Abstract
Introduction
Pregnancy registries and spontaneous reports are essential pharmacovigilance tools to evaluate drug safety during pregnancy.
Objectives
The aim of this study was to evaluate postmarket capture of exposed pregnancies.
Methods
Pregnancy registries for drugs and biologics were identified in a systematic review. Through a standardized questionnaire, manufacturers provided information on (1) pregnancy registry enrollment and retention, and (2) worldwide receipt of spontaneous reports for exposed pregnancies. A validated algorithm for live-birth pregnancies allowed calculation of exposure rates per 100,000 live births using claims data.
Results
Among 34 products with a pregnancy registry, median (interquartile range) registry enrollment was 36 pregnancies (5–258) and median spontaneous report capture was 450 pregnancies (89–1192). Products used in >20/100,000 live births had a median registry enrollment of 490 pregnancies and median capture of 1061 spontaneously reported exposed pregnancies. Lower median registry enrollment and spontaneous report capture was observed for products used in 0.5–20/100,000 live births (36 from registries, 541 spontaneous reports) and <0.5/100,000 live births (3 from registries, 41 spontaneous reports). Among 24 registries enrolling ≥10 pregnancies, median capture of pregnancy outcomes (e.g. live birth, spontaneous abortion) was 83.9%. For 19 registries enrolling ≥10 infants, the median proportion of infants achieving protocol-specified follow-up was 89.9% for up to 4 weeks post-birth, 75.0% for 1–5 months, and 57.1% for ≥6 months.
Conclusions
Relatively higher product utilization among pregnant women predicted greater pregnancy registry enrollment. For products rarely used during pregnancy, registry enrollment was low and differences in registry enrollment compared with worldwide spontaneous report receipt were most pronounced. Products with very low utilization levels during pregnancy may require a combination of worldwide pharmacovigilance, pregnancy registries, and additional study methods to achieve adequate surveillance.
1. Introduction
The safety of new medical products in pregnant women is often poorly characterized. In the preapproval stage, animal data are often the only evidentiary source to guide use of medical products in pregnancy. Postapproval, passive surveillance of real-world exposure to medical products is often a primary source of new safety information in pregnancy. Passive data collection relies on health professionals and patients to submit spontaneous reports for exposed pregnancies and birth defects to the US FDA’s Adverse Event Reporting System (FAERS) or the product manufacturers’ proprietary pharmacovigilance databases.
In 2002, the FDA released a Guidance to Industry for Establishing Pregnancy Exposure Registries [1]. Pregnancy registries may be conducted for drug or biologic products with a concerning or unknown safety profile at approval when there is potential for exposure among pregnant women. Pregnancy registries prospectively enroll pregnant women who have a medical product exposure and follow them through delivery, and often postpartum, to assess adverse effects in exposed infants. Rates of malformations and other adverse outcomes among exposed pregnancies can be calculated, and comparator groups can be enrolled or identified to quantify relative risks for pregnant women and their offspring. Adequate recruitment of exposed pregnancies and complete ascertainment of pregnancy and infant outcomes are critical to a properly executed registry.
Both pregnancy registries [2, 3] and spontaneous reports [4, 5] have produced new safety data for the use of drugs in pregnancy. In order to better focus available resources and efforts in postmarket pregnancy surveillance, it is important to quantify the total number of exposures captured in pregnancy registries and pharmacovigilance databases. Our study sought to (1) quantify pregnancy registry enrollment and the number of spontaneous reports received by the drug manufacturer; (2) assess retention of pregnant women and their infants in pregnancy registries; (3) calculate pregnancy registry enrollment per 100 total spontaneous reports identified; and (4) assess whether pregnancy registry enrollment and spontaneous report capture can be predicted based on certain factors.
2. Materials and Methods
2.1. Systematic Review of Pregnancy Exposure Registries
Our systematic review for pregnancy registry identification has been previously described in detail [6]. Briefly, we identified pregnancy registries for drug or biologic products registered on http://www.clinicaltrials.gov, presented on the FDA’s Office of Women’s Health webpage [7], or submitted to the FDA for product safety evaluation. Pregnancy registries were included if they were designed with the primary objective to study the safety of drugs or biologics in pregnancy, they sought to enroll pregnancies prior to knowledge of the study outcome, the product was approved in the US, and the registry started prior to January 2014 to allow a minimum of 1 year of patient accrual for this analysis. Pharmacovigilance plans designed to analyze spontaneous reports of birth defects were not included because they did not meet our definition of a prospectively enrolling registry. For the purpose of analyzing enrollment, multiproduct registries contributed only one product to the analysis. In this case, we applied the following hierarchical prioritization schedule to select a single product for inclusion in the analysis: (1) products included in the registry based on an FDA postmarket requirement or commitment; (2) products approved after 2001; and (3) random selection.
