Abstract
Background
BRCA1 and BRCA2 pathogenic variants (PVs) are associated with prostate cancer (PCa) risk, but a wide range of relative risks (RRs) has been reported.
Methods
We systematically searched PubMed, Embase, MEDLINE and Cochrane Library in June 2021 for studies that estimated PCa RRs for male BRCA1/2 carriers, with no time or language restrictions. The literature search identified 27 studies (BRCA1: n = 20, BRCA2: n = 21).
Results
The heterogeneity between the published estimates was high (BRCA1: I2 = 30%, BRCA2: I2 = 83%); this could partly be explained by selection for age, family history or aggressive disease, and study-level differences in ethnicity composition, use of historical controls, and location of PVs within BRCA2. The pooled RRs were 2.08 (95% CI 1.38–3.12) for Ashkenazi Jewish BRCA2 carriers, 4.35 (95% CI 3.50–5.41) for non-Ashkenazi European ancestry BRCA2 carriers, and 1.18 (95% CI 0.95–1.47) for BRCA1 carriers. At ages <65 years, the RRs were 7.14 (95% CI 5.33–9.56) for non-Ashkenazi European ancestry BRCA2 and 1.78 (95% CI 1.09–2.91) for BRCA1 carriers.
Conclusions
These PCa risk estimates will assist in guiding clinical management. The study-level subgroup analyses indicate that risks may be modified by age and ethnicity, and for BRCA2 carriers by PV location within the gene, which may guide future risk-estimation studies.
Subject terms: Cancer epidemiology, Cancer epigenetics, Prostate cancer, Risk factors
Introduction
Pathogenic variants (PVs) in BRCA1 and BRCA2 are associated with prostate cancer (PCa) risk, but a wide range of relative risk (RR) estimates has been reported [1–26]. A systematic review and meta-analysis on PCa risks for men with germline BRCA1/2 PVs (henceforth, “BRCA1/2 carriers”) was published in 2019, and estimated pooled RRs of 1.35 (95% CI 1.03–1.76) for BRCA1 and 2.64 (95% CI 2.03–3.47) for BRCA2 carriers [27]. However, that meta-analysis did not consider variation in the RRs by age, PCa family history, ethnicity or PV location despite evidence of variation by these factors [1–8, 10–12, 14–17, 23, 28–33], and did not include two subsequent studies that reported prospective RR estimates for BRCA1/2 carriers: the IMPACT screening trial [20] and the EMBRACE study [23].
Study aims
This systematic review and meta-analysis aimed to synthesise the available evidence on the RRs of PCa for male BRCA1 and BRCA2 carriers, overall and by age groups, and to explore potential explanatory factors for the variation in the reported estimates by study-level covariates. Secondarily, we aimed to estimate RRs of PCa applicable to BRCA1/2 carriers with a PCa family history, and RRs of aggressive PCa.
Methods
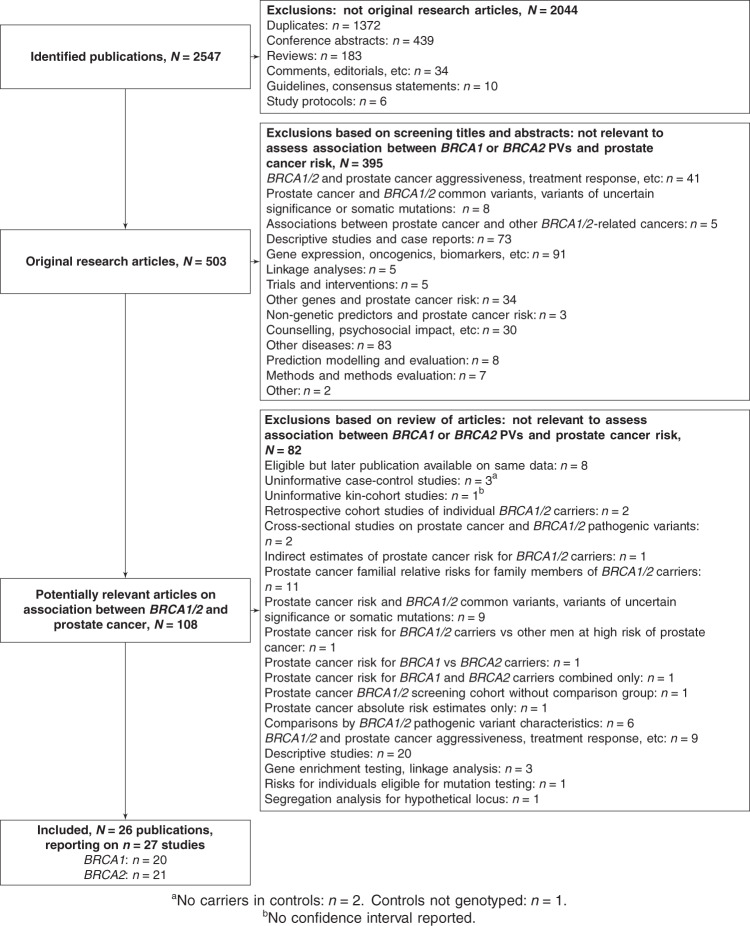
We sought to identify all available estimates of the RRs of PCa for BRCA1/2 carriers, based on valid study designs [34]. On June 19, 2021, the first author (TN) searched PubMed, Embase, MEDLINE and Cochrane Library with no time or language restrictions. The search query is available in the Supplementary Material. The first author removed duplicates, conference abstracts and publications that did not report original data, and screened the remaining publications based on their titles and abstracts to identify those potentially relevant. The first author thereafter screened these articles in their entirety. We contacted the authors of five articles to ask for clarifications.
We included case–control, prospective cohort and family-based retrospective cohort studies [34] that estimated the RR and 95% CI of diagnosed PCa (regardless of histopathology) for carriers of rare PVs in BRCA1 and/or BRCA2 compared to the general population or to non-carriers, or studies where RRs and/or CIs were not reported but the study provided sufficient information to allow calculation of the missing measures. Whenever available, we used estimates adjusted for age and/or ancestry as reported in the publications. PVs were defined as any deleterious variants as determined by the study investigators or in a clinical setting to be clinically actionable based on established clinical guidelines. Studies that only reported on PVs in the two genes together, without providing separate risk estimates for BRCA1 and BRCA2 PVs, were not included. We did not include retrospective cohort studies that recruited PV carriers in clinical settings and assessed association with previous cancer diagnoses, because of the likely ascertainment bias associated with such study designs; [34] nor cross-sectional studies that compared frequencies of prevalent PCa between PV carriers and non-carriers, because prevalence ratios are unbiased RR estimates only under strong assumptions about the population incidence [35]. When data from the same study had been published more than once, we only included the most recent publication.
Statistical analysis
We used the DerSimonian—Laird method for the between-study variance [36] and derived pooled estimates according to both fixed-effects and random-effects models. To assess heterogeneity between RR estimates, we used the DerSimonian—Laird heterogeneity of effects chi-square test and reported the corresponding I2 statistic [37]. We assessed whether the study estimates varied by covariate moderators using nested chi-square tests for categorical moderators or meta-regression for quantitative moderators [38]. To assess potential publication bias, we used funnel plots and tested for funnel plot asymmetry using the rank correlation test [39]. To assess the impact of individual studies on the results, we performed leave-one-out sensitivity analyses by omitting one of the included studies at a time and refitting the models.
For the meta-analysis by age groups, we initially considered all reported estimates by age at diagnosis with no restriction on age cutpoints considered, and also specifically those that used an age cutpoint of 65 years. For the meta-analysis of aggressive PCa, we considered studies that had exclusively or preferentially included participants with aggressive PCa, or studies that reported aggressive PCa-specific RRs, with PCa aggressiveness as defined by the study authors. In addition, when no RR of aggressive PCa had been reported but sufficient data were available within a study (e.g. Gleason score frequencies for PCa cases by PV status), we estimated the RR of Gleason score ≥7 PCa. We explored whether the variability between the estimates could be explained by the following study-level covariates (defined in Supplementary Table S1): study design; the majority ethnic ancestry of the study participants; age-adjustment approach; participant, case or control selection; use of historical or external controls; and the proportion of observed BRCA2 PVs that were located within the wide definition ovarian cancer cluster region (OCCR) [8, 16, 23, 29, 30, 32, 40]. We performed the meta-analyses using R software [41], with the meta [42] and metafor [38] packages.
