Abstract
Purpose of Review
Cardiac magnetic resonance imaging provides radiation-free, 3-dimensional soft tissue visualization with adjunct hemodynamic data, making it a promising candidate for image-guided transcatheter interventions. This review focuses on the benefits and background of real-time magnetic resonance imaging (MRI)-guided cardiac catheterization, guidance on starting a clinical program, and recent research developments.
Recent Findings
Interventional cardiac magnetic resonance (iCMR) has an established track record with the first entirely MRI-guided cardiac catheterization for congenital heart disease reported nearly 20 years ago. Since then, many centers have embarked upon clinical iCMR programs primarily performing diagnostic MRI-guided cardiac catheterization. There have also been limited reports of successful real-time MRI-guided transcatheter interventions. Growing experience in performing cardiac catheterization in the magnetic resonance environment has facilitated practical workflows appropriate for efficiency-focused cardiac catheterization laboratories. Most exciting developments in imaging technology, MRI-compatible equipment and MRI-guided novel transcatheter interventions have been limited to preclinical research. Many of these research developments are ready for clinical translation.
Summary
With increasing iCMR clinical experience and translation of preclinical research innovations, the time to make the leap to radiation-free procedures is now.
Keywords: Interventional cardiac magnetic resonance, Magnetic resonance imaging, Cardiac catheterization, Congenital heart disease
Introduction
Since its inception, cardiac catheterization has been performed with X-ray guidance to guide movement of wires, catheters, and deployment of devices in the heart [1]. This imaging guidance is suboptimal. It provides visualization of radio-opaque objects (wires and devices) within the patient’s X-ray which only provides gross detail of the cardiac silhouette, trachea, bronchi and bony structures rather than blood vessels or cardiac anatomy. Vessels and intracardiac structures must be delineated by direct injection of iodinated contrast to perform angiography of the blood pool and the surrounding soft tissue is inferred. The inherent nature of this imaging modality exposes the patient and operators to radiation associated health risks and operator orthopedic injuries associated with wearing protective lead [2–4]. Adjunctive non-radiating ultrasound and echocardiographic guidance have proven useful and convenient. Nevertheless, it is inadequate for full procedural guidance of complex interventions. Radiation-free, real-time cardiac MRI (CMR) with 3-dimensional (3D) soft tissue imaging and superior hemodynamic data has potential to be the preferred catheterization laboratory imaging modality of the future.
Why Pursue iCMR for Congenital Heart Disease?
CMR catheterization was first introduced nearly 20 years ago [5]. The logic behind migration toward CMR guidance is compelling. First, CMR provides excellent soft tissue visualization, 3-dimensional (3D) reconstructions of complex anatomy, and delineation of surrounding structures. CMR also permits characterization of tissue properties, such as edema, fibrosis, and iron content. For the congenital heart disease population, the superior soft tissue imaging afforded by CMR catheterization is of great importance given the frequency with which complex congenital and post-surgical anatomy is encountered. This is not only beneficial for anatomic catheter navigation. It may optimize diagnostic and interventional procedures such as improved endomyocardial biopsy yield using an MRI-compatible and visible bioptome and better ablation lesion visualization during real-time MRI-guided electrophysiology studies [6–9, 10•]. Second, CMR hemodynamics, namely cardiac function and blood flow, are better than traditional X-ray catheterization-derived hemodynamics. CMR-derived hemodynamics, particularly those involving complex shunt calculations, are more accurate than traditional Fick-derived calculations for congenital heart disease patients [11, 12]. CMR also offers useful hemodynamics not available with traditional X-ray such as regurgitant valve fractions and volumetric or flow analysis of any chamber or vessel. Real-time CMR-guided diagnostic right, and in some cases left, heart catheterization has been performed with similar procedure times to X-ray–guided procedures with the added benefit of additional and superior hemodynamic data from cardiac MRI [11, 13•, 14, 15••, 16]. Physiologic provocation states including vasodilator testing with inhaled nitric oxide, intravenous fluid or inotrope challenge and exercise study with MRI-compatible bicycle are feasible as part of real-time CMR-guided cardiac catheterization, while maintaining accuracy of hemodynamic evaluation [11, 14, 15••]. Third, CMR catheterization is radiation-free in growing and developing children with congenital heart disease that face true radiation exposure risk [2]. Fourth, interventional CMR offers the opportunity of real-time visual assessment of transcatheter interventions and deployed devices on the surrounding valves, vessels and extracardiac structures in addition to the hemodynamic effect of the intervention [17, 18].
Pre-clinical
Early experience was focused on preclinical proof of concept work and novel interventions taking advantage of CMR. Interventions commonly performed by pediatric and adult congenital interventional cardiologists under X-ray and echocardiographic guidance have been reported in animal models for many years including pericardiocentesis, balloon angioplasty, balloon valvuloplasty, atrial septal defect device closure, pulmonary artery stent angioplasty, coarctation stent angioplasty, transcatheter aortic valve replacement [17–23]. Real-time CMR guidance may allow more precise diagnostic studies as shown in improved targeted biopsy yield with real-time CMR guidance compared with X-ray [6]. Real-time CMR-guided chemoablation targeting the conductive isthmus between scarred ventricular myocardium regions susceptible to ventricular tachycardia is promising [24]. Moreover, real-time CMR-guidance may facilitate novel transcatheter interventions such as those that require transthoracic access to the heart or transcatheter vessel anastomosis: closed-chest percutaneous transthoracic ventricular septal defect (VSD) device closure, closed-chest percutaneous transthoracic ventricular access, posterior percutaneous transthoracic left atrium access for mitral valve interventions and transcatheter cavopulmonary shunt [25–29]. These preclinical demonstrations have illustrated the significant promise of CMR-guidance.
Clinical
In recent years, multiple new and long standing interested groups have pushed forward building and growing clinical diagnostic CMR catheterization programs [5, 12, 13•, 14, 15••, 16]. Indications include pulmonary hypertension, post-heart transplant surveillance, cardiomyopathy, intracardiac shunt, congenital heart disease, and Fontan fenestration test occlusion [12, 15••, 30–32]. With cumulative experience, workflow solutions to the challenges of the MRI environment have been developed. After an initial learning curve, CMR-guided catheterization times even with adjunct MR imaging and MR hemodynamic data are comparable to traditional X-ray guided cardiac catheterization [5, 11, 13•, 14, 15••]. Video examples of real-time CMR-guided catheterization for congenital heart disease patients, with refined workflow and efficient process, are available at the online links detailed in Table 1. Clinical real-time CMR-guided electrophysiology studies and successful radiofrequency ablation experience continues to grow [33, 34].
Table 1.
