Abstract
Abstract
The potential use of biological agents has become a major public health concern worldwide. According to the CDC classification, Bacillus anthracis and Clostridium botulinum, the bacterial pathogens that cause anthrax and botulism, respectively, are considered to be the most dangerous potential biological agents. Currently, there is no licensed vaccine that is well suited for mass immunization in the event of an anthrax or botulism epidemic. In the present study, we developed a dual-expression system-based multipathogen DNA vaccine that encodes the PA-D4 gene of B. anthracis and the HCt gene of C. botulinum. When the multipathogen DNA vaccine was administered to mice and guinea pigs, high level antibody responses were elicited against both PA-D4 and HCt. Analysis of the serum IgG subtype implied a combined Th1/Th2 response to both antigens, but one that was Th2 skewed. In addition, immunization with the multipathogen DNA vaccine induced effective neutralizing antibody activity against both PA-D4 and HCt. Finally, the protection efficiency of the multipathogen DNA vaccine was determined by sequential challenge with 10 LD50 of B. anthracis spores and 10 LD50 of botulinum toxin, or vice versa, and the multipathogen DNA vaccine provided higher than 50% protection against lethal challenge with both high-risk biothreat agents. Our studies suggest the strategy used for this anthrax-botulinum multipathogen DNA vaccine as a prospective approach for developing emergency vaccines that can be immediately distributed on a massive scale in response to a biothreat emergency or infectious disease outbreak.
|
Key points • A novel multipathogen DNA vaccine was constructed against anthrax and botulism. • Robust immune responses were induced following vaccination. • Suggests a potential vaccine development strategy against biothreat agents. |
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00253-022-11812-6.
Keywords: Anthrax, Biothreat agent, Botulism, DNA vaccine, Multipathogen vaccine
Introduction
Anthrax and botulism are serious infectious diseases caused by toxin-producing bacteria. Anthrax is caused by Bacillus anthracis, a spore-forming, aerobic, gram-positive, and non-motile bacterium, and anthrax toxin is thought to play a critical role in the disease (Fribe et al. 2019; Moayeri et al. 2015; Young and Collier 2007). The toxin consists of three proteins: protective antigen (PA), lethal factor (LF), and edema factor (EF). PA binds to cellular receptors to translocate LF and EF into cells. Following anthrax inhalation, symptoms are mild and non-specific during the initial stage of infection, as patients infected with anthrax typically have fever, body pain, and a sore throat. However, if infected patients are not treated, death occurs rapidly. LF in combination with PA (lethal toxin, LeTx) impairs function of immune cells and leads cell lysis. Inflammatory mediators released in response to LeTx may contribute to the sudden death characteristic of systemic anthrax. (Artenstein and Opal 2012; Lowe and Glomski 2012).
Botulinum neurotoxin (BoNT) is produced by a rod-shaped, gram-positive, and spore-forming anaerobic bacterium called Clostridium botulinum (Simpson 2004). BoNT is the most poisonous naturally occurring compound (Wheeler and Smith 2013). The toxin causes a neuroparalytic syndrome characterized by descending, symmetric, and flaccid paralysis of voluntary muscles, which leads to respiratory arrest and death (Pirazzini et al. 2017).
The Centers for Disease Control and Prevention (CDC) has classified the major biological agents into three categories based on their potential impact on public health (Jansen et al. 2014). The CDC lists anthrax and botulism as category A agents, which are the highest priority, because both are highly lethal and are the most easily weaponized and disseminated. Because they have the potential to cause mass casualties that could provoke extensive social disruption, they require broad-based public health preparedness systems. Several other agents are assigned lower priority for specific preparations. These category B agents would have lower morbidity and mortality as compared to category A. Category C is the third highest priority and includes the emerging pathogens that have a potential for availability, production, and dissemination (Trull et al. 2007).
Vaccination is generally regarded as the best strategy to protect the population from biothreat agents. PA has been shown to be a key component of the currently licensed anthrax vaccines (Clark and Wolfe 2020). However, with this vaccine, multiple immunizations are required to evoke and maintain protective immunity, and the vaccine also shows considerable local and general reactogenicity. These vaccines were prepared from PA-containing sterile filtrates of culture supernatant from a non-capsulated B. anthracis strain. These disadvantages, such as the limited immunogenicity, safety issues, and technical hurdles in production, make the current licensed anthrax vaccines unsuitable for clinical use as emergency vaccines against biothreat agents (Greidanus and Honl 2002; Pittman et al. 2001; Wasserman et al. 2003). Currently, no vaccine against botulism has been licensed for general use. However, toxoid vaccines have been developed against botulism that were administered to people at risk for botulism, such as health care providers, first responders, and military personnel. However, the CDC discontinued this vaccine program because of its low efficiency (Sundeen and Barbieri 2017).
Among the many vaccine platforms, DNA vaccines are especially attractive for the development of vaccines against biothreat agents. Compared with the alternatives, the DNA platform is safe and stable, as DNA-based vaccines can be stored and delivered without a cold chain. More importantly, the development, manufacture, and scaling-up of these vaccines are simple and cost-effective (Dupuy and Schmaljohn 2009; Li and Petrovsky 2016). These characteristics favor the use of DNA-based vaccines against biothreat agents, as they would allow for rapid development and quick deployment in response to a biothreat emergency. Previous studies have shown that DNA vaccines have the potential to induce robust immunogenicity against both B. anthracis (Kim et al. 2015; Livingston et al. 2010) and C. botulinum (Kim et al. 2019; Scott et al. 2015; Trollet et al. 2009). In the case of B. anthracis, protective immunity against B. anthracis spores has been demonstrated in various animal models following administration of a PA-DNA vaccine (Hermanson et al, 2004; Midha and Bhatnagar 2009). It also found that in addition to full-length PA, truncated PA such as domain 4 of PA (PA-D4) plays an important role in generating immunity (Park et al. 2008; Kim et al. 2015). In addition, plasmids containing the gene encoding the BoNT heavy chains (HC) are an attractive DNA vaccine platform, as high antibody titers were obtained in rabbits using a BoNT HC-encoding DNA vaccine, and the neutralizing antibody titers were high enough to meet the criteria of the European Pharmacopeia (Burgain et al. 2013).
The development of multivalent vaccines is a novel approach for eliciting protection against several diseases. Such vaccines are highly desirable for biothreat agent vaccine applications because they simplify the manufacturing processes and reduce the number of required vaccinations, making them more cost-effective. Therefore, in this study, we constructed a multipathogen DNA vaccine against the pathogens B. anthracis and C. botulinum and investigated its immunogenicity using a dual challenge model in which mice were sequentially challenged with a lethal dose of B. anthracis and BoNT.
