Abstract
A liquid chromatography equipped with tandem mass spectrometric method using multi-stage flow rates was developed for the determination of donepezil in human plasma to support a randomized, crossover bioequivalence (BE) study in which healthy volunteers each received a single oral dose of the reference and test formulations of 10 mg donepezil hydrochloride. This integrated liquid chromatography with tandem mass spectrometry (LC-MS/MS) system with electrospray ionization and a deuterium-labeled internal standard (IS) were employed for the positive multiple-reaction-monitoring (MRM) analyses. The baseline separation using a high-resolution monolithic column under gradient and flexible flowrate conditions between donepezil and multiple interfering peaks from the extracted quality control, calibration standard and study plasma samples following simple protein precipitation extraction procedures was accomplished within 1.5 minutes. The ultrafast monolithic column performance in terms of chromatographic separation efficiency, peak asymmetry and resolution and retention time reproducibility was found to be sustainable. The linear dynamic range was detected over a concentration range of 0.2–50 ng/mL. The intra- and inter-day assay accuracy and precision were within 15% for the analyte in individual biological fluids. A positive correlation coefficient (r) greater than 0.995 for donepezil concentrations in study plasma samplers measured by the proposed and the other validated LC-MS/MS methods in support of a bioequivalence study was observed.
Keywords: Donepezil, Bioequivalence, Multi-stage Flow Rate, LC-MS/MS
INTRODUCTION
The investigation of efficient bioanalytical methodologies to accurately explore drug metabolism and pharmacokinetics studies plays a critical role in the drug development process. The objective of this study was to develop a high-efficiency liquid chromatography with tandem mass spectrometry (LC-MS/MS) method to support a comparative pharmacokinetics of the test and reference formulations of donepezil, an acetylcholinesterase (AChE) inhibitor as a selective treatment for Alzheimer’s disease [1], in healthy volunteers. In previous literature, high-performance liquid chromatography (HPLC) hyphenated with tandem mass spectrometry (MS/MS) methods have become the method of choice for donepezil quantitation in various biological fluids due to its inherent selectivity and high sensitivity [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. However, for the determination of donepezil in human plasma, these methods conventionally demanded lengthy sample preparation procedures such as solid-phase extraction (SPE) [8,14] or liquid extraction [6,7,10,12,17] followed by drying the final extract at an elevated temperature and reconstitution steps before injection into LC-MS/MS systems to avoid ionization suppression, the requirement for longer chromatographic run times (≥ 4 minutes run time) to resolve interference by endogenous constituents and/or for a longer lifespan of chromatographic columns. This study describes a multi-stage flow rate, ultrafast reversed phase type LC-MS/MS method for the determination of donepezil in human plasma with protein precipitation and for its application to a pilot bioequivalence study. The introduction of a high-resolution monolithic stationary phase column in combination with an automated diverter vale was an important element to enhance assay efficiency while making simple sample preparation possible to eliminate labor-intensive and time-consuming processes and consequently to improve productivity.
METHODS
Materials and reagents
Donepezil and Donepezil-d5 used as an internal standard (IS) in this study with purity > 98% (as shown in Fig. 1A and B, respectively) were obtained from National Institutes for Food and Drug Control (Beijing, China) and TLC Pharmaceutical Standards (Ontario, Canada), respectively. HPLC-grade methanol, acetonitrile and formic acid were purchased from Sigma-Aldrich (Beijing, China). All other reagents were of analytical grade. Ultrapure water was obtained from a Milli-Q® water purification system (Merck Millipore, Billerica, MA, USA). Pooled human control plasma (K2EDTA) was kindly donated from Phase I Clinical Center of Taizhou Hospital (Zhejiang, China).
Figure 1. Product ion mass spectra of [M+H]+ ions of (A) donepezil and (B) donepezil-d5.
Instrumentation and chromatographic conditions
The LC-MS/MS system consisted of an ExionLC™ AC liquid chromatography system consisted of a micro volume double plunger pump, an autosampler and a thermostatted column compartment integrated with a SCIEX Triple Quad™ 6500+ hybrid linear ion trap triple quadrupole mass spectrometer equipped with a Turbo Spray source and the diverter valve. The mass spectrometer was operated in positive ionization mode and data were acquired using the Analyst 1.7.0 software. The autosampler tray temperature was maintained at 15°C. The diverter valve automatically controlled by the integrated system is a two-position valve. At position B, the eluent is injected into mass spectrometer for further ionization and detection when the valve is set to switch to position A, the mobile phase flows through the external loop to waste. As shown in Fig. 1A and B (the product ion mass spectra of the [M+H]+ ion of donepezil and IS, respectively), quantitative MS/MS detection in the positive mode was performed using the transitions of m/z 380 to 91 for donepezil and m/z 385 to 96 for IS, respectively. The MS parameters used for monitoring donepezil and IS were as follows: ion spray = 5,500; ion source gas 1 = 60; source gas 2 = 60; temperature = 600; curtain gas = 30; declustering potential = 60; entrance potential = 10; CAD gas = medium; collision energy = 40; cell exit potential = 10; dwell Time (msec) = 100.0 and unit resolution for Q1 and Q3.
