Abstract
The livestock industry produces a large amount of greenhouse gases (GHG) that cause global warming. A high percentage of GHG emissions are derived from cattle and has been suggested to be a factor in global warming. With the global increase in the consumption of livestock products, the number of farm animals has increased. In addition, the reduction in productivity and reproductive capacity of cattle has resulted in accelerated GHG emissions. In a high-temperature environment, the pregnancy rate decreases, leading to an increase in animals that do not contribute to production. Consequently, GHG emission per unit product increases, thereby accelerating global warming. To reduce this environmental impact, it is important to improve the breeding efficiency of cattle by the use of reproductive technology and, thus, reduce the number of non-productive animals. Thus, reproductive biology plays a major role in mitigating global warming related to the livestock industry.
Keywords: Cattle, Global warming, Greenhouse gases, Reproductive capacity, Sustainable Development Goals (SDGs)
Introduction
In September 2015, a United Nations (UN) summit listed 17 goals to be achieved by 2030 as Sustainable Development Goals (SDGs, https://sdgs.un.org/goals). These 17 goals are expected to be achieved by the solidarity of nations and industries worldwide, including the agriculture and livestock industries. The Food and Agriculture Organization (FAO), which is a UN agency, is also engaged in awareness-raising activities such as issuing reports about the SDGs related to the achievements of the livestock industry [1]. This review article discusses the relationship between global warming and livestock industry/livestock production from the viewpoint of cattle reproductive biology, with regard to the 13th SDG, “Climate action: Take urgent action to combat climate change and its impacts”.
Global Warming and Cattle Production
Following the turn of this century, global warming has become a serious issue. Extreme climate events, such as high temperature, drought, heavy rain and typhoons, have been experienced frequently not only in Japan but also worldwide. Therefore, it is considered necessary to assess the present status and the prospects of global warming, which is considered responsible for the aforementioned extreme climate events in Japan.
The Japan Meteorological Agency publishes various meteorological data, including the annual mean temperature anomalies (change relative to the mean of 30 years from 1990 to 2020), in Japan from the 1890s to the present [2, 3]. According to the data, temperature anomalies are increasing progressively, and the difference between 1900 and 2020 is greater than 2°C. This means that the mean temperature has increased by over 2°C in 120 years. Furthermore, extremely hot days with a maximum temperature of ≥ 35°C have increased rapidly from the 1990s, and the mean annual number of extremely hot days, which was 0 at all 13 locations in Japan in 1910, exceeded 5 in 2020, indicating a marked temperature increase in summer. This global warming is caused by an increase in the emission of greenhouse gases (GHG), such as carbon dioxide (CO2), dinitrogen monoxide (N2O), and methane (CH4). The Intergovernmental Panel on Climate Change (IPCC) has issued global warming predictions on the basis of representative concentration pathways (RCP) of GHG emissions. Even RCP2.6, the stringent mitigation scenario in the 5th report of the IPCC (2014), predicts an increase in temperature of 0.3 to 1.7°C at the end of the 21st century compared with that at the end of the 20th century. This means that the temperature will increase by 3.5°C at the end of the 21st century compared with that of the early 1900s. The Paris Agreement adopted in 2015 by the Conference of the Parties to the UN Framework Convention on Climate Change (COP21) set the goal of GHG emission reduction at a level sufficiently lower than 2°C and to suppress it to 1.5°C, compared with the pre-industrial level. However, this is difficult to achieve. The 6th IPCC report (2021) warned that global warming has progressed more rapidly than the RCP2.6 scenario and the climate change typified by global warming needs urgent addressing in the SDGs.
