokinetic study by biochemical profiles of blood urea nitrogen (BUN), serum creatinine, potassium, magnesium, hepatic transaminases, total bilirubin, and alkaline phosphatase obtained on the first and last days of administration of the drug. All animals were clinically evaluated each day and weighed once weekly.
Processing of blood and tissue.
Blood samples were collected in heparinized syringes. Plasma was separated by centrifugation and stored at −80°C until assay.
Following autopsy, tissues (for solid tissues, the entire organ) and body fluids were stored at −80°C. Before assay, tissues were thawed and three aliquots of approximately 1 g were weighed out for each sample (Mettler AE 163; Mettler Instrument Corp., Hightstown, N.J.). The specimens were thoroughly rinsed with phosphate-buffered saline, pH 7.4 (Quality Biological, Inc., Gaithersburg, Md.). Buffer solution remaining on the tissue surface was blotted with Micro Wipes (Scott Paper Company, Philadelphia, Pa.). Specimens were then reweighed and homogenized with ice-cold high-performance liquid chromatography (HPLC)-grade methanol (1:2 [wt/wt]; Fisher Scientific, Fair Lawn, N.J.). Tissues were homogenized in a high-speed tissue homogenizer (Tekmar, Cincinnati, Ohio) with a 10 N head twice, for 30 s each time, with the sample being placed in an ice bucket. Standards and quality control samples for drug analysis in tissues were similarly prepared by homogenizing normal tissue in 1:2 [wt/wt] ice-cold HPLC-grade methanol and adding known amounts of compound. Homogenized samples were incubated for 30 min at 4°C and centrifuged at 2,000 × g for 10 min. A 1.5-ml portion of the methanolic supernatant was transferred to 1.5-ml polypropylene tubes and centrifuged at 10,000 × g for 4 min. Four hundred microliters of the resulting supernatant was transferred to 0.22-μm-pore-size Durapore filter tubes (Ultra-Free MC; Millipore, Bedford, Mass.), centrifuged at 4,000 × g for 4 min, and submitted to an assay. Drug concentrations in tissues were calculated to 1 g of tissue.
Extraction of drug from plasma and body fluids involved addition of 1 ml of HPLC-grade methanol to 500 μl of sample (2:1 [vol/vol]) and incubation at 4°C for 30 min. This was followed by centrifugation at 2,000 × g for 10 min, transfer of the supernatant to 1.5-ml polypropylene tubes, and a second centrifugation at 10,000 × g for 4 min. Four hundred microliters of the resulting supernatant was transferred to 0.22-μm-pore-size Durapore filter tubes (Ultra-Free MC; Millipore), centrifuged at 4,000 × g for 4 min, and submitted to an assay. Standards and quality control samples were similarly prepared by adding known amounts of bulk nystatin (Gist-Procades, Wilmington, Del.) to either normal rabbit serum (for serum and choroid; Gibco Laboratories, Grand Island, N.Y.), commercially available CSF standards (Instrumentation Laboratories, Lexington, Mass.), Hanks' balanced salt solution (for vitreous and aqueous humors; Mediatech, Herndon, Va.), or normal rabbit bile or urine, followed by precipitation with HPLC-grade methanol (1:2 [wt/wt]). Blank samples of all matrices also were extracted to ensure the absence of interfering peaks.
Analytical method.
Levels of drug in plasma, body fluids, and tissue homogenates were determined as total unassociated (free) nystatin using a fully validated HPLC method (7) and bulk nystatin (Gist-Procades) as the reference standard. The mobile phase consisted of 10 mM sodium phosphate, 1 mM EDTA, 30% HPLC-grade methanol, and 30% HPLC-grade acetonitrile (Fisher Scientific). Samples were kept at 4°C by a cooling module attached to the autosampler. The injection volume was 200 μl, and the flow rate was 2.0 ml/min. Nystatin was detected by UV absorbance at 305 nM using a C18 analytical column maintained at 30°C (μBondapak C18; 300 by 3.9 mm; internal diameter, 100Å; particle size, 10 μm; Waters Corp., Milford, Mass.) in conjunction with an in-line precolumn filter (NewGuard RP-18, Perkin-Elmer, Norwalk, Conn.).
Quantification was based on the sum of the peak area-concentration of the two major isomers of nystatin, which eluted at 6.4 to 6.5 and 8.1 to 8.2 min, and the peak area-concentration response of the external calibration standard. Seven-point standard curves (range of concentrations, 0 to 1.5 and 1.5 to 50 μg/ml for plasma; 0 to 1.5 μg/ml for CSF standards and Hanks' balanced salt solution; 0 to 20 μg per g or ml, respectively, for solid tissues and remaining body fluids) were linear with r2 values greater than 0.97. The lower limit of quantification (LLQ) was 0.050 μg/ml in plasma. Accuracies were within 11%, and intra- and interday variabilities (precision) were <10% for plasma and <13 and 12%, respectively, for all tissues and remaining body fluids with the exception of bile (≤13 and ≤45%), where numerous interfering peaks were present in blank matrix.
