Abstract
Purpose
PARP inhibitor resistance may be overcome by combinatorial strategies with agents that disrupt homologous recombination repair (HRR). Multiple HRR pathway components are HSP90 clients, so that HSP90 inhibition leads to abrogation of HRR and sensitisation to PARP inhibition. We performed in vivo preclinical studies of the HSP90 inhibitor onalespib with olaparib and conducted a Phase 1 combination study.
Patients and methods
Tolerability and efficacy studies were performed in patient-derived xenograft(PDX) models of ovarian cancer. Clinical safety, tolerability, steady-state pharmacokinetics and preliminary efficacy of olaparib and onalespib were evaluated using a standard 3 + 3 dose-escalation design.
Results
Olaparib/onalespib exhibited anti-tumour activity against BRCA1-mutated PDX models with acquired PARPi resistance and PDX models with RB-pathway alterations(CDKN2A loss and CCNE1 overexpression). Phase 1 evaluation revealed that dose levels up to olaparib 300 mg/onalespib 40 mg and olaparib 200 mg/onalespib 80 mg were safe without dose-limiting toxicities. Coadministration of olaparib and onalespib did not appear to affect the steady-state pharmacokinetics of either agent. There were no objective responses, but disease stabilisation ≥24 weeks was observed in 7/22 (32%) evaluable patients including patients with BRCA-mutated ovarian cancers and acquired PARPi resistance and patients with tumours harbouring RB-pathway alterations.
Conclusions
Combining onalespib and olaparib was feasible and demonstrated preliminary evidence of anti-tumour activity.
Subject terms: Gynaecological cancer, Cancer therapy
Introduction
Homologous recombination repair (HRR) deficient tumours are exquisitely sensitive to PARP inhibitors (PARPis) and this synthetic lethal interaction is being exploited therapeutically across multiple cancer types [1]. However, acquired PARPi resistance occurs commonly, mainly due to secondary genetic or epigenetic events that restore HRR proficiency, and represents a rapidly emerging unmet medical need. Combination of PARPis with agents that induce “BRCAness”, i.e. agents which directly or indirectly inhibit HRR, is a promising strategy to overcome acquired PARPi resistance in HRR-deficient tumours as well as de novo PARPi resistance in HRR-proficient tumours [2, 3].
Heat-shock protein 90 (HSP90) is a molecular chaperone that facilitates the stability, maturation and function of a number of substrates (also referred to as HSP90 clients) that are involved in diverse cellular processes, including DNA repair [4–7]. Multiple components of the HRR pathway are HSP90 clients, including BRCA1, BRCA2 and RAD51, suggesting that inhibition of HSP90 may abrogate HRR and therefore sensitise cancer cells to PARPi [8, 9]. In this regard, we have previously demonstrated that HSP90 inhibition (HSP90i) in HRR-proficient high-grade serous ovarian cancer cell lines leads to downregulation of BRCA1 and RAD51, compromise of HRR, increased DNA damage, and subsequent sensitisation to PARPis [10]. Importantly, in vitro synergism between HSP90i and PARPi was observed in HRR-proficient models even with sublethal concentrations of HSP90i [10]. Similar experiments have been conducted in HRR-proficient NCI-H1299 non-small cell lung cancer (NSCLC) cells, showing that HSP90i depletes BRCA2, BRCA1 and RAD51 and sensitises them to PARPi. HSP90 has also been implicated as a mechanism of acquired PARPi resistance in HRR-deficient cells. Specifically, in MDA-MB-436 triple-negative breast cancer cells harbouring a BRCA1 BRCT domain mutation that were rendered PARPi resistant via exposure to graded concentrations of PARPi over time, resistance was associated with HSP90-mediated stabilisation of the BRCA1-mutant protein, without evidence for BRCA1 reversion mutation [11]. In that setting, resistance was overcome with the application of an HSP90i, so that PARPi sensitivity was restored [11].
Onalespib (AT13387) is a synthetic, second-generation, non-ansamycin, small-molecule HSP90 inhibitor that exhibits a high affinity for the ATP-binding site at the N-terminal domain of HSP90 [12, 13]. Onalespib has been studied in advanced solid tumours both as monotherapy and in combination with targeted agents [12, 14, 15]. Here, we evaluated the synergism between HSP90i and PARPi in vivo by performing tolerability and efficacy studies of onalespib combined with olaparib in patient-derived xenograft (PDX) models of ovarian cancer and subsequently conducted a Phase 1 study of these agents in patients with advanced solid tumours.
Methods
Preclinical studies using ovarian cancer patient-derived xenograft (PDX) models
A panel of clinically and molecularly characterised PDX mouse models of high-grade serous ovarian cancer (HGSOC) generated from patient ascites as previously reported [16] was selected for preclinical studies of olaparib in combination with onalespib. These models have been luciferized thereby providing the ability to follow the growth of tumours by non-invasive bioluminescent imaging (BLI) technology. All models have been characterised for HRR proficiency status utilising a RAD51 immunohistochemical assay developed in the Dana-Farber Cancer Institute (DFCI) Center for DNA Damage and Repair [17, 18].
Approximately 5 × 106 cells derived from ascites were injected intraperitoneally into 8-week-old female NSG mice. One to 2 weeks post implantation, upon establishment of tumour burden as measured by BLI, mice were randomised to vehicle, olaparib 100 mg/kg/day p.o. once daily for 28 days, onalespib (AT13387) 45 mg/kg/day intraperitoneally (i.p.) for 2 days (D1, D2) on/5 days off × 4 weeks (i.e. days 1, 2, 8, 9, 15, 16, 22 and 23), and the combination of olaparib plus onalespib at the same doses and schedule of administration. Olaparib was formulated with 10% DMSO, 50% of 60% hydroxypropyl-beta-cyclodextrin (HPβCD) and 40% water, while onalespib was formulated with 17.5% HPβCD in water. Peritoneal disease dissemination in the mice was monitored serially once weekly for up to 6 weeks using a Xenogen IVIS-200 system (Xenogen). End of study mouse plasma CA125 levels was measured via a custom assay using BioScale’s Acoustic Membrane Micro Particle technology as previously described [19].
Study design and treatment
This was a Phase 1, multicenter, open-label, investigator-initiated study of olaparib and onalespib (NCT02898207) sponsored by the National Cancer Institute (NCI) Cancer Therapy Evaluation Program (NCI-CTEP). Patients were enrolled at three different centres (Dana-Farber Cancer Institute, Boston, MA, Mayo Clinic, Rochester, MN and Mayo Clinic, AZ) in the United States through the Experimental Therapeutics Clinical Trials Network (ETCTN). A standard 3 + 3 design was employed, with dose escalation if 0/3 or 1/6 participants experienced dose-limiting toxicity (DLT). The primary objective of this study was to establish the maximum tolerated dose (MTDs) of olaparib and onalespib administered in combination in patients with advanced solid tumours. Secondary objectives were to determine the dose-limiting toxicity (DLT), safety, recommended Phase 2 doses (RP2D), plasma pharmacokinetics and preliminary anti-tumour activity of the olaparib and onalespib combination as assessed by RECIST 1.1.
