Abstract
The pharmacokinetic properties of oral and intravenous artesunate (2 mg/kg of body weight) were studied in 19 adult patients with acute uncomplicated Plasmodium falciparum malaria by using a randomized crossover design. A sensitive bioassay was used to measure the antimalarial activity in plasma which results from artesunate and its principal metabolite, dihydroartemisinin. The oral study was repeated with 15 patients during convalescence. The mean absolute oral bioavailability of the antimalarial agent in patients with acute malaria was 61% (95% confidence interval [CI], 52 to 70%). The absorption and elimination of oral artesunate were rapid, with a mean elimination half-life of antimalarial activity of 43 min (95% CI, 33 to 53 min). Following oral administration to patients with acute falciparum malaria, peak antimalarial activity in plasma and the area under the plasma concentration-time curve were approximately double those during convalescence and the apparent volume of distribution and clearance were approximately half those during convalescence (P ≤ 0.005). Acute malaria is associated with a significant reduction in the clearance of artesunate-associated antimalarial activity.
Artemisinin (qinghaosu) and its derivatives are a major advance in antimalarial treatment (8). These drugs are increasingly used in southeast Asia for the treatment of multidrug-resistant Plasmodium falciparum malaria (2, 8, 15, 22). Artesunate, the most widely available of the artemisinin-related compounds, is a hemisuccinate derivative of dihydroartemisinin (DHA). It may be given parenterally, intravenously, intramuscularly, orally, or rectally. Oral artesunate is used either alone or in combination, usually with mefloquine (15). Despite considerable use in areas where malaria is endemic, there are relatively few data on the pharmacokinetics of artesunate in the treatment of malaria (2–4, 6, 9, 13, 21, 27). There are concerns that the various artesunate formulations may have different bioavailabilities and that the development of resistance will be accelerated if suboptimal doses are used (13, 24). Optimization of dosing recommendations is also important because of evidence that high doses of parenteral artemisinin derivatives (artemether, arteether) are neurotoxic in experimental mammals (16).
Oral artesunate and artemether, but not artemisinin, are hydrolyzed rapidly back to the common metabolite DHA, which is intrinsically more active as an antimalarial agent. Oral artesunate may be considered mainly a prodrug for DHA, as the metabolite is the main contributor to overall antimalarial activity (2, 6). Thus, in order to compare different formulations of these drugs accurately and to guide the accurate choice of compound, the bioavailability of the antimalarial agent must be assessed.
Chemical methods for the assay of DHA and the related derivatives have a limit of accurate quantitation above the range of concentrations which provide significant antimalarial effect. High-performance liquid chromatography (HPLC) with electrochemical detection (ECD) (14) is considered the “gold standard,” but this method is difficult, time-consuming, and expensive. HPLC methods with UV detection and pre- or postcolumn derivatization are simpler but have limits of detection some 10 times higher than the 50% inhibitory concentrations (IC50s) for antimalarial activity. Bioassay gives an alternative and considerably more sensitive measure and provides important clinical pharmacodynamic information. However, it does not distinguish between parent drugs and their active metabolites (20). We have used the sensitive bioassay, supplemented by HPLC-ECD, to assess the bioavailability and disposition of oral artesunate during acute uncomplicated falciparum malaria and during convalescence.
MATERIALS AND METHODS
Patients.
This study was conducted in Paholpolpayuhasena Hospital, Kanchanaburi, western Thailand, in 1993. Nonpregnant adults (age, >14 years) hospitalized with uncomplicated acute P. falciparum malaria (26) were included in the study, provided that they gave fully informed consent and had not received previous treatment with an artemisinin derivative. Pretreatment with quinine was checked by a urine dipstick screening method (19). The study was approved by the ethical and scientific review subcommittee of the Royal Thai Government Ministry of Public Health.
Clinical procedures.
On admission the patients were weighed; a full clinical examination was conducted; and venous hematocrit, urea and electrolytes, creatinine, liver enzymes, glucose, and lactate levels were measured. Samples for thick and thin blood smears were taken, and quantitative parasite counts were recorded.
Drug and sampling regimens.
Patients were initially randomized to receive either oral or intravenous artesunate at a dose of 2 mg/kg of body weight. The parenteral drug was dispensed as artesunic acid powder at 60 mg per ampoule (Guilin No. 2 Factory, Guangxi, People's Republic of China) and was dissolved in 1 ml of 5% sodium bicarbonate (to form sodium artesunate) and was then diluted to 5 ml in 5% dextrose and given by intravenous bolus injection. The 50-mg artesunate tablets (Guilin No. 1 Factory; Guangxi, People's Republic of China) were crushed, dissolved in water to provide the weight-adjusted dose (within ±2.5 mg), and immediately given to the patient. Blood samples were taken through an indwelling catheter in a forearm vein at 0, 5, 15, 30, 45, 60, 90, and 120 min and then at 3, 4, 6, 8, 12, 18, and 24 h following drug administration. A second dose of artesunate (2 mg/kg) was then given by the opposite route, i.e., if oral administration was given first, then intravenous administration was given second. Blood samples were again taken at the same time intervals at which they were taken on the first day. On day 3, mefloquine (25 mg/kg; Lariam; Roche) was given to complete the treatment. Vital signs were recorded every 4 h, and hematocrit and parasitemia levels were measured every 6 h until parasite clearance (defined as the first negative thick film, i.e., no parasites seen, after the counting of 200 white blood cells). Following recovery and discharge from hospital, the patients were asked to return for a convalescent-phase study. The hematocrit and blood film for malaria parasites were checked, and, provided the patient was fully recovered and blood smear negative for malaria parasites, the same oral artesunate dose (2 mg/kg) was readministered, followed by the same regimen of blood sampling described above.
