Abstract
The endometrial epidermal growth factor (EGF) profile is an indicator of uterine function and fertility in cattle. The present study aimed to investigate the effects of heat stress on the endometrial EGF profile and fertility in lactating Holstein cows. The endometrial EGF profiles of 365 cows in the Hokkaido and Kyushu regions were examined between June and September (heat stress period, n = 211) and between October and January (control period, n = 154). EGF profiles were investigated using uterine endometrial tissues obtained by biopsy 3 days after estrus (Day 3). The proportion of cows with an altered EGF profile was higher between June and September than between October and January (41.2 vs. 16.2%, P < 0.05). The effects of rectal temperature on Days 0 and 3 on the endometrial EGF profile were also assessed in cows (n = 79) between June and September in the Kyushu region. A single embryo was transferred to cow on Day 7 to evaluate fertility (n = 67). Regardless of the rectal temperature on Day 3, the proportion of cows with an altered EGF profile was higher (64.1 vs. 30.0%, P < 0.05) and the pregnancy rate after embryo transfer (ET) was lower (26.7 vs. 51.4%, P < 0.05) in cows with a rectal temperature ≥ 39.5°C on Day 0 than in cows with a rectal temperature < 39.5°C on Day 0. The present results indicate that alterations in the endometrial EGF profile induced by an elevated body temperature on Day 0 contributed to reductions in fertility in lactating dairy cows during the heat stress period.
Keywords: Dairy cow, Embryo transfer, Endometrial epidermal growth factor, Heat stress
Heat stress is one of the major contributing factors to low fertility in dairy cows. It is defined as an environment that increases body temperature to above the set-point temperature [1]. Ambient temperature (AT), humidity, wind, and solar radiation are some of the factors contributing to heat stress [2]. A negative correlation has been reported between an elevated body temperature and fertility in lactating dairy cows. The conception rate of artificial insemination (AI) begins to decline when uterine temperature at insemination increases by approximately 0.5°C above the normal range (38.3–38.6°C) [3]. The temperature-humidity index (THI), which is calculated from AT and relative humidity (RH), has been widely used as an indicator of heat stress in dairy cows. The typical stress threshold of THI is 72 [4]. THI higher than 72 may be reached in tropical and subtropical zones, and recently in temperate and some cold zones [5]. The effect of heat stress on fertility was not examined in detail in the latter zones approximately 3–4 decades ago, however, it is now becoming a major contributing factor for low fertility in high-yielding cows.
Summer heat stress decreases fertility through multifactorial causes, such as disturbed follicular growth and ovulation, impaired corpus luteum (CL) function, the suppressed expression of estrus, and embryonic loss [6, 7]. The detrimental effects of heat stress on oocytes and early embryos are considered to be the main cause of increased embryonic loss [8]. The exposure of cattle to heat stress between the follicular phase and within 3 days after AI at estrus in the natural cycle as well as after superovulatory treatment was found to decrease fertility [9,10,11,12]. Accordingly, studies using in vitro embryo production systems demonstrated that the developmental competence of oocytes obtained from cows exposed to heat stress [13,14,15] or a high temperature (41.0°C) during in vitro maturation cultures [16, 17] was reduced. Furthermore, the developmental competence of zygotes and two-cell stage embryos was reduced in an in vitro culture at a high temperature (41.0°C) [18, 19].
Early embryos after Day 3 were found to be less sensitive to heat stress [10, 18, 19]. Consequently, heat stress decreases the pregnancy rate of AI more than that of embryo transfer (ET) [20, 21] and, thus, ET has been used to compensate for low fertility during the hot season [22]. The effects of heat stress on pregnancy after ET currently remain unclear. A previous study reported a decreased pregnancy rate after ET between the hot and cool seasons [23], while other studies found no or only slight differences in pregnancy rates [24,25,26,27]. Nevertheless, changes in the production and circulating levels of ovarian steroid hormones [4], and the synthesis and secretion of proteins [28] and prostaglandins [29,30,31] in the endometrium by heat stress may increase the incidence of embryonic loss even after ET due to an improper endocrine environment [7] or uterine dysfunction [32, 33].
In cattle, the epidermal growth factor (EGF) profile in the uterine endometrium has been identified as an indicator of endometrial function and fertility [34, 35]. Endometrial EGF concentrations exhibit a cyclic change with two peaks on Days 2–4 and 13–14 during the estrous cycle [34, 36]. The loss of these peaks reduces fertility with an increase in embryonic loss [37] in repeat breeder (RB) and high-yielding dairy cows [35, 38, 39]. The normalization of the EGF profile by treatments with hormonal drugs [40] and seminal proteins [41] restored fertility in RB cows. Furthermore, the pregnancy rate was lower in apparently normal recipient cows with low EGF concentrations on Day 3 (< 4.70 ng/g tissue weight) than in those with EGF concentrations within the normal range (33.3 vs. 76.9%) [42].
Alterations in the endometrial EGF profile have been linked to changes in circulating estradiol (E2) and progesterone (P4) concentrations in RB and high-yielding cows [39]. In dairy cows, a high feed intake supporting a large amount of milk production increases liver blood flow and, in turn, the clearance of E2 and P4 from the circulation [43]. This may cause a slower increase and lower peaks in E2 and P4 concentrations in the circulation [44]. Although RB cows may not necessarily be high producers, they show similar alterations in ovarian steroid hormone profiles to those in high-yielding cows [35]. Since the expression of EGF in the endometrium is primarily regulated by E2 and P4 [45, 46], changes in circulating E2 and P4 concentrations may be amplified in the endometrium as an altered EGF profile [35]. Seasonal heat stress was also found to suppress the production and circulating concentrations of E2 and P4 in dairy cows [4]; therefore, reduced fertility during the heat stress period may be attributed, at least in part, to uterine dysfunction caused by alterations in the endometrial EGF profile.
The present study examined the relationship between decreased fertility during the heat stress period and uterine dysfunction caused by an altered endometrial EGF profile in dairy cows. We initially investigated the effects of seasons and regions on the EGF profile on Day 3. We then examined the effects of an elevated body temperature on Day 0 (estrus) and Day 3 on the EGF profile on Day 3 and pregnancy rate after ET.
Materials and Methods
Animals
A total of 444 Holstein cows (8,500–12,000 kg of 305-day fat-corrected milk) between 2 and 5 in parity in commercial farms in the Hokkaido (central area: 42–44°N, 141–142°E) and Kyushu (north-west area: 32–34°N, 130–131°E) regions in Japan were used. All cows were observed for estrus at least twice a day or estrus was detected using an automated activity monitor. All cows showed a normal inter-estrus interval (18–23 days) and ovulated within 48 h of the onset of estrus. In cows exhibiting weak signs of estrus, particularly during the heat stress period, estrus was confirmed by ovulation within 48 h and blood concentrations of E2 (≥ 5 pg/ml) and P4 (< 1 ng/ml). All experimental procedures were approved by the Hokkaido University Animal Care and Use Committee (No. 16-0071).