During cycle 0 (C0), olaparib was administered alone twice daily for 1 week (days 1–7). To test the hypothesis that olanespib sensitises to olaparib by inhibiting HRR, three optional biopsies were planned, one pretreatment/baseline, one after C0 when olaparib was administered alone and one after the combination of olaparib/onalespib with the expectation that there will be the induction of BRCA1 and RAD51 foci after administration of olaparib alone and reduced formation of these foci and downregulation of expression of HRR pathway genes after the combination of olaparib/onalespib. All biopsies were optional but, eventually, no patient agreed to undergo these biopsies. Cycle 1 and beyond, olaparib continued to be administered twice daily (days 1–28) and onalespib was administered IV for 2 consecutive days on/5 days off × 3 weeks of a 4-week cycle (i.e. days 1, 2, 8, 9, 15, 16 of a 28-day cycle). Per the dose-escalation schema, doses of olaparib ranging from 50 to 300 mg orally twice daily were combined with doses of onalespib ranging from 20 to 160 mg/m2 (20-40-80-120-160) IV using a ping-pong strategy. Tumour assessment by RECIST 1.1 occurred every two cycles ±7 days. Protocol treatment continued indefinitely until progression, unacceptable toxicity, patient refusal, the intercurrent illness that prevented further administration of treatment, or general or specific changes in the participant’s condition which rendered the participant unacceptable for further treatment in the opinion of the treating investigator.
The choice of the twice-weekly (days 1 and 2), i.e. consecutive-day dosing of onalespib, was supported by the tolerability and efficacy studies of olaparib/onalespib in our PDX models. Historically, many HSP90 inhibitors were developed with a twice-weekly intermittent schedule based on an initial depletion in HSP90 client protein expression with re-expression after approximately 72 h. Therefore, intermittent, twice-weekly dosing of onalespib was the chosen schedule for the initial clinical study in humans [20]. However, the alternative dosing schedule with daily administration for the first 2 days per week (QDx2/week, i.e., consecutive-day dosing) demonstrated consistently greater tumour growth inhibition and intratumoral drug accumulation in several human xenograft models [21]. Consecutive-day dosing also allowed increased magnitude and duration of drug exposures in tumours, while the drug was cleared rapidly from the plasma, potentially improving the therapeutic window with sustained client protein depletion and minimising damage to normal tissues. Additional preclinical work demonstrated similar results with ganetespib, where consecutive-day dosing resulted in superior tumour xenograft regressions, accompanied by more sustained pharmacodynamic effects compared to intermittent dosing [22]. Finally, in a second Phase 1 study of onalespib, consecutive-day dosing was well tolerated with pharmacodynamic effects demonstrated among nine tumour biopsy pairs [12]. For these reasons, the daily administration of onalespib for the first 2 days per week (QDx2/week) was chosen. Nonetheless, it is important to acknowledge that the optimal schedule of onalespib (as well as of HSP90 inhibitors in general) remains unclear and future studies randomising between different schedules are needed to address this question.
The clinical trial was approved by the NCI Central Institutional Review Board (CIRB) and the US Food and Drug Administration (NCT02898207). All procedures involving human participants were carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from patients or guardians before enrolment in the study. This study was funded by the NCI-Cancer Therapy Evaluation Program (CTEP) and conducted under the auspices of the Experimental Therapeutics Clinical Trials Network (ETCTN). Dana-Farber/Harvard Cancer Center served as the lead site for the study, supported by NIH grant UM1 CA186709. The drugs were provided by AstraZeneca (olaparib) and Astex Pharmaceuticals (onalespib) via the NCI. AstraZeneca and Astex Pharmaceuticals had no role in study design, data collection, data analysis, data interpretation or writing of the report. All authors had full access to all the data in the study, were involved in writing the report, and approved the final version for submission. The first and last authors had final responsibility for the decision to submit for publication.
Eligibility
Eligibility criteria included histologically or cytologically confirmed malignancy that was metastatic or unresectable and for which standard curative or palliative measures did not exist or were no longer effective. Patients may have received any number of prior therapies, including prior PARPi therapy. Other eligibility criteria included age ≥18 years, availability of a formalin-fixed, paraffin-embedded tumour specimen, ECOG performance status 0 or 1 (Karnofsky > 60%), life expectancy of greater than 12 weeks, normal organ and marrow function, QTcF ≤450 ms, ability to swallow tablets and no significant impairment in gastrointestinal absorption, and ability to understand and willingness to sign a written informed consent document. Women of child-bearing potential and men had to agree to use adequate contraception (hormonal or barrier method of birth control; abstinence) prior to study entry, for the duration of study participation, and for 3 months after the last dose of study drugs. Exclusion criteria included known active or history of brain metastases, known history of QT/QTc prolongation or Torsades de Pointes (TdP), and uncontrolled intercurrent illness including, but not limited to, ongoing or active infection, symptomatic congestive heart failure, unstable angina pectoris, cardiac arrhythmia, or psychiatric illness/social situations that would limit compliance with study requirements. Pregnant and HIV-positive patients were excluded. Finally, participants receiving any medications or substances that are strong inhibitors or inducers of CYP3A4 or moderate inhibitors of CYP3A4 or medications with a known risk to prolong the QT interval or inducing TdP were ineligible. All patients had to undergo a pre-study ophthalmologic exam by an ophthalmologist that included visual acuity testing, slit-lamp examination, and fundoscopic examination.