In the agriculture sector, livestock production accounts for a large share of GHG emissions and produced 7.1 gigatons GHG emissions or 14.5% of the total GHG emissions (CO2 equivalent) in 2013 [4]. Major sources of GHG emissions in the livestock industry are 1) CO2 associated with feed production, 2) N2O derived from the excrement of livestock/feed residue, and 3) CH4 associated with digestion (fermentation) by ruminants [4, 5]. As of 2019, the numbers of major livestock species worldwide, i.e., cattle, pigs, sheep/goats, and poultry, were 1.5, 0.85, 2.3, and 26 billion, respectively (2019, FAO). While GHG emissions from pigs and poultry, which are raised in larger numbers than cattle, are only 9% and 8%, respectively, GHG emissions from cattle account for approximately 60% of the emissions from the livestock industry [4]. One reason for the high GHG emissions from cattle is the emission of digestion-associated methane by ruminants. As shown in Fig. 1, the feed ingested by cattle is fermented/degraded by microorganisms in the rumen and converted to volatile fatty acids, which are used as a source of energy, and methane, which is not utilized. The methane generated in the ruminant is then emitted from the body through belches, and promotes global warming. According to the calculation by the author of the cited report, the mean daily GHG emissions from dairy cattle, including growing and dry cows, reached 14.1 kg in CO2 equivalent [6].
Fig. 1.
GHG emitted from cattle. Feed is digested and fermented by microorganisms in the rumen, and degraded into volatile fatty acids, which are utilized as a source of energy, and methane, which is not utilized. Methane is passed out of the body through belching. In addition, CO2 is emitted through expiration, and N2O and NH3 are emitted in excrement. * GHG emission (CO2 equivalent) calculated by the author according to the manual for calculating/reporting GHG emissions (livestock volume), Ministry of Agriculture, Forestry and Fisheries of Japan.
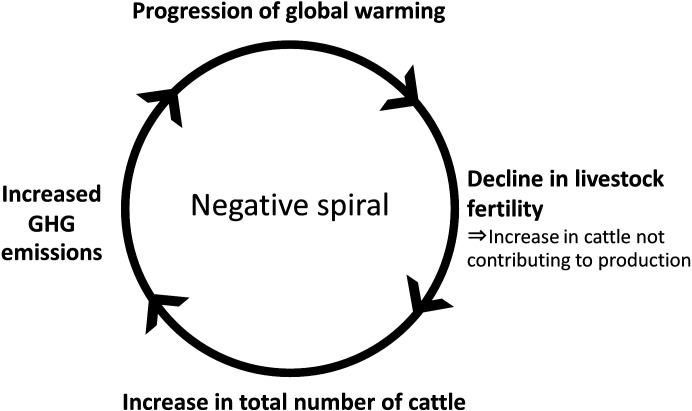
Recently, with a rise in income, the consumption of animal protein (meat, dairy products, eggs) has increased worldwide, and livestock production is increasing to meet the demand. The global population reached 7.6 billion in 2018 from 3 billion in 1961, representing an approximate 2.5-fold increase; however, the meat production increased more than five-fold during this period, thus, surpassing the rise in human population. In addition to the increase in the consumption of livestock products, increase in the number of livestock with the progression of global warming has been reported, which results in further rise in GHG emissions in a negative spiral (Fig. 2). The productivity and fertility of cattle in high-temperature environments is largely involved in this problem. The next section focuses on cattle fertility in terms of increased GHG emissions and global warming.
Fig. 2.
Diagram of global warming caused by the livestock industry (cattle).
Decline in Livestock Fertility and Global Warming
One reason for the increase in GHG emissions in the livestock industry is an increase in the total number of animals. As selective breeding of cattle has emphasized productivity, milk yield and the fertility of dairy cows have declined to two-third of that seen 40 years ago [7]. Mammals are generally homeothermic and their body temperature is maintained despite changes in the ambient temperature. However, the temperature-regulating mechanism collapses if the temperature exceeds a certain level (critical temperature), and the body temperature increases with high ambient temperature and decreases with low ambient temperature [8]. If the body temperature elevates in a hot environment, reflex suppression of thermogenesis associated with metabolism (temperature increase) is induced by reducing food intake [8]. Body weight gain, lactation, and reproduction occur when surplus energy can be secured. If the energy deficiency is caused by reduced food intake, the reproductive capacity is affected first, followed by adverse effects on body weight gain and lactation [9,10,11,12]. In a high-temperature environment, the decline in fertility is accelerated, leading to an increase in the number of animals not contributing to production, that is, an increase in the number of animals emitting only GHG. This results in an increase in total GHG emissions (Fig. 2).
