Abstract
Objectives
To evaluate the effect of urinary trypsin inhibitor (UTI) or Ulinastatin on postoperative course and clinical outcomes in patients with cardiopulmonary bypass.
Methods
We searched PubMed, Embase, Web of Science, and Cochrane Library for the keywords UTI and Cardiopulmonary bypass (CPB). The primary outcome measure was the intensive care unit length of stay (ICU LOS), and results were stratified for relevant subgroups (dosage of UTI). The effects of UTI on mechanical ventilation duration (MVD), hospital LOS, renal failure incidence (RFI), and all-cause mortality were studied as secondary outcomes.
Results
Twelve randomized controlled trials (enrolling 1620 patients) were evaluated. Eleven studies pooled for subgroup analysis showed that using UTI persistently or with a considerable amount would lead to a shorter ICU LOS (95% CI, − 0.69 to − 0.06; P = 0.0001). Ten studies showed that UTI could shorten MVD in patients (95% CI, − 1.505 to − 0.473; P < 0.0001). RFI generally showed a more favourable outcome with UTI treatment (95%CI, 0.18–1.17; P = 0.10). And the current evidence was insufficient to prove that UTI could reduce the hospital LOS (95% CI, − 0.22 to 0.16; P = 0.75) and the all-cause mortality rate (95% CI, 0.24–2.30; P = 0.60).
Conclusions
Various subsets of UTI treatment suggested that UTI could shorten ICU LOS, and it is associated with the dosage of UTI. Considering the substantial heterogeneity and lack of criteria for UTI dosage, more evidence is needed to establish a standard dosing guideline.
Keywords: Ulinastatin, Cardiopulmonary bypass, Clinical outcome, ICU length of stay, Prognosis, Acute inflammatory disorder
Introduction
Cardiopulmonary bypass (CPB) has been used for most cardiac surgeries for over half a century worldwide. CPB has been shown to provoke ischemia–reperfusion and subsequent cellular injury with inflammatory reaction [1]. Moreover, shear stress associated with cardiopulmonary bypass could induce the expression of inflammatory cytokines and necroptosis in monocytes [2].
On account of the CPB-induced damage, up to 10% of patients require prolonged postoperative care [3], with a more extended intensive care unit length of stay (ICU LOS) and mechanical ventilation duration (MVD) [4]. Recently, ICU-acquired weakness is evolving into a complication that cannot be neglected [5], and It is estimated that the ICU consumes 20%of hospital expenditures and approximately 1%of gross domestic product[6, 7]. Furthermore, patients with a long MVD often suffer many complications, such as ventilator-associated pneumonia [8] and pressure sores [9]. All of these resulted in the use of an incredible amount of medical resources. Hence, patients who underwent CPB are associated with a higher healthcare cost [10, 11].
Urinary trypsin inhibitor(UTI) or Ulinastatin was first identified in human blood, urine and other tissues in the 1980s [12], with inhibitory effects on a variety of proteases [13]. UTI has been testified to have the potential of reducing CPB-induced damage[14] and in turn may shorten ICU LOS and MVD. Based on recent studies, UTI not only could inhibit the activation of various pro-inflammatory cytokines but may also inhibit the release of neutrophil elastase [15]. Meanwhile, other studies have found that UTI attenuates inflammation and resists oxidative stress by inhibiting the JNK/NF-kappaB signalling pathway and PI3K/Akt/Nrf2 pathway [16, 17]. Besides, UTI could afford a certain degree of protection in alleviating cerebral ischemia–reperfusion injury by activating the Nrf-2/HO-1 signalling pathway and may improve myocardial ischemia–reperfusion injury through endoplasmic reticulum stress-induced apoptosis pathway [18, 19]. Furthermore, as a natural anti-inflammatory molecule, UTI could have excellent prospects in both pharmacologic prophylaxis of post-endoscopic retrograde cholangiopancreatography pancreatitis and the treatment of severe decompression sickness, and even in the therapy of SARS-CoV-2 infection [20–22]. In the regions of Asia, UTI is used as an essential drug to treat acute inflammatory disorders, including pancreatitis, sepsis and shock [23, 24].
Recent clinical trials conducted on CPB patients with UTI showed a beneficial trend in restraining inflammation[25]. And five meta-analyses on this topic have been published [26–30]; among which, one [26] evaluated the effect of UTI in reducing postoperative bleeding of CPB patients and did not analyze other clinical outcomes; four [27–30] analyzed the effect of UTI, but no significant benefit in reducing ICU LOS and MVD had been shown. More to the point, a retrospective study indicated that UTI did not improve postoperative outcomes in CPB patients [31]. It’s difficult to interpret why UTI is beneficial for reducing inflammation, but could not improve other clinical outcomes. Therefore, this meta-analysis was conducted to clarify the clinical effectiveness of UTI in CPB patients.
Materials and methods
According to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) statement for reporting systematic reviews and meta-analyses [29], we conducted the meta-analysis with the registration number CRD42020215640 (registered on 21 November 2020). Two groups of researchers (Hu Zhenyu and Yuan Qiaoli) independently conducted literature searches, established the study inclusion and exclusion criteria, performed quality assessment, and extracted data. Any disagreement between the two researchers was resolved by the senior authors (Wang Maohua). Eligibility criteria included randomized controlled trials (RCTs) which assessed the effect of UTI treatment on clinical outcomes in CPB patients.
Data sources and searches
The electronic databases PubMed, EMBASE, Web of Science, and Cochrane library were searched systematically (20 January 2022) with the following search strategy:”ulinastatin” OR “UTI68″ OR “acid-stable protease inhibitor” OR “MR 20 (magnetic powder) of urinastatin” AND "Cardiopulmonary Bypass" OR “cardiopulmonary”. Besides, personal records and reference lists of relevant articles were screened. We tried to contact the corresponding authors if information about important clinical outcome indicators was missing, but sadly there was no response.
Study selection
Studies were independently screened based on title and abstract by two authors (Hu Zhenyu and Yuan Qiaoli), and differences were resolved by consensus. Study inclusion criteria were: (1) patients who underwent surgery with CBP; (2) type of interventions: used UTI alone regardless of treatment duration; (3) research design: RCTs only. Exclusion criteria included: (1) pediatric studies; (2) review articles, case reports and letters; (3) animal experiment studies; (4) duplicate publications; (5) studies that did not describe correlation outcomes of interest. The eligibility of the remaining studies for final inclusion was further determined by reading the full text.
