Abstract
Background
Interval cancer (IC) is a critical issue in colorectal cancer (CRC) screening. We identified factors associated with ICs after faecal immunochemical test (FIT) screening and explored the impact of lowering FIT cut-off or shortening screening interval on FIT-ICs in Flanders.
Methods
FIT participants diagnosed with a CRC during 2013–2018 were included. Factors associated with FIT-ICs were identified using logistic regression. Distributions of FIT results among FIT-ICs were examined.
Results
In total, 10,122 screen-detected CRCs and 1534 FIT-ICs were included (FIT-IC proportion of 13%). FIT-ICs occurred more frequently in women (OR 1.58 [95% CI 1.41–1.76]) and ages 70–74 (OR 1.35 [1.14–1.59]). FIT-ICs were more often right-sided (OR 3.53 [2.98–4.20]), advanced stage (stage IV: OR 7.15 [5.76–8.88]), and high grade (poorly/undifferentiated: OR 2.57 [2.08–3.18]). The majority (83–92%) of FIT-ICs would still be missed if FIT cut-off was lowered from 15 to 10 µg Hb/g or screening interval was shortened from 2 to 1 year.
Conclusions
FIT-ICs were more common in women, older age, right-sided location, advanced stage and high grade. In Flanders, lowering FIT cut-off (to 10 µg Hb/g) or shortening screening interval (to 1 year) would have a minimal impact on FIT-ICs.
Subject terms: Cancer screening, Risk factors, Colorectal cancer
Background
Worldwide, colorectal cancer (CRC) accounts for one in every ten cancer cases and deaths. Between 2012 and 2018, the number of patients diagnosed with CRC in Europe increased from 447,000 to 500,000 while the number of those who died from this disease increased from 215,000 to 242,000 [1]. In Flanders (57% of the Belgian population), CRC is the second most common cancer in women and third in men. In 2018, the age-standardised (world standard population) CRC incidence rates for men and women were 33.8/100,000 and 24.1/100,000 person-years, respectively [2].
CRC screening helps to detect precancerous lesions and tumours at an early stage and can therefore reduce CRC-related mortality. Faecal occult blood test is recommended for organised CRC screening by the European guidelines [3]. Guaiac faecal occult blood test (gFOBT) has been shown to reduce CRC-related mortality by 15.0–33.0% [4–6]. In recent years, faecal immunochemical test (FIT) is a more preferred screening test by many CRC screening programmes since it offers a higher sensitivity compared to gFOBT [7]. Among the organised screening programmes that use FIT, each programme implements a different screening strategy: a different FIT cut-off (15–80 µg Hb/g) or screening interval (1-year or 2-year), depending on its desired diagnostic values and capacity of follow-up colonoscopy after a positive FIT [7]. Research is still ongoing to identify the optimal screening strategy for each programme.
The optimisation of a screening programme needs to be approached from different angles. The occurrence of FIT interval cancers (FIT-ICs) is an important quality indicator of any screening programme using FIT. FIT-IC is defined as CRC diagnosed after a negative FIT and before the next recommended examination [3]. The proportion of FIT-ICs ranged from 7 to 51% in previous studies with a FIT cut-off between 10 and 80 µg Hb/g (2-year screening interval) [8–13]. In addition, FIT-ICs have been shown to be associated with more advanced stage, higher grade and more aggressive histotype, resulting in reduced survival compared to screen-detected CRCs [8, 12, 14, 15]. The European guidelines recommend monitoring interval cancers as a parameter of programme effectiveness [3].
Prior research has pointed out several subgroups who are at a higher risk of having FIT-IC such as women [9, 13, 16–19] and older age [16, 20]. These individuals may be disadvantaged when only a single FIT cut-off is used for the whole screening population. Therefore, many studies have advocated individualising FIT usage in CRC screening to increase equity across subgroups and improve the performance of the screening programmes [13, 21–23]. Gender-specific FIT cut-offs have been introduced in several screening programmes to narrow the gap between men and women in the test’s diagnostic performance, especially sensitivity, such as 80 µg Hb/g for men and 40 µg Hb/g for women in Sweden [24, 25], or 70 µg Hb/g for men and 25 µg Hb/g for women in Finland [26]. Shortening screening interval has also been suggested as a possible measure for subgroups with a lower FIT sensitivity [21].
In Flanders, the organised CRC screening programme has been in place since 2013, which offers a free biennial FIT to all individuals in the target population using a centralised invitation procedure. During the study period (2013–2018), the programme used a uniform FIT cut-off of 75 ng Hb/ml (15 µg Hb/g) and a uniform screening interval (2-year) for all screening individuals aged 53–74 years. However, like other CRC screening programmes, the Flemish programme is attempting to determine the optimal screening strategy to optimise its efficacy. The objectives of the current study were to identify factors associated with the risk of having a FIT-IC versus a screen-detected CRC and explore the impact of lowering FIT cut-off or shortening screening interval on reducing FIT-ICs in the context of the Flemish CRC screening programme. These findings can provide valuable information on the directions of personalising CRC screening using FIT for the Flemish screening programme as well as for other countries and regions.
Methods
Flanders and its CRC screening programme
Flanders is the most populated region of Belgium (6.6 million inhabitants, 57% of the Belgian population) [27]. The CRC screening programme in Flanders has been in place since October 2013 and offers a free biennial quantitative FIT (OC-sensor, Eiken Chemical Co, Tokyo, Japan) to all citizens eligible for CRC screening. During the study period, the target screening ages were extended gradually from 56–74 in 2013 to 53–74 in 2018 (up to 50–74 in 2020). People were excluded from the screening invitation list if they had had a stool test in the past 2 years, a virtual colonoscopy in the past 4 years or a complete colonoscopy in the past ten years, had been diagnosed with CRC in the past ten years or had had a colectomy (excluded permanently). The positivity cut-off of FIT was ≥15 µg Hb/g [or 75 ng Hb/ml, conversion formula: µg Hb/g faeces = (ng Hb/ml buffer) × 2 mL buffer/10 mg faeces collected]. In 2018, the response rate of the Flemish CRC screening programme was 51.5% (∼670,000 invitations sent out). The FIT sensitivity values were 72.4% and 86.3%, positive predictive values were 3.7% and 4.1%, and detection rates were 0.17% and 0.19%, respectively, for invasive and in situ cancers [28].
