Abstract
Background
The objective of this study was to evaluate the efficacy and safety of induction chemotherapy (ICT), GOFL (gemcitabine, oxaliplatin plus fluorouracil (5-FU)/leucovorin) versus modified FOLFIRINOX (irinotecan, oxaliplatin plus 5-FU/leucovorin), followed by concurrent chemoradiotherapy (CCRT) in locally advanced pancreatic adenocarcinoma (LAPC).
Methods
Chemo-naive patients with measurable LAPC were eligible and randomly assigned to receive biweekly ICT with either mFOLFIRINOX or GOFL for 3 months. Patients without systemic progression would have 5-FU- or gemcitabine-based CCRT (5040 cGy/28 fractions) and were then subjected to surgery or continuation of chemotherapy until treatment failure. The primary endpoint was 9-month progression-free survival (PFS) rate.
Results
Between July 2013 and January 2019, 55 patients were enrolled. After ICT, 21 (77.8%) of 27 patients who received mFOLFIRINOX and 17 (60.7%) of 28 patients who received GOFL completed CCRT. Of them, one and five had per-protocol R0/R1 resection. On intent-to-treat analysis, the 9-month PFS rate, median PFS and overall survival in mFOLFIRINOX and GOFL arms were 30.5% versus 35.9%, 6.6 (95% confidence interval: 5.9–12.5) versus 7.6 months (3.9–12.3) and 19.6 (13.4–22.9) versus 17.9 months (13.4–23.9), respectively. Grade 3–4 neutropenia and diarrhoea during induction mFOLFIRINOX and GOFL were 37.0% versus 21.4% and 14.8% versus 3.6%, respectively.
Conclusion
Induction GOFL and mFOLFIRINOX followed by CCRT provided similar clinical outcomes in LAPC patients.
Clinicaltrial.gov identifier
Subject terms: Pancreatic cancer, Chemotherapy, Radiotherapy
Background
The management of locally advanced pancreatic adenocarcinoma (LAPC) has been challenging due to the heterogeneous spectrum of disease and the lack of consensus among the members of different expertise in the multidisciplinary team [1, 2]. However, cumulative evidence suggested the importance of primary or induction systemic chemotherapy for LAPC, as exemplified by 43.3% of patients who either developed metastatic diseases or died within 3 months after the initiation of upfront fluorouracil (5-FU)-based concurrent chemoradiotherapy (CCRT) in a large randomised phase 3 trial [2, 3].
The use of induction chemotherapy (ICT) to treat existing micro-metastases and to select appropriate patients who are most likely to benefit from CCRT has been extensively evaluated and reviewed [4]. In a randomised phase III LAP07 trial, despite a reduction in the incidence of local progression, the study failed to show the overall survival (OS) benefit of adding CCRT in patients with non-progressing LAPC after ICT with either gemcitabine alone or gemcitabine plus erlotinib [5]. The median OS of intent-to-treat (ITT) population was 12.8 months, while the median OS of those who had secondary randomisation after ICT was 15.2 months (95% confidence interval [CI], 13.9–17.3) in the CCRT group and 16.5 months (95% CI, 14.5–18.5) in the chemotherapy continuation group (p = 0.83). Enthusiasts for CCRT suggested that in the era of more effective systemic therapies and with newer radiotherapy techniques, further trials to evaluate CRT in LAPC are warranted [4–6].
Before the emergence of effective multi-agent combinations, we developed a biweekly triplet regimen consisting of gemcitabine and oxaliplatin plus 5-FU/leucovorin (LV) (GOFL) for advanced pancreatic adenocarcinoma (APC) based on the known synergisms among infusion 5-FU, gemcitabine and oxaliplatin in various GI cancers, including pancreatic cancer [7, 8]. In the phase II part study, the median OS of LAPC patients who had either consolidation or salvage CCRT after GOFL was 15.4 months [8]. Based on that, we further evaluated the efficacy and safety of 3-month ICT with GOFL followed by gemcitabine-based CCRT (Gem-CCRT) for LAPC in a multicentre, phase II study, the Taiwan Cooperative Oncology Group (TCOG) T1204 study [9]. The median OS of the ITT population and those who had CCRT after ICT, per-protocol (PP) population, was 14.5 (95% CI, 11.9–17.1) and 18.3 months (95% CI, 17.1–19.6), respectively, with acceptable toxicity profile.
Lately, both FOLFIRINOX (5-FU/LV, oxaliplatin and irinotecan) and Nab-P + Gem (nab-paclitaxel plus gemcitabine) have demonstrated significant survival benefit against gemcitabine in patients with metastatic pancreatic adenocarcinoma (mPC) and good performance status [10, 11]. Despite the absence of randomised trials, both regimens are also recommended as the primary treatment options for LAPC [12–14]. In a recent cross-continent survey for the preference management on LPAC, 86.9% of responders, mainly pancreatic surgeons from high-volume centres, would “always or often” recommend neo-adjuvant systemic therapy and FOLFIRINOX was the preferred neo-adjuvant regimen of 64.7% responders [15].
Herein, we report the results of a small, randomised phase II study, which aims to select a winner treatment option, gemcitabine-based induction GOFL followed by Gem-CCRT versus gemcitabine-free induction modified FOLFIRINOX followed by 5-FU-CCRT, based on 9-month progression-free survival (PFS) rate in patients with LAPC.
Methods
Patient and study design
This randomised phase II study, the TCOG T2212, was an open-label study conducted in nine TCOG member institutions.
Patients
Eligibility criteria included (1) cyto-/histologically confirmed, LAPC without systemic, including distant lymph node(s), dissemination. Unresectable diseases were defined by either radiographically evident tumour extension to the coeliac axis or superior mesenteric artery, occlusion of the superior mesenteric-portal venous confluence, and invasion or encasement of the aorta, inferior vena cava or of SMV below transverse mesocolon, or intraoperative decision; (2) at least one measurable lesion defined by Response Evaluation Criteria In Solid Tumours version 1.1 (RECIST 1.1) [16]; (3) age 20–70 years old; (4) Eastern Cooperative Oncology Group performance score (ECOG PS) 0–1; (5) adequate organ functions, with neutrophil count ≥1500/mL, platelet count ≥100,000/mL, serum creatinine within institutional upper normal limits (UNLs) or clearance ≥60 mL/min/1.73 m2 (estimated by Cockcroft–Gault formula), bilirubin ≤1.5× UNL and alanine aminotransferase ≤5× UNL; and (6) willingness to give signed informed consent.
Exclusion criteria mainly included (1) preexisting grade 2 or more peripheral neuropathy of any aetiology; (2) pregnant or breastfeeding woman, or those of childbearing age who were unable or not willing to undergo adequate contraception; (3) history of non-curable second malignancy within 5 years, except non-melanoma skin cancer; and (4) uncontrolled intercurrent illness, including chronic diarrhoea that might limited compliance to study requirement. The study was approved by the institutional review board of each participating hospital and of National Health Research Institutes, Taiwan, and conducted in accordance with the International Conference on Harmonization Guideline for Clinical Practice and general ethical principles of the Declaration of Helsinki. All patients provided written informed consent. The study was registered with clinicaltrials.gov identifier: NCT01867892.
Treatment plan
Enrolled patients were stratified by ECOG PS (0 versus 1) and location of the primary tumour (pancreatic head versus body/tail) and then randomly assigned on 1:1 to receive biweekly ICT with either mFOLFIRINOX or GOFL for 3 months. Patients who had responsive or stable disease (SD), as well as those with localised progressive disease, after ICT would have concurrent CCRT at least 4 weeks after the last dose of ICT. Surgical intervention would be evaluated and performed within 4–6 weeks after the completion of CCRT. Patients who had curative resection would receive additional 6 months of postoperative adjuvant chemotherapy. However, patients whose diseases remained unresectable or had non-curative resection would receive assigned ICT chemotherapy till disease progression or unacceptable toxicity. Randomisation was performed at the TCOG coordination centre using a computer-generated procedure.
