Abstract
Monoclonal antibodies are highly specific proteins that are cloned from a single B cell and bind to a single epitope on a pathogen. These laboratory-made molecules can serve as prophylactics or therapeutics for infectious diseases and have an impressive capacity to modulate the progression of disease, as demonstrated for the first time on a large scale during the COVID-19 pandemic. The high specificity and natural starting point of monoclonal antibodies afford an encouraging safety profile, yet the high cost of production remains a major limitation to their widespread use. While a monoclonal antibody approach to abrogating malaria infection is not yet available, the unique life cycle of the malaria parasite affords many opportunities for such proteins to act, and preliminary research into the efficacy of monoclonal antibodies in preventing malaria infection, disease, and transmission is encouraging. This review examines the current status and future outlook for monoclonal antibodies against malaria in the context of the complex life cycle and varied antigenic targets expressed in the human and mosquito hosts, and provides insight into the strengths and limitations of this approach to curtailing one of humanity’s oldest and deadliest diseases.
This review discusses the use of monoclonal antibodies for the prevention of malaria, describing the prospects and challenges of this strategy in the context of the complex Plasmodium life cycle. Several antibodies are discussed in detail, providing a thorough overview of this approach to malaria prevention.
Introduction
Monoclonal antibodies (mAbs), defined as a single antibody (Ab) cloned from a single B cell, have been in use for decades as immune modulators for transplantation, autoimmune diseases, and cancer.1 Most clinical uses take advantage of the high specificity of Abs which can safely target specific proteins to deplete cells or block receptor-ligand interactions. This specificity and the fact that Abs are naturally occurring proteins rather than foreign molecules make for an excellent clinical safety profile. It is curious, however, that although Abs were first discovered and used in the context of infectious disease, mAbs are only recently seeing a resurgence in their use for this purpose.2,3 This review will focus on mAb development for one of the oldest and deadliest infectious diseases that remains without an effective long-term vaccine or chemoprophylactic: malaria.
Considerations for monoclonal antibody development for infectious diseases
mAbs are currently being adopted for numerous infectious diseases including respiratory syncytial virus,4 anthrax,5 HIV,6, 7, 8 and Ebola.9 They have recently been approved for use against Ebola and COVID-19, with the latter proving that mAbs can be a rapid and highly effective means of responding to emerging pathogens. However, as highlighted by the COVID-19 pandemic, developing mAbs for infectious diseases is not amenable to a one-size-fits-all approach. Special considerations must be taken to consider the host-pathogen immunobiology and epidemiology of each disease as well as the market environment for novel interventions. For example, mAbs can be used as a prophylactic, therapeutic, or both. Which approach is best depends on a number of factors including the likelihood of the mAb in preventing infection or disease, the utility of the mAb at the individual and population level, the underlying cause of disease following infection, and the intended recipient population. In addition, it must be determined whether preventing infection, disease, or transmission is the priority. Finally, mAbs must be considered in the context of available or emerging drugs and/or vaccines which will compete on a public health and market level.
In the context of drugs and vaccines for infectious diseases, mAbs offer a number of potential benefits. They have an excellent safety profile with minimal off-target effects and can be used in combination with little to no interference.10 They can be delivered at effective doses in a single, directly observed injection or infusion and can persist at effective concentrations in the blood for longer than 1 year when using long-lasting variants.6,11,12 Unlike vaccines, mAbs do not depend on the host immune system for production and therefore should have less variability across populations in terms of immediate serum Ab concentration. However, genetic mutations in the Fc receptor can influence the downstream effector mechanisms13,14 and half-life15 of anti-cancer mAbs. How such variations contribute to mAb efficacy in infectious diseases has not been well defined and will be specific for each target pathogen. Finally, unlike vaccines that often require multiple doses and at least weeks to have an effect, mAbs are effective almost immediately upon administration.
Still, the road ahead for mAbs against infectious diseases contains several hurdles. The first and foremost concern is cost. The cost of a course of mAbs depends greatly on the intended market,16 but using COVID-19 as an example, the mAb therapy REGEN-COV is charged at ∼$2,100/dose to the US Government.17 This is small compared with the cost of even a short hospital stay but is orders of magnitude above the costs of COVID-19 vaccines. Thus, while COVID-19 has shown that mAbs can offer a cost-effective benefit in the absence of a vaccine, the cost of mAbs will need to decrease significantly before widespread use against infectious diseases is possible, particularly in low- and middle-income countries (LMIC). Another consideration is that, typically, tens or hundreds of mAbs need to be screened for function before advancing clinical candidates. This is best achieved using predictive and high-throughput in vitro assays (e.g., neutralization assays), which exist for some pathogens such as SARS-CoV-2 but do not exist for other infectious agents such as tuberculosis and for most stages of infection for the parasite that causes malaria. Pathogens are also adept at evolving past even the complex immunity in populations and thus will likely be able to evolve around most individual mAbs and even cocktail combinations of mAbs. Therefore, careful work is required to select either immutable targets or combinations of targets to prevent resistance.18 While a concern most relevant for dengue virus, the risk of Ab-dependent enhancement of disease due to the presence of non-neutralizing Abs must also be considered. Finally, even fully human monoclonals may be recognized as foreign by the recipient immune system, and “anti-drug antibodies” (ADAs) may form to either clear mAbs or reduce their efficacy. While ADA formation is dependent on many factors, experience with mAbs for infectious diseases have shown little evidence that ADAs will be an issue even following repeated injections.7,8,19,20
Considerations for monoclonal antibody development for malaria
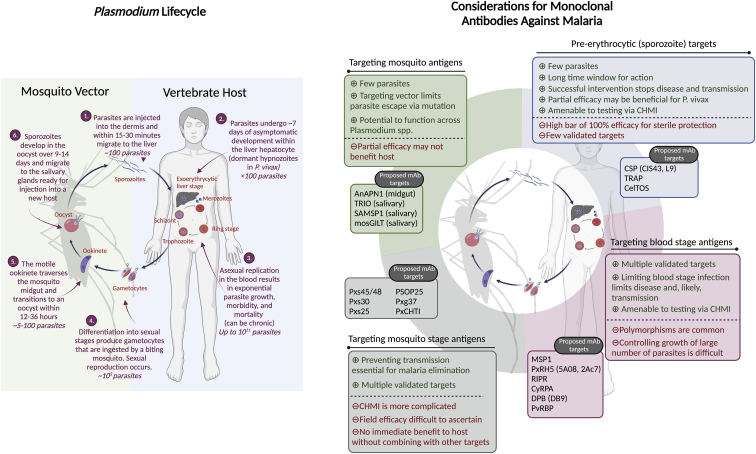
Malaria is the disease caused by infection with eukaryotic pathogens of the genus Plasmodium, which have a host range spanning from reptiles and birds to rodents, humans, and other mammals. There are multiple species of Plasmodium that infect humans, with the vast majority of disease caused by Plasmodium falciparum (Pf) common to Sub-Saharan Africa and Plasmodium vivax (Pv) which dominates in Southeast Asia and South America. Plasmodium parasites are transmitted via multiple species of Anopheline mosquitoes, and the mammalian portion of the parasite life cycle begins when an infected mosquito injects tens to hundreds of “sporozoite” forms of the parasite into the dermis. These sporozoites then actively migrate through the skin and into the blood where they will be carried to the liver. Here, the parasites replicate asymptomatically and asexually for ∼7 days within a single hepatocyte, although at this stage Pv also forms dormant stages in the liver called “hypnozoites” that can persist and reactivate for years. At the end of the liver stage the parasites emerge as red blood cell-infectious “merozoites.” These merozoites cyclically infect red blood cells, which rapidly expands the parasite burden and initiates the symptomatic stage of infection. Some parasites also undergo sexual replication to become male and female “gametocytes” which can then be picked up by a new mosquito vector. The invertebrate portion of the parasite life cycle starts in the mosquito midgut where the gametocytes will mate, forming a motile “ookinete” that invades the mosquito midgut, where new sporozoites will develop within the “oocyst” for approximately 2 weeks. These sporozoites will then emerge from the oocyst and migrate into the mosquito salivary gland where they can then be transmitted to a new host and complete the transmission cycle. In this review, we will divide this complex life cycle roughly into three phases: the skin-to-liver or “pre-erythrocytic” stage; the “erythrocytic” or blood stage; and the mosquito stage.
The Plasmodium life cycle is more complex than bacterial or viral infections, which, on the one hand, presents a challenge to mAb development but also serves as an opportunity, as Abs can function against each stage of infection (Figure 1). This susceptibility has driven the development of multiple prophylactic Ab-based vaccine candidates which have only recently achieved high levels of protection against disease.21 However, protection against infection has been much more difficult to achieve and will be necessary to disrupt the transmission cycle and achieve malaria eradication. Such high levels of infection-blocking protection have been achieved in controlled human malaria infection (CHMI) studies in malaria-naive volunteers,22,23 but this has not translated to field trials in endemic areas.24, 25, 26, 27 It is hypothesized that this is due in part to pre-existing malaria-specific immune modulation in previously infected persons. In this case, a long-acting prophylactic mAb would be ideal as it does not rely on the recipient immune system to produce Abs. However, parasite fitness and polymorphisms also play a pivotal role in vaccine efficacy,28,29 which will need to be considered in developing anti-malarial mAbs.
Figure 1.
The Plasmodium parasite lifecycle and considerations for monoclonal antibodies against malaria
Left: schematic of the Plasmodium life cycle, including the approximate number of parasites present in, and duration of, each stage. Right: considerations for monoclonal antibodies against malaria, by life cycle stage. Ab targets that are discussed in this paper are listed by their abbreviation, with specific antibody names included in parentheses where relevant. When the antigen typically includes reference to a specific Plasmodium species, x is used instead of species designation. Full antigen names can be found in the text, where relevant. CHMI, controlled human malaria infection. Created with Biorender.com.