Biopsy of endometrial tissues
Uterine endometrial tissues were obtained using a biopsy instrument (3050100, Fujihira Industry, Tokyo, Japan) under caudal epidural anesthesia with 3 ml of 2% lidocaine (2% xylocaine, AstraZeneca, Osaka, Japan) as previously described [38]. Two pieces of uterine endometrial tissues from the inter-caruncle region (25–50 mg) were obtained from the middle of 3 sections in the uterine horns, which were equally divided along the longitudinal axis. The caruncle region was distinguished from the inter-caruncle region as fluffy cut surface due to rich blood vessels. If the caruncle was greater than one-third of the tissue, another biopsy was collected. However, if the caruncle was approximately one-third or less of the biopsy, the caruncle was dissected out and the rest of the tissue was used [34]. All tissue samples were obtained from the uterine horns on the contralateral side to CL. Tissues were immediately frozen in liquid nitrogen and stored at –30°C for the EGF assay.
Measurement of EGF concentrations and judgement of the EGF profile
Uterine endometrial tissue samples were processed as previously described [38, 47] with a modification of changing the concentration of acetic acid (01021-70, Kanto Chemical Co., Inc., Tokyo, Japan) for extraction solution from 1 M to 0.1 M. EGF concentrations in uterine endometrial tissue extracts were assessed using double-antibody sandwich EIA with 96-well microtiter plates (Costar 3590, Corning, NY, USA) [38]. An anti-human EGF mouse monoclonal antibody (MAB636, R & D Systems, Inc., Minneapolis, MN, USA) was used as the solid-phase antibody and anti-human EGF rabbit antiserum (5022-100, Biogenesis, Poole, UK) for detection with a peroxidase-conjugated anti-rabbit IgG goat antibody (270335, Seikagaku, Tokyo, Japan). Neither of these antibodies showed significant cross-reactivity with other cytokines tested by the manufacturers. The assay system was verified using increasing concentrations of recombinant bovine EGF. A linear regression analysis of recombinant bovine EGF concentrations and assay results gave y = 0.96x + 0.39, r = 0.97 [41]. The sensitivity of the assay was 10 pg/well. Intra- and inter-assay CVs at 50 pg/well were 4.2 and 5.3%, respectively. The EGF profile was determined by the endometrial EGF concentration on Day 3; EGF concentration between 4.70 and 13.50 ng/g tissue weight (normal range) was considered to be normal, whereas that of lower than 4.70 and higher than 13.50 ng/g tissue weight was considered to be altered based on previous findings [36, 38].
Measurement of rectal temperature
Rectal temperature was measured using a clinical thermometer once a day between 1300 and 1700 h on the day of estrus (Day 0) and Day 3.
Measurement of plasma E2 and P4 concentrations
Plasma E2 and P4 concentrations were determined using competitive double-antibody enzyme immunoassays, as described previously [48]. The primary antibodies used for the E2 and P4 assays were anti-estradiol-17β-6-carboxymethiloxime (CMO)-BSA (FKA204; Cosmo Bio, Tokyo, Japan) and anti-progesterone-3-CMO-BSA (KZ-HS-P13; Cosmo Bio), respectively. Goat anti-rabbit serum (111-005-003; Jacson Immuno Research Laboratories, West Grove, PA, USA) was used as the secondary antibody. The inter- and intra-assay coefficients of variations were 9.7 and 3.5% for E2, and 4.7 and 6.5% for P4, respectively.
AT, RH, and THI
Data on hourly AT and RH during the study period (3 years; 2015–2017) were obtained from the local meteorological observatory in the Hokkaido region (Sapporo and Tomakomai) and Kyushu region (Fukuoka and Kumamoto), in which the commercial farms used in the present study are located. The following equation was used to calculate THI [49].
THI = (1.8 × AT + 32) – (0.55 – 0.0055 × RH) × (1.8 × AT – 26)
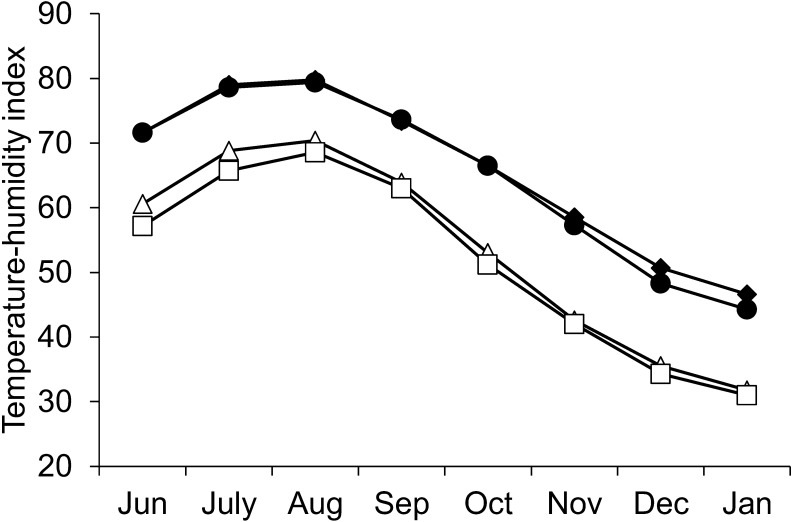
Monthly THI in the Kyushu region ranged from 44.3 to 79.8, whereas that in the Hokkaido region was from 31.0 to 70.4 (Fig. 1). Mean AT and THI between June and September and between October and January in each of the four areas are summarized in Table 1. In the four areas, mean THI between June and September ranged from 63.7 to 76.0, while that between October and January was from 39.6 to 55.5. The number of days when daily maximum THI exceeded 72 between June and September was from 26 to 118, and that between October and January was from 0 to 15.
Fig. 1.
Mean monthly temperature humidity index (THI) of the study period (2015–2017) in two regions (Hokkaido: Sapporo Δ and Tomakomai □; Kyushu: Fukuoka ♦ and Kumamoto ●).
Table 1. Ambient temperature (AT), temperature humidity index (THI), and number of days when daily maximum THI exceeded 72 between June and September and between October and January in two study regions, Hokkaido and Kyushu.
| Regions | Seasons | AT (°C) | THI | Number of days when daily maximum THI exceeded 72 |
|
|---|---|---|---|---|---|
| Hokkaido | Sapporo | Jun–Sep | 19.8 ± 4.3 | 66.0 ± 6.2 | 50 |
| Oct–Jan | 2.7 ± 6.8 | 40.7 ± 10.1 | 0 | ||
| Tomakomai | Jun–Sep | 17.9 ± 3.8 | 63.7 ± 6.2 | 26 | |
| Oct–Jan | 2.4 ± 6.9 | 39.6 ± 10.8 | 0 | ||
| Kyushu | Fukuoka | Jun–Sep | 26.2 ± 3.7 | 76.0 ± 5.1 | 116 |
| Oct–Jan | 12.7 ± 6.2 | 55.5 ± 9.3 | 9 | ||
| Kumamoto | Jun–Sep | 26.0 ± 3.9 | 75.8 ± 5.1 | 118 | |
| Oct–Jan | 12.0 ± 7.2 | 51.1 ± 11.1 | 15 | ||
Values are presented as means ± SDs.
Embryo transfer (ET)
ET was performed by one technician and two veterinarians. A frozen in vivo produced embryo (IETS standards; Codes 1–2) was transferred into the uterine horn ipsilateral to CL on Day 7.