ICT: modified FOLFIRINOX consisted of intravenous infusion of irinotecan 150 mg/m2 over 90 min followed by oxaliplatin 85 mg/m2 for 2 h and then a 48-h continuous infusion of 5-FU 3000 mg/m2 and LV 150 mg/m2; GOFL consisted of intravenous infusion of gemcitabine 800 mg/m2 over 80 min (10 mg/m2/min, fixed-dose rate [FDR]) followed by oxaliplatin 85 mg/m2 for 2 h and then a 48-h continuous infusion of 5-FU 3000 mg/m2 and LV 150 mg/m2. Both treatments were given biweekly on days 1 and 15 every 28 days per cycle, for three cycles.
CCRT: four or more weeks after the last dose of ICT, patients who had recovered from all ICT-associated adverse events to grade <2 and no radiographic evidence of distant dissemination would receive CCRT. CCRT after induction mFOLFIRINOX consisted of weekly 30 min infusion of 450 mg/m2 5-FU, while CCRT after GOFL consisted of weekly FDR infusion of 400 mg/m2 gemcitabine. The radio-sensitiser was administered 2 h before the scheduled radiation on days 1, 8, 15, 22, 29 and 36. Radiotherapy consisted of 28 fractions of 180 cGy up to 5040 cGy in 6 weeks to the pancreatic tumour and suspicious lymph nodes using intensity-modulated radiotherapy planning. The gross tumour volume was the primary tumour identifiable on computed tomography (CT) scan before ICT. The clinical target volume was defined as the gross tumour volume plus 1.0 cm. The planning target volume was the clinical target volume plus 0.5 cm for daily patient set-up variation. No prophylactic nodal irradiation would be given. In general, a three- to four-field beam arrangement (opposed laterals with posterior with or without anterior) was used.
Evaluations
Baseline evaluations included medical history, physical examination, complete blood count/differential count (CBC/DC), blood chemistry, tumour markers, chest X-ray, and abdominal CT or magnetic resonance imaging. After starting protocol treatment, detailed history, physical examinations and treatment-related adverse events were recorded, and CBC/DC was examined before each session of chemotherapy. Blood chemistry and tumour markers, CEA and CA19-9, were examined every 4 and 8 weeks, respectively. Tumour response assessment imaging study was performed after 8 weeks of ICT, before and 4 weeks after CCRT, and then every 8 weeks until disease progression. Tumour response and adverse events were recorded according to the RECIST 1.1 and National Cancer Institute Common Toxicity Criteria version 4.03, respectively [16, 17]. The quality of life (QoL) was assessed using the EORTC-QLQ-C-30 Questionnaire before treatment, and every 8–10 weeks during the study period.
Statistical considerations
Based on the median PFS of 9.3 months in our previous TCOG 1204 phase II trial, a 9-month PFS rate was selected as the primary endpoint of the current randomised phase II trial [9]. In addition, at the time of this protocol writing, the only prospective trial with available survival data of FOLFIRINOX in LAPC was 15.7 months in a subgroup analysis of the original phase II study of Conroy and colleagues, as compared to the 15.9 months of the LAPC subgroup in GOFL phase II trial [8, 18]. In order to select the best treatment from these two triplet regimens, we used Selection Designs to determine the sample size based on a 9-month PFS rate [19]. The assumption was that one treatment arm might have a higher PFS rate than the other, which could be either mFOLFIRINOX or GOFL; the ratio between the 9-month PFS rate of the two arms is estimated to be 1.22 (0.55/0.45 that was relatively 10% above and below the 0.5 (50%) 9-month PFS rate in the T1204 trial) [9]. With the assuming exponential survival distributions and a correct selection probability of 0.8, the total sample size required for each study arm was 39. With an anticipated 10% dropout rate, 43 patients would be needed per arm. With an estimated 20 accruals per year, the study would complete recruitment within 4.5 years.
Secondary endpoints were objective response rate (ORR), disease control rate (DCR), toxicity, median PFS, OS, failure pattern and QoL. OS was defined as the duration of time from the start of treatment to patients’ death. PFS was defined as the time elapsed between treatment initiation and tumour progression or death from any cause, or censoring at the time of off-study for those treatment discontinuation due to toxicity, withdrawal of consent or data cut-off. The Kaplan–Meier curves for OS, PFS including the hazard ratio and 95% CI were compared between the two groups with the log-rank test. χ2 Tests were used to compare frequencies for categorical data. Fisher’s exact test was employed to test for the differences with sparse cell counts.
All statistical analyses were performed using SAS version 9.4 and R 4.1. In the meeting of December 2018, the Data and Safety Monitoring Committee decided to terminate this study due to significant recruitment lag and the opening of a new TCOG T5217 study that compared the efficacy and safety of mFOLFIRINOX versus gemcitabine, oxaliplatin and o ral S-1/LV, the SLOG regimen, in APC (ClinicalTrials.gov Identifier: NCT03443492). Patient enrolment was terminated in January 2019, but previously enrolled patients were kept on protocol-assigned treatments and followed until disease progression and/or death.
Results
Patients
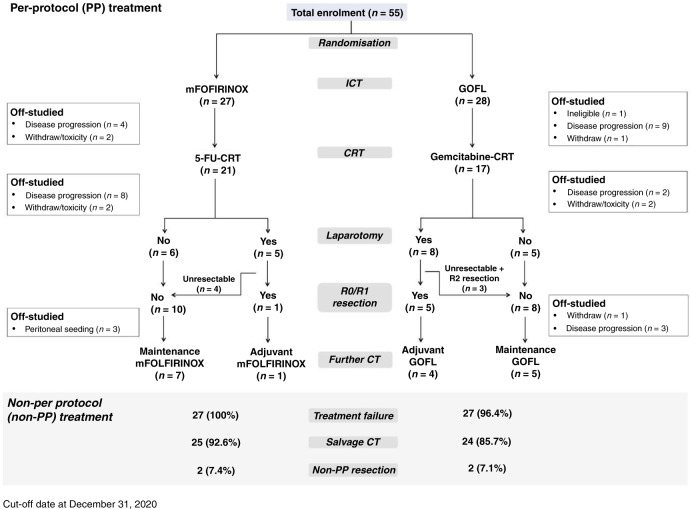
Between July 2013 and January 2019, 55 patients were included, of which 27 patients were randomised to have mFOLFIRINOX and 28 to have GOFL. The baseline characteristics of median age, primary tumour location (head versus body/tail), ECOG PS (0 versus 1) and prior bypass (yes versus no) were well balanced between the study arms, as shown in Table 1. However, significantly more patients in the GOFL arm were female (57.1 versus 22.2%, p = 0.013) and had baseline serum CA19.9 level ≥400 U/mL (60.7 versus 29.6%, p = 0.031) compared with that in the mFOLFIRINOX arm. One patient in the GOFL arm who was found to be ineligible after receiving the first cycle of medication was included in the safety and survival analysis, as shown in the schema of study flow (Fig. 1). Except for one patient in the GOFL arm who had a borderline resectable disease, all the rest 53 eligible patients had unresectable LAPC at study entry.
Table 1.