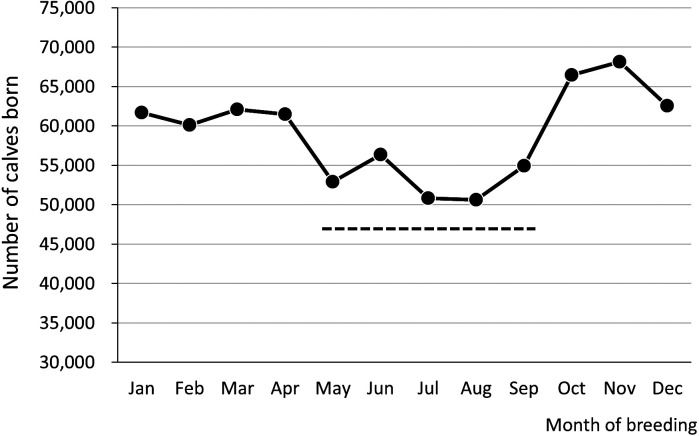
The dairy cattle in Japan are mainly of the Holstein breed that is native to cool European regions, and the beef cattle are mainly Japanese Black cattle, also called wagyu, that originate from domestic and European species. Therefore, they are relatively resistant to cold, but vulnerable to heat. In lactating dairy cows, the body temperature begins to increase when the ambient temperature exceeds 25°C [13] and they are subjected to heat stress when the temperature humidity index (THI) exceeds 72 [9, 10]. In Japan, days with a maximum temperature of ≥ 25°C are not rare from May to September, and dairy cattle suffer from heat stress for around half a year. In mid-summer, when the ambient temperature further elevates, the effects are greater. The effects of high temperature on fertility can be evaluated according to the relationship between the month of breeding and the number of calves born. The number of calves delivered by dairy cattle according to the month of breeding is shown in Fig. 3 (based on the national database provided by the National Livestock Breeding Center https://www.id.nlbc.go.jp/data/toukei.html). The number of calves born to dairy cattle bred in May–September is low, suggesting that fewer cows become pregnant in a high-temperature environment.
Fig. 3.
Number of calves delivered based on the month of breeding in dairy cattle (2019). Number of dairy/crossbred calves born from dairy cattle. Dash line: months with a daily maximum temperature of ≥ 25°C (May–September) (prepared from the statistics of the National Database of Individual Identification Information of Cattle 2019, National Livestock Breeding Center).
Furthermore, the reasons for the decrease in the number of pregnant cows under high-temperature conditions and the relation of this decrease to the increase in GHG emissions are discussed here. The decrease in the number of pregnant cattle is considered to be caused primarily by two factors. The first is a decrease in the number of cattle that could be bred. During summers, a negative energy balance is caused by a decrease in feed intake. As a result, steroid metabolism, which controls sex hormone secretion, is adversely affected, causing ovarian dysfunction [12, 14,15,16]. Breeding of cattle is mostly carried out by artificial insemination (AI) in Japan. Therefore, the detection of estrus is essential to determine the optimal time for AI. It is recognized that the ratio of estrogen and progesterone regulates the expression of estrus symptoms. Thus, the alteration in hormone secretion may cause weak estrus or anestrus and thus make accurate estrus detection difficult [14, 17, 18]. Artificial breeding is impossible if the optimal estrus time cannot be determined. Moreover, if follicular development or ova are affected, AI is not performed even if estrus is detected. According to the fertility survey in 2016 (Livestock Improvement Association of Japan), the number of dairy cattle in which AI was carried out during July–September was approximately 25% lower than that of cattle inseminated during January–March, indicating a decrease in the number of cattle bred in the summer season.