Materials and methods
Construction of a multipathogen DNA vaccine
The nucleotide sequences encoding B. anthracis PA-D4 and the C. botulinum toxin type E C-terminal fragment of the heavy chain (HCt/E) were obtained from GenBank (accession numbers MZ923834 and MZ923835, respectively). The full nucleotide sequence of gene is given in Tables S1 and S2. The monovalent DNA vaccine vector was constructed as described in previous studies (Kim et al. 2015, 2019). To generate a multipathogen DNA vaccine vector, the HCt fragment containing the enhancer, promoter, signal peptide, and poly A-tail was amplified from pHCt monovalent vector using the following primer set (F: 5′-ATAGATCTGATCTATGCTTTGCATA-3′ and R: 5′-ATAGATCTGAAGCCATAGAGCCCA-3′). Then, the amplified HCt fragment was inserted into the pD4 monovalent vector at BglII site and NruI site. The resulting plasmid, which contains genes encoding both the anthrax PA-D4 antigen and BoNT HCt/E antigen, was named pD4/HCt (Fig. 1A). The accuracy of all plasmid constructs was confirmed by restriction analysis and by sequence analysis (Cosmogenetech, Seoul, Korea).
Fig. 1.

Construction and in vitro expression of a multipathogen anthrax-botulinum DNA vaccine. A Schematic diagram of the pD4/HCt vaccine. Codon-optimized B. anthracis PA-D4 and C. botulinum HCt/E genes were cloned into a dual promoter plasmid for eukaryotic expression to generate pD4/HCt. In vitro expression of the antigens in the pD4/HCt vaccine in DNA-transfected 293 T cells by western blot analysis. Antigen expression was detected with anti-PA-D4 (B) and anti-HCt/E (C) antibodies
In vitro expression of the multipathogen DNA vaccine
The generated plasmids were expressed in 293 T cells following transient transfection using VivaMagic (Vivagen, Seoul, Korea), according to the manufacturer’s protocol. Cells were harvested at 48 h post-transfection and lysed for western blot analysis. Proteins were separated by 4–20% SDS-PAGE and then transferred to polyvinylidene difluoride (PVDF) membranes. The PA-D4 protein was detected using a rabbit anti-PA-D4 antibody, and the HCt/E protein was detected using a rabbit anti-HCt/E antibody (Cosmogenetech, Seoul, Korea). Horseradish peroxidase-conjugated goat anti-rabbit IgG (31460, Invitrogen, Waltham, USA) was used as the secondary antibody (dilution 1:5,000). Proteins were visualized using Western Lighting Plus-ECL (Perkin Elmer, Waltham, USA).
Animals and ethics statement
All the animals were maintained in a temperature (22 ± 0.5 °C)- and humidity (60 ± 2%)- controlled room on a 12-h light/dark cycle (light on at 06:00). The animals had free access to food and water. Animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of Seoul National University, Seoul, Korea (approval ID: SNU-190325–1), and Agency for Defense Development, Daejon, Korea (approval ID: ADD-IACUC-20–08).
Animal immunization
Female 6–8-week-old Balb/c mice and A/J mice were obtained from Samtako (Osan, Korea) and immunized (n = 10 per group). The Balb/c mice demonstrated strong immune response against pathogens such as B. anthracis and C. botulinum (Kim et al. 2015, 2019) and A/J mice have been used as small animal models for anthrax vaccine candidate screening (Flick-Smith et al. 2005). The mice were acclimated for 1 week prior to the experiments. The multipathogen DNA vaccine was administered by intramuscular electroporation as described in a previous study (Kim et al. 2015). The immunization schedule for mice and guinea pigs was based on previous our monovalent DNA vaccine studies (Kim et al. 2015, 2019). Briefly, 50 μg of plasmid DNA suspended in PBS was injected three times at 2-week intervals. As a control group, mice were injected with pcDNA 3.1, the empty vector, as described above. Before plasmid DNA injection, the mice were anesthetized intraperitoneally with Zoletile (2 mg/kg body weight) and the skin overlying the quadriceps muscles was shaved. Immediately after the mice were injected with the plasmid DNA, electroporation was performed with two-needle array electrodes (BTX, Hollison, USA), which were inserted into the muscle at the injection site. The electrodes were placed longitudinally relative to the muscle fibers at a 5 mm distance. Three electroporation pulses of 90 V/mm, with a 20-ms pulse length and reversal of polarity after each pulse, were administered with a BTX ECM-830 electroporator.
To test the efficiency of the vaccine in a species with a large body mass, guinea pigs were immunized. For guinea pig immunization, groups of guinea pigs (3–4-week old, female, 3 per group) were injected with 100, 200, 300, and 500 μg of plasmid DNA into the muscle three times at 3-week intervals. Immediately following plasmid DNA injection, a needle array electrode, with a 10 mm electro distance, was inserted into the injection site and a voltage was applied (three pulses, 90 V/mm, 25-ms pulse length). These electroporation protocols have been shown to induce significant immunity following administration of a DNA vaccine (Kim et al. 2015).
Antibody assessment
Serum was collected 2-week intervals and stored at − 20 °C until assay. To determine the PA-D4 and HCt/E-specific antibody titers, sera were serially diluted in PBS containing 2% skim milk and added to 96-well plates, which were coated with recombinant PA-D4 or HCt/E protein (1.0 μg/ml) as previously described (Kim et al. 2015, 2019). Bound antibodies were detected with HRP-conjugated goat anti-mouse IgG (31,430, Invitrogen, Waltham, USA) at dilution of 1:4,000 using a colorimetric assay with 3,3′,5,5′-tetramethylbenzidine (TMB) substrate. Endpoint titers were expressed as the highest dilution yielding an absorbance > twofold higher than the background values.
Determination of IgG subtype
IgG subtypes were identified using ELISA. A/J mouse serum was collected at 2 weeks after the last immunization, and the IgG subtypes were analyzed. An ELISA was performed using serum samples (diluted 1:10,000) as the primary antibody and goat anti-mouse IgG1-HRP (PA1-74421) or IgG2a-HRP (A-10685) as the secondary antibody (1:4,000, Invitrogen, Waltham, USA).
