Abstract
Autoimmunity, hyperstimulation of the immune system, can be caused by a variety of reasons. Viruses are thought to be important environmental elements that contribute to the development of autoimmune antibodies. It seems that viruses cause autoimmunity with mechanisms such as molecular mimicry, bystander activation of T cells, transient immunosuppression, and inflammation, which has also been seen in post-Covid-19 autoimmunity. Infection of respiratory epithelium by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) dysregulates the immune response, triggers both innate and acquired immunity that led to the immune system's hyperactivation, excessive cytokine secretion known as “cytokine storm,” and finally acute respiratory distress syndrome (ARDS) associated with high mortality. Any factor in the body that triggers chronic inflammation can contribute to autoimmune disease, which has been documented during the Covid-19 pandemic. It has been observed that some patients produce autoantibody and autoreactive CD4+ and CD8+ T cells, leading to the loss of self-tolerance. However, there is a scarcity of evidence defining the precise molecular interaction between the virus and the immune system to elicit autoreactivity. Here, we present a review of the relevant immunological findings in Covid-19 and the current reports of autoimmune disease associated with the disease.
Keywords: SARS-CoV-2, Covid-19, Autoimmune disease, Autoimmunity, Cytokine
1. Introduction
Beta-coronaviruses (β-CoVs) belong to the genus of coronaviruses (CoVs) and family of zoonotic viruses. After the incidence of severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) in 2003 and 2012, respectively, the newest of β-CoVs appeared in December 2019 in Wuhan city, China, was named SARS-CoV-2 [1]. SARS-CoV-2 has been identified as the cause of coronavirus disease 2019 (Covid-19), defined as an acute inflammatory infectious disease [2], [3], [4]. Over 80 million cases of Covid-19 have been confirmed universally, at a ∼2.2% case fatality rate that mostly happens in the 15–20% of the severe cases of the disease with bilateral interstitial pneumonia [5]. In these cases, acute respiratory distress syndrome (ARDS), characterized by the quick beginning of widespread inflammation in the lungs, is considered as the leading cause of mortality [4], [6], [7], [8], [9], [10].
Both innate and acquired immunity play a crucial role in response to SARS-CoV-2 and a disease progression [11]. It has been shown that Covid-19 disrupts normal protective antiviral immunity, particularly in patients with comorbidities, old age, and specific genetic background by causing lymphopenia (CD4+ T, CD8+ T, NK, and B cell), reduction of regulatory T cells (Treg), overactivation of T cell, overproduction of pro-inflammatory cytokines (IL-2R, IL-1, IL-6, IL-8, IL-10, IL-17, and TNF-α), exhaustion of T cell, and an increase in antibodies [12], [13], [14]. Studies show that defects in immune tolerance and homeostasis mechanisms lead to inappropriate activation of the interferon pathway and autoinflammation [15], [16]. It is now broadly acknowledged that autoimmune disorders are linked to autoinflammatory conditions [17], [18].
Although the precise etiology of such complicated diseases remains unclear [19], various factors including genetic susceptibility [20], epigenetic effects, the environmental stimuli such as microbial, fungal, parasitic, and viral pathogens can predispose a person to autoimmune disorders [21], [22], [23], [24], [25]. Viral infections can cause intolerance by different mechanisms, including molecular mimicry (cross-reacting epitope between pathogen-derived and the self-antigens), bystander killing (virus-specific CTLs migrating to the target tissues and exerting cytotoxicity), epitope spreading (polyclonal activation due to the constant presence of viral antigens driving immune-mediated injury), clearance deficiency and viral persistence that can increase the risk of autoreactivity. These factors contribute to autoinflammatory reactions and exacerbations of autoimmune disease [16], [26], [27], [28], [29]. Like as many viral infections [30], SARS-CoV-2 can lead to various autoimmune symptoms [31]. By entering the upper respiratory tract, virus multiplies in the respiratory mucosa's epithelial cells. The immune system eradicates the virus. Otherwise, the virus reaches the lungs with the possibility of over-activation of the innate and acquired immune system, followed by the entry of antibodies into the bloodstream. The antibodies produced against the virus can be reacted with the proteins expressed on human cells, causing systemic manifestations [32], [33]. Several autoimmune disorders such as Immune thrombocytopenic purpura (ITP), Guillian-Barrė syndrome (GBS), Miller Fisher syndrome (MFS), Kawasaki-like disease in children, etc. have been recorded in connection with COVID-19 [34], [35], [36], [37], [38].
Given the increasing mystery of SARS-COV.2 virus and its essential relationship to the autoimmune complications, we reviewed current advances in autoimmune conditions following COVID-19 disease.
2. Covid-19 and autoimmunity
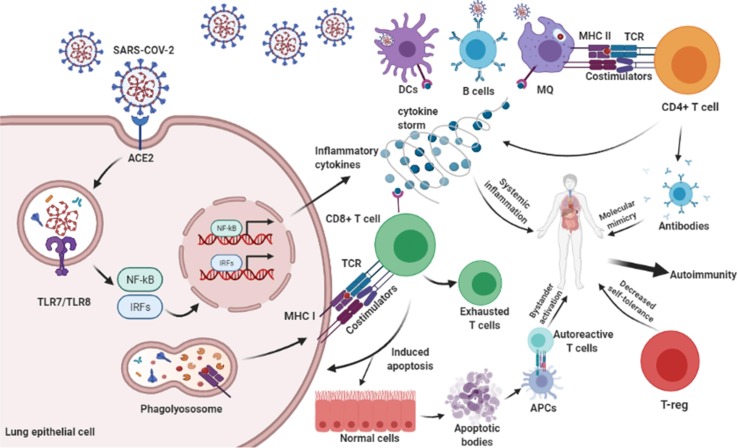
Entry and replication of SARS-CoV-2 in a host cell are mediated by the interaction of angiotensin-converting enzyme 2 (ACE-2) host receptor, expressed in respiratory epithelial cells, with the receptor-binding domain (RBD) region of virus Spike glycoprotein (S) [39]. Then, antigen-presenting cells (APCs) such as dendritic cells, macrophages, and B cells can endocytose-released viral particles and present peptide antigens to CD4+ T cells by MHC class II. Activated CD4+ T cells, as a central mediator, are involved in pro-inflammatory cytokine secretion, macrophage activation, CD8+ T cells priming, promoting B cells to antibody production and targeting the SARS-CoV-2 [40], [41]. The antibody often interacts misleadingly with the host surface proteins in a phenomenon called “Molecular mimicry” (Fig. 1 ) [42]. It usually happens when microbial peptides share the same antigenic sequences with host self-proteins [43]. Several studies have suggested that molecular mimicry may play an important role in autoimmunity generation in Covid-19 [44], [45]. It has been shown that hexapeptide sequences of SARS-CoV-2 (nucleocapsid (N), membrane (M), ORF7b, ORF7a, ORF71a, ORF71b, especially S glycoprotein) shared a similar sequence with human proteins that can cause a wide range of complications from pulmonary disorders, cardiac and vascular failure, neurological disorders to autoinflammatory syndrome and ADs [46], [47]. Hexapeptides of N and surface glycoprotein of SARS-CoV-2 have shown notable similarity with three human proteins, namely DAB1, AIFM, and SURF1, which are involved in neurons development and mitochondrial metabolism [48]. Angileri et al. revealed the cross-reactivity of SARS-COV-2 amino acid sequences with human proteins including OR7D4, PARP9, SLC12A6 on the plasma membrane of olfactory neurons, the cytoplasmic matrix of B cell and macrophage, endothelial cells of various organs, respectively, leading to a malfunction in target organs [44]. Also, Angileri et al. found Ankyrin 1 (ANK-1) amino acid sequence (323-LLLQY-327) similar to S glycoprotein of SARS-CoV-2 (752-LLLQY-756), describing autoimmune hemolytic anemia associated with SARS-CoV-2 disease [49]. Furthermore, shock protein (HSP)/chaperones with a high grade of similarity in all organisms can be targeted by the immune response, probably due to molecular mimicry. Cellular stress, followed by decreases in oxygen levels by SARS-CoV-2-induced pneumonia, can induce molecular chaperones to migrate to the plasma-cell membrane, probably after being modified and, consequently, can be recognized as foreign antigens by circulating antibodies made against cross-reactive microbial antigens, thus, creates the conditions for autoimmunity [32], [45]. Additionally, sequence analysis of the 41 human proteins associated with acute and chronic immune-mediated neuropathies showed that SARS-CoV-2 shares two immunologically relevant hexapeptides (KDKKKK and EIPKEE) with the human heat shock proteins 90 (HSP90B and HSP90B2) and 60 (HSP60), respectively, which are associated with Guillain-Barré syndrome, myasthenia gravis, and multiple sclerosis (MS) [42]. Vojdani et al. tested five SARS-CoV-2 IgG and IgM antibody positive blood samples and found that the monoclonal antibodies against S and N proteins of SARS-CoV-2 in three samples have strong reactions with human tissue antigens, including transglutaminase 3 (tTG3), transglutaminase 2 (tTG2), ENA, myelin basic protein (MBP), mitochondria, nuclear antigen (NA), α-myosin, thyroid peroxidase (TPO), collagen, claudin 5 + 6, and S100B [50]. Furthermore, in the clinical context, it is worth noting that SARS-CoV-2 shares 6 minimum immunological determinants (KTVLK, TPEEH, RETMS, PFVVS, GLEAP, ICLLQ) with the Kawasaki antigen Inositol-triphosphate 3-kinase [51]. A common heptapeptide sequence (LDKYFKN/Follistatin-related protein 1) between the SARS-CoV-2 spike and human proteins is found in the serum of rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, and systemic sclerosis patients [51], [52]. These outcomes highlight the cross-reaction of SARS-CoV-2 proteins and tissue antigens as a potential mechanism to onset autoimmunity disorders in the consequence of SARS-CoV-2 infection.