Finally, it is important to consider that multiple effective and affordable drugs exist to prevent and treat malaria, and that vaccines can be made relatively cheaply if one is developed for malaria. Therefore, the current costs of mAbs would preclude large-scale administration campaigns similar to mass drug administration or mass vaccination. Even at current cost estimates, however, mAbs offer advantages over chemoprophylaxis for members of the military or travelers making multi-week or multi-month visits to endemic areas. This is because long-term chemoprophylaxis is still expensive30 and suffers from low compliance (10%–50%) due to inconvenient schedules and side effects.31, 32, 33 The high cost of mAbs is also lower than the costs of a medical evacuation and therefore could be cost-effective for visitors at high risk of malaria. Compared with the protracted regimens of vaccines and drugs and the lag time between vaccine administration and efficacy, mAbs would also offer the benefit of a simplified regimen given in a single directly observed administration that has immediate efficacy for unplanned or short-notice trips. While an exact “target product profile” for an anti-malaria mAb will depend on the user and goal, most models suggest that we will need >80% infection-blocking sterile protection for longer than a year to drive malaria toward elimination,34, 35, 36 similar to what has been proposed for long-lasting injectable chemoprophylactics.36 It is within this context that we will discuss the current state of mAbs for malaria as well as the short-term outlook for achieving the first competitive malaria mAb product capable of achieving high levels of infection-blocking protection. This will be discussed through the lens of the Plasmodium life cycle, given the profound impact of the distinct nature of each stage of infection on mAb development (see Figure 1 for summary of life cycle and potential mAb targets).
“Pre-erythrocytic” stage targeted monoclonal antibodies
As a bottleneck in the life cycle, the numbers of sporozoites injected by the mosquito at the skin-to-liver, or pre-erythrocytic (PE), stages are relatively small37, 38, 39 and the time between injection, invasion of the vasculature, and transit to the liver is minutes to hours,40, 41, 42, 43 which provides a large window for infection-blocking Ab activity. The circumsporozoite protein (CSP) is the most abundant surface antigen present in the sporozoite stage of the Plasmodium parasite44 and is critical to the normal development of sporozoites in the mosquito salivary gland45 as well as their ability to invade and infect hepatocytes once inside the vertebrate host.46 The two most successful and advanced malaria vaccines, RTS,S and R21, are both designed to elicit an immune response against CSP47 and are thought to provide protection by neutralizing Abs. This is supported by studies showing the capacity of mAbs against Pf CSP (PfCSP) and Plasmodium yoelii CSP (PyCSP) to prevent hepatocyte invasion both in vitro and in vivo.11,48, 49, 50, 51, 52, 53 Using Pv infection of liver-humanized mice, Schäfer et al. recently demonstrated that an mAb against PvCSP could also reduce the overall liver burden and in turn relapse infection via reduction of the number of dormant hypnozoites.54 This is critical, as the majority of Pv disease burden is driven by relapses from dormant liver hypnozoites.55
The mechanisms of anti-PfCSP mAbs have been well-studied. The CSP is composed of three domains: an N terminus, a C terminus, and a central region characterized by a repeating amino acid sequences.56 In Pf, this repeat region contains a major repeating sequence, NANP, and a minor repeat, NVDP. The CSP-specific mAb CIS43 was recently shown to be a “dual binder” in that it specifically binds to the central repeat region as well as a short junctional sequence that bridges the N terminus and the central repeat region,11 and has been reported to provide sterile protection in two different mouse models following passive transfer.48 This may be explained by the ability of CIS43 to bind multiple CSP epitopes, as such dual binding confers potent neutralizing capabilities to a number of other mAbs which target the major repeat and either the minor repeat or the junction sequence.49,57 Of note, the introduction of the “LS” mutation into the Fc domain of CIS43 increased the serum half-life of the Ab while maintaining a high level of protection in vivo.12 This modified CIS43LS has been taken into phase I clinical trials in malaria-naive adults, and this first-in-human study for malaria mAbs showed a very promising safety and pharmacokinetic profile after intravenous or subcutaneous administration.58 Furthermore, participants underwent CHMI challenge and all nine were sterilely protected against infection.58 However, the numbers of volunteers per dose was small and serum concentration at time of challenge ranged from ∼50 to 500 μg/mL, which is likely higher than what is feasible for long-term protection in the field due to cost. Still, the results from an ongoing field trial in Mali will be critical for understanding the potential for anti-malaria mAbs to perform against natural infection (ClinicalTrials.gov ID: NCT04329104). Encouragingly, a new dual-binding anti-PfCSP mAb, L9, outperformed six published neutralizing mAbs—including CIS43 and other dual binders—in mosquito bite challenges in vivo and therefore provides a path to improved potency should results with CIS43LS indicate the need for improvements.53 In summary, mAbs against PfCSP are paving the way to become the first anti-malarial mAb to prevent infection. However, low levels of sterile protection achieved by CSP recombinant vaccines in the field despite high titers of anti-PfCSP Abs21,25 and success in CHMI suggest that multiple avenues to improve mAb potency should continue to be pursued.
One such strategy is targeting additional PE antigens. Although no other PE Ab targets have been as well defined or as potent as CSP, the PE stages provide numerous points of possible intervention,59 and non-CSP polyclonal Abs have recently been shown as potent inhibitors of parasite liver infection in humanized liver mice.60 A leading target for PE mAb development is the thrombospondin-related adhesive protein (TRAP), a transmembrane protein essential for sporozoite motility and successful liver invasion.61,62 High levels of anti-TRAP Abs have been correlated with higher protection against malaria in children,63 suggesting that this protein may yield another promising mAb target. The idea of using TRAP in combination with CSP as an Ab target has been considered for decades, but studies have generated mixed results. One vaccine trial using TRAP in combination with RTS,S failed to show any significant protection, perhaps because of immune interference that reduced the anti-CSP Ab titers.64 Another clinical trial combining RTS,S with viral-vectored TRAP showed no benefit to adding TRAP, yet interpretation of this is complicated by the combination of vaccine platforms.65 This is in contrast to studies in mice where active vaccination with CSP and TRAP suggested the utility of adding TRAP,66 and a TRAP/CSP fusion protein conferred sterile protection for 6 months in mice.67 Concrete evidence of the utility of additional PE antigens would be best achieved by using passive transfer of polyclonal Abs or mAbs. A recent manuscript in preprint has shed some light on this and has demonstrated that anti-TRAP mAbs can significantly improve the protective efficacy of anti-CSP mAbs in both rodent models and liver-humanized mice to above 80% at low doses.68 However, whether this enhancement is additive or synergistic was not addressed and will be critical for determining the utility of such combinations. Together, these data suggest that combinations of PE mAbs may be a pathway to achieving high levels of protection at serum concentrations achievable over extended periods.
A critical part of Plasmodium motility and infection at the PE stage is called traversal, where the parasite actively crosses through host cells as it migrates from the skin to the liver in the vertebrate host. Traversal is also utilized during the mosquito stages when the parasite invades the mosquito midgut and salivary glands. This active process involves several proteins including the cell traversal protein for ookinetes and sporozoites (CelTOS), which is required at multiple stages.69 This antigen was first isolated in 2003, is highly immunogenic, and is highly conserved across Plasmodium species.70,71 Polyclonal Abs against CelTOS are able to suppress parasite motility, inhibit hepatocyte invasion, and provide sterile protection in rodent models.72 In addition, passive transfer of anti-CelTOS mAbs has been shown to reduce sporozoite infectivity in mice and decrease oocyst burden in mosquitoes.73 This suggests that multiple functions of CelTOS can be targeted to disrupt multiple points of Plasmodium motility within each host, and this may be achievable with a single mAb.
In summary, with CSP leading the way, future studies of PE Ab targets will require improvement of mAbs against existing targets by way of similar detailed mechanistic studies as have been conducted for CSP. This should be supplemented with the identification of additional Ab targets, as we have only begun to explore the >30 sporozoite surface or secreted proteins potentially accessible to Ab binding.
Blood stage targeted monoclonal antibodies
The gold standard for a prophylactic anti-malarial or vaccine is “sterile protection,” defined as the prevention of blood stage infection. Achieving sterile protection by exclusively targeting the blood stages has proved extremely difficult, as most simply reduce but do not eliminate parasite replication and many blood stage proteins exhibit substantial antigenic polymorphism.74 Sterile protection via vaccines has been rare and has been achieved in high proportions thus far only with vaccines and monoclonals targeting the PE stages. Given this difficulty in achieving sterile protection, a vaccine or mAb treatment that targets the asexual blood stages of the parasite could either more quickly alleviate or completely prevent symptomatic blood stage infection. Thus, antigens presented by the infective merozoites as well as those expressed on the red blood cells (RBCs) after infection offer appealing targets for mAbs either alone or in combination with PE targets.
The merozoite surface protein 1 (MSP1) complex is critical to the normal progression of the Pf life cycle and has been shown to be necessary for both RBC invasion and merozoite egress from infected erythrocytes.75,76 MSP1 is the most abundant protein on the surface of the merozoite, making it a viable target for vaccine and mAb interventions.77 It is proteolytically processed as the merozoite matures, resulting in a non-covalently linked complex of the fragments p83, p30, p38, and p42.78 It has been shown that MSP1 mediates Pf merozoite interactions with human erythrocytes, and Abs targeting various fragments can disrupt parasite growth.79,80 However, the prevalent polymorphisms within certain portions of MSP1 and relatively low levels of protection afforded by MSP1-based vaccines has likely limited enthusiasm for MSP1 as a mAb target, although detailed studies using mAbs targeting conserved epitopes are warranted.
A newer and more promising blood stage target with considerable mAb research is the Pf reticulocyte-binding protein homolog 5 (PfRH5). This protein binds the surface receptor basiginin on the erythrocyte membrane and has been shown to be essential to merozoite invasion.81,82 During invasion, PfRh5 forms a complex with the cysteine-rich protective antigen (CyRPA) and PfRh5 interacting protein (PfRIPR).83 Both CyRPA and PfRIPR are housed within parasite micronemes and are released during merozoite invasion to facilitate entrance into the erythrocyte via their assembly into a trimeric complex with PfRh5.83,84 Given the essential nature of this protein to the parasite invasion of RBCs and the association of anti-PfRH5 with protection in field studies, as well as promising data as a vaccine target,85,86 PfRh5 is leading the field as an mAb target at the blood stage. A variety of potent neutralizing mAbs targeting PfRH5 have been identified, three of which have been demonstrated to be capable of inhibiting merozoite invasion by >95% at low concentrations in vitro.87 This work identified 5A08, an Ab that recognizes a highly immunogenic epitope on PfRh5, which has shed light on the mechanism of anti-PfRh5 mAb inhibition.87 A more recent study showed that the anti-PfRH5 mAb 2Ac7 can provide sterilizing protection against stringent Pf blood stage challenge in non-human primates and also established the in vitro growth inhibition assay as predictive of protection in vivo.88
Still, the concentrations needed to provide protection are too high for direct clinical use, and the potency of anti-PfRh5 mAbs will need to be improved. One path to increasing potency is to disrupt the assembly of the PfRh5/RIPR/CyRPA complex rather than solely targeting PfRH5. This has been achieved using mAbs targeting both Rh5 and CyRPA that prevent the formation of the trimeric complex, and using Abs against PfCyRPA and PfRIPR that act synergistically to reduce merozoite invasion in vitro.89 These results show the need for further research on these antigens and support mAbs targeting the entire complex as a viable path forward in improving the potency of blood stage mAbs.