Pharmacokinetic (PK) evaluation
The PK study was designed to assess the effect of onalespib on the steady-state PKs of olaparib and if the PKs of onalespib were affected by the concurrent administration of olaparib. PK sampling was performed in all patients enrolled in the dose escalation to define the plasma concentration-time profile of olaparib over the dosing interval for the day 7 dose when given alone in cycle 0. Pharmacokinetic samples were also collected over a 24-h interval on days 1 and 15 of cycle 1 to define the plasma profiles for olaparib and onalespib. Blood samples were collected in 4 mL plastic Vacutainer tubes with spray-dried K2EDTA (Becton, Dickinson and Co., Franklin Lakes, NJ) and promptly centrifuged to harvest the plasma which was stored in cryovials at −80 °C. The concentrations of olaparib and onalespib in the plasma were determined independently using analytical methods based upon reversed-phase high-performance liquid chromatography with tandem mass spectrometric detection. The analytical methods were validated and applied to the routine analysis of study samples as recommended in the current U.S. Food and Drug Administration Guidance for Industry (https://www.fda.gov/media/70858/download). At the lowest concentration (free base equivalent) included in the calibration curves, which was 1.0 ng/mL for olaparib and 2.5 ng/mL for onalespib, interday accuracy was within 1.0% of the nominal concentration and the precision was ≤3.4% for both analytes. Interday accuracy ranged from 95.5 to 103.5% and the precision ranged from 2.9 to 6.8% for all other calibration standards for both analytes. Time points were determined as the difference between the blood sample collection time and the time that olaparib was taken or the starting time of the onalespib infusion. The plasma concentration (free base equivalent)–time data were analysed by noncompartmental methods using WinNonlin Professional version 5.0.1 (Pharsight Corp, Mountain View, CA). Pharmacokinetic parameters are reported as the geometric mean (geometric %CV) of the values for individual patients at each dose level. GraphPad Prism for Windows, version 8.3.0 (GraphPad Software, La Jolla, CA) was used for the statistical comparison of mean pharmacokinetic parameters using the paired or unpaired two-tailed t test, as appropriate, after logarithmic transformation of the data. P < 0.05 was the criterion for statistical significance.
Targeted next-generation sequencing of archival EOC
Tumour DNA from archival, formalin-fixed tissues was analysed at the Dana-Farber Cancer Institute (DFCI)/Brigham and Women’s Hospital (BWH) Center for Advanced Molecular Diagnostics (CAMD) using targeted panel next-generation sequencing (Oncopanel) covering exons of 447 cancer-associated genes, plus intronic regions of genes involved in somatic rearrangements [23–25].
Statistical analysis
Activity was measured as an objective response using RECIST 1.1. Safety data were described by the number and proportion of patients who had treatment-related adverse events using CTCAE v5.0. Patient characteristics and adverse event frequencies were summarised using descriptive statistics.
Results
Olaparib/onalespib in HGSOC PDX models
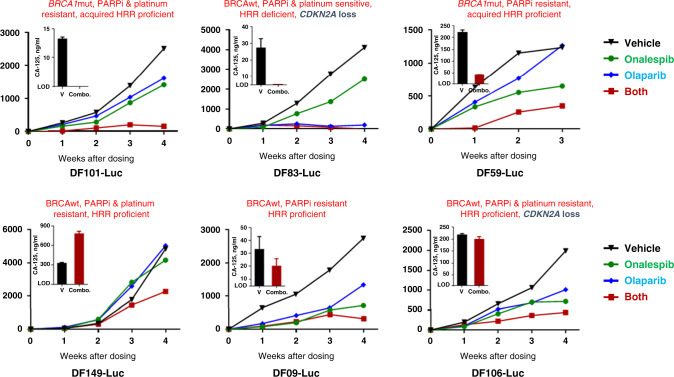
Before embarking on the clinical trial of olaparib plus onalespib, we assessed the tolerability and efficacy of this combination in HGSOC PDX models. We initially performed tolerability studies. As shown in Supplement Fig. S1, doses of olaparib 100 mg/kg/day p.o. daily × 3 weeks and onalespib 45 mg/kg i.p. for 2 days (D1, D2) on/5 days off × 3 weeks (i.e. days 1, 2, 8, 9, 15, 16) were well tolerated without weight loss in the mice. Efficacy studies revealed that the combination of onalespib and olaparib induced inhibition of tumour growth in a variety of ovarian high-grade serous PDX models, including (i) BRCA1/2 wild-type (wt), PARPi-resistant, HRR-proficient models (DF149, DF09, DF106, Fig. 1, bottom panel); (ii) the BRCA1/2 wild-type, PARPi and platinum-sensitive, HRR-deficient DF83 model (Fig. 1, top panel) and (iii) BRCA1-mutated, PARPi-resistant DF101 and DF59 models with acquired HRR proficiency (Fig. 1, top panel). In five of these six models, treatment with olaparib/onalespib was associated with a reduction in the CA125 (DF101, DF83, DF59, DF09 and DF106) while in one model (DF149) CA125 increased (Fig. 1). The phenomenon whereby there is a decrease in tumour burden but the rise in the CA125 is not unusual as it is well described that, particularly in platinum-resistant disease, CA125 is not a reliable marker for assessment of response and progression [26].
Fig. 1. Olaparib and onalespib combination in ovarian PDX models.
NSG mice bearing luciferized PDXs were treated with vehicle, olaparib (100 mg/kg, daily), onalespib (45 mg/kg 2×/week) or their combination for 4 weeks with n = 1 per group per model. Tumour growth was monitored by weekly bioluminescence imaging. Fold change in the bioluminescence from day 0 is shown. Inlay bar graph shows CA125 in mouse plasma at the end of treatment performed in triplicate.
The PARPi (olaparib)-resistant DF149, DF09, DF106, DF101 and DF59 models were determined to be HRR proficient using a RAD51-based IHC assay whereby freshly derived tumour cells from PDX-bearing mice were exposed to radiation and stained with an anti-RAD51 antibody. Consistent with the olaparib response, these five olaparib-resistant models exhibited DNA damage-induced RAD51 foci suggestive of intact HRR. The presence of RAD51 foci in the BRCA1-mutant DF101 and DF59 models suggested that restoration of HRR had occurred thereby explaining the olaparib resistance of these models. Conversely, the olaparib- and platinum-sensitive DF83 model showed the absence of RAD51 foci, consistent with HRR deficiency (this model harbours RAD51C promoter hypermethylation) as previously described [18]. Of note, two of the models (DF83 and DF106) that responded to the combination of onalespib and olaparib, harboured CDKN2A loss while four models exhibited elevated CCNE1 mRNA expression (DF106, DF09, DF149 and DF101) as previously described [18]; no models exhibited CCNE1 amplification. Taken together, activity was observed in both HRR-deficient and -proficient models, BRCAwt and BRCA1-mutated models, as well as PARPi-sensitive and PARPi-resistant models.
Patient accrual
Between September 8, 2017 and November 12, 2019, a total of 28 patients were enrolled into the study; 26 patients were female (Table 1). In total, 18 patients had ovarian cancer, 5 uterine cancer, 2 colon cancer and the remaining 3 had breast cancer (n = 1), melanoma (n = 1) and malignant solitary fibrous tumour (n = 1). The median number of prior lines of therapy was 5.5 (range 1–15). Twenty-one patients were evaluable for DLT; 7 patients were not evaluable for DLT assessment and were replaced. Of these seven patients, two patients developed small bowel obstruction related to disease progression during cycle 0 and 1, two patients withdrew consent during cycle 1 because of advanced disease, two patients could not receive at least 75% of cycle 1 dosing because of advanced disease, and one patient assigned to dose level DL3 was erroneously treated with DL2. This patient was replaced but continued treatment on DL2, was followed for radiographic response and exhibited no DLT (Table 2).