Anthrax lethal toxin neutralization assay
The anthrax lethal toxin neutralization assay was performed as previously described (Kim et al. 2014) with minor modifications. A/J mice were immunized three times at 2-week intervals, and sera were collected at 2 weeks after the last immunization. Briefly, 150 ng of PA and 50 ng of LF per ml were prepared in RPMI medium, and sera were diluted with the toxin mixture (1:20) and pre-incubated for 30 min at 37 °C. Then, the toxin-sera mixtures were transferred to J774A.1 macrophage cells grown to confluence in 96-well plates at a density of 1 × 105 cells/well and incubated for 3 h. Cell viability was assessed by the MTT assay. Briefly, MTT solution was added to each well at final concentration of 0.5 mg/ml. After incubating at 37 °C for additional 1 h, cell culture medium was removed. The insoluble formazan was dissolved in solubilization solution. Finally, the optical density was measured at 540 nm with a spectrometer (Molecular Devices, San Jose, USA). The recombinant PA and LF were purified from Escherichia coli as described previously (Wu et al. 2010; Arora and Leppla 1994).
BoNT/E in vivo neutralization assay
To evaluate the neutralization capability of the HCt/E antibody, A/J mice (n = 5 per group) were intraperitoneally injected with BoNT/E toxin and serum mixture. Serum was collected at 2 weeks after the final immunization. Fifty microliters of serum from pD4/HCt-vaccinated mice was added to mouse 10 LD50 of BoNT/E toxin in a total volume of 0.2 ml of phosphate buffer and incubated at 25 °C for 1 h. Then, the toxin-serum mixture was injected intraperitoneally into mice. The mice were observed daily. Final survival was determined at 10 days after injection. Serum from vector-treated mice was used as a control. C. botulinum type E (Alaska E43) was grown and BoNT/E toxin was purified as described in the previous study (Gessler 2005).
Sequential mouse challenge
At 2 weeks after the last immunization, the immunized A/J mice were challenged with 10 LD50 (1 LD50 = 1.8 × 103 spores) of B. anthracis Sterne (34F2) strain subcutaneously (Kim et al. 2015) and monitored daily for symptoms of intoxication and mortality for 14 days post challenge. The surviving mice were further challenged with 10 LD50 (1 LD50 = 1.377 ng) of BoNT/E by intraperitoneal injection and monitored for another 14 days for mortality and symptoms of intoxication. In a separate experiment, mice were challenged sequentially with 10 LD50 of BoNT/E and then 10 LD50 of anthrax spores as described above. Mice in the control group were injected with pcDNA 3.1 plasmid. Anthrax spores were prepared as described previously (Skoble et al. 2009).
Statistical analysis
Results are expressed as mean ± standard deviation (SD). To compare mouse antibody production at different time points, Friedman test with Dunn’s correction for multiple comparisons was used. Differences in antibody titers, IgG types and neutralization activity were analyzed statistically using the Student’s t-test. Statistical analyses for guinea pig antibody production were performed using the Kruskal–Wallis test. Mann–Whitney test was used to test for differences in guinea pig body weight. The level of significance was set at p < 0.05.
Results
Construction and characterization of the multipathogen anthrax-botulinum vaccine
To develop a multipathogen, anthrax-botulinum DNA vaccine, we constructed a dual promoter expression vector that allows for simultaneous expression of two antigen proteins. After confirming the correct orientation of the cassettes in the recombinant vector by restriction enzyme digestion and the sequences of the genes encoding the antigens by sequencing, the plasmid was transfected into 293 T cells, and antigen expression was analyzed by western blotting with an anti-PA-D4 or anti-HCt/E antibody. As shown in Fig. 1B, cells transfected with the pD4/HCt plasmid expressed a protein of approximately 20 kDa, which corresponds to the size of the PA-D4 domain fused to a 20 amino acid IgM signal peptide. In the experiment using the anti-HCt/E antibody, as expected, a higher molecular weight of glycosylated protein band of HCt/E was detected in cells transfected with the pD4/HCt plasmid (Fig. 1C). However, control cells that were transfected with the pcDNA 3.1 empty vector failed to produce these proteins.
Humoral immune responses in mice immunized with the multipathogen vaccine
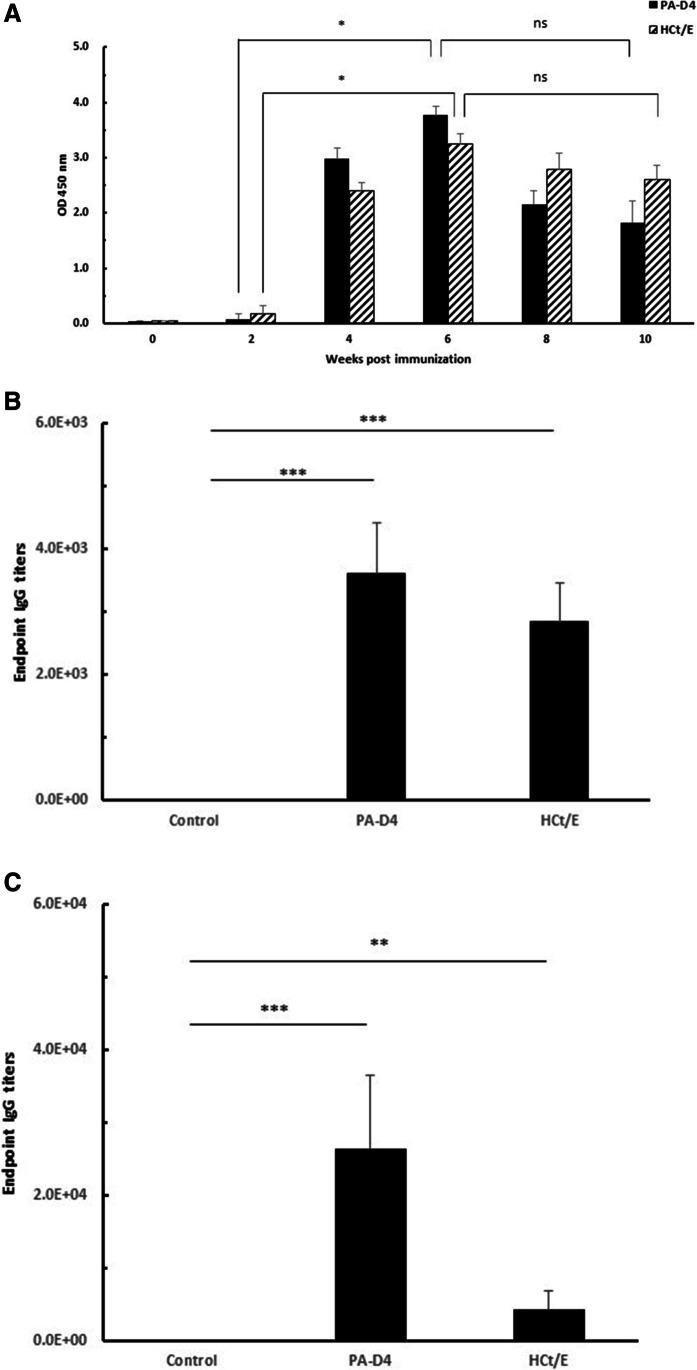
To examine whether inoculation with the multipathogen DNA vaccine can induce an antigen-specific immune response in vivo, Balb/c mice were immunized intramuscularly with pD4/HCt or pcDNA 3.1 (control). Mice were immunized three times at 2-week intervals, and sera were collected to analyze antigen-specific IgG levels by ELISA. As shown in Fig. 2A, the levels of PA-D4- and HCt/E-specific IgG showed increasing trends after the second immunization, reached the highest levels after the third immunization, and then slowly declined. The level of IgG was significantly higher at 6 weeks than at 2 weeks (p < 0.05). However, there was no significant difference in IgG level between at 6 weeks and at 10 weeks (p > 0.05). To quantify antibody production induced by pD4/HCt immunization, an endpoint titer assay was performed. As shown in Fig. 2B, at 2 weeks after all three doses of vaccines, PA-D4- or HCt/E-specific antibodies were detected in all Balb/c mice immunized with pD4/HCt. The geometric mean titers of the PA-D4- and HCt/E-specific antibodies induced by the pD4/HCt vaccine were 3.6 × 103 and 2.8 × 103, respectively, which were significantly different from those in the control group (p < 0.001).