Clinicopathologic characteristics.
| mFOLFIRINOX (N = 27) | GOFL (N = 28) | P value | |
|---|---|---|---|
| Age | 0.385 | ||
| Mean ± SD | 58.2 ± 9.8 | 56.5 ± 10 | |
| Median | 60 | 59.5 | |
| Gender | 0.013 | ||
| Male | 21 (077.8%) | 12 (042.9%) | |
| Female | 6 (022.2%) | 16 (057.1%) | |
| Performance status (ECOG) | 1.000 | ||
| 0 | 9 (033.3%) | 10 (035.7%) | |
| 1 | 18 (066.7%) | 18 (064.3%) | |
| Location of the primary tumour | 0.790 | ||
| Head | 13 (48.1%) | 15 (53.6%) | |
| Body and tail | 14 (51.9%) | 13 (46.4%) | |
| Tumour clinical stage | 1.000 | ||
| T2–3 | 1 (003.7%) | 3 (0010.7%) | |
| T4 | 26 (096.3%) | 25 (089.3%) | |
| Lymph node | 0.089 | ||
| N0 | 12 (044.4%) | 6 (021.4%) | |
| N1 | 15 (055.6%) | 22 (078.6%) | |
| Baseline CA19-9 | 0.031 | ||
| <400 U/mL | 19 (070.4%) | 11 (039.3%) | |
| ≥400 U/mL | 8 (029.6%) | 17 (060.7%) | |
| Bypass surgery | |||
| Yes | 1 (003.7%) | 3 (010.7%) | 0.611 |
| No | 26 (096.3%) | 25 (089.3%) | |
| Resectability | 1.000 | ||
| Borderline | 0 (000.0%) | 1 (003.6%) | |
| Unresectable | 27 (100.0%) | 27 (096.4%) |
Statistically significant p-values are in bold.
Fig. 1. Schema of study flow.
Flow chart illustrates the number of clinical trial participants randomized to each treatment group.
Treatment delivery
All 55 patients received assigned ICT; the best tumour response was partial response (PR) in 6 (22.2%) and SD in 20 (74.1%) patients with a DCR of 96.3% after induction mFOLFIRINOX, and PR in 4 (14.3%) and SD in 18 (64.3%) patients with a DCR of 78.6% after induction GOFL. Although only 22 (81.5%) and 21 (75%) patients in mFOLFIRINOX and GOFL arms, respectively, received all three cycles of ICT, 21 (77.8%) among them in the mFOLFIRINOX arm and 17 (60.7%) in the GOFL arm proceeded to and completed the full course of 5-FU-CCRT and Gem-CCRT, respectively. After completion of per-protocol ICT-CCRT treatment, the DCR was similar between both arms, 51.9 versus 53.6%. No further responder was observed after CCRT in both arms (Table 2).
Table 2.
Therapeutic efficacies.
| mFOLFIRINOX (N = 27) | GOFL (N = 28) | Total (N = 55) | |
|---|---|---|---|
| Best response to ICT | |||
| Partial response | 6 (22.2%) | 4 (14.3%) | |
| Stable disease | 20 (74.1%) | 18 (64.3%) | |
| Progressive disease | 1 (3.7%) | 6 (21.4%) | |
| Disease control rate | 26 (96.3%) | 22 (78.6%) | |
| Response after CCRT | |||
| Partial response | 6 | 4 | |
| Stable disease | 7 | 11 | |
| Progressive disease | 8 | 2 | |
| Per-protocol R0/R1 resection | 1 (3.7%) | 5 (17.8%) | 6 (10.9%) |
| Median (95% CI) PFS, months | 6.6 (5.9–12.5) | 7.6 (3.9–12.3) | 6.7 (6.2–8.3) |
| 9-month PFS rate | 30.5% | 35.9% | 33.3% |
| Median (95% CI) OS, months | 19.6 (13.4–22.9) | 17.9 (13.4–23.9) | 18.7 (15.8–21.9) |
| 12-month OS rate (%) | 88.9 (69.4–96.3) | 82.1 (62.3–92.2) | 85.5 (73.0–92.4) |
| 24-month OS rate (%) | 29.6 (14.1–47.0) | 31.8 (15.7–49.1) | 30.8 (19.2–43.1) |
Among 54 eligible patients, 6 of 13 patients who underwent laparotomy had per-protocol R0/R1 resection, one (3.7%) after mFOLFIRINOX followed by 5-FU-CCRT and 5 (18.5%) after GOFL followed by Gem-CCRT. Three patients in the GOFL arm had margin-free (R0) resection in histology, while the other three had margin-positive resection (R1). Pathologic complete remission was noted in one patient in the GOFL arm. The assigned ICT regimen was continued as maintenance chemotherapy to 12 patients with non-progressed, unresectable localised disease defined by either post-CCRT imaging in 11 or by laparotomy finding in one and as an adjuvant to five patients with per-protocol R0/R1 resection. The schema of treatment flow is shown in Fig. 1.
Of the 54 eligible patients who had disease progression or intolerable toxicities after assigned treatment, 52 (96.3%) received various subsequent systemic chemotherapies. The most frequently administered second-line chemotherapy in both arms was gemcitabine plus oral S-1-based regimens including gemcitabine plus oral S-1 (GS) in 13 (48.1%), gemcitabine plus oxaliplatin and oral S-1/LV (SLOG) in 5 (18.5%) and nab-P + Gem or S-1 alone in 2 (7.4%) in the mFOLFIRINOX arm, while in the GOFL arm were GS in 6 (22.2%), liposomal irinotecan plus 5-FU/LV (nal-IRI + 5-FU/LV) or SLOG in 5 (18.5%), nab-P + Gem or gemcitabine alone in 2 (7.4%) and mFOLFIRINOX in 1 (Supplementary Fig. 1). Additional four patients underwent non-per-protocol R0/R1 resection after maintenance or salvage treatment. R0 resection was achieved after salvage GS in two patients who failed induction mFOLFIRINOX for poor compliance, while R1 resection was performed after S-1-based CCRT for local progression disease and maintenance S-1 after per-protocol CCRT in each patient in the GOFL arm.
Survival and patterns of relapse
At the cut-off date of December 31, 2020, 2 years after the last patient’s first visit, four patients were alive, two in each arm, while only one patient with GOFL remained progression-free. The median follow-up time was 18.9 months (range 4.1–64.0). On ITT analysis, the median PFS and OS of the entire study population were 6.7 months (95% CI: 6.2–8.3) and 18.7 months (95% CI, 15.8–21.9), respectively (Fig. 2a). The median PFS was 6.6 months (95% CI: 5.9–12.5) in the mFOLFIRINOX arm and 7.6 months (95% CI: 3.9–12.3) in the GOFL arm (p = 0.62, Fig. 2b) with a 9-month PFS rate of 30.5% and 35.9%, respectively, in the corresponding arm (p = 0.70). The median OS was 19.6 months (95% CI: 13.4–22.9) and 17.9 months (95% CI: 13.4–23.9) (p = 0.66, Fig. 2c), respectively (Table 2).
Fig. 2. Kaplan–Meier curves of entire population and different treatment arms.