For the method routinely practiced in this laboratory, chromatographic separation was achieved using an InfinityLab Poroshell 120 EC-C18 column (2.1 × 50 mm 2.7-Micron; Agilent Technologies, Santa Clara, CA, USA) maintained at 45°C. The mobile phases consisted of A (5 mM ammonium formate/0.1% formic acid in 95:5 water: acetonitrile, v/v) and B (5 mM ammonium formate/0.1% formic acid in 5:95 water: acetonitrile, v/v) delivered at a constant flow rate of 0.4 mL/min. The mobile phase initial conditions were solvent A (78%) and solvent B (22%) held for 0.5 minutes then ramped to solvent A (90%) by 1.7 minutes held until 2.5 minutes and followed by an equilibration with solvent A (78%) and solvent B (22%) for 1.5 minutes with a total run time of 4.0 minutes.
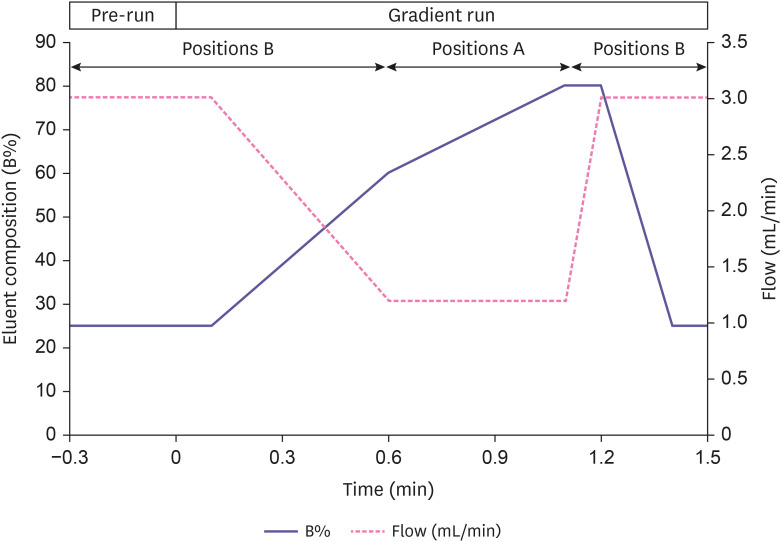
For this proposed method, chromatographic separation was optimized using a Chromolith high resolution RP-18e monolithic column (50 × 4.6 mm; MilliporeSigma, Burlington, MA, USA) maintained at room temperature. Mobile phase A (0.1% formic acid in water, v/v) and mobile phase B (0.1% formic acid in acetonitrile, v/v) were delivered at multiple-stage flow rates. The mobile phase initial conditions were solvent A (75%) and solvent B (25%) held for 0.1 minute then ramped to solvent A (40%) by 0.6 minutes, then solvent A (20%) by 1.1 minutes, held until 1.2 minutes and followed by an equilibration with solvent A (75%) and solvent B (25%) for 1.5 minutes. The column eluent flow rates were set initially at 3 mL/min held for 0.1 minutes then reduced down to 1.2 mL/min by 0.6 minutes, held until 1.1 minutes and followed by an equilibration flow rate at 3 mL/min for 0.4 minutes with a total run time of 1.5 minutes. As shown in Fig. 2, the diverter valve was sustained to position A and automatically switched to position B when the eluent flow rate was reduced down to 1.2 mL/min during each run cycle.
Figure 2. Gradient run and flow rates schemes with additional pre-run phase and injection position modes for fast equilibration, chromatographic separation and mass spectrometric detection.
Sample preparation
Donepezil and IS stock solutions were prepared in 50% methanol. All stock solutions were stored in brown glass vials at 4°C. Donepezil working solution was prepared freshly by serial dilutions of the stock solution with 50% methanol on the day of analysis. For quantitation of donepezil in calibration standards and quality control (QC) samples were prepared in blank human plasma at the concentrations of 0.2–50 ng/mL. QC samples were prepared in blank human plasma at the concentrations of 0.2 (lower limit of quantification; LLOQ), 0.6, 9, 20 and 35 ng/mL. All standards and QCs were prepared fresh daily. For long-term and freeze-thaw stability studies, QC samples were prepared as a batch and stored at −80°C.