After a positive FIT, patients are advised to undergo a colonoscopy ordered either through their GP or by consulting a gastroenterologist. During the study period, follow-up colonoscopy was not included as part of the population-based CRC screening programme in Flanders and there was also no centralised colonoscopy quality register in Belgium. Data on the performance of a follow-up colonoscopy after a positive FIT, based on the reimbursement data from health insurance companies, was available at the Belgian Cancer Registry and used to create an exclusion list (as detailed above) for the CRC screening programme for the next invitation round [16].
Study population and data sources
The study population included all eligible individuals for CRC screening (53–74 years) who participated in the Flemish CRC screening programme between October 2013 (start of the programme) and December 2018 (the latest year for which all required data were complete) and were subsequently diagnosed with either a screen-detected CRC or FIT-IC in the same period. A screen-detected CRC and FIT-IC were defined by the screening programme as follows:
Screen-detected CRC was defined as a CRC diagnosed after a positive FIT, within 6 months after the first follow-up colonoscopy and before the next recommended FIT invitation (24 months).
FIT-IC was defined as a CRC diagnosed after a negative FIT and before the next recommended FIT invitation (24 months).
Data on individuals’ screening history (FIT result and follow-up colonoscopy) were retrieved from the database of the Flemish Centre for Cancer Detection and were linked with data on tumour characteristics (location, stage and differentiation grade) from the population-based Belgian Cancer Registry. The Belgian Cancer Registry collects information regarding new CRC diagnoses based on obligatory notifications provided by the oncological care programmes and the laboratories for pathological anatomy. Validated data are currently available for Flanders from 2001 to 2018 with an estimated >98% completeness. In the case of multiple lesions, only the most advanced finding was retained (e.g., prioritising invasive lesions over in situ lesions). The applicable TNM edition at the time of diagnosis was used (TNM 7th edition for incidence years 2013–2016 and TNM 8th edition for incidence years 2017–2018) [29, 30]. A combined TNM stage was determined by prioritising pathological staging over clinical staging, except in the presence of clinical distant metastases which were always considered stage IV. Tumour location was classified as right side (from the cecum to the transverse colon), left side (from the splenic flexure to the sigmoid colon) or rectum [8, 9, 18]. Differentiation grade was classified as well-differentiated (grade 1), moderately differentiated (grade 2) and poorly/undifferentiated (grade 3–4) [8, 14, 31, 32].
Statistical analysis
Sample size
All 11,656 FIT participants between October 2013 and December 2018 who were subsequently diagnosed with either a screen-detected CRC (N = 10,122) or FIT-IC (N = 1,534) in the same period were included for all the analyses; except in the analyses regarding tumour location where we only included 10,111 screen-detected CRCs and 1528 FIT-ICs because two CRCs with an overlapping location and 15 CRCs in the right side of the colon but detected with an incomplete colonoscopy were removed.
Missing data
Data on gender, age at FIT screening and cancer diagnosis were known for all study subjects. About 14% of the tumours had an unknown stage, 38% had an unknown location and 47% had an unknown differentiation grade. In such cases, the data providers (oncological care programmes and/or laboratories for pathological anatomy) filled in the variables with an unspecified code or left them blank (although the fields were mandatory in the registration form). In our data analyses, these observations were included under the “unknown” category.
Main analysis
Continuous variables were described with medians (interquartile ranges) and categorical variables were described with numbers (percentages). Logistic regression was used to assess the associations between individuals’ and tumours’ characteristics and the risk of having a FIT-IC versus a screen-detected CRC. Crude and adjusted odds ratios (for age and gender) with 95% confidence intervals were reported. In this study, stage I was used as the reference to enable the comparison with other studies where only stages I–IV were included [8, 10–12, 31]. FIT-IC proportions for different profiles combining individuals’ and tumours’ characteristics were presented. FIT-IC proportion was calculated as the number of FIT-ICs divided by the total number of FIT-ICs and screen-detected CRCs and presented as percentage to enhance comprehension [11, 12, 16]. We also examined the distributions of FIT results among FIT-ICs in the first/second year of the screening interval and by patients’ and tumours’ characteristics to explore the impact of shortening FIT screening interval or lowering FIT cut-off on reducing FIT-ICs. There is discrepancy among guidelines regarding whether to include in situ cancers in the definition of colorectal carcinoma (TNM and Japanese classification systems) or not (European and US classification systems) [3, 30, 33]. To facilitate the comparison of our findings with those of other studies, we present, where it is possible, the results for in situ cancers as a separate group from invasive cancers.
P-values less than 0.05 (two-sided) were considered statistically significant. All analyses were performed with RStudio (version 1.3.1056; RStudio, PBC, Boston, MA).
Privacy and ethics
When participating in the Flemish CRC screening programme, each person fills out a written informed consent stating that personal information can be used for evaluating and improving the screening programme and for scientific research. Data used in the current study relied on recurrent data exchanges between the Flemish Centre for Cancer Detection and Belgian Cancer Registry, for which approval was given by the Belgian Privacy Commission on 17 September 2013 and amended on 2 July 2019, with reference IVC/KSZG/19/236, number 13/091 [34]. Only pseudonymized data were used for this study, and results are reported in an aggregated way. The study protocol conforms to the principles of the Declaration of Helsinki. Our reporting adheres to the STROBE guidelines for observational studies (Supplementary Table 1) [35].
Results
Characteristics of the study population
In total, 11,656 CRCs diagnosed after FIT screening were included, with a FIT-IC proportion of 13%. The number of CRCs decreased gradually each year from 3174 in 2014 to 1524 in 2018. Most of the study subjects were male (64.5%). The median age at FIT screening was 66 years. A large proportion of the tumours were classified as “unknown” for location (38.3%), stage (14.0%) or differentiation grade (46.7%). Among the tumours with known categories, the majority presented in the left side of the colon or rectum (5680/7,188; 79.0%), at stage I or in situ (7184/10,019; 72.0%) and were moderately differentiated (3577/6209; 57.0%) (Table 1).
Table 1.