Table 1.
Clinicopathologic characteristics of patients enrolled in the study.
| Overall (n = 28) | ||
|---|---|---|
| N | % | |
| Gender | ||
| Female | 26 | 92.9% |
| Male | 2 | 7.1% |
| Age | ||
| Mean (SD) | 61.5 (9.2) | |
| Median (IQR) [min, max] | 65 (11.3) [45,78] | |
| Race | ||
| White | 25 | 89.3% |
| Asian | 1 | 3.6% |
| Unknown | 2 | 7.1% |
| Ethnicity | ||
| Not Hispanic or Latino | 28 | 100% |
| ECOG performance status | ||
| 0 = asymptomatic and fully active | 17 | 60.7% |
| 1 = symptomatic; fully ambulatory; restricted in physically strenuous activity | 11 | 39.3% |
| Primary site of disease | ||
| Ovary | 18 | 64.3% |
| Uterus | 5 | 17.9% |
| Colon | 2 | 7.1% |
| Breast | 1 | 3.6% |
| Melanoma | 1 | 3.6% |
| Malignant solitary fibrous tumour | 1 | 3.6% |
| Stage at diagnosis | ||
| IC | 1 | 3.6% |
| IIC | 1 | 3.6% |
| III | 3 | 10.7% |
| IIIC | 5 | 17.9% |
| IV | 14 | 50% |
| IVB | 4 | 14.3% |
| Histology | ||
| Well differentiated | 2 | 7.1% |
| Moderately differentiated | 2 | 7.1% |
| Poorly differentiated | 15 | 53.6% |
| Grade cannot be assessed/unknown | 9 | 32.1% |
| Lines of prior therapy | ||
| Median [min, max] | 5.5 | 1–15 |
Table 2.
Dose levels explored, number of patients on each dose level, and whether DLTs were observed.
| Dose level | Cycle 0 dose (olaparib p.o. b.i.d.) | Cycle 1 + dose (olaparib p.o. b.i.d.; onalespib i.v. on days 1, 2, 8, 9, 15, and 16) | Total patients | Evaluable patients for DLTa | DLTs (aetiology) |
|---|---|---|---|---|---|
| DL0 | Olaparib 200 mg | Olaparib 200 mg; onalespib 20 mg/m2 | 3 | 3 | 0 |
| DL1 | Olaparib 200 mg | Olaparib 200 mg; onalespib 40 mg/m2 | 4 | 3 | 0 |
| DL2 | Olaparib 300 mg | Olaparib 300 mg; onalespib 40 mg/m2 | 6 | 3 | 0 |
| DL3 | Olaparib 300 mg | Olaparib 300 mg; onalespib 80 mg/m2 | 7 | 5 | 2 (both Gr3 anaemia) |
| DL2a | Olaparib 200 mg | Olaparib 200 mg; onalespib 80 mg/m2 | 3 | 3 | 0 |
| DL3a | Olaparib 200 mg | Olaparib 200 mg; onalespib 120 mg/m2 | 5 | 4 | 1 (Gr3 thrombocytopenia) |
aSeven patients were not evaluable for DLT assessment and were replaced. Of these seven patients, two patients developed small bowel obstruction related to disease progression during cycle 0 and 1 (DL1 and DL2), two patients withdrew consent during cycle 1 because of advanced disease (DL2 and DL3), two patients could not receive at least 75% of cycle 1 dosing because of advanced disease (DL2 and DL3a), and one patient assigned to dose level DL3 was erroneously treated with DL2 (this patient was replaced but remained on treatment on DL2, was followed for radiographic response and exhibited no DLT).
Dose escalation and toxicities
Six dose levels were evaluated (Table 2). The intended starting dose (defined as dose level (DL) 0) was olaparib 200 mg p.o. twice daily and onalespib 20 mg/m2 and was well tolerated without DLTs (0 DLTs out of three evaluable patients). The next dose level (DL1) of onalespib 40 mg/m2 plus olaparib 200 mg b.i.d. was also well-tolerated without DLTs (0 DLTs among three evaluable patients). The next dose level (DL2) of onalespib 40 mg/m2 plus olaparib 300 mg b.i.d. was also well-tolerated without DLTs (0 DLTs among three evaluable patients). However, when the onalespib was escalated to 80 mg/m2 with olaparib 300 mg b.i.d. (DL3) there were 2 DLTs (both anaemia) in five evaluable patients. When olaparib was de-escalated back to 200 mg b.i.d. with onalespib 80 mg/m2 (DL2a), this dose level was well-tolerated without DLTs (0 DLTs among three evaluable patients). The next dose level (DL3a) of olaparib 200 mg b.i.d. with onalespib 120 mg/m2 did not complete evaluation (1 DLT (thrombocytopenia) among four evaluable patients) as enrolment was suspended due to discontinuation of further development of onalespib.
Treatment-related non-haematologic and haematologic toxicities that were Grade 3+ or occurred in ≥10% of all treated patients are listed in Table 3. There were no unexpected toxicities observed based on the known toxicities of olaparib and onalespib and no irreversible toxicities. Diarrhoea (expected toxicity of onalespib), nausea and anaemia (expected toxicities of both olaparib and onalespib) were the most common treatment-related toxicities, predominantly Grades 1 and 2. Grade 3 anaemia was observed in five patients (18%). There was only one Grade 4 toxicity (neutropenia) and no Grade 5 toxicities.
Table 3.
Treatment-related toxicities that are grade 3 + or occurring in ≥ 10% of patients (n = 28).
| Toxicity | Maximum grade | ||
|---|---|---|---|
| 1–2 | 3 | 4 | |
| Diarrhoea | 21 (75%) [3–3-3–5-3–4]a | 1 (4%) DL3a | — |
| Nausea | 18 (64%) [1–2–4–6-3–2] | 1 (4%) DL0 | — |
| Anaemia | 13 (46%) [2–3–2–4-1–1] | 5 (18%) 2 DL3, 3 DL3a | — |
| Thrombocytopenia/platelet count decrease | 10 (36%) [0–3-1–3-0–3] | 2 (7%) DL3, DL3a | — |
| Fatigue | 12 (43%) [1–3–3–2-1–2] | — | — |
| Vomiting | 8 (29%) [1–1–3–2-1-0] | 1 (4%) DL0 | — |
| Abdominal pain | 4 (14%) [1–1-0-0-2–0] | 1 (4%) DL2 | — |
| Anorexia | 5 (18%) [1–1-1-0-1–1] | — | — |
| Neutrophil count decreased | 3 (11%) [0-0-1–1-0-1] | — | 1 (4%) DL3a |
| Lymphocyte count decreased | 3 (11%) [0-0-0-1–1-1] | 1 (4%) DL3a | — |
| Floaters | 4 (14%) [0-1-0-1-0-2] | — | — |
| Dysgeusia | 4 (14%) [0-1-0-1-0-2] | — | — |
aCorresponding to DL0-DL1-DL2-DL3-DL2a-DL3a, respectively.
