Non-pharmaceutical interventions (NPIs) and vaccination programs have played crucial roles in mitigating a novel coronavirus disease 2019 (COVID-19) pandemic. The isolation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-infected individuals is one of the important and effective NPIs1. Many studies revealed that patients with mild-to-moderate illness confirmed with SARS-CoV-2 could not be infectious beyond 10 days after symptom onset2. Therefore, the guidelines were published for releasing COVID-19 patients from isolation 10 days after symptom onset, plus at least 3 additional days without symptoms. For severely ill COVID-19 patients, extending the duration of isolation and precautions up to 20 days after symptom onset and after resolution of fever and improvement of other symptoms may be warranted.
The emergence of SARS-CoV-2 variants of concern (VOCs) harboring mutations in the spike (S) protein has raised concerns about higher transmissibility and potential immune escape after vaccination and natural infection. The B.1.617.2 (delta) variant was detected in India in December 2020. New variants and SARS-CoV-2 vaccination strategies may influence the duration of viable virus shedding in confirmed patients3. In addition, corticosteroid and interleukin (IL)−6 blockade, tocilizumab, which have been recommended regimens in severe patients, may be associated with prolonged viral shedding4. Therefore, any recommendations which use a fixed number of days should incorporate various influences on viral shedding such as host susceptibility, vaccination status, and virus strains. In addition, more studies are needed to fully understand virus transmission related to the delta variant among severe patients. Since virus culture is important to assess the viability, and could be a surrogate for transmissibility, we aimed to identify the transmissibility of SARS-CoV-2 in severe patients who were infected with the delta variant in comparison to the original virus (Wuhan-hu-1).
Twenty-six patients who were hospitalized and diagnosed with delta variant infections between June and August 2021 were consecutively enrolled as the delta variant cohort. The cohort was compared to the original strain cohort, which comprised twenty-one original virus-infected patients who were consecutively hospitalized between February and June 2020. It was a single center cohort study at a referral hospital where COVID-19 patients at higher risk of severe infection were transferred, and antiviral therapy as well as other medication, including immunomodulator, was administered based on the guideline available at the time and clinical judgement. Our team has previously reported the result of the original virus culture to suggest the duration of transmissibility of the virus5. According to the study, samples from patients with original virus were collected 1, 3, 5, 7, 10, and 14 days after admission. In delta variant patients, samples from nasopharyngeal (NP) swabs and sputum were obtained at times decided by an attending physician. The study was approved by the Institutional Review Board of Severance Hospital (4–2020–0076) and written informed consent was obtained from all subjects.
We defined severity of the COVID-19 infection that mild patients required hospitalization without oxygen therapy, moderate patients required low flow oxygen therapy, severe patients required high-flow oxygen therapy, and critically ill patients required mechanical ventilation or extracorporeal membrane oxygenation. The methods of confirmation, identification and culture of SARS-CoV-2 methods were described in Supplementary method.
In the delta variant cohort, the patients whose samples were collected beyond 14 days was further divided in two groups: “late clearance (LC)” group which is defined as virus culture positive beyond 14 days after symptom onset and “early clearance (EC)” whose samples beyond 14 days but were culture negative.
The demographics and clinical characteristics of COVID-19 patients who were consecutively enrolled during different period are shown in Supplementary Table. The median age of patients infected with delta variants and original strains was 58 and 72, respectively. 77% of the delta variant group had a severe form of infection requiring high flow oxygen therapy, while only 28.6% patients were severe in original strain group. In addition, according to the guideline at the time, only 28.6% of patients with the original virus received the antiviral agent, remdesivir, but all delta variant patients were administered remdesivir. For the purpose of anti-inflammation, all and 24 out of 26 (92.3%) patients infected with delta variants whereas 8 out of 21 (38.1%) and no patients with original virus received steroids and IL-6 blockade, respectively.