2.2. Targeted Information Request for Identified Pregnancy Exposure Registries
We developed a standard information request form to obtain study protocols and targeted data from each registry. We requested total and annual registry enrollment as well as data on the retention of pregnant women to allow for the capture of birth outcomes (e.g. live birth, stillbirth, spontaneous or elective abortion). In addition, manufacturers responsible for included registries were asked to provide information about the planned length of the postpartum period during which the infants of exposed mothers would be followed and success rates of achieving prespecified follow-up. We further requested from each manufacturer the number of postmarketing spontaneous reports of exposed pregnancies, excluding those in the registry, received from worldwide sources since market approval, by country of origin. A study protocol or statistical analysis plan was requested for each registry. See electronic supplementary material 1 for the information request.
2.3. Predictors for Capture of Pregnancy Exposures
Our study sought to quantify how the prevalence of utilization during pregnancy impacted enrollment in pregnancy registries. We obtained utilization levels of products with pregnancy registries from a sample of 1.9 million pregnancies resulting in live-birth deliveries in the Sentinel Distributed Database, which contains electronic healthcare data from a distributed network of data partners in the US, mostly large commercial health insurers, who contribute data using a common data model (CDM) [8]. The CDM allows for standardized queries across data partners and includes a robust data quality assurance process. Using data from 15 data partners, we identified live-birth pregnancies among women aged 10 through 54 years at delivery using a validated algorithm based on International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes [9, 10]. Eligible pregnancies required continuous health plan enrollment with medical and pharmacy benefits for a minimum 480 days prior to delivery admission to capture drug product exposures during and prior to pregnancy. The start and end of pregnancy were estimated using diagnosis codes to determine gestational age at birth [11]. A pregnancy was considered exposed to a drug or biologic product if there was a dispensing or active supply of medication between the start of pregnancy and the hospital admission for delivery. The prevalence for utilization of each product with a pregnancy registry was calculated using the number of exposed pregnancies as the numerator and the total number of live-birth pregnancies identified since the approval date of the given product as the denominator. Products were then grouped into one of three utilization categories: >20 exposed pregnancies per 100,000 live-birth pregnancies, 0.5–20/100,000 live-birth pregnancies, and <0.5/100,000 live-birth pregnancies.
Because anticipated utilization rates during pregnancy are often unknown at the time of product approval when pregnancy registries are typically issued by the FDA, we also evaluated the primary distribution channel as a candidate predictor for capture of exposed pregnancies. The predominant distribution channel was defined for each study product using the IMS National Sales Perspectives™ database [12], which provides national sales estimates of product packages sold from manufacturers to all channels of distribution in the US. Distribution channels were defined as (1) outpatient retail pharmacies (chain stores, food stores, and independent); (2) mail-order pharmacies; and (3) non-retail pharmacies (e.g. hospitals, clinics, home health, and long-term care).
Finally, we evaluated the countries included in the pregnancy registry (i.e. US only or multinational) as a predictor of capturing exposed pregnancies. We reviewed each study protocol and classified registries on whether patient enrollment was limited to the US only or also included enrollment from at least one other country (i.e. multinational).
2.4. Statistical Analysis
Data obtained from our information requests are proprietary, were intended for internal regulatory evaluation of pregnancy registries, and can only be shared in aggregate. We used descriptive statistics, primarily median and interquartile ranges (IQRs), to present enrollment in registries and the number of spontaneous reports received by the manufacturer’s proprietary pharmacovigilance databases. Median enrollment was contrasted descriptively, using the above predictors. Our primary analyses present the total capture of exposed pregnancies in pregnancy registries and spontaneous reports by study predictor. Secondary analyses evaluated enrollment per calendar year to account for different enrollment lengths per registry. These comparisons were justified on the basis that, for the most part, all pregnancy reports are derived from a similar passive reporting process. Enrollment in the registry versus inclusion in a pharmacovigilance database is generally determined by whether the birth outcome has yet to occur (prospective reporting), the effectiveness of registry enrollment efforts, and the women or healthcare providers’ willingness to participate.
We further contrasted the raw capture of enrolled pregnancies in pregnancy registries with the total number of spontaneous reports in both the registry and the manufacturer’s proprietary pharmacovigilance database combined, after duplicate pregnancies included in both data sources were removed. We reported it as the number of exposed pregnancies enrolled in pregnancy registries per 100 total known pregnancy exposures identified (i.e. registry + spontaneous reports). For the predictor ‘countries included’, registries enrolling in the ‘US only’ were only compared with spontaneous reports from the US as the denominator.