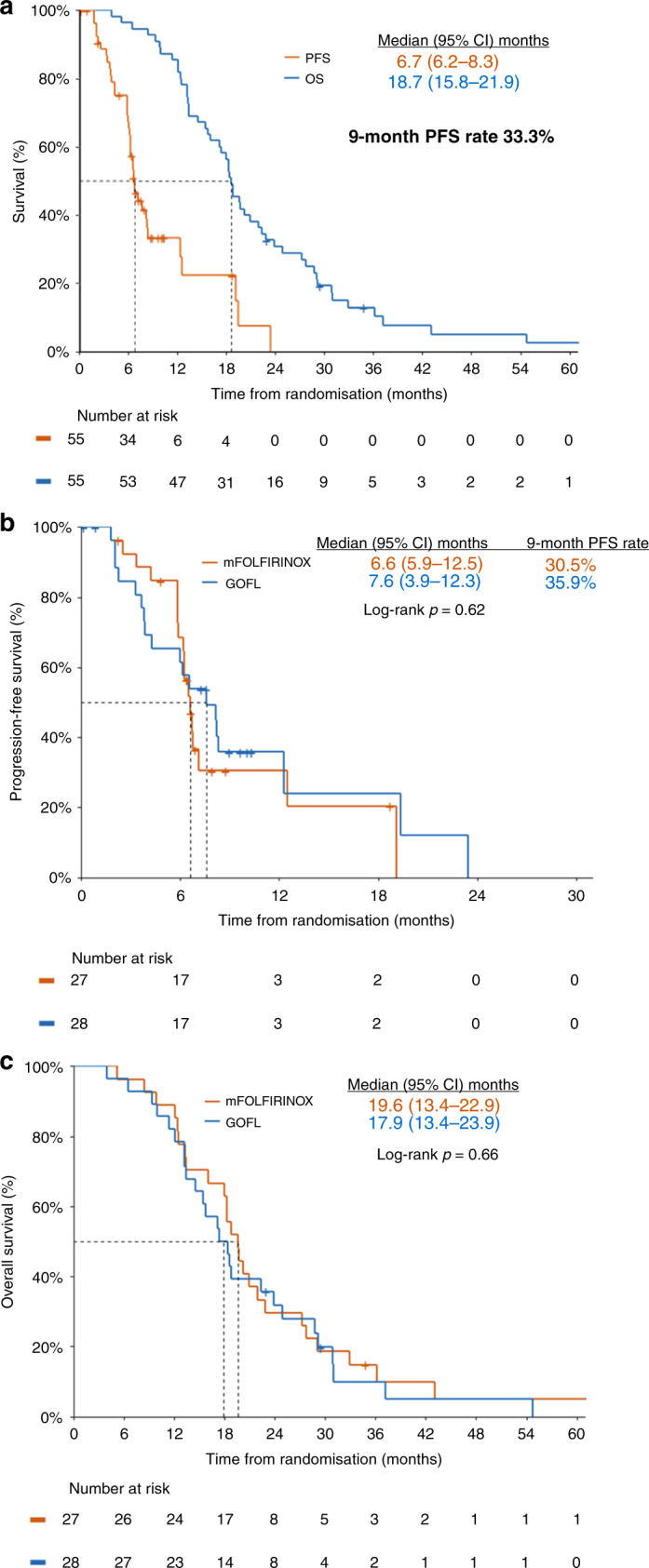
a Progression-free survival and overall survival of the entire population. Progression-free survival (b) Progression-free survival of different treatment arms. c Overall survival of different treatment arms.
In a post hoc analysis, we explored the treatment outcomes of patients with and without per-protocol CCRT, as we did in the T1204 trial. The median PFS and OS of 38 patients with CCRT were 8.3 months (95% CI: 6.7–12.5) and 18.8 months (95% CI: 17.1–21.9), respectively, and 3.4 (95% CI: 2.1–3.9) and 14.5 months (95% CI: 9.4–29.1), respectively, for the 17 patients without CCRT (Supplementary Fig. 2A, B). The median PFS of patients without and with CCRT in mFOLFIRINOX and GOFL arms were 3.4 months (95% CI: 1.8–not reached [NR]) versus 3.3 months (95% CI: 1.8–3.9) and 6.7 months (95% CI: 6.2–12.5) versus 12.3 months (95% CI: 7.6–NR), respectively (Supplementary Fig. 3A). However, the median OS of patients without and with CCRT in mFOLFIRINOX and GOFL arms were 31.0 months (95% CI: 5.3–NR) versus 13.2 months (95% CI: 6.6–18.7) and 18.3 months (95% CI: 13.4–20.3) versus 23.9 months (95% CI: 15.8–29.2), respectively (Supplementary Fig. 3B). The unexpected long survival of the six patients who had no CCRT after induction mFOLFIRINOX failure could result from their extraordinary response to subsequent treatment, as listed in Supplementary Table 1.
The median OS of six patients with per-protocol R0/R1 resection was 24.9 months as compared to the 18.3 months of the rest 49 patients (p = 0.12) (Supplementary Fig. 4A). However, the median OS of all ten patients who underwent R0/R1 resection regardless of per protocol or not was 29.2 months (95% CI: 17.3–NR) versus 18.0 months (95% CI: 13.4–18.8) for those without resection (p = 0.0024) (Supplementary Fig. 4B). The median OS of 7 (25.0%) patients with and 21 (77.8%) patients without conversion R0/R1 resection was 29.2 (95% CI: 17.3–NR) and 15.4 (95% CI: 12.2–18.7) months, respectively, in the GOFL arm, while that of the 3 (11.1%) patients with and 24 (88.8%) patients without conversion R0/R1 resection was not reached (95% CI: 20.3–NR) and 18.6 months (95% CI: 13.4–21.9), respectively, in the mFOLFIRINOX arm (Supplementary Fig. 5).
Excluding one ineligible patient in the GOFL arm, nine patients in the mFOLFIRINOX arm and eight patients in the GOFL arm were off study due to adverse events or withdrawal of consent during the study period. Patterns of disease progression of the rest of the patients were somehow different between the two study arms. Local progression alone and distant metastases with or without local progression as the initial manifestation of progression in mFOLFIRINOX and GOFL arms were 16.7% versus 42.1% and 83.3% versus 52.6%, respectively (Table 3). Of note, ten patients had distant metastases during the period of local therapy with weekly bolus 5-FU-CCRT and laparotomy after 3 months of ICT mFOLFIRINOX as demonstrated by a steep decline in the metastasis-free survival curve at 6 months (Supplementary Fig. 6), while it only occurred in four patients in the GOFL arm. Local progression was rare during CCRT in both study arms, but occurred in seven patients while receiving GOFL treatment, with four during ICT and three during adjuvant or maintenance therapy (Table 3).
Table 3.
Failure patterns during treatment courses.
| mFOLFIRINOX | GOFL | |||||||
|---|---|---|---|---|---|---|---|---|
| Induction CT (N = 27) | Peri-5-FU CCRTa (N = 21) | Maintenance/adjuvant CT (N = 8) | Subtotal | Induction CT (N = 27)b | Peri-Gem-CCRTa (N = 17) | Maintenance/adjuvant CT (N = 9) | Subtotal | |
| Withdrawal/AE | 2 | 2 | 5 | 9 | 1 | 3 (1c) | 4 | 8 |
| Local PD only | 1 | 1 | 1 | 3 | 4 | 1 (1) | 3 | 8 |
| Distant ± local PD | 3 | 10 (3d) | 2 | 15 | 5 | 4 (2e) | 1 | 10 |
| Progression-free | – | – | – | – | – | – | 1 | 1 |
| Subtotal | 6 | 13 | 8 | 27 | 10 | 8 | 9 | 27 |
AE adverse events, CT chemotherapy, CCRT concurrent chemoradiotherapy, PD disease progression.
aPeri-CCRT including those detected during laparotomy, total case (detected during laparotomy).
bExclude one ineligible patient who had metastatic disease upon diagnosis.
cWithdrawal due to unresectable disease with arterial encasement for subsequent therapy.
dPeritoneal seeding was noted during laparotomy in all three patients.
ePeritoneal seeding was noted during laparotomy in one and liver metastases within 4 weeks after R1 resection in one.