Pharmacokinetic analysis.
Pharmacokinetic parameters for liposomal nystatin were determined using compartmental analysis. Experimental plasma concentration-time data were fit to a two-compartment open model with i.v. bolus input and elimination from the central compartment using iterative weighted nonlinear least-squares regression with the ADAPT II computer program (4). Weighting was by maximum a posteriori probability, and model selection was guided by Akaike's information criterion (27). The model fit the data well with a mean coefficient of determinations (r2) of 0.991 (range, 0.975 to 0.999), and the regression lines through the plot of observed versus estimated concentrations did not differ from the line of identity. Similar fits were obtained for an open three-compartment model with i.v. bolus input and elimination from the central compartment; however, according to standard practices, the less-complex model was chosen for final data analysis. Fitted parameters included maximum plasma concentrations (Cmax), the area under the concentration-vs.-time curve from time zero (start of the i.v. bolus) to 24 h (AUC0–24); total clearance (CLt), distributional clearance (CLd), volume of distribution (VD), volume of distribution of the central (VDc) and the peripheral (VDp) compartments, distributional half-life (t1/2α), and elimination half-life (t1/2β). Given that the last measurable time point was different among dosage groups, and assuming that all values below the LLQ were 0.05 (i.e., the LLQ), the fraction of the extrapolated AUC was less than 7% for all dosage groups.
Statistical analysis.
Differences between the means of pharmacokinetic parameters across dosage levels were evaluated by analysis of variance (ANOVA) with Bonferroni's correction for multiple comparisons. For comparison of two dosage levels, Student's t test or Welch's t test was used, as appropriate. Simple linear regression and one-way ANOVA were utilized to assess dose linearity. A two-tailed P value of <0.05 was considered statistically significant.
RESULTS
Single-dose studies.
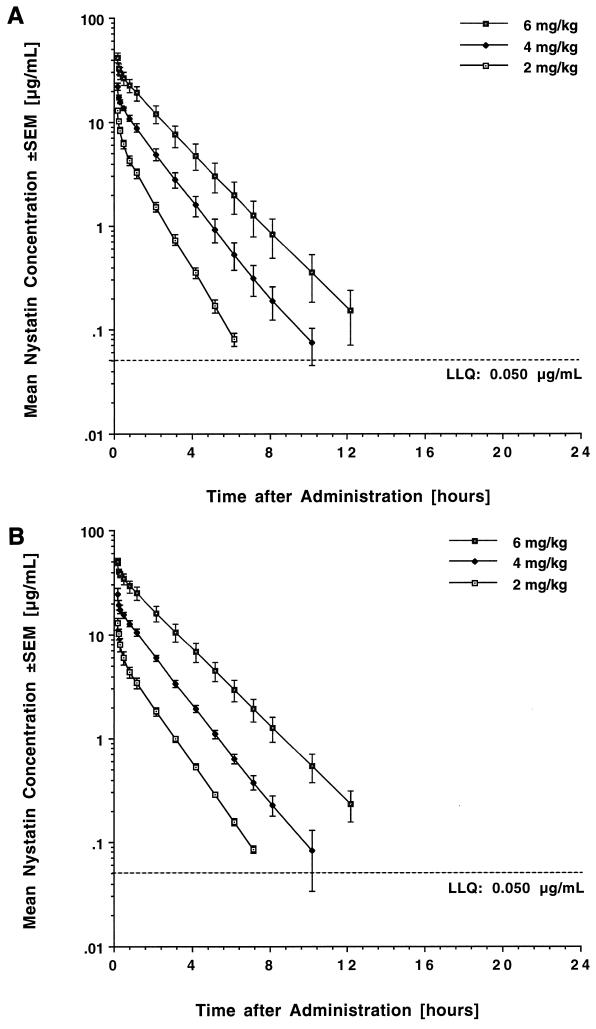
The estimated plasma concentration-versus-time curves following single-dose administration of liposomal nystatin are shown in Fig. 1A, and corresponding mean pharmacokinetic parameters for the three dosage levels are listed in Table 1.
FIG. 1.
Concentration-versus-time profiles in plasma after intravenous administration of liposomal nystatin over 10 min. (A) Single-dose profiles after administration of 2, 4, and 6 mg/kg. (B) Profiles after administration of 2, 4, and 6 mg/kg over 14 days. Each point plots the mean level of four rabbits at that time point.
TABLE 1.