Fig. 1.
The SARS-CoV-2 proliferates by binding to ACE-2 receptor on the surface of host epithelial cell. Intracellular signaling pathways are activated and produce inflammatory cytokines such as TNF-α, IFN-β, IL-6, IL-1β, IL-17, and IL-18 that may cause autoimmunity via systemic inflammation. APCs process the antigens and present them to TCD4+ cells. Activated TCD4+ cells have a role in inflammatory cytokine secretion, MQ activation, and antibody formation. Antibody against the viral antigen can recognize host tissue antigens that cause autoimmunity. Also, TCD8+ cells can detect viral antigens via TCR-MHC I and cause apoptosis in virus-infected and uninfected cells mainly via perforin-granzyme and Fas and its ligand. This procedure is named bystander activation and causes autoimmunity. The apoptotic bodies which contain self-antigens are presented to autoreactive T cells and cause autoimmunity. Given that autoimmunity is driven by recognition of self-antigen and CD8 T-cell exhaustion dependent on chronic antigen stimulation, T-cell exhaustion could facilitate the retention of antigen-specific T cells in the repertoire under chronic stimulation. Also, the reduction of T-reg by the SARS-CoV-2 reduces self-tolerance and leads to autoimmunity.
Studies show that despite lymphopenia during SARS-CoV-2 infection, CD4+ and CD8+ T cells are active with high proportions of HLA-DR (CD3), CD 28, CD38, and increase the expression of the proliferation marker Ki67 [53], [54], [55]. CD8+ T-cells showed a notable ability to clearance SARS-CoV-2 infected cells with long-lasting immunity [56]. Bystander killing in the elimination of infected cells is another probable mechanism to promote autoimmunity followed by COVID-19 (Fig. 1). Also, during the clonal expansion of reactive T-cells to an infection, a significant proportion of self-reactive T-cells may be increased in Covid-19 [57].
In the pathogenesis of autoimmune disease, cytokines may also play an essential role. SARS-CoV-2 infection stimulates the secretion of serum cytokines majorly TNF-α, IFN-β, IL-6, IL-1β, IL-17, and IL-18 [53], leading to an aberrant innate and acquired immune response [58], [59] and loss of tolerance to self-antigens, ADs, and deterioration patients with ADs [60]. CD4+ T-regulatory cells suppress the immunity at the end of an immune response, control immune homeostasis via inhibiting the pro-inflammatory function of effector T cells [61]. They are identified by FOXPE expression as a specific transcription factor, and also, high levels of variable inhibitory molecules include CTLA-4, GITR, CD39, and CD73. T-regs can also be characterized based on the secretion of immunosuppressive cytokines that can prevent autoimmune phenomena, including TGF-beta, IL-10, and IL-35 [62]. Studies indicate the diminished and dysfunctional T-regs in severe Covid-19 patients [63], [64]. Besides, a proportion of Treg will shift their phenotype to Th17 cells under an inflammatory environment, which will enhance the inflammatory response and trigger neutrophils [65], [66]. So, the balance between these two cell populations is a hallmark factor to immune-homeostasis, and disturbance of this balance is known to be associated with ADs pathogenesis [67]. Additionally, IL-6, which is the most prominent cytokine produced in Covid-19 patients, inhibits CD4+ CD25+ FOXP3 T-reg cells, therefore attributing to the development of various ADs [68]. Increased expression of inhibitory receptors (CD57, PD-1, and Tim-3) in CD8+ T cell is associated with prolonged infections, and T cell exhaustion has been observed in patients with Covid-19 [69], [70], [71]. The binding of PD-L1 to PD-1 on the surface of CD8 T cell inhibits the proliferation of CD8+ T cell, inflammation, results in apoptosis of infiltrating lymphocytes, diminished cytotoxicity, and prevents autoimmune tissue damaging [72]. T-cell exhaustion is a characteristic of chronic illness that prevents the immune response and encourages viral retention [73]. On the other hand, auto-reactive immune cells followed by a persistent chronic inflammatory response exert major autoimmune diseases [43]. Cañas CA et al have put forward a hypothesis that the development of post-COVID-19 autoimmunity may be a consequence of transient immunosuppression of both innate and acquired immunity leading to a lack of self-tolerance and an insufficient process of immune reconstitution [74]. It seems that the immune response may not be well regulated in some infected individuals, paving the way for the development of secondary autoimmunity to SARS-CoV-2, which is summarized in Table 1.
2.1. Post-SARS-CoV-2 Idiopathic thrombocytopenic purpura (ITP)
Idiopathic thrombocytopenic purpura (ITP) is a hematological disorder affecting the total number of blood platelets and thrombocytopenia (platelet count < 100 × 109/L, normal range 150–450 × 109/L) [75]. Two-thirds of ITP cases can be secondary to infections that induce autoantibodies cross-reactive to glycoproteins IIb-IIIa or Ib on platelets and megakaryocytes, causing reduced longevity of circulating platelets and impaired platelet production [76]. CD4+ T helper cells reacting with platelet antigens are correlated with B cells' differentiation into autoantibody secreting plasma cells in ITP. Opsonization and phagocytosis of platelet by splenic macrophages, dendritic cells induce platelet apoptosis. Also, disrupted function and decreased number of Treg cells, cytotoxic T cells, and Th2 that are important to the immune homeostasis contribute to the development of ITP [77].
In a case series study, Bomhof et al. showed that the ITP occurred not only during active COVID-19 infection but also up to 10 days after the reduction of the clinical symptoms of COVID-19 [78]. In a case study report, a 48-year-old man with a history of type 2 diabetes, obesity, and obstructive sleep apnea was diagnosed with Covid-19 according to a chest X-ray and a reverse transcription-polymerase chain reaction (RT-PCR) test of a nasopharyngeal swab. CBC results on the ninth day of hospitalization revealed a thrombocytopenia (96,000/mm3) with macroscopic hematuria and minor bleeding from oral mucosa. A further decline was observed on the 12th day (2,000/mm3) with petechial bleeding appeared on the trunk. Based on the clinical and laboratory evaluations with the exception of other possible causes of thrombocytopenia, including DIC, bacterial sepsis, and medication, COVID-19-associated ITP was suspected. The direct Coombs test was positive for IgG; the indirect coombs test was negative, showing antibodies against red blood cells. Intravenous dexamethasone and IVIG therapy at the end of the corticosteroid therapy improved platelet count and oxygenation [79].