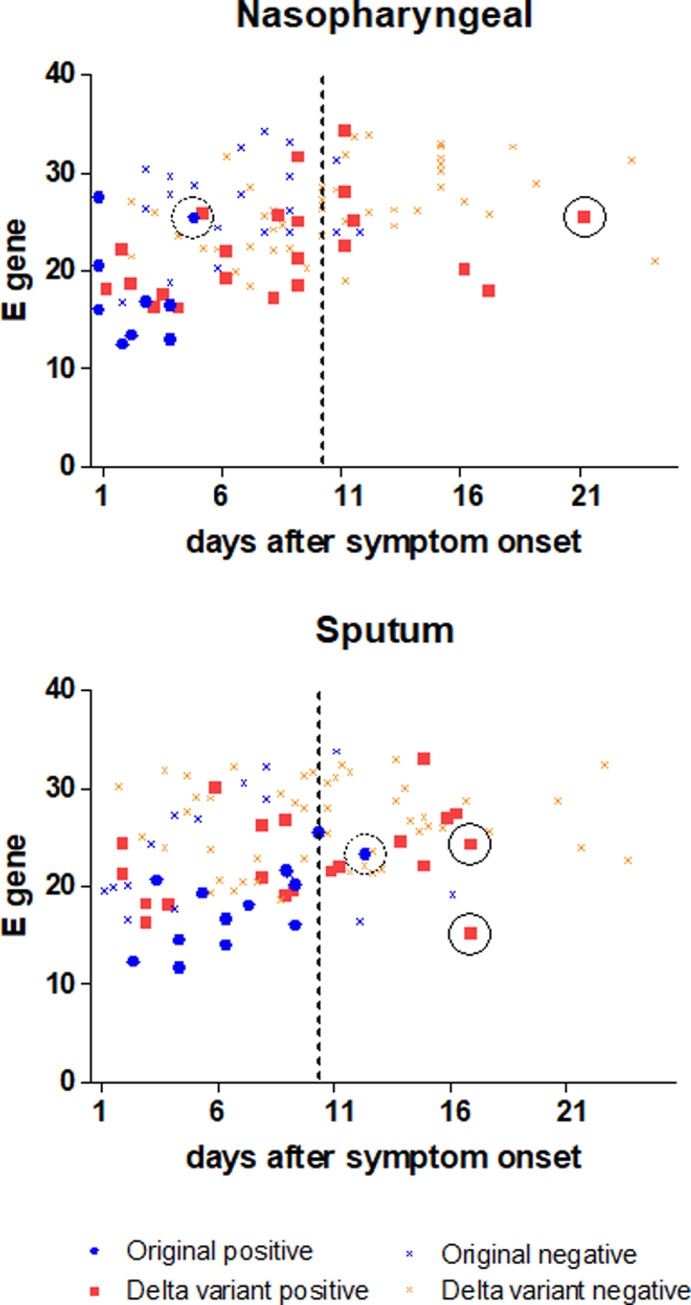
In the original virus group, 29 and 28 samples were from NP and sputum samples and 9 (31.0%) and 13 (46.4%) were culture-positive in collected NP and sputum samples, respectively. In the delta virus group, 67 and 68 samples, and 22 (32.8%), and 20 (29.4%) samples were cultured in NP and sputum, respectively. Cyclic threshold (Ct) values of collected samples from the delta variant and original SARS-CoV-2 virus with culture tests is described in Fig. 1 . We provided a Ct value for E gene of copy number quantification.
Fig. 1.
Cyclic threshold (Ct) value of collected samples with culture tests at time point after symptom onset in (A) nasopharyngeal, (B) sputum samples. Negative and positive mean culture-negative and culture-positive, respectively. The dashed lines indicate the general time of releasing COVID-19 patients who are not seriously ill, the solid circles show the latest presence of the viable Delta variant, and the dotted circles represent the latest presence of the viable original virus.
In the original virus group, all viable viruses in the NP samples was seen within 10 days after symptom onset, and three (10.7%) were culture positive beyond 10 days in the sputum samples5. In the delta variant group, seven (10.4%) and nine (13.2%) samples presented viable virus beyond 10 days in NP and sputum samples, respectively, implying delta-variant patients showed longer duration of viable virus in both nasopharyngeal and sputum samples than the original virus. In addition, Ct values of four from culture-positive samples in delta variant group were over 30 in culture-positive samples. One patient who was hospitalized with mechanical ventilation produced a viable virus from a NP swab 21 days after symptom onset.