Drug assays.
Immediately after they were taken, the blood samples were centrifuged and the plasma was stored at −50°C for up to 1 month and then at −80°C for ≤48 months until assay. Antimalarial activity in plasma was measured, as described previously, by an in vitro bioassay for P. falciparum in which antimalarial activity is expressed as DHA equivalents (20). The lower limit of quantitation of the bioassay was 2.5 ng/ml, and interassay coefficients of variation were 9 to 13% for DHA concentrations in the range from 5 to 50 ng/ml. Dilutions were used for samples with concentrations of >100 ng/ml. Plasma samples from three patients (six data series) were also analyzed at the National Centre for Drug Research, Universiti Sains Malaysia, by HPLC with ECD in the reductive mode for separate quantification of artesunate and DHA (14). The lower limit of detection of HPLC-ECD was 4 ng/ml for both artesunate and DHA, and interassay coefficients of variation were 3.1% for artesunate and 5.9% for DHA at concentrations of 30 and 60 ng/ml, respectively.
Pharmacokinetic and statistical analysis.
Open one- and two-compartment models were fitted to the plasma concentration-time data, and standard pharmacokinetic parameters were derived. Curve fitting was performed with WinNonlin (User's guide for WinNonlin; Scientific Consulting, Inc., Cary, N.C.), which is a weighted, iterative, nonlinear regression procedure. Compartmental models were chosen on the basis of the Akaike Information Criterion (AIC) and standard pharmacokinetic equations applied (User's guide for WinNonlin; Scientific Consulting, Inc.). The area under the curve (AUC) from 0 to 24 h (AUC0–24) was calculated by using the linear trapezoidal rule. Clearance (CL) was calculated from the model-independent equation CL/f = dose/AUC0–24, where f is the fraction of the oral dose that is absorbed, and bioavailability was calculated from the equation (AUC0–24oral × dosei.v.)/(AUC0–24i.v. × doseoral), where oral is oral administration and i.v. is intravenous administration.
It was assumed that artesunate was completely converted to DHA (i.e., there was no other significant route of artesunate elimination) for determination of the dose in pharmacokinetic analysis of HPLC data for DHA. Visual examination of frequency plots and, if necessary, the Shapiro-Wilks test were used to determine the appropriateness of using parametric statistical tests (Student's t test) or nonparametric statistical tests (Mann-Whitney and Wilcoxon signed rank tests). Correlations were assessed by using Pearson's and Spearman's correlation coefficients. Analyses were performed by using SPSS 8.0 (SPSS Inc., Chicago, Ill.).
RESULTS
Clinical responses.
Twenty adult patients (17 males and 3 females) hospitalized with uncomplicated falciparum malaria were enrolled in the study. One patient was excluded from the subsequent analysis because insufficient clinical data were recorded. At the time of presentation the patients had been ill for a median of 3 days (range, 1 to 10 days) with fever, headache, anorexia, nausea, and vomiting. The clinical and laboratory findings upon admission are shown in Table 1. The median parasite clearance time was 32 h (range, 8 to 88 h). All patients made a rapid and uncomplicated recovery. There were no significant differences in clinical or laboratory features between those patients who received oral or intravenous artesunate first (P > 0.04 for all comparisons). Two patients vomited after administration of the oral dose during the acute phase, at 3 and 0.75 h, respectively, after their individual maximum concentrations in serum (Cmaxs) had been reached. Fifteen patients returned for the convalescent-phase oral dose study a median of 7 days (range 5 to 31 days) after initial admission. No patient had malaria parasites on thick films at follow-up. The characteristics of the 15 patients who returned for follow-up did not differ from those of the 19 patients studied during the acute phase (P > 0.05). No adverse effects of the study drug were noted.
TABLE 1.
Clinical and laboratory findings upon admission
| Parameter | Median | Range |
|---|---|---|
| Age (yr) | 27 | 18–41 |
| Wt (kg) | 53 | 41–64 |
| Temp (°C) | 38.3 | 36.5–41.0 |
| Hematocrit (%) | 38 | 21–44 |
| Parasite count (no./μl)a | 24,289b | 832–185,888 |
| Total serum bilirubin concn (μmol/liter)c | 21.1 | 6.8–48.2 |
| Serum creatinine concn (μmol/liter)d | 115 | 97–141 |
| Plasma lactate concn (mmol/liter)e | 2.7 | 1.3–4.4 |
| Serum albumin concn (g/liter)f | 33 | 24–39 |
Counts are (number of asexual parasites/1,000 erythrocytes on thin film × percent hematocrit × 125.6) or (number of asexual parasites/200 white blood cells on thick film × 40), assuming that the peripheral white blood cell count is 8.0 × 109/liter.