Nesr et al. show the role of COVID-19 in the ITP flare. They reported a 34-year-old pregnant woman, known to have ITP since 2013, diagnosed with mild Covid-19 with a 1-day history of dry cough, fever, petechiae, and gum bleeding. Initial CBC shows a platelet count of 13 × 109/L on admission. The normal kidney and liver functions, and the absence of red cell fragments, excluded thrombotic microangiopathies. One day after IVIG and oral prednisolone therapy, the platelet count reached 34 × 109/L, and the patient reported improvement in gum bleeding and no new petechiae elevated platelet and response to IVIG and steroids make a diagnosis of gestational thrombocytopenia unlikely, and the diagnosis of ITP flare-up was instated [80].
Artru et al. reported a 38-year- old COVID-19 obese patient with decompensated cirrhosis and IgA nephropathy that was treated with hydroxychloroquine and co-amoxicillin during the first four days and showed a rapid decrease of platelet count to 1 × 109/L and severe epistaxis one day after Covid-19 treatment. A blood smear confirmed severe thrombocytopenia and did not show any schistocytes. Other causes of thrombocytopenia were excluded, and SARS-CoV-2-related ITP diagnosis was approved. Although in this case, the autoantibody level was not measured, IVIG and high-dose dexamethasone administration resulted in rapid platelet counts and epistaxis [81]. The response to IVIG and corticosteroids therapy is a fair indicator of immune origin in thrombocytopenia and has a beneficial effect on excessive SRAS-CoV-2 inflammatory reactions.
2.2. Post-SARS-CoV-2 Graves’ disease (GD)
Graves' disease (GD) is an autoimmune hyperthyroidismis that onsets in the consequence of immune imbalance and the development of auto-antibodies against the thyrotropin receptor (TSHR) [82]. Other antibodies, including thyroid peroxidase (TPO) and thyroglobulin (TG) antibodies, may be significantly elevated but are not specific to GD [82]. In an active GD, T-lymphocytes are sensitized to thyroid antigens and promote the B lymphocytes to produce antibodies. Also, T-lymphocyte suppressor function is diminished in GD [83].
Studies show that SARS-CoV-2 can be directly involved in the pituitary-thyroid axis via infecting target cells, including thyroid cells on thyroid follicular and pituitary cells, or indirectly through systemic inflammation in immune responses to the virus [84]. Also, the increased cytokine and hyperactivation of Th1/Th17 of immune responses to virus infection may have a role in the pathogenesis of thyroid disorders following SARS-CoV-2 [85]. Infection of the thyroid gland and increased concentrations of interleukins, especially IL-6, lead to thyrotoxicosis that was reported in SARS-CoV-2-related subacute thyroiditis [86]. Mateu-Salat et al describe two autoimmune hyperthyroidism cases identified after SARS-CoV-2 infection in Italy. One of them had a previous history of GD with remission for more than 30 years, and another was known to have no history of thyroid disease. These hyperthyroidism cases were diagnosed 1 and 2 months after the clinical onset of Covid-19, respectively. TSHR, TPO, and TG antibodies were positive for both cases. Administration of thiamazole and propranolol improves the symptoms and thyroid function in both cases [87].
2.3. Post-SARS-CoV-2 multiple sclerosis (MS)
Multiple sclerosis (MS) is a central nervous system (CNS) autoimmune disease that induces serious neurological impairment. Demyelination, inflammation, and axonal loss in the CNS are the pathological conditions characterized in MS [88], [89], [90]. Although the precise mechanism of MS has been remaining unclear, activation of Toll-like receptors (TLRs) on microglia/macrophages and dendritic cells by viral particles can disrupt self-tolerance and promote auto-immunity [91]. The autoreactive CD4+ T lymphocytes in MS patients are related to an upregulation of adhesion molecules and chemokines on endothelial cells and immune cells, pro-inflammatory cytokines, matrix metalloproteinases (MMPs), which contribute to increased permeability and crossing of antibodies in the blood–brain barrier (BBB) [92]. Nerve demyelination does not seem to occur directly by virus particles, but can be caused by active T lymphocytes and inflammatory mediators. Several evidence provided the association between viral infections and demyelinating disease progression. For example, infection with the Epstein-Barr virus is considered a significant risk factor for MS development and the viral respiratory infections may also exacerbate MS [93], [94].
It has been suggested that SARS-CoV-2 can induce brain and spine demyelinating lesions. Palao et al reported the first case of CNS demyelinating shortly after SARS-CoV-2 manifestation. The case was a 29-year-old woman with a history of asthma and rhino-conjunctivitis who had not previously experienced any neurological symptoms. SARS-CoV-2 PCR analysis in nasopharyngeal and CSF was negative, whereas immunological testing was positive for IgM and IgG, indicating a previous infection. The MRI showed inflammation in the right optic nerve and demyelinating lesions in the CNS. Laboratory results showed the presence of oligoclonal IgG bands in the CSF and no detectable anti-AQP4-IgG and anti-MOG antibodies in serum samples. The clinical and serological studies in blood and CSF met the criteria for MS and ruled out other etiologies. The improvement of ocular pain and visual acuity patient was observed after intravenous methylprednisolone therapy. The researchers suggested that SARS-CoV-2 acted as an accelerating factor for MS manifestation [95].
Neuromyelitis optica spectrum disorder (NMOSD) is a rare severe demyelinating autoimmune inflammatory disorder of the CNS, which was thought to be a variant of MS, predominantly affecting the optic nerves and spinal cord [96]. De Ruijter et al. reported a case of a 15-year-old boy who had been ill for about ten days with fever, nausea, and a cough followed by developed subacute visual loss at the hospital presentation. The presence of anti-MOG-IgG in serum and optic nerve swelling was confirmed following a supposed SARS-CoV-2 infection. Serum anti-AQP4-IgG was negative, and cerebrospinal fluid analysis showed no oligoclonal bands. He was diagnosed with NMOSD and was treated with intravenous methylprednisolone, leading to a significant resolution of symptoms [97].
2.4. Post-SARS-CoV-2 Guillain–Barré syndrome (GBS)
Guillain Barré syndrome (GBS) is a worsening neuromuscular disorder that tends to destroy the nerves and leads to muscle paralysis [98]. The precise cause of GBS is unknown, but post-respiratory or gastrointestinal infection, and rarely recent vaccination can develop the disease [99], [100]. Immunological research indicates that GBS pathogenesis requires both cell-mediated and humoral immunity. The production of cross-reactive nerve ganglioside antibodies like anti-GM1 and anti-GD1a can cause complement activation and membrane attack complex formation at the surface of nerve cells that have increased myelin invasions by macrophages [101].
From March 2020 to April 2020, the reported number of GBS cases was fivefolds higher than the number of reported cases in the last three years [102]. The GBS caused by SARS-CoV-2 was found in a case study in Italy [103]. Korem et al. reported a 58-year-old woman with three years history of cervical spondylosis and disc herniation. The COVID-19 test was positive, and she was treated with azithromycin at home. Two weeks later, after resolving her upper respiratory tract symptoms, she developed symmetric ascending quadriparesis and paresthesia. The diagnosis of GBS was made through cerebrospinal fluid analysis, and she was successfully treated with IVIG [104]. Hirayama et al reported a 54-year-old female patient with a history of asthma who showed numbness and weakness in her limbs twenty days after the positive results for SARS-CoV-2. The patient was diagnosed with GBS based on neurological and electrophysiological findings. Blood tests showed no abnormalities in glucose levels and no results suggestive of collagen disease, thyroid disease, or vitamin abnormalities [105], [106]. Although the role of antibodies in developing GBS remains unclear, antibody-independent pathways such as hyperinflammation following macrophage activation and vascular endothelial damage of the brain may lead to neurological symptoms in patients with SARS-CoV-2 infection [107].
2.5. Post-SARS-CoV-2 myasthenia gravis (MG)
Myasthenia gravis (MG) is an autoimmune disorder characterized by fluctuating fatigability and muscle weakness. MG can appear with extraocular muscle involvement as the initial symptom, usually progressing to other bulbar muscles and limb muscle groups, resulting in generalized MG. It may be associated with thymoma, and anti-neuromuscular junction autoantibodies, or it may appear without autoantibodies [108]. Thymic impairment and peripheral self-tolerance mechanisms followed by different factors such as viral infection promote CD4+ T cell-mediated B cell activation and synthesis of pathogenic high-affinity autoantibodies. The autoantibodies bind to the acetylcholine receptor (AchR) and lead to impaired neuromuscular transmission and clinical manifestation of the disease [109], [110].