Characteristics of individuals who participated in the Flemish colorectal cancer screening programme during 2013–2018 and were subsequently diagnosed with either a screen-detected or interval colorectal cancer.
| Characteristics | Number (%) |
|---|---|
| N = 11,656 | |
| Screen-detected CRCs + FIT-ICs per incidence year | |
| 2013 | 143 (1.2%) |
| 2014 | 3174 (27.2%) |
| 2015 | 2687 (23.1%) |
| 2016 | 2192 (18.8%) |
| 2017 | 1936 (16.6%) |
| 2018 | 1524 (13.1%) |
| Gender | |
| Male | 7516 (64.5%) |
| Age at FIT screening | |
| Median (IQR) | 66 (61–70) |
| 53–59 | 2035 (17.5%) |
| 60–69 | 6290 (54.0%) |
| 70–74 | 3331 (28.6%) |
| Tumour location | |
| Right side | 1506 (12.9%) |
| Left side | 2761 (23.7%) |
| Rectum | 2919 (25.0%) |
| Unknown | 4468 (38.3%) |
| Overlap | 2 (~0%) |
| Tumour stage | |
| In situ | 4470 (38.3%) |
| I | 2714 (23.3%) |
| II | 1060 (9.1%) |
| III | 1269 (10.9%) |
| IV | 506 (4.3%) |
| Unknown | 1637 (14.0%) |
| Differentiation grade | |
| Well differentiated | 1752 (15.0%) |
| Moderately differentiated | 3577 (30.7%) |
| Poorly and undifferentiated | 880 (7.5%) |
| Unknown | 5447 (46.7%) |
| Outcome | |
| FIT-ICs | 1534 (13.2%) |
CRC Colorectal cancer, FIT faecal immunochemical test, IC interval cancer, IQR interquartile range.
Factors associated with the risk of having a FIT-IC versus a screen-detected CRC
The risk of having a FIT-IC versus a screen-detected CRC was 1.6 times higher in women vs. men (OR = 1.58 [1.41–1.76]) and 1.4 times higher in people aged 70–74 compared to ages 53–59 (OR = 1.35 [1.14–1.59]). Regarding tumours’ characteristics, the risk of having FIT-IC was 3.5 times higher for tumours in the right side of the colon (OR = 3.53 [2.98–4.20]) and twice higher for those in the rectum (OR = 2.01 [1.72–2.37]) compared to those in the left side; 7.2 times higher for stage IV compared to stage I (OR = 7.15 [5.76–8.88]); and 2.6 times higher for poorly/undifferentiated lesions compared to well-differentiated lesions (OR = 2.57 [2.08–3.18]) (Table 2).
Table 2.
Univariable and multivariable associations between individuals’ and tumours’ characteristics and risk of having an interval cancer versus a screen-detected cancer after screening with a faecal immunochemical test.
| Characteristics | Category | Screen-detected CRC (%) N = 10,122c | Interval CRCs (%) N = 1534c | Crude OR | p-value | aOR (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| Gendera | Male | 6670 (66%) | 846 (55%) | Ref | Ref | ||
| Female | 3452 (34%) | 688 (45%) | 1.57 (1.41–1.75) | <0.001* | 1.58 (1.41–1.76) | <0.001* | |
| Age at FIT screeninga | 53–59 | 1800 (18%) | 235 (15%) | Ref | Ref | ||
| 60–69 | 5483 (54%) | 807 (53%) | 1.13 (0.97–1.32) | 0.129 | 1.16 (0.99–1.35) | 0.067 | |
| 70–74 | 2839 (28%) | 492 (32%) | 1.33 (1.13–1.57) | <0.001* | 1.35 (1.14–1.59) | <0.001* | |
| Locationb | Left | 2503 (25%) | 258 (17%) | Ref | Ref | ||
| Right | 1077 (11%) | 414 (27%) | 3.73 (3.15–4.43) | <0.001* | 3.53 (2.98–4.20) | <0.001* | |
| Rectum | 2431 (24%) | 488 (32%) | 1.95 (1.66–2.29) | <0.001* | 2.01 (1.72–2.37) | <0.001* | |
| Unknown | 4100 (41%) | 368 (24%) | 0.87 (0.74–1.03) | 0.104 | 0.89 (0.75–1.05) | 0.171 | |
| Stageb | I | 2438 (24%) | 276 (18%) | Ref | Ref | ||
| II | 881 (9%) | 179 (12%) | 1.80 (1.46–2.20) | <0.001* | 1.75 (1.42–2.14) | <0.001* | |
| III | 1031 (10%) | 238 (16%) | 2.04 (1.69–2.46) | <0.001* | 2.04 (1.69–2.47) | <0.001* | |
| IV | 278 (3%) | 228 (15%) | 7.25 (5.85–8.99) | <0.001* | 7.15 (5.76–8.88) | <0.001* | |
| Unknown | 1434 (14%) | 203 (13%) | 1.25 (1.03–1.52) | 0.023* | 1.28 (1.06–1.55) | 0.012* | |
| In situ | 4060 (40%) | 410 (27%) | 0.89 (0.76–1.05) | 0.163 | 0.91 (0.78–1.08) | 0.296 | |
| Differentiation gradeb | Well differentiated | 1551 (15%) | 201 (13%) | Ref | Ref | ||
| Moderately differentiated | 3078 (30%) | 499 (33%) | 1.25 (1.05–1.49) | 0.012* | 1.23 (1.03–1.46) | 0.023* | |
| Poorly/undifferentiated | 657 (6%) | 223 (15%) | 2.62 (2.12–3.24) | <0.001* | 2.57 (2.08–3.18) | <0.001* | |
| Unknown | 4836 (48%) | 611 (40%) | 0.98 (0.83–1.16) | 0.769 | 0.99 (0.83–1.17) | 0.874 |
FIT faecal immunochemical test, CRC colorectal cancer, OR odds ratio, aOR adjusted odds ratio.
aFor the variables “gender” and “age at FIT screening” that were assessed at the time of FIT screening, “age at FIT screening” and “gender” were adjusted for in the multivariable analyses, respectively.
bFor the variables “location”, “stage” and “differentiation grade” that were assessed at the time of cancer diagnosis, age at cancer diagnosis and gender were adjusted for in the multivariable analyses.
cIn the analysis of tumour location, only 10,111 screen-detected CRCs and 1,528 FIT-ICs were included. Two CRCs with overlapping location and 15 CRCs in the right side of the colon but detected with an incomplete colonoscopy were excluded from the analysis.