Online resources
| Link | Content |
|---|---|
| https://www.youtube.com/watch?v=7JxBNIoJLrQ | - Real-time MRI-guided right and transeptal left heart catheterization at Children’s National Heart Institute, Washington, DC |
| https://icmr.nhlbi.nih.gov | - Presentations from the iCMR workshops 2015 – 2019 at the Society for Cardiovascular Magnetic Resonance Scientific Sessions |
| https://www.optoacoustics.com/medical/imroc-ir | - Opto-acoustics IMROC IR Wireless communication with demonstration of real-time MRI-guided right heart catheterization (conference proceedings, Society for Cardiovascular Magnetic Resonance Scientific Sessions 2017) |
| https://nhlbi-mr.github.io/PRiME | - PRIME (Physiologic Recording in MRI Environment) system |
| https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5585983/bin/12968_2017_374_MOESM3_ESM.tif | - “Postage stamp” views to pre-select prior to real-time imaging guidance (supplement to [29] PMID: 28874164 [15••]) |
Clinical interventional CMR has encountred slow progress. In humans, there have been limited reports of successful transcatheter CMR-guided interventions including pulmonary balloon valvuloplasty and aortic coarctation angioplasty [20, 35]. The limiting factor to more widespread clinical translation of real-time MRI-guided interventions has primarily been availability of MRI-compatible transcatheter devices approved for clinical use.
How to Start an iCMR Program
Team Building
Most important to start and successfully run an iCMR program is recruiting an invested, experienced, enthusiastic and adaptable team. Key team members are detailed in Table 2. The cardiac catheterization team will require introduction to working in the MR environment and the MRI team will require acquaintance to cardiac catheterization to gain mutual understanding of the combined iCMR environment. CMR technologists require training and practice to refine real-time protocols and switching between pre-selected views required for real-time guidance of interventional procedures [36, 37]. Reaching out to other services who may wish to use an interventional MRI-suite including interventional radiology, neurosurgery and other surgical sub-specialties can help to build momentum and expand applications to benefit overall patient care [38]. The entire team should be invited to give input on design of potential iCMR facilities. Institutional programatic investment is crucial.
Table 2.
Suggeted iCMR team members
| Cardiac catheterization team |
|---|
|
- Interventional cardiologist with experience in congenital heart catheterization - Assistants (nurse practitioners, fellows, or additional physicians) - Catheterization laboratory nurses - Catheterization laboratory technologists |
| Cardiac MRI team |
|
- Imaging specialist: CMR imaging trained pediatric cardiologist or radiologist - MRI technologists: At least one technologist to train in real-time image guidance protocols - MRI physicists - MRI nurse |
| Procedural clinical and administrative support team |
|
- Pediatric cardiac anesthesia team with experience of patient care in the MRI-environment - MRI safety officer - Vendor representative - Cardiac catheterization and MRI administrative staff (for procedure scheduling) - Other services with potential interest in performing MRI-guided procedures (pediatric cardiac electrophysiology, interventional radiology, neurosurgery, and other surgical subspecialties) |
CMR, cardiac magnetic resonance; MRI, magnetic resonance imaging
Starting with Limited Facilities and Resources
Some centers have proactively planned and built iCMR suites (typically with a biplane X-Ray room adjoining a MRI room separated by shielded sliding doors for independent or combined operation; Fig. 1). Other centers have taken advantage of upcoming hospital construction to plan iCMR suites. The costs associated with building a new iCMR suite, particularly when hospital construction is not already planned, may be prohibitive. Many centers must garner enthusiasm, consensus and experience by initiating experience with existing limited facilities. For example, centers with only a 3T magnet may not be able to achieve ideal real-time cardiac imaging precluding guidance of catheters within the heart and equipment is more susceptible to heating at higher magnet power. Centers with only 3T scanners can still reduce radiation exposure and invasive procedure time by performing most diagnostic evaluation via CMR including flow and shunt calculations with superior anatomic delineation of cardiac structures compared to angiography, followed by invasive X-ray cardiac catheterization to obtain direct pressure measurements and perform transcatheter interventions. Similarly, invasive catheters can be pre-placed under X-ray guidance before transfer to MRI. Initially performed at 1.5T, this has been referred to as “MRI-augmented” catheterization [39]. This type of combined sequential CMR and invasive cardiac catheterization evaluation initiates the process of working together as an iCMR team increasing familiarity of all involved parties with both modalities. The likely increase in CMR volume may also help to advocate for institutional funding of further iCMR related resources. Another starting point for iCMR programs is embracing 3D CMR-derived overlay. Ideally obtained immediately before same setting X-ray cardiac catheterization, CMR overlays directly onto live X-ray fluoroscopy serve as a roadmap with the potential to simplify complex transcatheter interventions, decrease radiation exposure/contrast burden/procedure time, add diagnostic value, increase operator confidence, and enable novel procedures [40–43].
Fig. 1.
iCMR suite at Rady Children’s Hospital, University of California - San Diego. A co-localized biplane X-Ray fluoroscopy room and 1.5T cardiac MRI room are separated by radiofrequency shielded sliding doors allowing independent and combined room use. A second biplane X-Ray fluoroscopy room is adjoining a room to house a future imaging modality also intended for independent and combined room use
Where to Perform iCMR Procedures: Purpose-built Suites versus Adapting Existing MRI Rooms
The major consideration for centers embarking on an iCMR program is whether to use existing diagnostic CMR facilities to start performing real-time CMR-guided procedures or to purpose build an iCMR suite with adjacent combined X-ray fluoroscopy and CMR rooms (Fig. 1) [44•, 45]. Centers in the process of hospital construction and designing new facilities can use that opportunity to advocate for X-ray and CMR rooms to be co-localized. To perform iCMR procedures, the CMR room is ideally located adjacent or in proximity to a room suitable for patient preparation and may require non-MRI-compatible equipment prior to patient transfer into the CMR room. Once in the CMR room, there must be adequate space for patient monitoring and hemodynamic recording equipment, projection screens or monitors and sterile table setup with cardiac catherization equipment next to the MRI scanner. As with conventional X-ray–guided cardiac catheterization, the operator must be able to view live real-time CMR during device navigation in addition to the contemporaneous hemodynamic data while performing the procedure. This can be achieved by installation of shielded projector systems or LCD monitors in the CMR room (Table 3) and demonstrated in the online resource link to recorded CMR-guided cases (Table 1) [10•, 15••]. The risk of unstable arrhythmia during congenital cardiac catheterization is rare but a response plan must be in place. MRI-conditional defibrillators are under development, but not yet approved for clinical use [46]. The preparation room or another CMR adjacent room, such as the X-Ray fluoroscopy portion of an iCMR suite, must be available for patient evacuation from the CMR room for pacing or defibrillation in case of unstable arrhythmia or code [44•]. Proximity of the CMR-room to the X-ray fluoroscopy room limits time taken to transfer the patient between modalities for any X-ray dependent interventions. Patient transfer, often with multiple infusion lines and ventilator tubing, is simplified by iCMR suites with adjoining CMR and X-ray fluoroscopy rooms in a straight line configuration. Radiofrequency-shielded sliding doors (Table 2, Fig. 1), between the X-ray fluoroscopy and CMR rooms in a combined iCMR suite maintain the ability for efficient patient transfer between rooms. Importantly, it allows for simultaneous use of the MRI room for diagnostic studies when non iCMR cardiac catheterization cases are scheduled in the X-ray fluoroscopy room. This optimizes clinical use of the suite. Costs can preclude ability to build a dedicated iCMR only suite in centers with limited space and funding. Mobile MRI systems eliminating the need for patient transport exist primarily for neuroimaging applications [47]. Theoretically attractive for iCMR procedures, programs exploring dual use to date have found roadblocks in practicality for iCMR procedures and additive costs unattractive. A growing number of centers have reported successful diagnostic focused iCMR programs using existing CMR rooms. After installing required iCMR hardware and software (Table 3), this is a viable option to get an iCMR program off the ground [13•].
