Abstract
Blood-sucking arthropods transmit a variety of human pathogens acting as disseminators of the so-called vector-borne diseases. Leishmaniasis is a spectrum of diseases caused by different Leishmania species, transmitted quasi worldwide by sand flies. However, whereas many laboratories focus on the disease(s) and etiological agents, considerably less study the respective vectors. In fact, information on sand flies is neither abundant nor easy to find; aspects including basic biology, ecology, and sand-fly-Leishmania interactions are usually reported separately. Here, we compile elemental information on sand flies, in the context of leishmaniasis. We discuss the biology, distribution, and life cycle, the blood-feeding process, and the Leishmania-sand fly interactions that govern parasite transmission. Additionally, we highlight some outstanding questions that need to be answered for the complete understanding of parasite–vector–host interactions in leishmaniasis.
Subject terms: Entomology, Parasitic infection, Parasite development
In this review, numerous aspects of sand flies as vectors of Leishmania parasites—from biology to the vector parasite interactions—are discussed.
Introduction
Estimates point to the existence of 200 million insects alive per each human at any given point; among them, around 14,000 species feed on blood1, some, with potentially severe implications for human health. In fact, diseases associated with arthropod vectors (generally known as vector-borne diseases) account for more than 17% of all infectious diseases, and cause at least 700,000 deaths annually, as per the most recent estimates2,3. Of note, since most of these diseases disproportionally affect individuals in resource-poor countries of the tropics and subtropics, they are considered Neglected Tropical Diseases (NTDs)3,4. In line with this notion, vector research has focused disproportionally on a few species (mostly mosquitoes associated with malaria and other diseases), and overlooked other arthropods still associated with a fairly high disease burden.
Among the abovementioned NTDs, leishmaniasis is associated with significant incidence, morbidity, and mortality (the deadliest NTD, according to recent global estimates)5,6. Leishmaniasis is a spectrum of diseases caused by around 20 Leishmania species, transmitted by different phlebotomine sand fly species (Table 1). Of note, many peculiarities of leishmaniasis were highlighted through the years, some of them, still without a clear explanation. For instance, while different Leishmania species are associated with similar clinical manifestations7–9, not infrequently, the same parasite species is linked to distinct clinical pictures10–12, suggesting that parasite tropism and/or virulence may not be the only pathogenesis determinants. This is supported by the many times repeated statement saying that infection and disease progression depends on “complex interactions between the parasite and the host’s immune response”13. Still, while the “atypical leishmaniasis” presentations described in the context of immunocompromised individuals (e.g., malnourished, HIV positive, and pharmacologically immunosuppressed) are easier to explain14–17, the same is not true considering disease in immunocompetent individuals, suggesting that the parasite, vector, and host determinants that condition infection and/or disease are largely unknown. This said, each Leishmania species is associated primarily with one type of disease, not many incriminated vectors, and frequently qualified as either dermotropic or viscerotropic (Table 1). This aligns with the notion that leishmaniasis endemicity depends on active and sustained parasite transmission. Since neither parasite nor vector species are ubiquitous, it is not surprising that specific parasite species are associated with specific areas of the globe and, consequently with particular vector species of the same defined areas, that are permissive to infection (Table 1)7,8.
Table 1.
Leishmaniasis: etiological, clinical, and epidemiological aspects, including the most relevant incriminated vectors.
| Leishmania species | Clinical form | Main clinical features | Natural progression | Risk groups | Main reservoir | Transmission | Main vectors& | OW vs. NW | High-burden countries or regions | Estimated annual worldwide incidence |
|---|---|---|---|---|---|---|---|---|---|---|
| Leishmania (Leishmania) donovani | VL and PKDL | Persistent fever, splenomegaly, weight loss, and anemia in VL; multiple painless macular, papular, or nodular lesions in PKDL | VL is fatal within 2 years if untreated; PKDL lesions self-heal in up to 85% of cases in Africa but rarely in Asia | Predominantly adolescents and young adults for VL; young children in Sudan and no clearly established risk factors for PKDL | Humans | Epidemic anthroponotic | Phlebotomus argentipes, Phlebotomus orientalis, Phlebotomus martini, Phlebotomus alexandri | OW | India, Bangladesh, Ethiopia, Sudan, and South Sudan | 50,000–90,000 VL cases; unknown number of PKDL cases |
| Leishmania (Leishmania) tropica | CL, LR, and rarely VL | Ulcerating dry lesions, painless, and frequently multiple | CL lesions often self-heal within 1 year | No well-defined risk groups | Humans, hyraxes | Urban anthroponotic | Phlebotomus sergenti, Phlebotomus arabicus | OW | Eastern Mediterranean, the Middle East, and northeastern and southern Africa | 200,000–400 000 CL |
| Leishmania (Leishmania) aethiopica | CL, DCL, DsCL, and oronasal CL | Localized cutaneous nodular lesions; occasionally oronasal; rarely ulcerates | Self-healing, except for DCL, within 2–5 years | Limited evidence; adolescents | Hyraxes | Rural zoonotic | Phlebotomus longipes, Phlebotomus pedifer, Phlebotomus sergenti | OW | Ethiopia and Kenya | 20,000–40,000 CL |
| Leishmania (Leishmania) major | CL | Rapid necrosis, multiple wet sores, and severe inflammation | Self-healing in >50% of cases within 2–8 months; multiple lesions slow to heal, and severe scarring | No well-defined risk groups | Rodents | Rural zoonotic | Phlebotomus papatasi, Phlebotomus duboscqi, Phlebotomus salehi, Phlebotomus caucasicus | OW | Iran, Saudi Arabia, north Africa, the Middle East, central Asia, and west Africa | 230,000–430,000 CL |
| Leishmania (Leishmania) infantum | VL and CL | Persistent fever and splenomegaly in VL; typically single nodules and minimal inflammation in CL | VL is fatal within 2 years if untreated; CL lesions self-heal within 1 year conferring individual immunity | Children under 5 years and immunocompromised adults for VL; older children and young adults for CL | Dogs, rodents, rabbits and hares, foxes, opossums, and humans | Peridomestic zoonotic | Phlebotomus Larroussius subgenus (e.g., P. ariasi, P. Perniciosus), Lutzomyia longipalpis, Lutzomyia cruzi | OW and NW | China, southern Europe, Brazil, and South America for VL and CL; Central America for CL | 6200–12,000 cases of Old World VL and 4500–6800 cases of New World VL; unknown number of CL cases |
| Leishmania (Leishmania) mexicana | CL, DCL, and DsCL | Ulcerating lesions, single or multiple | Often self-healing within 3–4 months | No well-defined risk groups | Rodents and marsupials | Sylvatic zoonotic | Lutzomyia olmeca, Lutzomyia ayacuchensis | NW | South America | Limited number of cases, included in the 187,200–300,000 total cases of New World CL |
| Leishmania (Leishmania) venezuelensis | CL | Ulcerating lesions | Not well described | No well-defined risk groups | Unknown | Zoonotic | Lutzomyia olmeca? Lutzomyia bicolor? | NW | Venezuela | Limited number of cases, included in the 187,200–300,000 total cases of New World CL |
| Leishmania (Leishmania) amazonensis | CL, DCL, and DsCL | Ulcerating lesions, single or multiple | Not well described | No well-defined risk groups | Opossums and rodents | Sylvatic zoonotic | Lutzomyia flaviscutellata | NW | South America | Limited number of cases, included in the 187,200–300,000 total cases of New World CL |
| Leishmania (Viannia) braziliensis | CL, MCL, DCL, and LR | Ulcerating lesions can progress to mucocutaneous form; local lymph nodes are palpable before and early on in the onset of the lesions | Might self-heal within 6 months; 2·5% of cases progress to MCL | No well-defined risk groups | Dogs, humans, rodents, and horses | Sylvatic zoonotic | Lutzomyia wellcomei, Lutzomyia migonei, Lutzomyia neivai, Lutzomyia carrerai | NW | South America | Majority of the 187 200–300 000 total cases of New World CL |
| Leishmania (Viannia) guyanensis | CL, DsCL, and MCL | Ulcerating lesions, single or multiple that can progress to mucocutaneous form; palpable lymph nodes | Might self-heal within 6 months | No well-defined risk groups | Opossums, sloths, and anteaters | Sylvatic zoonotic | Lutzomyia whitmani, Lutzomyia shawi, Lutzomyia anduzei, Lutzomyia ayacuchensis | NW | South America | Limited number of cases, included in the 187,200–300,000 total cases of New World CL |
| Leishmania (Viannia) peruviana | CL | Ulcerating lesions, single or multiple | Not well described | No well-defined risk groups | Unknown, dogs? | Zoonotic | Lutzomyia peruensis, Lutzomyia verrucarum | NW | Peru, Bolivia | Limited number of cases, included in the 187,200–300,000 total cases of New World CL |
| Leishmania (Viannia) panamensis | CL, MCL and DCL | Ulcerating lesions, single or multiple that can progress to mucocutaneous form | Not well described | No well-defined risk groups | Rodents, dogs? | Sylvatic zoonotic | Lutzomyia gomezi, Lutzomyia hartmanni, Lutzomyia trapidoi, Lutzomyia yuilli | NW | Central and South America | Limited number of cases, included in the 187,200–300,000 total cases of New World CL |
| Leishmania (Viannia) lainsoni | CL | Ulcerating lesions, single or multiple | Not well described | No well-defined risk groups | Rodents, porcupines | Sylvatic zoonotic | Lutzomyia ubiquitalis | NW | Brazil, Bolivia, Peru, Ecuador | Limited number of cases, included in the 187,200–300,000 total cases of New World CL |
| Leishmania (Viannia) lindenbergi | CL | Ulcerating lesions | Not well described | No well-defined risk groups | Unknown | Zoonotic | Lutzomyia atunesi? | NW | Brazil | Limited number of cases, included in the 187,200–300,000 total cases of New World CL |
| Leishmania (Viannia) naiffi | CL | Ulcerating lesions, single and small | Not well described | No well-defined risk groups | Rodents, Anteaters | Sylvatic zoonotic | Lutzomyia ayrozai, Lutzomyia squamiventris | NW | Brazil, French Guyana | Limited number of cases, included in the 187,200–300,000 total cases of New World CL |
| Leishmania (Viannia) shawi | CL | Ulcerating lesions | Not well described | No well-defined risk groups | Rodents, sloths | Sylvatic zoonotic | Lutzomyia whitmani | NW | Brazil | Limited number of cases, included in the 187,200–300,000 total cases of New World CL |
| Leishmania colombiensis # | CL and VL | Not well described | Not well described | No well-defined risk groups | Sloths | Sylvatic zoonotic | Lutzomyia hartmanni | NW | Colombia | Limited number of cases |
| Leishmania (Mundinia) martiniquensis | CL and VL | Not well described | Not well described | No well-defined risk groups | Unknown | Likely sylvatic zoonotic | Unknown | OW and NW | Martinique, Thailand, Central Europe, USA | Limited number of cases |
| Leishmania (Mundinia) orientalis | CL and VL | Not well described | Not well described | No well-defined risk groups | Unknown | Likely sylvatic zoonotic | Unknown | OW and NW | Thailand | Limited number of cases |
Adapted from7–9,31,123–128. Legend: CL cutaneous leishmaniasis; DCL diffuse cutaneous leishmaniasis; DsCL disseminated cutaneous leishmaniasis; LR leishmaniasis recidivans; MCL mucocutaneous leishmaniasis; NW New World; OW Old World; PKDL post-kala-azar dermal leishmaniasis; VL visceral leishmaniasis. Notes: &For a more comprehensive list of vectors, including suspected ones, please check two previous Review Articles7,31. # L. colombiensis has been included in the genus Endotrypanum129.