Retention of pregnancies and infants of exposed mothers is also presented using descriptive statistics. We limited assessment of pregnancy retention to registries enrolling ≥10 pregnancies to reduce the impact of rates based on very small denominators. For the primary assessment, we calculated the percentage of pregnancies in each registry that were successfully followed through completion and the percentage of pregnancies with ongoing follow-up at the time of our analysis. Results are described as the median (IQR) percentage for retention of pregnancies through the birth outcomes for enrolled patients. Similarly, registries with data on ≥10 infants of exposed mothers were characterized based on the median (IQR) percentage of registries achieving their prespecified postnatal follow-up goal (i.e. up to 4 weeks post-birth, 1–5 months, or ≥6 months).
This study protocol was waived from review by the FDA’s Research in Human Subjects Committee because no individual patient-level data were obtained. The analysis conducted in Sentinel was considered a public health surveillance activity that was not under the purview of Institutional Review Boards [13]. Data were analyzed using Statistical Analysis Software version 9.3 (SAS Institute, Inc., Cary, NC, USA).
3. Results
Our search identified 254 potential registries, 80 of which were determined to be pregnancy exposure registries, 35 of which met the criteria for inclusion into the study (Fig. 1). Of these 35 pregnancy registries, 11 were multiproduct registries for which one product was identified for analysis, as described above. See electronic supplementary material 2 for a list of study registries, ordered by drug utilization category, providing product name, registry name, year of initial FDA approval, and primary distribution channel according to IMS’s National Sales Perspective database. A protocol or other similar documentation was obtained from the product manufacturer or registry investigators for each of the 34 included registries.
Fig. 1.
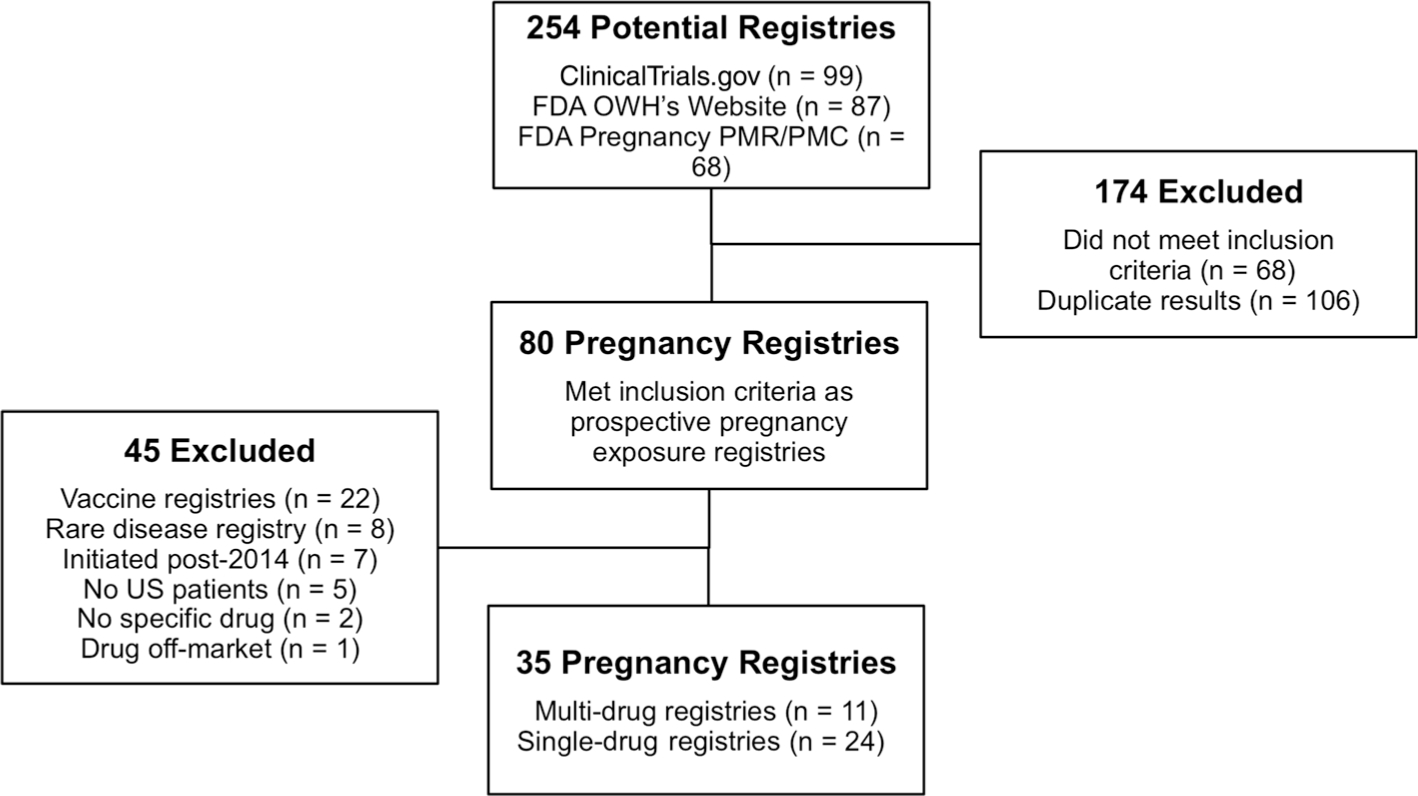
Search results and selection of pregnancy exposure registries. FDA Food and Drug Administration, OWH Office of Women’s Health, PMR/PMC postmarket requirement or commitment