Table 3.
Infrastructure and equipment to start an iCMR program. Commercially available products are listed to share knowledge of available resources used by centers referenced in this review. This does not reflect product endorsement, the authors do not have conflicts of interest with any companies, and other examples may be available
| Equipment/infrastructure | Examples |
|---|---|
| 1.5 T wide-short-bore scanner optimized for cardiovascular imaging | - Vendor specific |
| CMR surface coils for cardiovascular imaging | - Vendor specific |
| Vendor specific real-time scanner control and visualization software |
- RTHawk by GE - Monte Carlo prototype software by Siemens, - iSuite by Philips |
| In-room display | |
|
- Shielded projector systems - LCD monitor |
- Gaven Industries - Sentient |
| In-suite cameras | - Gaven Industries |
| Audio communication noise cancelling headsets | - IMROC by OptoAcoustics |
| Patient monitoring | - Expression MRI Patient Monitoring System, Invivo Corporation |
| Hemodynamic recording system augmentation |
- Physiological Recording in MRI Environment [PriME] system - Wireless telemetry system, PELEX-MAX, PinMed Inc |
| Nitric oxide delivery system | - INOmax, Mallinckrodt Pharmaceuticals |
| Electrophysiology equipment specific to MRI-guided arrhythmia ablation | |
| - Recording system and simulator | - Advantage MR EP Recorder/Simulator, Imricor Medical Systems, USA |
| - Ablation catheter | - Vision MRTM ablation catheter, Imricor Medical Systems |
| Sterile procedure maintenance in the MRI-room |
- Large sterile drapes for MRI-scanner - Long extension tubing for infusions - MRI-compatible stand to serve as a procedure table |
| Commercially available “off the shelf” standard catheterization equipment that is also MR-compatible | |
| - Balloon wedge catheter and 1% dilute gadolinium solution for balloon inflation | - Arrow balloon wedge catheter, Teleflex Medical |
| Purpose built MRI-conditional catheterization equipment | |
| - MR-conditional guidewire | - Angled-tip Emeryglide MRWire, Nano4Imaging |
Imaging Hardware and Software
Wide, short-bore MRI scanners facilitate operator proximity to reach vascular access sites for cardiac catheterization. This is particularly relevant in the setting of increased ergonomic challenges with children and infants who may be completely within the central bore of the scanner [15••, 38]. The major imaging vendors each have their own MRI scanner options suitable for iCMR applications that can accommodate rapid real-time imaging that demands fast gradient hardware and dedicated phased-array torso coils, including coils for pediatric patients. In addition, each of the major imaging vendors provides accompanying software (Table 3) to facilitate multi-planar or 3D volume imaging, and interactive sequence modification to aid real-time MRI-guided procedures. MRI scanners are not specifically designed for the procedural environment but can be easily draped in a straightforward manner to create sterile conditions suitable for cardiac catheterization [15••, 36]. The current recommended magnet strength for clinical iCMR procedures is 1.5T providing excellent temporal and spatial resolution despite expected cardiorespiratory motion at fast heart rates in the pediatric population [10•]. Image acquisition by cardiac MRI designed for diagnostic data collection over multiple heartbeats does not translate well for real-time imaging guidance. iCMR procedures require rapid image acquisition, reconstruction and display to visualize catheters moving through the heart in an acceptable timeframe for procedural guidance. Real-time imaging typically uses balanced steady state free-precession (bSSFP) for suitable blood-myocardium contrast, magnetization preparation pulses for improved contrast, and parallel imaging reconstruction for imaging speed. The capability to interactively modify slice orientation and position, as well as other imaging parameters, during continuous imaging is critical for procedural guidance with real-time MRI. With multiple imaging protocol and reconstruction modifications, real-time magnetic-resonance imaging has progressed to facilitate guidance of up to ten frames per second which is comparable to modern conventional X-ray guidance but with superior soft tissue data with single or multiplanar viewing options [10•, 15••, 37]. Visibility of MRI-compatible equipment in the heart and vessels with real-time imaging is often limited without modifications to the equipment and imaging parameters as detailed in the discussion of cardiac catheterization equipment below. Dilute gadolinium contrast for balloon inflation of commercially available balloon wedge catheters together with imaging pre-pulses make this contrast filled balloon more visible in real-time imaging in comparison to the blood pool [10•, 48]. This is one example of a simple equipment and imaging parameter modification to aid in facilitating iCMR clinical translation.
Patient Monitoring and Hemodynamics
MRI-conditional anesthesia equipment, ventilators, infusion pumps/tubing and patient monitoring equipment perform well for routine diagnostic MRI clinical practice. This equipment can be used for iCMR procedures with pre-planned consideration for ideal placement on the patient and location in the room. During iCMR procedures, limited patient access in the MRI bore, dimmed room lights, and sterile coverage drapes necessitates special consideration for optimal access to intravenous lines and patient airway [49]. iCMR studies require clear electrocardiogram and invasive blood pressure tracings for patient monitoring and interpretation of pressure tracings. MRI-compatible hemodynamic recording systems can be outfitted with signal optimization to filter noise and signal distortion caused by MRI scanning and allow for clinically acceptable electrocardiogram and invasive blood pressure tracings (Table 3) [50]. MRI-conditional nitric oxide delivery systems are available as detailed in Table 3. There are also unique patient considerations. Personal patient pumps, relevant to pulmonary hypertension patients receiving subcutaneous treprostinil do not have long tubing and are not MRI compatible. Infusions must be discontinued for the duration of time in the MRI room if clinically acceptable or switched to the intravenous route.