The second is an increase in the number of cattle that do not conceive even after insemination. The conception rate of Holstein cows is 25% lower in summer than in other seasons [12, 19]. The causes of infertility are multiple, including abnormal follicular development and deterioration of oocyte quality due to high temperatures [20], a decrease in the fertilization rate due to exposure of both ova and sperm to high temperatures [21, 22], and embryonic death at an early stage of embryonic development [23, 24]. With the elevation of body temperature, there is an increase in harmful reactive oxygen species (ROS) in cells, which can damage DNA and organelles, interfere with the normal functions of cells, and lead to cell death. Germ cells, such as ova and sperm, and embryos are no exception [20, 22,23,24,25]. As a result, conception fails, and the number of non-pregnant cattle increases during summer.
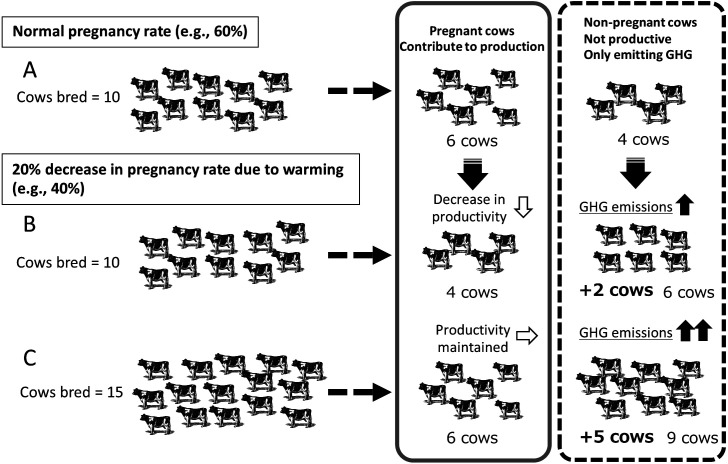
An example of an increase in GHG emissions associated with an increase in cows under high temperatures is shown in Fig. 4. This may be evaluated with the formula “pregnancy rate = estrus detection rate × conception rate” in consideration of the two effects described above. If the normal pregnancy rate is 60% and 10 cows are inseminated, 6 cows become pregnant and contribute to production, whereas 4 do not, and only emit GHG (Fig. 4A). If the pregnancy rate decreases to 40% under high temperature conditions, the number of cows that do not contribute to production and only emit GHG increases to six (Fig. 4B). However, if more cows are bred to obtain the same number of pregnant cows to maintain production, the number of non-pregnant cows becomes nine at a pregnancy rate of 40% (Fig. 4C). Thus, a 20% decrease in the pregnancy rate causes a 1.5-fold increase in the number of cattle necessary to maintain the production and a 2.25-fold increase in those that only emit GHG. With the progression of global warming, the pregnancy rate will decrease further with an increasing number of cattle not contributing to production. This phenomenon sets off a negative spiral of increase in GHG emissions and acceleration of global warming (Fig. 2).
Fig. 4.
An example of a decrease in fertility and increase in GHG emissions due to global warming. A: Normal. B: The pregnancy rate is reduced by global warming/the number of bred cows is the same as that of Normal. C: The pregnancy rate is reduced by global warming/productivity is maintained by increasing the number of bred cows.
Reproductive Strategies for the Mitigation of Global Warming
There are a few reproductive strategies that can be used to mitigate GHG emissions from cattle. In consideration of the current global trend of livestock product consumption, it is difficult to reduce livestock production. Therefore, the target is to reduce unnecessary GHG emissions by improving production efficiency.