Fig. 2.
Generation of anti-PA-D4 and anti-HCt/E IgG in immunized mice (n = 10). A IgG production in Balb/c mice. Sera were collected at indicated time points, and IgG levels were analyzed by ELISA. Titers of anti-PA-D4 and anti-HCt/E antibodies in Balb/c (B) and A/J mice (C). Sera were collected at 2 weeks after the last immunization. Antibody levels are expressed as the endpoint titers. Statistical analysis was conducted using the Friedman test with Dunn’s correction for multiple comparisons (A) and t-test (B and C). *, p < 0.05; **, p < 0.01; ***, p < 0.001. ns not significant
To further assess the immunogenicity of the multipathogen DNA vaccine, A/J mice were immunized with pD4/HCt, and the antigen-specific antibody titers were analyzed. Similar to that observed in Balb/c mice, the antigens induced high levels of antigen-specific IgG. As shown in Fig. 2C, high levels of anti-PA-D4 antibodies were observed in the immunized group, with a geometric mean titer of 2.6 × 104, while the geometric mean titer of anti-HCt/E antibodies in the immunized group was 4.2 × 103. There were significant differences in the anti-PA-D4 or anti-HCt/E titers between the immunized and control groups (p < 0.001 and p < 0.01, respectively).
Antibody isotype profiles following immunization with the multipathogen vaccine
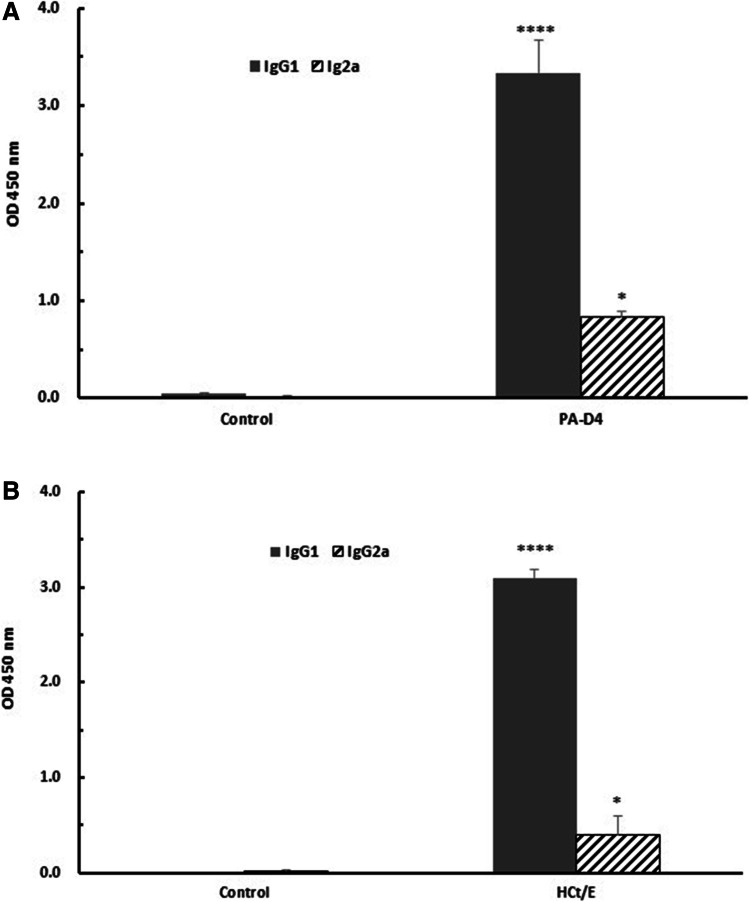
To better understand the nature of the immune response elicited by pD4/HCt, the IgG subclass distribution in immunized mice was analyzed. The levels of the antigen-specific IgG subclasses were determined by ELISA at 2 weeks after the last immunization. It is well known that IgG1 and IgG2a isotypes are associated with Th2 and Th1 immune responses, respectively. Immunized mice showed higher levels of PA-D4-specific IgG1 and IgG2a than the control mice (p < 0.0001 and p < 0.05, respectively; Fig. 3A). The HCt/E-specific IgG1 and IgG2a responses were also significantly greater than those in the control group (p < 0.0001 and p < 0.05, respectively; Fig. 3B). The IgG1/IgG2a ratio is used as an indicator of Th1 or Th2 skew. The immunized mice showed a PA-D4-specific IgG1/IgG2a ratio of 3.99 and a HCt/E-specific antibody IgG1/IgG2a ratio of 7.77, suggesting that immunization with pD4/HCt induced a bias toward a Th2 immune response.
Fig. 3.