Results
The literature search identified 27 studies that reported PCa RR estimates for BRCA1 (n = 20) and/or BRCA2 carriers (n = 21; Fig. 1). These included 20 case–control studies from 19 publications [1, 3, 4, 6, 7, 10–15, 17–19, 21, 22, 24–26], two prospective cohort studies [20, 23], and five family-based retrospective cohort studies [2, 5, 8, 9, 16] (Tables 1 and 2). Full details are available in the Supplementary Material.
Fig. 1. Flowchart.
Flowchart detailing the identification of original research articles on the relative risk of prostate cancer for carriers of BRCA1 and BRCA2 pathogenic variants.
Table 1.
Case–control studies.
| Publication | Population, dataset | Period | Study design | Selection | Cases average age | Controls average age | Age-adjustment | Gene | Considered PVs | % PVs located in BRCA2 OCCR | Cases PV carriers/total (%) | Controls PV carriers/total (%) | OR (95% CI)a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Johannesdottir [1] | Iceland | 1983–1992 | Cases vs controls from the same population | Cases: men diagnosed with PCa at age <65 at a single clinic (University Hospital of Iceland). Controls: participants in an unrelated public health study. | Not stated (all <65) | Not stated | None | BRCA2 | c.771_775del | 0% by design | 2/75 (2.67%) | 2/499 (0.40%) | 6.6 (0.81–56.9) |
| Hubert [3] | Israel | Not stated | Cases vs controls from the same population | Cases: unselected men diagnosed with PCa at a single clinic (Sharett Institute, Hadassah Hebrew University Hospital). Controls: recruited from homes for the elderly. | Median: 71 | Median: 72 | None, but cases and controls were of comparable ages | BRCA1 | c.68_69delAG | 2/87 (2.30%) | 2/87 (2.30%) | Not reported | |
| BRCA2 | c.5946delT | 100% by design | 1/87 (1.15%) | 1/87 (1.15%) | Not reported | ||||||||
| Vazina [4] | Israel | 1998 | Cases vs historical controls | Cases: unselected men diagnosed with PCa at three clinics (Rabin, Sheba or the Wolfson Medical Centers). Controls: historical US Ashkenazi controls [50]. | Median: 66 | Not stated (historical controls) | None | BRCA1 | c.68_69delAG | 4/87 (4.60%) | 61/5318 (1.15%) | Not reported | |
| BRCA2 | c.5946delT | 100% by design | 1/86 (1.16%) | 59/5087(1.16%) | Not reported | ||||||||
| Giusti [6] | Israel | 1994–1995 | Cases vs historical controls | Cases: unselected men diagnosed with PCa at 16 clinics. Controls: historical controls from the US Ashkenazi population [50] and an Israeli colorectal cancer case–control study [51]. | Mean: 73.6 | Not stated (historical controls) | None | BRCA1 | c.68_69delAG and c.5266dupC | 16/940 (1.70%) | 11/1344 (0.82%) | Not reported | |
| BRCA2 | c.5946delT | 100% by design | 14/940 (1.49%) | 10/1344 (0.74%) | 2.02 (0.89–4.56) | ||||||||
| Hamel [7]b | Canadian Ashkenazi | 1991–2002 | Cases vs historical controls | Cases: unselected Ashkenazi men diagnosed with PCa at three clinics in Montreal. Controls: historical controls from five studies with Ashkenazi general population or study control groups. | Mean: 67.9 | Not stated (historical controls) | None | BRCA1 | c.68_69delAG and c.5266dupC | 0/146 (0.00%) | 109/9371 (1.16%) | Not reported | |
| BRCA2 | c.5946delT | 100% by design | 2/146 (1.37%) | 119/9514 (1.25%) | Not reported | ||||||||
| Agalliu[10] | USA (predominantly European ancestry) | 1993–1996, 2002–2005 | Cases vs population frequency estimate | Cases: men diagnosed with PCa at age <55 in two case–control studies. No controls; comparison to a previous population BRCA2 frequency estimate for US Caucasians. | Median: 49.5 | -- | None | BRCA2 | c.3847_3848del and c.4398_4402del | 2/2 (100%) | 2/257 (0.78%) | Population frequency: 0.1% | 7.78 (1.80–9.37) |
| Agalliu [11] | US Ashkenazi | 1998–2005 | Cases vs controls from the same population | Cases and controls: self-selected Ashkenazi volunteers who were recruited through advertisements. The participants provided self-reported case/control status. | Mean: 69.4 | Mean: 68.3 | Covariate adjustment for age | BRCA1 | c.68_69delAG and c.5266dupC | 12/978 (1.23%) | 11/1247 (0.88%) | 1.39 (0.60–3.22) | |
| BRCA2 | c.5946delT | 100% by design | 18/969 (1.86%) | 12/1240 (0.97%) | 1.92 (0.91–4.07) | ||||||||
| Gallagher [12] | US Ashkenazi, MSKCC | 1988–2007 | Cases vs controls from the same population | Cases: unselected Ashkenazi men treated with PCa at a single clinic (Memorial Sloan-Kettering Cancer Center, New York). Controls: Ashkenazi healthy volunteers from a prospective study in New York. | Median: 68 | Median: 42 | Covariate adjustment for age | BRCA1 | c.68_69delAG | 6/832 (0.72%) | 4/454 (0.88%) | 0.38 (0.05–2.75) | |
| BRCA2 | c.5946delT | 100% by design | 20/832 (2.40%) | 3/454 (0.66%) | 3.18 (1.52–6.66) | ||||||||
| Fachal [13] | Spain | 2006–2009 | Cases vs controls from the same population | Cases: unselected men treated for PCa at one clinic (Santiago de Compostela). Controls: healthy men aged >44 (selection unclear). | Median: 68 | Median: 60 | Covariate adjustment for age | BRCA1 | c.211 A > G | 1/905 (0.11%) | 3/936 (0.32%) | 0.27 (0.01–2.36) | |
| Kote-Jarai [14] | UK, UKGPCS | Not stated | Cases vs population frequency estimate | Cases: men with PCa recruited nationwide due to being diagnosed with PCa at age <65 years (87% of the study sample), or due to having a family history of PCa (13% of the study sample). No controls; comparison to a previous UK population BRCA2 frequency estimate. | Not stated (87% <65) | -- | None | BRCA2 | Any pathogenic variant | 11/19 (58%) | 19/1832 (1.04%) | Population frequency: 0.16% | 8.6 (5.1–12.6) |
| Leongamornlert [15] | UK, UKGPCS | Not stated | Cases vs population frequency estimate | Cases: men with PCa recruited nationwide due to being diagnosed with PCa at age <65 years (90% of the study sample), or due to having a family history of PCa (10% of the study sample). No controls; comparison to a previous UK population BRCA1 frequency estimate. | Not stated (90% <65) | -- | None | BRCA1 | Any pathogenic variant | 4/886 (0.45%) | Population frequency: 0.12% | 3.75 (1.02–9.60) | |
| Cybulski [17] | Poland | 1999–2012 | Cases vs controls from the same population | Cases: unselected men with PCa from 14 centres. Controls: population controls from four sources (a random clinic record sample, a population-based study, PSA-screen negative men, colonoscopy screening participants). | Mean: 68.8 | Mean: 61.2 | None | BRCA1 | c.181 T > G, c.4035del and c.5266dupC | 14/3750 (0.37%) | 17/3956 (0.43%) | 0.9 (0.4–1.8) | |
| Akbari [18] | Canada (predominantly European ancestry) | 1998–2010 | Cases vs controls from the same population | Cases and controls: unselected men who had a biopsy because of elevated PSA or abnormal DRE at two clinics; cases were those biopsy-positive, controls were those biopsy-negative. | Mean: 65 | Not stated | None, but cases and controls were likely of comparable ages | BRCA2 | Any pathogenic variant | Not specified | 26/1904 (1.37%) | 9/2283 (0.39%) | 3.5 (1.63–7.48) |
| Pritchard [19] | UK and USA (predominantly European ancestry) | Not stated | Cases vs population frequency estimate | Cases: men with metastatic PCa from seven case series. No controls; comparison to carrier frequency in the Exome Aggregation Consortium database. | Not stated | Not stated (external estimate) | None | BRCA1 | Any pathogenic variant | 6/692 (0.87%) | 104/53105 (0.20%) | 3.9 (1.4–8.5) | |
| BRCA2 | Any pathogenic variant | 24/37 (65%) | 37/692 (5.35%) | 153/53105 (0.29%) | 18.6 (13.2–25.3) | ||||||||
| Matejcic [21] | US African Americans (AA) | 1993–2015 | Cases vs controls from the same population | Cases: men with PCa “overselected for high stage and Gleason score” from incident cases from a US prospective cohort study and two US case series of African American participants. Controls: unaffected African American participants in the US prospective cohort study. | Mean: 66.71 | Mean: 71.52 | Covariate adjustment for age and genetic ancestry | BRCA1 | Any pathogenic variant | 3/1447 (0.21%) | 1/995 (0.10%) | 2.84 (0.26–30.59) | |