For this purpose, two major methods are considered promising: 1) reducing the number of cattle that do not contribute to production by improving the pregnancy rate (breeding efficiency and conception rate), and 2) shortening the non-pregnant period (days open) and reducing the non-productive period. According to provisional calculations, GHG emissions can be reduced by approximately 10% with a 10% improvement in the conception rate [26]. This means that a 10% improvement in the conception rate during summer (one-third of the year) enables an approximate 3% reduction of annual GHG emissions. Moreover, if the parturition interval of dairy cows is shortened by 10 days, GHG emissions can be reduced by approximately 3%. An FAO report reviewing livestock breeding management techniques suggested that modification of breeding methods, assisted reproduction technologies, and mitigation of stress in cattle are effective not only for reducing GHG emissions but also for improving livestock productivity [26, 27].
In Japan, as most cattle are bred artificially, the detection of estrus is important to know the optimal time for breeding. In summer, the estrus cycle becomes irregular, its duration is shortened, and the estrus behavior is weakened, making its detection difficult by standard methods [17, 28]. In addition, the herd sizes of dairy farms are on the rise. Therefore, an estrus detection method using sensing techniques has recently begun to be used widely in the livestock industry. When sensors were used in cattle farms to monitor the activity level (acceleration), step count, and body temperature, estrus was reported to be detected more accurately than by visual observation [29]. Furthermore, breeding using sensors has been reported to cause no difference in the pregnancy rate of beef cattle with weak signs of estrus [30]; the estrus detection rate and accuracy and the breeding rate can in fact be improved by the use of sensors. As sensing is also available for health management, sensors are expected to be useful for fertility improvement by promoting not only estrus detection but also health protection of the herd. In addition, estrus synchronization technique using hormone treatments was reported to promote estrus symptoms and improve the conception rate after AI [31]. A modified method, timed-AI, by which cattle can be inseminated without checking for estrus, can increase the number of cows to be inseminated and improve the breeding coefficient [32].
Selective breeding also assumes significance as a method for improving the conception rate. In Japanese Black cattle, the inbreeding coefficient has been increasing and there are concerns over its effects on fertility. Expanding diversity and breeding selection may be good in terms of fertility and health, but this is not easy to achieve. By contrast, assisted reproductive technologies, especially embryo transfer (ET), are considered effective as relatively simple methods to improve the conception rate [27]. Blastocysts at a more advanced stage of development, which are usually used in ET, would be more resistant to stress than 2- or 8-cell embryos at an early stage of development [23, 24]. ET is advantageous in that the embryo is less likely to be adversely affected in the subsequent stages of development because it bypasses the stress in the oocyte or early embryonic stage. Therefore, conception is more likely to occur because an assuredly developing embryo is introduced to the uterus directly [24, 33]. Transfer of unfrozen fresh embryos ensures a stable conception rate throughout the year [33, 34]. However, as mentioned earlier, it is difficult to secure a large number of fresh embryos in the summer, because of the qualitative deterioration of oocytes and embryos. In addition, as frozen embryos are subjected to stress (damage) in the process of freezing, their heat resistance after thawing is lower than that of fresh embryos, and they result in lower rates of confirmed conception [33, 35, 36]. For this reason, further technical advancements, such as improving the quality of frozen embryos and enhancing their heat resistance, are necessary to improve the conception rate by ET, during summer. However, embryo-freezing techniques have also been enhanced, such as the application of the vitrification method and other improvements in the freezing agents and methods; it is expected to be leveraged in the future when a conception rate comparable to that with fresh embryos is achieved under standard conditions.
Furthermore, there are methods to mitigate maternal stress and improve the pregnancy rate. The maternal body is likely to fall into a state of malnutrition after delivery. By preventing this malnutrition and the consequent diseases, it may be possible to conduct breeding early after delivery [27]. The decline in fertility in a high-temperature environment is associated with increase in the maternal body temperature. There have been attempts to prevent such decline in fertility by feeding management to control the body temperature elevation. A high body temperature at the time of insemination exerts adverse effects on both oocytes and sperm, and hinders fertilization [21, 22]. A negative correlation is observed between the recipient body temperature and conception rate, even in the case of ET [37]. The body temperature was controlled and the conception rate was improved by cooling the maternal body for only a few hours before and after AI [38], and the possibility of improving fertility by mitigating stress on the maternal body itself has been suggested. The mitigation of maternal stress has immediate favorable effects, and also results in future sustainability and normality of the industry; therefore, it can be an important approach with regard to long-term GHG emissions and SDGs. However, from the viewpoint of GHG emissions, the use of cooling facilities (sprinklers and fans) that involves extra CO2 emissions, must somehow be balanced.