In ad hoc analysis of the delta variant group, the demographics of both LC and EC group is described in Table 1 . All patients received steroid and IL-6 blockade, and the doses of corticosteroid were similar in both groups. In the LC group, critically ill patients represented half of the patients, but the severity between groups did not show statistical differences. The median age in the LC group was significantly higher than that in the EC group (p<0.001) and female has high portion in the LC group (p = 0.014). Epidemiologic studies show that the delta variant might be more virulent, increasing risk of hospitalization, intensive care unit (ICU) admission, and death6. In our study, most of the delta variant-infected patients had received critical care in ICU, ten of whom needed ventilation support and three, extracorporeal membrane oxygenation. They produced more proportion of replication-competent virus than original virus-infected patients beyond 10 days after symptom onset (10.4% vs. 0, 13.23% vs. 10.7% in nasopharyngeal and sputum samples, respectively), implying late viral clearance; one critically ill patient had viable virus in the sputum 15 days after symptom onset with a Ct value of 32.96. Few samples were obtained 20 days after symptom onset according to the study protocol, but a replication-competent virus was confirmed 21 days after symptom onset from a nasopharyngeal sample of 77-year-old male with underlying bladder cancer and coronary artery disease. Because of this culture result, the patient could have infected others via the viable virus beyond 20 days.
Table 1.
Demographics of late clearance (LC) and early clearance (EC) groups in delta variant confirmed patients.
| LC (n = 8) | EC (n = 13) | P-value | |
|---|---|---|---|
| Sex, male | 3 (37.5) | 12 (92.3) | 0.014 |
| Age, years, median [IQR] | 63 [56–73.3] | 58 [50–63] | <0.001 |
| Comorbidities | |||
| HTN | 4 (50) | 2 (23.1) | 0.346 |
| DM | 2 (25) | 2 (15.4) | 0.618 |
| Heart failure | 1 (12.5) | 2 (15.4) | 1.0 |
| Malignancy | 1 (12.5) | 1 (7.7) | 1.0 |
| Immunosuppressed | 0 | 1 (7.7) | 1.0 |
| Severity1 | 0.67 | ||
| Moderate | 2 (25) | 4 (30.8) | |
| Severe | 2 (25) | 4 (30.8) | |
| Critically ill | 4 (50) | 5 (38.5) | |
| Medication | |||
| Corticosteroid | 8 (100) | 13 (100) | 1.0 |
| steroid total dose2 | 197.9 | 190 | 0.75 |
| Tocilizumab | 8 (100) | 13 (100) | 1.0 |
| Mortality | 3 (37.5) | 0 | 0.042 |
Values were presented as numbers (%), unless other described.
IQR=interquartile range; HTN=hypertension; DM=diabetes mellitus.
moderate patients required low flow oxygen therapy, severe patients required high flow oxygen therapy, and critically ill patients required mechanical ventilation or extracorporeal membrane oxygenation.
The doses were adjusted to equivalent doses of prednisolone (mg).
A recent study suggested SARS-CoV-2 viral dynamics for some VOCs, indicating that peak Ct was lower, but posterior trajectories had higher proportions of Ct count less than 15 in the delta variant compared to the original or other variant of SARS-CoV-27. Other study revealed that individual infections during which viral replication is initially fastest generate the highest peak viral load and see the slowest viral clearance, but vaccinations accelerate viral clearance3. In our study, only three patients received partial vaccination (primer dose of vaccination), so other factors such as strains, medication could impact the viral clearance. Although interleukin (IL)−6 blockade is associated with clinical improvements for COVID-19 patients8, it could suppress pathogenic T-cells and inflammatory monocytes, while inducing a reduction in the peripheral memory B cells and suppressing the viral clearance4. Therefore, compromised immunity due to immunomodulatory combination therapy for severe infection may allow the virus to continually replicate and progressively accrue genetic diversity9. In addition, older age and female are related to prolonged viral shedding in subsequent analysis, which is consistent with the previous results and difference in immune responses to the infection10. In fact, some severe patients confirmed with delta variant have prolonged viral shedding regardless of viral load of the samples, indicating that Ct values would not be specific for viability of the delta variant SARS-CoV-2.