| BRCA2 | Any pathogenic variant | Not stated | 9/1447 (0.62%) | 3/995 (0.30%) | 1.91 (0.48–7.59) | ||||||||
| Uganda | 2010–2016 | Cases vs controls from the same population | Cases: men with prostate cancer from 13 clinics in Uganda. Controls: patients recruited from non-urologic clinics in Uganda. | Mean: 70.77 | Mean: 65.04 | Covariate adjustment for age and genetic ancestry | BRCA1 | Any pathogenic variant | 2/651 (0.31%) | 1/486 (0.21%) | 1.11 (0.09–13.54) | ||
| BRCA2 | Any pathogenic variant | Not stated | 12/651 (1.84%) | 1/486 (0.21%) | 10.30 (1.28–82.58) | ||||||||
| Momozawa [22] | Japan, BioBank Japan | 2003–2018 | Cases vs controls from the same population | Cases: unselected men with PCa from a nationwide hospital-based biobank. Controls: male non-cancer patients from the same biobank older than 60 and with no personal or family history of cancer in first- or second-degree relatives. | Mean: 71.0 | Mean: 70.4 | None, but cases and controls were of comparable ages | BRCA1 | Any pathogenic variant | 14/7636 (0.18%) | 10/12366 (0.08%) | 2.27 (0.94–5.71) | |
| BRCA2 | Any pathogenic variant | Not specified | 83/7636 (1.09%) | 24/12366 (0.19%) | 5.65 (3.55–9.32) | ||||||||
| Oak [24] | USA (predominantly European ancestry), The Cancer Genome Atlas | 2005–2013 | Cases vs controls from the same population | Cases: men with PCa from a nationwide biobank. Controls: patients with non-prostate cancers from the same biobank. | Not stated | Not stated | Covariate adjustment for age and genetic ancestry | BRCA1 | Any pathogenic variant | 3/409 (0.73%) | Not stated (total: 7711 non-PCa patients) | 2.20 (0.62–7.83) | |
| Wokolorczyk [25] | Poland | 2000–2017 | Cases vs controls from the same population | Cases: men with PCa who had a family history of PCa in first- or second-degree relatives (three or more relatives with PCa, or two affected relatives of whom at least one was diagnosed before age 60). Controls: participants in an unrelated population-based study. | Mean: 61.6 | Mean: 59.4 | None, but cases and controls were of comparable ages | BRCA1 | Any pathogenic variant | 5/390 (1.28%) | 1/308 (0.32%) | 4.0 (0.5–34.3) | |
| BRCA2 | Any pathogenic variant | 0/4 (0%) | 4/390 (1.03%) | 0/308 (0.00%) | -- | ||||||||
| Nguyen-Dumont [26] | Australia | Not stated | Cases vs controls from the same population | Cases: men with aggressive prostate cancer (T4, M1, N1 or Gleason score≥8) from four cohort studies and case series. Controls: male participants in an unrelated trial.c | Median: 65–69 | Not stated (all ≥70) | Covariate adjustment for age | BRCA1 | Any pathogenic variant | 5/833 (0.60%) | 10/5356 (0.19%) | 2.9 (0.66–12.5) | |
| BRCA2 | Any pathogenic variant | 6/21 (29%) | 19/833 (2.28%) | 17/5356 (0.32%) | 3.9 (1.1–13) |
PCa prostate cancer, PV pathogenic variant, OCCR ovarian cancer cluster region, OR odds ratio, CI confidence interval.
aWhen available, the table includes adjusted odds ratio estimates (as indicated in the “age-adjustment” field). Otherwise, the reported unadjusted odds ratio estimates by each study are included. For studies that did not report odds ratios, unadjusted odds ratio estimates calculated from the frequencies of case and control PV carriers were used in the meta-analysis (not shown in this descriptive table but included in the forest plots).
bReported on both BRCA1 and BRCA2, but is not included in the BRCA1 meta-analysis due to observing no BRCA1 PVs in the cases which hence did not enable estimation of a 95% CI for the RR.
cThe main analysis in this study compared cases with aggressive prostate cancer to a combined comparison group comprising cases with non-aggressive prostate cancer and unaffected men. The meta-analysis includes the supplementary analysis of cases with aggressive prostate cancer versus unaffected men.
Table 2.
Cohort studies.
| Publication | Population, dataset | Period | Study design | Selection | Average age | Age-adjustment | Gene | Considered PVs | % PVs located in BRCA2 OCCR | N | RR (95% CI)a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BCLC [2] | Europe and North America (predominantly European ancestry), BCLC | Not stated | Kin-cohort | Families with a history of breast and/or ovarian cancer and at least one known BRCA2 carrier, recruited through genetics clinics. | Not stated | Comparison to age-specific population incidence | BRCA2 | Any pathogenic variant | Not specified | 29 PCa in male BRCA2 carriers from 173 breast-ovarian cancer families | 4.65 (3.48–6.22) |
| Thompson [5] | Europe and North America (predominantly European ancestry), BCLC | Until 1999 | Kin-cohort | Families with a history of breast and/or ovarian cancer and at least one known BRCA1 carrier, recruited through genetics clinics. | Not stated | Comparison to age-specific population incidence | BRCA1 | Any pathogenic variant | 11 PCa in male BRCA1 carriers and 7 PCa in non-carriers from 699 families | 1.07 (0.75–1.54) | |
| van Asperen [8] | The Netherlands, GEO-HEBON | 1998–2003 | Kin-cohort | Relatives of breast or ovarian cancer cases who had undergone breast and ovarian cancer counselling in 8 clinics in the Netherlands and who tested positive for BRCA2 PVs. | Not stated | Comparison to age-specific population incidence | BRCA2 | Any pathogenic variant | 92/139 (66%) of family PVs | 24 PCa in 803 men from 139 BRCA2 families | 2.5 (1.6–3.8) |
| Risch [9] | Canada (predominantly European ancestry) | 1995–1999 | Kin-cohort | Probands with ovarian cancer who were identified through a cancer register and who provided cancer family history information. | Not stated | Comparison to age-specific population incidence | BRCA1 | Any pathogenic variant | 4 PCa in 75 BRCA1 families and 89 PCa in 1042 non-carrier families | 0.65 (0.051–8.3) | |
| BRCA2 | Any pathogenic variant | 27/54 (50%) of family PVs | 9 PCa in 54 BRCA2 families and 89 PCa in 1042 non-carrier families | 2.7 (1.1–7.1) | |||||||
| Moran [16] | UK | 1996 and after | Family-based retrospective cohort | Families seeking genetic counselling in two clinics in England from which at least one individual tested positive for BRCA1/2 PVs. | Not stated | Comparison to age-specific population incidence | BRCA1 | Any pathogenic variant | 6.1 standardised PCa observations in male BRCA1 carriers from 268 BRCA1 families | 1.0 (0.4–2.3) | |
| BRCA2 | Any pathogenic variant | 90/222 (41%) of family PVsb | 31.7 standardised PCa observations in male BRCA2 carriers from 222 BRCA2 families | 6.3 (4.3–9.0) | |||||||
| Page [20] | International (predominantly European ancestry), IMPACT | 2005–2015 | Prospective screening cohort | BRCA1/2-positive and BRCA1/2-negative men aged 40–69 from families with BRCA1/2 PV, recruited through 65 centres in 20 countries. | Not stated (median enrolment age across all participants: 54) | Covariate adjustment for age, ethnicity and country | BRCA1 | Any pathogenic variant | 19 PCa in 919 BRCA1 carriers, 14 PCa in 709 non-carriers | 1.36 (0.75–2.45) | |
| BRCA2 | Any pathogenic variant | 42%b | 57 PCa in 902 BRCA2 carriers, 20 PCa in 497 non-carriers | 1.95 (1.06–3.56) | |||||||
| Nyberg [23] | UK and Ireland, EMBRACE | 1999–2016 | Prospective cohort | Unaffected men with BRCA1/2 PVs recruited nationwide through genetics centres and followed prospectively for PCa development. | Median: 54.0 BRCA1; 51.4 BRCA2 | Comparison to age-specific population incidence | BRCA1 | Any pathogenic variant | 16 PCa in 376 BRCA1 carriers | 2.35 (1.43–3.88) | |
| BRCA2 | Any pathogenic variant | 178/445 (40%) | 26 PCa in 447 BRCA2 carriers | 4.45 (2.99–6.61) |
PCa prostate cancer, PV pathogenic variant, OCCR ovarian cancer cluster region, RR relative risk, CI confidence interval.
aIn all studies except Page et al. [20], the RR represents the estimated standardised incidence ratio, comparing pathogenic variant carriers to age-specific population cancer incidences. Page et al. [20] adjusted for age, ethnicity and country.
bProvided by the study authors on request.