The plasma samples preserved at −80°C were thawed at room temperature. Donepezil extraction from plasma was conducted by a simple protein precipitation technique where proteins in the mixture for each human plasma sample (200 μL) spiked with 50 μL of IS in the 96-well polypropylene plate were precipitated and extracted with methanol (500 μL). After vortexing, the samples were centrifuged at 3,500 g for 5 minutes at 10°C. The supernatant (200 μL) was transferred to another 96-well plates added with additional 400 μL water, from which an aliquot (5 μL) of the solution was injected for further LC-MS/MS analysis.
Method validation
The validation of the quantification method was performed based on the “Bioanalytical Method Validation-Guidance for Industry” published by National Medical Products Administration China and US Food and Drug administration (FDA) [18]. The following items were evaluated in this study: sensitivity, carryover check, linearity, precision, accuracy, recovery, ion suppression/enhancement, matrix effect, cross specificity and stability under various conditions. The specificity of the method was investigated by comparing LC-MS/MS extracted-ion chromatograms of blank plasma to blank plasma spiked with working standard stocks at the lowest concentration of calibration standards (LLOQ) with a signal-to-noise ratio (S/N) greater than 5, an accuracy of 80–120%, and an imprecision of ≤ 20%. Selectivity was carried out in 6 different lots of blank plasma collected with K2EDTA as anticoagulant. The linearity was determined by analysis of standard plots associated with an eight-point standard calibration curve. The plasma concentrations of donepezil were calculated from calibration curve using linear regression analysis with reciprocate of the drug concentration as a weighing factor (1/χ2). The intra-day accuracy and precision were determined by replicate analyses of the QCs on the same day. The inter-day accuracy and precision were determined by replicate analyses of the QCs on three separate days. The extraction recovery was determined by comparing peak area ratios of donepezil vs. IS obtained from QC samples that were spiked with donepezil prior to extraction to those obtained from blank plasma extracts that were spiked with known amounts of donepezil following extraction. Matrix effects were evaluated by comparing peak areas of donepezil from blank plasma extracts that were spiked with known amounts of donepezil to peak areas from samples prepared in the solvent with known amounts of donepezil. The stability experiments were performed to evaluate the stability of donepezil in stock solutions and in plasma samples under different conditions. Freeze-thaw stability was evaluated by exposing QCs to three freeze (−20°C and −80°C)-thaw (room temperature) cycles prior to sample preparation. Long-term storage stability was evaluated by storing QCs at −80°C for two months.
Application of the methods in a bioequivalence study
The objective of an open label, randomized, single-dose, two-sequence, two-period cross-over, comparative evaluation of the test and reference formulations (10 mg donepezil hydrochloride tablets) was to explore the bioequivalence under fasting and fed conditions in healthy adult human volunteers. All the subjects were informed of the aim and risk involved in the study and written consent were obtained. The inclusion criteria for volunteer selection were based on the age (18 or above), body mass index, general physical examination, electrocardiogram and laboratory tests. The healthy volunteers with allergic responses to donepezil, history of alcoholism, smokers were excluded. The clinical protocol was approved by Human Institutional Ethics Committee. Subjects were separated by a washout period of 14 days. Venous blood samples (5 mL) were withdrawn from each volunteer and placed into K2EDTA tubes according to the following time schedule: before (1.0 hour) and at 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.25, 3.5, 3.75, 4.0, 4.5, 5.0, 6.0, 8.0, 12.0, 24.0, 48.0 and 72.0 hours of administration of drug. Blood samples were centrifuged, and plasma was separated, then stored at −80°C (± 10°C) until analysis for donepezil using 2 validated LC-MS/MS methods. The plasma concentration–time profiles and pharmaco-kinetic parameters (maximum plasma concentration [Cmax], the time-to-maximum concentration [Tmax], the area under the curve [AUC0-t], apparent elimination half-life [t1/2], and the terminal elimination rate constant [ke]) obtained from the individual subjects were derived by a non-compartmental approach using Phoenix WinNonlin software from Certara (Princeton, NJ, USA). Reanalysis of incurred sample was conducted by random selection of the study plasma samples. Subjects completed both periods were included in the pharmacokinetic and statistical calculations based on the actual time of sample collection.
RESULTS
Method development
Although a few LC-MS/MS methods for quantitative determination of donepezil in human plasma samples had been demonstrated in the literatures, there is no report yet on performing LC-MS/MS in combination with both a simple extraction procedure and a short chromatographic run time. Thus, in this work the goal for method development was to accomplish an efficient LC-MS/MS method to shorten overall analysis time for the quantification of donepezil in human plasma. Sample preparation is one of the rate-limiting steps in a quantitative mass spectrometry-based assay. Protein crash technique by adding precipitating reagents to the plasma samplers without evaporation and re-constitution steps provides a quick and straightforward approach for removal of proteins from biological fluids before directly introducing the supernatant to the mass spectrometry-based systems. However, protein precipitation yields relatively poor sample cleanup resulting in not only negative changes in ionization efficiency by the matrix components but potential peak interferences with the target analytes from co-eluting components carrying the same multiple reaction monitoring (MRM) transitions [19,20]. As an example, for the LC-MS/MS method using a sub-3-μm C18 column described in this manuscript for the determination of donepezil in human plasma, a longer chromatographic run time was demanded to resolve the other two notable interference peaks for the endogenous constitute present in the blank plasma extract of a mixture after protein precipitation procedure which shared the same MRM (m/z 380 to 91) with the analyte (data not shown).