One limitation of our study is, since it is an observational cohort study, two separate cohorts show different baseline characteristics, which are not comparable to analyze. In addition, the relatively small samples may limit the ability to verify the difference between the delta variant and other SARS-CoV-2. Stratified matching with a large sample size would be warranted to determine the isolation period and risk factors in severe patients. A culturable virus does not necessarily mean a transmissible virus. Lastly, in real-world, large-scale vaccination programs and emerging different variants (eg, Omicron variant) could complicate the viral kinetics and the timing of when to end isolation. However, our study suggests that severe form of SARS-CoV-2 infected patients, in particular, who were administered immunomodulators such as IL-6 blockade or old, should be considered for longer periods of isolation and precautions on the basis of virus culture results.
Conflict of interests
The authors declare no conflict of interest.
Acknowledgments
This work was supported by a research program funded by the Korea Disease Control and Prevention Agency (2021-ER1902–0), a grant from the Ministry of Health & Welfare, Republic of Korea (grant no. HI14C1324), and the 2020 Joint Research Project of Institutes of Science and Technology.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.04.003.
Appendix. Supplementary materials
Reference
- 1.Jeong Y.D., Ejima K., Kim K.S., Iwanami S., Bento A.I., Fujita Y., et al. Revisiting the guidelines for ending isolation for COVID-19 patients. Elife. 2021;10 doi: 10.7554/eLife.69340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh K.A., Spillane S., Comber L., Cardwell K., Harrington P., Connell J., et al. The duration of infectiousness of individuals infected with SARS-CoV-2. J Infect. 2020;81(6):847–856. doi: 10.1016/j.jinf.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singanayagam A., Hakki S., Dunning J., Madon K.J., Crone M.A., Koycheva A., et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cogliati Dezza F., Oliva A., Cancelli F., Savelloni G., Valeri S., Mauro V., et al. Determinants of prolonged viral RNA shedding in hospitalized patients with SARS-CoV-2 infection. Diagn Microbiol Infect Dis. 2021;100(2) doi: 10.1016/j.diagmicrobio.2021.115347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baek Y.J., Lee Y.J., Yoon J.S., Sohn Y., Cho Y., Kim M.H., et al. Duration of culturable SARS-CoV-2 within different specimens among mild and severe COVID-19 patients: a longitudinal study. J Infect. 2021;83(1):e29–e31. doi: 10.1016/j.jinf.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisman D., Tuite A. Progressive Increase in Virulence of Novel SARS-CoV-2 Variants in Ontario, Canada, February to June 2021. MedRxiv. 2021.
- 7.Kissler S.M., Fauver J.R., Mack C., Tai C.G., Breban M.I., Watkins A.E., et al. Viral Dynamics of SARS-CoV-2 Variants in Vaccinated and Unvaccinated Persons. N Engl J Med. 2021;385(26):2489–2491. doi: 10.1056/NEJMc2102507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo L., Luo T., Du M., Mei H., Hu Y. Efficacy and safety of tocilizumab in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Infect. 2022;84(3):418–467. doi: 10.1016/j.jinf.2021.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koeckerling D., Barker J. Accelerating the Evolution of Severe Acute Respiratory Syndrome Coronavirus 2: a Risk of Combining Dexamethasone and Tocilizumab for Severe Coronavirus Disease 2019. J Infect Dis. 2021;224(6):934–937. doi: 10.1093/infdis/jiab328. [DOI] [PubMed] [Google Scholar]
- 10.Long H., Zhao J., Zeng H.-.L., Lu Q.-.B., Fang Ll-Q, Wang Q., et al. Prolonged viral shedding of SARS-CoV-2 and related factors in symptomatic COVID-19 patients: a prospective study. BMC Infect. Dis. 2021;21(1):1–10. doi: 10.1186/s12879-021-07002-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.