*Statistically significant (p-value < 0.05).
Figure 1 illustrates the increase in FIT-IC proportion when the tumour stage (the strongest factor associated with an increased risk of having a FIT-IC) was combined with other factors. FIT-IC proportion was 45% when considering stage IV alone but increased to 49% when stage IV was combined with age 70–74; 55–56% when stage IV was combined with female gender or poorly/undifferentiated grade and to 63% when stage IV was combined with right side location.
Fig. 1. Proportions of interval cancer after a faecal immunochemical test (FIT-IC) for different profiles combining individuals' and tumours' characteristics.
Proportions of FIT-IC when tumour stage is combined with gender, age group, tumour location and differentiation grade.
Distribution of FIT results among FIT-ICs in the first/second year of screening interval and by patients’ and tumours’ characteristics
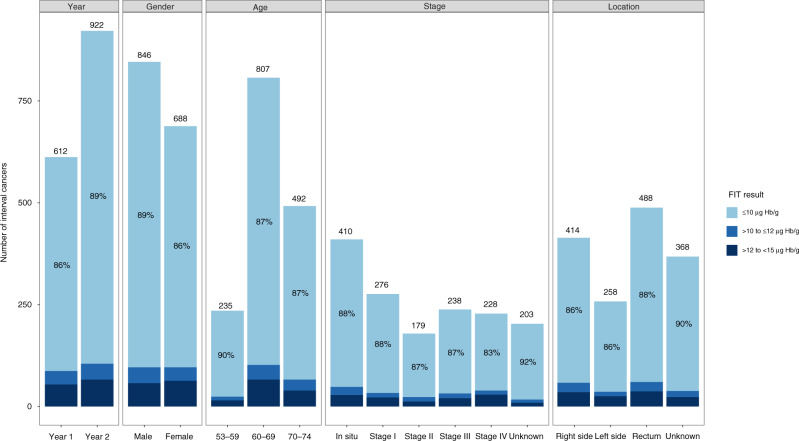
The number of FIT-ICs increased during the 2-year screening interval (Fig. 2). More than sixty percent (922/1534) of FIT-ICs were detected in the second year. According to the distributions of FIT results (Fig. 3 and Supplementary Table 2), 83–92% FIT-ICs in both sexes, all age groups, tumour locations and stages had a low quantitative FIT result of ≤10 µg Hb/g. This majority of FIT-ICs would not be picked up if the FIT cut-off was lowered from 15 to 10 µg Hb/g. Similarly, 89% of the FIT-ICs detected in the second year of the 2-year screening interval also had a low FIT result of ≤10 µg Hb/g, suggesting that most FIT-ICs would still be missed even if the screening interval was shortened from 2 to 1 year, given the current FIT cut-off of 15 µg Hb/g.
Fig. 2. Number of interval cancers after a faecal immunochemical test (FIT-ICs) during the 2-year screening interval.
The numbers of invasive, in situ and total FIT-ICs in the first and second year of the 2-year screening interval (M1–M24: month 1 to month 24 after FIT screening).
Fig. 3. Distributions of quantitative results of faecal immunochemical test (FIT) among FIT-interval cancers (FIT-ICs).
Distributions of FIT quantitative results among FIT-ICs in the first/second year of the screening interval and by genders, age groups, tumour stages and tumour locations.
Discussion
Using data of all screen-detected CRCs and FIT-ICs diagnosed in FIT participants for CRC screening in Flanders during 2013–2018, we identified several factors associated with a higher risk of having a FIT-IC versus a screen-detected CRC. These include female gender, older age, right side and rectum locations, advanced stage and high grade. The majority (83–92%) of FIT-ICs in both the first and second year of the 2-year screening interval and all subgroups had a low FIT result of ≤10 µg Hb/g, indicating a minimal impact of shortening the screening interval from 2 years to 1 year or lowering the FIT cut-off from 15 µg Hb/g (the current cut-off) to 10 µg Hb/g on reducing FIT-ICs.
The FIT-IC proportion in Flanders during 2013–2018 was 13%, which lies within the range of FIT-IC proportion of 7–23% in other screening programmes using a FIT cut-off between 10 and 20 µg Hb/g [8–12]. Our study also supports previous findings that ICs after a negative faecal occult blood test are more common in women, [9, 13, 16–19] older people [16, 20, 36], in the right side of the colon [8, 9, 11, 16–19, 22] or in the rectum [10, 17, 37], at a more advanced stage [8–14, 16, 22, 31, 37] and with a higher grade [14, 31], compared to screen-detected CRCs. Prior literature has proposed several explanations for these associations but definitive conclusions have not been reached.
The fact that women have a higher risk of having FIT-IC than men might be due to lower blood haemoglobin concentrations [17, 20, 38], a longer colonic transit time leading to a greater degree of haemoglobin degradation [39], or a higher proportion of harder-to-detect, right-sided cancers [17, 20, 22, 40]. The last proposed reason might contribute modestly to the explanation since after adjusting for tumour location, we found almost the same association between gender and the risk of having a FIT-IC versus a screen-detected CRC (OR 1.53 [1.36–1.71]), showing 1.53 times higher risk of having a FIT-IC in women, independently of location.
Although a number of studies have shown a lower FIT sensitivity in older people [16, 20, 36], no possible explanations for this association have been given. Fraser et al. (2014) reported increasing faecal haemoglobin concentrations with age (50–69 years) for both men and women in the screening populations for CRC [39]. With a higher faecal haemoglobin concentration and a higher CRC incidence rate, we would normally expect a higher FIT sensitivity in the older age group; it is interesting that studies have found the inverse. We also tested the possibility that CRCs diagnosed in older people presented at a more advanced stage and therefore were missed more by FIT. However, this hypothesis was not supported by our data since the association between the oldest age group (70–74) and the risk of having a FIT-IC versus a screen-detected CRC remained (OR 1.31 [1.11–1.56]) after we adjusted for tumour stage in addition to gender. It is a question of future research to investigate the possible reasons for the lower sensitivity of FIT in the older age group.