Adding up to these notions, most of the Leishmania species that are pathogenic to humans are associated with zoonotic transmission. Only for Leishmania donovani parasites, no animals other than man have been incriminated as a reservoir [although evidence suggests that domestic dogs and mongooses (Herpestes ichneumon) may be reservoirs of this parasite species, as per studies from India18 and East Africa19,20; for all of the remaining Leishmania species that cause disease in humans at least one animal reservoir (frequently sylvatic) is recognized (Table 1)8,21. All of the abovementioned justify the epidemiological complexity of leishmaniasis, whose control is, consequently, extremely difficult to accomplish22. In fact, there is a real risk of the disease(s) spreading to non-endemic regions, a consequence of the arrival of parasites and/or vector species to new areas, driven by changes in weather patterns and/or migrations21,23,24. It is, therefore, not unreasonable to qualify global warming, globalization, and war/conflicts as major potential risk factors of leishmaniasis emergence21,25–27.
Therefore, the complete understanding of leishmaniasis (as a “whole”), depends not only on the dissection of the clinical aspects (parasite-host interactions) but also on the comprehension of the sand fly vectors and their interactions with Leishmania parasites and the animal/human hosts. However, most laboratories around the globe focus exclusively on parasite-host interactions, disregarding the sand fly vectors. In fact, information in the literature on sand flies is not easily accessible, at least in a comprehensible fashion. Therefore, the familiarization of new researchers with the vectors of Leishmania parasites can be a challenging task. To address this issue, here, we compile the basic information on sand flies, including the taxonomy (at a glance), biology, distribution, and life cycle, the blood-feeding process, and the Leishmania-sand fly interactions important for parasite transmission, as a resource for the scientific community in general. Moreover, we also discuss the outstanding questions in the field, answers to which are essential for the complete understanding of the parasite-vector-host interactions that lead to leishmaniasis
Taxonomy at a glance, biology, distribution, and life cycle
Sand flies are arthropods and insects included in the order Diptera (two-winged flies), suborder Nematocera, family Psychodidae, and subfamily Phlebotominae7. Around 1000 sand fly species/subspecies were validated/described thus far around the world28. Initially, the taxonomical classification of sand flies was based on morphological analyses, including first an external analysis also known as phlebotometry (e.g., observation of the male genitalia, and determination of the wing venation indices…), and then the investigation of internal structures such as the spermathecae, cibarium, and the pharynx7,29. More recently, modern methods including chromosome analysis, isoenzyme analysis, molecular and phylogenetic analyses (DNA barcoding and Next-Generation Sequencing), and mass spectrometry (MALDI-TOF), allowed the better identification and classification of sand fly specimens and consequently the clarification of some variations within sand fly subgenera/populations7,30. Many classification systems have been proposed over the years including those of Abonnenc, Davidson, Fairchild, Galati, Leng, Lewis, Quate, Rispail & Légerand, Secombe, Theodor, and Young & Duncan7,28,30,31. However, with respect to taxonomy and the classification of sand flies, there is still no consensus, reason why we decided not to describe each of the aforementioned classification systems in this Review; for more details, as well as a historical perspective on the taxonomy and systematics of sand flies, please check a few comprehensive reviews on the subject7,28,30,31. Instead, for the sake of simplicity, here we adopted the subdivision of the Phlebotominae into six genera, as per the widely accepted classification based on a conservative approach: Phlebotomus (13 subgenera), Sergentomyia (10 subgenera), and Chinius (four species) from the Old World, and Lutzomyia (26 subgenera and groups), Brumptomyia (24 species), and Warileya (six species) from the New World (Fig. 1)7,30. Of note, female sand flies (with the exception of a few autogenous species), apart from plant sugars (for flight energy and longevity), need to take a blood meal in order to develop and lay eggs. Importantly, the genera Lutzomyia and Phlebotomus are the ones that include the anthropophagous species (although some Sergentomyia may also feed on humans), and therefore, those relevant for the transmission of human disease (Table 1)29.
Fig. 1. Sand fly distribution map by genera/subspecies.
Sand flies have a global distribution between latitude 50° N and latitude 40° S (demarked by the gray horizontal lines), excluding New Zealand and the Pacific islands. In the map, the relevant sand fly genera/subspecies (as per the widely accepted classification based on a conservative approach) are listed based on their presence in defined zoogeographical regions: Palearctic (purple), Nearctic (red), Neotropic (dark blue), Afrotropic (green), Malagasy (orange), Australia (light blue), and Indian (yellow). Adapted from7. Courtesy NIAID.
In contrast to mosquitoes and other Diptera, sand flies do not have an aquatic stage in their life cycle32. Still, humidity is an important factor that together with temperature, are detrimental for, and influence sand fly development33–35. This justifies the fact that the sand fly distribution is limited to areas having temperatures above 15.6 °C for at least three months of the year32, which still corresponds to the greatest portion of the world—from latitude 50° N to latitude 40° S (although they are absent from New Zealand and the Pacific islands) (Fig. 1)36.
The sand fly life cycle comprises four major stages: eggs, larvae, pupae, and adults (Fig. 2). On average, a female sand fly deposits 30 to 70 eggs in protected places chosen based on humidity and the presence of organic matter (e.g., cracks and holes in the ground, animal burrows/dens, termite mounds, leaf litter…)37,38. Typically, the eggs hatch between four and 20 days after oviposition, although this timing may be extended in cooler weather—eggs may diapause under unfavorable conditions38,39. There are four larval instars, and larval development is usually completed in 20–30 days, depending on the sand fly species40, as well as on the temperature and availability of food. However, this period may be prolonged to several months in sand fly species that diapause to cope with winter (only full-grown larvae diapause—instar four)37,38. Pupation usually takes from six to 13 days with adults emerging during the hours of darkness, often just before dawn38. Males usually emerge before females. Adult life expectancy in the wild has hardly been determined for sand flies, particularly considering females. However, it is known that while males may live only about a week in the wild, females may live longer as they undergo more than one gonotrophic cycle (some as many as three)41. Normally, oviposition occurs between five and eight days after blood-feeding, although some species are known to feed multiple times before successfully developing viable eggs41. Of note, most sand fly species are exophagic (feed outside of dwellings), although some are known to be endophagic and endophilic (feeding and resting in human and animal dwellings), commonly referred to as domestic or peridomestic species. A more detailed description of sand fly biology, behavior, and morphology (used to distinguish sand fly species) can be found elsewhere29–31,37,38,41. Still, much remains to be learned regarding these subjects.
Fig. 2. Schematic representation of the sand flies’ life cycle.
The sand fly life cycle comprises four major stages: eggs (orange background), larvae (four instars: green background), pupae (yellow background), and adults (blue background). In the latter two stages, different morphological features (highlighted within the circles) can be used to distinguish the gender. The most important characteristics with respect to each stage (sub-stage), are listed near the images, as are the average timings of development. Adapted from7,130,131. Courtesy NIAID.