Of particular concern in malaria-endemic areas is pregnancy-associated malaria (PAM), which threatens 125 million women per year and is a significant cause of maternal and infant mortality.90 Pf-infected erythrocytes are known to sequester in the placenta owing to their ability to bind chondroitin sulfate A (CSA), and it has been shown that the parasite protein VAR2CSA, a member of the Pf erythrocyte membrane protein 1 (PfEMP1) family,91 is upregulated in placental infected erythrocytes.92,93 One study showed that women with high levels of anti-VAR2CSA immunoglobulin G gave birth to heavier infants and were at a significantly lower risk of delivering low-birth-weight children in comparison with mothers with low levels of circulating Ab.92 Therefore, VAR2CSA is a logical vaccine target with the potential to protect pregnant women and their children. However, safety considerations have prevented pregnant women from receiving an experimental malaria immunization, let alone one targeted to preventing PAM.94 Furthermore, antigenic variation in VAR2CSA complicates the development of a VAR2CSA vaccine, especially given the difficulty in developing such a vaccine in a vulnerable population.95 This provides an interesting case use for mAbs given their safety profile in pregnant women96 with the possibility that a broadly neutralizing mAb could be administered during pregnancy, likely with only a single dose, in the absence of an effective vaccine with an anti-PAM component. Such an mAb could even be administered on top of a partially effective vaccine targeting other stages (e.g., RTS,S) to provide additional protection during pregnancy.
In Pv, the blood stage parasites are unique in that they infect immature reticulocytes rather than the mature erythrocytes targeted by Pf. Thus, there are unique invasion proteins to consider for Pv blood stage mAbs, including the erythrocyte-binding ligand family that is essential for Pv merozoite entry into the reticulocyte.97 These proteins contain a cysteine-rich binding domain at the N-terminal region called the Duffy binding-like domain, which is the functional portion of the Duffy binding protein (DBP) ligand. This ligand must engage with the Duffy antigen receptor for chemokines (DARC) expressed on the host reticulocyte membrane surface in order for the parasite to begin invasion.98 Natural exposure to malaria elicits DBP-specific Abs that inhibit the binding of the parasite99 and are associated with clinical protection,100 possibly due to the highly polymorphic capacity of the molecule that allows it to evade the host immune response.101 Thus, DBP is a logical target for mAb development.
Moreover, an artificial DBPII immunogen consisting of the DARC-binding region II of the protein optimized for functional and non-polymorphic targets102 was used to produce a panel of mAbs in BALB/c mice. A total of ten of these mAbs showed significant inhibition of parasite invasion in vitro.103 Rawlinson et al. isolated mAbs from volunteers immunized with a PvDBPII vaccine candidate and found a promising mAb, DB9, that inhibits parasite invasion in vitro and prevents the binding of five variant alleles of PvDBPII to DARC.100 Other groups have successfully isolated mAbs to PvDBPII from individuals with natural immunity to Pv, which may show enhanced inhibition and can transcend wild-type Pv strains.104,105 Interestingly, mAbs that bind close to or at the DB9 epitope can provide additive inhibition while mAbs that bind different epitopes elsewhere in the PvDBPII molecule are antagonistic.
Pv reticulocyte invasion also requires the interaction between the Pv reticulocyte protein (PvRBP) and transferrin receptor on the host reticulocyte.106,107 Four anti-PvRBP mAbs have been identified thus far that can prevent reticulocyte invasion in vitro, therefore providing another encouraging Pv blood stage target.107 The ability of these mAbs to target unique proteins and invasion pathways to work in additivity or synergy, and the impact of any such mAb in vivo, will be critical data. However, this is difficult research to conduct given that blood stage culture of the Pv blood stages is limited to using fresh field isolates in short-term assays and that Pv blood stage challenge in vivo is only possible as a CHMI.108, 109, 110, 111, 112 However, a manuscript in preprint at the time of this review suggests that a non-human primate model of Pv blood stage infection may be near and thus could fill a significant gap in the preclinical assessment of Pv blood stage mAbs.113
Mosquito stage targeted monoclonal antibodies
The transmission of parasites between the human host and mosquito vector is an appealing target for Abs, as these stages present another bottleneck in the parasite life cycle37,38,114, 115, 116, 117 and display minimal polymorphisms, likely due to the lack of evolutionary pressure by the human immune system,118, 119, 120, 121 and Abs against these stages are especially potent.122, 123, 124 While Abs targeting solely the transmission of an established blood stage infection to mosquitoes offers no direct benefit to the individual, sufficient coverage of a local population with effective transmission-blocking Abs could have drastic effects on the burden of disease,117,125,126 and preliminary data suggest that they may be readily accepted in affected communities.127, 128, 129 Much of the data available on such transmission-blocking targets concern vaccine development, yet these data have clear applicability to the development of mAbs for passive immunization.
Abs against two proteins expressed on the transmissible gametocyte, Pfs48/45121,130 and Pfs230,121,131,132 have demonstrated substantial blocking of parasite development in the mosquito at concentrations as low as 1–3 μg/mL.121 Notably, one such mAb, TB31F, is currently in a clinical trial (ClinicalTrials.gov ID: NCT04238689) aimed to test the safety and pharmacokinetics of intravenous and subcutaneous administration, down to 0.1 mg/kg. Importantly, antigens on the gametocyte may act synergistically as dual-antigen Ab targets that neutralize the gametocyte prior to fertilization in the mosquito midgut.132,133 Once the parasite has begun to transition into a zygote and then ookinete, additional proteins, notably Pfs/Pvs25 and Pfs230, are expressed on the surface and can be targeted to prevent subsequent invasion and development within the midgut. These proteins—Pfs25, Pfs230, and Pfs48/45—are the only parasite antigens currently in clinical trials as a transmission-blocking vaccine candidate125 (and ClinicalTrials.gov ID: NCT04862416). Additional targets of the parasite at early stages of mosquito development include PSOP25, Pbg37, and PfCHT1, which have yet to be validated for human parasite species, but suggest that the list of potential transmission-blocking candidates may be more extensive than those currently being developed as vaccine candidates.134, 135, 136, 137
In addition to targeting the parasite, an intriguing strategy is to target mosquito proteins involved in parasite transmission. While in its infancy, targeting mosquito proteins is an especially appealing avenue because Abs against these proteins could disrupt transmission in a manner that transcends malaria species and is more resistant to evolutionary circumvention by the parasite itself. To this end, the mosquito midgut protein, AnAPN1, shows considerable promise as a nanoparticle vaccine in animal models that functions by blocking ookinete invasion of the mosquito midgut.138, 139, 140 Another interesting approach targets the other side of mosquito transmission: the saliva proteins that are injected with the parasite during probing. These proteins have a myriad of functions, including immunomodulation during normal probing feeding.141 Abs against the Anopheles gambiae TRIO salivary gland protein can provide partial protection against mosquito bite challenge with multiple Plasmodium species and have the potential to work in tandem with anti-sporozoite Abs.142 Other components of the mosquito saliva, including SAMSP1 and mosGILT, have been shown to affect sporozoite motility to either aid or hinder the progress of the sporozoite,143,144 suggesting that Abs raised to novel mosquito saliva proteins may be promising avenues for research. This approach is not unique to malaria control efforts, and ideas can be borrowed from strategies being pursued for arboviruses. For example, Abs to proteins in the Aedes aegypti saliva may prevent successful infection by flaviviruses145 and one construct, AGS-v, has recently been shown to be safe and immunogenic in clinical trials.146 In summary, mAb approaches to malaria need not be limited to classic parasite antigens, and a combination of both “traditional” and novel targets should be pursued to achieve high levels of protection and eradication.
Future outlook
As with mAbs for many infectious diseases, mAbs for malaria are poised to become a paradigm-shifting intervention. The numerous lines of research in the preceding discussion indicate that they are indeed a promising avenue for clinical intervention at a number of stages across the parasite life cycle, and the small first-in-human trial is encouraging. Yet for malaria and other diseases that overwhelmingly affect people in LMIC, low investment in research paired with the need for a low cost of goods will be a major impediment. The latter is a technological barrier that is likely easier to overcome than the former, which is an impediment of will and interest by wealthy nations and funders. Optimistically, the COVID-19 pandemic has proved that mAbs can be developed faster, are better tolerated, are as efficacious, and are at least as adaptable compared with vaccines and drugs when it comes to battling infectious diseases. The high demand for and apparent profitability of mAbs in the COVID-19 pandemic will hopefully usher in a new wave of interest in improving the production of mAbs at scale and at lower costs for other diseases. With any luck, these accelerated technological advances to reduce cost will coincide with improved mAb efficacy for malaria that increases potency and reduces the dose required to achieve the high threshold of protection needed.
Such improvements in efficacy are likely to come in both detailed and iterative investigations of structure-function biology as has been performed for PfCSP and PfRh5. Yet it is perhaps too optimistic to assume that a single mAb targeting a single epitope will achieve sufficient efficacy to warrant stand-alone use as a prophylactic or therapeutic. Furthermore, it is likely unwise to use such a single-antigen approach given concerns over resistance and breakthrough infection. Therefore, combinations of mAbs that target multiple epitopes and multiple proteins will likely be needed. Yet the utility of such a combinatorial approach has lacked extensive evidence. Indeed, the blood stage anti-RIPR complex Abs have demonstrated efficacy and even synergy in vitro,147,148 and combining PE targets such as CSP and TRAP may hold promise despite mixed results. However, it remains to be seen whether additional gains can be made from combining mAbs targeting different stages. A major hurdle in developing such multi-stage approaches is that the infection cycle spanning the mosquito, PE, and blood stages is impossible to replicate in vitro, let alone in a high-throughput manner. Therefore, each mAb targeting each stage will need to be vetted individually in their respective assays and combined for final assessment in vivo. The preclinical model that is best poised to assess multiple stages—mosquito bite challenge of humanized liver mice repopulated with exogenous RBCs54,149,150—is tractable but expensive, lacks a gametocyte-transmission component, and is not high throughput. Even CHMI of actively or passively immunized volunteers has yet to be developed for such a multi-stage approach although the PE, blood stages, and transmission can be assessed independently.151 Even with these limitations, whether the existing in vitro and in vivo models predict clinical success will require clinical testing of both optimal and suboptimal mAb regimens. This will stretch already limited funds which to date have been reserved for only the safest and most highly promising interventions.