Prior research has reported a higher proportion of nonpolypoid (flat) tumours in the right side compared to the left side of the colon [21, 41]. These tumours tend to have a higher risk of malignant transformation and invasiveness at a relatively smaller size [41, 42]. Due to the smaller areas in contact with faeces and sparser vasculature in the mucosa, they bleed less and are less sensitive to FIT [21, 43]. A longer transit time from the right side may also lead to a greater degree of haemoglobin degradation, and therefore more false-negative results occur with right-sided tumours [17, 19, 21, 44]. Selby et al. reported a significantly lower faecal haemoglobin level of the right-sided cancers among FIT screenees compared to that of the left-sided cancers (12.4 versus 60.0 μg Hb/g; p < 0.001) [20].
Regarding a higher risk of being a FIT-IC for tumours in the rectum compared to the left side location, erythrocytes in blood released from rectum lesions may not have been sufficiently haemolysed during a short passage through the rectum, and therefore do not yield a positive result to a FIT [13, 37]. In the same study by Selby et al. the faecal haemoglobin level of the rectal cancers was also significantly lower than that of the left-sided cancers (24.4 versus 60.0 μg Hb/g, p < 0.001) [20]. Another possible explanation is that rectal bleeding more often presents with bright red blood in faeces, which is easier to notice. Screening participants generally have a heightened awareness of signs of blood in their faeces. Once they notice bright red blood in faeces, they tend to consult with primary care promptly [22]. It cannot be ruled out that low FIT effectiveness for tumours in the right colon or rectum may also stem from lesions that grow more rapidly [41, 42, 45].
Compared to screen-detected CRCs, FIT-ICs exhibited a higher grade and aggressive histotype (signet ring cell and mucinous carcinomas) [14]. However, it is still unclear to what degree FIT-ICs are due to FIT false-negative tests or a faster growth pathway of high-grade and aggressive histotype tumours. A recent study by Steel et al. reported a mean time of 10.9 ± 2.9 months from FIT screening to diagnoses of all ICs and 11.6 ± 7.2 months from FIT screening to diagnoses of high-grade and aggressive histotype ICs [14]. The more advanced stage of FIT-ICs compared to screen-detected CRCs might be because FIT is known to be less sensitive for flat, right-sided and poorly or undifferentiated lesions, [14, 46–48] tumours with these characteristics may be missed more often by FIT. Once these tumours are detected later on when symptoms appear, they are already at an advanced stage. A proportion of these ICs may also originate from tumours with more aggressive characteristics and worse behaviour, which actually arose after a true negative FIT [14, 15]. Our findings reinforce the current advice to screening participants not to regard a negative FIT result as a “certificate of health”. Instead, they need to be vigilant and seek primary care promptly when any signs or symptoms appear [22, 49, 50].
To reduce FIT-ICs, previous studies have also suggested personalising FIT cut-offs, for example, using a lower cut-off in the subgroups with a lower FIT sensitivity such as women and older people [13, 21–23]. However, our data showed that in Flanders, lowering FIT cut-off from 15 to 10 μg Hb/g would only have a limited impact on reducing FIT-ICs since more than 83% of FIT-ICs had a low FIT result of ≤10 μg Hb/g across genders, age groups, tumour locations and stages. Although the FIT test used in the Flemish screening programme could theoretically detect up to 3 μg Hb/g faeces, the quantitative results between 3–10 μg Hb/g were considered (quantitatively) unreliable due to large deviations. Therefore, lowering FIT cut-off to below 10 μg Hb/g would not be a suitable option for the test used. Prior research has reported around 75% of FIT-ICs with a low level of haemoglobin (<10 μg Hb/g) and 19.4–44% with an undetectable level (0 μg Hb/g) [11–13]. This implies that the majority of FIT-ICs would still be missed even with a drastic reduction in the FIT cut-off.
Gender-specific cut-offs have also been introduced/piloted in several screening programmes, for example, 40 µg Hb/g for women and 80 µg Hb/g for men in Sweden [24, 25]; or 25 µg Hg/g for women and 70 µg Hg/g for men in Finland [26]. In Flanders, a much lower FIT cut-off of 15 µg Hg/g has already been applied for all screening individuals. Our results suggest that 15 µg Hg/g should be the lowest FIT cut-off that a CRC screening programme should aim for, regardless of the patient’s gender and age. This recommendation is supported by a recent study by Vanaclocha-Espi et al. which found the optimal FIT cut-off of around 15 µg Hg/g for the subgroup of women aged 60–69, which had the lowest FIT sensitivity among the subgroups evaluated (women and men, aged 50–59 and 60–69) [51].
Many studies have also highlighted a substantial increase in colonoscopy demand for only a marginal gain in sensitivity by lowering FIT cut-off [13, 20, 52]. For example, the Scottish Bowel Screening Programme reported that halving the FIT cut-off in their programme from 80 μg Hb/g to 40 μg Hb/g faeces would reduce the FIT-IC proportion from 50.8 to 45.9%, but would increase the number of colonoscopies required by 58.6% [13]. An American screening programme predicted that lowering the FIT cut-off in their programme from 20 μg Hb/g to 10 μg Hb/g would increase the programme’s sensitivity by only 3% (from 76.3 to 79.3%) while increasing the number of positive results per one cancer case detected from 52 to 85 [20].
In agreement with Giorgi Rossi et al. [18], we also observed more FIT-ICs in the second year than the first year of the 2-year screening interval (60% of all ICs). The difference in the number of FIT-ICs between the second and the first year was 310 cases. It seems, at first glance, that shortening the FIT screening interval from 2 to 1 year during the study period might have helped to reduce this number of FIT-ICs [21]. However, 89% of FIT-ICs in the second year were found to have a low FIT result at screening of ≤10 µg Hb/g, suggesting that the majority of ICs would still be missed even when the screening interval was shortened to 1 year. One might also argue that the FIT results were obtained at the time of screening and as tumours progressed between the first and second year, FIT results might get higher and reach the positive cut-off. The proportion of such tumours seemed to be modest since our data showed that 83–92% of FIT-ICs across all tumour stages had a low FIT result of ≤10 µg Hb/g. Thus, in the programmes where a low FIT cut-off of 15 µg Hb/g is implemented, shortening screening interval from 2 to 1 year seems to produce only a marginal impact on reducing FIT-ICs.