Data synthesis and analysis
Data from individual clinical studies adopting an intention to treat design were used for this meta-analysis. A random-effects model that used the DerSimonian and Laird method was applied for all individual pooling estimates. For dichotomous outcomes (e.g., renal failure), the selected effect size was risk ratio (RR) with 95% confidence interval (CI) calculated from the two-by-two table. For continuous outcome (i.e., ICU LOS), the effect size was standard deviation (STD) because the constant outcome units were miscellaneous in the included studies. The median (quartile interval) data were conversed to mean ± standard deviation using Shi et.al.’s methods [32], so all continuous data were expressed as mean ± standard deviation.
Heterogeneity was assessed by using the I2 statistics, chi-square test, Tau2, and visualization in a funnel plot. The effects of UTI were independent of many confounding factors. To explore heterogeneity, the subgroup design was based on the dosage of UTI and analyzed using a meta-regression framework. We performed sensitivity analyses to explore the potential impact of a single trial on the overall results by omitting one trial from the meta-analysis at a time. Studies were conducted with Review Manager software 5.4 and Statistic/Data Analysis 12.0.
Results
Search results and study characteristics
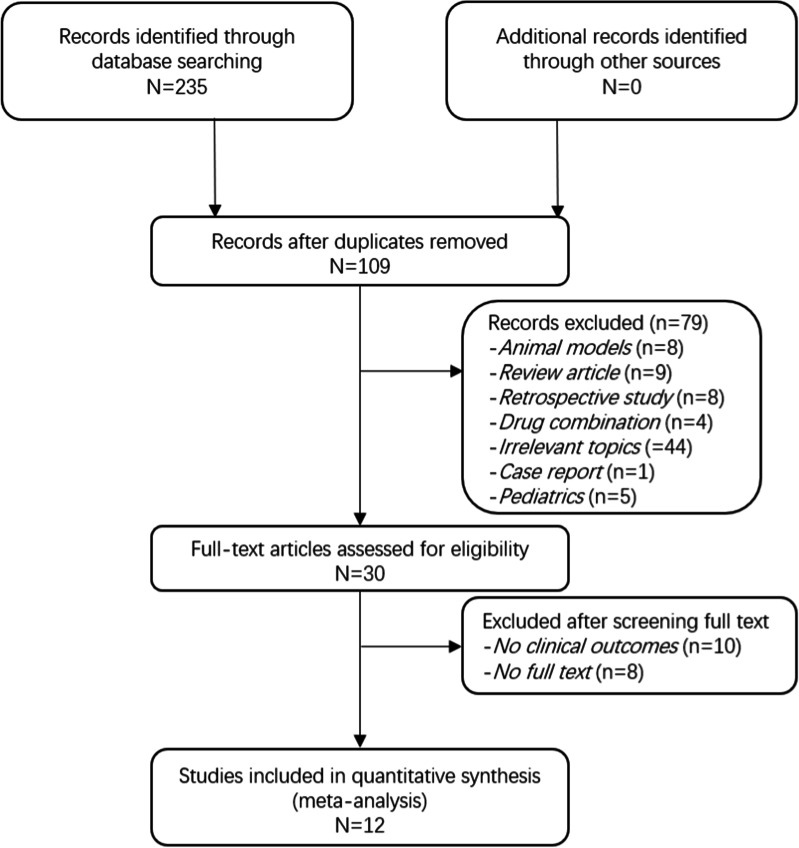
Figure 1 is the PRISMA flow diagram that summarizes the search and selection strategy. Our search strategy resulted in considering 235 studies for inclusion. After removing duplicates, 109 reviews were screened for titles and abstracts, and 58 studies were excluded. Finally, 51 full-text articles were assessed for eligibility. In total, twelve RCTs were included for this meta-analysis, of which one study [33] was fit only for secondary outcomes. Due to the data of ICU LOS were described as median (quartile interval) in this article, it’s hard to combine them with other data. We had tried to contact the authors for complete data, yet no response was heard. The included articles were published from 2006 to 2020 [33–44], and data collection was performed between 1998 and 2016. Three studies included patients who underwent coronary artery bypass graft (CABG) surgery, four included patients with valve replacement or valve repairment, three included patients receiving combined surgery (valve relevant surgery and CABG) which was collectively called open-heart surgery, and the remaining two studies included aortic arch replacement (AAR) patients. No adverse event existed among these studies. The population characteristics of included patients were summed up in Table1.
Fig. 1.
Meta-analysis flowchart for selecting eligible studies
Table 1.
Characteristics of the clinical trials included in the meta-analysis
| Reference, years, country of origin | Number of patients | Mean age | Gender (F/M) | Surgery | Interventions | CPB time (min) | Adverse effects | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UTI group | Control group | UTI group | Control group | UTI group | Control group | UTI group | Control group | UTI group | Control group | ||||