Geometric mean.
Normal range, 3 to 17 μmol/liter.
Normal range, 70 to 150 μmol/liter.
Normal range, <4 mmol/liter.
Normal range, 35 to 50 g/liter.
Drug measurements.
Three patients were found to have received quinine, one patient was found to have received chloroquine, and one patient was found to have received tetracycline before the study. The bioassay method was able to control for these potential confounders by calibrating the baseline plasma sample as having zero DHA equivalents. In these cases the considerably lower levels of antimalarial activity from quinine, chloroquine, and tetracycline (half-lives, 16 to 18 h, 30 to 60 days, and 8 h, respectively [21, 23]) over the 4 to 8 h during which artesunate concentrations were measurable was assumed not to have changed. The same approach accommodates the antimalarial action of mefloquine (half-life, 2 to 3 weeks [23]) in the convalescent-phase study samples. The only other oral drugs taken by the patients immediately before or during the study were acetaminophen (paracetamol; n = 8), ferrous sulfate (n = 5), folic acid (n = 3), chlorpheniramine (n = 3), vitamins B1, B6, and B12 (n = 2), metoclopramide (n = 2), primaquine (n = 1), mebendazole (n = 1), and thiabendazole (n = 1). None of these drugs are known to interact with artesunate.
Pharmacokinetics. (i) Models.
An open two-compartment model with first-order kinetics gave a good fit for 17 of the data sets for bioassay with intravenous administration, with a mean AIC of 147 (95% confidence interval [CI], 137 to 157) (Fig. 1; Table 2). Fitting of the one-compartment models to these data sets gave significantly higher mean AIC values (190 [95% CI, 182 to 198]; P < 0.0001). The two remaining data sets, for bioassay with intravenous administration and HPLC with intravenous administration, could be modeled only with a one-compartment model, with AIC values of ≤160 and ≤205, respectively. An open one-compartment model with first-order absorption and elimination provided a good fit to the plasma concentration-time data sets for bioassay following oral administration (Table 3) and the acute-phase oral and convalescent-phase data sets for the HPLC assay (median AIC, 143; range, 86 to 180). No significant differences were found between the pharmacokinetic parameters if artesunate was given intravenously first or second (P > 0.05 for all comparisons).
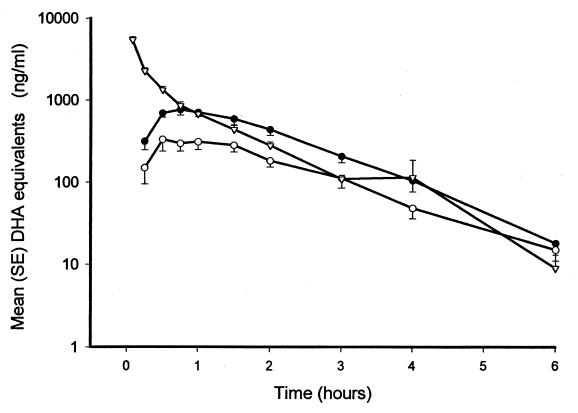
FIG. 1.
Mean (standard error [SE]) log antimalarial activity in DHA equivalents following acute-phase intravenous (▿), acute-phase oral (●), and convalescent-phase oral (○) artesunate administration in patients with acute falciparum malaria.
TABLE 2.
Means and 95% CIs or medians and ranges for pharmacokinetic variables for acute-phase intravenous administrationa
| Variable | Value for acute-phase intravenous administrationb |
|---|---|
| Cmax (ng/ml) | 8,240 (2,188–70,763)c |
| k10 (/h)d | 3.15 (1.08–20.69)c |
| k12 (/h)d | 3.12 (0.61–17.03)c |
| k21 (/h)d | 2.37 (2.02–2.72) |
| t1/2 (h) | 0.73 (0.62–0.83) |
| α (h−1)d | 7.24 (2.64–38.84)c |
| β (h−1)d | 1.01 (0.88–1.11) |
| MRT (h−1) | 0.76 (0.64–0.88) |
| Vc (liter/kg)d | 0.27 (0.20–0.34) |
| VSS (liter/kg) | 0.61 (0.50–0.72) |
| CL (1/kg/h) | 0.83 (0.70–0.96) |
| AUC0–24 (ng · h/ml) | 3,013 (2,482–3,544) |
Bioassay results are in DHA equivalents (n = 19 subjects). Abbreviations: Cmax, predicted concentration at time zero; k10, elimination rate constant; k12, rate constant, central to peripheral compartment; k21, rate constant, peripheral to central compartment; t1/2, half-life (0.693/β and 0.693/k10 for two- and one-compartment models, respectively); α, alpha rate constant; β, beta rate constant; MRT, mean residence time; Vc, volume of distribution of central compartment per kilogram of body weight); VSS, volume of distribution at steady state per kilogram of body weight; CL, clearance per kilogram of body weight; AUC0–24, area under the curve from 0 to 24 h. Values are means (95% CIs) unless indicated otherwise.