The reported RR estimates showed a high degree of variability, particularly those for BRCA2 carriers (BRCA1: I2 = 30%, BRCA2: I2 = 83%; Figs. 2 and 3). The funnel plots indicated both high and low RR estimates as outliers and that smaller BRCA2 studies generally reported lower RR estimates than larger studies. However, there was no statistically significant funnel plot asymmetry (Supplementary Figs. S1 and S2).
Fig. 2. Forest plots of overall BRCA1 RR estimates.
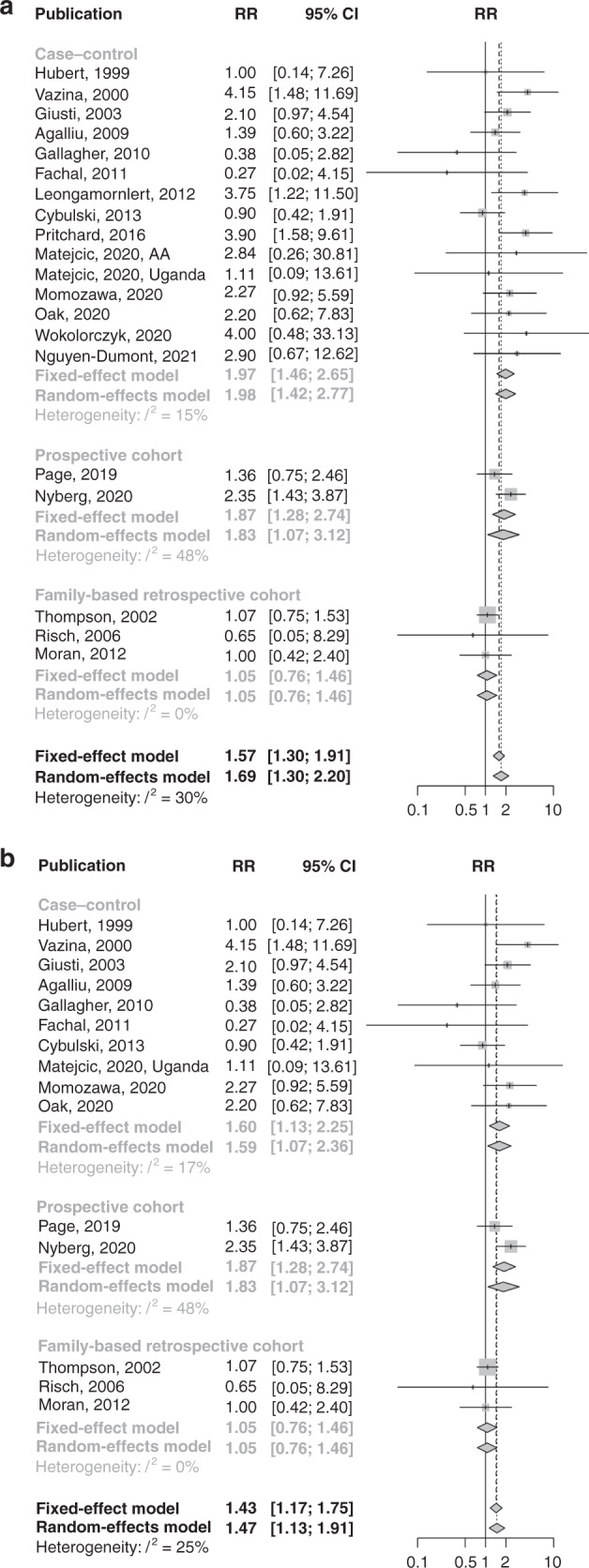
a All initially considered studies; b after restriction to studies unselected for age at diagnosis, family history or aggressive disease.
Fig. 3. Forest plots of overall BRCA2 RR estimates.
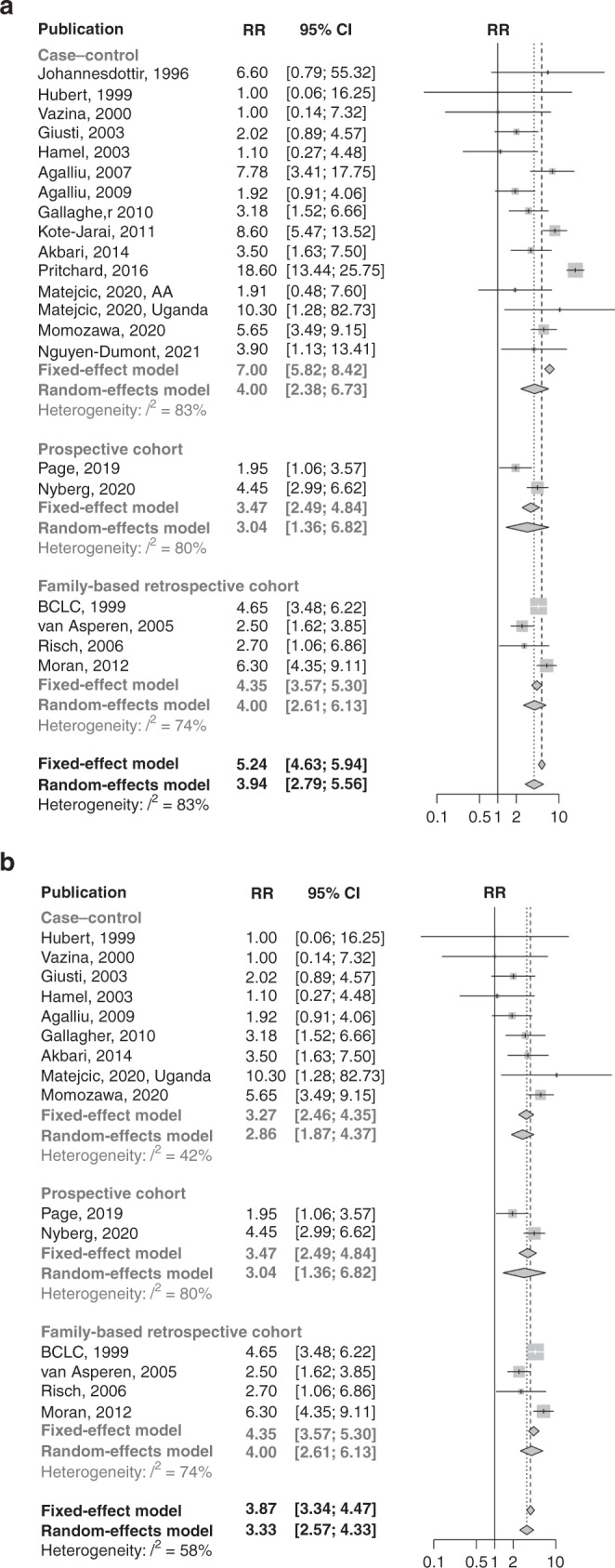
a All initially considered studies; b after restriction to studies unselected for age at diagnosis, family history or aggressive disease.
The RR estimates from studies that selected participants for PCa diagnosis at a young age, PCa family history or aggressive PCa were higher than estimates from studies in unselected participants (BRCA1: test for subgroup differences, P = 0.056, BRCA2: test for subgroup differences, P < 0.001; Supplementary Table S2). We restricted the main meta-analysis to studies unselected for age at PCa diagnosis, PCa family history or aggressive PCa, but separately analysed these subgroups. Table 3 summarises the pooled RR estimates from the further restrictions, subgroup analyses and adjustments made in the meta-analysis.
Table 3.