| Zhang et al. 2020 China | 142 | 141 | 49.0 ± 14.3 | 50.3 ± 12.4 | 63/79 | 71/70 | Open heart surgery | 1,000,000 U iv, the half after anesthesia induction and the rest prime into CPB | Equivalent normal saline | 102.87 ± 42.7 | 98.69 ± 47.76 | No | |
| Xu et al. 2017 China | 20 | 20 | 53.4 ± 5.2 | 53.2 ± 5.1 | 12/8 | 13/7 | Open heart surgery | 5000 U/kg iv before CPB and at 1–3 days postoperatively | Equivalent normal saline | Unclear | No | ||
| Wang et al. 2016 China | 36 | 30 | 48.4 ± 11.3 | 49.3 ± 11.7 | 32/4 | 26/4 | AAR | 300,000 U/8 h iv before 3 days postoperatively and 300,000 U/2 h during surgery | NR | 228.6 ± 69.1 | 22.4 ± 62.5 | No | |
| Pang et al. 2015 China | 30 | 30 | 53.5 ± 9.8 | 50.2 ± 9.1 | 15/15 | 21/9 | Open heart surgery | 5000 U/Kg iv after anesthesia induction | Equivalent normal Saline | 215.3 ± 57.9 | 221.6 ± 76.1 | No | |
| Xu et al. 2013 China | 18 | 18 | 54.8 ± 8.9 | 53.2 ± 7.3 | 16/2 | 15/3 | AAR | 20,000 U/Kg iv after anesthesia induction | Equivalent placebo | 235.5 ± 25.9 | 247.2 ± 20.3 | No | |
| Hao et al. 2013 China | 20 | 20 | 56.36 ± 10.81 | 54.27 ± 10.90 | 22/18 | MVR, DVR, AVR | 1000 U/Kg iv during the preoperative period | NR | 98.36 ± 9.87 | 105.55 ± 44.16 | No | ||
| Chen et al. 2012 China | 30 | 30 | 49.8 ± 10.8 | 50.4 ± 10.0 | 14/16 | 12/18 | VR | 12,000 U/Kg iv after anesthesia induction | Equivalent normal Saline | 102.5 ± 20.7 | 105.9 ± 20.2 | No | |
| Oh et al. 2012 Korea | 30 | 30 | 67 ± 19 | 60 ± 12 | 20/10 | 16/14 | AVR | 1,000,000 U iv during surgery | Equivalent normal Saline | 99 ± 25 | 96 ± 22 | No | |
| ONG et al. 2011 Korea | 24 | 24 | 51.9 ± 17.3 | 52.7 ± 18.9 | 8/16 | 9/15 | Aortic valve repair | 5000 U/Kg iv before CPB | Equivalent normal Saline | 164.3 ± 31.5 | 173.4 ± 28.4 | No | |
| Zhou et al. 2010 China | 20 | 20 | 61.1 ± 8.7 | 28/12 | CABG | 15,000 U/Kg iv during surgery | Equivalent normal Saline | 83.5 ± 23.1 | 79.6 ± 25.7 | No | |||
| Jiang et al. 2007 China | 15 | 15 | 57.4 ± 6.6 | 56.8 ± 6.1 | 11/9 | 11/9 | CABG | 1,000,000 U iv during surgery | Equivalent placebo | 108 ± 29 | 117 ± 34 | No | |
| Nakanishi et al. 2006 Japan | 14 | 14 | 62 ± 9 | 61 ± 10 | 12/2 | 11/3 | CABG | 5000 U/Kg iv before aortic cannulation for CBP | Equivalent normal Saline | 150 ± 37 | 135 ± 39 | No | |
CBP = Cardiopulmonary Bypass; TAR = Total aortic arch replacement; AAR = Aortic Arch Replacement; VR = Valve Replacement, including Aortic Valve Replacement, Mitral Valve Replacement; MVR = Mitral Valve Replacement; DVR = Double (aortic and mitral) Valve Replacement; AVR = Aortic Valve Replacement; CABG = Coronary Artery Bypass Graft
Data extraction and quality assessment
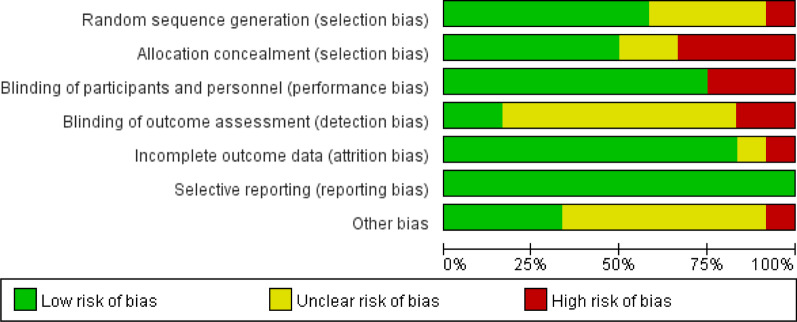