To convert DHA equivalents in nanograms per milliliter to nanomoles per liter, multiply by 3.517.
Values are medians (ranges).
Refers only to data from the 17 two-compartmental models; the remaining variables refer to the 19 one- and two-compartmental models combined.
TABLE 3.
Means and 95% CIs or medians and ranges for pharmacokinetic variables for acute- and convalescent-phase oral administrationa
| Variable | Acute phaseb | Convalescent phaseb | P value |
|---|---|---|---|
| No. of subjects | 19 | 15 | |
| AIC | 158 (148–168) | 130 (118–142) | |
| Tmax (h) | 0.75 (0.50–4.00)c | 1.00 (0.50–4.00)c | NS |
| Cmax (ng/ml) | 1,021 (775–1267) | 546 (379–715) | 0.013 |
| Tlag (h) | 0.30 (0.16–0.44) | 0.36 (0.18–0.54) | NS |
| t1/2 (h) | 0.71 (0.55–0.87) | 0.84 (0.49–1.09) | NS |
| k01 (h−1) | 1.86 (0.80–20.87)c | 3.44 (0.60–29.52)c | NS |
| k10 (/h−1) | 1.13d (0.96–1.30) | 1.08d (0.78–1.38) | NS |
| V/f (liters/kg) | 1.33d (1.02–1.64) | 3.15d (2.02–4.28) | 0.005 |
| CL/f (liters/kg/h) | 1.38d (1.03–1.73) | 2.54d (2.10–2.98) | 0.003 |
| AUC0–24 (ng · h/ml) | 1,738d (1,412–2,064) | 886d (733–1,039) | 0.0005 |
Bioassay results are in DHA equivalents. Abbreviations are the same as defined in footnote a of Table 2 and as follows: AIC, Akaike Information Criterion; Tmax, observed time to Cmax; Cmax, maximum observed concentration; Tlag, absorption lag time; k01, absorption rate constant; V/f, total apparent volume of distribution per kilogram of body weight; f, fraction of the oral drug that is absorbed; NS, not significant. Values are means (95% CIs) unless indicated otherwise.
To convert DHA equivalents in nanograms to milliliter to nanomoles per liter, multiply by 3.517.
Values are medians (ranges).
Significantly (P < 0.01) different from mean for acute-phase intravenous administration (Table 2). For oral and convalescent-phase volume of distribution data compared with the estimated steady-state volume of distribution data, P was not significant P > 0.02.
(ii) Absorption.
Oral artesunate was absorbed rapidly, reaching peak antimalarial concentrations in a median of 0.75 h (range 0.5 to 4.0 h) and 1.00 h (range, 0.5 to 4.00 h) for acute- and convalescent-phase oral doses, respectively (P = 0.9). Cmaxs ranged between 268 and 2,506 ng of DHA equivalents per ml (mean, 1,021 ng/ml) after acute-phase oral administration but ranged between 137 and 1,040 ng/ml (mean, 546 ng/ml) after administration during the convalescent phase (Table 3). Antimalarial activity 5 min after intravenous injection in patients with acute malaria was considerably higher, ranging between 2,809 and 9,757 ng/ml (median, 5,100 ng/ml) (Fig. 1 and 2; Tables 2 and 3). The calculated mean absolute antimalarial bioavailability of the acute oral dose was 61% (95% CI, 52 to 70%; range, 30 to 104%).
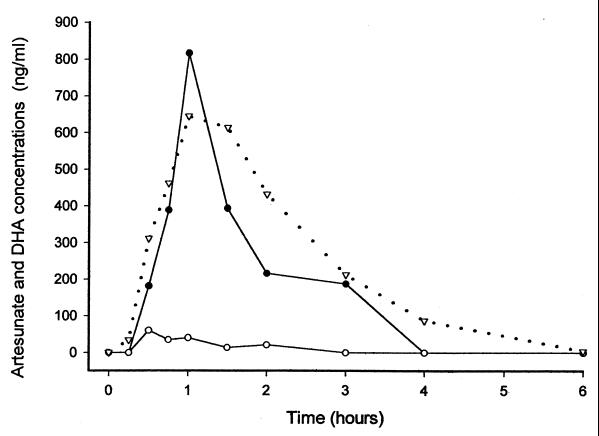
FIG. 2.
Relationship between total plasma antimalarial activity by bioassay (▿) in DHA equivalents and concentrations of artesunate (○) and DHA (●) in plasma measured by HPLC-ECD following acute-phase oral artesunate administration for one patient.
(iii) Disposition.