Study design
Study 1: Study 1 was conducted between 2015 and 2017. Lactating Holstein cows (n = 365) between 60 and 90 days postpartum in the Hokkaido and Kyushu regions were used to examine the effects of seasons and regions on the proportion of cows with an altered EGF profile and the endometrial EGF concentration on Day3. Hokkaido is located in the northeastern region of Japan and has a cool and dry climate, whereas Kyushu is in the southwestern region and has a hot and humid climate (Table 1 and Fig. 1). During the heat stress period (between June and September), endometrial tissues were obtained for the EGF assay on Day 3 of the estrous cycle from 211 cows (90 cows in the Hokkaido region and 121 cows in the Kyushu region). During the control (cool) period (between October and January), endometrial tissues were obtained from 154 cows (86 cows in the Hokkaido region and 68 cows in the Kyushu region).
Study 2: Study 2 was performed between June and September in 2017. Lactating Holstein cows (n = 79) between 60 and 90 days postpartum in the Kyushu region were used to examine the effects of rectal temperature on Days 0 and 3 on the proportion of cows with an altered EGF profile and the pregnancy rate after ET. Rectal temperature on Days 0 and 3 and the endometrial EGF concentration on Day 3 were measured in all cows. ET was performed on Day 7 of the same estrous cycle (n = 67). Pregnancy was diagnosed by palpation of the uterine tract per rectum between Days 56 and 60.
Data analysis
In study 1, the proportion of cows with an altered EGF profile was compared between the different regions and seasons using the chi-squared test. The effects of seasons (June-September and October-January), regions (Hokkaido and Kyushu) and EGF profile (normal and altered) on endometrial EGF concentrations were evaluated by the three-way ANOVA. In study 2, cows were divided into four groups based in combination of rectal temperature; 39.5°C or higher (≥ 39.5°C) and lower than 39.5°C (< 39.5°C), on Days 0 and 3. The effects of rectal temperature on Days 0 and 3 on the proportion of cows with an altered EGF profile and the pregnancy rate after ET were evaluated using Fisher’s exact test. Endometrial EGF concentrations were not normally distributed based on the Shapiro-Wilk test, and, thus, were transformed to ranks. The effects of the rectal temperature category (≥ 39.5°C and < 39.5°C) and days of heat stress (Days 0 and 3) on endometrial EGF concentrations were evaluated by a nonparametric two-way ANOVA. EGF concentrations were compared between cows with a rectal temperature ≥ 39.5°C and < 39.5°C on Day 0 using the Mann-Whitney U test. Pregnancy rates were compared between cows with normal and altered EGF profiles by Fisher’s exact test. All statistical analyses were performed using JMP software version 14.0.0 (SAS Institute Japan, Tokyo, Japan) or SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
Study 1
In the present study, the endometrial EGF concentrations on Day 3 were within or lower than the lower limit of the normal range (4.70 ng/g tissue weight) and, thus, all altered EGF profiles were characterized with a suppressed EGF peak [36, 38]. The proportion of cows with an altered EGF profile was higher between June and September than between October and January in both regions (P < 0.05) (Table 2). The proportion of cows with an altered EGF profile increased by approximately 2- and 3-fold in the Hokkaido and Kyushu regions, respectively, during the heat stress period. No significant differences were observed in the proportion of cows with an altered EGF profile between the two regions in each seasonal period; however, the proportion of cows with an altered EGF profile was slightly higher in the Kyushu region than in the Hokkaido region throughout the study period (P = 0.07). The three-way ANOVA for seasons (June–September and October–January), regions (Hokkaido and Kyushu) and EGF profile (normal and altered) indicated only main effect of EGF profile for the endometrial EGF concentrations (P < 0.01). EGF concentrations in cows with normal and altered EGF profiles did not differ between the seasons in both regions. On the other hand, EGF concentrations in all cows (subtotal) were lower between June and September than between October to January in both regions (P < 0.05), reflecting the higher proportion of cows showing an altered EGF profile with low EGF concentrations in June to September than October to January.
Table 2. Proportion of dairy cows showing the normal and altered epidermal growth factor (EGF) profile and their EGF concentrations on day 3 in Hokkaido and Kyusyu regions.
| Region | EGF profile | June–September |
October–January |
Total |
|||
|---|---|---|---|---|---|---|---|
| No. (%) of cows showing indicated profile |
EGF concentrations (ng/g tissue weight) |
No. (%) of cows showing indicated profile |
EGF concentrations (ng/g tissue weight) |
No. (%) of cows showing indicated profile |
EGF concentrations (ng/g tissue weight) |
||
| Hokkaido | Normal | 58 (64.4) a | 6.71 ± 0.97 | 72 (83.7) b | 6.71 ± 1.04 | 130 (73.9) | 6.71 ± 0.99 |
| Altered | 32 (35.6) a | 1.66 ± 0.73 | 14 (16.3) b | 1.42 ± 0.70 | 46 (26.1) A | 1.59 ± 0.71 | |
| Subtotal | 90 (100) | 4.92 ± 2.59 a | 86 (100) | 5.81 ± 1.83 b | 176 (100) | 5.37 ± 2.11 | |
| Kyushu | Normal | 66 (54.5) a | 7.01 ± 1.32 | 57 (83.8) b | 6.76 ± 0.62 | 123 (65.1) | 6.89 ± 0.44 |
| Altered | 55 (45.5) a | 1.12 ± 0.48 | 11 (16.2) b | 1.68 ± 0.66 | 66 (34.9) B | 1.21 ± 0.53 | |
| Subtotal | 121 (100) | 4.33 ± 2.61 a | 68 (100) | 5.97 ± 2.34 b | 189 (100) | 5.01 ± 2.42 | |
| Total | Normal | 124 (58.8) a | 6.87 ± 1.12 | 129 (83.8) b | 6.73 ± 0.92 | 253 (69.3) | 6.80 ± 0.99 |
| Altered | 87 (41.2) a | 1.32 ± 0.68 | 25 (16.2) b | 1.53 ± 0.68 | 112 (30.7) | 1.37 ± 0.68 | |
| Subtotal | 211 (100) | 4.58 ± 2.60 a | 154 (100) | 5.88 ± 2.06 b | 365 (100) | 5.13 ± 2.32 | |
a, b Values with different letters within the same row significantly differ (P < 0.05). A, B Values with different letters within the same column slightly differ (P = 0.07). The three-way ANOVA for seasons (June–September and October–January), regions (Hokkaido and Kyushu) and EGF profile (normal and altered) indicated only main effect of EGF profile on the endometrial EGF concentrations (P < 0.01). None of the interactions were significant. EGF concentrations are presented as means ± SDs. EGF profile was determined by the value of endometrial EGF concentration on Day 3; EGF concentration of between 4.7 and 13.5 ng/g tissue weight (normal range) was considered to be normal, whereas that of < 4.70 and 13.5 < ng/g tissue weight was considered to be altered based on previous findings [36, 38].