Of the registries contacted, 34 of 35 (97.1%) completed the information request and were included in the analyses. The median (IQR) duration of registry enrollment at the time of the questionnaire was 6 years (4.7–8.8), and the median (IQR) enrollment in study registries was 36 pregnancies (5–258), with three registries enrolling no pregnancies and three registries enrolling more than 1500 pregnancies. The median (IQR) number of infants enrolled was 12 (2–119), and the median (IQR) capture of spontaneous reports for exposed pregnancies outside the registry was 450 (89–1192). Of 31 pregnancy registries for products that received at least one spontaneous report, 22 identified a majority of spontaneous reports from countries outside the US, with a median (IQR) of 37% (25.3–55.2%) of spontaneous reports having a US origin.
Figure 2 depicts the total number of products with pregnancy registries (total n = 34). For each registry, the total number of drug exposures is shown within ranges of exposures captured (e.g. no exposures, 1–20 exposures). This is shown for women exposed to drug products during pregnancy, infants exposed to drug products in utero, and spontaneous reports of pregnancy exposure. In this figure, it can be observed that most registries have a total capture of women and infants in the smaller exposure categories (e.g. 1–20, 21–100), but a total capture of spontaneous reports in the larger exposure categories (e.g. 501–1500, >1500).
Fig. 2.
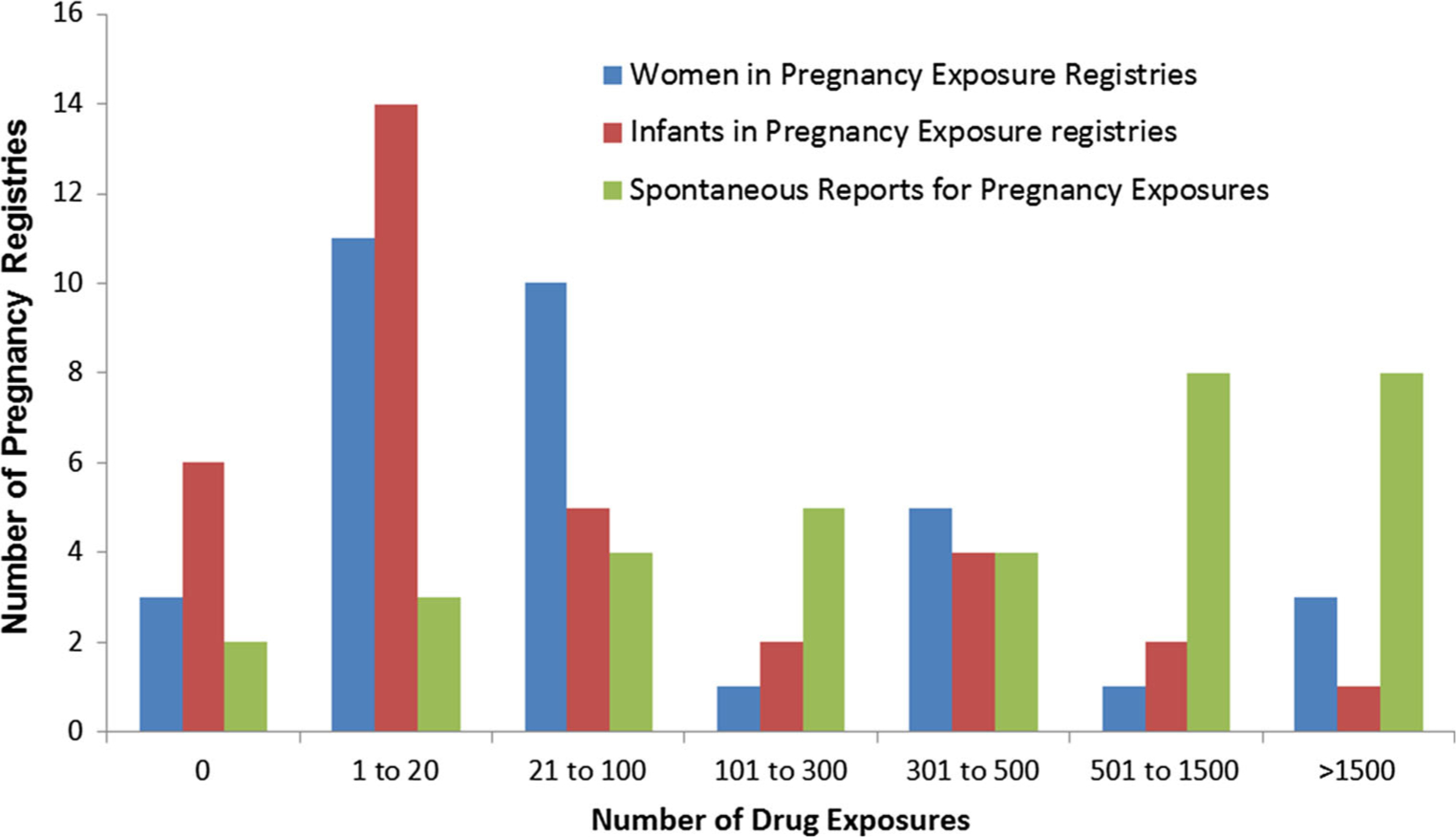
Number of pregnancy registries within each range of exposed pregnancies and infants captured (n = 34 total products). This figure represents the number of products with pregnancy registries (total n = 34), and describes the distribution of the number of exposed pregnancies and resultant infants captured, using six discrete categories for the total number of drug exposures. The distributions are provided for a blue bars: women exposed to drug products during pregnancy; b red bar: infants exposed to drug products in utero; and c green bar: spontaneous reports of pregnancy exposure by the sponsor
Although all products in this study had low levels of utilization among live-birth pregnancies, products with relatively greater utilization during pregnancy had markedly greater registry enrollment (Table 1), with median enrollment of 490 pregnancies for products used in >20 pregnancies per 100,000 live-birth pregnancies, 36 enrolled pregnancies for products used in 0.5–20/ 100,000 live-birth pregnancies, and 3 enrolled pregnancies for products used in <0.5/100,000 live-birth pregnancies. A secondary analysis evaluating enrollment per calendar year found a similar trend [>20/100,000: n = 36 (13–164) pregnancies/year; 0.5–20/100,000: n = 9 (3–33) pregnancies/year; <0.5/100,000: n = 0.3 (0.0–0.7) pregnancies/year]. The number of total spontaneous reports received by the manufacturer followed a