The mean elimination half-lives of antimalarial activity were 44 min (95% CI, (38 to 50 min), 43 min (95% CI, 33 to 53 min) and 50 min (95% CI, 35 to 65 min) for intravenous, acute-phase oral, and convalescent-phase oral administrations, respectively (P > 0.2 for all comparisons).
After intravenous, acute-phase oral, and convalescent-phase oral administrations for one, two, and three patients, respectively, the antimalarial activity had not reached zero 24 h after dosing. Assuming ∼70% in vivo drug protein binding (11), the residual low concentrations (median, 4.0 ng of DHA equivalents per ml [range, 1 to 25 ng/ml]) are just below the current range of in vitro IC50s of DHA for P. falciparum in western Thailand (A. Brockman, personal communication). Among the remaining patients the earliest time at which no antimalarial activity was detected was a median of 8 h (range, 6 to 24 h) for intravenous administration, 8 h (range, 6 to 18 h) for acute-phase oral administration, and 8 h (range, 3 to 14 h) for convalescent-phase oral administration.
The estimated CL of antimalarial activity following acute-phase oral artesunate administration was positively correlated with that following acute-phase intravenous administration (r = 0.65; P = 0.003) but not with that following convalescent-phase oral artesunate administration (P = 0.8). There were no significant relationships between any of the derived pharmacokinetic variables and clinical and laboratory measurements upon admission (P > 0.05).
Acute-phase oral administration gave peak antimalarial activities and AUC0–24 values which were approximately twice as high as those during the convalescent phase and, thus, corresponding lower estimates for apparent volume of distribution and CL that were approximately half those during the convalescent phase (Table 3). The convalescent-phase AUC0–24 was a mean of 61% (95% CI, 40 to 82%) of the acute-phase AUC0–24 after oral administration. The ratios of convalescent-phase oral AUC0–24/acute-phase AUC0–24 after oral administration did not correlate significantly with any clinical or laboratory measurements (P > 0.05). There were no significant differences between acute-phase and convalescent-phase oral treatment regimens in times to Cmax absorption rate constants, and lag times (P ≥ 0.19).
(iv) HPLC data.
The HPLC assays for artesunate and DHA for three patients yielded 12 data sets (Table 4). Although the sample size is small, the data suggest that artesunate is rapidly and largely converted to DHA (Fig. 2). For the three patients the AUC0–24s for DHA after oral administration during the acute phase as a percentage of the AUC0–24 determined by the antimalarial bioassay were 72, 95, and 102%, respectively. For each of these patients the time to Cmax after drug administration was longer for DHA than for artesunate.
TABLE 4.
Summary of results of HPLC assays for artesunate and DHAa
| Variable | Oral administration
|
Convalescent phase
|
Intravenous administration
|
|||
|---|---|---|---|---|---|---|
| Artesunateb | DHAc | Artesunate | DHA | Artesunate | DHA | |
| No. of patients | 3 | 3 | 1 | 1 | 2 | 2 |
| AUC0–24 (ng · h/ml) | 210 (70–667) | 1,334 (1,018–2,673) | 317 | 829 | 1,056 (322–1789) | 3,999 (2,360–5,637) |
| k01 (h−1) | 10.50 (2.55–47.91) | 9.47 (2.36–9.69) | 0.618 | 11.95 | ||
| k10 (h−1) | 1.29 (1.05–1.97) | 1.06 (1.02–1.46) | 0.623 | 0.248 | 3.00 (0.96–5.05) | 1.93 (1.02–2.84) |
| thalf (h) | 0.54 (0.35–0.66) | 0.65 (0.47–0.68) | 1.11 | 2.79 | 0.43 (0.14–0.72) | 0.46 (0.24–0.68) |
| Tlag (h) | 0.19 (0.00–0.25) | 0.22 (0.14–0.45) | 0.27 | 0.25 | ||
| Tmax (h) | 0.50 (0.25–0.50) | 0.75 (0.5–1.0) | 3 | 1 | ||
| Cmax (ng/ml)d | 198 (61–510) | 1,052 (817–2,853) | 98 | 294 | ||
| V (liters/kg) | 7.1 (1.8–31.4) | 1.49 (0.68–1.69) | 8.75 | 7.75 | 3.23 (0.62–5.84) | 0.50 (0.36–0.64) |
| CL (liters/kg/h) | 9.51 (2.99–32.80) | 1.50 (0.75–2.24) | 6.32 | 2.41 | 4.38 (3.19–5.58) | 0.84 (0.66–1.01) |
To convert artesunate in nanograms per milliliter to nanomoles per liter, multiply by 2.601.
To convert DHA in nanograms per milliliter to nanomoles per liter, multiply by 3.517.
Cmax is the observed value for the oral administration data sets and the extrapolated value for the intravenous administration data sets.
DISCUSSION
Oral artesunate is a widely used, very well tolerated, and highly effective antimalarial agent. Its rapid and consistent activity against multidrug-resistant strains of P. falciparum has led to its increasing use in areas such as southeast Asia where there is widespread resistance to other antimalarial drugs (8, 15). Other members of this class, artemisinin, artemether, arteether, and dihydroartemisinin, are also in clinical use. There are several different pharmaceutical formulations of each drug, and they may have significantly different pharmacokinetic properties (13). The present pharmacokinetic data on the original Chinese oral artesunate formulation, which is by far the most widely used formulation and which has been used in most of the clinical trials of artesunate, provide a benchmark against which other formulations and derivatives may be compared.