Relevant data were extracted using a standardized data extraction sheet. The primary outcome measure was ICU LOS. MVD, hospital LOS, and renal failure incidence (RFI) were also noted as secondary outcomes. The research methodology quality of each RCT was assessed following the Cochrane Risk of Bias (RoB 2.0) Tool for randomized controlled trials [45]. The Cochrane tool assesses five domains, including bias arising from the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selecting the reported result. For each domain, bias was classified as either low, uncertain, or high. The risk of bias of included studies and the overall risk of bias are shown in Figs. 2 and 3, respectively. Furthermore, the studies are substantially different in methods in terms of study population and study design. Hence, results were stratified and, if possible, analyzed for subsets. Two authors independently screened, reviewed, and scored each trial using this method and extracted data for analysis. Disagreements about scoring or extracting data were resolved through discussion or consulting with a third expert.
Fig. 2.
Risk of bias of included studies
Fig. 3.
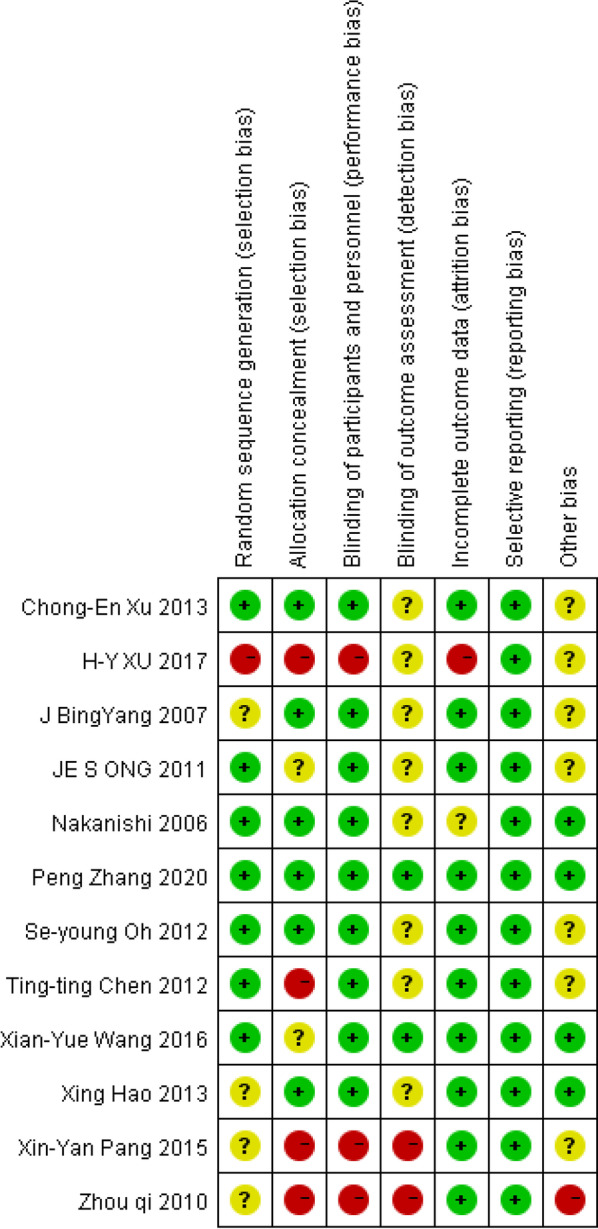
Risk of bias summary
Outcomes
ICU length of stay
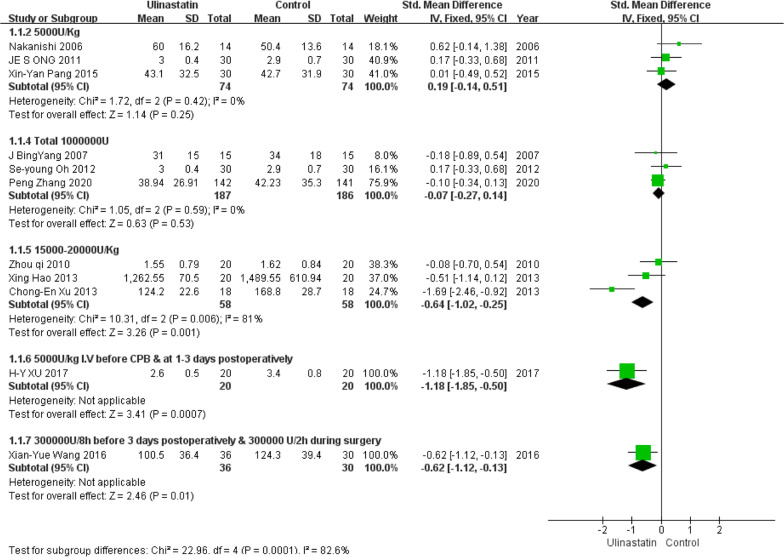
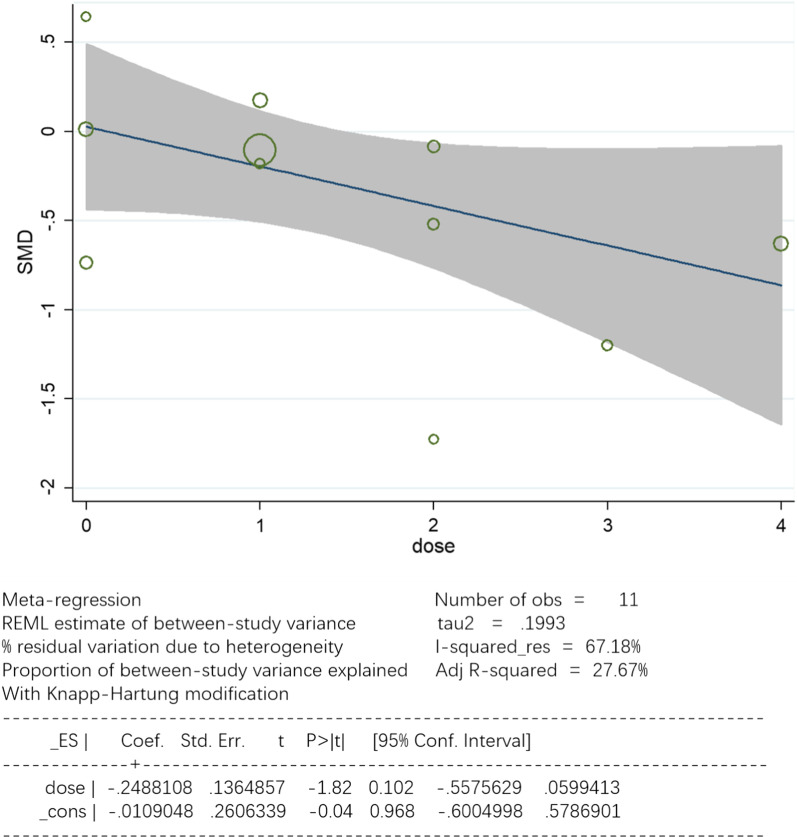
Eleven studies with a total of 743 patients, including 375 in the control group and 368 in the UTI group, were available for analysis. A forest plot is presented in Fig. 4. Pooled analysis showed that the control groups and UTI groups differed significantly in ICU LOS (95% CI, − 0.69 to − 0.06; P = 0.0001). The heterogeneity among studies was conspicuous(I2 = 82.7%). Based on different UTI dosages and methods of administration, a single factor regression analysis was used. As shown in Fig. 5, the result indicates that UTI dosage might be one of the sources of heterogeneity (tau2 = 0.1993; I-squared res = 67.18%; Adj R-squared = 27.67%; P = 0.102).
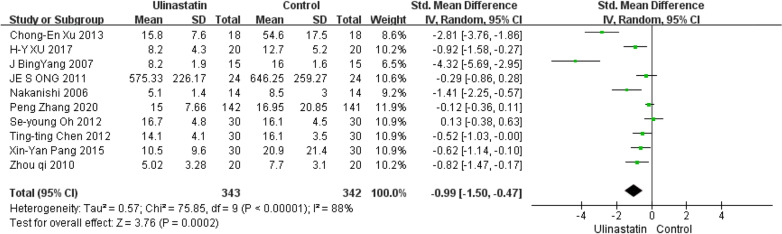
Fig. 4.
Forest plots of ICU LOS in the control groups and UTI groups. CI = confidence interval; IV = inverse variance; Std = standardized mean difference
Fig. 5.
Meta-regression analysis of effects on ICU LOS by dosages of UTI
Mechanical ventilation duration
Ten studies enrolling 685 patients, including 343 in the UTI group and 342 in the control group, analyzed MVD. A forest plot is presented in Fig. 6, which shows a significantly longer ventilation time in the Control group than in the UTI group (95% CI, − 1.505 to − 0.473; P < 0.0001). Pooled data synthesis of this outcome showed marked heterogeneity(I2 = 88.1%).
Fig. 6.
Forest plot for MVD in the control groups and UTI groups
Hospital length of stay
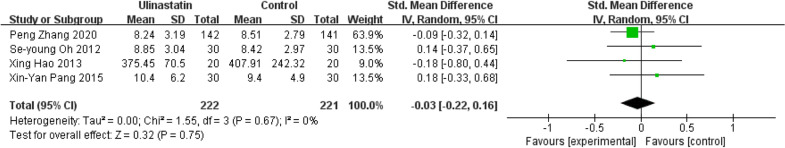
Four studies (443 patients) including 222 in the UTI group and 221 in the control group, analyzed hospital LOS. A forest plot is presented in Fig. 7, and it seems that the evidence was insufficient to prove the efficiency of UTI in shortening hospital LOS (95% CI, − 0.22 to 0.16; P = 0.75). Pooled data synthesis of this outcome showed no heterogeneity(I2 = 0%).
Fig. 7.
Forest plot for hospital LOS in the control groups and UTI groups
Renal failure incidence
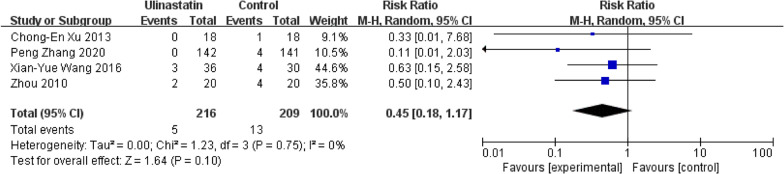
Four studies enrolling 385 patients were taken in the analysis of RFI. Pooled analysis showed a significantly higher rate of renal failure in the control group than in the UTI group (95% CI, 0.24–2.30; P = 0.10) with no heterogeneity(I2 = 0%). The results are shown in Fig. 8.