similar trend [>20/100,000: n = 1061 spontaneous reports; 0.5–20/100,000: n = 541 spontaneous reports; <0.5/100,000: n = 41 spontaneous reports]. For exposures >20/100,000 live-birth pregnancies, the median number of registry-enrolled pregnancies per 100 total pregnancy exposures (44/100 total) far exceeded that for exposures 0.5–20/100,000 live-birth pregnancies (6/100 total) or exposures <0.5/100,000 live-birth pregnancies (5/100 total).
Table 1.
Data capture in pregnancy exposure registries and number of spontaneous reports by enrollment predictor
| No. of registries | Registry enrollment [median (IQR)] | SRs [median (IQR)] | Registry enrollment per 100 SRsb [median (IQR)] | |
|---|---|---|---|---|
|
| ||||
| Drug utilization categories | ||||
| >20/100,000 live-birth pregnancies | 9 | 490 (92–1597) | 1061 (743–1224) | 44 (14–52) |
| 0.5–20/100,000 live-birth pregnancies | 16 | 36 (16–150) | 541 (291–2186) | 6 (3–9) |
| <0.5/100,000 live-birth pregnancies | 9 | 3 (0–4) | 41 (5–72) | 5 (0–9) |
| Primary distribution channel | ||||
| Retail | 14 | 59 (15–639) | 564 (164–1183) | 11 (6–52) |
| Mail order | 12 | 39 (3–150) | 541 (39–2186) | 6 (1–14) |
| Non-retail | 8 | 18 (13–124) | 367 (188–760) | 6 (5–9) |
| Included countriesa | ||||
| US only | 19 | 31 (4–113) | 85 (26–417) | 16 (9–48) |
| Multinationalc | 15 | 43 (11–593) | 743 (182–1143) | 7 (3–52) |
IQR interquartile range, SRs spontaneous reports (total)
For the ‘included countries’ predictors, registries enrolling in the US were only compared with spontaneous reports captured in the US. Multinational registries were compared with the multinational capture of spontaneous reports
Median registry enrollment per 100 total pregnancy exposures is calculated as the median value across all registries, not directly from the median registry enrollment and spontaneous report capture reported in this table
Multinational refers to registries enrolling in the US and at least one other country
Products primarily dispensed in retail pharmacies had greater median registry enrollment (59 pregnancies) and a larger enrollment per 100 total pregnancy exposures (11/ 100 total) than mail order (39 pregnancies; 6/100 total) or non-retail (18 pregnancies; 6/100 total) distribution pathways. Pregnancy registries with multinational enrollment had a larger median enrollment (43 pregnancies) than registries enrolling only in the US (31 pregnancies). Among registries enrolling only US patients (n = 19), on average 15.8 pregnancies were enrolled in the registry per 100 total spontaneous reports from the US.