Comparison of bioactivity and HPLC results suggests that oral artesunate is largely converted to the more active antimalarial metabolite DHA, as has been documented for oral artemether (20). Oral artesunate is absorbed rapidly, with absolute bioavailability averaging 61%, although there is considerable interpatient variation. The coefficient of variation of the antimalarial activity AUC following oral administration was similar in patients with acute malaria (42%) and in patients in the convalescence phase (34%) (Table 3), which indicates that much of the interindividual variation is due to patient factors and not variation in disease severity. The absolute bioavailability of artesunate cannot be compared with those of other artemisinin derivatives as there are no intravenous formulations of these other drugs.
The absolute antimalarial bioavailability of artesunate found in this study is lower than the DHA bioavailability reported recently for Vietnamese adults with uncomplicated falciparum malaria (mean, 82%; 95% CI, 71 to 92%) and vivax malaria (mean, 85%; 95% CI, 68 to 101%) as determined by a less sensitive but reliable HPLC assay with UV detection (3, 4). The bioavailability reported here represents total antimalarial activity, whereas the estimates of Batty et al. (3, 4) represent only the bioavailability of DHA. Assuming that artesunate is entirely converted to DHA, the sum of published AUCs for artesunate and DHA after intravenous and oral administration (3, 4), corrected for their molecular weights, can be used to estimate the corresponding total antimalarial bioavailability. This yields antimalarial bioavailability estimates of 56 and 71% for falciparum and vivax malaria patients, respectively, consistent with the results presented here. The derived pharmacokinetic parameters were also similar in both studies. Four factors may confound comparisons between pharmacokinetic studies of artesunate. First, the calculation of the AUC0–24 after intravenous administration is highly dependent on the extrapolated concentration at time zero, and therefore, errors in the concentration at time zero will have a profound effect on the calculated bioavailability. Indeed, the AUC for the first 15 min after dosing is nearly half (mean, 42%; 95% CI, 37 to 47%) of the total AUC in the first 24 h. Second, disease severity may vary and the patients recruited into this study may have had more severe disease than those described earlier (4), with higher levels of parasitemia and higher serum creatinine and bilirubin concentrations. Third, the study drugs may have different contents. Batty et al. (3, 4) found that 50-mg artesunate tablets (which were from the same source as those used in this study) had a mean artesunate concentration of 44.8 mg (95% CI, 42.8 to 46.7 mg). If this correction is applied to our data, the estimated mean absolute bioavailability of oral artesunate was 68% (95% CI, 58 to 78%). Fourth, different assay methods may give different results.
Vietnamese children who had moderately severe malaria and who were given 3 mg of oral artesunate per kg had lower mean Cmax (664 ng of DHA equivalents per ml) and AUC (1,286 ng · h/ml) values, as determined by the same bioassay, compared with those achieved in the present study. Children may have higher levels of drug clearance than adults (6).
The significantly lower AUC0–24 during the convalescent phase probably did not result from a reduction in absolute oral bioavailability per se; indeed, the opposite would have been more likely as visceral blood flow and intestinal absorption are reduced in patients with acute malaria and should have returned to normal during the convalescent phase (12, 17). The reduction in AUC probably results from expansion in the apparent volume of distribution and improved systemic clearance on recovery with increased presystemic (first-pass), intestinal, and hepatic metabolism. In patients with acute malaria the apparent volume of distribution of protein-bound basic drugs, such as quinine and presumably the artemisinin derivatives, is reduced as a consequence of increased binding to α-1-acid glycoprotein (11, 18). It is not clear whether hepatic artesunate and DHA metabolism is autoinduced, as has been described for artemisinin itself (1, 9). This study cannot distinguish between the pharmacokinetic effects of disease and autoinduction.
Artesunate is readily hydrolyzed to DHA, probably by blood esterases and the hepatic cytochrome P450 3A4, as is the case with the closely related compounds artelinic acid and arteether (7, 25). Artemether is also probably biotransformed by intestinal cytochrome P450 3A4 (M. A. van Agtmael, V. Gupta, and C. J. van Boxtel, Abstr. 38th Intersci. Conf. Antimicrob Agents Chemother., abstr. A-081, p. 26, 1998). If artesunate is similarly metabolized, interindividual variability in intestinal P450 3A4 activity may be important in determining bioavailability. Comparison of bioassay and HPLC results suggests that the majority of antimalarial activity can be explained by DHA, which is cleared predominantly by hepatic biotransformation either to biologically inert glucuronides (such as DHA-glucuronide (with the glucuronide at the 12 position) or to metabolites which lack the peroxide bridge necessary for antimalarial activity (5, 7, 10, 11; K. Ilett, T. M. E. Davis, K. T. Batty, J. L. Maggs, B. K. Park, T. Q. Binh, L. T. A. Thu, N. C. Hung, and G. Edwards, Abstr. 5th Int. ISSX Meet., 1998). DHA clearance may be reduced in patients with malaria as the disease affects the function of a broad range of hepatic and possibly intestinal drug biotransformation pathways (5, 7, 12, 17; Ilett et al., Abstr. 5th Int. ISSX Meet., 1998).