The quest for blood and the blood-feeding process at a glance
The need for blood to give rise to a new sand fly generation (for the perpetuation of the species), associated with the fact that sand flies are weak fliers (reports state that adults usually disperse 100 meters or less from their larval habitats32), makes feeding-preferences relevant only in the context of high (and diverse) host abundance38. In other words, most times, feeding depends on host availability; sand flies will take blood from the closest permissive available source (although the engorgement outcome may vary)32,42. Of note, this aligns with the many times repeated idea that humans are generally accidental Leishmania hosts (obviously excluding those infected with the anthroponotic L. donovani parasites)43,44. Interestingly, contrarily to mosquitoes which are usually vessel feeders, sand flies are blood-pool feeders. They use their toothed mandibles in a scissors-like manner to lacerate the host’s skin, disrupting cells and causing an extravascular pool of blood from which they ingest the blood meal29,45. Importantly, during this process, through salivation, sand flies introduce several pharmacologically active molecules into the skin, to facilitate feeding. The sand fly salivary proteome, usually less than 40 secreted proteins according to transcriptomic studies, is quite diverse in function46. While a vasodilator molecule promotes the increase of local blood circulation, a molecule with apyrase function inhibits platelet aggregation through the destruction of the agonist adenosine diphosphate (ADP), and together with molecules that inhibit the blood coagulation cascade and the classical pathway of the complement system, counteract an efficient hemostatic response46. Additionally, other proteins have relevant immunomodulatory properties, that are, however, not important for the feeding process46.
Sand flies as vectors of multiple diseases
Although sand flies are mostly recognized as Leishmania vectors, they transmit other pathogens, such as bacteria and viruses. Carrion’s disease is a sand fly transmitted biphasic illness caused by Bartonella bacilliformis bacteria in Central/South America47,48. It is characterized by either intermittent febrile states (called Oroya fever), sometimes with hepatic involvement that can lead to death in the absence of (or delayed) treatment (when infected individuals are naïve); or by cutaneous lesions called Peruvian warts (when infected individuals were previously exposed to the bacteria)48. Some arboviruses are also human pathogens transmitted by sand flies, particularly those belonging to the Phlebovirus genus (order Bunyavirales, family Phenuiviridae)—enveloped single-stranded RNA(-) viruses49. At least nine virus “species” are recognized, containing 70 antigenically distinct viruses; of note, 33 other viruses, yet to be classified, are not included in the previous numbers50. Most of the sand fly transmitted viruses cause uncomplicated to moderate fever states known (when identified) as “sand fly fever” or “pappataci fever”. However, one particular agent, Toscana virus has a marked tropism for the central and peripheral nervous systems, potentially causing neuro-invasive conditions such as meningitis or encephalitis49,51–53.
Sand flies as vectors of leishmaniasis: permissive versus restrictive
Among the 1000 sand fly species/subspecies validated/described thus far around the world28, only one-tenth (10%) are proven or suspected vectors of Leishmania parasites31,54. These meet all (proven vectors) or almost all of the vector incrimination criteria proposed by Killick-Kendrick55 and the WHO Expert Committee on the control of Leishmaniases56: (i) they feed on humans (are anthropophilic), (ii) they also feed on the relevant reservoir hosts in the case of zoonotic agents, (iii) they are found in nature infected with the same parasites (Leishmania species) circulating in humans (from the same geographical area); (iv) they support the complete development of the Leishmania parasites circulating in humans, including after the defecation of the bloodmeal remnants; and (v) they are able to transmit those parasites to susceptible hosts when they take a bloodmeal30,31. Importantly, with respect to the sand fly vectors incriminated thus far, Leishmania-sand fly interactions studied under laboratory conditions led to their separation into two major groups: restrictive and permissive vectors57. As the names indicate, while the first group displays a remarkable specificity for the Leishmania species they transmit in nature (e.g., Phlebotomus papatasi and Phlebotomus sergenti), the second permits the development of distinct Leishmania species (e.g., Phlebotomus arabicus and Lutzomyia longipalpis)57. For instance, recently, we reported that L. longipalpis sand flies, vectors of Leishmania infantum parasites in nature, are competent vectors of Leishmania major parasites under laboratory conditions. We demonstrated that L. longipalpis sand flies are able to acquire L. major parasites from cutaneous leishmaniasis active lesions, to sustain mature infections, and to transmit the parasites to naïve hosts, causing disease58. This permissive versus restrictive dichotomy is thought to be related to parasite attachment to the sand fly midgut, defined as an essential mechanism for infections to proceed within the sand fly—discussed in detail in the following section59,60. However, while we understand these interactions in restrictive vectors (mediated by very specific ligand-receptor interactions), a lot is yet unknown regarding permissive ones59. What we know in this regard considering restrictive (or specific) vectors comes from the study of L. major development within P. papatasi sand flies. In this context, the attachment of parasites to the midgut is mediated by the binding of L. major lipophosphoglycan (LPG) molecules to a specific sand fly midgut receptor, a galectin (β-galactoside binding family of lectins)61. Importantly, although LPG molecules are very abundant surface proteins, found in all Leishmania species, they are also polymorphic (particularly the 10–30 phosphoglycan repeating units) and different, not only considering parasite species but also parasite strains, and even stages61,62. Interestingly, these differences explain both vector restrictiveness and the attachment-detachment processes of L. major parasites to the midgut of P. papatasi, obviously dependent on the specificity of ligand–receptor interactions61. On the other hand, vector permissiveness suggests broader or even non-specific binding processes. Although these processes are yet to be fully understood, the involvement of sand fly midgut “sticky proteins” is a considered hypothesis (e.g., O-linked glycoproteins with mucine-like properties)63.
Sand fly-Leishmania interactions toward the development of mature infections
The development of Leishmania parasites within the sand fly vector is quite complex (Fig. 3), with distinct differentiation processes that are required for the establishment of a successful infection. Of note, contrary to many vector-borne agents (including some Trypanosomatids), the development of Leishmania parasites is confined to the sand fly digestive tract (there is no crossing/disruption of the epithelial barrier59,64), simplistically divided here (excluding the crop) into: (i) the foregut— the most anterior portion, from the mouth to the cardia, which includes the stomodeal valve; (ii) the (thoracic and abdominal) midgut—from the cardia to the pylorus; and (iii) the hindgut—the most posterior portion, from the pylorus to the rectum65.
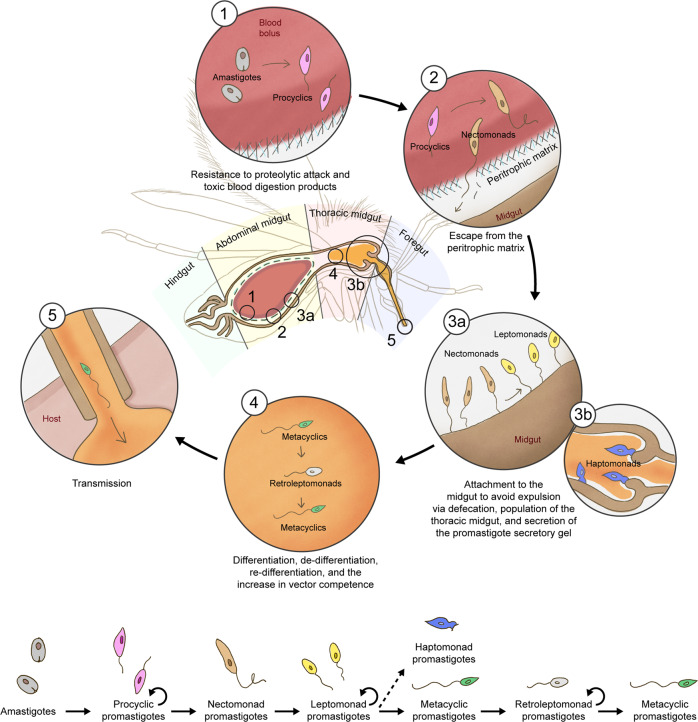
Fig. 3. Leishmania development within the sand fly midgut.
Schematic representation of the different forms of Leishmania parasites within the sand fly vector and of the major barriers they must overcome to establish a productive infection—including the resistance to proteolytic attack/toxic byproducts of the digestion of blood (1), “escape” from the perothrophic matrix (2), attachment to the midgut to avoid expulsion (3a), attachment to (and impairment of) the stomodeal valve (3b), and (de-)differentiation and replication dynamics (4)—and ensure their transmission (5) to a new host. A linear life cycle with the different parasite forms within the vector is also represented; the circular arrows highlight the replicative parasite forms. Adapted from60,86. Courtesy NIAID.
Although most Leishmania species are suprapylarian (development restricted to the midgut), species of the subgenus Viannia colonize the hindgut before migrating forward to the midgut (peripylarian parasites)65. The first differentiation step occurs not long after ingestion of the infected blood meal by sand flies. Due to changes in conditions (such as the decrease of temperature and the increase in the pH), the amastigotes differentiate into procyclic promastigotes, weakly motile forms59,66. This first differentiation step was proposed to be extremely important; it was postulated that parasites within the blood meal need to resist the effect of digestive proteases, the first and one of the most significant barriers to parasite survival67. In one study, L. major procyclic promastigotes were demonstrated to be more resistant to proteolytic attack than the parasites in the transitional state (from amastigote to promastigote forms)68; a possible explanation for such a phenotype is the known dynamic changes of the parasites’ glycocalyx components: e.g., comparing amastigotes with promastigotes, the latter have a higher content of LPG in their membrane62,67. On the other hand, a more recent study showed contrary findings, and the authors suggested parasite killing (L. major and L. donovani) could be due to the toxic products of blood meal digestion69. Importantly, although contradictory, these studies both suggest the impact of midgut proteases in the establishment of Leishmania parasites within the vector (either directly or indirectly); this notion is further supported by studies focusing on other vector-parasite pairings (Lu. longipalpis – L. mexicana/L. infantum) showing that Leishmania parasites thrive after the downregulation of the proteolytic activity in the sand fly midgut70–72. Of note, procyclic promastigotes are also the first replicative form found within the sand fly vector, increasing parasite numbers, important to the next step of the vector infection cycle59.