Communication
While operators can enjoy being free from the weight and constraints of wearing lead, they have to contend with the loud noise emitted by the MRI scanner necessitating noise cancelling protective headsets. These headsets can serve a dual purpose, when paired with an MR-compatible microphone, allowing communication between the operators, anesthesia team, MR-technologist and team in the control room. Wireless solutions are optimal. Potential communication systems and examples of use are available in online video links (Table 1). There must also be a separate communication channel between the patient and the MRI control room and cardiac catheterization operators for patients undergoing procedures under conscious sedation.
Patient Safety Considerations
Widespread implementation of routine iCMR has been challenging for many reasons including the inherent hurdles of working in the magnetic resonance environment and limited availability of MRI-compatible equipment for cardiac catheterization. MRI scanners generate a magnetic field powerful enough to turn standard medical equipment into dangerous projectiles such as oxygen tanks. Equipment specifically designed for routine anesthesia care in the magnetic field is commonplace including MRI-compatible nitric oxide delivery systems. Cardiac catheterization equipment primarily composed of metal are susceptible to becoming projectiles when pulled toward the magnet. Typical standard cardiac catheterization wires, catheters and device delivery systems are susceptible to significant heating under real-time CMR-guidance. Safety protocols and standard operating procedures can easily mitigate these risks. One example is if anesthesia induction, vascular access or any portion of the catheterization case is performed prior to entering the CMR room, a time-out metal safety check should be performed prior to patient transfer to the CMR room. This guarantees that all non-compatible objects, such as stainless-steel wires and needles, have been removed from the field. Likewise, and similar to non-iCMR CMR cases, each team member entering the CMR room should perform a personal check to make sure they are not inadvertently carrying or wearing items likely to become projectiles. The radio-frequency field risk of the MRI environment can also generate currents that interfere with pacemakers and with hemodynamic monitoring systems. As per routine for any diagnostic MRI scan, the compatibility of the patient’s existing implanted devices is determined prior to approving the study on an individual case basis. Consideration should be given to suturing in place any vascular access sheaths prior to transfer to the CMR room as transfer between the X-ray fluoroscopy and CMR rooms introduces the potential risk of sheath dislodgement [15••]. Frequent simulations and drills for routine and emergency clinical scenarios including cardiac arrest in the iCMR environment can reduce the risk of patient-safety related events [44•, 49].
Cardiac Catheterization Equipment
Much of the standard equipment for a cardiac catheterization is MRI-compatible including most sterile drapes, transducers and tubing, access sheaths and non-braided diagnostic catheters such as balloon wedge catheters. Availability of specifically designed MRI-compatible and MRI-visible equipment to facilitate real-time MRI-guided cases is continually increasing. A brief summary of useful existing equipment, including specific MRI-guided ablation procedure equipment, is listed in Table 3. There are limitiations in the MRI-visibility of most standard catheterization wires, catheters, sheaths and devices. In addition, long metallic devices such as guidewires and braided catheters are susceptible to radiofrequency-induced heating during real-time MRI and should be avoided [51, 52]. Cardiac catheteterization equipment used in CMR-guided procedures is classed as passive or active depending on method of visualization. Passive device visualization relies on intrinsic device properties such as air or Gadolinium filled balloons (balloon wedge catheters) or signal voids created by metallic devices, for example nitinol guidewires. Some purpose built devices for MRI-guided interventions have additional passive markers, such as iron oxide bands, for signal void creation and improved MRI visualization. An MR-conditional approved guidewire with passive markers exists and has been reported for iCMR procedures in patients with congenital heart disease [53]. A much larger number of approved MR-conditional devices is needed to expand the potential diagnostic and interventional reach of iCMR procedures. Active devices incorporate electronic components connected to the scanner such that devices themselves can receive local MRI signal for improved visualization, for example, generating a bright-colored overlay to highlight the device on real-time imaging [6, 24–29]. Clinical translation of these active devices has been limited. Radiofrequency receiver coils can be embedded into the device to allow for active tracking of the device during imaging rather than relying on manually adjusting to the exact slice plane that shows the device and have been used for MRI-guided arrhythmia studies [9].
Future Directions
Rather than rely on development of purpose-built equipment for the MRI environment, advances in imaging may be a better route to widespread iCMR feasibility. Modification of imaging techniques has made it possible to investigate off-label use of commercially available X-ray equipment such as hydrophilic glidewires at 1.5T for real-time MRI-guided right heart catheterization with visualization and without significant heating [54, 55]. Lower field MRI, for example the modification of a commercial 1.5T scanner to operate at 0.55T, as demonstrated in Fig. 2, is an exciting advance for iCMR procedures explored in the last few years [56]. At this lower magnet strength, even with preferred real-time imaging techniques, there is less heating of catheterization equipment traditionally used for X-ray-guided cardiac catheterization. Low field imaging may rapidly expand the equipment available for use in iCMR procedures from the armamentarium of existing traditional cardiac catheterization equipment, while also reducing artifacts caused by post-intervention metallic implants and maintaining diagnostic CMR image quality [56–58]. With these advances in imaging technology in addition to purpose-modified MR compatible devices, CMR-guidance could dramatically increase the scope of transcatheter congenital heart interventions particularly those that require visualization of soft tissues such as anastomosis of vessels and other procedures currently undertaken solely by open heart surgery.
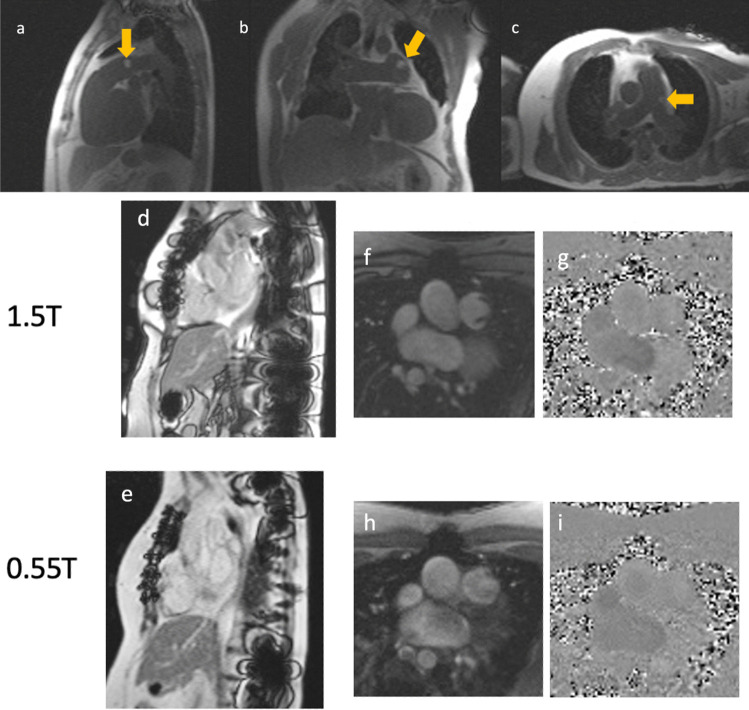
Fig. 2.