Conclusion
The answer to the title phrase “Will increase in cattle numbers progress to global warming?” is indeed affirmative, because an increase in cattle numbers leads to an increase in GHG emissions. However, the animal protein provided by livestock is important for nutrition, and compost produced from their excretions is a fertilizer important for agriculture. These aspects of livestock farming are considered crucial for the SDGs “2. Zero hunger” and “3. Good health and well-being”. Thus, the future livestock industry must be developed in a manner harmonious with SDGs.
In this review article, the causes and countermeasures of global warming were discussed from the perspective of cattle reproduction science by focusing on GHG emissions. Improvements in the reproductive capacity and productivity of cattle may enable control of their numbers, while maintaining productivity. The reduction in cattle population may lead to control of GHG emissions from cattle and deceleration of global warming. Thus, the negative spiral that has progressed to the present scenario might be stopped. By contrast, the clarification of factors related to the decline in fertility due to global warming, and mitigation strategies and technologies to counteract the same, are still at the research level and remain unclear. Further advances in research on global warming and livestock breeding are awaited, which may lead to efficient breeding management methods and technologies with potential to reduce cattle-associated global warming.
Conflict of interests
The author declares that there are no conflicts of interest.
Acknowledgments
This review article is a summary of a public online seminar titled “The Role of Reproductive Biology in SDGs” held by the Society for Reproduction and Development in May 2021. The author expresses sincere appreciation to the Society for Reproduction and Development for the opportunity to publish this review article. This manuscript was supported by JSPS KAKENHI Grant Number 17HP2009.
References
- 1.World Livestock. Transforming the livestock sector through the Sustainable Development Goals. Food and Agriculture organization of the United Nations (FAO), Rome2018http://www.fao.org/3/CA1201EN/ca1201en.pdf.
- 2.Japan Meteorological Agency. Mean annual temperature anomalies in Japan. 2021https://www.data.jma.go.jp/cpdinfo/temp/list/an_jpn.html (In Japanese).
- 3.Japan Meteorological Agency. Changes in extreme weather event from 1910 to 2020. 2021 https://www.data.jma.go.jp/cpdinfo/extreme/extreme_p.html (In Japanese).
- 4.Gerber PJ, Steinfeld H, Henderson B, Motter A, Opio C, Dijkman J, Falcucci A, Tempio G. Tackling climate change through livestock–A global assessment of emissions and mitigation opportunities. Food and Agriculture organization of the United Nations (FAO), Rome2013. http://www.fao.org/3/i3437e/i3437e.pdf.
- 5.Rotz CA. Modeling greenhouse gas emissions from dairy farms. J Dairy Sci 2018; 101: 6675–6690. [DOI] [PubMed] [Google Scholar]
- 6.Livestock Industry Department. Production Bureau, Ministry of Agriculture, Forestry and Fisheries: Manual for calculating/reporting GHG emissions (livestock volume) 2011https://www.maff.go.jp/j/chikusan/kankyo/taisaku/pdf/ontaihou_manu.pdf (In Japanese).