Pharmacokinetic (PK) studies
Mean values of selected steady-state pharmacokinetic parameters for olaparib and onalespib are presented in Tables 4 and 5, respectively. At each dose level evaluated, there were no significant differences between the mean values of the olaparib pharmacokinetic parameters determined when it was given alone on cycle 0 day 7 as compared to days 1 and 15 of cycle 1, when olaparib was taken immediately after completing the IV infusion of onalespib. In addition, there was no evidence of a trend in the mean apparent oral clearance of olaparib with respect to the onalespib dose. There were no significant differences in the mean onalespib pharmacokinetic parameters on cycle 1 day 15 as compared to cycle 1 day 1. Mean values of the olaparib steady-state pharmacokinetic parameters determined in this study were in very good agreement with data reported for the drug when given alone [27]. In addition, values of the mean CL of onalespib at each dose level were within the range previously reported in the initial Phase I clinical trial of onalespib given as a single agent to solid tumour patients, which was 40.9 to 69.1 L/h/m2 [20]. Accordingly, the coadministration of olaparib and onalespib does not appear to affect the steady-state pharmacokinetics of either agent.
Table 4.
Mean steady-state pharmacokinetic parameters for olaparib given alone (cycle 0) and combined with onalespib (cycle 1).a
| Dose | Dose | No. of | Cmin | Cmax | AUCt | CL/F | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| level | (mg) | Visit | patients | (ng/mL) | (ng/mL) | (ng h/mL) | (L/h) | ||||
| 0 | 200 | C0D7 | 3 | 1134 | (35.8) | 5793 | (12.2) | 34,481 | (28.7) | 5.80 | (28.7) |
| 0 | 200 | C1D1 | 3 | 783 | (97.2) | 5173 | (74.9) | 30,525 | (77.6) | 6.55 | (77.6) |
| 0 | 200 | C1D15 | 3 | 1350 | (195) | 6218 | (37.5) | 37,072 | (75.1) | 5.39 | (75.1) |
| 1 | 200 | C0D7 | 4 | 955 | (27.4) | 7058 | (11.0) | 36,972 | (12.4) | 5.41 | (12.4) |
| 1 | 200 | C1D1 | 4 | 1069 | (38.6) | 6155 | (18.2) | 36,004 | (19.3) | 5.55 | (19.3) |
| 1 | 200 | C1D15 | 3 | 895 | (56.3) | 6135 | (8.8) | 31,830 | (22.1) | 6.28 | (22.1) |
| 2 | 300 | C0D7 | 7b | 2189 | (51.8) | 9598 | (24.6) | 60,713 | (28.8) | 4.94 | (28.8) |
| 2 | 300 | C1D1 | 7b | 2230 | (50.5) | 9920 | (20.7) | 62,622 | (22.7) | 4.79 | (22.7) |
| 2 | 300 | C1D15 | 4 | 1891 | (56.4) | 8232 | (44.7) | 54,057 | (46.6) | 5.55 | (46.6) |
| 3 | 300 | C0D7 | 5 | 2408 | (111) | 9092 | (50.5) | 50,493 | (56.6) | 5.94 | (56.6) |
| 3 | 300 | C1D1 | 5 | 1229 | (29.9) | 8095 | (48.8) | 47,415 | (35.0) | 6.33 | (35.0) |
| 3 | 300 | C1D15 | 3 | 1326 | (34.3) | 6734 | (9.3) | 44,184 | (12.6) | 6.79 | (12.6) |
| 2a | 200 | C0D7 | 3 | 1837 | (1.6) | 7755 | (9.3) | 46,710 | (14.0) | 4.28 | (14.0) |
| 2a | 200 | C1D1 | 3 | 1579 | (41.8) | 7516 | (29.0) | 47,319 | (25.2) | 4.23 | (25.2) |
| 2a | 200 | C1D15 | 3 | 1567 | (49.7) | 8267 | (20.3) | 49,677 | (21.3) | 4.03 | (21.3) |
| 3a | 200 | C0D7 | 5 | 1380 | (106) | 7670 | (24.3) | 45,266 | (25.8) | 4.42 | (25.8) |
| 3a | 200 | C1D1 | 5 | 1507 | (25.1) | 8644 | (13.5) | 50,651 | (28.2) | 3.95 | (28.2) |
| 3a | 200 | C1D15 | 2 | 1327 | (5.0) | 7085 | (2.1) | 42,602 | (0.7) | 4.69 | (0.7) |
aData are reported as the geometric mean (geometric %CV).
bOne patient assigned to DL3 was erroneously treated with DL2 and included in the DL2 for the PK studies.
Table 5.
Mean steady-state pharmacokinetic parameters for onalespiba.
| Dose | Dose | No. of | Cmax | AUC24 | CL | Vz | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| level | (mg/m2) | Visit | patients | (ng/mL) | (ng h/mL) | (L/h/m2) | (L/m2) | ||||
| 0 | 20 | C1D1 | 3 | 51.2 | (62.9) | 312.8 | (32.8) | 52.3 | (34.3) | 777 | (28.9) |
| 0 | 20 | C1D15 | 2 | 46.7 | (8.2) | 305.8 | (12.3) | 49.7 | (21.6) | 899 | (1.6) |
| 1 | 40 | C1D1 | 4 | 128.2 | (61.3) | 672.8 | (10.9) | 49.4 | (13.4) | 1,290 | (179) |
| 1 | 40 | C1D15 | 3 | 116.8 | (78.4) | 657.5 | (6.8) | 51.1 | (5.4) | 732 | (3.2) |
| 2 | 40 | C1D1 | 4 | 140.6 | (188.5) | 602.4 | (48.9) | 57.0 | (48.2) | 786 | (34.8) |
| 2 | 40 | C1D15 | 3 | 196.7 | (115) | 782.7 | (53.4) | 44.7 | (52.8) | 561 | (53.6) |
| 3 | 80 | C1D1 | 5 | 238.4 | (94.4) | 1207.0 | (17.3) | 58.3 | (14.7) | 718 | (15.4) |
| 3 | 80 | C1D15 | 3 | 278.2 | (64.1) | 1073.2 | (23.6) | 69.2 | (25.2) | 662 | (28.4) |
| 2a | 80 | C1D1 | 3 | 271.6 | (127) | 1461.7 | (59.0) | 47.1 | (57.7) | 624 | (50.2) |
| 2a | 80 | C1D15 | 3 | 267.0 | (175) | 1241.7 | (52.6) | 57.0 | (46.9) | 684 | (57.1) |
| 3a | 120 | C1D1 | 5 | 560.3 | (99.1) | 2082.8 | (34.2) | 49.4 | (33.4) | 614 | (59.4) |
| 3a | 120 | C1D15 | 2 | 390.3 | (38.5) | 2003.1 | (11.6) | 53.7 | (8.0) | 597 | (16.6) |
aData are reported as the geometric mean (geometric %CV).