Meanwhile, a CRC screening programme should also consider the impact of screening interval on screen-detected CRCs and the related costs. Specifically, when the screening interval of a 2-year programme was shortened to 1 year, the number of prevalent cases detected among individuals entering the screening programme for the first time would be almost similar (same population and same target screening ages). The main difference is that after the first screening round, the population would repeat screening right the next year in the 1-year programme, instead of waiting for 2 years in the 2-year programme. A proportion of the screen-detected incident CRCs in the 2-year programme would be diagnosed 1 year earlier in the 1-year programme. This, however, comes at the cost of having the whole screening population undergo FIT every year instead of every 2 years (Flanders: ~850,000 individuals in 2020) [28], meaning doubling the entire process of screening invitation, FIT provision, result analyses and follow-up with colonoscopy after a positive FIT.
With data from a population-based screening programme of the largest region in Belgium, which used a FIT cut-off within the common range of 10–20 µg Hb/g, our results can be widely generalised to other CRC screening programmes. The large sample size allowed us to stratify our results by multiple participants’ and tumours’ characteristics.
Several limitations need to be acknowledged. Firstly, since data on both FIT participation and cancers were retrieved between October 2013 and December 2018, data on screen-detected CRCs and FIT-ICs following FITs taken in the latest years (2017–2018) might be incomplete, resulting in an underestimation of both screen-detected CRC and FIT-ICs, with an expected larger extent for FIT-ICs. As a result, FIT-IC proportion might be underestimated, especially for the latest screening years. Secondly, the administrative data used in this study contained sizable proportions of tumours with unknown location, stage or differentiation grade. Future research can benefit from using pathology reports to supplement missing or non-specific information. Lastly, data on molecular characteristics of tumours are lacking in the current study. We plan to analyse pathology reports to study the difference in molecular characteristics between screen-detected CRCs and FIT-ICs, especially those at an advanced stage, in the next step.
Conclusions
We identified several factors associated with a higher risk of having a FIT-IC versus a screen-detected CRC, including female gender, older age, right side and rectum locations, advanced stage and high grade. Our findings suggest that 15 µg Hg/g should be the lowest FIT cut-off (OC-sensor) that one CRC screening programme should go for, regardless of individuals’ characteristics. In the Flemish CRC screening programme where a low FIT cut-off (15 µg Hg/g) is implemented, shortening the screening interval from 2 years to 1 year is likely to have only a marginal impact on reducing FIT-ICs. With the current screening strategy, cancers may still appear after a negative FIT, often at a more advanced stage and with a higher grade compared to screen-detected CRCs. It is important to empower and inform the target population that despite a negative FIT result, they should carefully monitor the symptoms of CRC and visit their GPs when symptoms appear.
Supplementary information
Acknowledgements
We acknowledge the Flemish Colorectal Cancer Screening Task Force for functioning as a sounding board.
Author contributions
TNT, MP, HDS, SH and GVH conceived the research idea and designed the study. SJ, HDS and TNT acquired data. TNT performed data analysis and drafted the initial version of the manuscript. All the authors interpreted data; reviewed and revised the manuscript; and approved the final version.
Funding
The authors received no specific funding for this work.
Data availability
The cancer cohort data used and analysed during the study are available from the corresponding author upon reasonable request. The pseudonymized data can be provided within the secured environment of the Belgian Cancer Registry after having been guaranteed that the applicable GDPR regulations are applied.
Code availability
Code used for data analysis is available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
When participating in the Flemish CRC screening programme, each person fills out a written informed consent stating that personal information can be used for evaluating and improving the screening programme and for scientific research. Data used in the current study relied on recurrent data exchanges between the Flemish Centre for Cancer Detection and Belgian Cancer Registry, for which approval was given by the Belgian Privacy Commission on 17 September 2013 and amended on 2 July 2019, with reference IVC/KSZG/19/236, number 13/091. Only pseudonymized data were used for this study, and results are reported in an aggregated way. The study protocol conforms to the principles of the Declaration of Helsinki.
Consent to publish
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01694-2.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Belgian Cancer Registry (2020). Cancer Fact Sheet Colorectal Cancer: Belgium 2018, https://kankerregister.org/media/docs/CancerFactSheets/2018/Cancer_Fact_Sheet_ColorectalCancer_2018.pdf Accessed 30 August 2021.
- 3.Segnan N, Patnick J, von Karsa, L (eds). European guidelines for quality assurance in colorectal cancer screening and diagnosis, 1st edn. (Publications Office of the European Union: Luxembourg, 2010).