Within the sand fly midgut, the blood meal is enveloped by a type I peritrophic matrix. This structure, found in blood-fed insects, has mainly a semi-barrier protective role: (i) against the possible damage caused to midgut microvilli by the concentrated digestive environment; (ii) against the potentially devastating effects of one of the blood digestion by-products, heme; and (iii) against potential pathogens73. Leishmania parasites must “escape” from this structure in order to establish an infection. Around 48 h after the blood meal ingestion, procyclic promastigotes begin to slow their replication and differentiate into strongly motile and long forms called nectomonad promastigotes. These developmental forms are the ones that “escape” from the peritrophic matrix into the midgut lumen59. Furthermore, these parasite forms are also responsible for the attachment to the sand fly midgut, another crucial step for the completion of the Leishmania cycle within the vector, since it prevents the parasites from being eliminated together with the blood meal remnants during defecation59,74,75. Of note, even in refractory vectors parasites can differentiate into promastigotes and multiply within the blood bolus, but are then eliminated via defecation69,74; therefore, the “escape” from the peritrophic matrix and the attachment to the sand fly midgut to avoid the defecation-mediated elimination are detrimental for the establishment of Leishmania parasites within permissive sand flies. In line with this, it is important to repeat here one of the criteria that need to be met for the incrimination of sand flies as vectors of Leishmania parasites—sand flies support the complete development of the Leishmania parasites circulating in humans, including after the defecation of the bloodmeal remnants—and stress the fact that the detection of parasites or their DNA in engorged sand fly females (before the defecation) is insufficient for vector incrimination76.
The life cycle then continues with the migration of nectomonad promastigotes towards the anterior midgut and their differentiation into leptomonad promastigotes. These shorter parasite forms are another replicative stage in the insect, responsible for the population of the sand fly anterior midgut as well as for the secretion of the promastigote secretory gel (PSG), important for the transmission process77,78. Eventually, the leptomonads undergo another differentiation step called metacyclogenesis giving rise to the parasite stage infective to vertebrate hosts, the metacyclic promastigote59,64. Although the metacyclogenesis determinants are yet to be fully understood, the nutritional deprivation hypothesis (and probably a resultant quorum sensing mechanism) makes sense and is supported by at least one study that shows that the absence of purines promotes parasite differentiation into metacyclic forms79. Metacyclic parasites have a smaller body, and a long flagellum, responsible for their extremely fast motility64. Additionally, leptomonad promastigotes are also thought to attach to the sand fly stomodeal valve and give rise to haptomonad promastigotes, the less studied (and thus the most “neglected”) vector-derived parasite form, whose role is not completely clear80. This said, the attachment of these parasite forms may be important for the loss of the integrity of the stomodeal valve (together with the action of parasite-derived chitinolytic enzymes), which is relevant for the transmission process81–83. Importantly, more than morphologically, all of these parasite stages were demonstrated to be transcriptionally distinct to varying degrees84.
In nature, sand flies are expected to take a blood meal every five to six days, to complete as many gonotrophic cycles as possible85. Consequently, the nutrient-deprived environment that develops in the sand fly midgut as the Leishmania infection progresses, is expected to be transient. This notion consequently changes the traditionally envisioned Leishmania cycle within the vector (linear), which needs to be adapted to the ecological reality (with an associated dynamicity). Serafim et al. showed the consequences of a second blood meal (non-infected) in experimental sand fly infections. The most important one led to the breaking of the dogma that metacyclics are the last stage in the life cycle of Leishmania within the vector. The revisited life cycle within the vector includes a new parasite stage called retroleptomonad, originated by the de-differentiation of metacyclic parasites in the presence of newly available nutrients, a consequence of blood intake by infected sand flies86. As the name implies, this newly described parasite form is morphologically closer to leptomonad promastigotes, as it appears to be functionally: contrary to metacyclics and similar to leptomonad promastigotes, retroleptomonads are replicative forms86. Eventually, when the stress conditions are re-established in the midgut (with the defecation of the second blood meal remnants), retroleptomonad parasites “re-differentiate” into metacyclic promastigotes86. Importantly, the retroleptomonad replication has as a consequence, better vector infections, both quantitatively (increased parasite numbers per midgut) and qualitatively (more homogeneous populations of metacyclic promastigotes) (Fig. 4)86.
Fig. 4. The impact of multiple blood-meals on the maturation of Leishmania infections within the sand fly vector.
In nature, sand flies are expected to feed on blood multiple times for the completion of more than one gonotrophic cycle. Importantly, the intake of multiple bloodmeals (represented by the red blood drops) is expected to impact the vector competence, promoting not only the increase in the absolute parasite numbers (yellow) but also in both the percentage and number of the metacyclic infectious forms (green) in the sand fly midgut. Importantly, such an increase in total parasite numbers (A), and particularly in the number of metacyclic promastigotes (B) in the midgut of infected sand flies that take subsequent blood meals, compared with single-fed flies (blue lines), results in a higher probability of transmission of Leishmania parasites (purple gradient). Courtesy NIAID.
The above-mentioned findings, aligned with the ecological context, may suggest that the development of a successful infection in wild sand flies is a gradual process, dependent on parasite amplification boosted by the intake of multiple blood meals by the sand fly vector (Fig. 4)86. Even if initially the parasite numbers are very few, the intake of a second blood meal will boost the replication of leptomonads, increasing parasite numbers up to a point that favors their differentiation into metacyclics; a third blood meal and another round of parasite replication may be necessary, depending on the initial infectious inoculum. Still, this may not be enough to originate a productive infection, known to be dependent on the infectious inoculum (at least experimentally)86,87. Nevertheless, the de-differentiation of metacyclic promastigotes into replicative retroleptomonads upon the intake of subsequent blood meals by infected sand flies will potentiate the development of “better” infections and increase vector competence up to a point that transmission is the most likely scenario (Fig. 4), assuming the sand fly survives long enough.
Leishmania transmission: the infectious inoculum
The deposition of metacyclic parasites into the host’s skin is dependent on their regurgitation by the sand fly vector. Importantly, this process is probably the result of a clever adaptation of Leishmania spp. parasites. The filamentous proteophosphoglycans secreted by leptomonad promastigotes form a gel-like plug called PSG plug, as mentioned above, that impairs the sand fly feeding process. Because the infected sand fly digestive tract is clogged, to an extent dependent on the parasite burden (the more parasites, the bigger the plug86), to facilitate blood intake (trying to unclog the anterior midgut), sand flies regurgitate8. Importantly, such a regurgitation is also facilitated by the Leishmania-induced damage of the sand fly stomodeal valve, known to be permanently opened in the context of heavy infections81,83. Of note, as an indirect consequence of the formation of the PSG plug/damage of the stomodeal valve, the behavior of infected sand flies is also altered toward an increase in the feeding persistence, with the potential to favor infection. Researchers have shown that heavily infected flies (with larger PSG plugs - “blocked sand fly” phenotype) had more difficulty in taking a full blood meal, and thus attempted to re-feed more often and on multiple hosts, positively impacting transmission78,88. Having the above in mind, in the end, mostly metacyclic promastigotes are egested into the skin of (multiple) hosts (in the context of a mature sand fly infection), but not alone. We now know that the infectious inoculum is composed of many relevant factors, both parasite- and vector-derived.
The Leishmania-derived proteophosphoglycans, part of the PSG plug, that is regurgitated together with parasites, were demonstrated to contribute to disease exacerbation (in the context of both cutaneous and visceral leishmaniasis—CL and VL, respectively)67. A possible mechanism proposed was the modulation of early innate pathways involved in response to a wound. The PSG was shown to potentially accelerate wound healing in the skin89, which in turn is known to be by itself a potential infection-enhancer stimulus90,91. Additionally, parasite-derived exosomes were also shown to be part of the infectious inoculum, and to potentiate disease, in both CL and VL animal models92,93. The alteration of cell recruitment patterns, and the modulation of cell behavior, were mechanisms shown to be involved in the exosome-mediated potentiation of infection92,93.
With respect to the vector-derived infection enhancers, both the sand fly gut microbiota and sand fly saliva were demonstrated to play a role94–96. Similar to almost every known gastrointestinal tract in nature, sand fly midguts harbor a diverse microbiological community. Importantly, the colonization of the vector intestinal tract was shown to be extremely important for the development of Leishmania parasites within the sand fly midgut97. Additionally, these sand fly bacterial midgut colonizers were shown to be co-egested together with Leishmania parasites and the abovementioned parasite-derived infection enhancers during transmission, additively contributing to infection establishment98. The egested microbes were shown to trigger the inflammasome, leading to a rapid production of IL-1β and a sustained neutrophil infiltration at the site of the vector bite98. Of note, the essential role of neutrophils in the context of Leishmania infection-establishment was shown for L. major parasites more than a decade ago99, although some studies point to an infection-protective role100. Last but not least, all of these midgut-derived immunomodulatory factors (and Leishmania parasites) will join the sand fly saliva within the host skin. And more than to impact host hemostasis, sand fly saliva was shown to modulate host immunity. Sand fly salivary proteins were shown to favor (in most contexts) anti-inflammatory local immune responses, beneficial for the establishment of parasites, and consequently, to potentiate infection and disease101. Of note, such an infection-enhancing effect of sand fly saliva was demonstrated in vivo both in the context of transmission (the establishment of infection)102,103, and of active disease (cutaneous leishmaniasis mouse model)104. Interestingly, recently, a family of insect-derived neutrophil chemoattractant proteins was identified, for the first time, in the saliva of sand flies, with infection-enhancer characteristics105.