Low-field CMR catheterization. Low field, 0.55T, real-time MRI-guided right heart catheterization using commercially available metallic hydrophilic coated guidewire (invisible with partial saturation pulse) and gadolinium filled commercially available balloon (yellow arrows) tipped wedge catheter in the sagittal (a), coronal (b) and axial (c) planes. For patients post cardiac interventions with metallic devices and implants including sternal wires, as illustrated for a patient post tetralogy of Fallot repair (d–g), metal artifact at 1.5T (d) is reduced at 0.55T (e) without compromising the ability to determine flow via the turbulent pulmonary valve at 0.55T (h and i) compared to 1.5T (f and g)
Conclusions
Cardiac magnetic resonance is an appealing modality for congenital heart disease cardiac catheterization image guidance as it provides radiation-free 3D soft tissue visualization and additional hemodynamic data for complex congenital anatomy and interventions. Multiple review articles have been published over the last two decades on the promise and progress of iCMR for standard and future transcatheter interventions to replace existing suboptimal X-ray imaging guidance with associated patient and occupational hazards [59–65]. Nevertheless, despite the promise and compelling logic supporting iCMR catheterization, clinical translation has been slow. There has been growing experience building iCMR suites and working in the magnetic resonance environment at multiple centers around the world. With continued experience of starting and running iCMR programs, building consensus and knowledge sharing in conjunction with translation of preclinical research innovations, iCMR could potentially facilitate routine radiation-free transcatheter interventions to become the standard operating modality of the future. The time to make the leap away from radiation emitting procedures is now.
Author Contribution
EA, ACW and KR conceptualized, wrote and edited the manuscript and figures.
Funding
Kanishka Ratnayaka, MD, receives funding from National Heart, Lung, and Blood Institute for development of novel treatments for congenital heart disease (Contract No: 75N92019P00353). Adrienne Campbell Washburn, PhD, receives funding from National Heart, Lung, and Blood Institute (Z01-HL006257, Z01-HL006213).
Compliance with Ethical Standards
Conflict of Interest
Adrienne Campbell Washburn reports that she is an investigator on a US Government Cooperative Research and Development Agreement (CRADA) with Siemens Healthcare. Siemens participated in the modification of the MRI system to operate at 0.55T. The other authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
Studies performed by the authors referenced in this review have been approved by the research ethics committee at the institution at which the studies were conducted.
Footnotes
This article is part of the Topical Collection on Congenital Heart Disease
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elena K. Amin, Email: elena.amin@ucsf.edu
Adrienne Campbell-Washburn, Email: adrienne.campbell@nih.gov.
Kanishka Ratnayaka, Email: kratnayaka@rchsd.org.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.King S. The development of interventional cardiology. JACC. 1998;31(4):64B–88B. doi: 10.1016/s0735-1097(97)00558-5. [DOI] [PubMed] [Google Scholar]
- 2.Johnson JN, Hornik CP, Li JS, Benjamin DK, Yoshizumi TT, Reiman RE, Frush DP, Hill KD. Cumulative radiation exposure and cancer risk estimation in children with heart disease. Circulation. 2014;130:161–167. doi: 10.1161/CIRCULATIONAHA.113.005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreassi MG, Ait-Ali L, Botto N, Manfredi S, Mottola G, Picano E. Cardiac catheterization and long-term chromosomal damage in children with congenital heart disease. Eur Heart J. 2006;27:2703–8. 10.1093/eurheartj/ehl014. [DOI] [PubMed]
- 4.Klein LW, Tra Y, Garratt KN, Powell W, Lopez-Cruz G, Chambers C, Goldstein JA. Occupational health hazards of interventional cardiologists in the current decade: results of the 2014 SCAI membership survey. Catheter Cardiovasc Interv 2015;86:913–24. 10.1002/ccd.25927. [DOI] [PubMed]
- 5.Razavi R, Hill DL, Keevil SF, et al. Cardiac catheterization guided by MRI in children and adults with congenital heart disease. The Lancet. 2003;362:1877–1882. doi: 10.1016/S0140-6736(03)14956-2. [DOI] [PubMed] [Google Scholar]
- 6.Rogers T, Ratnayaka K, Karmarkar P, Campbell-Washburn AE, Schenke WH, Mazal JR, Kocaturk O, Faranesh AZ, Lederman RJ. Real-time magnetic resonance imaging guidance improves the diagnostic yield of endomyocardial biopsy. JACC Basic Transl Sci. 2016;1:376–383. doi: 10.1016/j.jacbts.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paestch I, Sommer P, Jahnke C, et al. Clinical workflow and applicability of electrophysiological cardiovascular magnetic resonance-guided radiofrequency ablation of isthmus-dependent atrial flutter. Eur Heart J Cardiovasc Imaging. 2019;20(2):147–156. doi: 10.1093/ehjci/jey143. [DOI] [PubMed] [Google Scholar]
- 8.Grant EK, Berul CI, CrossRR, Moak JP, Hamann KS, Sumihara K, Cronin I, O’Brien KJ, Ratnayaka K, Hansen MS, Kellman P, Olivieri LJ. Acute cardiac MRI assessment of radiofrequency ablation lesions for pediatric ventricular arrhythmia: feasibility and clinical correlation. J Cardiovasc Electrophysiol. 2017;28:517–22. 10.1111/jce.13197. [DOI] [PMC free article] [PubMed]
- 9.Hilbert S, Sommer P, Gutberlet M, Gaspar T, Foldyna B, Piorkowski C, Weiss S, Lloyd T, Schnackenburg B, Krueger S, Fleeter C, Paetsch I, Jahnke C, Hindricks G, Grothoff M. Real-time magnetic resonance-guided ablation of typical right atrial flutter using a combination of active catheter tracking and passive catheter visualization in man: initial results from a consecutive patient series. Europace 201618:572–7. 10.1093/europace/euv249. [DOI] [PubMed]