- 4.Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–71. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 5.Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–71. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 6.Hardcastle JD, Chamberlain JO, Robinson MHE, Moss SM, Amar SS, Balfour TW, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–7. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 7.Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637–49. doi: 10.1136/gutjnl-2014-309086. [DOI] [PubMed] [Google Scholar]
- 8.Portillo I, Arana-Arri E, Idigoras I, Bilbao I, Martínez-Indart L, Bujanda L, et al. Colorectal and interval cancers of the Colorectal Cancer Screening Program in the Basque Country (Spain) World J Gastroenterol. 2017;23:2731–42. doi: 10.3748/wjg.v23.i15.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zorzi M, Fedato C, Grazzini G, Stocco FC, Banovich F, Bortoli A, et al. High sensitivity of five colorectal screening programmes with faecal immunochemical test in the Veneto Region, Italy. Gut. 2011;60:944–9. doi: 10.1136/gut.2010.223982. [DOI] [PubMed] [Google Scholar]
- 10.Garcia M, Domenech X, Vidal C, Torne E, Mila N, Binefa G, et al. Interval cancers in a population-based screening program for colorectal cancer in catalonia, Spain. Gastroenterol Res Pract. 2015;2015:672410. doi: 10.1155/2015/672410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mlakar DN, Bric TK, Škrjanec AL, Krajc M. Interval cancers after negative immunochemical test compared to screen and non-responders’ detected cancers in Slovenian colorectal cancer screening programme. Radio Oncol. 2018;52:413–21. doi: 10.2478/raon-2018-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Vlugt M, Grobbee EJ, Bossuyt PMM, Bos A, Bongers E, Spijker W, et al. Interval colorectal cancer incidence among subjects undergoing multiple rounds of fecal immunochemical testing. Gastroenterology. 2017;153:439–47 e432. doi: 10.1053/j.gastro.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Digby J, Fraser CG, Carey FA, Lang J, Stanners G, Steele RJ. Interval cancers using a quantitative faecal immunochemical test (FIT) for haemoglobin when colonoscopy capacity is limited. J Med Screen. 2016;23:130–4. doi: 10.1177/0969141315609634. [DOI] [PubMed] [Google Scholar]
- 14.Steel MJ, Bukhari H, Gentile L, Telford J, Schaeffer DF. Colorectal adenocarcinomas diagnosed following a negative faecal immunochemical test show high-risk pathological features in a colon screening programme. Histopathology. 2021;78:710–6. doi: 10.1111/his.14278. [DOI] [PubMed] [Google Scholar]
- 15.Richter JM, Pino MS, Austin TR, Campbell E, Szymonifka J, Russo AL, et al. Genetic mechanisms in interval colon cancers. Dig Dis Sci. 2014;59:2255–63. doi: 10.1007/s10620-014-3134-2. [DOI] [PubMed] [Google Scholar]
- 16.van de Veerdonk W, Hoeck S, Peeters M, Van Hal G, Francart J, De Brabander I. Occurrence and characteristics of faecal immunochemical screen-detected cancers vs non-screen-detected cancers: Results from a Flemish colorectal cancer screening programme. U Eur Gastroenterol J. 2020;8:185–94. doi: 10.1177/2050640619882157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steele RJ, McClements P, Watling C, Libby G, Weller D, Brewster DH, et al. Interval cancers in a FOBT-based colorectal cancer population screening programme: implications for stage, gender and tumour site. Gut. 2012;61:576–81. doi: 10.1136/gutjnl-2011-300535. [DOI] [PubMed] [Google Scholar]
- 18.Giorgi Rossi P, Carretta E, Mangone L, Baracco S, Serraino D, Zorzi M. Incidence of interval cancers in faecal immunochemical test colorectal screening programmes in Italy. J Med Screen. 2017;25:32–39. doi: 10.1177/0969141316686391. [DOI] [PubMed] [Google Scholar]
- 19.Morris EJ, Whitehouse LE, Farrell T, Nickerson C, Thomas JD, Quirke P, et al. A retrospective observational study examining the characteristics and outcomes of tumours diagnosed within and without of the English NHS Bowel Cancer Screening Programme. Br J Cancer. 2012;107:757–64. doi: 10.1038/bjc.2012.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selby K, Jensen CD, Lee JK, Doubeni CA, Schottinger JE, Zhao WK, et al. Influence of varying quantitative fecal immunochemical test positivity thresholds on colorectal cancer detection: a community-based cohort study. Ann Intern Med. 2018;169:439–47. doi: 10.7326/M18-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiu HM, Lee YC, Tu CH, Chen CC, Tseng PH, Liang JT, et al. Association between early stage colon neoplasms and false-negative results from the fecal immunochemical test. Clin Gastroenterol Hepatol. 2013;11:832–8 e831-832. doi: 10.1016/j.cgh.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Steele RJ, Stanners G, Lang J, Brewster DH, Carey FA, Fraser CG. Interval cancers in a national colorectal cancer screening programme. U Eur Gastroenterol J. 2016;4:587–94. doi: 10.1177/2050640615624294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selby K, Levine EH, Doan C, Gies A, Brenner H, Quesenberry C, et al. Effect of Sex, Age, and Positivity Threshold on Fecal Immunochemical Test Accuracy: A Systematic Review and Meta-analysis. Gastroenterology. 2019;157:1494–505. doi: 10.1053/j.gastro.2019.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribbing Wilen H, Saraste D, Blom J. Gender-specific cut-off levels in colorectal cancer screening with fecal immunochemical test: A population-based study of colonoscopy findings and costs. J Med Screen. 2021;28:439–47. doi: 10.1177/09691413211020035. [DOI] [PubMed] [Google Scholar]
- 25.Blom J, Lowbeer C, Elfstrom KM, Sventelius M, Ohman D, Saraste D, et al. Gender-specific cut-offs in colorectal cancer screening with FIT: Increased compliance and equal positivity rate. J Med Screen. 2019;26:92–97. doi: 10.1177/0969141318804843. [DOI] [PubMed] [Google Scholar]
- 26.Sarkeala T, Farkkila M, Anttila A, Hyoty M, Kairaluoma M, Rautio T, et al. Piloting gender-oriented colorectal cancer screening with a faecal immunochemical test: population-based registry study from Finland. BMJ Open. 2021;11:e046667. doi: 10.1136/bmjopen-2020-046667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.STATBEL (2020). Structure of the population, https://statbel.fgov.be/en/themes/population/structure-population Accessed 16 Feb 2021.
- 28.Centre for Cancer Detection (2021). Monitoring Report of the Flemish Colorectal Cancer Screening Programme, https://www.bevolkingsonderzoek.be/sites/default/files/atoms/files/Jaarrapport%202021%20BVO%20naar%20kanker.pdf Accessed 3 December 2021.
- 29.Sobin, LH, Gospodarowicz, MK, Wittekind, C (eds). TNM Classification of Malignant Tumours, 7th edn. Wiley-Blackwell: Chichester, West Sussex, UK, 2009.
- 30.Brierley, JD, Gospodarowicz, MK, Wittekind, C (eds). TNM Classification of Malignant Tumours, 8th edn. John Wiley & Sons, Inc.: Oxford, UK; Hoboken, NJ, 2017.