Single-dose compartmental pharmacokinetic parameters of liposomal nystatin in plasmaa
| Drug dose (mg/kg) | Cmaxb (μg/ml) | Cmin (μg/ml) | AUC0–24b (μg · h/ml) | VD (liters/kg) | VDc (liters/kg) | VDp (liters/kg) | CLtc (liters/kg · h) | CLd (liters/kg · h) | t1/2α (h) | t1/2βd (h) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 13.07 ± 0.52 | 0.00 ± 0.00 | 11.65 ± 0.82 | 0.205 ± 0.011 | 0.121 ± 0.004 | 0.084 ± 0.008 | 0.173 ± 0.011 | 0.205 ± 0.011 | 0.140 ± 0.008 | 0.962 ± 0.040 |
| 4 | 22.39 ± 1.05 | 0.00 ± 0.00 | 29.77 ± 2.80 | 0.225 ± 0.007 | 0.146 ± 0.008 | 0.079 ± 0.002 | 0.137 ± 0.011 | 0.284 ± 0.017 | 0.117 ± 0.006 | 1.240 ± 0.087 |
| 6 | 41.91 ± 4.15 | 0.00 ± 0.00 | 67.44 ± 13.09 | 0.184 ± 0.025 | 0.119 ± 0.011 | 0.074 ± 0.010 | 0.101 ± 0.022 | 0.258 ± 0.030 | 0.116 ± 0.012 | 1.510 ± 0.161 |
All values are expressed as means ± standard errors of the means. Cmin, plasma concentration at the end of the dosing interval.
P < 0.001 by ANOVA.
P = 0.03 by ANOVA.
P = 0.01 by ANOVA.
Administration of the compound at dosages ranging from 2 to 6 mg/kg resulted in escalating peak plasma levels ranging from 13.07 ± 0.52 to 401.91 ± 4.15 μg/ml. Liposomal nystatin was rapidly distributed, followed by a slower elimination phase with a terminal half-life ranging from 0.96 to 1.51 h. Mean plasma levels fell below the LLQ in a dose-dependent manner at 7, 12, and 18 h postdosing. Over the investigated dose range, increases in dosage resulted in more than proportional increases in the AUC0–24 (P < 0.05 for the comparison of dose-normalized AUC0–24 values among groups). This coincided with a decrease in total clearance and an increased elimination half-life, consistent with nonlinear disposition of the compound. The volume of distribution was close to that of plasma and, although not statistically significant, appeared to decrease at the highest dosage level.
Multiple-dose studies.
The estimated plasma concentration-versus-time profiles of liposomal nystatin following once-daily administration of 2, 4, and 6 mg/kg over 14 days are shown in Fig. 1B, and the corresponding mean pharmacokinetic parameters are listed in Table 2.
TABLE 2.
Compartmental pharmacokinetic parameters of liposomal nystatin in plasma after multiple dosing over 14 daysa
| Drug dose (mg/kg · day) | Cmaxb (μg/ml) | Cmin (μg/ml) | AUC0–24b (μg · h/ml) | VD (liters/kg) | VDc (liters/kg) | VDp (liters/kg) | CLtc (liters/kg · h) | CLd (liters/kg · h) | t1/2α (h) | t1/2βd (h) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 12.86 ± 1.34 | 0.00 ± 0.00 | 11.15 ± 1.23 | 0.249 ± 0.039 | 0.123 ± 0.011 | 0.126 ± 0.030 | 0.168 ± 0.019 | 0.272 ± 0.077 | 0.141 ± 0.013 | 1.200 ± 0.054 |
| 4 | 24.65 ± 2.10 | 0.00 ± 0.00 | 32.00 ± 2.25 | 0.230 ± 0.024 | 0.137 ± 0.011 | 0.092 ± 0.012 | 0.118 ± 0.008 | 0.205 ± 0.043 | 0.182 ± 0.045 | 1.520 ± 0.189 |
| 6 | 50.40 ± 3.79 | 0.00 ± 0.00 | 88.20 ± 14.06 | 0.159 ± 0.017 | 0.101 ± 0.008 | 0.057 ± 0.008 | 0.071 ± 0.013 | 0.186 ± 0.004 | 0.128 ± 0.013 | 1.700 ± 0.097 |
All values are expressed as means ± standard errors of the means. Cmin, plasma concentration at the end of the dosing interval.
P < 0.001 by ANOVA.
P = 0.005 by ANOVA.
P = 0.03 by ANOVA.
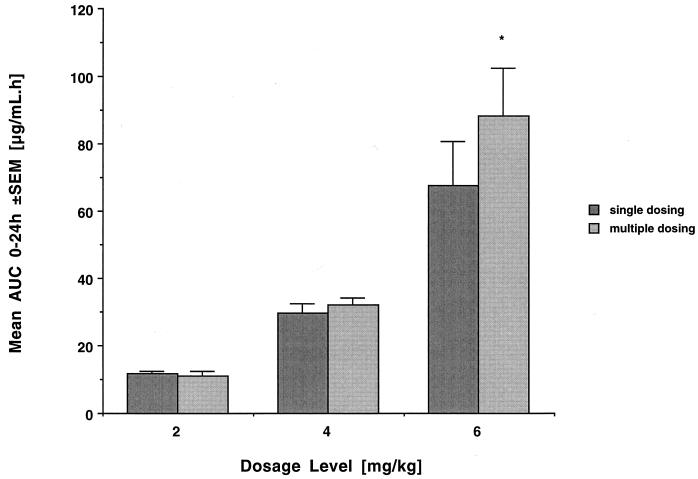
Plasma levels immediately prior to dosing were below the LLQ. Peak plasma concentrations immediately after dosing were not significantly different from those observed after administration of a single dose. As with single dosing, mean plasma levels fell below the LLQ in a dose-dependent manner at 8, 12, and 18 h postdosing. There were no significant differences in AUC0–24 (Fig. 2), CLt, and half-life compared to those with single dosing. Accordingly, drug disposition after multiple dosing retained its nonlinear character (P < 0.005 for the comparison of dose-normalized AUC0–24 values among groups). This again coincided with a decrease in total clearance, an increase in half-life, and a trend toward a decrease in the volume of distribution at the highest dosage level.