Fig. 8.
Forest plot for RFI in the control groups and UTI groups
All-cause mortality rate
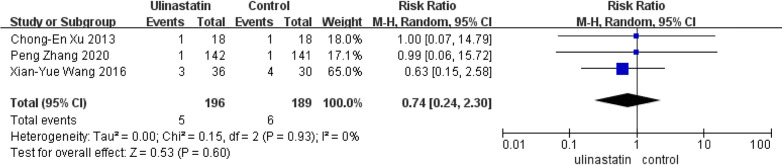
Only three studies reported the all-cause mortality rate [34, 36, 38]. So We took the three studies enrolling 385 patients into the analysis of all-cause mortality. As presented in Fig. 9, the current evidence could not prove the efficiency of UTI in reducing the all-cause mortality rate (95% CI, 0.24–2.30; P = 0.60). Pooled data synthesis of this outcome showed no heterogeneity (I2 = 0%).
Fig. 9.
Forest plot for all-cause mortality rate in the control groups and UTI groups
Publication bias and sensitivity analysis
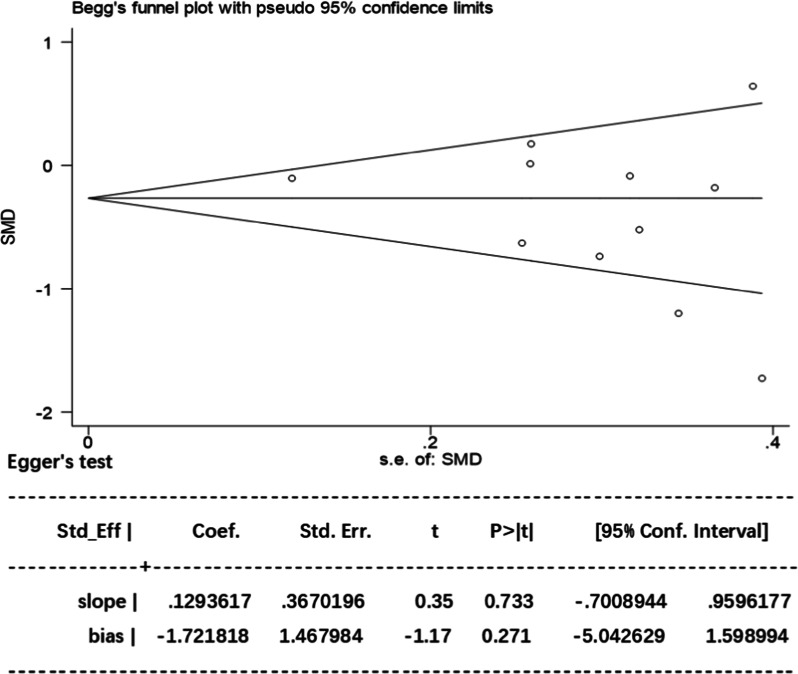
Unmeasured bias may impose a further limitation inherent to analyses in these RCTs. All the included studies involved the use of UTI for treating CPB patients. Using ICU LOS as the primary variable, the included studies were evaluated for the effect of study size. The funnel plot demonstrated an approximate symmetrical shape, suggesting that substantial publication bias is remote. Besides, Egger’s test also revealed a statistically substantial symmetry (p = 0.733). Therefore, potential publication bias had no significant influence on the results (Fig. 10).
Fig. 10.
The Begg’s test and Egger’s test for ICU LOS. Begg’s test: rank correlation test; Egger’s test: linear regression method; SMD: standardized mean difference; 95% CI confidence interval
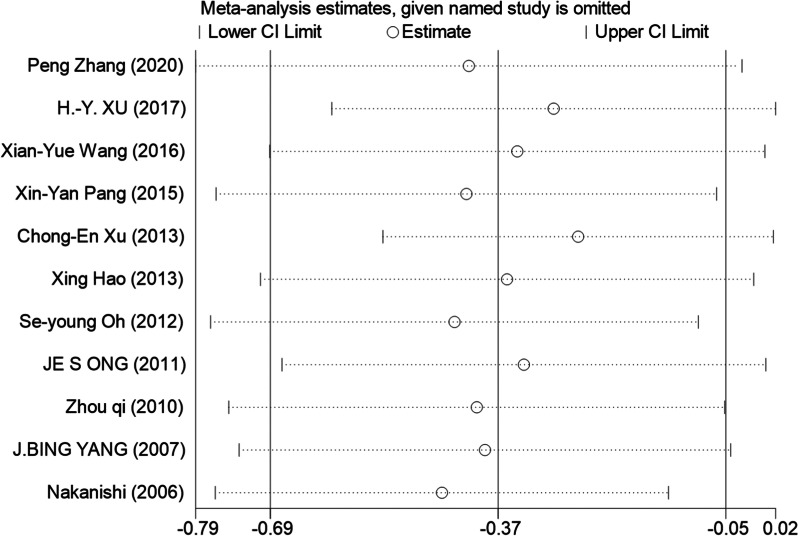
As to ICU LOS, sensitivity analysis showed that omitting specific trails had no significant impact on this outcome (Fig. 11). We could not entirely rule out the possibility that our findings are impacted by publication bias from the funnel plot. Three studies were outside of the confidence interval in Begg's funnel plot with pseudo 95% confidence limits. And we believe that the high heterogeneity may arise from many factors such as sample size, different design methods and study population.
Fig. 11.
The form of sensitivity analysis
Discussion
Commonly associated with systematic inflammatory, cardiac surgery with CPB is inimical to clinical outcomes of the patients [46]. Meanwhile, Ulinastatin, a protease inhibitor emanating from human urine and blood, has potent anti-inflammatory and anticoagulant activity [47] which has been shown to ameliorate inflammatory response to CPB [22] significantly. And several immunomodulatory strategies for UTI have been evaluated accordingly. Nonetheless, what is much less clear is the impact of UTI on the postoperative course and overall outcomes in CPB patients [48].
This meta-analysis identified 11 RCTs investigating the effect of UTI on ICU LOS of CPB patients. Despite the limitations of RCTs included in this meta-analysis and the existence of considerable heterogeneity, with data pooled from all these patients, we could still see that UTI could be associated with ICU LOS. After meta-regression was analyzed, we discovered the source of heterogeneity might be UTI dosage, MVD was found to be associated with UTI too. However, considering the significant heterogeneity, profound conclusions and causal inferences had been hampered.