Among registries enrolling ≥10 pregnancies (n = 24), the median (IQR) retention rate to capture the pregnancy outcomes (e.g. live birth) was 83.9% (72.5–94.5%). For registries with data on ≥10 infants of exposed mothers, the median retention rate to achieve protocol-specified follow-up was as follows: up to 4 weeks post-birth, 83.9% (72.5–94.5%); 1–5 months, 75.0% (72.0–82.1%); and ≥6 months, 57.1% (35.0–71.6). While registries with longer protocol-specified follow-up had lower rates of achieved follow-up, this was partially accounted for by the larger percentage of pregnancies with follow-up ongoing (Table 2).
Table 2.
Data capture of women and infants of exposed mothers in pregnancy exposure registries
| Exposure | No. of registries | Achieved follow-up, % [median (IQR)] | Achieved and ongoing follow-up, % [median (IQR)] |
|---|---|---|---|
|
| |||
| Womena | 24 | 83.9 (72.5–94.5) | 94.1 (75.7–97.3) |
| Infantsb | |||
| Up to 4 weeks post-birth | 4 | 89.9 (81.1–97.8) | 92.1 (84.5–97.8) |
| 1–5 months | 4 | 75.0 (72.0–82.1) | 77.4 (75.2–82.6) |
| >6 months | 11 | 57.1 (35.0–71.6) | 83.0 (67.7–92.6) |
IQR interquartile range
Follow-up of exposed women was only reported for pregnancy exposure registries enrolling ≥10 pregnancies
Follow-up of infants of exposed mothers was only reported for pregnancy exposure registries enrolling ≥10 infants
In our primary analysis, all predictors were evaluated independently. To explore relationships between predictors, we created a matrix of the presence of each predictor within all other predictors (see electronic supplementary material III). A high degree of collinearity was observed between exposures in >20/100,000 live-birth pregnancies and retail distribution (i.e. 8 of 14 retail exposures in >20/ 100,000 pregnancies), but not among other predictors.
4. Discussion
We observed a median enrollment of 36 pregnancies and 12 infants across included registries in comparison with a median of 450 spontaneous reports of exposed pregnancies received by manufacturers. This sizable discrepancy indicates both a need to improve enrollment in pregnancy registries and to place additional focus on analysis of spontaneous reports which currently provide the largest amount of safety data describing medical product exposure during pregnancy. In a prior review of protocols for registries included in this analysis, we identified a median target enrollment of 300 women (range 150–500) [6]. This sample size was typically justified as having the ability to detect a teratogen associated with a to a two- to threefold increased relative risk for major malformations overall. In our study, nine registries successfully enrolled 300 or more women (three ongoing, six closed), while the remainder fell short or have yet to reach this target (16 ongoing, 9 closed). A well-conducted pregnancy registry may identify a rare adverse effect, even with limited power, which was the case in the example of topiramate and oral clefts [3]. However, a registry that successfully achieves 300 enrolled pregnancies is not adequately powered for, and often may be unable to detect, a rare specific malformation [6].
We determined several key predictors for registry enrollment. Greater product utilization among pregnant women was the strongest predictor of registry enrollment. Three products with pregnancy registries achieved enrollment of >1500 pregnant women—bupropion [14], lamotrigine [15], and Truvada (emtricitabine and tenofovir) [16]. Bupropion and lamotrigine had the first and third highest rates of utilization among our sample of pregnant women in the US, likely contributing to their successful enrollment. Truvada prevents mother–baby transmission of HIV and has a unique scenario where a select number of clinics prescribe a high volume of prescriptions to pregnant women as a focus for recruitment efforts. Across all nine registries with products used in <0.5/100,000 live-birth pregnancies, only 59 total pregnancies were enrolled, most of which came from a single registry. Retail-dispensed products and multinational recruitment were also found to have larger pregnancy registry enrollment. These factors may help frame expectations when initiating a registry.