Determination of the antibody isotypes produced by mice immunized with the pD4/HCt DNA vaccine. A The levels of PA-D4-specific IgG1 and IgG2a. B The levels of HCt/E-specific IgG1 and IgG2a. Sera were collected at 2 weeks after the last immunization, and antibody subclasses were analyzed by ELISA. Statistical analysis was conducted using t-test. *, p < 0.05; ****, p < 0.0001
Immunization of guinea pigs with the multipathogen vaccine
To further evaluate the efficiency of the pD4/HCt vaccine, guinea pigs were immunized with different dose of the vaccine. As shown in Fig. 4A, guinea pigs immunized with pD4/HCt produced high levels of anti-PA-D4-specific IgG antibodies, and the endpoint titers in the immunized animals were 1.0 × 103–5.2 × 103. Similar trends were observed for anti-HCt/E-specific IgG antibodies; the endpoint titers in the immunized animals were 1.7 × 103–6.3 × 103 (Fig. 4B). However, there were no significant differences in the anti-PA-D4- or anti-HCt-specific titer levels among the different dose groups.
Fig. 4.
Antibody responses in guinea pigs (n = 3) immunized with various doses of the pD4/HCt DNA vaccine. Anti-PA-D4 (A) and anti-HCt/E (B) IgG levels are expressed as the endpoint titers. Control guinea pigs (0 μg) were injected with pcDNA 3.1. Statistical analysis was conducted using Kruskal–Wallis test. ns not significant
Neutralization assay of multipathogen vaccine-induced antibodies
Next, to determine the functionality of the anti-PA-D4 and anti-HCt antibodies elicited by immunization with pD4/HCt, in vitro and in vivo neutralization assays were performed. As shown in Fig. 5, a 1:20 dilution of sera from pD4/HCt immunized mice conferred 100% protection against the cytotoxic effects of LeTx, suggesting that these anti-PA-D4 antibodies can neutralize the toxicity of LeTx. The neutralization activity of anti-HCt/E antibodies was also evaluated in vivo using a mouse assay in which pD4/HCt-immunized mouse serum and toxin were premixed and injected, and the number of surviving mice was evaluated. In this assay, 50 μl of serum was mixed with 10 LD50 BoNT/E toxin and injected intraperitoneally. As shown in the results, all mice that received the immunized mice serum-toxin mixture survived. In contrast, mice that were administered control mouse serum with toxin died within 1 day after injection, indicating that the anti-HCt/E antibodies have potent neutralization activity (Table S3).
Fig. 5.
Anthrax toxin LeTx neutralization assay. J774A.1 macrophages were incubated with anthrax lethal toxin (LeTx) with or without sera from mice taken at 2 weeks after the last immunization. Statistical analysis was conducted using t-test. **, p < 0.01
Protection ability of the multipathogen vaccine against challenge with anthrax spores and BoNT
Finally, to confirm the efficiency of our multipathogen vaccine, its protective ability was tested by exposing animals to both pathogens. Two different sequential challenge studies were performed that differed regarding the order of the challenge agents and are referred to as challenges 1 and 2.
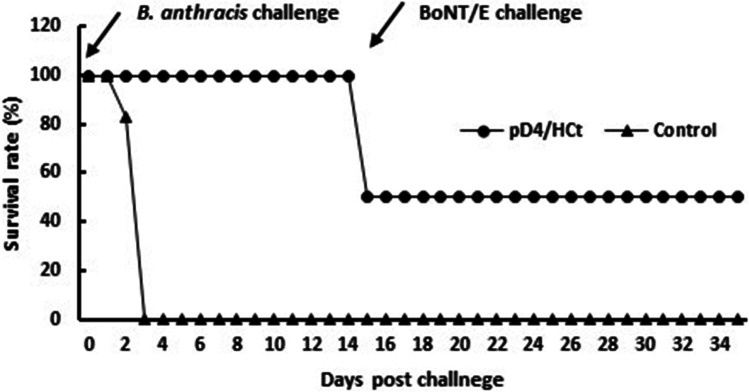
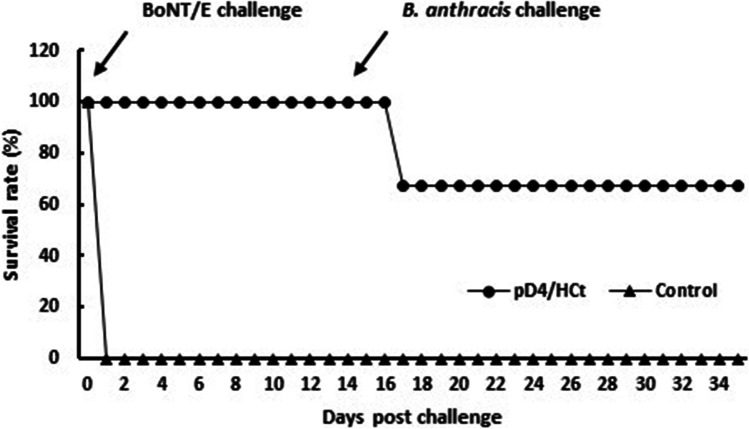
In challenge 1, A/J mice were immunized and challenged with agents. Two weeks after all three doses of vaccines, mice were challenged with a 10 LD50 dose of B. anthracis spores and then challenged with a 10 LD50 dose of BoNT/E on day 14 post-B. anthracis challenge. As shown in Fig. 6, pD4/HCt protected up to 50% of the immunized mice, whereas all mice in the vector control group died within 3 days post challenge. In the challenge 2 study, the mice were challenged with BoNT/E first, followed by B. anthracis 14 days. In challenge 2, the results showed a similar trend in protection to that observed in challenge 1. The protection rate against both agents was 66% in the immunized mice (Fig. 7). These results imply that the pD4/HCt vaccine can provide partial protection against both anthrax and botulism.
Fig. 6.
Survival of mice immunized with the pD4/HCt DNA vaccine against sequential challenge with anthrax spores and botulinum toxin. A/J mice (n = 6) were immunized and challenged with sequentially with 10 LD50 of anthrax spores and 10 LD50 of BoNT/E. The survival rates are shown as the percentage of survivors
Fig. 7.
Survival of mice immunized with the pD4/HCt DNA vaccine against sequential challenge with botulinum toxin and anthrax spores. A/J mice (n = 6) were immunized and challenged with sequentially with 10 LD50 of BoNT/E and 10 LD50 of anthrax spores. The survival rates are shown as the percentage of survivors
Discussion
Multipathogen vaccines can elicit protection against multiple diseases with fewer injections over a short period of time. The major benefits of this type of vaccine are simplified vaccine administration and fewer visits to medical institutions, resulting in reduced public health costs. Other advantages of multipathogen vaccines include reduced cost of stocking and facilitating the addition of new vaccines into immunization programs (Lauer et al. 2017). Multipathogen vaccines are highly desirable for biodefense applications because they reduce the number of vaccinations an individual must obtain in a bioterrorism or other emergency situation where vaccines should be rapidly deployed on a massive scale. Anthrax and botulism are two of the most dangerous bioterrorism agents and are a potential threat to both the military and civilians (Trull et al. 2007). There have been extremely limited studies on the development of anthrax/botulism multipathogen vaccines. However, those studies relied on a simple combination of monovalent vaccine (Lee et al. 2006). In the current study, we developed and evaluated a novel multipathogen vaccine for B. anthracis and C. botulinum based on co-expression DNA vaccine technology. We used the technique of electroporation to facilitate DNA delivery in vivo. Prior and current clinical studies have revealed that electroporation can be successfully used in humans (ClinicalTrials.gov).