Following artesunate administration, antimalarial activity was eliminated rapidly, with terminal half-lives of approximately 45 min. Despite this, once-daily administration to patients with acute malaria has proved highly effective and gives parasite and fever clearance times equivalent to those achieved with twice-daily administration (15). Transient exposure to parasiticidal concentrations of the drug twice per parasite asexual life cycle are sufficient for an optimum pharmacodynamic effect. However, the considerable interindividual variability in the profile of the concentration of the drug in blood argues in favor of using a dose higher than 2 mg/kg, at least initially, for the treatment of acute uncomplicated falciparum malaria.
ACKNOWLEDGMENTS
We are very grateful to the director and staff of Paholpolpayuhasena Hospital and to Duangsuda Keeratithakul, Maneerat Rasameesoraj, Richard Newton, Kamolrat Silamut, and Julie Simpson for help. Alan Brockman kindly provided the IC50 data.
The bioassay was supported by the U.S. Army Medical Component, Armed Forces Research Institute of Medical Science, Bangkok, Thailand, and the U.S. Army Medical Research and Materiel Command, Fort Detrick, Frederick, Md., and the HPLC assay was supported by the Tropical Diseases Research Programme of the World Health Organization. This study was part of the Wellcome Mahidol University Oxford Tropical Medicine Research Programme funded by The Wellcome Trust of Great Britain.
REFERENCES
- 1.Ashton M, Hai T N, Sy N D, Huong D X, Huong N V, Nieu N T, Cong L D. Artemisinin pharmacokinetics is time-dependent during repeated oral administration in healthy male adults. Drug Metab Dispos. 1998;26:25–27. [PubMed] [Google Scholar]
- 2.Barradell L B, Fitton A. Artesunate: a review of its pharmacology and therapeutic efficacy in the treatment of malaria. Drugs. 1995;50:714–741. doi: 10.2165/00003495-199550040-00009. [DOI] [PubMed] [Google Scholar]
- 3.Batty K T, Thu L T A, Illett K F, Mai T X, Hung N C, Tien N P, Powell S M, Thien H V, Binh T Q, Kim N V, Davis T M E. A pharmacokinetic and pharmacodynamic study of artesunate in vivax malaria. Am J Trop Med Hyg. 1998;59:823–827. doi: 10.4269/ajtmh.1998.59.823. [DOI] [PubMed] [Google Scholar]
- 4.Batty K T, Thu L T A, Davis T M E, Illett K F, Mai T X, Hung N C, Tien N P, Powell S M, Thien H V, Binh T Q, Kim N V. A pharmacokinetic and pharmacodynamic study of intravenous vs oral artesunate in uncomplicated falciparum malaria. Br J Clin Pharmacol. 1998;45:123–129. doi: 10.1046/j.1365-2125.1998.00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batty K T, Ilett K F, Edwards G, Powell S M, Maggs J L, Park B K, Davis T M E. Assessment of the effect of malaria infection on hepatic microsomes of dihydroartemisinin using rat liver perfusions and microsomes. Br J Pharmacol. 1998;125:159–167. doi: 10.1038/sj.bjp.0702023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bethell D B, Teja-Isavadharm P, Phuong C X, Thuy P T, Mai T T, Thuy T T, Ha N T, Phuong P T, Kyle D, Day N P J, White N J. Pharmacokinetics of oral artesunate in children with moderately severe Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 1997;91:195–198. doi: 10.1016/s0035-9203(97)90222-4. [DOI] [PubMed] [Google Scholar]
- 7.Grace J M, Skanchy D J, Aguilar A J. Metabolism of artelinic acid to dihydroqinghaosu by human liver cytochrome P450 3A. Xenobiotica. 1999;29:703–717. doi: 10.1080/004982599238335. [DOI] [PubMed] [Google Scholar]
- 8.Hien T T, White N J. Qinghaosu. Lancet. 1993;341:603–608. doi: 10.1016/0140-6736(93)90362-k. [DOI] [PubMed] [Google Scholar]
- 9.Khanh N X, de Vries P J, Ha L D, van Boxtel C J, Koopmans R, Kager P A. Declining concentrations of dihydroartemisinin in plasma during 5-day oral treatment with artesunate for falciparum malaria. Antimicrob Agents Chemother. 1999;43:690–692. doi: 10.1128/aac.43.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maggs J L, Madden S, Bishop L P, O'Neill P M, Park B K. The rat biliary metabolites of dihydroartemisinin, an antimalarial endoperoxide. Drug Metab Dispos. 1997;25:1200–1204. [PubMed] [Google Scholar]