Outstanding questions
Over the past few decades, our understanding of sand fly vector–parasite–host interactions has considerably improved. However, many gaps in knowledge must still be addressed in the field, in leishmaniasis endemic areas.
Most of what we know about Leishmania transmission by sand flies is based on laboratory evidence. Therefore, the translation of these notions to the natural context, or, in other words, their validation is still needed. The clarification of the transmission determinants is essential. Many questions still need to be answered, such as “Is there a threshold of infection (both related to the prevalence of infected flies and the average infection burden) associated with effective transmission?”, and “Can the bite of a single infected fly lead to the development of visceral leishmaniasis?”. Of note, some interesting theories have been proposed106; however, they are again mainly supported by laboratory findings.
In fact, the ecological context is mostly unknown, and probably different considering the distinct vector–parasite–reservoir combinations found in nature. Only the dissection of such interactions considering each particular combination will enable us to fully understand disease transmission and either optimize the control strategies available or develop better-suited ones. With this respect, considering that most disease-causing Leishmania species are zoonotic agents, to investigate the interactions between sand flies and the (sylvatic) Leishmania reservoirs is of paramount importance. For instance, it is essential to understand whether in nature the interaction between sand flies and reservoirs boosts each other’s competence, in a vicious cycle that ultimately leads to the perpetuation of Leishmania parasites, as was somehow suggested in a laboratory study107.
Also related to the above topic the complete disclosure of the competent vectors at a given location (including the potential incrimination of new sand fly species), is vital for the development of vector control strategies with real impact on disease control108. As a speculative exercise, thinking on the Mediterranean Basin, where at least eight different sand fly species were incriminated as vectors of L. infantum parasites109, to focus the control strategy on the main vector species in the area, but not in all permissive sand fly species may have little/limited impact on disease control, considering the possibility of redundancy. Other vector species, either endemic or emergent [global warming is expected to change the sand fly distribution landscape109,110] in the region, may assume the role of “main disease vector”.
Additionally, also on the topic of vector control, it is of paramount importance to disclose the immune responses in sand flies, in the context of Leishmania infection. And although more than a few breakthroughs were made on this topic in the last years, as recently reviewed in detail by Telleria and colleagues96, much is yet unknown, particularly with respect to the existence of immune-related determinants of vector refractoriness. The fact that both the modulation of the gut microbiota97,111,112 and infection with particular viral agents113 impact the establishment of Leishmania parasites within the sand fly vector, makes us wonder whether more than the simple competition, this is due to some kind of “immune priming”, as reported for other relevant vectors of human disease114,115; this hypothesis is worth to be explored in the future. Importantly, only when we comprehensively understand the sand fly immune responses detrimental for the elimination of Leishmania parasites, can we start trying to answer the question: can we modulate immunity in sand flies to make them refractory to Leishmania parasites and use this as a vector-based strategy for the control of leishmaniasis?
The deep knowledge of sand fly biology and behavior is also vital for the complete understanding of leishmaniasis and the rational development of prophylactic and even therapeutic interventions. Substantial progress has been made in this respect. For instance, the definition of the infectious inoculum discussed above is quite recent. Additionally, more and more sand fly-based vaccine candidates against leishmaniasis116,117 are being proposed as essential disease control tools that can be used in combination with the Leishmania-derived ones118,119. The same is true considering the exploitation of sand fly salivary proteins as markers of exposure, as a tool for the control of leishmaniasis46,95. However, much still remains to be done; many questions are still unanswered. For instance, to what extent do vector-derived factors impact host immunity and how can we overcome such responses in a significant fashion? Additionally, should we revisit the aim of vector-derived vaccines - inducing Th1 DTH immune responses versus blocking the activity of infection-enhancer molecules including neutrophil chemoattractant proteins, hyaluronidases, and endonucleases120,121?
Last but not least, the acknowledgment of the vector as part of the equation is also indispensable for a complete understanding of leishmaniasis. As mentioned in the Introduction, most laboratories interested in leishmaniasis do not have access to sand flies. Therefore, most of the information generated may not be translatable to the natural context. A good example of the negative impact of the disregard of the vector in experimental studies was published by Peters et al.; these authors showed in vivo that a previously defined effective anti-Leishmania vaccine lost its protective potential in the context of natural Leishmania transmission via sand fly bites122. Therefore, one question that deserves to be answered is: “Are there any established dogmas based on the incomplete focus on the parasite–host interactions that are not valid in the context of the vector? Also in line with this limitation, we also dare to ask whether surrogate models (closer to the natural context than the traditionally used leishmaniasis in vitro/animal models) can be developed and widely employed?
Conclusion
As a vector-borne disease, leishmaniasis is the result of an intricate web of vector–parasite–host interactions. However, while the hosts and the parasites are the traditional focus of the studies, the vectors are often overlooked. In this review, we tried to compile information, in our opinion, essential for new researchers to become familiarized with sand flies, in the context of leishmaniasis. Importantly, only when we understand sand flies as well as we do Leishmania parasites and their hosts, will we be able to establish the determinants of transmission and disease and to implement strategies to effectively control leishmaniasis.
Supplementary information
Acknowledgements
We are grateful to all of our laboratory colleagues for the stimulating discussions along the years. We are also grateful to Shaden Kamhawi (NIAID, NIH, USA), Claudio Meneses (NIAID, NIH, USA), Philip Lawyer (Brigham Young University, USA), Claudia Brodskyn (Fundação Oswaldo Cruz-CPqGM, Brazil), John Andersen (NIAID, NIH), and Janneth Rogrigues (GSK, Tres Cantos, Spain) for the valuable feedback. Finally, we acknowledge Rose Perry-Gottschalk (Research Technologies Branch, NIAID, NIH), who created the illustrations. This study received funding from the project NORTE-01-0145-FEDER-000012, supported by Norte Portugal Regional Operational Program (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund - ERDF (A.C.-d.-S.). This study was also supported in part by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases (F.O.). P.C. was supported by Foundation for Science and Technology (FCT), Portugal, through the individual Grant SFRH/BD/121252/2016. P.C. was also supported by Fulbright Portugal. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
P.C. and F.O. envisioned the structure of the manuscript. P.C. conducted the bibliographic search and wrote the initial draft of the manuscript. A.C.-d.-S. and F.O. reviewed and edited the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Peer review
Peer review information
Communications Biology thanks Yara Traub-Csekö and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Manuel Breuer. Peer reviewer reports are available.
Funding
Open Access Funding provided by the National Institutes of Health (NIH).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pedro Cecílio, Email: pedro.amadocecilio@nih.gov.
Fabiano Oliveira, Email: loliveira@niaid.nih.gov.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-022-03240-z.
References
- 1.Lehane, M. J. Biology of Blood-Sucking Insects 2nd edn (ed M. J. Lehane) (Cambridge University Press, 2005).
- 2.WHO. Fact Sheet: Vector-Borne Diseases, https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (2020).
- 3.Müller, R. R. F., Kendrovski, V. & Montag, D. Biodiversity and Health in the Face of Climate Change (eds Marselle, R., Stadler, J., Korn, H., Irvine, K. & Bonn, A.) (Springer, 2019).