The methodology used in include studies may substantially influence our findings. What’s more, UTI dosage, age range, complications of patients and other confounders might also be the heterogeneity source. According to the meta-regression analysis, UTI dosage may be the most critical factor determining the effect size (I-squared_res = 67.18%). In our finding, three studies[38, 39, 42] used a higher dosage (15,000–20,000 U/Kg) and two studies (5000U/Kg I.V before CPB & at 1-3 days postoperatively [35] and 300,000U/8 h from admission to 3 days postoperatively & 300,000 U/2 h during surgery [36]) used a considerable amount of UTI persistently, and based on subgroup analysis, all of them effectively reduced ICU LOS. So we find that maintaining the blood concentration of UTI is critical for CPB patients, as giving a high dose at one time or giving amounts of dose over a period of time for CPB patients could achieve better clinical outcomes, but the best dosage warrants further investigation. Previous studies explored the effects of different dosages of UTI on CPB patients, and a study [49] compared the effect of 5000U/Kg UTI to 20,000U/Kg UTI on pulmonary protection after CPB, and results showed a better outcome with the higher dose of UTI. Another study [50] established three different UTI dosage groups (20,000 U/Kg, 40,000 U/Kg, 60,000 U/Kg) and one control group (100,000 U during surgery & 100,000U/8 h postoperatively for 2 days). They discovered that the inflammatory cytokine levels were lower in the control group than in the 20,000 U/Kg group, and 60,000 IU/Kg of UTI exhibited the lowest inflammatory cytokine levels. These results were in line with our findings and could be associated with UTI pharmacokinetics. UTI is mainly eliminated in the kidneys with a half-life of about 33 min [51], while the duration of CPB often exceeds 40 min and activation of leukocytes and release of proinflammatory factors peak at 4-6 h after surgery[45]. Nevertheless, the study by Nakanishi et al. [44] contradicts most of our other findings. It is possibly an outlier, as it is the only study that came from Japan with a sample size that is too small (n = 28).
Our results regarding MVD should be interpreted with caution because of the high heterogeneity (I2 = 88.1%). Owing to the discrepancies in the definition of MVD, the high heterogeneity in ventilation time may be artificial, similar to ICU LOS, so we need more studies to analyse factors affecting the results. Therefore, the marked heterogeneity among the studies impeded the drawing of a powerful conclusion.
Our results about RFI shows that four studies with a marginal significance (P = 0.10, I2 = 0%) recorded RFI, which means that UTI treatment could protect renal function in CPB patients, this finding is consistent with a retrospective study [52]. previous studies [53, 54] proved that the systematic inflammatory response syndrome in CPB plays a vital role in the development of RFI, and other studies had found a correlation between UTI treatment and multiple organ function protection [55].
Our study could not prove that hospital LOS is related to UTI treatment. Four studies (443 patients) considered hospital LOS, but the sample was too small to have a high confidence level, although the heterogeneity was negligible (I2 = 0%). And our study on the all-cause mortality rate did not show a significant effect on UTI treatment.
Limitation
Some limitations in this meta-analysis warrant further consideration. Firstly, an additional restriction inherent to analyses is that some short reviews concerning UTI treatment and ICU LOS with negative results may not be submitted or accepted for publication. Therefore, the number of studies included in this meta-analysis is quite small. Secondly, the definition of clinical outcomes was not consistent among these studies, which may substantially influence our findings:
ICU LOS: only one study [41] described their ICU discharged standard;
MVD: seven of the included studies defined this parameter as the postoperative duration of mechanical ventilation [34, 37, 38, 40–43], and the other three circumscribed it as extubation time with no declaration of whether it is postoperative or not [33, 35, 44];
RFI: two studies [36, 38] described their RFI as”Postoperative renal failure needing ultrafiltration”, one described RFI as “Renal dysfunction according to blood routine results” [42], and the last one did not clarify how RFI was defined [34];
The rest of the clinical outcomes have no definite criterion.
These differences in methodology may conduce to heterogeneity. Thirdly, there is a lack of sufficient evidence to prove that UTI treatment could shorten hospital LOS or reduce the all-cause mortality rate. In the absence of studies specifically addressing the effects of UTI in other surgeries, such as dissecting aneurysms of ascending aorta, heart or lung transplantation, the vast majority of current analyses consist of patients with CABG, valve replacement, AAR, and combined surgery. Fourthly, large RCTs with a patient population of more than 1,000 have traditionally been considered the “ gold standard” for evaluating the reliability of clinical interventions [56], ergo hindered by the small sample size in included studies, a large number of high-quality RCTs are called for. Lastly, since UTI is not widely used in the West, the clinical relevance of this meta-analysis is confined.
Conclusion
This meta-analysis demonstrates that using UTI is associated with a shorter ICU LOS and MVD in CPB patients despite methodological limitations, and RFI generally showed a more favourable outcome with UTI treatment. According to the meta-regression and subgroup analysis, a higher UTI dosage seems to be correlated with better clinical outcomes. In summary, to better verify the beneficial effects of UTI treatment for CPB patients, further large, multicenter clinical trials and a standard dosing guideline are needed.
Acknowledgements
Not applicable.
Abbreviations
- UTI
Urinary trypsin inhibitor
- CPB
Cardiopulmonary bypass
- ICU
Intensive care unit
- LOS
Length of stay
- MVD
Mechanical ventilation duration
- RFI
Renal failure incidence
- CABG
Coronary artery bypass graft
- AAR
Aortic arch replacement
- VR
Valve replacement, including aortic valve replacement, mitral valve replacement
- TAR
Total aortic arch replacement
- MVR
Mitral valve replacement
- DVR
Double (aortic and mitral) valve replacement
- AVR
Aortic Valve Replacement
- CI
Confidence interval
- IV
Inverse variance
- Std
Standardized mean difference
Authors’ contributions
HZ: comprehensive search, data screening, data collection, data analysis, and writing the paper. YQ: data screening, collection and analysis. CG: meta analysis. WM: supervision, data analysis, review and editing the paper. The authors read and approved the final manuscript.
Funding
The work was funded by the joint foundation of Luzhou government and Southwest Medical University (2021LZXNYD-J28).
Availability of data and materials
Please contact the author for data requests.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hu Zhenyu, Email: simaweiwei@foxmail.com.
Yuan Qiaoli, Email: 772485722@qq.com.
Chen Guangxiang, Email: cgx23ly2002@163.com.
Wang Maohua, Email: wangmaohua@swmu.edu.cn.