FIG. 2.
AUC0–24 at the three investigated dosage levels after single and multiple administration of liposomal nystatin (mean values for four rabbits each ± standard errors of the means). Note the disproportional increases in AUC at the highest dosage level but the lack of significant accumulation of drug in plasma over time (asterisk, not significant).
Tissue distribution.
Mean tissue levels at near-peak plasma concentrations after multiple dosing over 15 days are shown in Table 3. The highest drug levels were detected in the lungs, liver, and spleen. Relative to the AUC0–24, the disposition in the lungs and liver decreased with increasing dosage, whereas it appeared proportional to the AUC0–24 in spleen tissue. Drug levels in the kidney were relatively low and proportional to the AUC at the 2- and 4-mg/kg dosage level but increased more than proportionally in relation to the AUC at the 6-mg/kg dose level. Notably, compared to concurrent plasma concentrations, high drug levels were found in the urine at all dosage levels. Levels in bile fluid were lower and highly variable at the 2- and 4-mg/kg dosage levels but increased substantially at the highest dosage level. Although nystatin was undetectable in CSF, it was detectable in brain tissue in all rabbits receiving 4 and 6 mg/kg/day. There was considerable drug distribution into the choroidal layer of the eye; low but detectable levels were found in aqueous humor, but not in vitreous humor.
TABLE 3.
Tissue levels after daily dosing with liposomal nystatin over 15 daysa
| Tissue | Level of drug at the indicated drug dose [mg/kg · day]
|
||
|---|---|---|---|
| 2.0 | 4.0 | 6.0 | |
| Lung (μg/g) | 20.84 ± 4.28 (1.631) | 32.83 ± 10.09 (0.911) | 72.84 ± 10.78 (0.757) |
| Liver (μg/g) | 13.21 ± 2.17 (1.034) | 18.52 ± 0.54 (0.514) | 41.26 ± 2.32 (0.429) |
| Spleen (μg/g) | 6.95 ± 1.07 (0.544) | 17.11 ± 1.38 (0.475) | 46.57 ± 3.07 (0.484) |
| Kidney (μg/g) | 1.79 ± 0.19 (0.140) | 5.18 ± 0.43 (0.143) | 22.85 ± 4.22 (0.237) |
| Bone marrow (μg/g) | 1.04 ± 0.19 (0.081) | 2.49 ± 0.36 (0.069) | 7.46 ± 0.76 (0.077) |
| Skeletal muscle (μg/g) | 0.95 ± 0.01 (0.074) | 1.17 ± 0.04 (0.032) | 1.98 ± 0.17 (0.020) |
| Perirenal fat (μg/g) | 0.33 ± 0.02 (0.025) | 0.97 ± 0.19 (0.026) | 2.75 ± 0.33 (0.028) |
| Bile (μg/ml) | 3.26 ± 1.67 (0.255) | 1.73 ± 0.20 (0.048) | 17.30 ± 4.02 (0.179) |
| Urine (μg/ml) | 14.52 ± 4.04 (1.137) | 19.80 ± 4.83 (0.550) | 24.50 ± 7.99 (0.254) |
| Brain (μg/g) | <LLQ | 0.21 ± 0.02 (0.005) | 0.85 ± 0.14 (0.008) |
| CSF (μg/ml) | <LLQ | <LLQ | <LLQ |
| Choroid (μg/g) | 3.11 ± 0.61 (0.243) | 7.65 ± 2.29 (0.212) | 12.51 ± 3.9 (0.130) |
| Aqueous humor (μg/ml) | 0.20 ± 0.00 (0.015) | 0.34 ± 0.06 (0.009) | 0.49 ± 0.07 (0.005) |
| Vitreous humor (μg/ml) | <LLQ | <LLQ | <LLQ |
| Plasma (μg/ml) | 6.09 ± 0.86 | 15.57 ± 1.11 | 34.74 ± 3.74 |
All values are expressed as means ± standard errors of the means. Values in parentheses are ratios of mean tissue levels to mean plasma AUC0–24 after multiple dosing. For comparison, concurrent plasma levels are listed at the bottom of the table.
Toxicity.