In summary, the path to malaria elimination will require a long-lasting, effective, and simple intervention that can prevent infection in a high proportion of people. If this intervention is based on mAbs, it will require: (1) iterative improvements of mAbs against existing targets that can function at lower doses; (2) identification of novel targets and mechanisms that can be incorporated into next-generation mAb regimens; (3) the identification of additive or synergistic combinations of mAbs that improve efficacy and guard against resistance; (4) the improvement of preclinical assays to assess multi-stage interventions; and (5) simultaneous changes in the production of mAbs to make them affordable for global health use. Thus, while the challenges remain large, the components of a path to the first protective mAb product for malaria has been made clear by the impressive work reviewed here and can become a reality with the addition of sufficient interest, financial investment, and time.
References
- 1.Liu J.K. The history of monoclonal antibody development - progress, remaining challenges and future innovations. Ann. Med. Surg. (Lond). 2014;3:113–116. doi: 10.1016/J.AMSU.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pelfrene E., Mura M., Cavaleiro Sanches A., Cavaleri M. Monoclonal antibodies as anti-infective products: a promising future? Clin. Microbiol. Infect. 2019;25:60–64. doi: 10.1016/J.CMI.2018.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laustsen A.H. How can monoclonal antibodies be harnessed against neglected tropical diseases and other infectious diseases? Expert Opin. Drug Discov. 2019;14:1103–1112. doi: 10.1080/17460441.2019.1646723. [DOI] [PubMed] [Google Scholar]
- 4.Soto J.A., Gálvez N.M.S., Pacheco G.A., Bueno S.M., Kalergis A.M. Antibody development for preventing the human respiratory syncytial virus pathology. Mol. Med. 2020;26:1–10. doi: 10.1186/S10020-020-00162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sparrow E., Friede M., Sheikh M., Torvaldsen S. Therapeutic antibodies for infectious diseases. Bull. World Health Organ. 2017;95:235–237. doi: 10.2471/BLT.16.178061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaudinski M.R., Coates E.E., Houser K.V., Chen G.L., Yamshchikov G., Saunders J.G., Holman L.A., Gordon I., Plummer S., Hendel C.S., et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a Phase 1 open-label clinical trial in healthy adults. PLoS Med. 2018;15:e1002493. doi: 10.1371/JOURNAL.PMED.1002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ledgerwood J.E., Coates E.E., Yamshchikov G., Saunders J.G., Holman L., Enama M.E., DeZure A., Lynch R.M., Gordon I., Plummer S., et al. Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin. Exp. Immunol. 2015;182:289–301. doi: 10.1111/CEI.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lynch R.M., Boritz E., Coates E.E., DeZure A., Madden P., Costner P., Enama M.E., Plummer S., Holman L., Hendel C.S., et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci. Transl. Med. 2015;7:319ra206. doi: 10.1126/SCITRANSLMED.AAD5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maxmen A. Two Ebola drugs show promise amid ongoing outbreak. Nature. 2019 doi: 10.1038/D41586-019-02442-6. [DOI] [PubMed] [Google Scholar]
- 10.Hansel T.T., Kropshofer H., Singer T., Mitchell J.A., George A.J. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 2010;9:325–338. doi: 10.1038/NRD3003. [DOI] [PubMed] [Google Scholar]
- 11.Livingstone M.C., Bitzer A.A., Giri A., Luo K., Sankhala R.S., Choe M., Zou X., Dennison S.M., Li Y., Washington W., et al. In vitro and in vivo inhibition of malaria parasite infection by monoclonal antibodies against Plasmodium falciparum circumsporozoite protein (CSP) Sci. Rep. 2021;11:5318. doi: 10.1038/S41598-021-84622-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kisalu N.K., Pereira L.D., Ernste K., Flores-Garcia Y., Idris A.H., Asokan M., Dillon M., MacDonald S., Shi W., Chen X., et al. Enhancing durability of CIS43 monoclonal antibody by Fc mutation or AAV delivery for malaria prevention. JCI Insight. 2021;6:e143958. doi: 10.1172/JCI.INSIGHT.143958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musolino A., Naldi N., Bortesi B., Pezzuolo D., Capelletti M., Missale G., Laccabue D., Zerbini A., Camisa R., Bisagni G., et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J. Clin. Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 14.Musolino A., Gradishar W.J., Rugo H.S., Nordstrom J.L., Rock E.P., Arnaldez F., Pegram M.D. Role of Fcγ receptors in HER2-targeted breast cancer therapy. J. Immunother. Cancer. 2022;10:e003171. doi: 10.1136/JITC-2021-003171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passot C., Azzopardi N., Renault S., Baroukh N., Arnoult C., Ohresser M., Boisdron-Celle M., Gamelin E., Watier H., Paintaud G., Gouilleux-Gruart V. Influence of FCGRT gene polymorphisms on pharmacokinetics of therapeutic antibodies. MAbs. 2013;5:614–619. doi: 10.4161/MABS.24815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez I., Bott S.W., Patel A.S., Wolf C.G., Hospodar A.R., Sampathkumar S., Shrank W.H. Pricing of monoclonal antibody therapies: higher if used for cancer? Am. J. Manag. Care. 2018;24:109–112. [PubMed] [Google Scholar]
- 17.Regeneron Announces New U.S. Regeneron Pharmaceuticals Inc; 2022. Government Agreement to Purchase Additional Doses of REGEN-COV™ (Casirivimab and Imdevimab) Antibody Cocktail.https://investor.regeneron.com/news-releases/news-release-details/regeneron-announces-new-us-government-agreement-purchase [Google Scholar]
- 18.Dussupt V., Sankhala R.S., Mendez-Rivera L., Townsley S.M., Schmidt F., Wieczorek L., Lal K.G., Donofrio G.C., Tran U., Jackson N.D., et al. Low-dose in vivo protection and neutralization across SARS-CoV-2 variants by monoclonal antibody combinations. Nat. Immunol. 2021;22:1503–1514. doi: 10.1038/S41590-021-01068-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh S.R., Seaman M.S. Broadly neutralizing antibodies for HIV-1 prevention. Front. Immunol. 2021;12:712122. doi: 10.3389/FIMMU.2021.712122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer K.H., Seaton K.E., Huang Y., Grunenberg N., Isaacs A., Allen M., Ledgerwood J.E., Frank I., Sobieszczyk M.E., Baden L.R., et al. Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: results of a phase 1 randomized trial. PLoS Med. 2017;14:e1002435. doi: 10.1371/JOURNAL.PMED.1002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Datoo M.S., Natama M.H., Somé A., Traoré O., Rouamba T., Bellamy D., Yameogo P., Valia D., Tegneri M., Ouedraogo F., et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: a randomised controlled trial. Lancet. 2021;397:1809–1818. doi: 10.1016/S0140-6736(21)00943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epstein J.E., Paolino K.M., Richie T.L., Sedegah M., Singer A., Ruben A.J., Chakravarty S., Stafford A., Ruck R.C., Eappen A.G., et al. Protection against plasmodium falciparum malaria by PfSPZ vaccine. JCI Insight. 2017;2:e89154. doi: 10.1172/jci.insight.89154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rampling T., Ewer K.J., Bowyer G., Bliss C.M., Edwards N.J., Wright D., Payne R.O., Venkatraman N., de Barra E., Snudden C.M., et al. Safety and high level efficacy of the combination malaria vaccine regimen of RTS,S/AS01B with chimpanzee adenovirus 63 and modified vaccinia ankara vectored vaccines expressing ME-TRAP. J. Infect. Dis. 2016;214:772–781. doi: 10.1093/INFDIS/JIW244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jongo S.A., Shekalaghe S.A., Church L.W.P., Ruben A.J., Schindler T., Zenklusen I., Rutishauser T., Rothen J., Tumbo A., Mkindi C., et al. Safety, immunogenicity, and protective efficacy against controlled human malaria infection of plasmodium falciparum sporozoite vaccine in Tanzanian adults. Am. J. Trop. Med. Hyg. 2018;99:338–349. doi: 10.4269/ajtmh.17-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tinto H., Otieno W., Gesase S., Sorgho H., Otieno L., Liheluka E., Valéa I., Sing'oei V., Malabeja A., Valia D., et al. Long-term incidence of severe malaria following RTS,S/AS01 vaccination in children and infants in Africa: an open-label 3-year extension study of a phase 3 randomised controlled trial. Lancet Infect. Dis. 2019;19:821–832. doi: 10.1016/S1473-3099(19)30300-7. [DOI] [PubMed] [Google Scholar]
- 26.Tiono A.B., Nébié I., Anagnostou N., Coulibaly A.S., Bowyer G., Lam E., Bougouma E.C., Ouedraogo A., Yaro J.B.B., Barry A., et al. First field efficacy trial of the Chad63 MVA ME-TRAP vectored malaria vaccine candidate in 5-17 months old infants and children. PLoS One. 2018;13:e0208328. doi: 10.1371/journal.pone.0208328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oneko M., Steinhardt L.C., Yego R., Wiegand R.E., Swanson P.A., Kc N., Akach D., Sang T., Gutman J.R., Nzuu E.L., et al. Safety, immunogenicity and efficacy of PfSPZ vaccine against malaria in infants in western Kenya: a double-blind, randomized, placebo-controlled phase 2 trial. Nat. Med. 2021;27:1636–1645. doi: 10.1038/S41591-021-01470-Y. [DOI] [PubMed] [Google Scholar]
- 28.McCall M.B.B., Wammes L.J., Langenberg M.C.C., van Gemert G.J., Walk J., Hermsen C.C., Graumans W., Koelewijn R., Franetich J.F., Chishimba S., et al. Infectivity of Plasmodium falciparum sporozoites determines emerging parasitemia in infected volunteers. Sci. Transl. Med. 2017;9:eaag2490. doi: 10.1126/scitranslmed.aag2490. [DOI] [PubMed] [Google Scholar]