Study 2
Rectal temperature between Days 0 and 3 was similar in both rectal temperature groups (Table 3). Regardless of rectal temperature on Day 3, the proportion of cows with an altered EGF profile was higher in the cows with a rectal temperature ≥ 39.5°C on Day 0 than in the cows with a rectal temperature < 39.5°C on Day 0 (P < 0.05) (Table 4). EGF concentrations in all cows, and in cows with normal and altered EGF profiles indicated the significant main effects of rectal temperature on Day 0 (P < 0.05). EGF concentrations were lower in the cows with a rectal temperature ≥ 39.5°C on Day 0 than in the cows with a rectal temperature < 39.5°C (P < 0.05), regardless of the EGF profie. Regardless of rectal temperature on Day 3, pregnancy rates after ET were lower in the cows with a rectal temperature ≥ 39.5°C on Day 0 than in the cows with a rectal temperature < 39.5°C on Day 0 (P < 0.05) (Table 5). EGF concentrations in all recipient cows and in recipient cows with a normal EGF profile indicated the significant main effects of rectal temperature on Day 0 (P < 0.05). EGF concentrations in cows with a normal EGF profile were lower in the cows with a rectal temperature ≥ 39.5°C on Day 0 than in the cows with a rectal temperature < 39.5°C on Day 0 (P < 0.05). EGF concentrations in cows with an altered EGF profile tended to low in the cows with a rectal temperature ≥ 39.5°C on Day 0 than in the cows with a rectal temperature < 39.5°C on Day 0 (P = 0.09). However, in cows with the normal EGF profile, no significant differences were observed in the pregnancy rate after ET between the two rectal temperature groups on Day 0. In cows with an altered EGF profile, no difference was observed in the pregnancy rate after ET between the cows with a rectal temperature ≥ 39.5°C and < 39.5°C on Day 0. The pregnancy rate after ET was markedly lower in cows with an altered EGF profile (6.3%, n = 32) than in those with a normal EGF profile (71.4%, n = 35) (P < 0.05). The overall conception rate of all recipient cows throughout the present study (n = 67) was 40.3%.
Table 3. Rectal temperature on Days 0 and 3 in cows with a rectal temperature ≥ 39.5ºC and < 39.5ºC in Study 2.
| Rectal temperature ≥ 39.5ºC | (n) | Rectal temperature < 39.5ºC | (n) | Total | (n) | |
|---|---|---|---|---|---|---|
| Day 0 | 40.0 ± 0.32 (39.6–40.8) | 39 | 39.0 ± 0.27 (38.2–39.4) | 40 | 39.5 ± 0.59 (38.2–40.8) | 79 |
| Day 3 | 40.0 ± 0.26 (39.5–40.4) | 34 | 39.0 ± 0.28 (38.3–39.4) | 45 | 39.4 ± 0.54 (38.3–40.4) | 79 |
Rectal temperatures are presented as means ± SDs. Numbers in parentheses show the ranges of rectal temperature.
Table 4. Effects of rectal temperature on Days 0 and 3 on endometrial epidermal growth factor (EGF) concentrations in dairy cows.
| Rectal temperature |
No. of cows | Proportion of cows with an altered EGF profile † |
EGF concentrations |
|||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Altered | Normal | All | ||
| ≥ 39.5ºC | ≥ 39.5ºC | 18 | 66.7 | 2.48 ± 1.03 (12) | 6.62 ± 1.45 (6) | 3.86 ± 2.28 (18) |
| ≥ 39.5ºC | < 39.5ºC | 21 | 61.9 | 2.09 ± 1.01 (13) | 6.82 ± 1.42 (8) | 3.89 ± 2.58 (21) |
| Sub total | 39 | 64.1 a | 2.28 ± 1.04 a (25) | 6.73 ± 1.44 a (14) | 3.88 ± 2.45 a (39) | |
| < 39.5ºC | ≥ 39.5ºC | 16 | 31.3 | 3.63 ± 0.40 (5) | 7.74 ± 1.38 (11) | 6.46 ± 2.23 (16) |
| < 39.5ºC | < 39.5ºC | 24 | 29.2 | 2.86 ± 1.30 (7) | 8.02 ± 1.15 (17) | 6.51 ± 2.63 (24) |
| Sub total | 40 | 30.0 b | 3.18 ± 1.10 b (12) | 7.91 ± 1.25 b (28) | 6.49 ± 2.48 b (40) | |
a, b Values with different letters significantly differ between cows with a rectal temperature of 39.5°C or higher (≥ 39.5°C) and lower than 39.5°C (< 39.5°C) on Day 0 (P < 0.05). EGF concentrations are presented as means ± SDs. Numbers in parentheses show the number of cows. † EGF profile was determined by the value of endometrial EGF concentration on Day 3; EGF concentration of between 4.7 and 13.5 ng/g tissue weight (normal range) was considered to be normal, whereas that of < 4.70 and 13.5 < ng/g tissue weight was considered to be altered based on previous findings [36, 38].
Table 5. Effects of rectal temperature on Days 0 and 3 on endometrial epidermal growth factor (EGF) concentrations and conception rates after embryo transfer (ET) in dairy cows.
| Rectal temperature |
EGF profile † (n) | EGF conc. | Conception (%) | |
|---|---|---|---|---|
| Day 0 | Day 3 | |||
| ≥ 39.5ºC | ≥ 39.5ºC | Normal (3) | 6.05 ± 0.54 | 2/3 (66.7) |
| Altered (9) | 2.76 ± 0.98 | 1/9 (11.1) | ||
| ≥ 39.5ºC | < 39.5ºC | Normal (7) | 6.68 ± 0.55 | 5/7 (71.4) |
| Altered (11) | 2.22 ± 1.03 | 0/11 (0.0) | ||
| Sub total | Normal (10) | 6.49 ± 1.35 a | 7/10 (70.0) | |
| Altered (20) | 2.47 ± 1.05 A | 1/20 (5.0) | ||
| All (30) | 3.81 ± 2.22 a | 8/30 (26.7 a) | ||
| < 39.5ºC | ≥ 39.5ºC | Normal (10) | 7.95 ± 1.28 | 6/10 (60.0) |
| Altered (5) | 3.63 ± 0.40 | 1/5 (20.0) | ||
| < 39.5ºC | < 39.5ºC | Normal (15) | 7.93 ± 1.18 | 12/15 (80.0) |
| Altered (7) | 2.86 ± 1.30 | 0/7 (0.0) | ||
| Sub total | Normal (25) | 7.93 ± 1.18 b | 18/25 (72.0) | |
| Altered (12) | 3.18 ± 1.10 B | 1/12 (8.3) | ||
| All (37) | 6.39 ± 2.52 b | 19/37 (51.4 b) | ||
| Total | Normal (35) | 7.52 ± 1.42 | 25/35 (71.4 x) | |
| Altered (32) | 2.73 ± 1.12 | 2/32 (6.3 y) | ||
| All (67) | 5.23 ± 2.71 | 27/67 (40.3) | ||
a, b Values with different letters significantly differ between cows with a rectal temperature of 39.5°C or higher (≥ 39.5°C) and lower than 39.5°C (< 39.5°C) on Day 0 (P < 0.05). A, B Values with different letters within the same column slightly differ between cows with a rectal temperature ≥ 39.5°C and < 39.5°C on Day 0 (P = 0.09). x, y Values with different letters significantly differ between cows with normal and altered EGF profiles (P < 0.05). EGF concentrations are presented as means ± SDs. † EGF profile was determined by the value of endometrial EGF concentration on Day 3; EGF concentration of between 4.7 and 13.5 ng/g tissue weight (normal range) was considered to be normal, whereas that of < 4.70 and 13.5 < ng/g tissue weight was considered to be altered based on previous findings [36, 38].