Heterogeneity and pooled RR estimates by study subgroups.
| Gene, age group | Subgroup | Selection | No. of studies | Fixed-effect pooled RR (95% CI) | Random-effect pooled RR (95% CI) | I2 |
|---|---|---|---|---|---|---|
| BRCA1, overall | All | All estimates | 20 | 1.57 (1.30–1.91) | 1.69 (1.30–2.20) | 30% |
| Studies unselected for age, aggressive prostate cancer, or family history of prostate cancer | All estimates | 15 | 1.43 (1.17–1.75) | 1.47 (1.13–1.91) | 25% | |
| Studies unselected for age, aggressive prostate cancer, or family history of prostate cancer; and, that did not use historical controls | All estimates | 13 | 1.32 (1.07–1.64) | 1.33 (1.05–1.69) | 8% | |
| All estimates: EMBRACE prospective RR estimate adjusted for potential screening effectsa | 13 | 1.18 (0.95–1.46) | 1.18 (0.95–1.46) | 0% | ||
| Ashkenazi ancestry | 3 | 1.12 (0.55–2.31) | 1.12 (0.55–2.31) | 0% | ||
| Non-Ashkenazi European ancestry | 8 | 1.30 (1.03–1.64) | 1.30 (0.95–1.79) | 30% | ||
| Non-Ashkenazi European ancestry: EMBRACE prospective RR estimate adjusted for potential screening effectsa | 8 | 1.13 (0.89–1.44) | 1.13 (0.89–1.44) | 0% | ||
| African ancestry | 1 | 1.11 (0.09–13.61) | 1.11 (0.09–13.61) | -- | ||
| Asian ancestry | 1 | 2.27 (0.92–5.59) | 2.27 (0.92–5.59) | -- | ||
| BRCA2, overall | All | All estimates | 21 | 5.24 (4.63–5.94) | 3.94 (2.79–5.56) | 83% |
| Studies unselected for age, aggressive prostate cancer, or family history of prostate cancer | All estimates | 15 | 3.87 (3.34–4.47) | 3.33 (2.57–4.33) | 58% | |
| Studies unselected for age, aggressive prostate cancer, or family history of prostate cancer; by ethnicity | Ashkenazi ancestry: all estimates | 6 | 2.08 (1.38–3.12) | 2.08 (1.38–3.12) | 0% | |
| Non-Ashkenazi European ancestry: all estimates | 7 | 4.07 (3.45–4.80) | 3.69 (2.71–5.04) | 66% | ||
| Non-Ashkenazi European ancestry: excluding two outliersb | 5 | 3.89 (3.20–4.73) | 3.71 (2.82–4.89) | 39% | ||
| Non-Ashkenazi European ancestry: excluding three outliersc | 4 | 4.35 (3.50–5.41) | 4.35 (3.50–5.41) | 0% | ||
| African ancestry: all estimates | 1 | 10.30 (1.28–82.73) | 10.30 (1.28–82.73) | -- | ||
| Asian ancestry: all estimates | 1 | 5.65 (3.49–9.15) | 5.65 (3.49–9.15) | -- | ||
| Studies unselected for age, aggressive prostate cancer, or family history of prostate cancer; by the proportion of PVs located in the OCCR | ≥50% OCCR PVs: all estimates | 8 | 2.30 (1.74–3.06) | 2.30 (1.74–3.06) | 0% | |
| ≥50% OCCR PVs: using separate OCCR estimates when available | 9 | 2.15 (1.61–2.88) | 2.15 (1.61–2.88) | 0% | ||
| OCCR PVsd | 8 | 2.10 (1.55–2.86) | 2.10 (1.55–2.86) | 0% | ||
| <50% OCCR PVs: all estimates | 4 | 4.74 (3.81–5.91) | 4.38 (2.83–6.77) | 73% | ||
| <50% OCCR PVs: excluding one outliere | 3 | 5.43 (4.29–6.87) | 5.43 (4.29–6.87) | 0% | ||
| <50% OCCR PVs: using separate non-OCCR estimates when available | 4 | 5.65 (4.49–7.12) | 5.65 (4.49–7.12) | 0% | ||
| Non-OCCR PVsd | 2 | 5.06 (3.48–7.36) | 4.93 (3.10–7.82) | 28% | ||
| Proportion of PVs located in OCCR not determinable: all estimates | 3 | 4.55 (3.48–5.95) | 4.55 (3.48–5.95) | 0% | ||
| BRCA1, age <65 years | All | All estimates | 4 | 2.21 (1.47–3.30) | 2.19 (1.21–3.98) | 47% |
| Excluding one outlierf | 3 | 2.52 (1.64–3.87) | 2.59 (1.58–4.24) | 19% | ||
| EMBRACE prospective RR estimate adjusted for potential screening effectsa | 4 | 1.79 (1.17–2.72) | 1.78 (1.12–2.85) | 14% | ||
| Studies that did not use historical controls or external population estimates | 3 | 2.04 (1.32–3.14) | 1.92 (0.94–3.92) | 57% | ||
| Excluding one outlierf, EMBRACE prospective RR estimate adjusted for potential screening effectsa, studies that did not use historical controls or external population estimates | 2 | 1.78 (1.09–2.91) | 1.78 (1.09–2.91) | 0% | ||
| BRCA1, age ≥65 years | All | All estimates | 3 | 1.18 (0.83–1.70) | 1.43 (0.71–2.87) | 65% |
| Excluding one outlierf | 2 | 1.09 (0.75–1.59) | 1.21 (0.55–2.62) | 73% | ||
| EMBRACE RR estimate adjusted for potential screening effectsa | 3 | 1.01 (0.70–1.45) | 1.10 (0.65–1.86) | 39% | ||
| Studies that did not use historical controls or external population estimates | 3 | 1.18 (0.83–1.70) | 1.43 (0.71–2.87) | 65% | ||
| Excluding one outlierf, EMBRACE prospective RR estimate adjusted for potential screening effectsa, studies that did not use historical controls or external population estimates | 2 | 0.91 (0.62–1.33) | 0.91 (0.62–1.33) | 0% | ||
| BRCA2, age <65 years | All | All estimates | 5 | 6.37 (4.81–8.43) | 5.28 (3.10–9.00) | 63% |
| By ethnicity | Ashkenazi ancestry | 1 | 1.58 (0.57–4.38) | 1.58 (0.57–4.38) | -- | |
| Non-Ashkenazi European ancestry | 4 | 7.14 (5.33–9.56) | 7.14 (5.33–9.56) | 0% | ||
| BRCA2, age ≥65 years | All | All estimates | 3 | 3.74 (2.82–4.96) | 3.74 (2.82–4.96) | 0% |
| By ethnicity | Ashkenazi ancestry | 1 | 2.63 (0.85–8.16) | 2.63 (0.85–8.16) | -- | |
| Non-Ashkenazi European ancestry | 2 | 3.83 (2.86–5.12) | 3.84 (2.84–5.18) | 6% |
RR relative risk, CI confidence interval, PV pathogenic variant, OCCR ovarian cancer cluster region.
aUsing a 6 month landmark and compared to population incidences adjusted by a factor of 1.6 [23].
bExcluding the studies by Page and coworkers [20] and Moran and coworkers [16].
cExcluding the studies by Page and coworkers [20], Moran and coworkers [16] and van Asperen and coworkers [8].
dRestricted to studies that reported separate RR estimates for OCCR and non-OCCR PVs, or where all reported PVs were located in the OCCR.
eExcluding the study by Page and coworkers [20].
fExcluding the study by Agalliu and coworkers [11].
BRCA1
Studies on BRCA1 carriers that relied on historical controls reported higher RR estimates than other studies (test for subgroup differences, P = 0.044; Supplementary Table S3).
BRCA1: studies without historical controls
Restricted to studies of BRCA1 carriers that did not use historical controls, the heterogeneity between estimates was low (I2 = 8%; Supplementary Figs. S3 and S4; Supplementary Table S4). A leave-one-out analysis identified the prospective EMBRACE study [23] as a high outlier (P = 0.013; Supplementary Table S5). The EMBRACE study reported a screening-bias-corrected estimate; [23] Table 3 shows the pooled RR when this estimate was used instead (Supplementary Figs. S3 and S4 and Table 3).
BRCA2
BRCA2 studies in Ashkenazi Jewish men reported lower RR estimates than studies in other populations (test for subgroup differences, P = 0.011). The RR estimates were lower in studies where ≥50% of the reported PVs were located in the OCCR (test for subgroup differences, P = 0.002; Supplementary Table S3).
BRCA2: prostate cancer risk by ethnicity
Table 3 shows pooled RR estimates based on studies in Ashkenazi Jewish populations (Supplementary Figs. S5 and S6), where the heterogeneity between estimates was low (I2 = 0%; Supplementary Tables S6 and S7).