- 11.Meshnick S R, Taylor T E, Kamchonwongpaisan S. Artemisinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiol Rev. 1996;60:301–315. doi: 10.1128/mr.60.2.301-315.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molyneux M E, Looareesuwan S, Menzies I S, Grainger S L, Phillips R E, Wattangoon Y, Thompson R P H, Warrell D A. Reduced hepatic blood flow and intestinal malabsorption in severe falciparum malaria. Am J Trop Med Hyg. 1989;40:470–476. doi: 10.4269/ajtmh.1989.40.470. [DOI] [PubMed] [Google Scholar]
- 13.Na-Bangchang K, Karbwang J, Congpoung K, Thanavibul A, Ubalee R. Pharmacokinetic and bioequivalence evaluation of two generic formulations of oral artesunate. Eur J Clin Pharmacol. 1998;53:375–376. doi: 10.1007/s002280050397. [DOI] [PubMed] [Google Scholar]
- 14.Navaratnam V, Mordi M N, Mansor S M. Simultaneous determination of artesunic acid and dihydroartemisinin in blood plasma by high-performance liquid chromatography for application in clinical pharmacological studies. J Chromatogr Ser B Biomed Sci Appl. 1997;692:157–162. doi: 10.1016/s0378-4347(96)00505-1. [DOI] [PubMed] [Google Scholar]
- 15.Nosten F, Luxemburger C, ter Kuile F O, Woodrow C, Pa Eh J, Chongsuphajaisiddhi T, White N J. Treatment of multidrug resistant falciparum malaria with a 3 day artesunate-mefloquine combination. J Infect Dis. 1994;170:971–977. doi: 10.1093/infdis/170.4.971. [DOI] [PubMed] [Google Scholar]
- 16.Petras J M, Kyle D E, Gettayacamin M, Young G D, Bauman R A, Webster H K, Corcoran K D, Peggins J O, Vane M A, Brewer T G. Arteether: risks of two week administration in Macaca mulatta. Am J Trop Med Hyg. 1997;56:390–396. doi: 10.4269/ajtmh.1997.56.390. [DOI] [PubMed] [Google Scholar]
- 17.Pukrittayakamee S, Looareesuwan S, Keeratithakul D, Davis T M E, Teja Isavadharm P, Nagachinta B, Weber A, Smith A, Kyle D, White N J. A study of the factors affecting the metabolic clearance of quinine in malaria. Eur J Clin Pharm. 1997;52:487–493. doi: 10.1007/s002280050323. [DOI] [PubMed] [Google Scholar]
- 18.Silamut K, Molunto P, Ho M, Davis T M E, White N J. Alpha 1-acid glycoprotein (orosomucoid) and plasma protein binding of quinine in falciparum malaria. Br J Clin Pharmol. 1991;32:311–315. doi: 10.1111/j.1365-2125.1991.tb03904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silamut K, Hough R, Eggelte T, Pukrittayakamee S, Angus B, White N J. A simple method for assessing quinine pre-treatment in acute malaria. Trans R Soc Trop Med Hyg. 1995;89:665–667. doi: 10.1016/0035-9203(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 20.Teja-Isavadharm P, Nosten F, Kyle D E, Luxemberger C, ter Kuile F, Peggins J O, Brewer T G, White N J. Comparative bioavailability of oral, rectal, and intramuscular artemether in healthy subjects—use of simultaneous measurement by high performance liquid chromatography with electrochemical detection and bioassay. Br J Clin Pharmacol. 1996;42:599–604. doi: 10.1111/j.1365-2125.1996.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 21.White N J. Antimalarial pharmacokinetics and treatment regimens. Br J Clin Pharmacol. 1992;34:1–10. doi: 10.1111/j.1365-2125.1992.tb04100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White N J. Clinical pharmacokinetics and pharmacodynamics of artemisinin and its derivatives. Trans R Soc Trop Med Hyg. 1994;88(Suppl. 1):41–43. doi: 10.1016/0035-9203(94)90471-5. [DOI] [PubMed] [Google Scholar]
- 23.White N J. Malaria. In: Cook G, editor. Manson's tropical diseases. W. B. London, United Kingdom: Saunders Co.; 1996. pp. 1087–1164. [Google Scholar]
- 24.White N J. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother. 1997;41:1413–1422. doi: 10.1128/aac.41.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White N J, van Vugt M, Ezzet F. Clinical pharmacokinetics and pharmacodynamics of artemether—lumefantrine. Clin Pharmacokinet. 1999;37:105–125. doi: 10.2165/00003088-199937020-00002. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organisation, Division of Control of Tropical Diseases. Severe and complicated malaria. Trans R Soc Trop Med Hyg. 1990;84(Suppl. 2):1–65. [PubMed] [Google Scholar]
- 27.Yang S D, Ma J M, Sub J H, Chen D X, Song Z Y. Clinical pharmacokinetics of a new effective anti-malarial artesunate, a qinghaosu derivative. Chin J Clin Pharmacol. 1986;1:106–109. [Google Scholar]