Discussion
The present results demonstrated that an elevated body temperature on the day of estrus caused by heat stress increased the incidence of abnormalities in the uterine endometrial EGF profile and reduced fertility. This may be one of the mechanisms contributing to reduced fertility in summer.
The proportion of cows with an altered EGF profile (i.e. lowered EGF peak on Day 3) was similar in both regions (approximately 16%) during the control period and increased by approximately 2- and 3-fold in the Hokkaido and Kyushu regions, respectively, during the heat stress period. An altered endometrial EGF profile has been linked to reduced fertility [35]; therefore, greater alterations in endometrial EGF profiles may explain, at least partly, the reductions observed in conception rates in summer. The degree of summer heat stress is milder in the Hokkaido region than in the Kyushu region. Kyushu is classified as a temperate zone. The number of days on which daily maximum THI exceeded 72 in this region was more than 115 and the monthly average of daily maximum THI ranged from 75.4 to 83.4 between June and September during the study period. Hokkaido is classified as a cold zone. The number of days on which daily maximum THI exceeded 72 in this region was approximately 40 and the monthly average of daily maximum THI ranged from 61.5 to 74.5 during the same period. The present results indicate that even the milder heat stress in Hokkaido was sufficient to alter the EGF profile. This may be attributed to differences in the cooling management of herds. In the Kyushu region, the majority of farms use intensive cooling management typically involving a combination of fan cooling and intermittent sprinklers, while cooling management in the Hokkaido region is limited to a less intensive fan cooling system.
The present study revealed that a rectal temperature of 39.5°C and higher on the day of estrus (Day 0), regardless of that on Day 3, resulted in the suppression of EGF concentrations on Day 3. The underlying mechanisms by which heat stress on Day 0 impairs the endometrial EGF profile may be multifactorial. Most importantly, an elevated body temperature on Day 0 may induce similar changes in E2 and P4 concentrations to those found in RB and high-yielding cows [35]. The alterations in plasma steroid hormones suppresses the expression of EGF in the uterus since E2 and P4 are the primary regulators of EGF in the endometrium [45, 46]. Heat stress suppresses ovarian steroid hormone production by inhibiting the systemic endocrine system and ovarian cell activity [4]. Heat stress was previously shown to reduce the number of luteinizing hormone (LH) pulses in lactating dairy cows [50]. This may lead to a decline in E2 secretion by granulosa cells. The exposure of cultured follicle tissues from dominant follicles to a high temperature (41.0°C) decreased E2 production by approximately 30% from that in the control (37°C) [51]. Therefore, plasma concentrations of E2 at the time of luteolysis [52] and estrus [53] decrease under heat stress conditions. Furthermore, heat stress was found to suppress the LH surge during the natural estrous cycle in Guernsey heifers [54] and its release in response to gonadotropin-releasing hormone administration in dairy cows [55]. The suppressed LH surge may delay the time of ovulation and CL formation; therefore, increases in the plasma concentration of P4 may be delayed.
A reduced blood flow to the uterus may also be one of the mechanisms by which heat stress decreased the endometrial EGF concentrations on Day 3. Blood flow to the uterus increases, particularly on the day of estrus (Day 0) with positive correlations to the increased estrogen concentration and the ratio of plasma E2/P4 concentrations [56, 57]. However, the redistribution of blood flow from visceral organs, including the ovary and uterus, to the periphery occurs for thermoregulation during heat stress [32]. An elevated uterine blood flow in response to treatment with E2 in ovariectomized cows decreased under the heat stressed condition [58]. Decreased blood flow to the uterus under the heat stressed condition at estrus may reduce the supply of hormones including E2 to the endometrial tissues [32] and alter the endometrial EGF profile.
Elevations induced in body temperature by heat stress may have a direct adverse effect on the expression of EGF. A high body temperature has been suggested to exert detrimental effects on uterine cell functions [32, 59]. The exposure of the cultured bovine endometrium to a high temperature increased the synthesis of heat shock protein (HSP) 70 and HSP90 [28]. Since HSPs are part of the complex of proteins that associate with P4 and estrogen receptors [60,61,62], changes in HSP synthesis may alter the assembly, transport, or binding activities of steroid receptors. Therefore, heat stress may inhibit the effects of ovarian steroid hormones on EGF production in the uterus. A previous study demonstrated that an increase in the cellular levels of HSP90 at an elevated temperature negatively interfered with ER-dependent transcription [62].
The rectal temperature showed a relatively wide range from 38.2°C to 40.8°C in the present study. This may be due to the differences in cooling management of farms or the daily variation of ambient temperature and relative humidity. Difference in rectal temperature can also be attributed to the difference in susceptibility to heat stress of individual cows associated with the levels of milk yield [63]. Further, genetic variation for tolerance of heat stress in dairy cows [64] could be a potential cause since the specific single nucleotide polymorphisms of genes for tolerance to heat stress has been identified [65, 66].
The pregnancy rate of ET recipients showing an altered EGF profile during the summer months in the present study (6.3%, n = 32) was lower than that in a previous study that reported year-round ET results (33.3%, n = 87) [42]. However, the pregnancy rate of recipients with a normal EGF profile in the present study was similar to that in the previously reported (71.4 vs. 76.9%, respectively). Differences in pregnancy rates in recipients with an altered EGF profile may be associated with a combination of potential role of EGF in the regulation of luteal function via prostaglandin synthesis in the endometrium and heat stress-induced enhancements in luteolytic effects. The EGF peak on Days 13–14 (the second peak) appeared to be important for the maintenance of CL. Although the EGF concentration at the second peak was not examined in the present study, the absence and recovery of the first and second peaks coincided in approximately 90% of cows [38]. The absence of EGF peaks would be associated with enhanced luteolytic effects because EGF increases the production ratio of PGE2/PGF2α [67] in the cultured endometrium and PGE2 functions as a luteotropic agent [68]. Moreover, the adverse effect of the absence of EGF peak on luteolysis may become apparent since an elevated temperature enhances the secretion of PGF2α (i.e., luteolytic factor) from a cultured bovine endometrium collected on Day 17 of the estrous cycle [69, 70].
The pregnancy rate after ET between June and September (the summer period) in the present study (40.3%) was similar to that in a previous study, which was performed during the same season in the same region (43.7%, n = 197) [71]. The pregnancy rate in the present study was within the range of previously reported pregnancy rates after ET during the hot season (14.3–55.4%) [20, 21, 23,24,25,26, 72,73,74,75], and higher than that of AI during the summer period in the commercial farms used in the present study (24.5%, n = 3863, data were obtained between 2016 and 2017) (unpublished data). Therefore, the present pregnancy rate after ET may be acceptable in summer trials. However, the present results indicated that heat stress, particularly on the day of estrus, decreased the pregnancy rate after ET through improper uterine functions that may be attributed to alterations in EGF profile. The pregnancy rate of ET in summer may be further improved by treatment targeting the uterine EGF expression [40, 41] or an intensive cooling around the day of estrus, which has been shown to increase the conception rate of AI during the summer period [76].