- 10.• Campbell-Washburn AE, Tavallaei MA, Pop M, Grant EK, Chubb H, Rhode K, Wright GA. Real-time MRI guidance of cardiac interventions. J Magn Reson Imaging. 2017;46:935–50. 10.1002/jmri.25749. Comprehensive review of imaging advances for iCMR procedures. [DOI] [PMC free article] [PubMed]
- 11.Muthurangu V, Atkinson D, Sermesant M, et al. Measurement of total pulmonary arterial compliance using invasive pressure monitoring and MR flow quantification during MR-guided cardiac catheterization. Am J Physiol Heart Circ Physiol. 2005;289(3):H1301–306. doi: 10.1152/ajpheart.00957.2004. [DOI] [PubMed] [Google Scholar]
- 12.Arar Y, Hussain T, Abou Zahr R, Gooty V, Greer JS, Huang R, Hernandez J, King J, Greil G, Veeram Reddy SR. Fick versus flow: a real-time invasive cardiovascular magnetic resonance (iCMR) reproducibility study. J Cardiovasc Magn Reson. 2021;23(1)95. 10.1186/s12968-021-00784-7. [DOI] [PMC free article] [PubMed]
- 13.• Knight DS, Kotecha T, Martinez-Naharro A, Brown JT, Bertelli M, Fontana M, Muthurangu V, Coghlan JG. Cardiovascular magnetic resonance-guided right heart catheterization in a conventional CMR environment — predictors of procedure success and duration in pulmonary artery hypertension. J Cardiovasc Magn Reson. 2019 Sep 9;21(1):57. 10.1186/s12968-019-0569-9. Example of an iCMR program using existing diagnostic MRI facilities with details of learning curve and workflow tips. [DOI] [PMC free article] [PubMed]
- 14.Rogers T, Ratnayaka K, Khan J, et al. CMR fluoroscopy right heart catheterization for cardiac output and pulmonary vascular resistance: results in 102 patients. JCMR. 2017;19(1):54. doi: 10.1186/s12968-017-0366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.•• Ratnayaka K, Kanter JP, Faranesh AZ, Grant EK, Olivieri LJ, Cross RR, Cronin IF, Hamann KS, Campbell-Washburn AE, O’Brien KJ, Rogers T, Hansen MS, Lederman RJ. Radiation-free CMR diagnostic heart catheterization in children. J Cardiovasc Magn Reson. 2017;19:1–65. 10.1186/s12968-017-0374-2. Workflow and outcomes for real-time MRI-guided cardiac catheterization in pediatric patients with details of iCMR team and suite. [DOI] [PMC free article] [PubMed]
- 16.Velasco Forte MN, Roujol S, Ruijsink B, Valverde I, Duong P, Byrne N, Krueger S, Weiss S, Arar Y, Reddy SRV, Schaeffter T, Hussain T, Razavi R, Pushparajah K. MRI for guided right and left heart cardiac catheterization: a prospective study in congenital heart disease. J Magn Reson Imaging. 2021;53:1446–1457. doi: 10.1002/jmri.27426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raval AN, Telep JD, Guttman MA, Ozturk C, Jones M, Thompson RB, Wright VJ, Schenke WH, DeSilva R, Aviles RJ, Raman VK, Slack MC, Lederman RJ. Real-time magnetic resonance imaging-guided stenting of aortic coarctation with commercially available catheter devices in Swine. Circulation. 2005;112(5):699–706. doi: 10.1161/CIRCULATIONAHA.105.542647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuehne T, Saeed M, Higgins CB, Gleason K, Krombach GA, Weber OM, et al. Endovascular stents in pulmonary valve and artery in swine: feasibility study of MR imaging-guided deployment and postinterventional assessment. Radiology. 2003;226(2):475–81. doi: 10.1148/radio.2262011639. [DOI] [PubMed] [Google Scholar]
- 19.Halabi M, Faranesh AZ, Schenke WH, Wright VJ, Hansen MS, Saikus CE, Kocaturk O, Lederman RJ, Ratnayaka K. Real-time cardiovascular magnetic resonance subxiphoid pericardial access and pericardiocentesis using off-the-shelf devices in swine. J Cardiovasc Magn Resonan. 2013;15(1):61. doi: 10.1186/1532-429X-15-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tzifa A, Krombach GA, Kramer N, Kruger S, Schutte A, von Walter M, Schaeffter T, Qureshi S, Krasemann T, Rosenthal E, Schwartz CA, Varma G, Buhl A, Kohlmeier A, Bucker A, Gunther RW, Razavi R. Magnetic resonance-guided cardiac interventions using magnetic resonance-compatible devices: a preclinical study and first-in-man congenital interventions. Circ Cariovasc Interv. 2010;3(6):585–592. doi: 10.1161/CIRCINTERVENTIONS.110.957209. [DOI] [PubMed] [Google Scholar]
- 21.Schalla S, Saeed M, Higgins CB, Weber O, Martin A, Moore P. Balloon sizing and transcatheter closure of atrial septal defects guided by magnetic resonance fluoroscopy: assessment and validation in a large animal model. J Magn Reson Imaging. 2005;21(3):204–11. 10.1002/jmri.20267. [DOI] [PubMed]
- 22.Rickers C, Jerosch-Herold M, Hu X, Murthy N, Wang X, Kong H, Seethamraju RT, Weil J, Wilke NM. Magnetic resonance-guided transcatheter closure of atrial septal defects. Circulation. 2003;107(1):132–138. doi: 10.1161/01.cir.0000039343.95540.cf. [DOI] [PubMed] [Google Scholar]
- 23.Kahlert P, Parohl N, Albert J, Schafer L, Reinhardt R, Kaiser GM, et al. Real-time magnetic resonance imaging-guided transarterial aortic valve implantation: in vivo evaluation in swine. J Am Coll Cardiol. 2012;59(2):192–3. doi: 10.1016/j.jacc.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 24.Rogers T, Mahapatra S, Kim S, Eckhaus MA, Schenke WH, Mazal JR, Campbell-Washburn, Sonmez M, Faranesh AZ, Ratnayaka K, Lederman RJ. Transcatheter myocardial needle chemoablation during real-time magnetic resonance imaging: a new approach to ablation therapy for rhythm disorders. Circ Arrhythm Electrophysiol. 2016;9(4):e003926. 10.1161/CIRCP.115.003926. [DOI] [PMC free article] [PubMed]