Toxicity
During the 3 months of ICT, the incidence of grade 3–4 neutropenia, diarrhoea and vomiting were numerically higher in patients receiving mFOLFIRINOX compared with those receiving GOFL, 37.0% versus 21.4%, 14.8% versus 3.6% and 11.1% versus 0%, respectively, while the incidence of grade 3–4 thrombocytopenia was 7.1% with GOFL and none with mFOLFIRINOX. Of the 38 patients who proceeded to CCRT, Gem-CCRT was associated with a significantly higher incidence of grade 3–4 leukopenia than 5-FU-CCRT, 29.4% versus 0% (p = 0.01). The adverse events for the 17 patients with maintenance or adjuvant chemotherapy were relatively less as compared to during ICT, with grade 3–4 neutropenia noted in 25.0% (2/8) and 11.1% (1/9) with mFOLFIRINOX and GOFL, respectively (Table 4). Ten (37.0%) and 15 (53.6%) patients experienced severe adverse events in the mFOLFIRINOX and GOFL arms, respectively. One patient died of sepsis accompanied by disease progression 4 weeks after completion of 3 months of ICT GOFL (Supplementary Table 2).
Table 4.
Haematological and >5% grade 3/4 non-haematological toxicities.
| Induction chemotherapy | Concurrent chemoradiotherapy | Maintenance/adjuvant chemotherapy | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mFOLFIRINOX (n = 27) | GOFL (n = 28) | P value* | 5-FU-CCRT (n = 21) | Gem-CCRT (n = 17) | P value* | mFOLFIRINOX (n = 8) | GOFL (n = 9) | P value* | |||||||||
| Adverse events (%) | Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | |||||
| Leukopenia | 48.1 | 7.4 | 32.1 | 10.7 | 1.00 | 38.1 | 0 | 58.8 | 29.4 | 0.01 | 37.5 | 25.0 | 44.4 | 0 | 0.20 | ||
| Neutropenia | 18.5 | 37.0 | 25.0 | 21.4 | 0.24 | 14.3 | 0 | 47.1 | 17.6 | 0.08 | 25.0 | 25.0 | 33.3 | 11.1 | 0.58 | ||
| Febrile neutropenia | 0 | 3.7 | 0 | 3.6 | 1.00 | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | – | ||
| Anaemia | 55.6 | 3.7 | 57.1 | 0 | 0.49 | 33.3 | 0 | 70.6 | 5.9 | 0.45 | 75.0 | 0 | 55.6 | 11.1 | 1.00 | ||
| Thrombocytopenia | 51.9 | 0 | 53.6 | 7.1 | 0.49 | 66.7 | 0 | 88.2 | 5.9 | 0.45 | 50.0 | 0 | 33.3 | 0 | – | ||
| Diarrhoea | 63.0 | 14.8 | 46.4 | 3.6 | 0.19 | 57.1 | 0 | 35.3 | 0 | – | 37.5 | 0 | 11.1 | 0 | – | ||
| Nausea | 77.8 | 7.4 | 82.1 | 0 | 0.24 | 57.1 | 0 | 47.1 | 5.9 | 0.45 | 75.0 | 0 | 77.8 | 0 | – | ||
| Vomiting | 48.1 | 11.1 | 71.4 | 0 | 0.11 | 19.0 | 4.8 | 17.6 | 0 | 1.00 | 50.0 | 0 | 33.3 | 11.1 | 1.00 | ||
| Anorexia | 77.8 | 0 | 71.4 | 0 | – | 52.4 | 0 | 58.8 | 5.9 | 0.45 | 62.5 | 0 | 55.6 | 0 | – | ||
| Weight loss | 25.9 | 0 | 10.7 | 0 | – | 4.8 | 0 | 11.8 | 5.9 | 0.45 | 25.0 | 0 | 33.3 | 0 | – | ||
| PSN | 51.9 | 0 | 42.9 | 0 | – | 57.1 | 0 | 58.8 | 0 | – | 62.5 | 0 | 66.7 | 0 | – | ||
PSN peripheral sensory neuropathy.
*P value of grade 3 or higher toxicity; grading according to NCI version 4.03.
Quality of Life
A significant decrease of QoL due to diarrhoea of patients receiving mFOLFIRINOX during ICT (p = 0.028) and financial difficulties of patients during 5-FU-CCRT (p = 0.029) was noted (Supplementary Table 3). There was no significant difference in global health status and other parameters of QoL between both arms during ICT, CCRT or after CCRT.
Discussion
In this randomised phase II selection design study, we demonstrated that 3-month triplet chemotherapy followed by CCRT could achieve a 9-month PFS rate of 33.3% and median OS of 18.7 months (95% CI, 15.8–21.9) in all 55 enrolled patients (Fig. 2a). The goal of this study was to select a winner between two triplets of ICT followed by CCRT, which could be either mFOLFIRINOX or GOFL. The clinical outcomes of patients in either study arm were comparable with 9-month PFS rate, median PFS and median OS in mFOLFIRINOX and GOFL arms were 30.5% versus 35.9%, 6.6 months (95% CI: 5.9–12.5) versus 7.6 months (95% CI: 3.9–12.3) and 19.6 months (95% CI: 13.4–22.9) versus 17.9 months (95% CI: 13.4–23.9), respectively.
With a nearly identical study design, except that patient recruitment was limited to age ≤70 years old, ECOG PS 0 or 1 and total bilirubin <1.5× UNL in considering the requirement of more fit patients for mFOLFIRINOX, 15.4% (26 evaluable patients) of ORR after induction GOFL, 60% patients with per-protocol CCRT and median PFS of 3.3 months (95% CI: 1.8–3.9) and 12.3 months (95% CI: 7.6–NR) in patients without and with per-protocol Gem-CCRT, respectively, in the current study were consistent with the 22.2%, 60%, 2.4 months (95% CI: 1.5–3.3) and 14.5 months (95% CI: 11.9–17.1), respectively, in our previous T1204 single-arm phase II study [9]. However, the median OS of patients in the current GOFL arm without and with CCRT was longer than the T1204 study, 13.2 months (95% CI: 6.6–18.7) versus 8.6 months (95% CI: 5.3–11.9) and 23.9 months (95% CI: 15.8–29.2) versus 18.3 months (95% CI: 17.1–19.6), respectively. The potential reasons that led to such improvement could be the inclusion of more fit patients and the higher percentage of patients who received salvage systemic chemotherapy after failure of study treatment, 92.6 versus 25% in T1204, due to the approval of national health insurance reimbursement for oral S-1 in June 2014 and nal-IRI + 5-FU/LV in August 2018 [20, 21]. The higher per-protocol R0/R1 resection rate in the GOFL arm of the current study, 5 of 27 eligible patients (18.5%) versus 8% in T1204, might be attributed to the evolving concept and techniques of our participating pancreatic surgeons [22]. On ITT analysis, the median OS of patients receiving ICT GOFL with and without conversion R0/R1 resection was 29.2 months (95% CI: 17.3–NR) and 15.4 months (95% CI: 12.2–18.7), respectively, as compared to the 27.5 months (95% CI: 17.2–NR) and 13.9 months (95% CI: 12.1–17.7), respectively, in recently reported randomised phase II NEOLAP trial [23].