- 4.Hotez PJ, Aksoy S, Brindley PJ, Kamhawi S. What constitutes a neglected tropical disease? PLoS Negl. Trop. Dis. 2020;14:e0008001. doi: 10.1371/journal.pntd.0008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez-Hernandez DA, Rivero-Zambrano L, Martinez-Juarez LA, Garcia-Rodriguez-Arana R. Overcoming the global burden of neglected tropical diseases. Ther. Adv. Infect. Dis. 2020;7:2049936120966449. doi: 10.1177/2049936120966449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herricks JR, et al. The global burden of disease study 2013: What does it mean for the NTDs? PLoS Negl. Trop. Dis. 2017;11:e0005424. doi: 10.1371/journal.pntd.0005424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akhoundi M, et al. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLoS Negl. Trop. Dis. 2016;10:e0004349. doi: 10.1371/journal.pntd.0004349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bates PA. Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies. Int. J. Parasitol. 2007;37:1097–1106. doi: 10.1016/j.ijpara.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018;392:951–970. doi: 10.1016/S0140-6736(18)31204-2. [DOI] [PubMed] [Google Scholar]
- 10.Cunha J, et al. Characterization of the biology and infectivity of Leishmania infantum viscerotropic and dermotropic strains isolated from HIV+ and HIV− patients in the murine model of visceral Leishmaniasis. Parasit. Vectors. 2013;6:122. doi: 10.1186/1756-3305-6-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranasinghe S, et al. Leishmania donovani zymodeme MON-37 isolated from an autochthonous visceral Leishmaniasis patient in Sri Lanka. Pathog. Glob. Health. 2012;106:421–424. doi: 10.1179/2047773212Y.0000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss F, Vogenthaler N, Franco-Paredes C, Parker SR. Leishmania tropica-induced cutaneous and presumptive concomitant viscerotropic Leishmaniasis with prolonged incubation. Arch. Dermatol. 2009;145:1023–1026. doi: 10.1001/archdermatol.2009.181. [DOI] [PubMed] [Google Scholar]
- 13.Cecilio P, et al. Deception and manipulation: the arms of Leishmania, a successful parasite. Front. Immunol. 2014;5:480. doi: 10.3389/fimmu.2014.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belli A, et al. Widespread atypical cutaneous Leishmaniasis caused by Leishmania (L.) Chagasi in Nicaragua. Am. J. Trop. Med. Hyg. 1999;61:380–385. doi: 10.4269/ajtmh.1999.61.380. [DOI] [PubMed] [Google Scholar]
- 15.Clemente WT, Couto CA, Ribeiro DD, de Medeiros Chaves Franca M, Sanches MD. An atypical course of visceral Leishmaniasis (Kala-azar) in a liver transplant recipient. Transplantation. 2007;83:368–369. doi: 10.1097/01.tp.0000251810.61080.33. [DOI] [PubMed] [Google Scholar]
- 16.Diro E, et al. Atypical manifestations of visceral Leishmaniasis in patients with HIV in north Ethiopia: A gap in guidelines for the management of opportunistic infections in resource poor settings. Lancet Infect. Dis. 2015;15:122–129. doi: 10.1016/S1473-3099(14)70833-3. [DOI] [PubMed] [Google Scholar]
- 17.van Griensven J, Carrillo E, Lopez-Velez R, Lynen L, Moreno J. Leishmaniasis in immunosuppressed individuals. Clin. Microbiol. Infect. 2014;20:286–299. doi: 10.1111/1469-0691.12556. [DOI] [PubMed] [Google Scholar]
- 18.Jambulingam P, Pradeep Kumar N, Nandakumar S, Paily KP, Srinivasan R. Domestic dogs as reservoir hosts for Leishmania donovani in the southernmost Western Ghats in India. Acta Trop. 2017;171:64–67. doi: 10.1016/j.actatropica.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Dereure J, et al. Visceral Leishmaniasis in eastern Sudan: Parasite identification in humans and dogs; host−parasite relationships. Microbes Infect. 2003;5:1103–1108. doi: 10.1016/j.micinf.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Elnaiem DA, et al. The Egyptian mongoose, Herpestes ichneumon, is a possible reservoir host of visceral Leishmaniasis in eastern Sudan. Parasitology. 2001;122:531–536. doi: 10.1017/s0031182001007594. [DOI] [PubMed] [Google Scholar]
- 21.Gradoni, L. The Leishmaniases: Old Neglected Tropical Diseases (eds Bruschi, F. & Gradoni, L.) 1–13 (Springer International Publishing, 2018).
- 22.Kamhawi S. The yin and yang of Leishmaniasis control. PLoS Negl. Trop. Dis. 2017;11:e0005529. doi: 10.1371/journal.pntd.0005529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oryan A, Akbari M. Worldwide risk factors in Leishmaniasis. Asian Pac. J. Trop. Med. 2016;9:925–932. doi: 10.1016/j.apjtm.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Pavli A, Maltezou HC. Leishmaniasis, an emerging infection in travelers. Int. J. Infect. Dis. 2010;14:e1032–e1039. doi: 10.1016/j.ijid.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Berry I, Berrang-Ford L. Leishmaniasis, conflict, and political terror: A spatio-temporal analysis. Soc. Sci. Med. 2016;167:140–149. doi: 10.1016/j.socscimed.2016.04.038. [DOI] [PubMed] [Google Scholar]
- 26.Dujardin JC, et al. Spread of vector-borne diseases and neglect of Leishmaniasis, Europe. Emerg. Infect. Dis. 2008;14:1013–1018. doi: 10.3201/eid1407.071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kholoud, K., Denis, S., Lahouari, B., El Hidan, M. A. & Souad, B. Management of Leishmaniases in the era of climate change in Morocco. Int. J. Environ. Res. Public Health10.3390/ijerph15071542 (2018). [DOI] [PMC free article] [PubMed]
- 28.Shimabukuro, P. H. F., de Andrade, A. J. & Galati, E. A. B. Checklist of American sand flies (Diptera, Psychodidae, Phlebotominae): genera, species, and their distribution. Zookeys660, 67–106 (2017). [DOI] [PMC free article] [PubMed]
- 29.Lane, R. P. Medical Insects and Arachnids (Springer, 1993).
- 30.Dvorak, V., Shaw, J. & Volf, P. The Leishmaniases: Old Neglected Tropical Diseases (eds Bruschi, F. & Gradoni, F.) 31–77 (Springer International Publishing, 2018).
- 31.Maroli M, Feliciangeli MD, Bichaud L, Charrel RN, Gradoni L. Phlebotomine sandflies and the spreading of Leishmaniases and other diseases of public health concern. Med. Vet. Entomol. 2013;27:123–147. doi: 10.1111/j.1365-2915.2012.01034.x. [DOI] [PubMed] [Google Scholar]
- 32.ECDC. Phlebotomine sand flies—factsheet for experts, https://www.ecdc.europa.eu/en/disease-vectors/facts/phlebotomine-sand-flies (2014).
- 33.Benkova I, Volf P. Effect of temperature on metabolism of Phlebotomus papatasi (Diptera: Psychodidae) J. Med. Entomol. 2007;44:150–154. doi: 10.1603/0022-2585(2007)44[150:eotomo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 34.Erguler K, et al. A climate-driven and field data-assimilated population dynamics model of sand flies. Sci. Rep. 2019;9:2469. doi: 10.1038/s41598-019-38994-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kasap OE, Alten B. Laboratory estimation of degree-day developmental requirements of Phlebotomus papatasi (Diptera: Psychodidae) J. Vector Ecol. 2005;30:328–333. [PubMed] [Google Scholar]
- 36.Killick-Kendrick R. The biology and control of phlebotomine sand flies. Clin. Dermatol. 1999;17:279–289. doi: 10.1016/s0738-081x(99)00046-2. [DOI] [PubMed] [Google Scholar]
- 37.Killick-Kendrick, R. World Class Parasites: Leishmania (ed Farrell, J. P.) (Springer, 2001).
- 38.Service, M. Medical Entomology for Students (Cambridge University Press, 2012).
- 39.Lawyer P, Killick-Kendrick M, Rowland T, Rowton E, Volf P. Laboratory colonization and mass rearing of phlebotomine sand flies (Diptera, Psychodidae) Parasite. 2017;24:42. doi: 10.1051/parasite/2017041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volf P, Volfova V. Establishment and maintenance of sand fly colonies. J. Vector Ecol. 2011;36:S1–S9. doi: 10.1111/j.1948-7134.2011.00106.x. [DOI] [PubMed] [Google Scholar]
- 41.Rutledge, L. C. & Gupta, R. K. Medical and Veterinary Entomology (eds Mullen, G. & Durden, L.) (Academic Press, 2002).
- 42.Gebresilassie A, et al. Host-feeding preference of Phlebotomus orientalis (Diptera: Psychodidae) in an endemic focus of visceral Leishmaniasis in northern Ethiopia. Parasit. Vectors. 2015;8:270. doi: 10.1186/s13071-015-0883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Handman E, Bullen DV. Interaction of Leishmania with the host macrophage. Trends Parasitol. 2002;18:332–334. doi: 10.1016/s1471-4922(02)02352-8. [DOI] [PubMed] [Google Scholar]
- 44.Quinnell RJ, Courtenay O. Transmission, reservoir hosts and control of zoonotic visceral Leishmaniasis. Parasitology. 2009;136:1915–1934. doi: 10.1017/S0031182009991156. [DOI] [PubMed] [Google Scholar]
- 45.USA_Department_of_Defense. Sand Flies - Significance, Surveillance, and Control in ContingencyOperations (Armed Forces Pest Management Board, 2015).