References
- 1.Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest. 1997;112(3):676–692. doi: 10.1378/chest.112.3.676. [DOI] [PubMed] [Google Scholar]
- 2.Tu LN et al., Shear stress associated with cardiopulmonary bypass induces expression of inflammatory cytokines and necroptosis in monocytes. JCI Insight, 2020. [DOI] [PMC free article] [PubMed]
- 3.Ghotkar SV, et al. Preoperative calculation of risk for prolonged intensive care unit stay following coronary artery bypass grafting. J Cardiothorac Surg. 2006;1:14. doi: 10.1186/1749-8090-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pivatto Júnior F, et al. Morbimortality in octogenarian patients submitted to coronary artery bypass graft surgery. Arq Bras Cardiol. 2010;95(1):41–46. doi: 10.1590/s0066-782x2010005000071. [DOI] [PubMed] [Google Scholar]
- 5.Vanhorebeek I, Latronico N, Van den Berghe G. ICU-acquired weakness. Intensive Care Med. 2020;46(4):637–653. doi: 10.1007/s00134-020-05944-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oye RK, Bellamy PE. Patterns of resource consumption in medical intensive care. Chest. 1991;99(3):685–689. doi: 10.1378/chest.99.3.685. [DOI] [PubMed] [Google Scholar]
- 7.Luce JM, Rubenfeld GD. Can health care costs be reduced by limiting intensive care at the end of life? Am J Respir Crit Care Med. 2002;165(6):750–754. doi: 10.1164/ajrccm.165.6.2109045. [DOI] [PubMed] [Google Scholar]
- 8.Kalanuria AA, Ziai W, Mirski M. Ventilator-associated pneumonia in the ICU. Crit Care. 2014;18(2):208. doi: 10.1186/cc13775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cushing CA, Phillips LG. Evidence-based medicine: pressure sores. Plast Reconstr Surg. 2013;132(6):1720–1732. doi: 10.1097/PRS.0b013e3182a808ba. [DOI] [PubMed] [Google Scholar]
- 10.Scott BH, et al. Octogenarians undergoing coronary artery bypass graft surgery: resource utilization, postoperative mortality, and morbidity. J Cardiothorac Vasc Anesth. 2005;19(5):583–588. doi: 10.1053/j.jvca.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 11.Aya HD, et al. Goal-directed therapy in cardiac surgery: a systematic review and meta-analysis. Br J Anaesth. 2013;110(4):510–517. doi: 10.1093/bja/aet020. [DOI] [PubMed] [Google Scholar]
- 12.Muramatu M, et al. Purification and characterization of urinary trypsin inhibitor, UTI68, from normal human urine, and its cleavage by human uropepsin. J Biochem. 1980;88(5):1317–1329. doi: 10.1093/oxfordjournals.jbchem.a133100. [DOI] [PubMed] [Google Scholar]
- 13.Linder A, Russell JA. An exciting candidate therapy for sepsis: ulinastatin, a urinary protease inhibitor. Intensive Care Med. 2014;40(8):1164–1167. doi: 10.1007/s00134-014-3366-9. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, et al. Effect of urinary protease inhibitor (ulinastatin) on cardiopulmonary bypass: a meta-analysis for China and Japan. PLOS ONE. 2014;9(12):e113973. doi: 10.1371/journal.pone.0113973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue K, et al. Urinary trypsin inhibitor protects against systemic inflammation induced by lipopolysaccharide. Mol Pharmacol. 2005;67(3):673–680. doi: 10.1124/mol.104.005967. [DOI] [PubMed] [Google Scholar]
- 16.Xie X, Li T, Yuan H. Protective effects of Ulinastatin on oxidative stress and inflammation of rat-derived cardiomyocytes H9c2. Am J Transl Res. 2019;11(11):7094–7103. [PMC free article] [PubMed] [Google Scholar]
- 17.Li ST, et al. Ulinastatin attenuates LPS-induced inflammation in mouse macrophage RAW264.7 cells by inhibiting the JNK/NF-κB signaling pathway and activating the PI3K/Akt/Nrf2 pathway. Acta Pharmacol Sin. 2018;39(8):1294–1304. doi: 10.1038/aps.2017.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lun MH, et al. Ulinastatin improves myocardial ischemia-reperfusion injury in rats through endoplasmic reticulum stress-induced apoptosis pathway. Eur Rev Med Pharmacol Sci. 2020;24(10):5742–5749. doi: 10.26355/eurrev_202005_21366. [DOI] [PubMed] [Google Scholar]
- 19.Cui L, et al. Ulinastatin alleviates cerebral ischemia-reperfusion injury in rats by activating the Nrf-2/HO-1 signaling pathway. Ann Transl Med. 2020;8(18):1136. doi: 10.21037/atm-20-5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng WT, et al. Ulinastatin: a potential alternative to glucocorticoid in the treatment of severe decompression sickness. Front Physiol. 2020;11:273. doi: 10.3389/fphys.2020.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allegra A, et al. Immunopathology of SARS-CoV-2 infection: immune cells and mediators, prognostic factors, and immune-therapeutic implications. Int J Mol Sci. 2020;21(13):4782. doi: 10.3390/ijms21134782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuhara H, et al. Pharmacologic prophylaxis of post-endoscopic retrograde cholangiopancreatography pancreatitis: protease inhibitors and NSAIDs in a meta-analysis. J Gastroenterol. 2014;49(3):388–399. doi: 10.1007/s00535-013-0834-x. [DOI] [PubMed] [Google Scholar]
- 23.Wang LZ, et al. Effect of ulinastatin on serum inflammatory factors in Asian patients with acute pancreatitis before and after treatment: a meta-analysis. Int J Clin Pharmacol Ther. 2016;54(11):890–898. doi: 10.5414/CP202454. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, et al. Improvement of sepsis prognosis by ulinastatin: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2019;10:1370. doi: 10.3389/fphar.2019.01370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Effect of urinary trypsin inhibitor on inflammatory cytokines and organ function in patients with cardiopulmonary bypass. Eur Rev Med Pharmacol Sci 2017; 21(9): 2220–2225. [PubMed]
- 26.Yao YT, et al. Ulinastatin reduces postoperative bleeding and red blood cell transfusion in patients undergoing cardiac surgery: a PRISMA-compliant systematic review and meta-analysis. Medicine. 2020;99(7):e19184. doi: 10.1097/MD.0000000000019184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He G, et al. Effect of ulinastatin on interleukins and pulmonary function in bypass patients: a meta-analysis of randomized controlled trials. Herz. 2020;45(4):335–346. doi: 10.1007/s00059-018-4732-0. [DOI] [PubMed] [Google Scholar]
- 28.He S et al., Effect of the urinary tryptin inhibitor ulinastatin on cardiopulmonary bypass-related inflammatory response and clinical outcomes: a meta-analysis of randomized controlled trials. Clin Therape; 2015. [DOI] [PubMed]