This T2212 study is the first prospective study to evaluate the efficacy and safety of mFOLFIRINOX for APC patients in Taiwan. Three months of induction mFOLFIRINOX (150 mg/m2 irinotecan) was well tolerated with grade 3–4 neutropenia and diarrhoea observed in 37.0% and 14.8% of patients, respectively, as compared to that of 47.8% and 10.1%, respectively, in a Japanese multicenter study for chemo-naive mPC [24]. On the contrary, Caucasian patients experienced less grade 3–4 neutropenia even with western version mFOLFIRINOX (180 mg/m2 irinotecan), i.e. 28.4% in an adjuvant trial for resectable pancreatic cancer [25]. The 22.2% of ORR after mFOLFIRINOX in our study was comparable to the 17.2% in the LAPC cohort of a phase II study and 16.7% after sequential mFOLFIRINOX following nab-P + Gem in the NEOLAP study [23, 26]. On ITT analysis, the median OS after mFOLFIRINOX in the current study was 19.6 months (95% CI: 13.4–22.9) compared with 21.6 months (1.8 years, 95% CI: 1.5–2.0) in JCOG1407 [27]. However, despite a comparable 1-year survival rate of 88.9 versus 82.5% after mFOLFIRINOX, the parameters of long-term disease control were numerically inferior in the current study as compared to JCOG1407, with a 1-year PFS rate of 30.5 versus 39.7%, 1-year metastasis-free survival of 40.5 versus 64.2% and 2-year OS rate of 29.6 versus 40.2% [27].
The current study was proposed in 2012 and followed the original design of T1204 with 3 months of triplet ICT followed by CCRT. On the other hand, the JCOG1407 has continuous ICT, nab-P + Gem versus mFOLFIRINOX, until disease progression or unacceptable toxicity. Although the comparison between parallel studies should be done carefully, combining the observations of relative inferior long-term outcomes against JCOG1407 and the 10 (46.7%) of 21 patients who completed 5-FU-CCRT developing distant metastases before and during surgical exploration raised concerns regarding the use of weekly bolus 5-FU as radio-sensitiser in our mFOLFIRINOX arm and the role or optimal timing of CCRT after effective ICT in LAPC. The high incidence of systemic disseminating after 5-FU-CCRT in the current study concurs with a 43.3% of participants suffering from distant failure or death within 3 months after the start of 5-FU (200 mg/m2/day continuous infusion)-based CCRT in a randomised phase 3 trial [3]. It was also consistent with the finding of a previous randomised study, in which the median time to metastases of patients with upfront 5-FU-CCRT and Gem-CCRT followed by systemic gemcitabine was 3.1 and 6.1 months, respectively [28]. However, the median metastasis-free survival after front-line full-dose S-1-based CCRT followed by systemic gemcitabine in the JCOG1106 trial was 11.0 months (95% CI: 6.0–15.9) [29]. It seems to suggest full-dose S-1 that showed non-inferior single-agent activity against gemcitabine for APC in GEST trial, and the feasibility in combination with radiotherapy can be an attractive radio-sensitiser option for future investigation in Asia [20, 29, 30].
In the LAP07 study, patients who received capecitabine-CCRT after the second randomisation had significantly more systemic dissemination (66 versus 44%, p = 0.04) and numerically inferior OS (15.2 versus 16.5 months, p = 0.83) than those who continued another 2 months of gemcitabine or gemcitabine plus erlotinib [5]. Recently, consolidation CCRT was rarely included as part of per-protocol treatment following standard multi-agent ICT in LAPC. For example, the NEOLAP study directly compared the conversion resection rate after four cycles of nab-P + Gem versus two cycles of nab-P + Gem plus four cycles of FOLFIRINOX, while the JCOG1407 compared the OS of patients receiving mFOLFIRINOX versus nab-P + Gem until disease progression or unacceptable toxicity [23, 27]. The LAPACT study evaluated the time-to-treatment failure with six cycles of nab-P + Gem followed by subsequent treatment per investigator’s choice [31].
Of the 27 eligible patients in the GOFL arm, the conversion R0/R1 resection was performed in 5 (18.5%) after per-protocol treatment and in 7 (25.9%) during the survival follow-up period, as compared to the 17 (15.9%) and 28 (26.2%) in the LAPACT study by ITT analysis [31]. However, the R0/R1 resection rate of the 165 enrolled and treated patients was 32% in the NEOLAP study [23]. The median OS of ITT populations in the GOFL arm of current T2212, LAPACT and NEOLAP studies was 17.9 months (95% CI: 13.4–23.9), 18.8 months (95% CI: 15.0–24.0) and 17.1 months (95% CI: 14.1–20.3), respectively [23, 31]. As previously described, the median OS of patients with and without R0/R1 resection in our GOFL arm and NEOLAP study were similar as well [23]. The data suggest that adding CCRT to short-term chemotherapy may not affect the survival of LPAC patients receiving modern ICT. However, the observation that 5 per 18 patients who had CCRT after 6 months of nab-P + Gem underwent surgical resection in the LAPACT study highlights the role of CCRT in chemotherapy-selected patients with long-term disease control [31]. On the other hand, 49% of ORR after 4 months of FOLFIRINOX plus losartan and the 60% of R0 resection after subsequent individualised CCRT seems to support the value of consolidation CCRT in facilitating R0 resection in ICT-responsive LAPC [32].
The safety of induction GOFL was consistent with our previous phase II studies for APC and LAPC with grade ≥3 neutropenia and diarrhoea of 25–30% and 3.8–8%, respectively, which was numerically better than that of 37.0 and 14.8% after mFOLFIRINOX in the current study [8, 9]. In addition, due to the early stopping rule in the presence of grade 2 peripheral sensory neuropathy (PSN), oxaliplatin-associated grade 3 PSN was rare in the current and our previous GOFL studies [8, 9]. On the contrary, nab-P + Gem treatment was associated with 70.1% and 11.8% of grade ≥3 neutropenia and PSN, respectively, in a Japanese mPC cohort [33]. However, a drawback of GOFL and mFOLFIRINOX compared to nab-P + Gem is the inclusion of an inconvenient 48-h infusion of 5-FU. To overcome such a hurdle and to enhance the activity, we used oral S-1/LV 1 week–on/1 week-off to substitute the biweekly infusion 5-FU and LV of GOFL regimen to develop a new version triplet, the SLOG regimen, which achieved a 40% ORR and median PFS and OS of 7.6 and 11.4 months, respectively, in TCOG T1211 phase II study [34]. The activity and safety of SLOG were recently evaluated in a randomised phase II study against mFOLFIRINOX in APC patients (ClinicalTrials.gov NCT03443492) [35]. On the other hand, considering the ethnic difference in compliance to S-1, GOFL can be a potential regimen for Western APC patients who are less fit or not suitable to have FOLFIRINOX and/or nab-P + Gem regimen.
Up to now, nal-IRI + 5-FU is the only approved treatment for mPC after previous gemcitabine-based therapy, while most of recommended second-line treatments after (modified) FOLFIRINOX were based on informal consensus with low evidence quality [14, 36]. For the delayed reimbursement and limited to first-line treatment use of FOLFIRINOX and nab-P + Gem for mPC in Taiwan, GS and SLOG became the main subsequent treatment option in the current study. In our previous study, we have demonstrated the feasibility and activity of GS and SLOG in the first-line setting for APC, but such regimens have not been evaluated as subsequent or salvage treatment after failure to modern multi-agent combinations [34, 37]. More than 10–12 months of survival after failure, including disease progression, to mFOLFIRINOX and GOFL in the current study supports the potential efficacies of GS-based regimen for second-line treatment in APC, which deserves further investigation.