SARS-CoV-2 may trigger an MG crisis [111]. Restivo et al. describe three patients (64- and 68-year-old men and a 71–year–old woman) without previous neurologic or autoimmune disorders. They were diagnosed with myasthenia gravis 5–7 days after the onset of COVID-19 symptoms such as fever and cough. RT-PCR testing for COVID-19 of two patients showed a positive result. The neurologic examination, differential diagnosis, elevated concentration of AChR antibodies (mean = 28.6 pmol/L; normal value, <0.4 pmol/L) confirmed the MG. The patients were treated with the administration of pyridostigmine bromide and prednisone, and IVIG. A patient with plasmapheresis and hydroxychloroquine treatment improved and was extubated [112]. Huber et al. reported a case of 21-year-old female who, four weeks after mild respiratory symptoms, showed subacute ocular manifestation, no pyrexia, temperate exhaustion with the pain in limbs and head. At the same time, a negative oropharyngeal swab testing suggested no acute SARS-CoV-2 infection, while SARS-CoV-2 IgA and IgG were positive in serum and negative in CSF. There was no sign of acute infection in further analysis and additional examinations of thoracic X-ray and MRI did not show a thymoma, and inflammatory modifications in lung parenchyma. Additional antibodies associated with myasthenia (Agrin, LRP4, MuSK, Titin) were unremarkable. The MG syndrome was easily treated with IVIg and oral pyridostigmine [113]. Furthermore, Sriwastava et al. described a case of ocular MG-secondary to Covid-19 infection based on laboratory investigations and electrodiagnostic testing in a 65-year-old woman who demonstrated neuromuscular manifestations two weeks after positive results of the COVID-19 test. Laboratory results revealed elevated inflammatory markers (IL-6, serum CRP, ferritin, fibrinogen, and D-dimer) and AchR antibody [114]. According to the reviewed studies, the MG manifestation can be a particular complication of Covid-19 patients. However, further studies are needed to make a definitive statement and to find an exact link between the pathogenesis of Covid and myasthenia gravis.
2.6. Post-SARS-CoV-2 systemic lupus erythematosus (SLE)
Systemic lupus erythematosus (SLE) is a clinically heterogeneous autoimmune disorder that results from a multifaceted interaction of the immune system, genetics, and environmental factors [19], [115], [116]. SLE pathogenesis comprises the inherent and adaptive immune systems, autoantibody formation, complement system activation, cytokine dysregulation (especially interferons of type I) and disrupted apoptotic clearance [117], [118], [119].
Literature indicates that Covid-19 could possibly be correlated with SLE. Slimani et al. demonstrated the onset of SLE with antiphospholipid syndrome (APLS) in a formerly healthy 23-year-old woman who later developed a skin rash on the trunk 13 days after the Covid-19 diagnosis. SARS-CoV-2 RT-PCR test in a nasopharyngeal swab sample and a fresh skin biopsy sample was positive and negative, respectively. Serologic testing showed the presence of anti-nuclear antibodies (ANAs), anti-double-stranded DNA antibodies (anti-dsDNA), anti. Steroid therapy had been launched; however, the patient was dead resulting in poor respiratory outcome [120]. Cardoso et al present a new case of SLE concomitantly who was an 18-year-old female with Covid-19 and the development of antiphospholipid antibodies. In this case, SLE was diagnosed based on clinical signs, laboratory, and serology tests within a week after the onset of Covid-19 symptoms. She received ceftazidime, vancomycin, azithromycin, hydroxychloroquine, steroids and underwent plasmapheresis for a short time. She also evolved severe anemia and thrombocytopenia, which was resolved with supportive care and transfusions. She then developed thrombosis, raising concerns for antiphospholipid syndrome, and was started on anticoagulation. Unfortunately, on the seventeenth day of her hospitalization, she died of a cardiac arrest [121].
3. Conclusion
Studies showed the gradual recognition of complications of SARS-CoV-2 infection. Although the respiratory system is affected mainly by the SARS-CoV-2, other organs, including gastrointestinal, hematopoietic, genitourinary, nervous, and cardiovascular systems, may even be affected without respiratory symptoms [122], [123], [124], [125]. COVID-19-related complications are either directly caused by the virus attack or indirectly due to the antiviral immune response [126], [127]. An inadequate immune response can lead to the spread of infection, and a strong immune response can lead to serious immunopathologic reactions, in some cases contributing to autoimmunity [128]. The relationship between autoimmunity and viral infection is often clarified by molecular mimicry, bystander activation, and viral persistence [129].
The SARS-CoV-2 infection could cause or simulate a type of organ-specific autoimmunity in predisposed patients, according to the serological, radiological, and histopathological similarities between Covid-19-associated ARDS and lung manifestations of connective tissue disease in a study [130]. Production of autoantibody, higher levels of IL-6 and TNF-α, increased inflammatory phenotypes of CD4+ T cells including Th1, Th17, and Th22, increased numbers of CD8+ T cells, and reduced numbers of Treg cells can contribute to the autoimmunity [131]. In addition to virus infections, vaccination can also be a cause of autoimmunity, as the increased risk of Guillain Barre syndrome following influenza vaccine was reported [132]. The possible mechanisms that vaccines can induce autoimmune reactions are molecular mimicry and bystander activation [133]. Understanding molecular mimicry about SARS-CoV-2 peptide sequences and comprehensive human proteome researches can be effective in producing safe and high-quality vaccines [134]. Such studies are necessary to reduce the risk of acute autoimmune reactions to vaccination and the development of autoimmune diseases following COVID-19 disease.
Ethical statement
The current study is a review article and the manuscript complies with the Ethical Rules applicable to this journal.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors appreciate the cooperation of Mashhad University of Medical Sciences.