For studies of BRCA2 carriers in non-Ashkenazi European ancestry populations (Supplementary Figs. S5 and S6), the heterogeneity between estimates was high (I2 = 66%). A leave-one-out analysis identified three outliers (Supplementary Table S7): a UK family-based retrospective cohort study (P = 0.010) [16], the IMPACT screening trial (P = 0.013) [20], and a Dutch kin-cohort study (P = 0.017) [8]. Table 3 shows pooled RR estimates after excluding these studies. Notably, the main estimate from the EMBRACE study [23] was not an outlier among the estimates for BRCA2 carriers (P = 0.6), and if instead a screening-effect-adjusted estimate was used, the RR estimate was an outlier and significantly lower than the other estimates (P = 0.025).
BRCA2: prostate cancer risk by pathogenic variant location
Table 3 shows pooled RR estimates in studies split by OCCR proportion, before and after exclusion of the IMPACT study [20] which was a low outlier among studies with <50% OCCR PVs (P = 0.002; Supplementary Figs. S7 and S8; Supplementary Tables S8 and S9), and after restriction to the available OCCR- or non-OCCR-specific estimates.
Furthermore, a meta-regression model showed a trend towards linearly decreasing log-RR estimates with the increasing proportion of OCCR PVs in a study (P < 0.001). The regression model had low residual heterogeneity (I2 = 5%), and predicted RRs of 2.31 (95% CI 2.20–2.42) from studies with 100% OCCR PVs and 6.50 (95% CI 6.14–6.87) from studies with 0% OCCR PVs (Supplementary Fig. S9).
Prostate cancer risk by age group
Supplementary Figs. S10 and S11 show all reported RR estimates by the age cutpoints used to define age groups. Restricted to RR estimates by age groups younger or older than 65 years, the RRs were heterogeneous for both BRCA1 (age <65 years I2 = 47%, age ≥65 years I2 = 65%; Supplementary Figs. S12 and S13) and BRCA2 carriers (age <65 years I2 = 63%, age ≥65 years I2 = 0%; Supplementary Figs. S14 and S15).
BRCA1
The age-specific estimates from a large international kin-cohort study [5] were somewhat lower at age≥65 years than estimates from other studies (age <65 years P = 0.4, age ≥65 years P = 0.019; Supplementary Tables S10 and S11). However, we could not identify any likely methodological explanation for this outlying estimate and therefore retained the study. The age-specific RR estimates from one case–control study in Ashkenazi Jewish men [11] were somewhat lower at younger ages and somewhat higher at older ages than estimates from other studies (age <65 years P = 0.073, age ≥65 years P = 0.15; Supplementary Table S11) and the RR estimates from the EMBRACE study [23] were somewhat higher than estimates from other studies at both younger and older ages (age <65 years P = 0.14, age ≥65 years P = 0.11; Supplementary Table S11), but these differences were not significant. Table 3 shows the results when excluding the study in Ashkenazi men, including screening-effect-adjusted estimates from EMBRACE, or restricting to studies that did not rely on external population frequency estimates.
BRCA2
The RR estimate for younger BRCA2 carriers from one study of Ashkenazi Jewish men [11] was a low outlier (age <65 years P = 0.005, age ≥65 years P = 0.5; Supplementary Fig. S14; Supplementary Tables S12 and S13). Table 3 shows pooled RR estimates by age group before and after excluding this study.
Prostate cancer risk by family history of prostate cancer
The pooled RR estimate for BRCA1 carriers with PCa family history was 2.79 (95% CI 1.33–5.88; I2 = 0%). Only one study reported a RR specifically for BRCA2 carriers with a family history, of 7.31 (95% CI 3.40–15.7).
Risk of aggressive prostate cancer
The pooled random-effects RRs of aggressive PCa (any definition) were 1.98 (1.35–2.90; I2 = 0%) for BRCA1 carriers and 6.08 (3.44–10.8; I2 = 82%) for BRCA2 carriers (Supplementary Fig. S16). For BRCA2 carriers, the RR estimates differed significantly by the definition of aggressive PCa (P < 0.001), with higher RR estimates reported for metastatic or Gleason score≥8 PCa than Gleason score≥7 PCa. For BRCA1, there was no significant heterogeneity by the definition of aggressive PCa (P = 0.3). Restricted to estimates of the RR of Gleason score ≥7 PCa, the pooled random-effects RRs were 1.59 (95% CI 1.02–2.49; I2 = 0%) for BRCA1 carriers and 4.94 (95% CI 3.51–6.96; I2 = 0%) for BRCA2 carriers.
Discussion
A wide range of PCa RR estimates have been reported for BRCA1 and BRCA2 carriers. The results of this meta-analysis suggest that the heterogeneity may in part be explained by selection for age, family history or aggressive disease, and study-level differences in the age and ethnic ancestry composition of the study participants, the reliance of some studies on historical controls, and the proportion of the studied BRCA2 carriers who have PVs within the OCCR.
The pooled RR estimates indicate that male BRCA2 carriers are at higher than population risk of PCa at all ages, whereas BRCA1 carriers may be at somewhat increased risk with the increased risk restricted to younger ages. Based on the most restrictive inclusion criteria considered, the overall random-effects RR estimates were 2.08 (95% CI 1.38–3.12) for Ashkenazi Jewish BRCA2 carriers and 4.35 (95% CI 3.50–5.41) for non-Ashkenazi European ancestry BRCA2 carriers. This heterogeneity in BRCA2 PCa risks by ethnicity indicates the need for further research to explore ethnicity-specific risk estimates for male BRCA2 carriers. The reported RRs for African and Asian ancestry BRCA2 carriers were similar to those for non-Ashkenazi European ancestry men, but this was based on a small number of studies and should be interpreted with caution. However, even if the RRs are similar, this would translate to different absolute risks for BRCA2 carriers by ethnicity, because the baseline population risks differ between ethnic groups [43, 44]. For BRCA1 carriers, there was no significant difference in reported RRs by ethnicity and the overall RR was estimated to be 1.18 (95% CI 0.95–1.46). For both BRCA1 and BRCA2 carriers, the reported RRs were higher at younger ages. Based on the most restrictive inclusion criteria, the estimated age-specific RRs applicable to non-Ashkenazi European ancestry men were 7.14 (95% CI 5.33–9.56) at ages <65 and 3.84 (95% CI 2.84–5.18) at ages ≥65 years for BRCA2 carriers and 1.78 (95% CI 1.09–2.91) at ages <65 and 0.91 (95% CI 0.62–1.33) at ages ≥65 years for BRCA1 carriers.
The reported overall RR estimates for BRCA2 carriers were lower from studies where a majority of the BRCA2 PVs were located in the OCCR (pooled RR = 2.30, 95% CI 1.74–3.06). The meta-regression showed a trend towards decreasing RRs with increasing study-level proportions of PVs located in the BRCA2 OCCR, consistent with the observations that carriers of BRCA2 PVs within the OCCR have a lower risk of PCa than other BRCA2 PV carriers [8, 23, 29–32]. The Ashkenazi BRCA2 studies reported exclusively on the Ashkenazi founder PV c.5946delT that is located in the OCCR, and the RRs from these studies (pooled RR = 2.08, 95% CI 1.38–3.12) were comparable with the RRs reported from studies in non-Ashkenazi European ancestry populations where the majority of participants had PVs located in the OCCR (pooled RR = 2.53, 95% CI 1.71–3.75). Hence, as has previously been suggested [11], it is possible that the lower PCa risks observed for Ashkenazi BRCA2 carriers [3, 4, 6, 7, 11, 12, 30, 33, 45] is explained by risk variation by the location of PVs within the BRCA2 gene.
By contrast, there was no significant variation in the reported overall BRCA1 RR estimates by the ethnic ancestry of the study participants. The studies in Ashkenazi Jewish men reported exclusively on the two Ashkenazi founder PVs c.68_69delAG and/or c.5266dupC. A lack of variation in the PCa risk by specific founder PVs is consistent with previous findings of a lack of significant variation by the location of PVs within BRCA1 [31]. Moreover, the reported RR estimates were higher from two studies that compared Israeli PCa patients to controls from previous studies of US Ashkenazi individuals [4, 6]. The use of cases and controls from different settings and time periods make the studies susceptible to bias from population stratification, and place- and time-specific differences in e.g. opportunistic screening rates. Only one study in Ashkenazi Jewish BRCA1 carriers had reported age-specific RR estimates [11], and these were somewhat lower for younger carriers and somewhat higher for older carriers compared to estimates from studies in non-Ashkenazi European ancestry populations. This study was however limited by the use of a self-selected sample and ascertainment bias may be likely. Hence, the finding may not be inconsistent with the finding of no significant differences by ethnicity in the meta-analysis of overall RR estimates for BRCA1 carriers.