In conclusion, the present results indicate that impaired fertility under heat stress conditions is associated with an increase in the proportion of cows with an altered endometrial EGF profile. The pregnancy rate after ET was reduced in cows with a high body temperature on Day 0. This result cannot be explained by the direct effects of a high body temperature on periovulatory oocytes, sperm, and zygotes [32]. It suggests that heat stress causing an elevated body temperature (≥ 39.5°C) on Day 0, but not on Day 3, disturbed the endometrial EGF profile and increased embryonic loss.
Conflict of interests
The authors declare that there is no conflict of interests.
Supplementary
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (JP16H05032 and JP1900964) from the Japan Society for the Promotion of Science.
References
- 1.Hansen PJ. Effects of heat stress on mammalian reproduction. Philos Trans R Soc Lond B Biol Sci 2009; 364: 3341–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadokawa H, Sakatani M, Hansen PJ. Perspectives on improvement of reproduction in cattle during heat stress in a future Japan. Anim Sci J 2012; 83: 439–445. [DOI] [PubMed] [Google Scholar]
- 3.Gwazdauskas FC, Thatcher WW, Wilcox CJ. Physiological, environmental, and hormonal factors at insemination which may affect conception. J Dairy Sci 1973; 56: 873–877. [DOI] [PubMed] [Google Scholar]
- 4.De Rensis F, Lopez-Gatius F, García-Ispierto I, Morini G, Scaramuzzi RJ. Causes of declining fertility in dairy cows during the warm season. Theriogenology 2017; 91: 145–153. [DOI] [PubMed] [Google Scholar]
- 5.Bai H, Ukita H, Kawahara M, Mitani T, Furukawa E, Yanagawa Y, Yabuuchi N, Kim H, Takahashi M. Effect of summer heat stress on gene expression in bovine uterine endometrial tissues. Anim Sci J 2020; 91: e13474. [DOI] [PubMed] [Google Scholar]
- 6.Noakes DE, Parkinson TJ, England GCW. Veterinary Reproduction and Obstetrics. Elsevier, Ltd; 2019. [Google Scholar]
- 7.Wolfenson D, Roth Z. Impact of heat stress on cow reproduction and fertility. Anim Front 2018; 9: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen PJ. Cellular and molecular basis of therapies to ameliorate effects of heat stress on embryonic development in cattle. Anim Reprod 2018; 10: 322–333. [Google Scholar]
- 9.Putney DJ, Mullins S, Thatcher WW, Drost M, Gross TS. Embryonic development in superovulated dairy cattle exposed to elevated ambient temperatures between the onset of estrus and insemination. Anim Reprod Sci 1989; 19: 37–51. [DOI] [PubMed] [Google Scholar]
- 10.Ealy AD, Drost M, Hansen PJ. Developmental changes in embryonic resistance to adverse effects of maternal heat stress in cows. J Dairy Sci 1993; 76: 2899–2905. [DOI] [PubMed] [Google Scholar]
- 11.Chebel RC, Santos JEP, Reynolds JP, Cerri RLA, Juchem SO, Overton M. Factors affecting conception rate after artificial insemination and pregnancy loss in lactating dairy cows. Anim Reprod Sci 2004; 84: 239–255. [DOI] [PubMed] [Google Scholar]
- 12.Nabenishi H, Ohta H, Nishimoto T, Morita T, Ashizawa K, Tsuzuki Y. Effect of the temperature-humidity index on body temperature and conception rate of lactating dairy cows in southwestern Japan. J Reprod Dev 2011; 57: 450–456. [DOI] [PubMed] [Google Scholar]
- 13.Al-Katanani YM, Paula-Lopes FF, Hansen PJ. Effect of season and exposure to heat stress on oocyte competence in Holstein cows. J Dairy Sci 2002; 85: 390–396. [DOI] [PubMed] [Google Scholar]
- 14.Gendelman M, Aroyo A, Yavin S, Roth Z. Seasonal effects on gene expression, cleavage timing, and developmental competence of bovine preimplantation embryos. Reproduction 2010; 140: 73–82. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira RM, Ayres H, Chiaratti MR, Ferraz ML, Araújo AB, Rodrigues CA, Watanabe YF, Vireque AA, Joaquim DC, Smith LC, Meirelles FV, Baruselli PS. The low fertility of repeat-breeder cows during summer heat stress is related to a low oocyte competence to develop into blastocysts. J Dairy Sci 2011; 94: 2383–2392. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence JL, Payton RR, Godkin JD, Saxton AM, Schrick FN, Edwards JL. Retinol improves development of bovine oocytes compromised by heat stress during maturation. J Dairy Sci 2004; 87: 2449–2454. [DOI] [PubMed] [Google Scholar]
- 17.Edwards JL, Bogart AN, Rispoli LA, Saxton AM, Schrick FN. Developmental competence of bovine embryos from heat-stressed ova. J Dairy Sci 2009; 92: 563–570. [DOI] [PubMed] [Google Scholar]
- 18.Edwards JL, Hansen PJ. Differential responses of bovine oocytes and preimplantation embryos to heat shock. Mol Reprod Dev 1997; 46: 138–145. [DOI] [PubMed] [Google Scholar]
- 19.Sakatani M, Kobayashi S, Takahashi M. Effects of heat shock on in vitro development and intracellular oxidative state of bovine preimplantation embryos. Mol Reprod Dev 2004; 67: 77–82. [DOI] [PubMed] [Google Scholar]
- 20.Ambrose JD, Drost M, Monson RL, Rutledge JJ, Leibfried-Rutledge ML, Thatcher MJ, Kassa T, Binelli M, Hansen PJ, Chenoweth PJ, Thatcher WW. Efficacy of timed embryo transfer with fresh and frozen in vitro produced embryos to increase pregnancy rates in heat-stressed dairy cattle. J Dairy Sci 1999; 82: 2369–2376. [DOI] [PubMed] [Google Scholar]
- 21.Al-Katanani YM, Drost M, Monson RL, Rutledge JJ, Krininger CE, 3rd, Block J, Thatcher WW, Hansen PJ. Pregnancy rates following timed embryo transfer with fresh or vitrified in vitro produced embryos in lactating dairy cows under heat stress conditions. Theriogenology 2002; 58: 171–182. [DOI] [PubMed] [Google Scholar]
- 22.Hansen PJ. Reproductive physiology of the heat-stressed dairy cow: implications for fertility and assisted reproduction. Anim Reprod 2019; 16: 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Block J, Hansen PJ. Interaction between season and culture with insulin-like growth factor-1 on survival of in vitro produced embryos following transfer to lactating dairy cows. Theriogenology 2007; 67: 1518–1529. [DOI] [PubMed] [Google Scholar]
- 24.Chebel RC, Demétrio DGB, Metzger J. Factors affecting success of embryo collection and transfer in large dairy herds. Theriogenology 2008; 69: 98–106. [DOI] [PubMed] [Google Scholar]