Data availability
The dataset(s) supporting the conclusions of this article is (are) included within the article (and its additional file(s)).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cyto.2022.155873.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Tu H., Tu S., Gao S., Shao A., Sheng J. Current epidemiological and clinical features of COVID-19; a global perspective from China. J. Infect. 2020 doi: 10.1016/j.jinf.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ge H., Wang X., Yuan X., Xiao G., Wang C., Deng T., Yuan Q., Xiao X. The epidemiology and clinical information about COVID-19. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39(6):1011–1019. doi: 10.1007/s10096-020-03874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarda S.R., Tekale S.U., Kótai L., Domb A.J., Pawar R.P. COVID-19: A global pandemic. Eur. Chem. Bull. 2020;9(8):266–272. [Google Scholar]
- 4.Raheem R., Alsayed R., Yousif E., Hairunisa N. Coronavirus new variants: the mutations cause and the effect on the treatment and vaccination: Coronavirus new Variants: effect and treatments. Baghdad J. Biochem. Appl. Biol. Sci. 2021;2(02):70–78. [Google Scholar]
- 5.Jain V., Yuan J.-M. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int. J. Public Health. 2020;65(5):533–546. doi: 10.1007/s00038-020-01390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan E., Beitler J.R., Brochard L., Calfee C.S., Ferguson N.D., Slutsky A.S., Brodie D. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir. Med. 2020;8(8):816–821. doi: 10.1016/S2213-2600(20)30304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou J., Li Z., Meng H., Chang Y.-C., Peng N.-H., Wei B. Chinese parental awareness of Children's COVID-19 protective measures. Am. J. Health Behav. 2021;45(4):657–664. doi: 10.5993/AJHB.45.4.5. [DOI] [PubMed] [Google Scholar]
- 8.Weaver R.H., Jackson A., Lanigan J., Power T.G., Anderson A., Cox A.E., Eddy L., Parker L., Sano Y., Weybright E. Health behaviors at the onset of the COVID-19 pandemic. Am. J. Health Behav. 2021;45(1):44–61. doi: 10.5993/AJHB.45.1.4. [DOI] [PubMed] [Google Scholar]
- 9.Suleiman A., Rafaa T., Alrawi A., Dawood M. The impact of ACE2 genetic polymorphisms (rs2106809 and rs2074192) on gender susceptibility to COVID-19 infection and recovery: A systematic review. Baghdad J. Biochem. Appl. Biol. Sci. 2021;2(03):167–180. [Google Scholar]
- 10.Jafari M., Kolahdooz H., Mahmoudi M., Azarnaminy A.F., Mobasheri L., Esmaeili S.-A. The impact of lymphoid memory cells in different ages of COVID-19 patients. Gene Rep. 2022;26:101503. doi: 10.1016/j.genrep.2022.101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jesenak M., Brndiarova M., Urbancikova I., Rennerova Z., Vojtkova J., Bobcakova A., et al. Immune parameters and COVID-19 infection-associations with clinical severity and diseases prognosis. Front. Cell. Infect. Microbiol. 2020;10:364. doi: 10.3389/fcimb.2020.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L., Liu S., Liu J., Zhang Z., Wan X., Huang B., Chen Y., Zhang Y. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct. Target. Therapy. 2020;5(1) doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J., Li S., Liu J., Liang B., Wang X., Wang H., Li W., Tong Q., Yi J., Zhao L., Xiong L., Guo C., Tian J., Luo J., Yao J., Pang R., Shen H., Peng C., Liu T., Zhang Q., Wu J., Xu L., Lu S., Wang B., Weng Z., Han C., Zhu H., Zhou R., Zhou H., Chen X., Ye P., Zhu B., Wang L.u., Zhou W., He S., He Y., Jie S., Wei P., Zhang J., Lu Y., Wang W., Zhang L., Li L., Zhou F., Wang J., Dittmer U., Lu M., Hu Y., Yang D., Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L., Xu L., Lin C. T cell response in patients with COVID-19. Blood Sci. 2020;2(3):76–78. doi: 10.1097/BS9.0000000000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo B., Lenardo M.J. Editorial overview: Autoimmunity: New genomics approaches are improving our understanding of autoimmunity. Curr. Opin. Immunol. 2017;49:iv–vi. doi: 10.1016/j.coi.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smatti M.K., Cyprian F.S., Nasrallah G.K., Al Thani A.A., Almishal R.O., Yassine H.M. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019;11(8):762. doi: 10.3390/v11080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frizinsky S., Haj-Yahia S., Machnes Maayan D., Lifshitz Y., Maoz-Segal R., Offengenden I., et al. The innate immune perspective of autoimmune and autoinflammatory conditions. Rheumatology. 2019;58(Supplement_6):vi1–vi8. doi: 10.1093/rheumatology/kez387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suksatan W., Chupradit S., Yumashev A.V., Ravali S., Shalaby M.N., Mustafa Y.F., et al. Immunotherapy of multisystem inflammatory syndrome in children (MIS-C) following COVID-19 through mesenchymal stem cells. Int. Immunopharmacol. 2021;101:108217. doi: 10.1016/j.intimp.2021.108217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Momtazi-Borojeni A.A., Haftcheshmeh S.M., Esmaeili S.-A., Johnston T.P., Abdollahi E., Sahebkar A. Curcumin: A natural modulator of immune cells in systemic lupus erythematosus. Autoimmunity Rev. 2018;17(2):125–135. doi: 10.1016/j.autrev.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Esmaeili A., Rabe S.Z.T., Mahmoudi M., Rastin M. Frequencies of HLA-A, B and DRB1 alleles in a large normal population living in the city of Mashhad, Northeastern Iran. Iran. J. Basic Med. Sci. 2017;20(8):940. doi: 10.22038/IJBMS.2017.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parks C., Miller F., Pollard K., Selmi C., Germolec D., Joyce K., Rose N., Humble M. Expert panel workshop consensus statement on the role of the environment in the development of autoimmune disease. Int. J. Mol. Sci. 2014;15(8):14269–14297. doi: 10.3390/ijms150814269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedrich C.M., Surace A.E. The role of epigenetics in autoimmune/inflammatory disease. Front. Immunol. 2019;10:1525. doi: 10.3389/fimmu.2019.01525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper G.S., Miller F.W., Pandey J.P. The role of genetic factors in autoimmune disease: implications for environmental research. Environ. Health Perspect. 1999;107(suppl 5):693–700. doi: 10.1289/ehp.99107s5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esmaeili S.-A., Mahmoudi M., Rezaieyazdi Z., Sahebari M., Tabasi N., Sahebkar A., Rastin M. Generation of tolerogenic dendritic cells using Lactobacillus rhamnosus and Lactobacillus delbrueckii as tolerogenic probiotics. J. Cell. Biochem. 2018;119(9):7865–7872. doi: 10.1002/jcb.27203. [DOI] [PubMed] [Google Scholar]
- 25.Yazdanpanah E., Mahmoudi M., Sahebari M., Rezaieyazdi Z., Esmaeili S.-A., Tabasi N., Jaberi S., Sahebkar A., Rastin M. Vitamin D3 alters the expression of toll-like receptors in peripheral blood mononuclear cells of patients with systemic lupus erythematosus. J. Cell. Biochem. 2017;118(12):4831–4835. doi: 10.1002/jcb.26155. [DOI] [PubMed] [Google Scholar]
- 26.Muñoz L.E., Lauber K., Schiller M., Manfredi A.A., Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat. Rev. Rheumatol. 2010;6(5):280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan M.J., Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J. Immunol. 2012;189(6):2689–2695. doi: 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khorasani S., Mahmoudi M., Kalantari M.R., Lavi Arab F., Esmaeili S.-A., Mardani F., Tabasi N., Rastin M. Amelioration of regulatory T cells by Lactobacillus delbrueckii and Lactobacillus rhamnosus in pristane-induced lupus mice model. J. Cell. Physiol. 2019;234(6):9778–9786. doi: 10.1002/jcp.27663. [DOI] [PubMed] [Google Scholar]
- 29.Mardani F., Mahmoudi M., Esmaeili S.A., Khorasani S., Tabasi N., Rastin M. In vivo study: Th1–Th17 reduction in pristane-induced systemic lupus erythematosus mice after treatment with tolerogenic Lactobacillus probiotics. J. Cell. Physiol. 2019;234(1):642–649. doi: 10.1002/jcp.26819. [DOI] [PubMed] [Google Scholar]
- 30.Karimovna T.N. Predictive criteria for the severity of associated glomerulonephritis virus in children. Central Asian J. Med. Natural Sci. 2021;2(3):295–299. [Google Scholar]