The limitation of the current T2212 study was the small number of participants following early termination due to lag of accrual, a situation further exacerbated by the opening of a new T5217 study that compared the efficacy and safety of SLOG versus mFOLFIRINOX in APC [35]. However, the likelihood of futility of our study was supported by a post hoc predictive probability of success analysis based on 40 progression or death events (data not shown) [38]. Another limitation was the outcomes of our mFOLFIRINOX arm were likely hampered by the high distance failure rate during and immediately after weekly bolus 5-FU-based CCRT. The exceptional, prolonged survival after subsequent treatment of the six ICT mFOLFIRINOX failure patients likely resulting from non-selection bias could also jeopardise the data interpretation. The lack of translational research component in this multicenter trial was mainly due to scant residual tissue after routine pathologic diagnostic procedures on small, endoscopic ultrasonography- or CT-guided biopsy samples. However, part of the current study population has been included in a study investigating the inherent genetic variants of Taiwanese pancreatic cancer patients [39].
Conclusions
ICT with either GOFL or mFOLFIRINOX followed by CCRT provided similar clinical outcomes in the current study. The results were largely compatible with recently published, prospectively trials with upfront active multi-agent combination regimens, nab-P + Gem and/or mFOLFIRINOX, in LAPC without per-protocol consolidation CCRT [5, 23, 27, 31]. The role of CCRT for LAPC in the era of modern active chemotherapy should be rethought and reserved for patients who had either front-line chemotherapy-responsive tumours to increase the likelihood of curative resection or long-term disease control or sole local progression after ICT who will likely benefit from (definitive) local control, as recommended in major treatment guideline [12–14].
Supplementary information
Acknowledgements
We thank all the hospitals for enrolling patients in this trial including National Cheng Kung University Hospital, Tainan (to Y-SS, N-JC); Veterans General Hospital, Taipei City (to C-PL); Mackay Memorial Hospital, Taipei (to JL); National Taiwan University Hospital, Taipei City (to S-HY); Veterans General Hospital, Kaohsiung City (to S-JL, Ming-Sun Yu), Tri-Service General Hospital, Taipei (to P-YC, J-HC), Linkou Chang Gung Memorial Hospital, Taoyuan (to J-SC) and Taipei Medical University Hospital, Taipei City (to Her-Shyong Shiah). We are grateful for the participation of all the patients and their families and to make this study possible, and also for the help of research nurses and statisticians from the Taiwan Cooperative Oncology Group. In particular, we would like to thank Ms. Shu-Chuan Lai for her tremendous efforts in the comprehensive statistical analyses of this trial. Oxaliplatin (Oxalip®) and irinotecan (Irino®) were kindly sponsored by TTY Biopharm Company Limited, Taipei, Taiwan.
Author contributions
H-JC, Y-SS, L-TC and Y-YS: conceptualisation, funding acquisition and writing—review & editing; Y-YS, Y-SS, C-PL, S-HY, JL, S-JL, P-YC, N-JC, L-TC and H-JC: data curation; Y-YS, H-JC, Y-FC, L-TC: formal analysis, methodology and writing—original draft; H-JC: project administration; Y-FC, Y-YS: software; Y-SS and L-TC: supervision; Y-YS, Y-SS, C-PL, S-HY, JL, S-HY, S-HL, P-YC, N-JC and L-TC: validation.
Funding
This study was conducted by the Taiwan Cooperative Oncology Group with funding (number CA-101–108-PP 25) from National Health Research Institutes, Ministry of Health and Welfare, Executive Yuan, Taiwan.
Data availability
All data generated and analysed during this study are included in this article and its Supplementary information files.
Competing interests
L-TC reports research funding from Novartis, Merck, Serono, TTY, Polaris, SyncorePharm, Pfizer, BMS; honoraria from ONO, Eli Lilly, MSD, PharmaEngine, TTY, SyncorePharm, Novartis, Astra Zeneca, Ipsen; patents and royalties from ENO-1mAb/HuniLife; membership on Board of Directors of ScinoPharm, Taiwan, Ltd. and on Scientific Advisory Committee of PharmaEngine. All the remaining authors declare no competing interests.
Ethics approval and consent to participate
The study protocol and informed consent forms were approved by the Research Ethics Committee, National Health Research Institutes, Taiwan (reference number: EC1010805) and the Institutional Review Board of each participating hospital. All subjects provided written informed consent prior to participating in the study. The study was conducted in accordance with the Declaration of Helsinki.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yung-Yeh Su, Yen-Feng Chiu.
These authors jointly supervised this work: Yan-Shen Shan, Hui-Ju Ch’ang, Li-Tzong Chen.
Contributor Information
Yan-Shen Shan, Email: ysshan@mail.ncku.edu.tw.
Hui-Ju Ch’ang, Email: hjmc@nhri.org.tw.
Li-Tzong Chen, Email: leochen@nhri.org.tw.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01649-7.
References
- 1.Sultana A, Tudur Smith C, Cunningham D, Starling N, Tait D, Neoptolemos JP, et al. Systematic review, including meta-analyses, on the management of locally advanced pancreatic cancer using radiation/combined modality therapy. Br J Cancer. 2007;96:1183–90. doi: 10.1038/sj.bjc.6603719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seufferlein T, Hammel P, Delpero JR, Macarulla T, Pfeiffer P, Prager GW, et al. Optimizing the management of locally advanced pancreatic cancer with a focus on induction chemotherapy: Expert opinion based on a review of current evidence. Cancer Treat Rev. 2019;77:1–10. doi: 10.1016/j.ctrv.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Herman JM, Wild AT, Wang H, Tran PT, Chang KJ, Taylor GE, et al. Randomized phase III multi-institutional study of TNFerade biologic with flurorouracil and radiotherapy for locally advanced pancreatic cancer: final results. J Clin Oncol. 2013;31:886–94. doi: 10.1200/JCO.2012.44.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang JS, Chiu YF, Yu JC, Chen LT, Ch’ang HJ. The role of consolidation chemoradiotherapy in locally advanced pancreatic cancer receiving chemotherapy: an updated systemic review and meta-analysis. Cancer Res Treat. 2018;50:562–74. doi: 10.4143/crt.2017.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammel P, Huguet F, van Laethem JL, Goldstein D, Glimelius B, Artru P, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients With locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: The LAP07 randomized clinical trial. JAMA. 2016;315:1844–53. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 6.Mukherjee S, Hurt CN, Bridgewater J, Falk S, Cummins S, Wasan H, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol. 2013;14:317–26. doi: 10.1016/S1470-2045(13)70021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ch’ang HJ, Wang CC, Cheng AL, Hsu C, Lu YS, Chang MC, et al. Phase I study of biweekly gemcitabine followed by oxaliplatin and simplified 48-h infusion of fluorouracil/leucovorin for advanced pancreatic cancer. J Gastroenterol Hepatol. 2006;21:874–9. doi: 10.1111/j.1440-1746.2005.04022.x. [DOI] [PubMed] [Google Scholar]
- 8.Ch’ang HJ, Huang CL, Wang HP, Shiah HS, Chang MC, Jan CM, et al. Phase II study of biweekly gemcitabine followed by oxaliplatin and simplified 48-h infusion of 5-fluorouracil/leucovorin (GOFL) in advanced pancreatic cancer. Cancer Chemother Pharmacol. 2009;64:1173–9. doi: 10.1007/s00280-009-0980-2. [DOI] [PubMed] [Google Scholar]
- 9.Ch’ang HJ, Lin YL, Wang HP, Chiu YF, Chang MC, Hsu CH, et al. Induction chemotherapy with gemcitabine, oxaliplatin, and 5-fluorouracil/leucovorin followed by concomitant chemoradiotherapy in patients with locally advanced pancreatic cancer: a Taiwan Cooperative Oncology Group phase II study. Int J Radiat Oncol Biol Phys. 2011;81:e749–757. doi: 10.1016/j.ijrobp.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 10.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 11.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balaban EP, Mangu PB, Khorana AA. Locally advanced, unresectable pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:2654–68. doi: 10.1200/JCO.2016.67.5561. [DOI] [PubMed] [Google Scholar]
- 13.Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, et al. NCCN clinical practice guidelines in oncology: pancreatic adenocarcinoma, version 2021.2. J Natl Compr Cancer Netw. 2021;19:439–57. doi: 10.6004/jnccn.2021.0017. [DOI] [PubMed] [Google Scholar]
- 14.Okusaka T, Nakamura M, Yoshida M, Kitano M, Uesaka K, Ito Y, et al. Clinical practice guidelines for pancreatic cancer 2019 from the Japan Pancreas Society: a synopsis. Pancreas. 2020;49:326–35. doi: 10.1097/MPA.0000000000001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reames BN, Blair AB, Krell RW, Groot VP, Gemenetzis G, Padussis JC, et al. Management of locally advanced pancreatic cancer” results of an international survey of current practice. Ann Surg. 2021;273:1173–81. doi: 10.1097/SLA.0000000000003568. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.National Institutes of Health. National Cancer Institute: Common Terminology Criteria for Adverse Events version 4.03. 2009. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_4.03.xlsx Accessed 11 Oct 2021.