- 25.Ratnayaka K, Saikus CE, Faranesh AZ, Bell JA, Barbash IM, Kocaturk O, Reyes CA, Sonmez M, Schenke WH, Wright VJ, Hansen MS, Slack MC, Lederman RJ. Closed-chest transthoracic magnetic resonance imaging-guided ventricular septal defect closure in swine. JACC Cardiovasc Interv. 2011;4(12):1326–1334. doi: 10.1016/j.jcin.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halabi M, Ratnayaka K, Faranesh AZ, Hansen MS, Barbash IM, Eckhaus MA, Wilson JR, Chen MY, Slack MC, Kocaturk O, Schenke WH, Wright VJ, Lederman RJ. Transthoracic delivery of large devices into the left ventricle through the right ventricle and interventricular septum: preclinical feasibility. J Cardiovasc Magn Reson. 2013;15(1):10. doi: 10.1186/1532-429X-15-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbash IM, Saikus CE, Faranesh AZ, Ratnayaka K, Kocaturk O, Chen MY, Bell JA, Virmani R, Schenke WH, Hansen MS, Slack MC, Lederman RJ. Direct percutaneous left ventricular access and port closure: pre-clinical feasibility. JACC Cardiovasc Interv 2011;4:1318–25. 10.1016/j.jcin.2011.07.017. [DOI] [PMC free article] [PubMed]
- 28.Rogers T, Ratnayaka K, Schenke WH, Sonmez M, Kocaturk O, Mazal JR, Chen MY, Flugelman MY, Troendle JF, Faranesh AZ, Lederman RJ. Fully percutaneous transthoracic left atrial entry and closure as a potential access route for transcatheter mitral valve interventions. Circ Cardiovasc Interv. 2015;8(6):e002538. doi: 10.1161/CIRCINTERVENTIONS.114.002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ratnayaka K, Rogers T, Schenke WH, Mazal JR, Chen MY, Sonmez M, Hansen MS, Kocaturk O, Faranesh AZ, Lederman RJ. Magnetic resonance imaging-guided transcatheter cavopulmonary shunt. JACC Cardiovasc Interv. 2016;9(9):959–970. doi: 10.1016/j.jcin.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pushparajah K, Tzifa A, Bell A, Wong JK, Hussain T, Valverde I, Bellsham-Revell HR, Greil G, Simpson JM, Schaeffter T, Razavi R. Cardiovascular magnetic resonance catheterization derived pulmonary vascular resistance and medium-term outcomes in congenital heart disease. Cardiovasc Magn Reson. 2015;17(1):28. doi: 10.1186/s12968-015-0130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandya B, Quail MA, Steeden JA, McKEe A, Odille F, Taylor Am, Schulze-Neick I, Derrick G, Moledina S, Muthurangu V. Real-time magnetic resonance assessment of septal curvature accurately tracks acute hemodynamic changes in pediatric pulmonary hypertension. Circ Cardiovasc Imaging. 2014;7(4):706–13. 10.1161/CIRCIMAGING.113.001156. [DOI] [PubMed]
- 32.Tzifa A, Razavi R. Test occlusion of Fontan fenestration: unique contribution of interventional MRI. Heart. 2011;97(1):89. doi: 10.1136/hrt.2010.203687. [DOI] [PubMed] [Google Scholar]
- 33.Sommer P, Grothoff M, Eitel C, Gaspar T, Piorkowski C, Gutberlet M, Hindricks G. Feasibility of real-time magnetic resonance imaging-guided electrophysiology studies in humans. Europace. 2013;15(1):101–108. doi: 10.1093/europace/eus230. [DOI] [PubMed] [Google Scholar]
- 34.Piorkowski C, Grothoff M, Gasar T, Eitel C, Sommer P, Huo Y, John S, Gutberlet M, Hindricks G. Cavotricuspid isthmus ablation guided by real-time magnetic resonance imaging. Circ Arrhythm Electrophysiol. 2013;6:e7–10. 10.1161/CIRCEP.112.973719. [DOI] [PubMed]
- 35.Krueger JJ, Ewert P, Yilmaz S, Gelernter D, Peters B, Pietzner K, et al. Magnetic resonance imaging-guided balloon angioplasty of coarctation of the aorta: a pilot study. Circulation. 2006;113(8):1093–100. doi: 10.1161/CIRCULATIONAHA.105.578112. [DOI] [PubMed] [Google Scholar]
- 36.Mazal JR, Rogers T, Schenke WH, Faranesh AZ, Hansen M, O'Brien K, Ratnayaka K, Lederman RJ. Interventional-cardiovascular MR: role of the interventional MR technologist. Radiol Technol. 82016;7(3):261–70. [PMC free article] [PubMed]
- 37.Ratnayaka K, Faranesh AZ, Hansen MS, Stine AM, Halabi M, Barbash IM, Schenke WH, Wright VJ, Grant LP, Kellman P, Kocaturk O, Lederman RJ. Real-time MRI-guided right heart catheterization in adults using passive catheters. Eur Heart J. 2013;34(5):380–9. doi: 10.1093/eurheartj/ehs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nour SG, Lewin JS. Creating a clinical interventional MRI service. Topics in Magnetic Resonance Imaging. 2018;28(1):25–31. doi: 10.1097/RMR.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 39.Ntsinjana HN, Tann O, Taylor AM. Trends in pediatric cardiovascular magnetic resonance imaging. Acta Radiol. 2013;54(9):1063–1074. doi: 10.1177/0284185113475609. [DOI] [PubMed] [Google Scholar]
- 40.Grant EK, Kanter JP, Olivieri LJ, Cross RR, Campbell-Washburn A, Faranesh AZ, Cronin I, Hamann KS, O’Byrne ML, Slack MC, Lederman RJ, Ratnayaka K. X-ray fused with MRI guidance of pre-selected transcatheter congenital heart disease interventions. Catheter Cardiovasc Interv. 2019;94(3):399–408. doi: 10.1002/ccd.28324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abu Hazeem AA, Dori Y, Whitehead KK, et al. X-ray magnetic resonance fusion modality may reduce radiation exposure and contrast dose in diagnostic cardiac catheterization of congenital heart disease. Catheter Cardiovasc Interv. 2014;84:795–800. doi: 10.1002/ccd.25473. [DOI] [PubMed] [Google Scholar]
- 42.Glockler M, Halbfabeta J, Koch A, Achenbach S, Dittrich S. Multimodality 3D-roadmap for cardiovascular interventions in congenital heart disease-a single-center, retrospective analysis of 78 cases. Catheter Cardiovasc Interv. 2013;82:436–442. doi: 10.1002/ccd.24646. [DOI] [PubMed] [Google Scholar]
- 43.Ratnayaka K, Raman VK, Faranesh AZ, et al. Antegrade percutaneous closure of membranous ventricular septal defect using X-ray fused with magnetic resonance imaging. JACC Cardiovasc Interv. 2009;2:224–230. doi: 10.1016/j.jcin.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.• Cronin I, Kanter J, Deutsch N, Hamann K, Olivieri L, Cross RR. Magnetic resonance imaging-guided cardiac catheterization evacuation drills. Crit Care Nurse. 2021;41(3):e19–26. 10.4037/ccn2021229. Essential tips for running and efficient iCMR program while prioritizing patient-safety. [DOI] [PMC free article] [PubMed]