Clinical efficacy and targeted next-generation sequencing
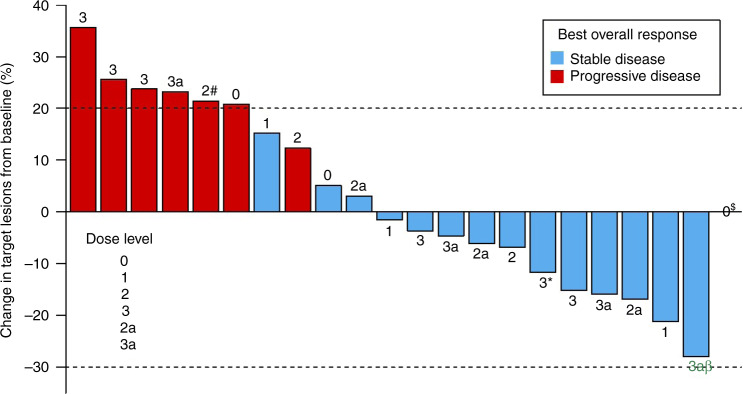
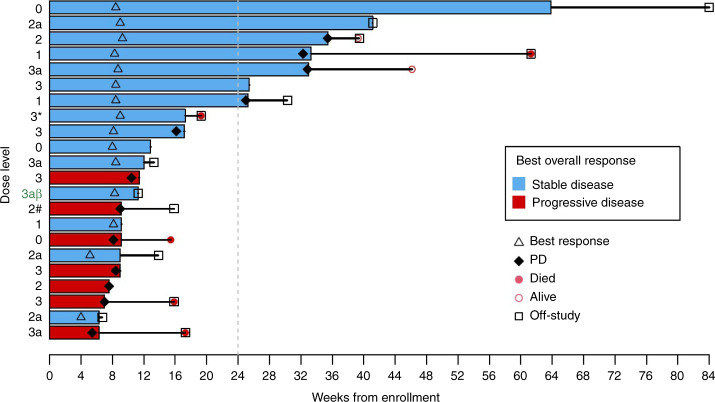
Among the 22 patients who had at least one restaging scan in the study, there were no objective responses by RECIST 1.1 (Fig. 2). One patient with ovarian carcinosarcoma experienced shrinkage of her tumour by 28%; this patient decided to withdraw after three cycles of protocol therapy. Fifteen patients (68%) had stable disease as a best radiologic response, while seven (32%) had progressive disease (PD) (Fig. 2). Seven (32%) patients derived clinical benefits from this regimen with stable disease for at least 24 weeks (Fig. 3).
Fig. 2. Response to study treatment (n = 22).
Summary of the maximum reduction in the sum of target lesion sizes. Dose level is indicated for each patient. $Patient in DL0 in the far right had no measurable disease. *This patient was replaced due to being enrolled under DL3 but treated at DL2. #This patient was replaced due to not having received 75% of Cycle 1 dosing. βThis patient had tumour shrinkage by 28%.
Fig. 3. Duration of treatment and time to progression (n = 22).
Duration and time to progression are defined as the interval from the date of enrolment to date of either progression or date of last disease assessment. *This patient was replaced due to being enrolled under DL3 but treated at DL2. #This patient was replaced due to not having received 75% of cycle 1 dosing. βThis patient had tumour shrinkage by 28%.
We performed targeted next-generation sequencing (Oncopanel) in archival, formalin-fixed tumour specimens from the seven patients who derived clinical benefit and the one patient who experienced tumour shrinkage by 28% (Table 6). Two patients had BRCA-mutated high-grade serous ovarian cancer (HGSOC) and had both progressed through prior PARP inhibitor therapy twice. The first patient with BRCA1-mutated HGSOC had progressed through olaparib and niraparib on two separate previous occasions and completed six cycles of olaparib/onalespib before progression. The second patient with BRCA2-mutated ovarian cancer had progressed through olaparib/alpelisib (PI3K inhibitor) and olaparib/prexasertib (CHK1 inhibitor) previously and completed ten cycles before withdrawing after learning that development of onalespib was discontinued. Two other patients among those with disease stabilisation ≥24 weeks exhibited alterations involving the RB pathway; one patient with uterine serous cancer had biallelic RB1 loss and completed eight cycles and one patient with HGSOC exhibited CCNE1 gain and completed six cycles. Finally, CCNE1 amplification was observed in the patient who experienced tumour shrinkage by 28%.
Table 6.
Genomic aberrations among patients with stable disease ≥24 weeks and in the patient with 28% tumour shrinkage.
| Patient | Tumour type | Best response | Cycles | Prior lines | Prior PARPi | Relevant genomic alterations |
|---|---|---|---|---|---|---|
| 1 | Ovarian (HGSOC) | SD | 14 | 11 | NO | ARID1A, TP53 mutations |
| 2 | Uterine serous | SD | 8 | 3 | NO | KRAS, PIK3CA, TP53 mutations, RB1 mutation and copy loss |
| 3 | HGSOC | SD | 6 | 7 | Yes (x2) | gBRCA1, TP53 mutations |
| 4 | Malignant solitary fibrous tumour | SD | 8 | 2 | NO | PIK3CA mutation |
| 5 | HGSOC | SD | 6 | 4 | NO | TP53 mutation, CCNE1 gain |
| 6 | HGSOC | SD | 10 | 7 | Yes (x2) | BRCA2, TP53 mutations |
| 7 | Uterine high-grade endometrioid | SD | 8 | 3 | NO | TP53, CTNNB1, PTEN mutations |
| 8 | Ovarian carcinosarcoma | SD (−28%) | 3 | 7 | NO | TP53 mutation, CCNE1 amplification |
Discussion
This Phase 1 dose-escalation study showed that combining the HSP90i onalespib and the PARPi olaparib is feasible, with no unexpected toxicities or safety signals identified. Although the majority of patients enrolled were heavily pretreated, the olaparib and onalespib combination was well tolerated and myelosuppression was not an issue. Adverse events that defined DLTs included anaemia in two patients and thrombocytopenia in one patient. Dose levels up to olaparib 300 mg/onalespib 40 mg (DL1) and olaparib 200 mg/onalespib 80 mg (DL2a) were deemed safe without any DLTs, but dose level DL3a (olaparib 200 mg/onalespib 120 mg) did not complete evaluation (one DLT in four evaluable patients) as enrolment was suspended due to discontinuation of further development of onalespib. PK analysis indicated that the coadministration of olaparib and onalespib does not appear to affect the steady-state pharmacokinetics of either agent.