Our aim for this work was to employ a rapid sample pretreatment procedure and to achieve a high-throughput and interference-free separation for LC-MS/MS analysis of donepezil in human plasma by using a sustainable and efficient analytical column. Ultra-high-pressure liquid chromatography (UHPLC) could offer advantages in chromatographic resolution and speed over conventional HPLC systems [21,22,23]. However, UHPLC requires an additional liquid handling system to manage the high backpressure resulting from the stationary phase with sub-2-µm particles. Monolithic columns are made via a sol-gel process leading to single silica rods with a higher total porosity and lower pressure drop to operate at faster flow rates (≥ 3 mL/min) and gradients to reduce separation time while maintaining column efficiency than traditional particulate including fused-core HPLC columns of the same dimensions [24,25,26,27,28]. Although turbulent flow extraction could also allow the operation at high flow rates with low backpressure, it normally offers insufficient chromatographic resolution and peak separation as compared with monolithic columns [28,29]. The low backpressure at high flow rates on a monolithic column combined with the small dependency of separation efficiency allows for adequate chromatographic resolving power in a reduced run time. In this work, Chromolith® HighResolution RP-18, the second-generation C18 bonded monolithic silica column with a characteristic bimodal pore structure, from Merck was adapted for the separation and elution of donepezil from all interferences under multi-stage flow conditions. The effluent flow rates were operated constantly at early stage of 3 mL/min for matrix removal, reduced down to 1.2 mL/min on gradient descent for MS detection and then shortly returned to the last stage at 3 mL/min for equilibrium within a total analysis time of one and half minutes. In addition to the use of monolithic column, other chromatographic conditions such as mobile-phase composition, volume ratio and selection, flow rate as well as injection volume were investigated and optimized to achieve an efficient separation and resolution of donepezil from interference peaks in human plasma extract after protein precipitation procedures.
The diverter valve in coordination with an automated injection program connected with post-monolithic column was switched to position A to divert LC effluent either away to waste for desalting, matrix removal and equilibrium at higher flow rates or back to position B to deliver the flow of mobile phase to MS for better ionization efficiency of the donepezil at the low flow rate. The systematic evaluation on whether potential metabolites [11] to be converted back to the dosed compound during the sample extraction and/or causing quantitation bias such as cross-talk effects was performed (data not shown). A stable isotope- labeled IS was used to minimize analytical variation due to extraction recovery, ionization efficiency and matrix effect potential for LC-MS/MS analysis.
Method validation
Linearity, precision, accuracy and extraction efficiency
An 8-point calibration curve with the spiked standard plasma samples was found to be linear over the concentration range of 0.2–50 ng/mL for donepezil with the mean correlation coefficient greater than 0.999. As shown in Table 1, the precision and accuracy of donepezil in the intra- and inter-runs were within ± 10% at all QCs and LLOQ concentrations. The extraction recoveries of donepezil with six replicates at QCs were found to be greater than 88% for both donepezil and IS.
Table 1. Precision and accuracy of the method for determining donepezil in human plasma samples.
| %Intra-run accuracy | Intra-run precision (%CV) | %Inter-run accuracy | Inter-run precision (%CV) | |
|---|---|---|---|---|
| 0.2 ng/mL | 100.8 | 4.9 | 100.8 | 4.6 |
| 0.6 ng/mL | 100.0 | 2.8 | 97.0 | 3.9 |
| 9.0 ng/mL | 97.7 | 1.7 | 96.0 | 3.4 |
| 20.0 ng/mL | 99.6 | 2.8 | 97.5 | 4.0 |
| 35.0 ng/mL | 99.1 | 1.3 | 97.0 | 3.5 |
| Number | 6 | 6 | 30 | 30 |
Selectivity, sensitivity, chromatography and matrix effect
As shown in Fig. 3, no significant direct interference by comparing the extracted LC-MS/MS chromatograms of six different batches of blank plasma from the same LLOQ plasma samples was observed from endogenous substances in blank plasma at the retention times of both donepezil and IS. Fig. 3A-C demonstrated the extracted LC-MS/MS chromatograms of the donepezil (left panel) and IS (right panel) in the blank plasma, LLOQ, and the study plasma sample from a healthy volunteer after receiving donepezil at 0.5 hours, respectively. As shown in Fig. 3, no endogenous peak was found to interfere with the quantitation of both donepezil and IS. The lowest limit of reliable quantification for donepezil was set at the concentration of the LLOQ. The precision and accuracy at LLOQ concentration were found to be 4.9% (coefficient of variation; CV) and 100.8%, respectively. No significant matrix effect due to co-eluted endogenous substances [19,20] was observed after evaluating all QCs and LLOQ in all the six batches of blank plasma for donepezil.