There were no significant differences in the mean BUN and plasma potassium, magnesium, bilirubin, alkaline phosphatase, and hepatic transaminase levels when values determined after 14 days of treatment were compared to those obtained at baseline (Table 4). A statistically significant increase in serum creatinine was observed at the highest dosage level, but this increase was mild and the mean end-of-treatment value did not exceed 1.5 times the mean baseline value. Throughout administration of the compound, no apparent infusion-related toxicity or other clinical abnormalities were observed and no abnormal weight changes were noted.
TABLE 4.
Effects of liposomal nystatin on laboratory values after multiple dosing over 14 daysa
| Drug dose (mg/kg · day) | Creatinine (mg/dl)
|
BUN (mg/dl)
|
K+ (mmol/liter)
|
Mg2+ (mmol/liter)
|
||||
|---|---|---|---|---|---|---|---|---|
| Baseline | End of treatment | Baseline | End of treatment | Baseline | End of treatment | Baseline | End of treatment | |
| 2.0 | 0.72 ± 0.02 | 0.92 ± 0.16 | 19.5 ± 1.3 | 21.7 ± 1.0 | 4.85 ± 0.19 | 4.45 ± 0.18 | 0.86 ± 0.01 | 0.87 ± 0.03 |
| 4.0 | 0.77 ± 0.07 | 0.96 ± 0.08 | 17.7 ± 2.0 | 20.0 ± 4.5 | 4.48 ± 0.14 | 4.33 ± 0.17 | 0.90 ± 0.28 | 0.89 ± 0.02 |
| 6.0 | 0.87 ± 0.04 | 1.33 ± 0.07b | 21.2 ± 1.6 | 23.7 ± 1.0 | 4.70 ± 0.10 | 4.47 ± 0.14 | 0.93 ± 0.05 | 0.97 ± 0.08 |
All values are expressed as means ± standard errors of the means for four rabbits per dosage cohort.
P < 0.05 by the Mann-Whitney U test.
DISCUSSION
The results of this study demonstrate nonlinear, dose-dependent plasma pharmacokinetics of liposomal nystatin over the investigated dosage range of 2 to 6 mg/kg/day. No differences in key pharmacokinetic parameters were noted between single-dose administration and once-daily dosing over 14 days. Plasma concentration data fit best with a two-compartment open model, with peak plasma levels that were severalfold above the MICs for susceptible fungi (13; C. J. Jessup, T. L. Wallace, and M. A. Ghannoum, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F88, p. 161, 1997), a very rapid initial distributive phase, and a somewhat slower elimination from plasma, resulting in a terminal half-life of 1 to 2 h. There were significant differences in the dose-normalized AUC0–24 across the three investigated dosage levels, revealing hyperproportional increases in the AUC0–24 with increasing dosages. This coincided with a decrease in total clearance and a trend toward an increased elimination half-life and a decreased volume of distribution, consistent with dose-dependent tissue uptake and/or dose-dependent elimination from the body. Assessment of tissue levels near peak plasma concentrations after multiple dosing over 15 days revealed the highest concentrations in the lungs, liver, and spleen, followed by the kidney. Apart from the detection of drug in bile, in comparison to peak plasma levels, high concentrations of nystatin were found in spot urine obtained at 30 min after dosing, indicating the possibility that renal excretion may be a major route of elimination for this liposomal polyene formulation.
The major rationale for performing the present animal study was provided by the challenges in evaluating the pharmacokinetics of liposomal nystatin in human subjects. As with the lipid formulations of amphotericin B, the potential toxicities in the parent compound interdict the assessment of the distribution of liposomal nystatin in human volunteers over the range of therapeutic dosages. As a consequence, the gathering of pharmacokinetic information often is restricted to severely ill patients with a wide variety of underlying conditions and concurrent drug therapies, resulting in large interpatient variability of pharmacokinetic parameters. Experimental animal studies, in contrast, may provide a systematic assessment of the disposition of a drug in vivo and may establish the foundation for understanding its pharmacokinetic and pharmacodynamic relationships and, ultimately, for rational drug therapy for patients with life-threatening fungal infections.
Our study demonstrates that such an inference is indeed valid for liposomal nystatin. The plasma pharmacokinetics of liposomal nystatin after intravenous administration to rabbits are comparable overall to those reported for human subjects in the blood (P. A. Cossum, J. Wyse, Y. Simmons, T. L. Wallace, and A. Rios, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A88, p. 17, 1996; Nyotran investigator brochure, Aronex Pharmaceuticals, 8 July 1998). In the latter study, after one single dose, the mean Cmax was 4.8 μg/ml, the mean AUC0–24 was 15 μg · h/ml, and mean elimination half-life was 3.5 h at 2 mg/kg. At 3 mg/kg, these parameters were 9.5 μg/ml, 27.3 μg · h/ml, and 1.9 h, respectively. Based on the AUC0–24 and the observation that concentrations in plasma are approximately twice as high as corresponding levels in blood (7), a 4-mg/kg dose in the rabbit approximately corresponds to a 2-mg/kg dose in humans, and a 6-mg/kg dose in the rabbit to a 3-mg/kg dose in humans. The up to twofold-lower projected mean peak plasma levels in humans at equivalent dosages may be accounted for mainly by a slower infusion rate (2 versus 10 mg/min in the rabbits). The close similarity between the plasma pharmacokinetics of liposomal nystatin in the two species may find further validation by the recently demonstrated dose-dependent antifungal activity of liposomal nystatin in a neutropenic rabbit model of disseminated candidiasis that showed 100% organ clearance at dosages of 4 mg/kg (8); for comparison, a 2-mg/kg dosage achieved an exceptionally good success rate of 83% in an open-label phase II study in nonneutropenic patients with candidemia (Williams and Moore, 39th ICAAC).