Compared with the pregnancy registry, the manufacturer’s proprietary pharmacovigilance databases captured approximately twice as many additional pregnancies for products used in >20/100,000 live-birth pregnancies, and 10–15 times as many pregnancies for products used in ≤20/100,000 live-birth pregnancies. This discordance likely results from a complex interplay of factors affecting timing of enrollment in the pregnancy registry, recruitment efforts, and the woman’s or healthcare provider’s willingness to participate. The large ratio of spontaneous reports captured to registry-enrolled exposed pregnancies, for products with very low utilization among pregnant women, is concerning. This observation suggests a need for worldwide safety data collection to achieve adequate numbers of exposed pregnancies for meaningful analysis of products that are very rarely used by pregnant women. Examples of additional approaches include enhanced pharmacovigilance strategies with follow-up questionnaires to exposed pregnant women who do not choose to enroll in a registry, population-based networks that capture birth defects and matched controls, and population-based studies employing mother–baby linkages in reimbursement claims or electronic medical records.
Improving outreach efforts is vital to improved registry enrollment. The expert panel at the 2014 FDA public meeting on pregnancy registries discussed the use of multiple methods for effective recruitment, including approaches that are based on health systems, healthcare providers, and patients [17]. A health-systems-based approach includes automated electronic health record alerts to inform physicians about the existence of a pregnancy registry, which are generated in response to positive pregnancy test results and prescription of a specific medical product. This approach has been employed by the Organization of Teratology Information Specialists (OTIS) MotherToBaby pregnancy registries. A healthcare provider approach includes targeting healthcare providers who are dedicated to encouraging their patients to participate in the registry. This was the case in the Truvada registry that recruited HIV clinics to participate. A patient approach can include recruitment through social media. The North American Antiepileptic Drug Pregnancy Registry has demonstrated improved enrollment through use of a social media campaign on Facebook [18].
The results of this study also demonstrated relatively high rates of retention for both enrolled pregnant women through delivery and enrolled infants through the prespecified follow-up goals. While many registries experienced low enrollment, efforts to ascertain outcome data on enrolled pregnancies and infants were largely successful. These high retention rates may serve as a basis for evaluating retention in future pregnancy registries.
To the best of our knowledge, this is the first study to quantify capture of exposed pregnancies in both pregnancy registries and spontaneous reports across a large number of drug products, with linkage to product utilization and distribution information to explore differences in enrollment. However, our study does have several limitations. We conducted independent analysis of each study predictor due to the limited sample size. To supplement these analyses, we evaluated the distribution of each predictor among the other predictors, noting a high correlation only between exposures in >20/100,000 live-birth pregnancies and the retail distribution pathway. In addition, because we sampled a single product from multiproduct registries, many of which were conducted independently of the sponsor, we cannot provide inference into whether these registries had differentially greater or lesser enrollment. Lastly, for comparison of multinational enrolling registries with worldwide spontaneous report capture, the countries included in the registry and pharmacovigilance database may not always match, and this predictor should be interpreted as a raw comparison of data capture rather than a percentage of spontaneous reports that enrolled in the registry.
5. Conclusion
Products with relatively greater utilization levels among pregnant women, those with primarily retail distribution, and multinational enrolling registries had more successful enrollment in pregnancy registries. Products with relatively lower use among pregnant women (≤20/100,000 live-birth pregnancies) had less successful enrollment in pregnancy registries, suggesting these products may especially benefit from a worldwide pregnancy surveillance approach with multiple study methods to achieve adequate surveillance. Pregnancy registries may benefit from more effective promotion of the registry to patients and healthcare providers for identification and enrollment of exposed pregnancies. Continued focus on optimizing the study of risks associated with medical product exposures during pregnancy is crucial to producing high-quality data in a reasonable time frame after approval to inform discussions between a pregnant woman and her healthcare provider.
Supplementary Material
Key Points.
Relatively higher utilization of a drug or biologic medical product in pregnant women predicted increased enrollment in that product’s pregnancy registry.
Enrollment in pregnancy registries was low relative to the total capture of spontaneous reports of pregnancy exposure for a given product.
Our study demonstrated relatively high rates of retention of enrolled women and their infants in pregnancy registries.
Funding
Sentinel is funded by the US FDA. No other funding was received for this study.
Footnotes
Compliance with ethical standards
Conflict of interest Steven Bird, Kate Gelperin, Lockwood Taylor, Leyla Sahin, Hoda Hammad, Susan Andrade, Mohamed Mohamoud, Sengwee Toh, and Christian Hampp have no conflicts of interest to declare that are directly relevant to the content of this study. This study represents the opinions of the authors and not necessarily those of the FDA. Susan Andrade received a grant from Pfizer Inc. unrelated to this study or any drugs evaluated in our study. No other conflicts are present.