The RR estimates from the EMBRACE study were identified as high outliers among the BRCA1 but not the BRCA2 estimates. The EMBRACE study was limited by potential confounding by screening effects [23]. BRCA2 PVs are associated with a more aggressive PCa phenotype than BRCA1 PVs [11, 12, 20, 23, 46], and the results may hence reflect that BRCA2 carriers are more likely than BRCA1 carriers to have clinically significant PCa which is diagnosed regardless of screening. When we instead included BRCA1 RR estimates from a sensitivity analysis that adjusted for potential screening effects, these RR estimates were consistent with those reported in other studies. The IMPACT screening trial reported an RR estimate for BRCA2 carriers that was significantly lower than estimates from other studies. Enhanced screening makes early diagnoses of indolent tumours likely in the trial arms. Hence, bias towards the null may be expected compared to the risk for the average BRCA1/2 carrier in the population, if overdiagnosis rates are similar in the carriers and non-carriers.
One case–control study included only cases with a family history of PCa and an unselected control group, and did not adjust for this family history-based ascertainment [25]. This is likely to lead to higher RR estimates compared to RRs based on case–control studies of unselected cases, because of likely enrichment of PCa PVs in subjects from PCa families. Although such designs may provide valid tests of association, they can lead to biased RR estimates [47]. Two family-based retrospective cohort studies in relatives of breast or ovarian cancer cases reported estimates that were significantly higher [16] or lower [8] than estimates from other studies. Assuming that no other shared genetic and familial risk factors besides BRCA1/2 PVs exist between PCa, breast and ovarian cancer, such ascertainment should in principle not introduce ascertainment bias. However, given the excess breast cancer risk in relatives of PCa cases [48] and the established associations between BRCA1/2 PVs and PCa, it cannot be ruled out that testing for BRCA1/2 PVs in individuals with breast cancer may in some instances have been influenced by the presence of PCa cases in the family. If so, failing to adjust for the PCa events that determined the ascertainment would bias the resulting PCa RRs away from the null. One study included biopsy-negative individuals as controls [18], one study used controls who had other cancers [24] and two studies used controls identified in healthcare settings [21, 22]. Such control selection might bias the corresponding RR estimates if the PV frequency among the controls differs systematically from the population. However, the meta-analysis did not suggest significant differences between these estimates and estimates from other studies.
The systematic review and meta-analysis has a number of strengths. Since the most recent previous systematic review and meta-analysis [27], seven studies [20–26] have been published, including two prospective studies [20, 23] and studies in African [21] and Asian [22] ancestry populations. By incorporating these studies, we update the available evidence. Furthermore, our meta-analysis expanded on previous meta-analyses by exploring variability in risks, which identified several possible explanatory factors for the heterogeneity between studies. We have provided estimates that synthesise all available data on the RRs of PCa for male BRCA1 and BRCA2 carriers.
The systematic review and meta-analysis also has limitations. Publication bias and selective reporting of significant outcomes within studies may bias meta-analysis estimates [49]. Because only a subset of the studies reported RRs by age, family history and PV location, and RRs of aggressive PCa, such bias cannot be ruled out. Funnel plots for the age-specific estimates showed no clear asymmetry, indicating that selective reporting is less likely. Another limitation is the potential overlap between the participants of different studies. As noted above some studies used the same historical controls, and the BRCA1/2 carrier participants partially overlapped between EMBRACE [23] and IMPACT [20]. This invalidates the assumption that the RRs are estimated based on independent samples, which may bias the pooled RR estimates and underestimate the width of the associated CIs. The meta-analysis of BRCA2 OCCR PVs was limited by a lack of separate estimates of the risks associated with OCCR and non-OCCR PVs. The analysis predominantly relied on study-level data on the proportion of reported PVs that were located within the OCCR. For some studies, this proportion was based on the family-level rather than the individual-level PV distribution. However, despite these limitations, the resulting RR estimate (pooled RR = 2.30, 95% CI 1.74–3.06) was consistent with the eight separate estimates reported for OCCR PVs (pooled RR = 2.10, 95% 1.55–2.86). Risk variation by the OCCR was however not present when split by age group. This might be due to the use of the study-level proportion of OCCR PV carriers, which may be a poor proxy for the proportion of OCCR PV carriers within age-stratified subgroups of the study participants. These study-level subgroup analyses are hypothesis-generating and larger studies are needed to estimate the age-specific risk associated with specific subgroups of BRCA1/2 carriers based on individual-level data, e.g. by ethnic ancestry and PV location. Finally, the literature search and review was performed by a single reviewer rather than several reviewers, and although the review assessed sources of study-specific bias, it did not use a standardised rating scale.
Conclusion
This meta-analysis has identified several potential effect modifiers that may guide future studies, and has provided pooled RR estimates, overall and by age group, of the risk of PCa for male BRCA1 and BRCA2 carriers that incorporate the current accumulated evidence. These risk estimates will be informative for the genetic counselling of male BRCA1 and BRCA2 carriers.
Supplementary information
Acknowledgements
We thank the IMPACT study collaborators and Steering Committee for providing additional data on their study, and Gareth Evans for helpful clarifications regarding one of the publications.
Author contributions
All authors conceived and designed the study. TN performed the literature review and the statistical analysis, and wrote the first draft of the manuscript. MT and ACA revised the manuscript and supervised the work. ACA obtained funding.
Funding
This work was funded by Cancer Research UK grants C12292/A20861, C12292/A22820 and PPRPGM-Nov20/100002; and supported by the National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. The sponsors played no direct role in the study.
Data availability
The literature review and meta-analysis datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable; this was a systematic review and meta-analysis of previously published research. It did not include any original data on research participants.
Consent to publish
None.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01675-5.
References
- 1.Johannesdottir G, Gudmundsson J, Bergthorsson JT, Arason A, Agnarsson BA, Eiriksdottir G, et al. High prevalence of the 999del5 mutation in Icelandic breast and ovarian cancer patients. Cancer Res. 1996;56:3663–5. [PubMed] [Google Scholar]
- 2.Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91:1310–6. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 3.Hubert A, Peretz T, Manor O, Kaduri L, Wienberg N, Lerer I, et al. The Jewish Ashkenazi founder mutations in the BRCA1/BRCA2 genes are not found at an increased frequency in Ashkenazi patients with prostate cancer. Am J Hum Genet. 1999;65:921–4. doi: 10.1086/302525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vazina A, Baniel J, Yaacobi Y, Shtriker A, Engelstein D, Leibovitz I, et al. The rate of the founder Jewish mutations in BRCA1 and BRCA2 in prostate cancer patients in Israel. Br J Cancer. 2000;83:463–6. doi: 10.1054/bjoc.2000.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson D, Easton DF, Breast Cancer Linkage Consortium Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94:1358–65. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 6.Giusti RM, Rutter JL, Duray PH, Freedman LS, Konichezky M, Fisher-Fischbein J, et al. A twofold increase in BRCA mutation related prostate cancer among Ashkenazi Israelis is not associated with distinctive histopathology. J Med Genet. 2003;40:787–92. doi: 10.1136/jmg.40.10.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamel N, Kotar K, Foulkes WD. Founder mutations in BRCA1/2 are not frequent in Canadian Ashkenazi Jewish men with prostate cancer. BMC Med Genet. 2003;4:7. doi: 10.1186/1471-2350-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Asperen CJ, Brohet RM, Meijers-Heijboer EJ, Hoogerbrugge N, Verhoef S, Vasen HFA, et al. Cancer risks in BRCA2 families: estimates for sites other than breast and ovary. J Med Genet. 2005;42:711–9. doi: 10.1136/jmg.2004.028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Risch HA, McLaughlin JR, Cole DEC, Rosen B, Bradley L, Fan I, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a Kin–cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98:1694–706. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 10.Agalliu I, Karlins E, Kwon EM, Iwasaki LM, Diamond A, Ostrander EA, et al. Rare germline mutations in the BRCA2 gene are associated with early-onset prostate cancer. Br J Cancer. 2007;97:826–31. doi: 10.1038/sj.bjc.6603929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agalliu I, Gern R, Leanza S, Burk RD. Associations of high-grade prostate cancer with BRCA1 and BRCA2 founder mutations. Clin Cancer Res. 2009;15:1112–20. doi: 10.1158/1078-0432.CCR-08-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallagher DJ, Gaudet MM, Pal P, Kirchhoff T, Balistreri L, Vora K, et al. Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin Cancer Res. 2010;16:2115–21. doi: 10.1158/1078-0432.CCR-09-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fachal L, Gómez-Caamaño A, Celeiro-Muñoz C, Peleteiro P, Blanco A, Carballo A, et al. BRCA1 mutations do not increase prostate cancer risk: Results from a meta-analysis including new data. Prostate. 2011;71:1768–79. doi: 10.1002/pros.21394. [DOI] [PubMed] [Google Scholar]