As is characteristic for multilamellar liposomal preparations (20), liposomal nystatin achieved high peak plasma levels followed by rapid distribution and elimination from plasma with a relatively short plasma half-life. The small volume of distribution is indicative of only minor tissue accumulation and/or binding to plasma proteins; the latter would be consistent with a rapid dissociation of nystatin from its liposome carrier in the bloodstream, as has been suggested by in vitro studies (23, 25), or with only minor drug accumulation in tissues. The pharmacokinetic profile of liposomal nystatin is thus markedly different from that of amphotericin B deoxycholate and from each of the three currently available lipid formulations of amphotericin B. Whereas amphotericin B deoxycholate achieves comparable peak plasma levels but is only slowly eliminated from the bloodstream and has a much higher VD, the lipid formulations either achieve comparatively lower peak concentrations, are rapidly taken up preferentially by organs of the mononuclear phagocytic system (MPS) but stay in the bloodstream at low concentrations (e.g., amphotericin B lipid complex and, to a lesser extent, amphotericin B colloidal dispersion), or achieve and maintain very high concentrations of amphotericin B in plasma over prolonged periods and are taken up more slowly by tissues (e.g., the small unilamellar formulation of amphotericin B) (9). It is still unclear, however, whether and how the distinct plasma pharmacokinetics of these different polyene formulations translate into unique antifungal pharmacodynamic properties.
At all dosages investigated, plasma levels immediately after infusion of liposomal nystatin exceeded the MICs reported for various clinical fungal isolates (13; Jessup et al., 37th ICAAC) and remained above these values for several hours before becoming undetectable. The exact pharmacokinetic and pharmacodynamic relationships of the antifungal polyenes in vivo remain to be explored. However, their antifungal activity in vitro is concentration dependent and becomes rapidly fungicidal within a narrow range of concentrations (15); furthermore, the Cmax/MIC ratio was the pharmacodynamic parameter most predictive of the antifungal efficacy of amphotericin B deoxycholate in a recently presented murine model of disseminated candidiasis (Andes, 39th ICAAC). Thus, for the antifungal polyenes, achieving high levels at the target over a relatively short period may be more important than the extent of drug exposure over the entire dosing interval. However, because the situation may be more complex with the novel lipid formulations, where carrier effects appear to play a pivotal role in drug distribution (2), the unique plasma pharmacokinetic of liposomal nystatin clearly warrant the exploration of the effects of dose fractionation on antifungal efficacy and safety.
Assessment of tissue levels near peak plasma levels after multiple dosing over 15 days demonstrated considerable concentrations of nystatin in the lungs, liver, spleen, and, to a lesser extent, the kidney. This distribution pattern appears similar to that reported for the multilamellar liposomal formulation of amphotericin B developed by Lopez-Berestein and coworkers in uninfected mice and rats (16, 24), and may reflect enhanced distribution to the lung and to the reticuloendothelial system shortly after infusion. However, peak drug concentrations in different tissues may be reached at different times during the dosing interval, and serial assessment of tissue concentrations over time and proper mass-balance and excretion studies are needed for a more complete description of the disposition of nystatin after administration of its liposomal formulation.
Drug levels in the kidney were comparatively low at the 2- and 4-mg/kg dosage levels, but increased disproportionally at the 6-mg/kg dosage level. A hypothesis for this observation may be derived from experimental findings on the binding and transport of nystatin in the aqueous environment of the bloodstream. When liposomal nystatin is incubated in plasma, the liposomal structure is lost relatively quickly. More than 50% of the compound distributes with the lipoprotein fraction, with the majority being associated with high-density lipoproteins (HDLs). Over time, nystatin then appears to slowly redistribute from HDLs to the lipoprotein-deficient plasma fraction, which is composed mainly of aqueous plasma proteins (23, 25). Unlike amphotericin B, free as well as liposome-encapsulated nystatin does not bind to a greater extent to low-density lipoproteins (LDLs). This association is believed to be a major contributor to the dose-limiting nephrotoxicity of amphotericin B due to the existence of high numbers of renal LDL receptors that mediate the cellular uptake of amphotericin B (26). Although hypothetical, it is conceivable that this distribution pattern is altered in the presence of high plasma concentrations, with a greater fraction of compound distributing with the LDL fraction, leading to increased distribution to the kidney. In this context, it may also be important to note that the distribution of nystatin in plasma lipoproteins may be different for different species, which could theoretically lead to certain interspecies differences in pharmacokinetics, antifungal efficacy, and nephrotoxicity (19). However, direct evidence for this hypothesis remains to be generated.