- 29.Shah Z., Naung M.T., Moser K.A., Adams M., Buchwald A.G., Dwivedi A., Ouattara A., Seydel K.B., Mathanga D.P., Barry A.E., et al. Whole-genome analysis of Malawian Plasmodium falciparum isolates identifies possible targets of allele-specific immunity to clinical malaria. PLoS Genet. 2021;17:e1009576. doi: 10.1371/journal.pgen.1009576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frosch A.E., Thielen B.K., Alpern J.D., Walz E.J., Volkman H.R., Smith M., Wanduragala D., Holder W., Boumi A.E., Stauffer W.M. Antimalarial chemoprophylaxis and treatment in the USA: limited access and extreme price variability. J. Trav. Med. 2021:taab117. doi: 10.1093/JTM/TAAB117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saunders D.L., Garges E., Manning J.E., Bennett K., Schäffer S., Kosmowski A.J., Magill A.J. Safety, tolerability, and compliance with long-term antimalarial chemoprophylaxis in American Soldiers in Afghanistan. Am. J. Trop. Med. Hyg. 2015;93:584–590. doi: 10.4269/ajtmh.15-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brisson M., Brisson P. Compliance with antimalaria chemoprophylaxis in a combat zone. Am. J. Trop. Med. Hyg. 2012;86:587–590. doi: 10.4269/AJTMH.2012.11-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitman T.J., Coyne P.E., Magill A.J., Blazes D.L., Green M.D., Milhous W.K., Burgess T.H., Freilich D., Tasker S.A., Azar R.G., et al. An outbreak of Plasmodium falciparum malaria in U.S. Marines deployed to Liberia. Am. J. Trop. Med. Hyg. 2010;83:258–265. doi: 10.4269/AJTMH.2010.09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White M.T., Verity R., Churcher T.S., Ghani A.C. Vaccine approaches to malaria control and elimination: insights from mathematical models. Vaccine. 2015;33:7544–7550. doi: 10.1016/J.VACCINE.2015.09.099. [DOI] [PubMed] [Google Scholar]
- 35.Penny M.A., Camponovo F., Chitnis N., Smith T.A., Tanner M. Future use-cases of vaccines in malaria control and elimination. Parasite Epidemiol. Control. 2020;10:e00145. doi: 10.1016/j.parepi.2020.e00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macintyre F., Ramachandruni H., Burrows J.N., Holm R., Thomas A., Möhrle J.J., Duparc S., Hooft van Huijsduijnen R., Greenwood B., Gutteridge W.E., et al. Injectable anti-malarials revisited: discovery and development of new agents to protect against malaria. Malar. J. 2018;17:402. doi: 10.1186/S12936-018-2549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graumans W., Jacobs E., Bousema T., Sinnis P. When is a plasmodium-infected mosquito an infectious mosquito? Trends Parasitol. 2020;36:705–716. doi: 10.1016/J.PT.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drexler A.L., Vodovotz Y., Luckhart S. Plasmodium development in the mosquito: biology bottlenecks and opportunities for mathematical modeling. Trends Parasitol. 2008;24:333–336. doi: 10.1016/J.PT.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ménard R., Tavares J., Cockburn I., Markus M., Zavala F., Amino R. Looking under the skin: the first steps in malarial infection and immunity. Nat. Rev. Microbiol. 2013;11:701–712. doi: 10.1038/nrmicro3111. [DOI] [PubMed] [Google Scholar]
- 40.Amino R., Thiberge S., Martin B., Celli S., Shorte S., Frischknecht F., Ménard R. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat. Med. 2006;12:220–224. doi: 10.1038/NM1350. [DOI] [PubMed] [Google Scholar]
- 41.Yamauchi L.M., Coppi A., Snounou G., Sinnis P. Plasmodium sporozoites trickle out of the injection site. Cell Microbiol. 2007;9:1215–1222. doi: 10.1111/J.1462-5822.2006.00861.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ejigiri I., Sinnis P. Plasmodium sporozoite-host interactions from the dermis to the hepatocyte. Curr. Opin. Microbiol. 2009;12:401–407. doi: 10.1016/J.MIB.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hopp C., Sinnis P. The ins and outs of sporozoite biology in the dermis. Malar. J. 2014;13(Suppl 1):O6. doi: 10.1186/1475-2875-13-S1-O6. [DOI] [Google Scholar]
- 44.Tewari R., Spaccapelo R., Bistoni F., Holder A.A., Crisanti A. Function of region I and II adhesive motifs of Plasmodium falciparum circumsporozoite protein in sporozoite motility and infectivity. J. Biol. Chem. 2002;277:47613–47618. doi: 10.1074/JBC.M208453200. [DOI] [PubMed] [Google Scholar]
- 45.Ménard R., Sultan A.A., Cortes C., Altszuler R., van Dijk M.R., Janse C.J., Waters A.P., Nussenzweig R.S., Nussenzweig V. Circumsporozoite protein is required for development of malaria sporozoites in mosquitoes. Nature. 1997;385:336–340. doi: 10.1038/385336A0. [DOI] [PubMed] [Google Scholar]
- 46.Rathore D., Sacci J.B., De La Vega P., McCutchan T.F. Binding and invasion of liver cells by Plasmodium falciparum sporozoites. Essential involvement of the amino terminus of circumsporozoite protein. J. Biol. Chem. 2002;277:7092–7098. doi: 10.1074/JBC.M106862200. [DOI] [PubMed] [Google Scholar]
- 47.Casares S., Brumeanu T.D., Richie T.L. The RTS,S malaria vaccine. Vaccine. 2010;28:4880–4894. doi: 10.1016/J.VACCINE.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 48.Kisalu N.K., Idris A.H., Weidle C., Flores-Garcia Y., Flynn B.J., Sack B.K., Murphy S., Schön A., Freire E., Francica J.R., et al. A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat. Med. 2018;24:408–416. doi: 10.1038/NM.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan J., Sack B.K., Oyen D., Zenklusen I., Piccoli L., Barbieri S., Foglierini M., Fregni C.S., Marcandalli J., Jongo S., et al. A public antibody lineage that potently inhibits malaria infection through dual binding to the circumsporozoite protein. Nat. Med. 2018;24:401–407. doi: 10.1038/nm.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oyen D., Torres J.L., Cottrell C.A., Richter King C., Wilson I.A., Ward A.B. Cryo-EM structure of P. falciparum circumsporozoite protein with a vaccine-elicited antibody is stabilized by somatically mutated inter-Fab contacts. Sci. Adv. 2018;4:eaau8529. doi: 10.1126/sciadv.aau8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foquet L., Hermsen C.C., Van Gemert G.J., Van Braeckel E., Weening K.E., Sauerwein R., Meuleman P., Leroux-Roels G. Vaccine-induced monoclonal antibodies targeting circumsporozoite protein prevent Plasmodium falciparum infection. J. Clin. Invest. 2014;124:140–144. doi: 10.1172/JCI70349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sack B.K., Miller J.L., Vaughan A.M., Douglass A., Kaushansky A., Mikolajczak S., Coppi A., Gonzalez-Aseguinolaza G., Tsuji M., Zavala F., et al. Model for in vivo assessment of humoral protection against malaria sporozoite challenge by passive transfer of monoclonal antibodies and immune serum. Infect. Immun. 2014;82:808–817. doi: 10.1128/IAI.01249-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L.T., Pereira L.S., Flores-Garcia Y., O'Connor J., Flynn B.J., Schön A., Hurlburt N.K., Dillon M., Yang A.S.P., Fabra-García A., et al. A potent anti-malarial human monoclonal antibody targets circumsporozoite protein minor repeats and neutralizes sporozoites in the liver. Immunity. 2020;53:733–744.e8. doi: 10.1016/J.IMMUNI.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schäfer C., Dambrauskas N., Reynolds L.M., Trakhimets O., Raappana A., Flannery E.L., Roobsoong W., Sattabongkot J., Mikolajczak S.A., Kappe S.H.I., Sather D.N. Partial protection against P. vivax infection diminishes hypnozoite burden and blood-stage relapses. Cell Host Microbe. 2021;29:752–756.e4. doi: 10.1016/j.chom.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 55.White M., Amino R., Mueller I. Theoretical implications of a pre-erythrocytic plasmodium vivax vaccine for preventing relapses. Trends Parasitol. 2017;33:260–263. doi: 10.1016/j.pt.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plassmeyer M.L., Reiter K., Shimp R.L., Kotova S., Smith P.D., Hurt D.E., House B., Zou X., Zhang Y., Hickman M., et al. Structure of the Plasmodium falciparum circumsporozoite protein, a leading malaria vaccine candidate. J. Biol. Chem. 2009;284:26951–26963. doi: 10.1074/JBC.M109.013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flores-Garcia Y., Wang L.T., Park M., Asady B., Idris A.H., Kisalu N.K., Muñoz C., Pereira L.S., Francica J.R., Seder R.A., Zavala F. The P. falciparum CSP repeat region contains three distinct epitopes required for protection by antibodies in vivo. PLoS Pathog. 2021;17:e1010042. doi: 10.1371/journal.ppat.1010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaudinski M.R., Berkowitz N.M., Idris A.H., Coates E.E., Holman L.A., Mendoza F., Gordon I.J., Plummer S.H., Trofymenko O., Hu Z., et al. A monoclonal antibody for malaria prevention. N. Engl. J. Med. 2021;385:803–814. doi: 10.1056/NEJMOA2034031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sack B., Kappe S.H., Sather D.N. Towards functional antibody-based vaccines to prevent pre-erythrocytic malaria infection. Expert Rev. Vaccin. 2017;16:403–414. doi: 10.1080/14760584.2017.1295853. [DOI] [PubMed] [Google Scholar]