- 14.Kote-Jarai Z, Leongamornlert D, Saunders E, Tymrakiewicz M, Castro E, Mahmud N, et al. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer. 2011;105:1230–4. doi: 10.1038/bjc.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leongamornlert D, Mahmud N, Tymrakiewicz M, Saunders E, Dadaev T, Castro E, et al. Germline BRCA1 mutations increase prostate cancer risk. Br J Cancer. 2012;106:1697–701. doi: 10.1038/bjc.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moran A, O’Hara C, Khan S, Shack L, Woodward E, Maher ER, et al. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam Cancer. 2012;11:235–42. doi: 10.1007/s10689-011-9506-2. [DOI] [PubMed] [Google Scholar]
- 17.Cybulski C, Wokołorczyk D, Kluźniak W, Jakubowska A, Górski B, Gronwald J, et al. An inherited NBN mutation is associated with poor prognosis prostate cancer. Br J Cancer. 2013;108:461–468. doi: 10.1038/bjc.2012.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akbari MR, Wallis CJD, Toi A, Trachtenberg J, Sun P, Narod SA, et al. The impact of a BRCA2 mutation on mortality from screen-detected prostate cancer. Br J Cancer. 2014;111:1238–40. doi: 10.1038/bjc.2014.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375:443–53. doi: 10.1056/NEJMoa1603144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page EC, Bancroft EK, Brook MN, Assel M, Al Battat MH, Thomas S, et al. Interim results from the IMPACT study: evidence for prostate-specific antigen screening in BRCA2 mutation carriers. Eur Urol. 2019;76:831–42. doi: 10.1016/j.eururo.2019.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matejcic M, Patel Y, Lilyquist J, Hu C, Lee KY, Gnanaolivu RD, et al. Pathogenic variants in cancer predisposition genes and prostate cancer risk in men of African ancestry. JCO Precis Oncol. 2020;4:32–43. doi: 10.1200/PO.19.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Momozawa Y, Iwasaki Y, Hirata M, Liu X, Kamatani Y, Takahashi A, et al. Germline pathogenic variants in 7636 Japanese patients with prostate cancer and 12 366 controls. J Natl Cancer Inst. 2020;112:369–76. [DOI] [PMC free article] [PubMed]
- 23.Nyberg T, Frost D, Barrowdale D, Evans DG, Bancroft E, Adlard J, et al. Prostate cancer risks for male BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Eur Urol. 2020;77:24–35. doi: 10.1016/j.eururo.2019.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oak N, Cherniack AD, Mashl RJ, Hirsch FR, Ding L, Beroukhim R, et al. Ancestry-specific predisposing germline variants in cancer. Genome Med. 2020;12:51. doi: 10.1186/s13073-020-00744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wokołorczyk D, Kluźniak W, Huzarski T, Gronwald J, Szymiczek A, Rusak B, et al. Mutations in ATM, NBN and BRCA2 predispose to aggressive prostate cancer in Poland. Int J Cancer. 2020;147:2793–2800. doi: 10.1002/ijc.33272. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen-Dumont T, Dowty JG, MacInnis RJ, Steen JA, Riaz M, Dugué PA, et al. Rare germline pathogenic variants identified by multigene panel testing and the risk of aggressive prostate cancer. Cancers. 2021;13:1495. doi: 10.3390/cancers13071495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh M, Alkhushaym N, Fallatah S, Althagafi A, Aljadeed R, Alsowaida Y, et al. The association of BRCA1 and BRCA2 mutations with prostate cancer risk, frequency, and mortality: a meta-analysis. Prostate. 2019;79:880–95. doi: 10.1002/pros.23795. [DOI] [PubMed] [Google Scholar]
- 28.Roed Nielsen H, Petersen J, Therkildsen C, Skytte AB, Nilbert M. Increased risk of male cancer and identification of a potential prostate cancer cluster region in BRCA2. Acta Oncologica. 2016;55:38–44. doi: 10.3109/0284186X.2015.1067714. [DOI] [PubMed] [Google Scholar]
- 29.Thompson D, Easton D. Breast Cancer Linkage Consortium. Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet. 2001;68:410–9. doi: 10.1086/318181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lubinski J, Phelan CM, Ghadirian P, Lynch HT, Garber J, Weber B, et al. Cancer variation associated with the position of the mutation in the BRCA2 gene. Fam Cancer. 2004;3:1–10. doi: 10.1023/B:FAME.0000026816.32400.45. [DOI] [PubMed] [Google Scholar]
- 31.Patel VL, Busch EL, Friebel TM, Cronin A, Leslie G, McGuffog L, et al. Association of genomic domains in BRCA1 and BRCA2 with prostate cancer risk and aggressiveness. Cancer Res. 2020;80:624–38.. doi: 10.1158/0008-5472.CAN-19-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nyberg T, Frost D, Barrowdale D, Evans DG, Bancroft E, Adlard J, et al. Prostate cancer risk by BRCA2 genomic regions. Eur Urol. 2020;78:494–7. doi: 10.1016/j.eururo.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laitman Y, Boker LK, Liphsitz I, Weissglas-Volkov D, Litz-Philipsborn S, Schayek H, et al. Cancer risks in Jewish male BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2015;150:631–5. doi: 10.1007/s10549-015-3340-4. [DOI] [PubMed] [Google Scholar]
- 34.Easton DF, Pharoah PD, Antoniou AC, Tischkowitz M, Tavtigian SV, Nathanson KL, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372:2243–57. doi: 10.1056/NEJMsr1501341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson ML, Myers JE, Kriebel D. Prevalence odds ratio or prevalence ratio in the analysis of cross-sectional data: what is to be done? Occup Environ Med. 1998;55:272–7. doi: 10.1136/oem.55.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 37.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48.. [Google Scholar]
- 39.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 40.Gayther SA, Mangion J, Russell P, Seal S, Barfoot R, Ponder BAJ, et al. Variation of risks of breast and ovarian cancer associated with different germline mutations of the BRCA2 gene. Nat Genet. 1997;15:103–5. doi: 10.1038/ng0197-103. [DOI] [PubMed] [Google Scholar]
- 41.R Core Team. R: a language and environment for statistical computing. Vienna, Austria. Available from: https://www.R-project.org/.
- 42.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–60. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ben-Shlomo Y, Evans S, Ibrahim F, Patel B, Anson K, Chinegwundoh F, et al. The risk of prostate cancer amongst black men in the United Kingdom: the PROCESS cohort study. Eur Urol. 2008;53:99–105. doi: 10.1016/j.eururo.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 44.Wild CP, Weiderpass E, Stewart BW, editors. World cancer report: cancer research for cancer prevention. Lyon: International Agency for Research on Cancer (World Health Organization); 2020.
- 45.Nastiuk KL, Mansukhani M, Terry MB, Kularatne P, Rubin MA, Melamed J, et al. Common mutations in BRCA1 and BRCA2 do not contribute to early prostate cancer in Jewish men. Prostate. 1999;40:172–7. doi: 10.1002/(sici)1097-0045(19990801)40:3<172::aid-pros5>3.0.co;2-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castro E, Goh C, Olmos D, Saunders E, Leongamornlert D, Tymrakiewicz M, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31:1748–57. doi: 10.1200/JCO.2012.43.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antoniou AC, Easton DF. Polygenic inheritance of breast cancer: implications for design of association studies. Genet Epidemiol. 2003;25:190–202. doi: 10.1002/gepi.10261. [DOI] [PubMed] [Google Scholar]
- 48.Anderson DE, Badzioch MD. Familial breast cancer risks. Effects of prostate and other cancers. Cancer. 1993;72:114–9. doi: 10.1002/1097-0142(19930701)72:1<114::aid-cncr2820720122>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 49.Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 50.Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401–8. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- 51.Gruber SB, Ellis NA, Rennert G, Offit K, Scott KK, Almog R, et al. BLM heterozygosity and the risk of colorectal cancer. Science. 2002;297:2013. doi: 10.1126/science.1074399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The literature review and meta-analysis datasets generated and analysed during the current study are available from the corresponding author on reasonable request.