An ideal multipathogen vaccine should induce a balanced immune response to all antigens. In the current study, the antibodies induced by the pD4/HCt vaccine against PA-D4 and HCt/E were preliminarily evaluated in Balb/c mice. These mice responded well to the two antigens and produced high levels of antigen-specific antibodies, based on the serum titers as measured by ELISA. In addition, comparison to our previous work (Kim et al. 2015, 2019) showed that the endpoint titers of anti-PA-D4 and anti-HCt/E antibodies in the pD4/HCt vaccine-immunized mice were similar to those induced following vaccination with each of the monovalent vaccines, indicating that the individual antigens do not interfere with each other. A/J strain mice, which are deficient in complement component C5, are more susceptible to a variety of infectious diseases (Nesbitt and Skamene 1999). Because of their susceptibility to uncapsulated anthrax strains, which cause death in a dose-dependent manner, A/J mice are excellent small-animal models for the screening of anthrax vaccines (Beedham et al. 2001; Kenney et al. 2004). Therefore, the immunity of the pD4/HCt vaccines was also evaluated using A/J mice as a second mouse strain. Antibody responses to both PA-D4 and HCt/E were detected, with a stronger response to PA-D4 than to HCt/E.
Previous studies have shown that robust humoral immunity was induced by inoculation with DNA vaccines expressing PA or recombinant BoNT/A vaccines in guinea pigs, which are a large mammalian animal model for evaluating vaccine efficiency (Chitlaru et al. 2007; Rosenthal and Zimmerman 2006). In the present study, a strong humoral immune response was also achieved when guinea pigs were immunized with the pD4/HCt vaccine. In addition, vaccination of guinea pigs with this vaccine did not result in significant weight loss, as confirmed by the body weight monitoring study (Fig. S1), indicating that the pD4/HCt vaccine was safe, which is a prerequisite for clinical application. Taken together, these results indicate that both antigens in the pD4/HCt vaccine formulation could elicit robust humoral immune responses in three different animal models, Balb/c mice, A/J mice, and guinea pigs, without interference between the antigens, implying that it may be possible to develop other multipathogen vaccines against biothreat agents using this vaccine formulation.
Because the type of immune response is critical to the effectiveness of a potential vaccine, a detailed analysis of the overall immune responses is crucial. For example, a Th1-type response indicates highly effective cell-mediated immunity, whereas a Th2-type response promotes the production of antibodies. Therefore, induction of both immune responses by the pD4/HCt vaccine indicates that it may be effective against B. anthracis and C. botulinum infections. In mice, IgG1 is associated with a Th2-like response, while induction of IgG2a is associated with a Th1 response (Hess et al. 2000). Our data showed that immunized mice had significantly higher levels of PA-D4- and HCt/E-specific IgG1 and IgG2a antibody titers when compared to the control mice, with a bias toward IgG1, implying a strong humoral response in the mice. These data indicate that the pD4/HCt vaccine has the ability to induce both Th1- and Th2-mediated immune responses, which will offer advantages for protection against an anthrax or botulinum toxin attack. Similarly, a Th1/Th2-mixed type of immune response has been reported following immunization with a DNA plasmid encoding PA (Gu et al. 1999) or HCt of BoNT/F (Jathoul et al. 2004).
The induction of neutralizing antibodies is crucial for the prevention of B. anthracis or C. botulinum infection. Therefore, we assessed neutralizing antibody activity in mice using an anthrax lethal toxin neutralization assay and an in vivo BoNT/E protection assay. Anthrax lethal toxin neutralizing activity was determined using a J774A.1 cell-based assay. We found that sera collected from immunized mice completely neutralized a lethal dose of anthrax toxin. BoNT/E-specific neutralization activity was also assessed using an in vivo protection assay in which sera from immunized or control mice were mixed with a lethal dose of BoNT/E toxin and then injected into naïve mice. The anti-HCt/E IgG elicited by the pD4/HCt vaccine showed high neutralization activity. Sera from vaccinated mice showed full protection against 10 LD50/mouse of BoNT/E. These neutralization assay results provide evidence that our pD4/HCt vaccine is immunogenic in animals and generates PA-D4 and HCt/E-specific antibodies, which have high functional affinity for the antigen.
Finally, to further investigate the potency of the multipathogen vaccine, the protective ability of the pD4/HCt vaccine was examined using a sequential challenge model. Immunized A/J mice that survived challenge with 10 LD50 of B. anthracis Sterne spores were then challenged 2 weeks later with 10 LD50 of BoNT/E. The immunized mice showed 100% survival against the primary anthrax spore challenge, whereas they showed 50% survival (three out of six survived) against the secondary challenge with BoNT/E. We also performed experiments in which mice that had been immunized with the pD4/HCt vaccine were first challenged with BoNT/E and then secondarily challenged with anthrax spores. As in the above challenge experiment, all immunized mice survived the primary BoNT/E challenge, while 66% (four out of six) survived the secondary challenge with anthrax spores, suggesting that our pD4/HCt vaccine induced substantial, although incomplete, protection against anthrax and BoNT sequential challenge.
Similar protection efficiency was reported in previous research that has shown the effectiveness of anthrax/botulism multivalent vaccine based on mixture of DNA (Lee et al 2006). A study demonstrated that mixture of PA and BoNT HCt/A viral replicon could protect 90% of mice from a Stern challenge and that had survived the Stern challenge, demonstrated that four of nine mice were protected from a BoNT/A challenge. This and our studies indicated that DNA-based anthrax/botulism multivalent vaccine is able to provide an effective level of protection against both biothreat agents. However, this strategy needs to be further optimized to obtain complete immunity. Meanwhile, it is noteworthy in our results that complete protection was obtained against the primary challenge, while incomplete protection was observed against the secondary challenge in both experiments. One possible explanation for this is that because the sequential challenges were only 2 weeks apart, this may be insufficient time for the mice to recover fully before the secondary challenge.