- 60.Fabra-García A., Yang A.S.P., Behet M.C., Yap X.Z., van Waardenburg Y., Kaviraj S., Lanke K., van Gemert G.-J., Jore M.M., Bousema T., Sauerwein R.W. Human antibodies against non-circumsporozoite proteins block Plasmodium falciparum parasite development in hepatocytes. JCI Insight. 2022;7:e153524. doi: 10.1172/JCI.INSIGHT.153524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sultan A.A., Thathy V., Frevert U., Robson K.J., Crisanti A., Nussenzweig V., Nussenzweig R.S., Ménard R. TRAP is necessary for gliding motility and infectivity of plasmodium sporozoites. Cell. 1997;90:511–522. doi: 10.1016/S0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- 62.Müller H.M., Reckmann I., Hollingdale M.R., Bujard H., Robson K.J., Crisanti A. Thrombospondin related anonymous protein (TRAP) of Plasmodium falciparum binds specifically to sulfated glycoconjugates and to HepG2 hepatoma cells suggesting a role for this molecule in sporozoite invasion of hepatocytes. EMBO J. 1993;12:2881–2889. doi: 10.1002/j.1460-2075.1993.tb05950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jimah J.R., Salinas N.D., Sala-Rabanal M., Jones N.G., Sibley L.D., Nichols C.G., Schlesinger P.H., Tolia N.H. Malaria parasite CelTOS targets the inner leaflet of cell membranes for pore-dependent disruption. Elife. 2016;5:e20621. doi: 10.7554/eLife.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kester K.E., Gray Heppner D., Moris P., Ofori-Anyinam O., Krzych U., Tornieporth N., McKinney D., Delchambre M., Ockenhouse C.F., Voss G., et al. Sequential Phase 1 and Phase 2 randomized, controlled trials of the safety, immunogenicity and efficacy of combined pre-erythrocytic vaccine antigens RTS,S and TRAP formulated with AS02 Adjuvant System in healthy, malaria naïve adults. Vaccine. 2014;32:6683–6691. doi: 10.1016/J.VACCINE.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 65.Rampling T., Ewer K.J., Bowyer G., Edwards N.J., Wright D., Sridhar S., Payne R., Powlson J., Bliss C., Venkatraman N., et al. Safety and efficacy of novel malaria vaccine regimens of RTS,S/AS01B alone, or with concomitant Chad63-MVA-vectored vaccines expressing ME-TRAP. NPJ Vaccin. 2018;3:49. doi: 10.1038/s41541-018-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Atcheson E., Bauza K., Salman A.M., Alves E., Blight J., Viveros-Sandoval M.E., Janse C.J., Khan S.M., Hill A.V.S., Reyes-Sandoval A. Tailoring a plasmodium vivax vaccine to enhance efficacy through a combination of a CSP virus-like particle and TRAP viral vectors. Infect. Immun. 2018;86:e00114-18. doi: 10.1128/IAI.00114-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu C., Song G., Beale K., Yan J., Garst E., Feng J., Lund E., Catteruccia F., Springer T.A. Design and assessment of TRAP-CSP fusion antigens as effective malaria vaccines. PLoS One. 2020;15:e0216260. doi: 10.1371/JOURNAL.PONE.0216260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilder B.K., Vigdorovich V., Carbonetti S., Minkah N., Hertoghs N., Raappana A., Cardamone H., Oliver B.G., Trakhimets O., Kumar S., et al. Anti-TRAP/SSP2 monoclonal antibodies can inhibit sporozoite infection and 1 enhance protection of anti-CSP monoclonal antibodies 2 3. BioRxiv. 2021 doi: 10.1101/2021.10.15.464611. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kariu T., Ishino T., Yano K., Chinzei Y., Yuda M. CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts. Mol. Microbiol. 2006;59:1369–1379. doi: 10.1111/J.1365-2958.2005.05024.X. [DOI] [PubMed] [Google Scholar]
- 70.Bergmann-Leitner E.S., Legler P.M., Savranskaya T., Ockenhouse C.F., Angov E. Cellular and humoral immune effector mechanisms required for sterile protection against sporozoite challenge induced with the novel malaria vaccine candidate CelTOS. Vaccine. 2011;29:5940–5949. doi: 10.1016/J.VACCINE.2011.06.053. [DOI] [PubMed] [Google Scholar]
- 71.Doolan D.L., Southwood S., Freilich D.A., Sidney J., Graber N.L., Shatney L., Bebris L., Florens L., Dobano C., Witney A.A., et al. Identification of Plasmodium falciparum antigens by antigenic analysis of genomic and proteomic data. Proc. Natl. Acad. Sci. U S A. 2003;100:9952–9957. doi: 10.1073/pnas.1633254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bergmann-Leitner E.S., Mease R.M., de la Vega P., Savranskaya T., Polhemus M., Ockenhouse C., Angov E. Immunization with pre-erythrocytic antigen CelTOS from Plasmodium falciparum elicits cross-species protection against heterologous challenge with Plasmodium berghei. PLoS One. 2010;5:e12294. doi: 10.1371/JOURNAL.PONE.0012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Espinosa D.A., Vega-Rodriguez J., Flores-Garcia Y., Noe A.R., Muñoz C., Coleman R., Bruck T., Haney K., Stevens A., Retallack D., et al. The Plasmodium falciparum cell-traversal protein for ookinetes and sporozoites as a candidate for preerythrocytic and transmission-blocking vaccines. Infect. Immun. 2017;85:e00498-16. doi: 10.1128/IAI.00498-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miura K. Progress and prospects for blood-stage malaria vaccines. Expert Rev. Vaccin. 2016;15:765–781. doi: 10.1586/14760584.2016.1141680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Das S., Hertrich N., Perrin A.J., Withers-Martinez C., Collins C.R., Jones M.L., Watermeyer J.M., Fobes E.T., Martin S.R., Saibil H.R., et al. Processing of plasmodium falciparum merozoite surface protein MSP1 activates a spectrin-binding function enabling parasite egress from RBCs. Cell Host Microbe. 2015;18:433–444. doi: 10.1016/j.chom.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blackman M.J., Scott-Finnigan T.J., Shai S., Holder A.A. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J. Exp. Med. 1994;180:389–393. doi: 10.1084/jem.180.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin C.S., Uboldi A.D., Marapana D., Czabotar P.E., Epp C., Bujard H., Taylor N.L., Perugini M.A., Hodder A.N., Cowman A.F. The merozoite surface protein 1 complex is a platform for binding to human erythrocytes by plasmodium falciparum. J. Biol. Chem. 2014;289:25655–25669. doi: 10.1074/jbc.M114.586495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McBride J.S., Heidrich H.G. Fragments of the polymorphic Mr 185,000 glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol. Biochem. Parasitol. 1987;23:71–84. doi: 10.1016/0166-6851(87)90189-7. [DOI] [PubMed] [Google Scholar]
- 79.Lin C.S., Uboldi A.D., Epp C., Bujard H., Tsuboi T., Czabotar P.E., Cowman A.F. Multiple plasmodium falciparum merozoite surface protein 1 complexes mediate merozoite binding to human erythrocytes. J. Biol. Chem. 2016;291:7703–7715. doi: 10.1074/jbc.M115.698282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Woehlbier U., Epp C., Kauth C.W., Lutz R., Long C.A., Coulibaly B., Kouyaté B., Arevalo-Herrera M., Herrera S., Bujard H. Analysis of antibodies directed against merozoite surface protein 1 of the human malaria parasite Plasmodium falciparum. Infect. Immun. 2006;74:1313–1322. doi: 10.1128/IAI.74.2.1313-1322.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baum J., Chen L., Healer J., Lopaticki S., Boyle M., Triglia T., Ehlgen F., Ralph S.A., Beeson J.G., Cowman A.F. Reticulocyte-binding protein homologue 5 - an essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. Int. J. Parasitol. 2009;39:371–380. doi: 10.1016/J.IJPARA.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 82.Payne R.O., Silk S.E., Elias S.C., Miura K., Diouf A., Galaway F., de Graaf H., Brendish N.J., Poulton I.D., Griffiths O.J., et al. Human vaccination against RH5 induces neutralizing antimalarial antibodies that inhibit RH5 invasion complex interactions. JCI Insight. 2017;2:e96381. doi: 10.1172/JCI.INSIGHT.96381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wong W., Huang R., Menant S., Hong C., Sandow J.J., Birkinshaw R.W., Healer J., Hodder A.N., Kanjee U., Tonkin C.J., et al. Structure of Plasmodium falciparum Rh5-CyRPA-Ripr invasion complex. Nature. 2019;565:118–121. doi: 10.1038/S41586-018-0779-6. [DOI] [PubMed] [Google Scholar]
- 84.Volz J.C., Yap A., Sisquella X., Thompson J.K., Lim N.T., Whitehead L.W., Chen L., Lampe M., Tham W.H., Wilson D., et al. Essential role of the PfRh5/PfRipr/CyRPA complex during plasmodium falciparum invasion of erythrocytes. Cell Host Microbe. 2016;20:60–71. doi: 10.1016/j.chom.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 85.Douglas A.D., Baldeviano G.C., Lucas C.M., Lugo-Roman L.A., Crosnier C., Bartholdson S.J., Diouf A., Miura K., Lambert L.E., Ventocilla J.A., et al. A PfRH5-based vaccine is efficacious against heterologous strain blood-stage plasmodium falciparum infection in Aotus monkeys. Cell Host Microbe. 2015;17:130–139. doi: 10.1016/j.chom.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Minassian A.M., Silk S.E., Barrett J.R., Nielsen C.M., Miura K., Diouf A., Loos C., Fallon J.K., Michell A.R., White M.T., et al. Reduced blood-stage malaria growth and immune correlates in humans following RH5 vaccination. Med. 2021;2:701–719.e19. doi: 10.1016/j.medj.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ord R.L., Caldeira J.C., Rodriguez M., Noe A., Chackerian B., Peabody D.S., Gutierrez G., Lobo C.A. A malaria vaccine candidate based on an epitope of the Plasmodium falciparum RH5 protein. Malar. J. 2014;13:326. doi: 10.1186/1475-2875-13-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Douglas A.D., Baldeviano G.C., Jin J., Miura K., Diouf A., Zenonos Z.A., Ventocilla J.A., Silk S.E., Marshall J.M., Alanine D.G.W., et al. A defined mechanistic correlate of protection against Plasmodium falciparum malaria in non-human primates. Nat. Commun. 2019;10:1953. doi: 10.1038/s41467-019-09894-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Healer J., Wong W., Thompson J.K., He W., Birkinshaw R.W., Miura K., Long C.A., Soroka V., Søgaard T.M.M., Jørgensen T., et al. Neutralising antibodies block the function of Rh5/Ripr/CyRPA complex during invasion of Plasmodium falciparum into human erythrocytes. Cell Microbiol. 2019;21:e13030. doi: 10.1111/cmi.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moya-Alvarez V., Abellana R., Cot M. Pregnancy-associated malaria and malaria in infants: an old problem with present consequences. Malar. J. 2014;13:271. doi: 10.1186/1475-2875-13-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Srivastava A., Gangnard S., Round A., Dechavanne S., Juillerat A., Raynal B., Faure G., Baron B., Ramboarina S., Singh S.K., et al. Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA. Proc. Natl. Acad. Sci. U S A. 2010;107:4884–4889. doi: 10.1073/pnas.1000951107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salanti A., Dahlbäck M., Turner L., Nielsen M.A., Barfod L., Magistrado P., Jensen A.T., Lavstsen T., Ofori M.F., Marsh K., et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 2004;200:1197–1203. doi: 10.1084/JEM.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Salanti A., Staalsoe T., Lavstsen T., Jensen A.T., Sowa M.P., Arnot D.E., Hviid L., Theander T.G. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 2003;49:179–191. doi: 10.1046/J.1365-2958.2003.03570.X. [DOI] [PubMed] [Google Scholar]