- 7.Huang W, Kirkpatrick BW, Rosa GJM, Khatib H. A genome-wide association study using selective DNA pooling identifies candidate markers for fertility in Holstein cattle. Anim Genet 2010; 41: 570–578. [DOI] [PubMed] [Google Scholar]
- 8.Ehrlemark AG, Sallvik KG. A model of heat and moisture dissipation from cattle based on thermal properties. Trans ASAE 1996; 39: 187–194. [Google Scholar]
- 9.West JW. Effects of heat-stress on production in dairy cattle. J Dairy Sci 2003; 86: 2131–2144. [DOI] [PubMed] [Google Scholar]
- 10.Soriani N, Panella G, Calamari L. Rumination time during the summer season and its relationships with metabolic conditions and milk production. J Dairy Sci 2013; 96: 5082–5094. [DOI] [PubMed] [Google Scholar]
- 11.Polsky L, von Keyserlingk MAG. Invited review: Effects of heat stress on dairy cattle welfare. J Dairy Sci 2017; 100: 8645–8657. [DOI] [PubMed] [Google Scholar]
- 12.Roth Z. Reproductive physiology and endocrinology responses of cows exposed to environmental heat stress - Experiences from the past and lessons for the present. Theriogenology 2020; 155: 150–156. [DOI] [PubMed] [Google Scholar]
- 13.Berman A, Folman Y, Kaim M, Mamen M, Herz Z, Wolfenson D, Arieli A, Graber Y. Upper critical temperatures and forced ventilation effects for high-yielding dairy cows in a subtropical climate. J Dairy Sci 1985; 68: 1488–1495. [DOI] [PubMed] [Google Scholar]
- 14.De Rensis F, Scaramuzzi RJ. Heat stress and seasonal effects on reproduction in the dairy cow—a review. Theriogenology 2003; 60: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 15.Takuma T, Sakai S, Ezoe D, Ichimaru H, Jinnouchi T, Kaedei Y, Nagai T, Otoi T. Effects of season and reproductive phase on the quality, quantity and developmental competence of oocytes aspirated from Japanese black cows. J Reprod Dev 2010; 56: 55–59. [DOI] [PubMed] [Google Scholar]
- 16.Sakatani M, Yamanaka K, Balboula AZ, Takahashi M. Different thermotolerances in in vitro-produced embryos derived from different maternal and paternal genetic backgrounds. Anim Sci J 2017; 88: 1934–1942. [DOI] [PubMed] [Google Scholar]
- 17.Takenouchi N, Fukujyu N. Estrous behavior/weak estrus behavior. Jpn J Embryo Transfer 2013; 35: 97–108 (In Japanese). [Google Scholar]
- 18.Sakatani M, Takahashi M, Takenouchi N. The efficiency of vaginal temperature measurement for detection of estrus in Japanese Black cows. J Reprod Dev 2016; 62: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C, Tsuruta S, Bertrand JK, Misztal I, Lawlor TJ, Clay JS. Trends for conception rate of Holsteins over time in the southeastern United States. J Dairy Sci 2009; 92: 4641–4647. [DOI] [PubMed] [Google Scholar]
- 20.Roth Z. Stress-induced alterations in oocyte transcripts are further expressed in the developing blastocyst. Mol Reprod Dev 2018; 85: 821–835. [DOI] [PubMed] [Google Scholar]
- 21.Sugasawa S, Kato M, Mori K, Kawana K, Masudo H, Suzuki N, Yako J. Relationships of conception performance with the facility and environment in dairy cows during hot season. Artif Insemin Livest 2001; 204: 55–61 (In Japanese). [Google Scholar]
- 22.Sakatani M, Yamanaka K, Balboula AZ, Takenouchi N, Takahashi M. Heat stress during in vitro fertilization decreases fertilization success by disrupting anti-polyspermy systems of the oocytes. Mol Reprod Dev 2015; 82: 36–47. [DOI] [PubMed] [Google Scholar]
- 23.Sakatani M, Kobayashi S, Takahashi M. Effects of heat shock on in vitro development and intracellular oxidative state of bovine preimplantation embryos. Mol Reprod Dev 2004; 67: 77–82. [DOI] [PubMed] [Google Scholar]