Although there were no objective responses by RECIST, 7 (32%) patients exhibited disease stabilisation for ≥24 weeks and one additional patient experienced shrinkage of her tumour by 28%; this patient decided to withdraw from the study after three cycles of protocol therapy. This activity is clinically meaningful considering that most of these patients were heavily pretreated (four patients with ≥7 prior lines and three with ≥3 prior lines) and had no alternative treatment options. Among the seven patients with disease stabilisation, two patients had BRCA-mutated HGSOC (1 BRCA1-mutated and 1 BRCA2-mutated) that had progressed twice through prior PARPi therapy. Activity in that setting is consistent with our preclinical findings from the PDX models whereby olaparib/onalespib demonstrated efficacy against BRCA1-mutated models with acquired PARPi resistance due to restoration of HRR proficiency (DF101 and DF59 models, Fig. 1, top panel).
Of note, alterations involving the RB pathway were identified in two patients among those with disease stabilisation ≥24 weeks (one patient with HGSOC exhibited CCNE1 gain and one patient with uterine serous cancer had biallelic RB1 loss) and in the patient who experienced tumour shrinkage by 28% (HGSOC with CCNE1 amplification). Loss of RB-pathway regulation is common in both uterine and ovarian high-grade serous tumours, and may occur via CCNE1 amplification, RB1 alterations (mutations or homozygous deletion), and CDKN2A loss. CCNE1, RB1 and CDKN2A alterations are mutually exclusive, reflecting a strong selection pressure for loss of RB-pathway regulation and rapid G1- >S phase entry in these tumours [28]. These observations are again consistent with our preclinical findings from the PDX models whereby olaparib/onalespib also demonstrated activity in tumours with RB-pathway alterations, i.e. models with CDKN2A loss and CCNE1 overexpression. Mechanistically, cyclin E activates CDK2 which phosphorylates RB leading to activation of E2F transcriptional activity and G1- >S phase progression [29, 30]. Interestingly, cyclin E is a client protein of HSP90 raising the possibility that abrogation of its activity may contribute to the activity of olaparib/onalespib against CCNE1-amplified or overexpressing tumours [31, 32]. This activity may also be explained by the fact that CCNE1-amplified tumours are dependent on HRR and sensitive to abrogation of BRCA1 [33]; therefore, downregulation of BRCA1 and other HRR proteins by onalespib, may also explain the activity against CCNE1-amplified/overexpressing tumours. Loss of CDKN2A leads to activation of CDK4 and CDK6, which similarly phosphorylate RB leading to activation of E2F transcriptional activity and accelerated G1- >S phase transition [30, 34]. As with cyclin E, CDK4 and CDK6 are also client proteins of HSP90 suggesting that the activity against tumours with CDKN2A loss may be related to abrogation of CDK4 and CDK6 activity by HSP90i [32, 35–37]. Finally, activity in tumours with biallelic RB1 loss may be explained by increased E2F1 activity with subsequent increase in CCNE1 transcription in a feed-forward loop that would similarly facilitate susceptibility to HSP90i. Another mechanism may be relevant in HGSOC whereby there is the correlation (co-occurrence) between RB1 loss and HRR deficiency (i.e., RB1 loss co-occurs with HRR deficiency) [38]. Therefore, if tumours with RB1 loss are also HRR deficient, they should respond to PARPi alone; however, if they have become HRR proficient (due to prior platinum or PARPi exposure), then the activity may be explained by HSP90i reversal of acquired HRR proficiency and sensitisation to PARPi as discussed above.
Taken together, the combination of olaparib and onalespib was feasible, and exhibited some preliminary evidence of activity manifesting as prolonged disease stabilisation, in this heavily pretreated patient population with advanced solid tumours. Data from PDX models and clinical data from patients indicate that this combination may provide disease stabilisation in patients with BRCA-mutated HGSOCs and acquired PARPi resistance as well as patients with tumours harbouring RB-pathway alterations such as CCNE1 amplification, CDKN2A loss and RB1 loss. Mechanistically, abrogation of HRR may explain the activity against BRCA-mutated HGSOCs with acquired PARPi resistance, while destabilization of cyclin E and of CDK4/CDK6 may explain the activity against tumours with CCNE1 amplification and CDKN2A loss, respectively.
Moving forward, further development of onalespib has been discontinued due to its limited efficacy in monotherapy and combination studies. Therefore, we do not anticipate any further development of the olaparib/onalespib combination. However, it was recently announced that an alternative HSP90 inhibitor TAS-116 (pimitespib), which is administered orally, met its primary endpoint of prolongation of PFS in patients with previously treated gastrointestinal stromal tumours (GISTs) in a pivotal Phase III trial (CHAPTER-GIST-301 trial). Pimitespib is a novel, selective inhibitor of cytosolic HSP90a and b that does not inhibit HSP90 paralogs such as endoplasmic reticulum GRP94 or mitochondrial TRAP1 and has a different structure from other HSP90 inhibitors resulting in less hepatic and neurologic toxicity [39]. Furthermore, oral administration allows for a more flexible dosing schedule compared with intravenous administration. In addition, there is now interest in alternative ways of inhibiting HSP90 such as targeting cell division cycle 37 (CDC37), a ubiquitous co-chaperone of HSP90 that directs client proteins into the HSP90 chaperone cycle [5]. In this regard, small-molecule inhibitors of CDC37 or its interaction with HSP90 have shown promising preclinical activity against several tumour models [40–43]. Of interest, ATP competitive CDK4/6 inhibitors can also block CDC37 binding and deprive CDK4 and CDK6 access to the Hsp90 chaperone system [35]. Therefore, although further development of olaparib/onalespib is not anticipated, it is our hope that the preclinical and clinical data presented here may support future evaluation of novel combinations of olaparib (or other PARP inhibitors) with alternative HSP90 inhibitors such as pimitespib or novel agents that inhibit CDC37 or its interaction with HSP90.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank all the patients and their families for participation in the present trial.