- 94.Healy S.A., Fried M., Richie T., Bok K., Little M., August A., Riley L., Swamy G.K., Wylie B.J., Menendez C., et al. Malaria vaccine trials in pregnant women: an imperative without precedent. Vaccine. 2019;37:763–770. doi: 10.1016/J.VACCINE.2018.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bockhorst J., Lu F., Janes J.H., Keebler J., Gamain B., Awadalla P., Su X.Z., Samudrala R., Jojic N., Smith J.D. Structural polymorphism and diversifying selection on the pregnancy malaria vaccine candidate VAR2CSA. Mol. Biochem. Parasitol. 2007;155:103–112. doi: 10.1016/J.MOLBIOPARA.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 96.Pham-Huy A., Top K.A., Constantinescu C., Seow C.H., El-Chaâr D. The use and impact of monoclonal antibody biologics during pregnancy. CMAJ. 2021;193:E1129–E1136. doi: 10.1503/cmaj.202391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Adams J.H., Blair P.L., Kaneko O., Peterson D.S. An expanding ebl family of Plasmodium falciparum. Trends Parasitol. 2001;17:297–299. doi: 10.1016/S1471-4922(01)01948-1. [DOI] [PubMed] [Google Scholar]
- 98.Miller L.H., Mason S.J., Dvorak J.A., Mcginniss M.H., Rothman I.K. Erythrocyte receptors for (Plasmodium knowlesi) malaria: duffy blood group determinants. Science. 1975;189:561–563. doi: 10.1126/SCIENCE.1145213. [DOI] [PubMed] [Google Scholar]
- 99.Ceravolo I.P., Souza-Silva F.A., Fontes C.J., Braga E.M., Madureira A.P., Krettli A.U., Souza J.M., Brito C.F., Adams J.H., Carvalho L.H. Inhibitory properties of the antibody response to Plasmodium vivax Duffy binding protein in an area with unstable malaria transmission. Scand. J. Immunol. 2008;67:270–278. doi: 10.1111/J.1365-3083.2007.02059.X. [DOI] [PubMed] [Google Scholar]
- 100.Rawlinson T.A., Barber N.M., Mohring F., Cho J.S., Kosaisavee V., Gérard S.F., Alanine D.G.W., Labbé G.M., Elias S.C., Silk S.E., et al. Structural basis for inhibition of Plasmodium vivax invasion by a broadly neutralizing vaccine-induced human antibody. Nat. Microbiol. 2019;4:1497–1507. doi: 10.1038/s41564-019-0462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tsuboi T., Kappe S.H., Al-Yaman F., Prickett M.D., Alpers M., Adams J.H. Natural variation within the principal adhesion domain of the Plasmodium vivax duffy binding protein. Infect. Immun. 1994;62:5581–5586. doi: 10.1128/IAI.62.12.5581-5586.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ntumngia F.B., Adams J.H. Design and immunogenicity of a novel synthetic antigen based on the ligand domain of the Plasmodium vivax duffy binding protein. Clin. Vaccin. Immunol. 2012;19:30–36. doi: 10.1128/CVI.05466-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ntumngia F.B., Schloegel J., Barnes S.J., McHenry A.M., Singh S., King C.L., Adams J.H. Conserved and variant epitopes of Plasmodium vivax duffy binding protein as targets of inhibitory monoclonal antibodies. Infect. Immun. 2012;80:1203–1208. doi: 10.1128/IAI.05924-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carias L.L., Dechavanne S., Nicolete V.C., Sreng S., Suon S., Amaratunga C., Fairhurst R.M., Dechavanne C., Barnes S., Witkowski B., et al. Identification and characterization of functional human monoclonal antibodies to plasmodium vivax duffy-binding protein. J. Immunol. 2019;202:2648–2660. doi: 10.4049/jimmunol.1801631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Urusova D., Carias L., Huang Y., Nicolete V.C., Popovici J., Roesch C., Salinas N.D., Dechavanne S., Witkowski B., Ferreira M.U., et al. Structural basis for neutralization of Plasmodium vivax by naturally acquired human antibodies that target DBP. Nat. Microbiol. 2019;4:1486–1496. doi: 10.1038/s41564-019-0461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gruszczyk J., Huang R.K., Chan L.J., Menant S., Hong C., Murphy J.M., Mok Y.F., Griffin M.D.W., Pearson R.D., Wong W., et al. Cryo-EM structure of an essential Plasmodium vivax invasion complex. Nature. 2018;559:135–139. doi: 10.1038/s41586-018-0249-1. [DOI] [PubMed] [Google Scholar]
- 107.Gruszczyk J., Kanjee U., Chan L.J., Menant S., Malleret B., Lim N.T.Y., Schmidt C.Q., Mok Y.F., Lin K.M., Pearson R.D., et al. Transferrin receptor 1 is a reticulocyte-specific receptor for Plasmodium vivax. Science. 2018;359:48–55. doi: 10.1126/science.aan1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Minassian A.M., Themistocleous Y., Silk S.E., Barrett J.R., Kemp A., Quinkert D., Nielsen C.M., Edwards N.J., Rawlinson T.A., Ramos Lopez F., et al. Controlled human malaria infection with a clone of Plasmodium vivax with high-quality genome assembly. JCI Insight. 2021;6:e152465. doi: 10.1172/JCI.INSIGHT.152465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Collins K.A., Abd-Rahman A.N., Marquart L., Ballard E., Gobeau N., Griffin P., Chalon S., Möhrle J.J., McCarthy J.S. Antimalarial activity of artefenomel against asexual parasites and transmissible gametocytes during experimental blood-stage plasmodium vivax infection. J. Infect. Dis. 2022;225:1062–1069. doi: 10.1093/INFDIS/JIAA287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Collins K.A., Wang C.Y., Adams M., Mitchell H., Rampton M., Elliott S., Reuling I.J., Bousema T., Sauerwein R., Chalon S., et al. A controlled human malaria infection model enabling evaluation of transmission-blocking interventions. J. Clin. Invest. 2018;128:1551–1562. doi: 10.1172/JCI98012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Griffin P., Pasay C., Elliott S., Sekuloski S., Sikulu M., Hugo L., Khoury D., Cromer D., Davenport M., Sattabongkot J., et al. Safety and reproducibility of a clinical trial system using induced blood stage plasmodium vivax infection and its potential as a model to evaluate malaria transmission. PLoS Negl. Trop. Dis. 2016;10:e0005139. doi: 10.1371/journal.pntd.0005139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McCarthy J.S., Griffin P.M., Sekuloski S., Bright A.T., Rockett R., Looke D., Elliott S., Whiley D., Sloots T., Winzeler E.A., Trenholme K.R. Experimentally induced blood-stage Plasmodium vivax infection in healthy volunteers. J. Infect. Dis. 2013;208:1688–1694. doi: 10.1093/INFDIS/JIT394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ventocilla J., Tapia L.L., Sperling L., Ponce R., Franco A., Leelawong M., Aguiar J.C., Baldeviano G.C., Wilder B.K. Analysis of pre-erythrocytic immunity during plasmodium vivax infection reveals a diversity of responses that is partially due to blood stage cross-reactivity. BioRxiv. 2021 doi: 10.21203/RS.3.RS-518437/V1. Preprint at. [DOI] [Google Scholar]
- 114.Schneider P., Reece S.E. The private life of malaria parasites: strategies for sexual reproduction. Mol. Biochem. Parasitol. 2021;244:111375. doi: 10.1016/J.MOLBIOPARA.2021.111375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith R.C., Vega-Rodríguez J., Jacobs-Lorena M. The Plasmodium bottleneck: malaria parasite losses in the mosquito vector. Mem. Inst. Oswaldo Cruz. 2014;109:644–661. doi: 10.1590/0074-0276130597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kuehn A., Pradel G. The coming-out of malaria gametocytes. J. Biomed. Biotechnol. 2010;2010:976827. doi: 10.1155/2010/976827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Duffy P.E. Transmission-blocking vaccines: harnessing herd immunity for malaria elimination. Expert Rev. Vaccin. 2021;20:185–198. doi: 10.1080/14760584.2021.1878028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miura K., Tachibana M., Takashima E., Morita M., Kanoi B.N., Nagaoka H., Baba M., Torii M., Ishino T., Tsuboi T. Malaria transmission-blocking vaccines: wheat germ cell-free technology can accelerate vaccine development. Expert Rev. Vaccin. 2019;18:1017–1027. doi: 10.1080/14760584.2019.1674145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Niederwieser I., Felger I., Beck H.P. Limited polymorphism in Plasmodium falciparum sexual-stage antigens. Am. J. Trop. Med. Hyg. 2001;64:9–11. doi: 10.4269/AJTMH.2001.64.9. [DOI] [PubMed] [Google Scholar]