In conclusion, we developed a multipathogen DNA vaccine that induces immune responses to two category A biothreat agents, B. anthracis and C. botulinum. The pD4/HCt vaccine generated high endpoint serum IgG antibody titers to each antigen as well as toxin-neutralizing antibodies, which is the crucial determinant of protective immunity against anthrax and BoNT. In addition to inducing Th2 responses, the vaccine also elicited supportive Th1 immune responses, indicating that pD4/HCt vaccines induced a broad range of immune responses, which is advantageous for developing prophylactic vaccines against other biothreat agents. However, the partial protection suggests a need for improvement in the vaccine strategy, including dendritic cell-targeted vaccines and use of TLR ligands as adjuvants, and experiments are currently being conducted to address these issues in detail. Our approach may contribute to the development of a multipathogen vaccine for emerging pathogens, such as coronaviruses and Zika virus. The lack of available vaccines for many emerging infectious diseases demands the development of new strategies for rapid development and mass vaccination to prevent global pandemics.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Conceived and designed the experiments: NYK, GHH, and SS. Performed the experiments: NYK, WRS, MHL, YJS, CHY, DHS, and SYH. Analyzed the data: NYK, HSC, JYC, STC, YKS, and SS. Contributed reagents/materials/analysis tools: NYK, WRS, MHL, YJS, CHY, DHS, and SYH. Wrote the paper: SS.
Funding
This research was supported by a grant from the Agency for Defense Development, Republic of Korea (UD180041GD).
Data availability
All relevant data are within the manuscript.
Code availability
Not applicable.
Declarations
Ethics approval
All animal experiments were conducted according to the protocols approved by the Institutional Animal Care and Use Committee of Seoul National University, Seoul, Korea (approval ID: SNU-190325–1), and Agency for Defense Development, Daejon, Korea (approval ID: ADD-IACUC-20–08).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arora N, Leppla SH. Fusions of anthrax toxin lethal factor with shiga toxin and diphtheria toxin enzymatic domains are toxic to mammalian cells. Infect Immun. 1994;62:4955–4961. doi: 10.1128/iai.62.11.4955-4961.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artenstein AW, Opal SM. Novel approaches to the treatment of systemic anthrax. Clin Infect Dis. 2012;54:1148–1161. doi: 10.1093/cid/cis017. [DOI] [PubMed] [Google Scholar]
- Beedham RJ, Turnbull PCB, Williamson ED. Passive transfer of protection against Bacillus anthracis infection in a murine model. Vaccine. 2001;19:4409–4416. doi: 10.1016/S0264-410X(01)00197-9. [DOI] [PubMed] [Google Scholar]
- Burgain A, Rochard A, Trollet C, Mazuet C, Popoff MR, Escriou V, Scherman D, Bigey PI. DNA electroporation in rabbits as a method for generation of high-titer neutralizing antisera. Examples of the botulinum toxin types A, B, and E. Hum Vaccin Immunother. 2013;9:2147–2156. doi: 10.4161/hv.25192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitlaru T, Gat O, Grosfeld H, Inbar I, Gozlan Y, Shafferman A. Identification of in vivo-expressed immunogenic proteins by serological proteome analysis of Bacillus anthracis secretome. Infect Immun. 2007;75:2841–2852. doi: 10.1128/IAI.02029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A, Wolfe DN. Current state of anthrax vaccine and key R&D gaps moving forward. Microorganisms. 2020;8:651. doi: 10.3390/microorganisms8050651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy LC, Schmaljohn CS. DNA Vaccines for Biodefense. Expert Rev. 2009;8:1739–1754. doi: 10.1586/erv.09.132. [DOI] [PubMed] [Google Scholar]
- Flick-Smith HC, Waters EL, Walker NJ, Miler J, Stagg AJ, Green M, Williamson ED. Mouse model characterization for anthrax vaccine development: comparison of one bred and one outbred mouse strain. Microb Pathog. 2005;38:33–40. doi: 10.1016/j.micpath.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Fribe SF, Goot FG, Burgi J. The ins and outs of anthrax toxin. Toxins. 2019;8:69. doi: 10.3390/toxins8030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessler F. A new scaleable method for the purification of botulinum neurotoxin type E. J Biotech. 2005;119:204–211. doi: 10.1016/j.jbiotec.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Greidanus TG, Honl BA. Delayed-type hypersensitivity reaction to anthrax vaccine. Mil Med. 2002;167:74–75. doi: 10.1093/milmed/167.1.74. [DOI] [PubMed] [Google Scholar]
- Gu ML, Leppla SH, Klinman DM. Protection against anthrax toxin by vaccination with DNA plasmid encoding anthrax protective antigen. Vaccine. 1999;17:340–344. doi: 10.1016/S0264-410X(98)00210-2. [DOI] [PubMed] [Google Scholar]
- Hermanson G, Whitlow V, Parker S, Tonsky K, Rusalov D, Ferrari M, Lalor P, Komai M, Mere R, Bell M, Brenneman K, Mateczun A, Evans T, Kaslow D, Galloway D, Hobart P. A cationic lipid-formulated plasmid DNA vaccine confers sustained antibody-mediated protection against aerosolized anthrax spores. Proc Natl Acad Sci USA. 2004;101:13601–13606. doi: 10.1073/pnas.0405557101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J, Schaible U, Raupach B, Kaufmann SH. Exploiting the immune system: toward new vaccines against intracellular bacteria. Adv Immunol. 2000;75:1–88. doi: 10.1016/S0065-2776(00)75001-2. [DOI] [PubMed] [Google Scholar]
- Jansen HJ, Breeveld FJ, Stijnis C, Grobusch MP. Biological warfare, bioterrorism and biocrime. Clin Microbiol Infect. 2014;20:488–496. doi: 10.1111/1469-0691.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jathoul AP, Holley JL, Garmory HS. Efficacy of DNA vaccines expressing the type F botulinum toxin Hc fragment using different promoters. Vaccine. 2004;22:3942–3946. doi: 10.1016/j.vaccine.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Kenney RT, Yu J, Guebre-Xabier M, Frech SA, Lambert A, Heller BA, Ellingsworth LR, Eyles JE, Williamson ED, Glenn GM. Induction of protective immunity against lethal anthrax challenge with a patch. J Infect Dis. 2004;190:774–782. doi: 10.1086/422694. [DOI] [PubMed] [Google Scholar]