- 18.Conroy T, Paillot B, Fran.ois E, Bugat R, Jacob JH, Stein U, et al. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer—a Groupe Tumeurs Digestives of the Federation Nationale des Centres de Lutte Contre le Cancer study. J Clin Oncol. 2005;23:1228–36. doi: 10.1200/JCO.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 19.Liu PY, Dahlberg S, Crowley J. Selection designs for pilot studies based on survival. Biometrics. 1993;49:391–8. doi: 10.2307/2532552. [DOI] [PubMed] [Google Scholar]
- 20.Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31:1640–8. doi: 10.1200/JCO.2012.43.3680. [DOI] [PubMed] [Google Scholar]
- 21.Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545–57. doi: 10.1016/S0140-6736(15)00986-1. [DOI] [PubMed] [Google Scholar]
- 22.Chao YJ, Sy ED, Hsu HP, Shan YS. Predictors for resectability and survival in locally advanced pancreatic cancer after gemcitabine-based neoadjuvant therapy. BMC Surg. 2014;14:72. doi: 10.1186/1471-2482-14-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunzmann V, Siveke JT, Algu¨l H, Goekkurt E, Siegler G, Martens U, et al. Nab-paclitaxel plus gemcitabine versus nab-paclitaxel plus gemcitabine followed by FOLFIRINOX induction chemotherapy in locally advanced pancreatic cancer (NEOLAP-AIO-PAK-0113): a multicenter randomised phase II trial. Lancet Gastroenterol Hepatol. 2021;6:128–38. doi: 10.1016/S2468-1253(20)30330-7. [DOI] [PubMed] [Google Scholar]
- 24.Ozaka M, Ishii H, Sato T, Ueno M, Ikeda M, Uesugi K, et al. A phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2018;81:1017–23. doi: 10.1007/s00280-018-3577-9. [DOI] [PubMed] [Google Scholar]
- 25.Conroy T, Hammel P, Hebbar M, Abdelghani MB, Wei AC, Raoul JL, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl J Med. 2018;379:2395–406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 26.Stein SM, James ES, Deng Y, Cong X, Kortmansky JS, Li J, et al. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer. 2016;114:737–43. doi: 10.1038/bjc.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozaka M, Ueno M, Ishii H, Mizusawa J, Katayama H, Kataoka T, et al. Randomized phase II study of modified FOLFIRINOX versus gemcitabine plus nab-paclitaxel combination therapy for locally advanced pancreatic cancer (JCOG1407). J Clin Oncol. 2021;39 Suppl 15;abstract 4017.
- 28.Li CP, Chao Y, Chi KH, Chan WK, Teng HC, Lee RC, et al. Concurrent chemoradiotherapy treatment of locally advanced pancreatic cancer: gemcitabine versus 5-fluorouracil, a randomized controlled study. Int J Radiat Oncol Biol Phys. 2003;57:98–104. doi: 10.1016/S0360-3016(03)00435-8. [DOI] [PubMed] [Google Scholar]
- 29.Ioka T, Furuse J, Fukutomi A, Mizusawa J, Nakamura S, Hiraok N, et al. Randomized phase II study of chemoradiotherapy with versus without induction chemotherapy for locally advanced pancreatic cancer: Japan Clinical Oncology Group trial, JCOG1106. Jpn J Clin Oncol. 2021;51:235–43. doi: 10.1093/jjco/hyaa198. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda M, Ioka T, Ito Y, Yonemoto N, Nagase M, Yamao K, et al. A multicenter phase II trial of S-1 with concurrent radiation therapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2013;85:163–9. doi: 10.1016/j.ijrobp.2012.03.059. [DOI] [PubMed] [Google Scholar]
- 31.Philip PA, Lacy J, Portales F, Sobrero A, Pazo-Cid R, Mozo JLM, et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicenter, open-label phase 2 study. Lancet. Gastroenterol Hepatol. 2020;5:285–94. doi: 10.1016/S2468-1253(19)30327-9. [DOI] [PubMed] [Google Scholar]
- 32.Murphy JE, Wo JY, Ryan DP, Clark JW, Jiang W, Yeap BY, et al. Total neoadjuvant therapy with FOLFIRINOX in combination with Losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5:1020–7. doi: 10.1001/jamaoncol.2019.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueno H, Ikeda M, Ueno M, Mizuno N, Ioka T, Omuro Y, et al. Phase I/II study of nab-paclitaxel plus gemcitabine for chemonaïve Japanese patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2016;77:595–603. doi: 10.1007/s00280-016-2972-3. [DOI] [PubMed] [Google Scholar]
- 34.Chiang NJ, Tsai KK, Hsiao CF, Yang SH, Hsiao HH, Shen WC, et al. A multicenter, phase I/II trial of biweekly S-1, leucovorin, oxaliplatin and gemcitabine in metastatic pancreatic adenocarcinoma-TCOG T1211 study. Eur J Cancer. 2020;124:123–30. doi: 10.1016/j.ejca.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 35.Chiang NJ, Shan YS, Bai LY, Li CP, Chen JS, Yang SH, et al. TCOG T5217 trial: a phase II randomized study of SLOG vs modified FOLFIRINOX as first-line treatment in locally advanced or metastatic pancreatic ductal adenocarcinoma. J Clin Oncol. 2021;39 Suppl 15;abstract 4143.
- 36.Sohal DPS, Kennedy EB, Cinar P, Conroy T, Copur MS, Crane CH, et al. Metastatic pancreatic cancer: ASCO guideline update. J Clin Oncol. 2020;38:3217–30. doi: 10.1200/JCO.20.01364. [DOI] [PubMed] [Google Scholar]
- 37.Chen CY, Liang SH, Su YY, Chiang NJ, Wang HC, Chiu CF, et al. Modified gemcitabine, S-1, and leucovorin combination for patients with newly diagnosed locally advanced or metastatic pancreatic adenocarcinoma: a multi-center retrospective study in Taiwan. PLoS ONE. 2020;15:e0244487. doi: 10.1371/journal.pone.0244487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lan KK, Wittes J. The B-value: a tool for monitoring data. Biometrics. 1988;44:579–85. doi: 10.2307/2531870. [DOI] [PubMed] [Google Scholar]
- 39.Shan YS, Chen LT, Wu JS, Chang YF, Lee CT, Wu CH, et al. Validation of genome-wide association study-identified single nucleotide polymorphisms in a case-control study of pancreatic cancer from Taiwan. J Biomed Sci. 2020;27:69. doi: 10.1186/s12929-020-00664-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analysed during this study are included in this article and its Supplementary information files.