- 31.Asteria CR, Lucchini G, Guarda L, Ricci P, Pagani M, Boccia L. The detection of interval colorectal cancers following screening by fecal immunochemical test may predict worse outcomes and prompt ethical concerns: a 6-year population-based cohort study in a full district. Eur J Cancer Prev. 2019;28:17–26. doi: 10.1097/CEJ.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 32.Vicentini M, Zorzi M, Bovo E, Mancuso P, Zappa M, Manneschi G, et al. Impact of screening programme using the faecal immunochemical test on stage of colorectal cancer: Results from the IMPATTO study. Int J Cancer. 2019;145:110–21. doi: 10.1002/ijc.32089. [DOI] [PubMed] [Google Scholar]
- 33.Yao T, Shiono S. Differences in the pathological diagnosis of colorectal neoplasia between the East and the West: Present status and future perspectives from Japan. Dig Endosc. 2016;28:306–11. doi: 10.1111/den.12535. [DOI] [PubMed] [Google Scholar]
- 34.Belgian Privacy Commission (2019). IVC/KSZG/19/236, https://www.ehealth.fgov.be/ehealthplatform/file/view/AWvhE2PqnF_Mkwg-mMCV?filename=13-091-n236-bevolkingsonderzoek%20dikkedarmkanker-gewijzigd%20op%202%20juli%202019.pdf Accessed 3 December 2021.
- 35.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–8. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin A, Choi KS, Jun JK, Noh DK, Suh M, Jung KW, et al. Validity of fecal occult blood test in the national cancer screening program, Korea. PLoS ONE. 2013;8:e79292. doi: 10.1371/journal.pone.0079292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tazi MA, Faivre J, Lejeune C, Bolard P, Phelip JM, Benhamiche AM. Interval cancers in a community-based programme of colorectal cancer screening with faecal occult blood test. Eur J Cancer Prev. 1999;8:131–5. doi: 10.1097/00008469-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 38.McDonald PJ, Strachan JA, Digby J, Steele RJ, Fraser CG. Faecal haemoglobin concentrations by gender and age: implications for population-based screening for colorectal cancer. Clin Chem Lab Med. 2011;50:935–40. doi: 10.1515/CCLM.2011.815. [DOI] [PubMed] [Google Scholar]
- 39.Fraser CG, Rubeca T, Rapi S, Chen LS, Chen HH. Faecal haemoglobin concentrations vary with sex and age, but data are not transferable across geography for colorectal cancer screening. Clin Chem Lab Med. 2014;52:1211–6. doi: 10.1515/cclm-2014-0115. [DOI] [PubMed] [Google Scholar]
- 40.Kim SE, Paik HY, Yoon H, Lee JE, Kim N, Sung MK. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol. 2015;21:5167–75. doi: 10.3748/wjg.v21.i17.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiu HM, Lin JT, Chen CC, Lee YC, Liao WC, Liang JT, et al. Prevalence and characteristics of nonpolypoid colorectal neoplasm in an asymptomatic and average-risk Chinese population. Clin Gastroenterol Hepatol. 2009;7:463–70. doi: 10.1016/j.cgh.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 42.Soetikno RM, Kaltenbach T, Rouse RV, Park W, Maheshwari A, Sato T, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027–35. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 43.Doubeni CA, Corley DA, Quinn VP, Jensen CD, Zauber AG, Goodman M, et al. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: a large community-based study. Gut. 2018;67:291–8. doi: 10.1136/gutjnl-2016-312712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doubeni CA, Levin TR. In screening for colorectal cancer, is the FIT right for the right side of the colon? Ann Intern Med. 2018;169:650–1. doi: 10.7326/M18-2444. [DOI] [PubMed] [Google Scholar]
- 45.Launoy G, Smith TC, Duffy SW, Bouvier V. Colorectal cancer mass-screening: estimation of faecal occult blood test sensitivity, taking into account cancer mean sojourn time. Int J Cancer. 1997;73:220–4. doi: 10.1002/(sici)1097-0215(19971009)73:2<220::aid-ijc10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 46.Ciatto S, Martinelli F, Castiglione G, Mantellini P, Rubeca T, Grazzini G, et al. Association of FOBT-assessed faecal Hb content with colonic lesions detected in the Florence screening programme. Br J Cancer. 2007;96:218–21. doi: 10.1038/sj.bjc.6603534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cross AJ, Wooldrage K, Robbins EC, Kralj-Hans I, MacRae E, Piggott C, et al. Faecal immunochemical tests (FIT) versus colonoscopy for surveillance after screening and polypectomy: a diagnostic accuracy and cost-effectiveness study. Gut. 2019;68:1642–52. doi: 10.1136/gutjnl-2018-317297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, et al. Multitarget stool DNA testing for colorectal-cancer screening. N. Engl J Med. 2014;370:1287–97. doi: 10.1056/NEJMoa1311194. [DOI] [PubMed] [Google Scholar]
- 49.Barnett KN, Weller D, Smith S, Steele RJ, Vedsted P, Orbell S, et al. The contribution of a negative colorectal screening test result to symptom appraisal and help-seeking behaviour among patients subsequently diagnosed with an interval colorectal cancer. Health Expect. 2018;21:764–73. doi: 10.1111/hex.12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hallifax R, Lacey M, Bevis P, Borley NR, Brooklyn T, Wheeler JMD. Slipping through the bowel cancer screening programme. Colorectal Dis. 2012;14:844–7. doi: 10.1111/j.1463-1318.2011.02828.x. [DOI] [PubMed] [Google Scholar]
- 51.Vanaclocha-Espi M, Ibanez J, Molina-Barcelo A, Valverde-Roig MJ, Nolasco A, Perez-Riquelme F, et al. Optimal cut-off value for detecting colorectal cancer with fecal immunochemical tests according to age and sex. PLoS ONE. 2021;16:e0254021. doi: 10.1371/journal.pone.0254021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berry E, Miller S, Koch M, Balasubramanian B, Argenbright K, Gupta S. Lower Abnormal Fecal Immunochemical Test Cut-Off Values Improve Detection of Colorectal Cancer in System-Level Screens. Clin Gastroenterol Hepatol. 2020;18:647–53. doi: 10.1016/j.cgh.2019.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The cancer cohort data used and analysed during the study are available from the corresponding author upon reasonable request. The pseudonymized data can be provided within the secured environment of the Belgian Cancer Registry after having been guaranteed that the applicable GDPR regulations are applied.
Code used for data analysis is available from the corresponding author upon reasonable request.