- 29.Zhang Y, et al. Effect of urinary protease inhibitor (ulinastatin) on cardiopulmonary bypass: a meta-analysis for China and Japan. PLOS ONE. 2014;9(12):113973. doi: 10.1371/journal.pone.0113973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He QL et al., Does intraoperative ulinastatin improve postoperative clinical outcomes in patients undergoing cardiac surgery: a meta-analysis of randomized controlled trials. Biomed Res Int 2014; 2014. [DOI] [PMC free article] [PubMed]
- 31.Qiu Y, et al. Lack of efficacy of ulinastatin therapy during cardiopulmonary bypass surgery. Chin Med J (Engl) 2015;128(23):3138–3142. doi: 10.4103/0366-6999.170364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi J, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. 2020;11(5):641–654. doi: 10.1002/jrsm.1429. [DOI] [PubMed] [Google Scholar]
- 33.Chen TT, et al. Combined treatment of ulinastatin and tranexamic acid provides beneficial effects by inhibiting inflammatory and fibrinolytic response in patients undergoing heart valve replacement surgery. Heart Surgery Forum. 2013;16(1):E38–E47. doi: 10.1532/HSF98.20121072. [DOI] [PubMed] [Google Scholar]
- 34.Zhang P, et al. Effect of ulinastatin on post-operative blood loss and allogeneic transfusion in patients receiving cardiac surgery with cardiopulmonary bypass: a prospective randomized controlled study with 10-year follow-up. J Cardiothorac Surg. 2020;15(1):98. doi: 10.1186/s13019-020-01144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu HY, et al. Effect of urinary trypsin inhibitor on inflammatory cytokines and organ function in patients with cardiopulmonary bypass. Eur Rev Med Pharmacol Sci. 2017;21(9):2220–2225. [PubMed] [Google Scholar]
- 36.Wang XY, et al. Protective effects of high-dose ulinastatin on vital organs in patients receiving total arch replacement for type A aortic dissection. J Southern Med Univ. 2016;36(8):1085–1089. [PubMed] [Google Scholar]
- 37.Pang XY, et al. Effects of ulinastatin on perioperative inflammatory response and pulmonary function in cardiopulmonary bypass patients. Am J Ther. 2016;23(6):e1680–e1689. doi: 10.1097/MJT.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 38.Xu CE, et al. Effects of high-dose ulinastatin on inflammatory response and pulmonary function in patients with type-A aortic dissection after cardiopulmonary bypass under deep hypothermic circulatory arrest. J Cardiothorac Vasc Anesth. 2013;27(3):479–484. doi: 10.1053/j.jvca.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Hao X, et al. Urinary trypsin inhibitor attenuated inflammatory response of patients undergoing cardiopulmonary bypass by inducing activated treg cells. Inflammation. 2013;36(6):1279–1285. doi: 10.1007/s10753-013-9666-3. [DOI] [PubMed] [Google Scholar]
- 40.Oh SY, et al. Effects of ulinastatin treatment on myocardial and renal injury in patients undergoing aortic valve replacement with cardiopulmonary bypass. Korean J Anesthesiol. 2012;62(2):148–153. doi: 10.4097/kjae.2012.62.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song JE, et al. The effect of ulinastatin on postoperative blood loss in patients undergoing open heart surgery with cardiopulmonary bypass. J Int Med Res. 2011;39(4):1201–1210. doi: 10.1177/147323001103900408. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Q, et al. Effect of ulinastatin on perioperative inflammatory response to coronary artery bypass grafting with cardiopulmonary bypass. J Central South Univ (Med Sci) 2010;35(2):107–110. doi: 10.3969/j.issn.1672-7347.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Bingyang J, et al. Effects of urinary protease inhibitor on inflammatory response during on-pump coronary revascularisation. Effect of ulinastatin on inflammatory response. J Cardiovasc Surg. 2007;48(4):497–503. [PubMed] [Google Scholar]
- 44.Nakanishi K, et al. Effects of ulinastatin treatment on the cardiopulmonary bypass-induced hemodynamic instability and pulmonary dysfunction. Crit Care Med. 2006;34(5):1351–1357. doi: 10.1097/01.CCM.0000215110.55899.AE. [DOI] [PubMed] [Google Scholar]
- 45.Higgins JP, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bronicki RA, Hall M. Cardiopulmonary bypass-induced inflammatory response: pathophysiology and treatment. Pediatr Crit Care Med. 2016;17(8 Suppl 1):S272–S278. doi: 10.1097/PCC.0000000000000759. [DOI] [PubMed] [Google Scholar]
- 47.Inoue K, Takano H. Urinary trypsin inhibitor as a therapeutic option for endotoxin-related inflammatory disorders. Expert Opin Investig Drugs. 2010;19(4):513–520. doi: 10.1517/13543781003649533. [DOI] [PubMed] [Google Scholar]
- 48.Qiu Y, et al. Lack of efficacy of ulinastatin therapy during cardiopulmonary bypass surgery. Chin Med J. 2015;128(23):3138–3142. doi: 10.4103/0366-6999.170364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi XQ, et al. Protective effect of ulinastatin on pulmonary function after cardiopulmonary bypass. J Sichuan Univ (Med Sci Edn) 2013;44(5):752–755. [PubMed] [Google Scholar]
- 50.Liu Y, et al. Effect of high-dose ulinastatin on the cardiopulmonary bypass-induced inflammatory response in patients undergoing open-heart surgery. Chin Med J (Engl) 2020;133(12):1476–1478. doi: 10.1097/CM9.0000000000000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jönsson-Berling BM, Ohlsson K. Distribution and elimination of intravenously injected urinary trypsin inhibitor. Scand J Clin Lab Invest. 1991;51(6):549–557. doi: 10.3109/00365519109104564. [DOI] [PubMed] [Google Scholar]
- 52.Wan X, et al. Ulinastatin administration is associated with a lower incidence of acute kidney injury after cardiac surgery: a propensity score matched study. Crit Care. 2016;20:42. doi: 10.1186/s13054-016-1207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62(5):1539–1549. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- 54.Rosenberger C, Rosen S, Heyman SN. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357(21):2204–5. doi: 10.1056/NEJMc072781. [DOI] [PubMed] [Google Scholar]
- 55.Liu S, et al. Multi-organ protection of ulinastatin in traumatic cardiac arrest model. World J Emerg Surg. 2018;13:51. doi: 10.1186/s13017-018-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ioannidis JP, Cappelleri JC, Lau J. Issues in comparisons between meta-analyses and large trials. JAMA. 1998;279(14):1089–1093. doi: 10.1001/jama.279.14.1089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact the author for data requests.