- 25.Loureiro B, Bonilla L, Block J, Fear JM, Bonilla AQS, Hansen PJ. Colony-stimulating factor 2 (CSF-2) improves development and posttransfer survival of bovine embryos produced in vitro. Endocrinology 2009; 150: 5046–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baruselli PS, Ferreira RM, Sales JNS, Gimenes LU, Sá Filho MF, Martins CM, Rodrigues CA, Bó GA. Timed embryo transfer programs for management of donor and recipient cattle. Theriogenology 2011; 76: 1583–1593. [DOI] [PubMed] [Google Scholar]
- 27.Vasconcelos JLM, Jardina DTG, Sá Filho OG, Aragon FL, Veras MB. Comparison of progesterone-based protocols with gonadotropin-releasing hormone or estradiol benzoate for timed artificial insemination or embryo transfer in lactating dairy cows. Theriogenology 2011; 75: 1153–1160. [DOI] [PubMed] [Google Scholar]
- 28.Malayer JR, Hansen PJ, Buhi WC. Effect of day of the oestrous cycle, side of the reproductive tract and heat shock on in-vitro protein secretion by bovine endometrium. J Reprod Fertil 1988; 84: 567–578. [DOI] [PubMed] [Google Scholar]
- 29.Putney DJ, Malayer JR, Gross TS, Thatcher WW, Hansen PJ, Drost M. Heat stress-induced alterations in the synthesis and secretion of proteins and prostaglandins by cultured bovine conceptuses and uterine endometrium. Biol Reprod 1988; 39: 717–728. [DOI] [PubMed] [Google Scholar]
- 30.Putney DJ, Drost M, Thatcher WW. Embryonic development in superovulated dairy cattle exposed to elevated ambient temperatures between Days 1 to 7 post insemination. Theriogenology 1988; 30: 195–209. [DOI] [PubMed] [Google Scholar]
- 31.Putney DJ, Torres CAA, Gross TS, Thatcher WW, Plante C, Drost M. Modulation of uterine prostaglandin biosynthesis by pregnant and nonpregnant cows at day 17 post-estrus in response to in vivo and in vitro heat stress. Anim Reprod Sci 1989; 20: 31–47. [Google Scholar]
- 32.Hansen PJ, Aréchiga CF. Strategies for managing reproduction in the heat-stressed dairy cow. J Anim Sci 1999; 77(Suppl 2): 36–50. [DOI] [PubMed] [Google Scholar]
- 33.Wolfenson D, Roth Z, Meidan R. Impaired reproduction in heat-stressed cattle: basic and applied aspects. Anim Reprod Sci 2000; 60-61: 535–547. [DOI] [PubMed] [Google Scholar]
- 34.Katagiri S, Takahashi Y. Changes in EGF concentrations during estrous cycle in bovine endometrium and their alterations in repeat breeder cows. Theriogenology 2004; 62: 103–112. [DOI] [PubMed] [Google Scholar]
- 35.Katagiri S, Moriyoshi M. Alteration of the endometrial EGF profile as a potential mechanism connecting the alterations in the ovarian steroid hormone profile to embryonic loss in repeat breeders and high-producing cows. J Reprod Dev 2013; 59: 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katagiri S, Moriyoshi M, Yanagawa Y. Endometrial epidermal growth factor profile and its abnormalities in dairy cows. J Reprod Dev 2016; 62: 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katagiri S, Takahashi Y. Relationship between endometrial concentrations of epidermal growth factor (EGF) and preimplantation embryo development in dairy cattle. The 37th Annual Meeting of Society for the Study of Reproduction; 2004; Vancouver, Canada. 518.
- 38.Katagiri S, Takahashi Y. Potential relationship between normalization of endometrial epidermal growth factor profile and restoration of fertility in repeat breeder cows. Anim Reprod Sci 2006; 95: 54–66. [DOI] [PubMed] [Google Scholar]
- 39.Katagiri S, Moriyoshi M, Takahashi Y. Low incidence of an altered endometrial epidermal growth factor (EGF) profile in repeat breeder Holstein heifers and differential effect of parity on the EGF profile between fertile Holstein (dairy) and Japanese Black (beef) cattle. J Reprod Dev 2013; 59: 575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katagiri S, Takahashi Y. A progestin-based treatment with a high dose of estradiol benzoate normalizes cyclic changes in endometrial EGF concentrations and restores fertility in repeat breeder cows. J Reprod Dev 2008; 54: 473–479. [DOI] [PubMed] [Google Scholar]
- 41.Badrakh D, Yanagawa Y, Nagano M, Katagiri S. Effect of seminal plasma infusion into the vagina on the normalization of endometrial epidermal growth factor concentrations and fertility in repeat breeder dairy cows. J Reprod Dev 2020; 66: 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katagiri S. Relationship between endometrial epidermal growth factor and fertility after embryo transfer. J Reprod Dev 2006; 52(Suppl): 133–137. [Google Scholar]
- 43.Sangsritavong S, Combs DK, Sartori R, Armentano LE, Wiltbank MC. High feed intake increases liver blood flow and metabolism of progesterone and estradiol-17β in dairy cattle. J Dairy Sci 2002; 85: 2831–2842. [DOI] [PubMed] [Google Scholar]
- 44.Wiltbank M, Lopez H, Sartori R, Sangsritavong S, Gümen A. Changes in reproductive physiology of lactating dairy cows due to elevated steroid metabolism. Theriogenology 2006; 65: 17–29. [DOI] [PubMed] [Google Scholar]
- 45.Brigstock DR. Growth factors in the uterus: steroidal regulation and biological actions. Baillieres Clin Endocrinol Metab 1991; 5: 791–808. [DOI] [PubMed] [Google Scholar]
- 46.Paria BC, Song H, Dey SK. Implantation: molecular basis of embryo-uterine dialogue. Int J Dev Biol 2001; 45: 597–605. [PubMed] [Google Scholar]
- 47.Katagiri S, Moon YS, Yuen BH. The role for the uterine insulin-like growth factor I in early embryonic loss after superovulation in the rat. Fertil Steril 1996; 65: 426–436. [DOI] [PubMed] [Google Scholar]
- 48.Yanagawa Y, Matsuura Y, Suzuki M, Saga S, Okuyama H, Fukui D, Bando G, Nagano M, Katagiri S, Takahashi Y, Tsubota T. Accessory corpora lutea formation in pregnant Hokkaido sika deer (Cervus nippon yesoensis) investigated by examination of ovarian dynamics and steroid hormone concentrations. J Reprod Dev 2015; 61: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Honig H, Ofer L, Kaim M, Jacobi S, Shinder D, Gershon E. The effect of cooling management on blood flow to the dominant follicle and estrous cycle length at heat stress. Theriogenology 2016; 86: 626–634. [DOI] [PubMed] [Google Scholar]
- 50.Wise ME, Armstrong DV, Huber JT, Hunter R, Wiersma F. Hormonal alterations in the lactating dairy cow in response to thermal stress. J Dairy Sci 1988; 71: 2480–2485. [DOI] [PubMed] [Google Scholar]