Figure 3. Representative extracted-ion chromatograms for (A) blank plasma; (B) LLOQ spiked with 0.2 ng/mL of donepezil; and (C) a subject’s plasma sample at 0.5 hours after an oral dose of 10 mg donepezil hydrochloride. Left panel: donepezil (I); Right panel: donepezil-d5 (II).
LLOQ, lower limit of quantification.
Carry-over and stability
The carry-over effect evaluated by injection of blank plasma after the upper limit of quantification (ULOQ) samples was detected to be less than 20% of the LLOQ and 5% for the IS in the blank sample. Stability was investigated to ensure that donepezil remained intact in solutions and plasma samples under various experimental conditions for a certain time period. Stock solutions of donepezil were found to be stable for 22 days at 2–8°C and for 18 hours at room temperature. Donepezil in controlled plasma at room temperature up to 20 hours and for 6 freeze—thaw cycles was found stable. Bench-top stability of donepezil in the extracted plasma samples was also up to 73 hours. For long-term stability, the spiked QC plasma samples of donepezil stored at −80°C (± 10°C) were found no degradation for a minimum period of 70 days.
Application to the study plasma samples and a BE study
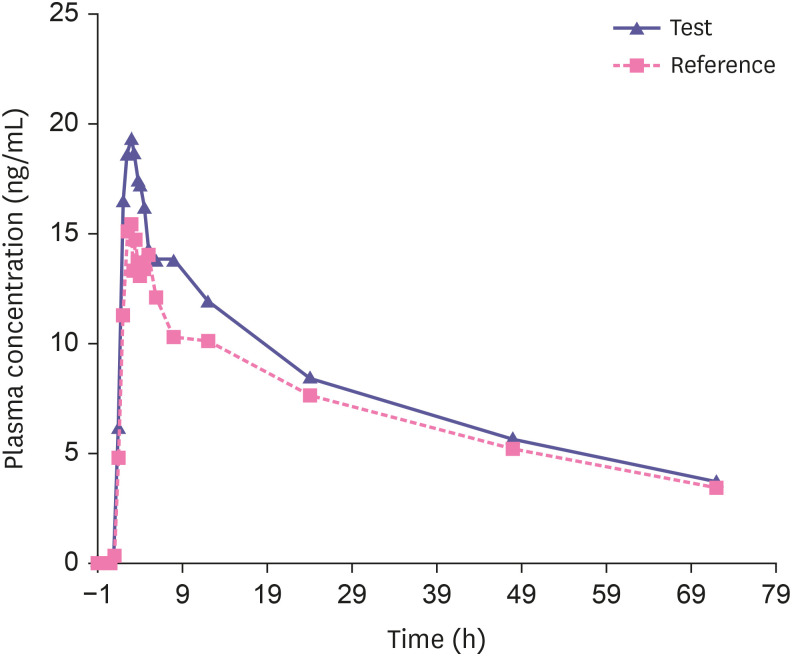
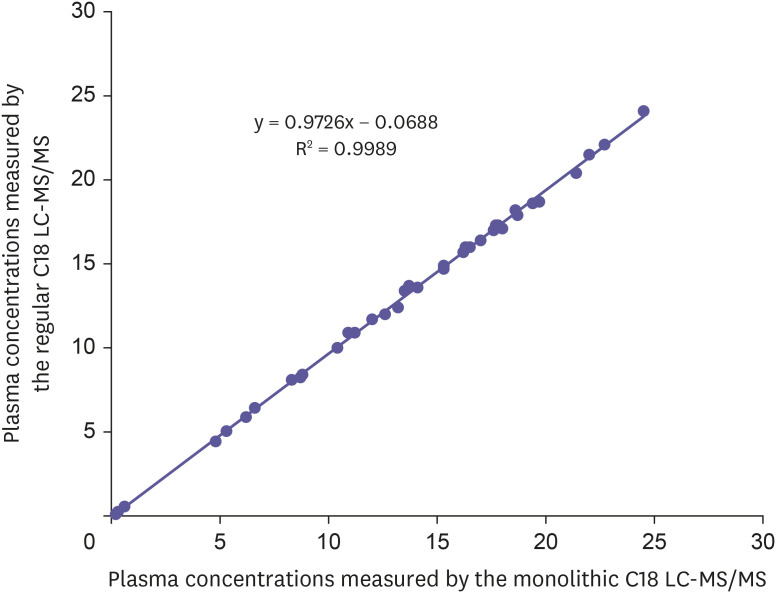
To verify the sensitivity and selectivity of the proposed method in a real-time situation, this monolithic LC-MS/MS method was used to determine the concentrations of donepezil in human plasma samples collected from healthy male volunteers (n = 26). The plasma concentrations vs time profiles of donepezil from a subject after receiving a test and a reference formulation is shown in Fig. 4. The plasma concentrations of donepezil in human evaluated by a validated ultra-performance LC-MS/MS method and monolithic LC-MS/MS method were found to be well correlated as indicated in Fig. 5. A bioequivalence study of donepezil comparing aforementioned formulations was conducted according to the approved protocol where an equal number of subjects were randomly assigned to each of the two possible dosing sequences. After the administration of a single dose of each formulation under fasting conditions, blood samples were collected at designated times and analyzed. The primary parameters were AUC0–72 and Cmax. Intra- and inter-subject CVs were calculated. Bioequivalence was to be concluded in the geometric least square mean (LSM) ratios (Test/Reference, %) and 90% confidence interval (CI) for the primary pharmacokinetic parameters Cmax and AUC0–72 of donepezil. Demographic data of the subjects completing this pilot bioequivalence studies with donepezil demonstrate that the ratio and corresponding 90% CI of the relative Cmax and AUC0–72 geometric LSM of the test and reference formulations were within the regulatory bioequivalence criterion of 80.00% to 125.00%. Mean pharmacokinetic parameters estimated from the raw concentration data are summarized in