The observation of drug concentrations in spot urine obtained from the urinary bladder 30 min after dosing that were comparatively high with respect to plasma levels also bears notice. This finding may indicate that renal excretion may be a major route of elimination for this liposomal polyene formulation. However, no formal studies have yet been performed on the quantitative recovery of nystatin in urine and the fractional distribution to major tissue sites after single- or multiple-dose administration.
Reaching the central nervous system is important for any antifungal drug. Like all four amphotericin B formulations (A. H. Groll, N. Giri, C. E. Gonzalez, T. Sein, J. Bacher, S. C. Piscitelli, and T. J. Walsh, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A90, p. 19, 1997), nystatin was undetectable in CSF and achieved relatively low levels in brain tissue compared to other sites. However, different equilibria may prevail at later time points of the dosing interval, and, as is true in the case of amphotericin B deoxycholate (12), therapeutically effective drug levels in brain tissue may be achieved in the state of tissue inflammation and/or necrosis.
Liposomal nystatin was well tolerated in the absence of severe illness, immunosuppression, or concurrent medication. Renal toxicity was limited to the 6-mg/kg dosage level, where a 1.5-fold increase in the mean serum creatinine levels of four rabbits was noted after treatment for 14 days. While this was not a formal toxicokinetic study, our findings are in agreement with results from a clinical phase I study of 32 neutropenic patients with hematological malignancies and persistent fever, who received the drug for a median of 8 days. In that study, liposomal nystatin was tolerated at dosages of up to 8 mg/kg/day without formally reaching the maximum tolerated dosage; elevated serum creatinine levels occurred in approximately 30% of patients but never exceeded grade II toxicity (Boutati et al., 35th ICAAC). Similarly, in an open-label study with 75 nonneutropenic patients with candidemia, liposomal nystatin was not discontinued in any patient due to nephrotoxicity (K. Rolston, I. Baird, D. R. Graham, and L. Jauregui, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. J79, p. 473, 1998).
In conclusion, over the investigated dose range of 2 to 6 mg/kg per day, liposomal nystatin demonstrated nonlinear pharmacokinetics, with a dose-dependent decrease in total clearance and preferential initial distribution into lung, liver, and spleen. Liposomal nystatin was well tolerated, with mild increases in serum creatinine levels at the end of therapy only at the highest dosage level. Demonstrated safety and efficacy in vivo, predictable plasma pharmacokinetics, and substantial tissue distribution support the further clinical investigation of this novel liposomal polyene formulation.
ACKNOWLEDGMENTS
We thank the staff of the Laboratory Animal Science Branch and of the Veterinary Resources Program of the Division of Research Resources, National Institutes of Health, for expert assistance.
REFERENCES
- 1.Andriole V T. History of antifungal therapy. Infect Dis Clin Pract. 1998;7(Suppl. 1):S2–S7. [Google Scholar]
- 2.Brajtburg J, Bolard J. Carrier effects on biological activity of amphotericin B. Clin Microbiol Rev. 1996;9:512–531. doi: 10.1128/cmr.9.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the care and use of laboratory animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- 4.D'Argenio D Z, Schumitzky A. Adapt II user's guide. Biomedical Simulations Resource. Los Angeles, Calif: University of Southern California; 1990. [Google Scholar]
- 5.Denning D W, Warn P. Dose range evaluation of liposomal nystatin and comparison with amphotericin B and amphotericin B lipid complex in temporarily neutropenic mice infected with an isolate of Aspergillus fumigatus with reduced susceptibility to amphotericin B. Antimicrob Agents Chemother. 1999;43:2592–2599. doi: 10.1128/aac.43.11.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groll A H, Gonzalez C E, Giri N, Kligys K, Love W, Peter J, Feuerstein E, Bacher J, Piscitelli S C, Walsh T J. Liposomal nystatin against experimental pulmonary aspergillosis in persistently neutropenic rabbits: efficacy, safety, and non-compartmental pharmacokinetics. J Antimicrob Chemother. 1999;43:95–103. doi: 10.1093/jac/43.1.95. [DOI] [PubMed] [Google Scholar]
- 7.Groll, A. H., D. Mickiene, K. Werner, S. C. Piscitelli, and T. J. Walsh. 1999. High-performance liquid chromatographic determination of liposomal nystatin in plasma and tissues for pharmacokinetic and tissue distribution studies. J. Chromatography B, in press. [DOI] [PubMed]