Electronic supplementary material The online version of this article (doi:10.1007/s40264-017-0591-5) contains supplementary material, which is available to authorized users.
References
- 1.US Food and Drug Administration. Guidance for Industry. Establishing Pregnancy Exposure Registries. Aug 2002. Available at: https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071639.pdf. Accessed 10 June 2016.
- 2.Vannappagari V, Albano JD, Koram N, Tilson H, Scheuerle AE, Napier MD. Prenatal exposure to zidovudine and risk for ventricular septal defects and congenital heart defects: data from the Antiretroviral Pregnancy Registry. Eur J Obstet Gynecol Reprod Biol. 2016;197:6–10. [DOI] [PubMed] [Google Scholar]
- 3.Hernández-Díaz S, Smith CR, Shen A, Mittendorf R, Hauser WA, Yerby M, Holmes LB. North American AED Pregnancy Registry. Comparative safety of antiepileptic drugs during pregnancy. Neurology. 2012;78(21):1692–9. [DOI] [PubMed] [Google Scholar]
- 4.Zagouri F, Sergentanis TN, Chrysikos D, Papadimitriou CA, Dimopoulos MA, Bartsch R. Trastuzumab administration during pregnancy: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;137:349–57. [DOI] [PubMed] [Google Scholar]
- 5.Dai WS, LaBraico JM, Stern RS. Epidemiology of isotretinoin exposure during pregnancy. J Am Acad Dermatol. 1992;26(4):599–606. [DOI] [PubMed] [Google Scholar]
- 6.Gelperin K, Hammad H, Leishear K, Bird ST, Taylor L, Hampp C, Sahin L. A systematic review of pregnancy exposure registries: examination of protocol-specified pregnancy outcomes, target sample size, and comparator selection. Pharmacoepidemiol Drug Saf. 2017;26(2):208–14. [DOI] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration. Office of Women’s Health. List of pregnancy registries. Available at: https://www.fda.gov/ScienceResearch/SpecialTopics/WomensHealthResearch/ucm251314.htm. Accessed 10 June 2016.
- 8.Behrman RE, Benner JS, Brown JS, McClellan M, Woodcock J, Platt R. Developing the sentinel system: a national resource for evidence development. N Engl J Med. 2011;364(6):498–9. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Andrade SE, Cooper WO, et al. Validation of an algorithm to estimate gestational age in electronic health plan databases. Pharmacoepidemiol Drug Saf. 2013;22(5):524–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raebel MA, Ellis JL, Andrade SE. Evaluation of gestational age and admission date assumptions used to determine prenatal drug exposure from administrative data. Pharmacoepidemiol Drug Saf. 2005;14(12):829–36. [DOI] [PubMed] [Google Scholar]
- 11.Andrade SE, Toh S, Houstoun M, et al. Surveillance of medication use during pregnancy in the mini-sentinel program. Matern Child Health J. 2016;20(4):895–903. [DOI] [PubMed] [Google Scholar]
- 12.IMS Institute for Healthcare Informatics. NSRD Data Brief: National Sales Perspectives™. Available at: https://www.imshealth.com/files/web/IMSH%20Institute/NSP_Data_Brief-.pdf. Accessed 31 June 2016.
- 13.McGraw D, Rosati K, Evans B. A policy framework for public health uses of electronic health data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):18–22. [DOI] [PubMed] [Google Scholar]
- 14.GlaxoSmithKline. The Bupropion Pregnancy Registry Final Report. Issued Aug 2008. Available at: http://pregnancyregistry.gsk.com/documents/bup_report_final_2008.pdf. Accessed 20 July 2017. [Google Scholar]
- 15.Cunnington MC, Weil JG, Messenheimer JA, Ferber S, Yerby M, Tennis P. Final results from 18 years of the International Lamotrigine Pregnancy Registry. Neurology. 2011;76(21):1817–23. [DOI] [PubMed] [Google Scholar]
- 16.Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral Pregnancy Registry International Interim Report for 1 January 1989 through 31 January 2017. Wilmington: Registry Coordinating Center; 2017. Available at: http://www.APRegistry.com. Accessed 20 July 2017. [Google Scholar]
- 17.US Food and Drug Administration. 2014 meeting materials; Study approaches and methods to evaluate the safety of drugs and biological products during pregnancy in the post-approval setting; public meeting, request for comments. 2014. Available at: https://www.fda.gov/Drugs/NewsEvents/ucm386560.htm. Accessed 20 July 2017.
- 18.Chavez N The key role of social media in registry recruitment [abstract no. S5]. In: Presented at the 57th Annual Meeting of the Teratology Society: Denver; 24–28 June 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