Table 2. Safety was evaluated through the assessment of adverse events (AEs) associated with the use of the test and reference products. There were no deaths or serious adverse events reported and all other observed AEs were resolved. All the drugs administered orally appeared to be well-tolerated. The results of incurred sample re-analysis of human plasma for donepezil (n = 24) are within the acceptance range of ± 20% of original value suggesting a success rate not exceeding the 33% of the guidelines.
Figure 4. Plasma concentration-time profiles from a healthy volunteer after administration of the reference and test formulations of 10 mg donepezil hydrochloride.
Figure 5. Comparison of the donepezil concentrations in human plasma measured with two validated LC-MS/MS methods.
Table 2. Pharmacokinetic parameters of donepezil in 26 healthy subjects following a single oral dose of the test and reference formulations of 10 mg donepezil hydrochloride tablet.
| Parameter | Fasted (mean ± % R.S.D.) | Fed (mean ± % R.S.D.) | ||
|---|---|---|---|---|
| Test | Reference | Test | Reference | |
| Cmax (ngmL−1) | 21.11 ± 5.54 | 21.77 ± 5.52 | 24.02 ± 5.48 | 24.38 ± 6.93 |
| Tmax (h) | 2.49 ± 1.45 | 2.67 ± 1.32 | 3.11 ± 2.26 | 3.02 ± 2.10 |
| t1/2 (h) | 43.00 ± 11.65 | 41.73 ± 10.18 | 41.46 ± 5.99 | 40.57 ± 11.88 |
| AUC0–72 (h ngmL−1) | 505.17 ± 227.29 | 561.48 ± 119.80 | 557.37 ± 162.48 | 512.60 ± 217.15 |
| Kel (h−1) | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.05 |
R.S.D., residual standard deviation; Cmax, maximum plasma concentration; Tmax, time-to-maximum concentration; t1/2, apparent elimination half-life; AUC0-t, area under the curve; ke, terminal elimination rate constant.
DISCUSSION
A high-throughput LC-MS/MS method was developed and validated for the determination of donepezil in human plasma. The method requiring only a simple sample preparation procedure was efficient over the previously reported methods. The method was successfully applied to quantitate plasma donepezil concentrations in support of a bioequivalence study involving healthy volunteers. The pharmacokinetic outcomes conducted in this study were comparable to those reported in literature. The pharmacokinetic results of donepezil in human evaluated with cross validation procedures by both ultra-performance and monolithic LC-MS/MS methods were found to be well correlated. The BE results via PK analysis suggested that a single dose of the test and reference formulations of donepezil were well tolerated and met the regulatory requirements of bioequivalence, based on the FDA regulatory definition.
Footnotes
Funding: The authors thank the Laboratory of ANSER Medical Technology for providing financial supports.
Conflict of Interest: - Authors: Nothing to declare
- Reviewers: Nothing to declare
- Editors: Nothing to declare
Reviewer: This article was reviewed by peer experts who are not TCP editors.
- Investigation: Huang YW.
- Supervision: Ding L, Hsieh Y.