- 31.Shah S., Danda D., Kavadichanda C., Das S., Adarsh M.B., Negi V.S. Autoimmune and rheumatic musculoskeletal diseases as a consequence of SARS-CoV-2 infection and its treatment. Rheumatol. Int. 2020;40(10):1539–1554. doi: 10.1007/s00296-020-04639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cappello F., Marino Gammazza A., Dieli F., Conway de Macario E., Macario A.JL. Does SARS-CoV-2 trigger stress-induced autoimmunity by molecular mimicry? A hypothesis. J. Clin. Med. 2020;9(7):2038. doi: 10.3390/jcm9072038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sessa F., Bertozzi G., Cipolloni L., Baldari B., Cantatore S., D’Errico S., Di Mizio G., Asmundo A., Castorina S., Salerno M., Pomara C. Clinical-forensic autopsy findings to defeat COVID-19 disease: a literature review. J. Clin. Med. 2020;9(7):2026. doi: 10.3390/jcm9072026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsao H.S., Chason H.M., Fearon D.M. Immune thrombocytopenia (ITP) in a SARS-CoV-2 positive pediatric patient. Pediatrics. 2020 doi: 10.1542/peds.2020-1419. [DOI] [PubMed] [Google Scholar]
- 35.Gutiérrez-Ortiz C., Méndez-Guerrero A., Rodrigo-Rey S., San Pedro-Murillo E., Bermejo-Guerrero L., Gordo-Mañas R., de Aragón-Gómez F., Benito-León J. Miller Fisher Syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95(5):e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 36.Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., Bonanomi E., D'Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. The Lancet. 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hummig W., Cruz M.M. Bruxism as a clinical indicator of mental ilness: lessons from the COVID-19 to the future! Archiv. Clin. Psychiatry (São Paulo) 2021;48:127. [Google Scholar]
- 39.Lee K.-Y., Rhim J.-W., Kang J.-H. Immunopathogenesis of COVID-19 and early immunomodulators. Clin. Exp. Pediatr. 2020;63(7):239–250. doi: 10.3345/cep.2020.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., et al. Immunology of COVID-19: current state of the science. Immunity. 2020 doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Razeghian E., Margiana R., Chupradit S., Bokov D.O., Abdelbasset W.K., Marofi F., Shariatzadeh S., Tosan F., Jarahian M. Mesenchymal stem/stromal cells as a vehicle for cytokine delivery: an emerging approach for tumor immunotherapy. Front. Med. 2021;8 doi: 10.3389/fmed.2021.721174. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Lucchese G., Flöel A. SARS-CoV-2 and Guillain-Barré syndrome: molecular mimicry with human heat shock proteins as potential pathogenic mechanism. Cell Stress Chaperones. 2020;25(5):731–735. doi: 10.1007/s12192-020-01145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cusick M.F., Libbey J.E., Fujinami R.S. Molecular mimicry as a mechanism of autoimmune disease. Clin. Rev. Allergy Immunol. 2012;42(1):102–111. doi: 10.1007/s12016-011-8294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Angileri F., Legare S., Marino Gammazza A., Conway de Macario E., JL Macario A., Cappello F. Molecular mimicry may explain multi-organ damage in COVID-19. Autoimmun. Rev. 2020;19(8):102591. doi: 10.1016/j.autrev.2020.102591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marino Gammazza A., Légaré S., Lo Bosco G., Fucarino A., Angileri F., Conway de Macario E., Macario A.JL., Cappello F. Human molecular chaperones share with SARS-CoV-2 antigenic epitopes potentially capable of eliciting autoimmunity against endothelial cells: possible role of molecular mimicry in COVID-19. Cell Stress Chaperones. 2020;25(5):737–741. doi: 10.1007/s12192-020-01148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanduc D., Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol. Res. 2020;68(5):310–313. doi: 10.1007/s12026-020-09152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanduc D. From anti-SARS-CoV-2 immune responses to COVID-19 via molecular mimicry. Antibodies. 2020;9(3):33. doi: 10.3390/antib9030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lucchese G., Flöel A. Molecular mimicry between SARS-CoV-2 and respiratory pacemaker neurons. Autoimmun. Rev. 2020;19(7):102556. doi: 10.1016/j.autrev.2020.102556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Angileri F., Legare S., Marino Gammazza A., Conway de Macario E., Macario A.J., Cappello F. Is molecular mimicry the culprit in the autoimmune hemolytic anemia affecting COVID-19 patients? Br. J. Haematol. 2020 doi: 10.1111/bjh.16883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clinical Immunology (Orlando, Fla). 2020;217:108480. doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ehrenfeld M., Tincani A., Andreoli L., Cattalini M., Greenbaum A., Kanduc D., et al. Covid-19 and autoimmunity. Autoimmunity Rev. 2020;19(8):102597. doi: 10.1016/j.autrev.2020.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li D., Wang Y., Xu N., Wei Q., Wu M., Li X., Zheng P., Sun S., Jin Y., Zhang G., Liao R., Zhang P. Follistatin-like protein 1 is elevated in systemic autoimmune diseases and correlated with disease activity in patients with rheumatoid arthritis. Arthritis Res. Therapy. 2011;13(1) doi: 10.1186/ar3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.-S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respirat. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuri-Cervantes L., Pampena M.B., Meng W., Rosenfeld A.M., Ittner C.A.G., Weisman A.R., Agyekum R.S., Mathew D., Baxter A.E., Vella L.A., Kuthuru O., Apostolidis S.A., Bershaw L., Dougherty J., Greenplate A.R., Pattekar A., Kim J., Han N., Gouma S., Weirick M.E., Arevalo C.P., Bolton M.J., Goodwin E.C., Anderson E.M., Hensley S.E., Jones T.K., Mangalmurti N.S., Luning Prak E.T., Wherry E.J., Meyer N.J., Betts M.R. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 2020;5(49) doi: 10.1126/sciimmunol.abd7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mathew D., Giles J.R., Baxter A.E., Oldridge D.A., Greenplate A.R., Wu J.E., et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369(6508) doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karlsson A.C., Humbert M., Buggert M. The known unknowns of T cell immunity to COVID-19. Sci. Immunol. 2020;5(53) doi: 10.1126/sciimmunol.abe8063. [DOI] [PubMed] [Google Scholar]
- 57.Kalfaoglu B., Almeida-Santos J., Tye C.A., Satou Y., Ono M. T-cell dysregulation in COVID-19. Biochem. Biophys. Res. Commun. 2021;538:204–210. doi: 10.1016/j.bbrc.2020.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Picchianti Diamanti A., Rosado M.M., Pioli C., Sesti G., Laganà B. Cytokine Release Syndrome in COVID-19 Patients, A New Scenario for an Old Concern: The Fragile Balance between Infections and Autoimmunity. Int. J. Mol. Sci. 2020;21(9):3330. doi: 10.3390/ijms21093330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Atabati H., Esmaeili S.-A., Saburi E., Akhlaghi M., Raoofi A., Rezaei N., Momtazi‐Borojeni A.A. Probiotics with ameliorating effects on the severity of skin inflammation in psoriasis: Evidence from experimental and clinical studies. J. Cell. Physiol. 2020;235(12):8925–8937. doi: 10.1002/jcp.29737. [DOI] [PubMed] [Google Scholar]
- 60.Najafi S., Rajaei E., Moallemian R., Nokhostin F. The potential similarities of COVID-19 and autoimmune disease pathogenesis and therapeutic options: new insights approach. Clin. Rheumatol. 2020:1–13. doi: 10.1007/s10067-020-05376-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kondelkova K., Vokurková D., Krejsek J., Borská L., Fiala Z., Ctirad A. Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Med. (Hradec Kralove) 2010;53(2):73–77. doi: 10.14712/18059694.2016.63. [DOI] [PubMed] [Google Scholar]