- 120.Vallejo A.F., Martinez N.L., Tobon A., Alger J., Lacerda M.V., Kajava A.V., Arévalo-Herrera M., Herrera S. Global genetic diversity of the Plasmodium vivax transmission-blocking vaccine candidate Pvs48/45. Malar. J. 2016;15:202. doi: 10.1186/s12936-016-1263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patel P.N., Tolia N. Structural vaccinology of malaria transmission-blocking vaccines. Expert Rev. Vaccin. 2021;20:199–214. doi: 10.1080/14760584.2021.1873135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.de Jong R.M., Meerstein-Kessel L., Da D.F., Nsango S., Challenger J.D., van de Vegte-Bolmer M., van Gemert G.J., Duarte E., Teyssier N., Sauerwein R.W., et al. Monoclonal antibodies block transmission of genetically diverse Plasmodium falciparum strains to mosquitoes. NPJ Vaccin. 2021;6:101. doi: 10.1038/S41541-021-00366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.de Jong R.M., Tebeje S.K., Meerstein-Kessel L., Tadesse F.G., Jore M.M., Stone W., Bousema T. Immunity against sexual stage Plasmodium falciparum and Plasmodium vivax parasites. Immunol. Rev. 2020;293:190–215. doi: 10.1111/imr.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Singh S.K., Thrane S., Chourasia B.K., Teelen K., Graumans W., Stoter R., van Gemert G.J., van de Vegte-Bolmer M.G., Nielsen M.A., Salanti A., et al. Pfs230 and pfs48/45 fusion proteins elicit strong transmission-blocking antibody responses against Plasmodium falciparum. Front. Immunol. 2019;10:1256. doi: 10.3389/FIMMU.2019.01256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Challenger J.D., Olivera Mesa D., Da D.F., Yerbanga R.S., Lefèvre T., Cohuet A., Churcher T.S. Predicting the public health impact of a malaria transmission-blocking vaccine. Nat. Commun. 2021;12:1494. doi: 10.1038/s41467-021-21775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Teboh-Ewungkem M.I., Woldegerima W.A., Ngwa G.A. Mathematical assessment of the impact of human-antibodies on sporogony during the within-mosquito dynamics of Plasmodium falciparum parasites. J. Theor. Biol. 2021;515:110562. doi: 10.1016/J.JTBI.2020.110562. [DOI] [PubMed] [Google Scholar]
- 127.McCoy K.D., Weldon C.T., Ansumana R., Lamin J.M., Stenger D.A., Ryan S.J., Bardosh K., Jacobsen K.H., Dinglasan R.R. Are malaria transmission-blocking vaccines acceptable to high burden communities? Results from a mixed methods study in Bo, Sierra Leone. Malar. J. 2021;20:183. doi: 10.1186/S12936-021-03723-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nunes J.K., Woods C., Carter T., Raphael T., Morin M.J., Diallo D., Leboulleux D., Jain S., Loucq C., Kaslow D.C., Birkett A.J. Development of a transmission-blocking malaria vaccine: progress, challenges, and the path forward. Vaccine. 2014;32:5531–5539. doi: 10.1016/j.vaccine.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 129.White S.E., Harvey S.A., Meza G., Llanos A., Guzman M., Gamboa D., Vinetz J.M. Acceptability of a herd immunity-focused, transmission-blocking malaria vaccine in malaria-endemic communities in the Peruvian Amazon: an exploratory study. Malar. J. 2018;17:179. doi: 10.1186/S12936-018-2328-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Theisen M., Jore M.M., Sauerwein R. Towards clinical development of a Pfs48/45-based transmission blocking malaria vaccine. Expert Rev. Vaccin. 2017;16:329–336. doi: 10.1080/14760584.2017.1276833. [DOI] [PubMed] [Google Scholar]
- 131.Williamson K.C. Pfs230: from malaria transmission-blocking vaccine candidate toward function. Parasite Immunol. 2003;25:351–359. doi: 10.1046/j.1365-3024.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- 132.Singh K., Burkhardt M., Nakuchima S., Herrera R., Muratova O., Gittis A.G., Kelnhofer E., Reiter K., Smelkinson M., Veltri D., et al. Structure and function of a malaria transmission blocking vaccine targeting Pfs230 and Pfs230-Pfs48/45 proteins. Commun. Biol. 2020;3:395. doi: 10.1038/S42003-020-01123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Singh S.K., Plieskatt J., Chourasia B.K., Singh V., Bengtsson K.L., Reimer J.M., van Daalen R.C., Teelen K., van de Vegte-Bolmer M., van Gemert G.J., et al. Preclinical development of a Pfs230-Pfs48/45 chimeric malaria transmission-blocking vaccine. NPJ Vaccin. 2021;6:120. doi: 10.1038/S41541-021-00383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zheng W., Liu F., He Y., Liu Q., Humphreys G.B., Tsuboi T., Fan Q., Luo E., Cao Y., Cui L. Functional characterization of Plasmodium berghei PSOP25 during ookinete development and as a malaria transmission-blocking vaccine candidate. Parasites Vectors. 2017;10:8–11. doi: 10.1186/S13071-016-1932-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang F., Liu F., Yu X., Zheng W., Wu Y., Qiu Y., Jin Y., Cui L., Cao Y. Evaluation of two sexual-stage antigens as bivalent transmission-blocking vaccines in rodent malaria. Parasites Vectors. 2021;14:241. doi: 10.1186/s13071-021-04743-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Langer R.C., Li F., Popov V., Kurosky A., Vinetz J.M. Monoclonal antibody against the Plasmodium falciparum chitinase, PfCHT1, recognizes a malaria transmission-blocking epitope in Plasmodium gallinaceum ookinetes unrelated to the chitinase PgCHT1. Infect. Immun. 2002;70:1581–1590. doi: 10.1128/IAI.70.3.1581-1590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li F., Patra K.P., Vinetz J.M. An anti-Chitinase malaria transmission-blocking single-chain antibody as an effector molecule for creating a Plasmodium falciparum-refractory mosquito. J. Infect. Dis. 2005;192:878–887. doi: 10.1086/432552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dinglasan R.R., Kalume D.E., Kanzok S.M., Ghosh A.K., Muratova O., Pandey A., Jacobs-Lorena M. Disruption of Plasmodium falciparum development by antibodies against a conserved mosquito midgut antigen. Proc. Natl. Acad. Sci. U S A. 2007;104:13461–13466. doi: 10.1073/pnas.0702239104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Atkinson S.C., Armistead J.S., Mathias D.K., Sandeu M.M., Tao D., Borhani-Dizaji N., Tarimo B.B., Morlais I., Dinglasan R.R., Borg N.A. The Anopheles-midgut APN1 structure reveals a new malaria transmission-blocking vaccine epitope. Nat. Struct. Mol. Biol. 2015;22:532–539. doi: 10.1038/NSMB.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Howard G.P., Bender N.G., Khare P., López-Gutiérrez B., Nyasembe V., Weiss W.J., Simecka J.W., Hamerly T., Mao H.Q., Dinglasan R.R. Immunopotentiation by lymph-node targeting of a malaria transmission-blocking nanovaccine. Front. Immunol. 2021;12:729086. doi: 10.3389/FIMMU.2021.729086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Titus R.G., Bishop J.V., Mejia J.S. The immunomodulatory factors of arthropod saliva and the potential for these factors to serve as vaccine targets to prevent pathogen transmission. Parasite Immunol. 2006;28:131–141. doi: 10.1111/j.1365-3024.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 142.Dragovic S.M., Agunbiade T.A., Freudzon M., Yang J., Hastings A.K., Schleicher T.R., Zhou X., Craft S., Chuang Y.M., Gonzalez F., et al. Immunization with AgTRIO, a protein in Anopheles saliva, contributes to protection against plasmodium infection in mice. Cell Host Microbe. 2018;23:523–535.e5. doi: 10.1016/j.chom.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chuang Y.M., Agunbiade T.A., Tang X.D., Freudzon M., Almeras L., Fikrig E. The effects of a mosquito salivary protein on sporozoite traversal of host cells. J. Infect. Dis. 2021;224:544–553. doi: 10.1093/infdis/jiaa759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schleicher T.R., Yang J., Freudzon M., Rembisz A., Craft S., Hamilton M., Graham M., Mlambo G., Tripathi A.K., Li Y., et al. A mosquito salivary gland protein partially inhibits Plasmodium sporozoite cell traversal and transmission. Nat. Commun. 2018;9:2908. doi: 10.1038/s41467-018-05374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Manning J.E., Morens D.M., Kamhawi S., Valenzuela J.G., Memoli M. Mosquito saliva: the hope for a universal arbovirus vaccine? J. Infect. Dis. 2018;218:7–15. doi: 10.1093/INFDIS/JIY179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Manning J.E., Oliveira F., Coutinho-Abreu I.V., Herbert S., Meneses C., Kamhawi S., Baus H.A., Han A., Czajkowski L., Rosas L.A., et al. Safety and immunogenicity of a mosquito saliva peptide-based vaccine: a randomised, placebo-controlled, double-blind, phase 1 trial. Lancet. 2020;395:1998–2007. doi: 10.1016/S0140-6736(20)31048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Azasi Y., Gallagher S.K., Diouf A., Dabbs R.A., Jin J., Mian S.Y., Narum D.L., Long C.A., Gaur D., Draper S.J., et al. Bliss' and Loewe's additive and synergistic effects in Plasmodium falciparum growth inhibition by AMA1-RON2L, RH5, RIPR and CyRPA antibody combinations. Sci. Rep. 2020;10:11802. doi: 10.1038/S41598-020-67877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Alanine D.G.W., Quinkert D., Kumarasingha R., Mehmood S., Donnellan F.R., Minkah N.K., Dadonaite B., Diouf A., Galaway F., Silk S.E., et al. Human antibodies that slow erythrocyte invasion potentiate malaria-neutralizing antibodies. Cell. 2019;178:216–228.e21. doi: 10.1016/J.CELL.2019.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schäfer C., Roobsoong W., Kangwanrangsan N., Bardelli M., Rawlinson T.A., Dambrauskas N., Trakhimets O., Parthiban C., Goswami D., Reynolds L.M., et al. A humanized mouse model for plasmodium vivax to test interventions that block liver stage to blood stage transition and blood stage infection. iScience. 2020;23:101381. doi: 10.1016/J.ISCI.2020.101381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Foquet L., Schäfer C., Minkah N.K., Alanine D.G.W., Flannery E.L., Steel R.W.J., Sack B.K., Camargo N., Fishbaugher M., Betz W., et al. Plasmodium falciparum liver stage infection and transition to stable blood stage infection in liver-humanized and blood-humanized FRGN KO mice enables testing of blood stage inhibitory antibodies (reticulocyte-binding protein homolog 5) in vivo. Front. Immunol. 2018;9:524. doi: 10.3389/FIMMU.2018.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cooper M.M., Loiseau C., McCarthy J.S., Doolan D.L. Human challenge models: tools to accelerate the development of malaria vaccines. Expert Rev. Vaccin. 2019;18:241–251. doi: 10.1080/14760584.2019.1580577. [DOI] [PubMed] [Google Scholar]