- 8.Groll A H, Petraitis V, Petraitiene R, Field-Ridley A, Candelario M, Bacher J, Piscitelli S C, Walsh T J. Antifungal efficacy and safety of multilamellar liposomal nystatin against disseminated candidiasis in persistently neutropenic rabbits. Antimicrob Agents Chemother. 1999;43:2463–2467. doi: 10.1128/aac.43.10.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groll A H, Piscitelli S C, Walsh T J. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv Pharmacol. 1998;44:343–500. doi: 10.1016/s1054-3589(08)60129-5. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton-Miller J M T. Chemistry and biology of the polyene macrolide antibiotics. Bacteriol Rev. 1973;37:166–196. [PMC free article] [PubMed] [Google Scholar]
- 11.Hazen E L, Brown R. Two antifungal agents produced by a soil actinomycete. Science. 1950;112:423. [PubMed] [Google Scholar]
- 12.Jafari H S, Saez-Llorens X, Severien C, Parras F, Friedland I, Rinderknecht S, Ehrett S, Olsen K D, Abramowsky C, McCracken G H., Jr Effects of antifungal therapy on inflammation, sterilization, and histology in experimental Candida meningitis. Antimicrob Agents Chemother. 1994;38:83–89. doi: 10.1128/aac.38.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson E M, Ojwang J O, Szekely A, Wallace T L, Warnock D W. Comparison of in vitro antifungal activities of free and liposome-encapsulated nystatin with those of four amphotericin B formulations. Antimicrob Agents Chemother. 1998;42:1412–1416. doi: 10.1128/aac.42.6.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleinberg M E, Finkelstein A. Single-length and double-length channels formed by nystatin in lipid bilayer membranes. J Membr Biol. 1984;80:257–269. doi: 10.1007/BF01868444. [DOI] [PubMed] [Google Scholar]
- 15.Klepser M E, Wolfe E J, Jones R N, Nightingale C, Pfaller M A. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested against Candida albicans. Antimicrob Agents Chemother. 1997;41:1392–1395. doi: 10.1128/aac.41.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Berestein G, Rosenblum M G, Mehta R. Altered tissue distribution of amphotericin B by liposomal encapsulation: comparison of normal mice to mice infected with Candida albicans. Cancer Drug Delivery. 1984;1:199–205. doi: 10.1089/cdd.1984.1.199. [DOI] [PubMed] [Google Scholar]
- 17.Mehta R T, Hopfer R L, Gunner L A, Juliano R L, Lopez-Berestein G. Formulation, toxicity, and antifungal activity in vitro of liposome-encapsulated nystatin as a therapeutic agent for systemic candidiasis. Antimicrob Agents Chemother. 1987;31:1897–1900. doi: 10.1128/aac.31.12.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta R T, Hopfer R L, McQueen T, Juliano R L, Lopez-Berestein G. Toxicity and therapeutic effects in mice of liposome-encapsulated nystatin for systemic fungal infections. Antimicrob Agents Chemother. 1987;31:1901–1903. doi: 10.1128/aac.31.12.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramaswamy M, Wallace T L, Cossum P A, Wasan K M. Species differences in the proportion of plasma lipoprotein lipid carried by high-density lipoproteins influence the distribution of free and liposomal nystatin in human, dog, and rat plasma. Antimicrob Agents Chemother. 1999;43:1424–1428. doi: 10.1128/aac.43.6.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider M. Liposomes as drug carriers: 10 years of research. In: Buri P, Gumma A, editors. Drug targeting. New York, N.Y: Elsevier Science Publishers; 1985. pp. 119–134. [Google Scholar]
- 21.Wallace T L, Paetznick V, Cossum P A, Lopez-Berestein G, Rex J H, Anaissie E. Activity of liposomal nystatin against disseminated Aspergillus fumigatus infection in neutropenic mice. Antimicrob Agents Chemother. 1997;41:2238–2243. doi: 10.1128/aac.41.10.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh T J, Bacher P, Pizzo P A. Chronic silastic central venous catheterization for induction, maintenance, and support of persistent granulocytopenia in rabbits. Lab Anim Med. 1988;38:467–470. [PubMed] [Google Scholar]
- 23.Wasan K M, Lopez-Berestein G. Diversity of lipid-based polyene formulations and their behavior in biological systems. Eur J Clin Microbiol Infect Dis. 1997;16:81–92. doi: 10.1007/BF01575125. [DOI] [PubMed] [Google Scholar]
- 24.Wasan K M, Vadiei K, Lopez-Berestein G, Luke D R. Pharmacokinetics, tissue distribution, and toxicity of free and liposomal amphotericin B in diabetic rats. J Infect Dis. 1990;161:562–566. doi: 10.1093/infdis/161.3.562. [DOI] [PubMed] [Google Scholar]
- 25.Wasan K M, Ramaswami M, Cassidy S M, Kazemi M, Strobel F W, Thies R L. Physical characteristics and lipoprotein distribution of liposomal nystatin in human plasma. Antimicrob Agents Chemother. 1997;41:1871–1875. doi: 10.1128/aac.41.9.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wasan K M, Rosenblum M G, Cheung L, Lopez-Berestein G. Influence of lipoproteins on renal cytotoxicity and antifungal activity of amphotericin B. Antimicrob Agents Chemother. 1994;38:223–227. doi: 10.1128/aac.38.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaoka K, Nakagawa T, Uno T. Application of Akaike's information criterion in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978;6:165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]