- 46.Abdeladhim M, Kamhawi S, Valenzuela JG. What’s behind a sand fly bite? The profound effect of sand fly saliva on host hemostasis, inflammation, and immunity. Infect. Genet. Evol. 2014;28:691–703. doi: 10.1016/j.meegid.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minnick MF, et al. Oroya fever and verruga peruana: bartonelloses unique to South America. PLoS Negl. Trop. Dis. 2014;8:e2919. doi: 10.1371/journal.pntd.0002919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pons MJ, Gomes C, Del Valle-Mendoza J, Ruiz J. Carrion’s disease: More than a sand fly-vectored illness. PLoS Pathog. 2016;12:e1005863. doi: 10.1371/journal.ppat.1005863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moriconi M, et al. Phlebotomine sand fly-borne pathogens in the Mediterranean Basin: Human Leishmaniasis and phlebovirus infections. PLoS Negl. Trop. Dis. 2017;11:e0005660. doi: 10.1371/journal.pntd.0005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elliott RM, Brennan B. Emerging phleboviruses. Curr. Opin. Virol. 2014;5:50–57. doi: 10.1016/j.coviro.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alkan C, et al. Sandfly-borne phleboviruses of Eurasia and Africa: Epidemiology, genetic diversity, geographic range, control measures. Antivir. Res. 2013;100:54–74. doi: 10.1016/j.antiviral.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Howell BA, Azar MM, Landry ML, Shaw AC. Toscana virus encephalitis in a traveler returning to the United States. J. Clin. Microbiol. 2015;53:1445–1447. doi: 10.1128/JCM.03498-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peyrefitte CN, et al. Toscana virus and acute meningitis, France. Emerg. Infect. Dis. 2005;11:778–780. doi: 10.3201/eid1105.041122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bates PA. Leishmania sand fly interaction: Progress and challenges. Curr. Opin. Microbiol. 2008;11:340–344. doi: 10.1016/j.mib.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Killick-Kendrick R. Phlebotomine vectors of the Leishmaniases: a review. Med. Vet. Entomol. 1990;4:1–24. doi: 10.1111/j.1365-2915.1990.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 56.WHO. Control of the Leishmaniases. World Health Organ Tech Rep Ser xii-xiii, 1–186 (World Health Organization (WHO), 2010). [PubMed]
- 57.Volf P, Myskova J. Sand flies and Leishmania: Specific versus permissive vectors. Trends Parasitol. 2007;23:91–92. doi: 10.1016/j.pt.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cecilio P, et al. Exploring Lutzomyia longipalpis sand fly vector competence for Leishmania major parasites. J. Infect. Dis. 2020;222:1199–1203. doi: 10.1093/infdis/jiaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dostalova A, Volf P. Leishmania development in sand flies: Parasite–vector interactions overview. Parasit. Vectors. 2012;5:276. doi: 10.1186/1756-3305-5-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kamhawi S. Phlebotomine sand flies and Leishmania parasites: Friends or foes? Trends Parasitol. 2006;22:439–445. doi: 10.1016/j.pt.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 61.Kamhawi S, et al. A role for insect galectins in parasite survival. Cell. 2004;119:329–341. doi: 10.1016/j.cell.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 62.Forestier CL, Gao Q, Boons GJ. Leishmania lipophosphoglycan: How to establish structure-activity relationships for this highly complex and multifunctional glycoconjugate? Front. Cell Infect. Microbiol. 2014;4:193. doi: 10.3389/fcimb.2014.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Myskova J, et al. Characterization of a midgut mucin-like glycoconjugate of Lutzomyia longipalpis with a potential role in Leishmania attachment. Parasit. Vectors. 2016;9:413. doi: 10.1186/s13071-016-1695-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramalho-Ortigao M, Saraiva EM, Traub-Cseko YM. Sand fly-Leishmania interactions: long relationships are not necessarily easy. Open Parasitol. J. 2010;4:195–204. doi: 10.2174/1874421401004010195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nieves E, Pimenta PF. Development of Leishmania (Viannia) braziliensis and Leishmania (Leishmania) amazonensis in the sand fly Lutzomyia migonei (Diptera: Psychodidae) J. Med. Entomol. 2000;37:134–140. doi: 10.1603/0022-2585-37.1.134. [DOI] [PubMed] [Google Scholar]
- 66.Lawyer PG, et al. Development of Leishmania major in Phlebotomus duboscqi and Sergentomyia schwetzi (Diptera: Psychodidae) Am. J. Trop. Med. Hyg. 1990;43:31–43. doi: 10.4269/ajtmh.1990.43.31. [DOI] [PubMed] [Google Scholar]
- 67.Rogers ME. The role of leishmania proteophosphoglycans in sand fly transmission and infection of the Mammalian host. Front. Microbiol. 2012;3:223. doi: 10.3389/fmicb.2012.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pimenta PF, Modi GB, Pereira ST, Shahabuddin M, Sacks DL. A novel role for the peritrophic matrix in protecting Leishmania from the hydrolytic activities of the sand fly midgut. Parasitology. 1997;115:359–369. doi: 10.1017/s0031182097001510. [DOI] [PubMed] [Google Scholar]
- 69.Pruzinova K, et al. Leishmania mortality in sand fly blood meal is not species-specific and does not result from direct effect of proteinases. Parasit. Vectors. 2018;11:37. doi: 10.1186/s13071-018-2613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rego FD, Soares RP. Lutzomyia longipalpis: An update on this sand fly vector. Acad. Bras. Cienc. 2021;93:e20200254. doi: 10.1590/0001-37652021XXXX. [DOI] [PubMed] [Google Scholar]
- 71.Sant’anna MR, Diaz-Albiter H, Mubaraki M, Dillon RJ, Bates PA. Inhibition of trypsin expression in Lutzomyia longipalpis using RNAi enhances the survival of Leishmania. Parasit. Vectors. 2009;2:62. doi: 10.1186/1756-3305-2-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silva Fernandes TL, et al. Galactosamine reduces sandfly gut protease activity through TOR downregulation and increases Lutzomyia susceptibility to Leishmania. Insect Biochem. Mol. Biol. 2020;122:103393. doi: 10.1016/j.ibmb.2020.103393. [DOI] [PubMed] [Google Scholar]
- 73.Secundino NF, Eger-Mangrich I, Braga EM, Santoro MM, Pimenta PF. Lutzomyia longipalpis peritrophic matrix: formation, structure, and chemical composition. J. Med. Entomol. 2005;42:928–938. doi: 10.1093/jmedent/42.6.928. [DOI] [PubMed] [Google Scholar]
- 74.Sadlova J, Homola M, Myskova J, Jancarova M, Volf P. Refractoriness of Sergentomyia schwetzi to Leishmania spp. is mediated by the peritrophic matrix. PLoS Negl. Trop. Dis. 2018;12:e0006382. doi: 10.1371/journal.pntd.0006382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilson, R. et al. Stage-specific adhesion of Leishmania promastigotes to sand fly midguts assessed using an improved comparative binding assay. PLoS Negl. Trop. Dis.10.1371/journal.pntd.0000816 (2010). [DOI] [PMC free article] [PubMed]
- 76.Sadlova J, et al. Sergentomyia schwetzi is not a competent vector for Leishmania donovani and other Leishmania species pathogenic to humans. Parasit. Vectors. 2013;6:186. doi: 10.1186/1756-3305-6-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gossage SM, Rogers ME, Bates PA. Two separate growth phases during the development of Leishmania in sand flies: Implications for understanding the life cycle. Int. J. Parasitol. 2003;33:1027–1034. doi: 10.1016/s0020-7519(03)00142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rogers ME, Chance ML, Bates PA. The role of promastigote secretory gel in the origin and transmission of the infective stage of Leishmania mexicana by the sandfly Lutzomyia longipalpis. Parasitology. 2002;124:495–507. doi: 10.1017/s0031182002001439. [DOI] [PubMed] [Google Scholar]
- 79.Serafim TD, et al. Leishmania metacyclogenesis is promoted in the absence of purines. PLoS Negl. Trop. Dis. 2012;6:e1833. doi: 10.1371/journal.pntd.0001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sunter, J. & Gull, K. Shape, form, function and Leishmania pathogenicity: from textbook descriptions to biological understanding. Open Biol.10.1098/rsob.170165 (2017). [DOI] [PMC free article] [PubMed]
- 81.Rogers ME, et al. Leishmania chitinase facilitates colonization of sand fly vectors and enhances transmission to mice. Cell Microbiol. 2008;10:1363–1372. doi: 10.1111/j.1462-5822.2008.01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schlein Y, Jacobson RL, Messer G. Leishmania infections damage the feeding mechanism of the sandfly vector and implement parasite transmission by bite. Proc. Natl Acad. Sci. USA. 1992;89:9944–9948. doi: 10.1073/pnas.89.20.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Volf P, Hajmova M, Sadlova J, Votypka J. Blocked stomodeal valve of the insect vector: similar mechanism of transmission in two trypanosomatid models. Int. J. Parasitol. 2004;34:1221–1227. doi: 10.1016/j.ijpara.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 84.Coutinho-Abreu IV, et al. Distinct gene expression patterns in vector-residing Leishmania infantum identify parasite stage-enriched markers. PLoS Negl. Trop. Dis. 2020;14:e0008014. doi: 10.1371/journal.pntd.0008014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ready PD. Biology of phlebotomine sand flies as vectors of disease agents. Annu. Rev. Entomol. 2013;58:227–250. doi: 10.1146/annurev-ento-120811-153557. [DOI] [PubMed] [Google Scholar]
- 86.Serafim TD, et al. Sequential blood meals promote Leishmania replication and reverse metacyclogenesis augmenting vector infectivity. Nat. Microbiol. 2018;3:548–555. doi: 10.1038/s41564-018-0125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loeuillet C, Banuls AL, Hide M. Study of Leishmania pathogenesis in mice: Experimental considerations. Parasit. Vectors. 2016;9:144. doi: 10.1186/s13071-016-1413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rogers ME, Bates PA. Leishmania manipulation of sand fly feeding behavior results in enhanced transmission. PLoS Pathog. 2007;3:e91. doi: 10.1371/journal.ppat.0030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Giraud E, et al. Leishmania proteophosphoglycans regurgitated from infected sand flies accelerate dermal wound repair and exacerbate Leishmaniasis via insulin-like growth factor 1-dependent signalling. PLoS Pathog. 2018;14:e1006794. doi: 10.1371/journal.ppat.1006794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abdoli A, Maspi N, Ghaffarifar F. Wound healing in cutaneous Leishmaniasis: A double edged sword of IL-10 and TGF-beta. Comp. Immunol. Microbiol. Infect. Dis. 2017;51:15–26. doi: 10.1016/j.cimid.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 91.Baldwin T, et al. Wound healing response is a major contributor to the severity of cutaneous Leishmaniasis in the ear model of infection. Parasite Immunol. 2007;29:501–513. doi: 10.1111/j.1365-3024.2007.00969.x. [DOI] [PubMed] [Google Scholar]