- Kim NY, Ahn HB, Yu CH, Song DH, Hur GH, Shin YK, Shin S. Intradermal immunization with botulinum neurotoxin serotype E DNA vaccine induces humoral and cellular immunity and protects against lethal toxin challenge. Hum Vaccin Immunother. 2019;15:412–419. doi: 10.1080/21645515.2018.1526554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NY, Chang DS, Kim Y, Kim CH, Hur GH, Yang JM, Shin S. Enhanced immune response to DNA vaccine encoding Bacillus anthracis PA-D4 protects mice against anthrax spore challenge. PLoS One. 2015;10:e013967. doi: 10.1371/journal.pone.0139671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NY, Kang CI, Hur GH, Yang JM, Shin S. Bacillus anthracis lethal toxin induces cell-type-specific cytotoxicity in human lung cell lines. J App Microbiol. 2014;116:1334–1343. doi: 10.1111/jam.12457. [DOI] [PubMed] [Google Scholar]
- Lauer KB, Borrow R, Blanchard TJ. Multivalent and multipathogen viral vector vaccines. Clin Vaccine Immunol. 2017;24:e00298–e316. doi: 10.1128/CVI.00298-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Groebner JL, Hadjipanayis AG, Negley DL, Schmaljohn AL, Welkos SL, Smith LA, Smith JF. Multiagent vaccines vectored by Venezuelan equine encephalitis virus replicon elicits immune response to Marburg virus and protection against anthrax and botulinum neurotoxin in mice. Vaccine. 2006;24:6886–6892. doi: 10.1016/j.vaccine.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Li L, Petrovsky N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev Vaccines. 2016;15:313–329. doi: 10.1586/14760584.2016.1124762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston BD, Little SF, Luxembourg A, Ellefsen B, Hannaman D. Comparative performance of a licensed anthrax vaccine versus electroporation based delivery of a PA encoding DNA vaccine in rhesus macaques. Vaccine. 2010;28:1056–1061. doi: 10.1016/j.vaccine.2009.10.111. [DOI] [PubMed] [Google Scholar]
- Lowe DE, Glomski IJ. Cellular and physiological effects of anthrax exotoxin and its relevance to disease. Front Cell Infect Microbiol. 2012;2:76. doi: 10.3389/fcimb.2012.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midha S, Bhatnagar R. Anthrax protective antigen administered by DNA vaccination to distinct subcellular locations potentiates humoral and cellular immune responses. Eur J Immunol. 2009;39:159–177. doi: 10.1002/eji.200838058. [DOI] [PubMed] [Google Scholar]
- Moayeri M, Leppla SH, Vrentas C, Pomerantsev AP, Liu S. Anthrax pathogenesis. Annu Rev Microbiol. 2015;69:185–208. doi: 10.1146/annurev-micro-091014-104523. [DOI] [PubMed] [Google Scholar]
- Nesbitt MN, Skamene E. Recombinant inbreed mice strains derived from A/J and C57BL/6J: a tool for the study of genetic mechanism in host resistance to infection and malignancy. J Leukoc Biol. 1999;36:357–364. doi: 10.1002/jlb.36.3.357. [DOI] [PubMed] [Google Scholar]
- Park YS, Lee JH, Hung CF, Wu TC, Kim TW. Enhancement of antibody responses to Bacillus anthracis protective antigen domain IV by use of calreticulin as achimeric molecular adjuvant. Infect Immun. 2008;76:1952–1959. doi: 10.1128/IAI.01722-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirazzini M, Rossetto O, Eleopra R, Montecucco C. Botulinum neurotoxins: biology, pharmacology, and toxicology. Pharmacol Rev. 2017;69:200–235. doi: 10.1124/pr.116.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman PR, Gibbs PH, Cannon TL, Friedlander AM. Anthrax vaccine: short-term safety experience in humans. Vaccine. 2001;20:972–978. doi: 10.1016/S0264-410X(01)00387-5. [DOI] [PubMed] [Google Scholar]
- Rosenthal KS, Zimmerman DH. Vaccines: all things considered. Clin Vaccine Immunol. 2006;13:821–829. doi: 10.1128/CVI.00152-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott VL, Villarreal DO, Hutnick NA, Walters JN, Ragwan E, Bdeir K, Yan J, Sardesai NY, Finnefrock AC, Casimiro DR, Weiner DB. DNA vaccines targeting heavy chain C-terminal fragments of Clostridium botulinum neurotoxin serotypes A, B, and E induce potent humoral and cellular immunity and provide protection from lethal toxin challenge. Hum Vaccin Immunother. 2015;11:1961–1971. doi: 10.1080/21645515.2015.1066051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson LL. Identification of the major step in botulinum toxin action. Ann Rev Pharmacol Toxicol. 2004;44:167–193. doi: 10.1146/annurev.pharmtox.44.101802.121554. [DOI] [PubMed] [Google Scholar]
- Skoble J, Beaber JW, Gao Y, Lovchik JA, Sower LE, Liu W, Luckett W, Peterson JW, Calendar R, Portnoy DA, Lyons CR, Dubensky TW. Killed but metabolically active Bacillus anthracis vaccines induce broad and protective immunity against anthrax. Infect Immun. 2009;77:1649–1663. doi: 10.1128/IAI.00530-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundeen G, Barbieri JT. Vaccines against Botulism. Toxins. 2017;9:268. doi: 10.3390/toxins9090268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trollet C, Pereira Y, Burgain A, Litzler E, Mezrahi M, Seguin J, Manich M, Popoff MR, Scherman D, Bigey P. Generation of high-titer neutralizing antibodies against botulinum toxins A, B, and E by electrotransfer. Infect Immun. 2009;77:2221–2229. doi: 10.1128/IAI.01269-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trull MC, Laney TV, Dibner MD. Turning biodefense dollar into products. Nat Biotechnol. 2007;25:179–184. doi: 10.1038/nbt0207-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GM, Grabenstein JD, Pittman PR, Rubertone MV, Gibbs PP, Wang LZ, Golder LG. Analysis of adverse events after anthrax immunization in US Army medical personnel. J Occup Environ Med. 2003;45:222–233. doi: 10.1097/01.jom.0000058345.05741.6b. [DOI] [PubMed] [Google Scholar]
- Wheeler A, Smith HS. Botulinum toxins: mechanism of action, antinociception and clinical application. Toxicology. 2013;306:124–146. doi: 10.1016/j.tox.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Wu G, Feng C, Hong Y, Guo A, Cao S, Dong J, Lin L, Liu Z. Soluble expression and purification of the anthrax protective antigen in E. coli and identification of a novel dominant-negative mutant N435C. Appl Microbiol Biotechnol. 2010;87:609–616. doi: 10.1007/s00253-010-2495-5. [DOI] [PubMed] [Google Scholar]
- Young JAT, Collier RJA. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–265. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the manuscript.
Not applicable.