- 45.White MJ, Thornton JS, Hawkes DJ, Hill DL, Kitchen N, Mancini L, et al. Design, operation, and safety of single-room interventional MRI suites: practical experience from two centers. J Magn Resonance Imaging. 2014;41(1):34–43. doi: 10.1002/jmri.24577. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt EJ, Watkins RD, Zviman MM, et al. A magnetic resonance imaging-conditional external cardiac defibrillator for resuscitation within the magnetic resonance imaging scanner bore. Circ Cardiovasc Imaging. 2016;9(10):e005091. doi: 10.1161/CIRCIMAGING.116.005091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoult DI, Saunders JK, Sutherland GR, Sharp J, Gervin M, Kolansky HG, Kripiakevich DL, Procca A, Sebastian RA, Bombay A, Rayner DL, Roberts FA, Tomanek B. The engineering of an interventional MRI with a movable 1.5 Tesla magnet. J Magn Resonance Imaging: JMRI. 2001;13(1):78–86. 10.1002/1522-2586(200101)13:1/78::aid-jmri1012/3.0.co;2-1. [DOI] [PubMed]
- 48.Velasco-Forte MN, Pushparajah K, Schaeffter T, et al. Improved passive catheter tracking with positive contrast for CMR-guided cardiac catheterization using partial saturation (pSAT) J Cardiovasc Magn Reson. 2017;19(1):60. doi: 10.1186/s12968-017-0368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deutsch N, Swink J, Matisoff AJ, et al. Anesthetic considerations for magnetic resonance imaging-guided right heart catheterization in pediatric patients: a single institution experience. Pediatr Anesthes. 2019;29(1):8–15. doi: 10.1111/pan.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kakareka JW, Faranesh AZ, Pursley RH, Campbell-Washburn A, Herzka DA, Rogers T, Kanter J, Ratnayaka K, Lederman RJ, Pohida TJ. Physiological recording in the MRI environment (PriME): MRI-compatible hemodynamic recording system. IEEE J Transl Eng Health Med. 2018;6:4100112. doi: 10.1109/JTEHM.2018.2807813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Konings MK, Bartels LW, Smits HF, Bakker CJ. Heating around intravascular guidewires by resonating RF waves. J Magn Reson Imaging. 2000;12(1):79–85. doi: 10.1002/1522-2586(200007)12:1<79::aid-jmri9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 52.Yeung CJ, Susil RC, Atalar E. RF safety of wires in interventional MRI: using a safety index. Magn Reson Med. 2002;47(1):187–193. doi: 10.1002/mrm.10037. [DOI] [PubMed] [Google Scholar]
- 53.Veeram Reddy S, Arar Y, Abou Zahr R, et al. Invasive cardiovascular magnetic resonance (iCMR) for diagnostic right and left heart catheterization using an MR-conditional guidewire and passive visualization in congenital heart disease. JCMR. 2020;22(1):20. doi: 10.1186/s12968-020-0605-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campbell-Washburn AE, Rogers T, Stine AM, Khan JM, Ramasawmy R, Schenke WH, McGuirt DR, Mazal JR, Grant LP, Grant EK, Herzka DA, Lederman RJ. Right heart catheterization using metallic guidewires and low SAR cardiovascular magnetic resonance fluoroscopy at 1.5 Tesla: first in human experience. 2018;20(1):41. 10.1186/s12968-018. [DOI] [PMC free article] [PubMed]
- 55.Campbell-Washburn AE, Rogers T, Basar B, Sonmez M, Kocaturk O, Lederman RJ, Hansen MS, Faranehs AZ. Positive contrast spiral imaging for visualization of commercial nitinol guidewires with reduced heating. J Cardiovasc Magn Reson. 2015;17:114. doi: 10.1186/s12968-015-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell-Washburn AE, Ramasawmy R, Restivo MC, et al. Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI. Radiology. 2019;293(2):384–393. doi: 10.1148/radiol.2019190452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bandettini WP, Shanbhag SM, Mancini C, McGuirt DR, Kellman P, Xue H, Henry JL, Lowery M, Thein SL, Chen MY, Campbell-Washburn AE. A comparison of cine CMR imaging at 0.55T and 1.5T. J Cardiovasc Magn Reson. 2020;22(1):37. 10.1186/s12968-020-00618-y. [DOI] [PMC free article] [PubMed]
- 58.Bandettini WP, Shanbhag SM, Mancini C, Henry JL, Lowery M, Chen MY, Xue H, Kellman P, Campbell-Washburn AE. Evaluation of myocardial infarctation by cardiovascular magnetic resonance at 0.55-T compared to 1.5-T. JACC Cardiovasc Imaging. 2021;14(9):1866–8. 10.1016/j.jcmg.2021.02.024. [DOI] [PubMed]
- 59.Moore P. MRI-guided congenital cardiac catheterization and intervention: the future? Catheter Cardiovasc Interv. 2005;66:1–8. doi: 10.1002/ccd.20485. [DOI] [PubMed] [Google Scholar]
- 60.Ratnayaka K, Faranesh AZ, Guttman MA, Kocaturk O, Saikus CE, Lederman RJ. Interventional cardiovascular magnetic resonance: still tantalizing. JCMR. 2008;10(1):62. doi: 10.1186/1532-429X-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saikus C, Lederman R. Interventional cardiovascular magnetic resonance imaging: a new opportunity for image guided interventions. JACC Cardiovasc Imaging. 2009;2(11):1321–1331. doi: 10.1016/j.jcmg.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rogers T, Ratnayaka K, Lederman R. MRI catheterization in cardiopulmonary disease. Chest. 2014;145(1):30–36. doi: 10.1378/chest.13-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rogers T, Lederman RJ. Interventional CMR: Clinical applications and future directions. Curr Cardiol Rep. 2015;17:31. doi: 10.1007/s11886-015-0580-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pushparajah K, Chubb H, Razavi R. MR-guided cardiac interventions. TMRI. 2018;27(3):115–128. doi: 10.1097/RMR.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 65.Nageotte SJ, Lederman RJ, Ratnayaka K. MRI catheterization: ready for broad adoption. 2020;41(3):503–513. doi: 10.1007/s00246-020-02301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]