- Writing - original draft: Hsieh Y.
- Writing - review & editing: Hsieh Y.
References
- 1.Cacabelos R. Donepezil in Alzheimer’s disease: from conventional trials to pharmacogenetics. Neuropsychiatr Dis Treat. 2007;3:303–333. [PMC free article] [PubMed] [Google Scholar]
- 2.Lee CB, Min JS, Chae SU, Kim HM, Jang JH, Jung IH, et al. Simultaneous determination of donepezil, 6-O-desmethyl donepezil and spinosin in beagle dog plasma using liquid chromatography–tandem mass spectrometry and its application to a drug-drug interaction study. J Pharm Biomed Anal. 2020;178:112919. doi: 10.1016/j.jpba.2019.112919. [DOI] [PubMed] [Google Scholar]
- 3.Noetzli M, Ansermot N, Dobrinas M, Eap CB. Simultaneous determination of antidementia drugs in human plasma: procedure transfer from HPLC-MS to UPLC-MS/MS. J Pharm Biomed Anal. 2012;64-65:16–25. doi: 10.1016/j.jpba.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Jeong HC, Park JE, Hyun JY, Park MK, Shin DS, Shin KH. Determination of donepezil in human plasma using ultra performance liquid chromatography-tandem mass spectrometry. Transl Clin Pharmacol. 2018;26:64–72. doi: 10.12793/tcp.2018.26.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park EJ, Lee HW, Ji HY, Kim HY, Lee MH, Park ES, et al. Hydrophilic interaction chromatography-tandem mass spectrometry of donepezil in human plasma: application to a pharmacokinetic study of donepezil in volunteers. Arch Pharm Res. 2008;31:1205–1211. doi: 10.1007/s12272-001-1290-6. [DOI] [PubMed] [Google Scholar]
- 6.Xie Z, Liao Q, Xu X, Yao M, Wan J, Liu D. Rapid and sensitive determination of donepezil in human plasma by liquid chromatography/tandem mass spectrometry: application to a pharmacokinetic study. Rapid Commun Mass Spectrom. 2006;20:3193–3198. doi: 10.1002/rcm.2718. [DOI] [PubMed] [Google Scholar]
- 7.Apostolou C, Dotsikas Y, Kousoulos C, Loukas YL. Quantitative determination of donepezil in human plasma by liquid chromatography/tandem mass spectrometry employing an automated liquid-liquid extraction based on 96-well format plates. Application to a bioequivalence study. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;848:239–244. doi: 10.1016/j.jchromb.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 8.Patel BN, Sharma N, Sanyal M, Shrivastav PS. Quantitation of donepezil and its active metabolite 6-O-desmethyl donepezil in human plasma by a selective and sensitive liquid chromatography-tandem mass spectrometric method. Anal Chim Acta. 2008;629:145–157. doi: 10.1016/j.aca.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 9.Khuroo AH, Gurule SJ, Monif T, Goswami D, Saha A, Singh SK. ESI-MS/MS stability-indicating bioanalytical method development and validation for simultaneous estimation of donepezil, 5-desmethyl donepezil and 6-desmethyl donepezil in human plasma. Biomed Chromatogr. 2012;26:636–649. doi: 10.1002/bmc.1709. [DOI] [PubMed] [Google Scholar]
- 10.Matsui K, Oda Y, Nakata H, Yoshimura T. Simultaneous determination of donepezil (aricept) enantiomers in human plasma by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr B Biomed Sci Appl. 1999;729:147–155. doi: 10.1016/s0378-4347(99)00145-0. [DOI] [PubMed] [Google Scholar]
- 11.Mano Y, Hotta K, Kusano K. Simultaneous determination of donepezil and its three metabolites in human plasma using LC-MS-MS. J Chromatogr Sci. 2016;54:1328–1335. doi: 10.1093/chromsci/bmw075. [DOI] [PubMed] [Google Scholar]
- 12.Pilli NR, Inamadugu JK, Kondreddy N, Karra VK, Damaramadugu R, Rao JV. A rapid and sensitive LC-MS/MS method for quantification of donepezil and its active metabolite, 6-O-desmethyl donepezil in human plasma and its pharmacokinetic application. Biomed Chromatogr. 2011;25:943–951. doi: 10.1002/bmc.1552. [DOI] [PubMed] [Google Scholar]
- 13.Bhateria M, Ramakrishna R, Pakala DB, Bhatta RS. Development of an LC-MS/MS method for simultaneous determination of memantine and donepezil in rat plasma and its application to pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;1001:131–139. doi: 10.1016/j.jchromb.2015.07.042. [DOI] [PubMed] [Google Scholar]
- 14.Shah HJ, Kundlik ML, Pandya A, Prajapati S, Subbaiah G, Patel CN, et al. A rapid and specific approach for direct measurement of donepezil concentration in human plasma by LC-MS/MS employing solid-phase extraction. Biomed Chromatogr. 2009;23:141–151. doi: 10.1002/bmc.1095. [DOI] [PubMed] [Google Scholar]
- 15.Noetzli M, Ansermot N, Dobrinas M, Eap CB. Simultaneous determination of antidementia drugs in human plasma: procedure transfer from HPLC-MS to UPLC-MS/MS. J Pharm Biomed Anal. 2012;64-65:16–25. doi: 10.1016/j.jpba.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Meier-Davis SR, Meng M, Yuan W, Diehl L, Arjmand FM, Lucke RM, et al. Dried blood spot analysis of donepezil in support of a GLP 3-month dose-range finding study in rats. Int J Toxicol. 2012;31:337–347. doi: 10.1177/1091581812447957. [DOI] [PubMed] [Google Scholar]
- 17.Iordachescu A, Silvestro L, Tudoroniu A, Rizea SR, Ciuca V. LC-MS–MS method for the simultaneous determination of donepezil enantiomers in plasma. Chromatographia. 2012;75:857–866. [Google Scholar]
- 18.U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Veterinary Medicine (CVM) Bioanalytical Method Validation: Guidance for Industry [Internet] [Accessed May 2018]. https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf .
- 19.Mei H, Hsieh Y, Nardo C, Xu X, Wang S, Ng K, et al. Investigation of matrix effects in bioanalytical high-performance liquid chromatography/tandem mass spectrometric assays: application to drug discovery. Rapid Commun Mass Spectrom. 2003;17:97–103. doi: 10.1002/rcm.876. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh Y, Chintala M, Mei H, Agans J, Brisson JM, Ng K, et al. Quantitative screening and matrix effect studies of drug discovery compounds in monkey plasma using fast-gradient liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2001;15:2481–2487. doi: 10.1002/rcm.479. [DOI] [PubMed] [Google Scholar]
- 21.O’Connor D, Mortishire-Smith R, Morrison D, Davies A, Dominguez M. Ultra-performance liquid chromatography coupled to time-of-flight mass spectrometry for robust, high-throughput quantitative analysis of an automated metabolic stability assay, with simultaneous determination of metabolic data. Rapid Commun Mass Spectrom. 2006;20:851–857. doi: 10.1002/rcm.2385. [DOI] [PubMed] [Google Scholar]
- 22.Yu K, Little D, Plumb R, Smith B. High-throughput quantification for a drug mixture in rat plasma-a comparison of ultra performance liquid chromatography/tandem mass spectrometry with high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:544–552. doi: 10.1002/rcm.2336. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh Y, Duncan CJ, Brisson JM. Fused-core silica column high-performance liquid chromatography/tandem mass spectrometric determination of rimonabant in mouse plasma. Anal Chem. 2007;79:5668–5673. doi: 10.1021/ac070343g. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh Y, Wang G, Wang Y, Chackalamannil S, Korfmacher WA. Direct plasma analysis of drug compounds using monolithic column liquid chromatography and tandem mass spectrometry. Anal Chem. 2003;75:1812–1818. doi: 10.1021/ac020630e. [DOI] [PubMed] [Google Scholar]
- 25.Huang MQ, Mao Y, Jemal M, Arnold M. Increased productivity in quantitative bioanalysis using a monolithic column coupled with high-flow direct-injection liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:1709–1714. doi: 10.1002/rcm.2494. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh Y, Wang G, Wang Y, Chackalamannil S, Brisson JM, Ng K, et al. Simultaneous determination of a drug candidate and its metabolite in rat plasma samples using ultrafast monolithic column high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2002;16:944–950. doi: 10.1002/rcm.648. [DOI] [PubMed] [Google Scholar]
- 27.Wu JT, Zeng H, Deng Y, Unger SE. High-speed liquid chromatography/tandem mass spectrometry using a monolithic column for high-throughput bioanalysis. Rapid Commun Mass Spectrom. 2001;15:1113–1119. doi: 10.1002/rcm.348. [DOI] [PubMed] [Google Scholar]
- 28.Zhou S, Zhou H, Larson M, Miller DL, Mao D, Jiang X, et al. High-throughput biological sample analysis using on-line turbulent flow extraction combined with monolithic column liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:2144–2150. doi: 10.1002/rcm.2037. [DOI] [PubMed] [Google Scholar]
- 29.De Wilde L, Roels K, Van Eenoo P, Deventer K. Online turbulent flow extraction and column switching for the confirmatory analysis of stimulants in urine by liquid chromatography-mass spectrometry. J Anal Toxicol. 2021;45:666–678. doi: 10.1093/jat/bkaa136. [DOI] [PubMed] [Google Scholar]