- 62.Rodríguez-Perea A., Arcia E., Rueda C., Velilla P. Phenotypical characterization of regulatory T cells in humans and rodents. Clin. Exp. Immunol. 2016;185(3):281–291. doi: 10.1111/cei.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan. China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gladstone D.E., Kim B.S., Mooney K., Karaba A.H., D'Alessio F.R. Regulatory T cells for treating patients with covid-19 and acute respiratory distress syndrome: two case reports. Ann. Intern. Med. 2020;173(10):852–853. doi: 10.7326/L20-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moudgil K.D., Choubey D. Cytokines in autoimmunity: role in induction, regulation, and treatment. J. Interferon Cytokine Res. 2011;31(10):695–703. doi: 10.1089/jir.2011.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kleinewietfeld M., Hafler D.A. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin. Immunol. 2013;25(4):305–312. doi: 10.1016/j.smim.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Diller M.L., Kudchadkar R.R., Delman K.A., Lawson D.H., Ford M.L. Balancing inflammation: the link between Th17 and regulatory T cells. Mediators Inflamm. 2016;2016:1–8. doi: 10.1155/2016/6309219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fara A., Mitrev Z., Rosalia R.A., Assas B.M. Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol. 2020;10(9):200160. doi: 10.1098/rsob.200160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Biasi S., Meschiari M., Gibellini L., Bellinazzi C., Borella R., Fidanza L., Gozzi L., Iannone A., Lo Tartaro D., Mattioli M., Paolini A., Menozzi M., Milić J., Franceschi G., Fantini R., Tonelli R., Sita M., Sarti M., Trenti T., Brugioni L., Cicchetti L., Facchinetti F., Pietrangelo A., Clini E., Girardis M., Guaraldi G., Mussini C., Cossarizza A. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-17292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chiappelli F., Khakshooy A., Greenberg G. CoViD-19 Immunopathology and Immunotherapy. Bioinformation. 2020;16(3):219. doi: 10.6026/97320630016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zafari P., Rafiei A., Esmaeili S.A., Moonesi M., Taghadosi M. Survivin a pivotal antiapoptotic protein in rheumatoid arthritis. J. Cell. Physiol. 2019;234(12):21575–21587. doi: 10.1002/jcp.28784. [DOI] [PubMed] [Google Scholar]
- 72.Gambichler T., Reuther J., Scheel C.H., Becker J.C. On the use of immune checkpoint inhibitors in patients with viral infections including COVID-19. J. ImmunoTherapy Cancer. 2020;8(2):e001145. doi: 10.1136/jitc-2020-001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McKinney E.F., Lee J.C., Jayne D.R., Lyons P.A., Smith K.G. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. 2015;523(7562):612–616. doi: 10.1038/nature14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cañas C.A. The triggering of post-COVID-19 autoimmunity phenomena could be associated with both transient immunosuppression and an inappropriate form of immune reconstitution in susceptible individuals. Med. Hypotheses. 2020;145:110345. doi: 10.1016/j.mehy.2020.110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Swinkels M., Rijkers M., Voorberg J., Vidarsson G., Leebeek F.W.G., Jansen A.J.G. Emerging Concepts in Immune Thrombocytopenia. Front. Immunol. 2018;9:880. doi: 10.3389/fimmu.2018.00880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Onisâi M., Vlădăreanu A.-M., Spînu A., Găman M., Bumbea H. Idiopathic thrombocytopenic purpura (ITP)–new era for an old disease. Rom. J. Intern. Med. 2019;57(4):273–283. doi: 10.2478/rjim-2019-0014. [DOI] [PubMed] [Google Scholar]
- 77.Kayal L., Jayachandran S., Singh K. Idiopathic thrombocytopenic purpura. Contemp Clin Dent. 2014;5(3):410–414. doi: 10.4103/0976-237X.137976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bomhof G., Mutsaers P.G.N.J., Leebeek F.W.G., Boekhorst P.A.W., Hofland J., Croles F.N., Jansen A.J.G. COVID-19-associated immune thrombocytopenia. Br. J. Haematol. 2020;190(2) doi: 10.1111/bjh.16850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martincic Z., Skopec B., Rener K., Mavric M., Vovko T., Jereb M., Lukic M. Severe immune thrombocytopenia in a critically ill COVID-19 patient. Int. J. Infect. Dis. 2020;99:269–271. doi: 10.1016/j.ijid.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nesr G., Garnett C., Bailey C., Arami S. ITP flare with mild COVID-19 infection in pregnancy: A case report. Br. J. Haematol. 2020 doi: 10.1111/bjh.16928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Artru F., Alberio L., Moradpour D., Stalder G. Acute immune thrombocytopaenic purpura in a patient with COVID-19 and decompensated cirrhosis. BMJ Case Rep. CP. 2020;13(7):e236815. doi: 10.1136/bcr-2020-236815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Girgis C.M., Champion B.L., Wall J.R. Current concepts in Graves’ disease. Therapeut. Adv. Endocrinol. Metabolism. 2011;2(3):135–144. doi: 10.1177/2042018811408488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morshed S.A., Latif R., Davies T.F. Delineating the autoimmune mechanisms in Graves’ disease. Immunol. Res. 2012;54(1–3):191–203. doi: 10.1007/s12026-012-8312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caron P. Thyroid disorders and SARS-CoV-2 infection: From pathophysiological mechanism to patient management. Ann. Endocrinol. 2020;81(5):507–510. doi: 10.1016/j.ando.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scappaticcio L., Pitoia F., Esposito K., Piccardo A., Trimboli P. Impact of COVID-19 on the thyroid gland: an update. Rev. Endocrine Metabolic Disord. 2021;22(4):803–815. doi: 10.1007/s11154-020-09615-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Muller I., Cannavaro D., Dazzi D., Covelli D., Mantovani G., Muscatello A., Ferrante E., Orsi E., Resi V., Longari V., Cuzzocrea M., Bandera A., Lazzaroni E., Dolci A., Ceriotti F., Re T.E., Gori A., Arosio M., Salvi M. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020;8(9):739–741. doi: 10.1016/S2213-8587(20)30266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mateu-Salat M., Urgell E., Chico A. SARS-COV-2 as a trigger for autoimmune disease: report of two cases of Graves’ disease after COVID-19. J. Endocrinol. Invest. 2020;43(10):1527–1528. doi: 10.1007/s40618-020-01366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Comabella M., Khoury S.J. Immunopathogenesis of multiple sclerosis. Clin. Immunol. 2012;142(1):2–8. doi: 10.1016/j.clim.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 89.Atabati H., Yazdanpanah E., Mortazavi H., Bajestani S.G., Raoofi A., Esmaeili S.-A., et al. Immunoregulatory effects of tolerogenic probiotics in multiple sclerosis. Adv. Exp. Med. Biol. 2021;1286:87–105. doi: 10.1007/978-3-030-55035-6_6. [DOI] [PubMed] [Google Scholar]
- 90.Atabati H., Yazdanpanah E., Mortazavi H., Raoofi A., Esmaeili S.-A., Khaledi A., et al. Immunoregulatory effects of tolerogenic probiotics in multiple sclerosis. Rev. New Drug Targets Age-Related Disord. 2021:87–105. doi: 10.1007/978-3-030-55035-6_6. [DOI] [PubMed] [Google Scholar]
- 91.Agrawal S.M., Yong V.W. Immunopathogenesis of multiple sclerosis. Int. Rev. Neurobiol. 2007;79:99–126. doi: 10.1016/S0074-7742(07)79005-0. [DOI] [PubMed] [Google Scholar]
- 92.Sica F., Centonze D., Buttari F. Fingolimod immune effects beyond its sequestration ability. Neurol. Therapy. 2019;8(2):231–240. doi: 10.1007/s40120-019-00162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Savarin C., Bergmann C.C. Viral-induced suppression of self-reactive T cells: Lessons from neurotropic coronavirus-induced demyelination. J. Neuroimmunol. 2017;308:12–16. doi: 10.1016/j.jneuroim.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ryder E., Steelman A.J. Does upper respiratory infection exacerbate symptoms of multiple sclerosis? : Future Medicine. 2018;13(5):503–505. doi: 10.2217/fmb-2017-0271. [DOI] [PubMed] [Google Scholar]
- 95.Palao M., Fernández-Díaz E., Gracia-Gil J., Romero-Sánchez C., Díaz-Maroto I., Segura T. Multiple sclerosis following SARS-CoV-2 infection. Multiple Sclerosis Related Disord. 2020;45:102377. doi: 10.1016/j.msard.2020.102377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pandit L. Neuromyelitis optica spectrum disorders: An update. Ann. Indian Acad. Neurol. 2015;18(Suppl 1):S11. doi: 10.4103/0972-2327.164816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Ruijter N.S., Kramer G., Gons R.A.R., Hengstman G.J.D. Neuromyelitis optica spectrum disorder after presumed coronavirus (COVID-19) infection: A case report. Multiple Sclerosis Related Disord. 2020;46:102474. doi: 10.1016/j.msard.2020.102474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Winer J.B. Guillain Barre syndrome. Mol. Pathol. 2001;54(6):381. [PMC free article] [PubMed] [Google Scholar]
- 99.Ullah M.W., Qaseem A., Amray A. Post Vaccination Guillain Barre Syndrome: A Case Report. Cureus. 2018;10(4) doi: 10.7759/cureus.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Amanpour S. The rapid development and early success of Covid 19 vaccines have raised hopes for accelerating the cancer treatment mechanism. Arch. Razi Inst. 2021;76(1):1. doi: 10.22092/ari.2021.353761.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Van Den Berg B., Walgaard C., Drenthen J., Fokke C., Jacobs B.C., Van Doorn P.A. Guillain-Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nature Rev. Neurol. 2014;10(8):469–482. doi: 10.1038/nrneurol.2014.121. [DOI] [PubMed] [Google Scholar]