- 51.Bridges PJ, Brusie MA, Fortune JE. Elevated temperature (heat stress) in vitro reduces androstenedione and estradiol and increases progesterone secretion by follicular cells from bovine dominant follicles. Domest Anim Endocrinol 2005; 29: 508–522. [DOI] [PubMed] [Google Scholar]
- 52.Wilson SJ, Marion RS, Spain JN, Spiers DE, Keisler DH, Lucy MC. Effects of controlled heat stress on ovarian function of dairy cattle. 1. Lactating cows. J Dairy Sci 1998; 81: 2124–2131. [DOI] [PubMed] [Google Scholar]
- 53.Wilson SJ, Kirby CJ, Koenigsfeld AT, Keisler DH, Lucy MC. Effects of controlled heat stress on ovarian function of dairy cattle. 2. Heifers. J Dairy Sci 1998; 81: 2132–2138. [DOI] [PubMed] [Google Scholar]
- 54.Madan ML, Johnson HD. Environmental heat effects on bovine luteinizing hormone. J Dairy Sci 1973; 56: 1420–1423. [DOI] [PubMed] [Google Scholar]
- 55.Gilad E, Meidan R, Berman A, Graber Y, Wolfenson D. Effect of heat stress on tonic and GnRH-induced gonadotrophin secretion in relation to concentration of oestradiol in plasma of cyclic cows. J Reprod Fertil 1993; 99: 315–321. [DOI] [PubMed] [Google Scholar]
- 56.Ford SP, Chenault JR, Echternkamp SE. Uterine blood flow of cows during the oestrous cycle and early pregnancy: effect of the conceptus on the uterine blood supply. J Reprod Fertil 1979; 56: 53–62. [DOI] [PubMed] [Google Scholar]
- 57.Bollwein H, Meyer HHD, Maierl J, Weber F, Baumgartner U, Stolla R. Transrectal Doppler sonography of uterine blood flow. Theriogenology 2000; 53: 1541–1552. [DOI] [PubMed] [Google Scholar]
- 58.Roman-Ponce H, Thatcher WW, Caton D, Barron DH, Wilcox CJ. Thermal stress effects on uterine blood flow in dairy cows. J Anim Sci 1978; 46: 175–180. [DOI] [PubMed] [Google Scholar]
- 59.De Rensis F, Scaramuzzi RJ. Heat stress and seasonal effects on reproduction in the dairy cow—a review. Theriogenology 2003; 60: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 60.Johnson J, Corbisier R, Stensgard B, Toft D. The involvement of p23, hsp90, and immunophilins in the assembly of progesterone receptor complexes. J Steroid Biochem Mol Biol 1996; 56: 31–37. [DOI] [PubMed] [Google Scholar]
- 61.Nair SC, Toran EJ, Rimerman RA, Hjermstad S, Smithgall TE, Smith DF. A pathway of multi-chaperone interactions common to diverse regulatory proteins: estrogen receptor, Fes tyrosine kinase, heat shock transcription factor Hsf1, and the aryl hydrocarbon receptor. Cell Stress Chaperones 1996; 1: 237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sabbah M, Radanyi C, Redeuilh G, Baulieu EE. The 90 kDa heat-shock protein (hsp90) modulates the binding of the oestrogen receptor to its cognate DNA. Biochem J 1996; 314: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan G, Liu K, Hao Z, Shi Z, Li H. The effects of cow-related factors on rectal temperature, respiration rate, and temperature-humidity index thresholds for lactating cows exposed to heat stress. J Therm Biol 2021; 100: 103041. [DOI] [PubMed] [Google Scholar]
- 64.Dikmen S, Cole JB, Null DJ, Hansen PJ. Heritability of rectal temperature and genetic correlations with production and reproduction traits in dairy cattle. J Dairy Sci 2012; 95: 3401–3405. [DOI] [PubMed] [Google Scholar]
- 65.Dikmen S, Cole JB, Null DJ, Hansen PJ. Genome-wide association mapping for identification of quantitative trait loci for rectal temperature during heat stress in Holstein cattle. PLoS One 2013; 8: e69202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bohmanova J, Misztal I, Tsuruta S, Norman HD, Lawlor TJ. Short communication: genotype by environment interaction due to heat stress. J Dairy Sci 2008; 91: 840–846. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Z, Krause M, Davis DL. Epidermal growth factor receptors in porcine endometrium: binding characteristics and the regulation of prostaglandin E and F2 α production. Biol Reprod 1992; 46: 932–936. [DOI] [PubMed] [Google Scholar]
- 68.Shelton K, Parkinson TJ, Hunter MG, Kelly RW, Lamming GE. Prostaglandin E-2 as a potential luteotrophic agent during early pregnancy in cattle. J Reprod Fertil 1990; 90: 11–17. [DOI] [PubMed] [Google Scholar]
- 69.Putney DJ, Gross TS, Thatcher WW. Prostaglandin secretion by endometrium of pregnant and cyclic cattle at day 17 after oestrus in response to in-vitro heat stress. J Reprod Fertil 1988; 84: 475–483. [DOI] [PubMed] [Google Scholar]
- 70.Malayer JR, Hansen PJ, Gross TS, Thatcher WW. Regulation of heat shock-induced alterations in the release of prostaglandins by the uterine endometrium of cows. Theriogenology 1990; 34: 219–230. [DOI] [PubMed] [Google Scholar]
- 71.Nabenishi H, Sugino F, Konaka R, Yamazaki A. Conception rate of Holstein and Japanese Black cattle following embryo transfer in southwestern Japan. Anim Sci J 2018; 89: 1073–1078. [DOI] [PubMed] [Google Scholar]
- 72.Putney DJ, Drost M, Thatcher WW. Influence of summer heat stress on pregnancy rates of lactating dairy cattle following embryo transfer or artificial insemination. Theriogenology 1989; 31: 765–778. [DOI] [PubMed] [Google Scholar]
- 73.Drost M, Ambrose JD, Thatcher MJ, Cantrell CK, Wolfsdorf KE, Hasler JF, Thatcher WW. Conception rates after artificial insemination or embryo transfer in lactating dairy cows during summer in Florida. Theriogenology 1999; 52: 1161–1167. [DOI] [PubMed] [Google Scholar]
- 74.Stewart BM, Block J, Morelli P, Navarette AE, Amstalden M, Bonilla L, Hansen PJ, Bilby TR. Efficacy of embryo transfer in lactating dairy cows during summer using fresh or vitrified embryos produced in vitro with sex-sorted semen. J Dairy Sci 2011; 94: 3437–3445. [DOI] [PubMed] [Google Scholar]
- 75.Vasconcelos JLM, Sá Filho OG, Justolin PLT, Morelli P, Aragon FL, Veras MB, Soriano S. Effects of postbreeding gonadotropin treatments on conception rates of lactating dairy cows subjected to timed artificial insemination or embryo transfer in a tropical environment. J Dairy Sci 2011; 94: 223–234. [DOI] [PubMed] [Google Scholar]
- 76.Moghaddam A, Karimi I, Pooyanmehr M. Effects of short-term cooling on pregnancy rate of dairy heifers under summer heat stress. Vet Res Commun 2009; 33: 567–575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.