- 92.Atayde VD, et al. Exosome secretion by the parasitic protozoan Leishmania within the sand fly midgut. Cell Rep. 2015;13:957–967. doi: 10.1016/j.celrep.2015.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perez-Cabezas B, et al. More than just exosomes: Distinct Leishmania infantum extracellular products potentiate the establishment of infection. J. Extracell. Vesicles. 2019;8:1541708. doi: 10.1080/20013078.2018.1541708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Andrade BB, de Oliveira CI, Brodskyn CI, Barral A, Barral-Netto M. Role of sand fly saliva in human and experimental Leishmaniasis: Current insights. Scand. J. Immunol. 2007;66:122–127. doi: 10.1111/j.1365-3083.2007.01964.x. [DOI] [PubMed] [Google Scholar]
- 95.Lestinova T, Rohousova I, Sima M, de Oliveira CI, Volf P. Insights into the sand fly saliva: Blood-feeding and immune interactions between sand flies, hosts, and Leishmania. PLoS Negl. Trop. Dis. 2017;11:e0005600. doi: 10.1371/journal.pntd.0005600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Telleria EL, Martins-da-Silva A, Tempone AJ, Traub-Cseko YM. Leishmania, microbiota, and sand fly immunity. Parasitology. 2018;145:1336–1353. doi: 10.1017/S0031182018001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kelly, P. H. et al. The Gut microbiome of the vector lutzomyia longipalpis is essential for survival of Leishmania infantum. mBio10.1128/mBio.01121-16 (2017). [DOI] [PMC free article] [PubMed]
- 98.Dey R, et al. Gut microbes egested during bites of infected sand flies augment severity of Leishmaniasis via inflammasome-derived IL-1beta. Cell Host Microbe. 2018;23:134–143 e136. doi: 10.1016/j.chom.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peters NC, et al. In vivo imaging reveals an essential role for neutrophils in Leishmaniasis transmitted by sand flies. Science. 2008;321:970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carlsen ED, et al. Permissive and protective roles for neutrophils in Leishmaniasis. Clin. Exp. Immunol. 2015;182:109–118. doi: 10.1111/cei.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gomes R, Oliveira F. The immune response to sand fly salivary proteins and its influence on Leishmania immunity. Front. Immunol. 2012;3:110. doi: 10.3389/fimmu.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Belkaid Y, et al. Development of a natural model of cutaneous Leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J. Exp. Med. 1998;188:1941–1953. doi: 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Titus RG, Ribeiro JM. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988;239:1306–1308. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- 104.Vojtkova, B. et al. Repeated sand fly bites of infected BALB/c mice enhance the development of Leishmania lesions. Front. Trop. Dis.10.3389/fitd.2021.745104 (2021).
- 105.Guimaraes-Costa AB, et al. A sand fly salivary protein acts as a neutrophil chemoattractant. Nat. Commun. 2021;12:3213. doi: 10.1038/s41467-021-23002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Courtenay O, Peters NC, Rogers ME, Bern C. Combining epidemiology with basic biology of sand flies, parasites, and hosts to inform Leishmaniasis transmission dynamics and control. PLoS Pathog. 2017;13:e1006571. doi: 10.1371/journal.ppat.1006571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Valverde JG, et al. Increased transmissibility of Leishmania donovani from the mammalian host to vector sand flies after multiple exposures to sand fly bites. J. Infect. Dis. 2017;215:1285–1293. doi: 10.1093/infdis/jix115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilson AL, et al. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 2020;14:e0007831. doi: 10.1371/journal.pntd.0007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alten B, et al. Seasonal dynamics of phlebotomine sand fly species proven vectors of mediterranean Leishmaniasis caused by Leishmania infantum. PLoS Negl. Trop. Dis. 2016;10:e0004458. doi: 10.1371/journal.pntd.0004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chalghaf B, et al. Ecological niche modeling predicting the potential distribution of Leishmania vectors in the Mediterranean basin: Impact of climate change. Parasit. Vectors. 2018;11:461. doi: 10.1186/s13071-018-3019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Louradour, I. et al. The midgut microbiota plays an essential role in sand fly vector competence for Leishmania major. Cell Microbiol.10.1111/cmi.12755 (2017). [DOI] [PMC free article] [PubMed]
- 112.Sant’Anna MR, et al. Colonisation resistance in the sand fly gut: Leishmania protects Lutzomyia longipalpis from bacterial infection. Parasit. Vectors. 2014;7:329. doi: 10.1186/1756-3305-7-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Faucher B, et al. Presence of sandflies infected with Leishmania infantum and Massilia virus in the Marseille urban area. Clin. Microbiol Infect. 2014;20:O340–O343. doi: 10.1111/1469-0691.12404. [DOI] [PubMed] [Google Scholar]
- 114.Crompton PD, et al. Malaria immunity in man and mosquito: Insights into unsolved mysteries of a deadly infectious disease. Annu. Rev. Immunol. 2014;32:157–187. doi: 10.1146/annurev-immunol-032713-120220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vargas V, Cime-Castillo J, Lanz-Mendoza H. Immune priming with inactive dengue virus during the larval stage of Aedes aegypti protects against the infection in adult mosquitoes. Sci. Rep. 2020;10:6723. doi: 10.1038/s41598-020-63402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cecilio, P., Oliveira, F. & Cordeiro-da-Silva, A. Vaccines for Human Leishmaniasis: Where Do We Stand and What Is Still Missing? Leishmaniases as Re-emerging Diseases (ed. Afrin, F.) (IntechOpen, 2018).
- 117.Cecilio P, et al. Engineering a vector-based pan-Leishmania vaccine for humans: proof of principle. Sci. Rep. 2020;10:18653. doi: 10.1038/s41598-020-75410-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cecilio P, et al. Pre-clinical antigenicity studies of an innovative multivalent vaccine for human visceral Leishmaniasis. PLoS Negl. Trop. Dis. 2017;11:e0005951. doi: 10.1371/journal.pntd.0005951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fernandez, L. et al. Protective efficacy in a Hamster model of a multivalent vaccine for human visceral Leishmaniasis (MuLeVaClin) consisting of the KMP11, LEISH-F3+, and LJL143 antigens in virosomes, plus GLA-SE adjuvant. Microorganisms10.3390/microorganisms9112253 (2021). [DOI] [PMC free article] [PubMed]
- 120.Chagas AC, et al. Lundep, a sand fly salivary endonuclease increases Leishmania parasite survival in neutrophils and inhibits XIIa contact activation in human plasma. PLoS Pathog. 2014;10:e1003923. doi: 10.1371/journal.ppat.1003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martin-Martin I, et al. Immunity to LuloHya and Lundep, the salivary spreading factors from Lutzomyia longipalpis, protects against Leishmania major infection. PLoS Pathog. 2018;14:e1007006. doi: 10.1371/journal.ppat.1007006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Peters NC, et al. Vector transmission of Leishmania abrogates vaccine-induced protective immunity. PLoS Pathog. 2009;5:e1000484. doi: 10.1371/journal.ppat.1000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cotton JA. The expanding world of human Leishmaniasis. Trends Parasitol. 2017;33:341–344. doi: 10.1016/j.pt.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 124.Desbois N, Pratlong F, Quist D, Dedet JP. Leishmania (Leishmania) martiniquensis n. sp. (Kinetoplastida: Trypanosomatidae), description of the parasite responsible for cutaneous Leishmaniasis in Martinique Island (French West Indies) Parasite. 2014;21:12. doi: 10.1051/parasite/2014011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jariyapan N, et al. Leishmania (Mundinia) orientalis n. sp. (Trypanosomatidae), a parasite from Thailand responsible for localised cutaneous Leishmaniasis. Parasit. Vectors. 2018;11:351. doi: 10.1186/s13071-018-2908-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Olivo Freites C, et al. First case of diffuse Leishmaniasis associated with leishmania panamensis. Open Forum Infect. Dis. 2018;5:ofy281. doi: 10.1093/ofid/ofy281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pothirat T, et al. First isolation of Leishmania from Northern Thailand: Case report, identification as Leishmania martiniquensis and phylogenetic position within the Leishmania enriettii complex. PLoS Negl. Trop. Dis. 2014;8:e3339. doi: 10.1371/journal.pntd.0003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roque AL, Jansen AM. Wild and synanthropic reservoirs of Leishmania species in the Americas. Int. J. Parasitol. Parasites Wildl. 2014;3:251–262. doi: 10.1016/j.ijppaw.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Espinosa OA, Serrano MG, Camargo EP, Teixeira MMG, Shaw JJ. An appraisal of the taxonomy and nomenclature of trypanosomatids presently classified as Leishmania and Endotrypanum. Parasitology. 2018;145:430–442. doi: 10.1017/S0031182016002092. [DOI] [PubMed] [Google Scholar]
- 130.Dillon, R. Introduction to sand flies: life cycle, http://pcwww.liv.ac.uk/leishmania/life_cycle__habitats.htm (2008).
- 131.Martin-Martin I, Aryan A, Meneses C, Adelman ZN, Calvo E. Optimization of sand fly embryo microinjection for gene editing by CRISPR/Cas9. PLoS Negl. Trop. Dis. 2018;12:e0006769. doi: 10.1371/journal.pntd.0006769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.