- 102.Gigli G.L., Bax F., Marini A., Pellitteri G., Scalise A., Surcinelli A., Valente M. Guillain-Barré syndrome in the COVID-19 era: just an occasional cluster? J. Neurol. 2021;268(4):1195–1197. doi: 10.1007/s00415-020-09911-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G., Franciotta D., Baldanti F., Daturi R., Postorino P., Cavallini A., Micieli G. Guillain-Barré syndrome associated with SARS-CoV-2. N. Engl. J. Med. 2020;382(26):2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Korem S., Gandhi H., Dayag D.B. Guillain-Barré syndrome associated with COVID-19 disease. BMJ Case Rep. CP. 2020;13(9):e237215. doi: 10.1136/bcr-2020-237215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hirayama T., Hongo Y., Kaida K., Kano O. Guillain-Barré syndrome after COVID-19 in Japan. BMJ Case Rep. CP. 2020;13(10):e239218. doi: 10.1136/bcr-2020-239218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nasser F., Younis L., Abidreda K., Alyasiri I. Importance of Vitamin D3 in COVID-19 Patients. Arch. Razi Inst. 2021;76(5):1545–1549. doi: 10.22092/ari.2021.356070.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guadarrama-Ortiz P., Choreño-Parra J.A., Sánchez-Martínez C.M., Pacheco-Sánchez F.J., Rodríguez-Nava A.I., García-Quintero G. Neurological aspects of SARS-CoV-2 infection: mechanisms and manifestations. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.01039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jayam Trouth A., Dabi A., Solieman N., Kurukumbi M., Kalyanam J. Myasthenia gravis: a review. Autoimmune Dis. 2012;2012:1–10. doi: 10.1155/2012/874680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Melzer N., Ruck T., Fuhr P., Gold R., Hohlfeld R., Marx A., Melms A., Tackenberg B., Schalke B., Schneider-Gold C., Zimprich F., Meuth S.G., Wiendl H. Clinical features, pathogenesis, and treatment of myasthenia gravis: a supplement to the Guidelines of the German Neurological Society. J. Neurol. 2016;263(8):1473–1494. doi: 10.1007/s00415-016-8045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cavalcante P., Maggi L., Colleoni L., Caldara R., Motta T., Giardina C., Antozzi C., Berrih-Aknin S., Bernasconi P., Mantegazza R. Inflammation and Epstein-Barr virus infection are common features of myasthenia gravis thymus: possible roles in pathogenesis. Autoimmune Dis. 2011;2011:1–17. doi: 10.4061/2011/213092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fares E., Tayyar R., Pathak K., Damiano C., Kuntz C. Myasthenia gravis crisis triggered by COVID-19. Chest. 2020;158(4):A734. [Google Scholar]
- 112.Restivo D.A., Centonze D., Alesina A., Marchese-Ragona R. Myasthenia gravis associated with SARS-CoV-2 infection. Ann. Intern. Med. 2020;173(12):1027–1028. doi: 10.7326/L20-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huber M., Rogozinski S., Puppe W., Framme C., Höglinger G., Hufendiek K., Wegner F. Postinfectious onset of myasthenia gravis in a COVID-19 patient. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.576153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sriwastava S., Tandon M., Kataria S., Daimee M., Sultan S. New onset of ocular myasthenia gravis in a patient with COVID-19: a novel case report and literature review. J. Neurol. 2021;268(8):2690–2696. doi: 10.1007/s00415-020-10263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ali A., Sayyed Z., Ameer M.A., Arif A.W., Kiran F., Iftikhar A., et al. Systemic lupus erythematosus: An overview of the disease pathology and its management. Cureus. 2018;10(9) doi: 10.7759/cureus.3288. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 116.Esmaeili S.A., Mahmoudi M., Momtazi A.A., Sahebkar A., Doulabi H., Rastin M. Tolerogenic probiotics: potential immunoregulators in Systemic Lupus Erythematosus. J. Cell. Physiol. 2017;232(8):1994–2007. doi: 10.1002/jcp.25748. [DOI] [PubMed] [Google Scholar]
- 117.Fava A., Petri M. Systemic lupus erythematosus: diagnosis and clinical management. J. Autoimmun. 2019;96:1–13. doi: 10.1016/j.jaut.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Radmanesh F., Mahmoudi M., Yazdanpanah E., Keyvani V., Kia N., Nikpoor A.R., Zafari P., Esmaeili S.-A. The immunomodulatory effects of mesenchymal stromal cell-based therapy in human and animal models of systemic lupus erythematosus. IUBMB Life. 2020;72(11):2366–2381. doi: 10.1002/iub.2387. [DOI] [PubMed] [Google Scholar]
- 119.Vahidi Z., Samadi M., Mahmoudi M., RezaieYazdi Z., Sahebari M., Tabasi N., Esmaeili S.-A., Sahebkar A., Rastin M. Lactobacillus rhamnosus and Lactobacillus delbrueckii ameliorate the expression of miR-155 and miR-181a in SLE patients. J. Funct. Foods. 2018;48:228–233. [Google Scholar]
- 120.Slimani Y., Abbassi R., El Fatoiki F.-Z., Barrou L., Chiheb S. Systemic lupus erythematosus and varicella-like rash following COVID-19 in a previously healthy patient. J. Med. Virol. 2021;93(2):1184–1187. doi: 10.1002/jmv.26513. [DOI] [PubMed] [Google Scholar]
- 121.Cardoso E.M., Hundal J., Feterman D., Magaldi J. Concomitant new diagnosis of systemic lupus erythematosus and COVID-19 with possible antiphospholipid syndrome. Just a coincidence? A case report and review of intertwining pathophysiology. Clin. Rheumatol. 2020:1–5. doi: 10.1007/s10067-020-05310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Y., Geng X., Tan Y., Li Q., Xu C., Xu J., Hao L., Zeng Z., Luo X., Liu F., Wang H. New understanding of the damage of SARS-CoV-2 infection outside the respiratory system. Biomed. Pharmacother. 2020;127:110195. doi: 10.1016/j.biopha.2020.110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cheung S., Fuentes A.D., Fetterman A.D. Recurrent Acute Pancreatitis in a Patient with COVID-19 Infection. Am. J. Case Rep. 2020;21:e927076–e927081. doi: 10.12659/AJCR.927076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Luiz A., Serpa O., Costa D.D.S., Pinheiro M.I.C., Diaz A.P., Silva A.G. Brief symptom inventory: Reporting Brazilian populational parameters during COVID- 19 pandemics. Arch. Clin. Psychiatry (São Paulo) 2021:12. [Google Scholar]
- 125.Akriti K., Satpathy I., Patnaik B. Covid-19 and its impact on livelihood: An Indian perspective. Eurasian Chem. Commun. 2021:81–87. [Google Scholar]
- 126.Talotta R., Robertson E. Autoimmunity as the comet tail of COVID-19 pandemic. World J. Clin. Cases. 2020;8(17):3621–3644. doi: 10.12998/wjcc.v8.i17.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Danesh H., Barzegar F., Ismaili A., Horri E., Abdolrazaghnejad A. Pharmacological and Radiological Study of Patients with COVID-19 in Iran. J. Med. Chem. Sci. 2022:215–226. [Google Scholar]
- 128.Florindo H.F., Kleiner R., Vaskovich-Koubi D., Acúrcio R.C., Carreira B., Yeini E., Tiram G., Liubomirski Y., Satchi-Fainaro R. Immune-mediated approaches against COVID-19. Nat. Nanotechnol. 2020;15(8):630–645. doi: 10.1038/s41565-020-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fujinami R.S., von Herrath M.G., Christen U., Whitton J.L. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin. Microbiol. Rev. 2006;19(1):80–94. doi: 10.1128/CMR.19.1.80-94.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gagiannis D., Steinestel J., Hackenbroch C., Hannemann M., Umathum V.G., Gebauer N., et al. COVID-19-induced acute respiratory failure: an exacerbation of organ-specific autoimmunity? medRxiv. 2020 [Google Scholar]
- 131.Swinkels M., Rijkers M., Voorberg J., Vidarsson G., Leebeek F.W., Jansen A. Emerging concepts in immune thrombocytopenia. Front. Immunol. 2018;9:880. doi: 10.3389/fimmu.2018.00880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Geier M.R., Geier D.A., Zahalsky A.C. Influenza vaccination and Guillain Barre syndrome☆. Clin. Immunol. 2003;107(2):116–121. doi: 10.1016/s1521-6616(03)00046-9. [DOI] [PubMed] [Google Scholar]
- 133.Vadalà M., Poddighe D., Laurino C., Palmieri B. Vaccination and autoimmune diseases: is prevention of adverse health effects on the horizon? EPMA J. 2017;8(3):295–311. doi: 10.1007/s13167-017-0101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mahase E. Covid-19: Vaccine candidate may be more than 90% effective, interim results indicate. Br. Med. J. Publishing Group. 2020 doi: 10.1136/bmj.m4347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset(s) supporting the conclusions of this article is (are) included within the article (and its additional file(s)).