- 24.Sakatani M. Effects of heat stress on bovine preimplantation embryos produced in vitro. J Reprod Dev 2017; 63: 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balboula AZ, Yamanaka K-I, Sakatani M, Kawahara M, Hegab AO, Zaabel SM, Takahashi M. Cathepsin B activity has a crucial role in the developmental competence of bovine cumulus-oocyte complexes exposed to heat shock during in vitro maturation. Reproduction 2013; 146: 407–417. [DOI] [PubMed] [Google Scholar]
- 26.Garnsworthy PC. The environmental impact of fertility in dairy cows: a modelling approach to predict methane and ammonia emissions. Anim Feed Sci Technol 2004; 112: 211–223. [Google Scholar]
- 27.Hristov AN, Oh J, Lee C, Meinen R, Montes F, Ott T, Firkins J, Rotz A, Dell C, Adesogan A, Tang W, Tricarico J, Kebreab E, Waghorn G, Dijkstra J, Oosting S. Mitigation of greenhouse gas emissions in livestock production – A Review of technical options for non-CO2 emissions. Edited by Gerber PJ, Henderson B, Makkar HPS. FAO Animal Production and Health Paper2013; 177. [DOI] [PubMed] [Google Scholar]
- 28.Sakatani M, Balboula AZ, Yamanaka K, Takahashi M. Effect of summer heat environment on body temperature, estrous cycles and blood antioxidant levels in Japanese Black cow. Anim Sci J 2012; 83: 394–402. [DOI] [PubMed] [Google Scholar]
- 29.Saint-Dizier M, Chastant-Maillard S. Potential of connected devices to optimize cattle reproduction. Theriogenology 2018; 112: 53–62. [DOI] [PubMed] [Google Scholar]
- 30.Hojo T, Sakatani M, Takenouchi N. Efficiency of a pedometer device for detecting estrus in standing heat and silent heat in Japanese Black cattle. Anim Sci J 2018; 89: 1067–1072. [DOI] [PubMed] [Google Scholar]
- 31.Cerri RLA, Burnett TA, Madureira AML, Silper BF, Denis-Robichaud J, LeBlanc S, Cooke RF, Vasconcelos JLM. Symposium review: Linking activity-sensor data and physiology to improve dairy cow fertility. J Dairy Sci 2021; 104: 1220–1231. [DOI] [PubMed] [Google Scholar]
- 32.Wiltbank MC, Pursley JR. The cow as an induced ovulator: timed AI after synchronization of ovulation. Theriogenology 2014; 81: 170–185. [DOI] [PubMed] [Google Scholar]
- 33.Hansen PJ, Block J. Towards an embryocentric world: the current and potential uses of embryo technologies in dairy production. Reprod Fertil Dev 2004; 16: 1–14. [DOI] [PubMed] [Google Scholar]
- 34.Baruselli PS, Ferreira RM, Vieira LM, Souza AH, Bó GA, Rodrigues CA. Use of embryo transfer to alleviate infertility caused by heat stress. Theriogenology 2020; 155: 1–11. [DOI] [PubMed] [Google Scholar]
- 35.Drost M, Ambrose JD, Thatcher MJ, Cantrell CK, Wolfsdorf KE, Hasler JF, Thatcher WW. Conception rates after artificial insemination or embryo transfer in lactating dairy cows during summer in Florida. Theriogenology 1999; 52: 1161–1167. [DOI] [PubMed] [Google Scholar]
- 36.Mori M, Hayashi T, Isozaki Y, Takenouchi N, Sakatani M. Heat shock decreases the embryonic quality of frozen-thawed bovine blastocysts produced in vitro. J Reprod Dev 2015; 61: 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasconcelos JLM, Demétrio DGB, Santos RM, Chiari JR, Rodrigues CA, Sá Filho OG. Factors potentially affecting fertility of lactating dairy cow recipients. Theriogenology 2006; 65: 192–200. [DOI] [PubMed] [Google Scholar]
- 38.Moghaddam A, Karimi I, Pooyanmehr M. Effects of short-term cooling on pregnancy rate of dairy heifers under summer heat stress. Vet Res Commun 2009; 33: 567–575. [DOI] [PubMed] [Google Scholar]