Author contributions
PAK conceived, supervised, coordinated, and analysed all the data and wrote the manuscript; S-CC performed the statistical analysis and was the lead statistician of the study; JGS performed the PK analyses; MP, BB, HS, PB, MH and JC contributed to data collection and coordinated the processing and distribution of the clinical trial samples; AEW, PI, NH, AAW and SMC provided clinical data and contributed to the analysis of data; EVI, CPP, SP, JFL, ADD and DC performed and contributed to the preclinical studies and to the analysis of data; UAM and GIS supported and supervised the analyses of this study. All authors contributed to the writing and editing of the manuscript.
Funding
The preclinical work was supported in part by the Department of Defense Ovarian Cancer Research Program Award DOD W81XWH-15-0564/OC140632 (PAK, Principal Investigator). The trial (clinicalTrials.gov Identifier: NCT02898207) was sponsored by the NCI-Cancer Therapy Evaluation Program (CTEP) and conducted under the auspices of the Experimental Therapeutics Clinical Trials Network (ETCTN). Dana-Farber/Harvard Cancer Center served as the lead site for the study, supported by NIH grant UM1 CA186709 (GIS, Principal Investigator). SPI is an employee of NIH and a member of NCI-CTEP as Associate Chief of the Investigational Drug Branch and Program Director of the ETCTN.
Ethics approval and consent to participate
The clinical trial was approved by the NCI Central Institutional Review Board (CIRB) and the US Food and Drug Administration (NCT02898207). All procedures involving human participants were carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from patients or guardians before enrolment in the study.
Consent to publish
Not applicable.
Competing interests
Dr. Konstantinopoulos has participated in advisory boards for Alkermes, AstraZeneca, GSK, BMS, Repare, Artios and Kadmon, serves in the gynaecologic oncology scientific committee for AstraZeneca and has received institutional support as P.I. of clinical trials from AstraZeneca, Bayer, Eli Lilly, GSK, Merck, Merck KGaA, Pfizer and GSK. Dr. Cloud Paweletz has sponsored research agreements with Daiichi Sankyo, Bicycle Therapeutics, Transcenta, Bicara Therapeutics, AstraZeneca, Intellia Therapeutics, Janssen Pharmaceuticals, Array Biopharma, has stock and other ownership Interests in XSphera Biosciences, has received honoraria from Bio-Rad and ThermoFisher and has consulting or advisory role for DropWorks and XSphera Biosciences. Dr. Liu has participated in advisory boards from AstraZeneca, Clovis, Eisai, EpsilaBio, Genentech, GSK/Tesaro, Regeneron Pharmaceuticals and has received institutional support as P.I. of clinical trials from 2X Oncology, Aravive, Arch Oncology, AstraZeneca, Bristol-Myers Squibb, Clovis Oncology, CytomX Therapeutics, GlaxoSmithKline, Regeneron, Surface Oncology, Tesaro, Vigeo therapeutics. Dr. Campos has participated in the advisory board for AstraZeneca. Dr. Wright has participated in the advisory board for GSK. Dr. Matulonis has participated in advisory boards for Novartis, Blueprint Medicines, Trillium, in data safety monitoring boards for Advaxis and Symphogen and has been a consultant for NextCure, Astrazeneca and Merck. Dr. D’Andrea is a consultant/advisory board member for Lilly Oncology, Merck-EMD-Serono, Intellia Therapeutics, Sierra Oncology, Cyteir Therapeutics, Third Rock Ventures, AstraZeneca, Ideaya Inc., Cedilla Therapeutics Inc., a stockholder in Ideaya Inc., Cedilla Therapeutics Inc., and Cyteir, and reports receiving commercial research grants from Lilly Oncology and Merck-EMD-Serono. Dr. Shapiro has received research funding from Eli Lilly, Merck KGaA/EMD-Serono, Merck, and Sierra Oncology, has served on advisory boards for Pfizer, Eli Lilly, G1 Therapeutics, Roche, Merck KGaA/EMD-Serono, Sierra Oncology, Bicycle Therapeutics, Fusion Pharmaceuticals, Cybrexa Therapeutics, Astex, Almac, Ipsen, Bayer, Angiex, Daiichi Sankyo, Seattle Genetics, Boehringer Ingelheim, ImmunoMet, Asana, Artios, Atrin, Concarlo Holdings, Syros, Zentalis, CytomX Therapeutics and Blueprint Medicines and holds a patent entitled, “Dosage regimen for sapacitabine and seliciclib,” also issued to Cyclacel Pharmaceuticals, and a pending patent, entitled, “Compositions and Methods for Predicting Response and Resistance to CDK4/6 Inhibition,” together with Liam Cornell.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ursula A. Matulonis, Geoffrey I. Shapiro.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01664-8.
References
- 1.Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D’Andrea AD. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov. 2015;5:1137–54. doi: 10.1158/2159-8290.CD-15-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konstantinopoulos PA, Barry WT, Birrer M, Westin SN, Cadoo KA, Shapiro GI, et al. Olaparib and alpha-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: a dose-escalation and dose-expansion phase 1b trial. Lancet Oncol. 2019;20:570–80. doi: 10.1016/S1470-2045(18)30905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veneris JT, Matulonis UA, Liu JF, Konstantinopoulos PA. Choosing wisely: selecting PARP inhibitor combinations to promote anti-tumor immune responses beyond BRCA mutations. Gynecol Oncol. 2020;156:488–97. doi: 10.1016/j.ygyno.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 4.Barrott JJ, Haystead TA. Hsp90, an unlikely ally in the war on cancer. FEBS J. 2013;280:1381–96. doi: 10.1111/febs.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearl LH. Hsp90 and Cdc37—a chaperone cancer conspiracy. Curr Opin Genet Dev. 2005;15:55–61. doi: 10.1016/j.gde.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–94. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- 7.Konstantinopoulos PA, Papavassiliou AG. 17-AAG: mechanisms of antitumour activity. Expert Opin Investig Drugs. 2005;14:1471–4. doi: 10.1517/13543784.14.12.1471. [DOI] [PubMed] [Google Scholar]
- 8.Pennisi R, Ascenzi P, di Masi A. Hsp90: a new player in DNA repair? Biomolecules. 2015;5:2589–618. doi: 10.3390/biom5042589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stecklein SR, Kumaraswamy E, Behbod F, Wang W, Chaguturu V, Harlan-Williams LM, et al. BRCA1 and HSP90 cooperate in homologous and non-homologous DNA double-strand-break repair and G2/M checkpoint activation. Proc Natl Acad Sci USA. 2012;109:13650–5. doi: 10.1073/pnas.1203326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi YE, Battelli C, Watson J, Liu J, Curtis J, Morse AN, et al. Sublethal concentrations of 17-AAG suppress homologous recombination DNA repair and enhance sensitivity to carboplatin and olaparib in HR proficient ovarian cancer cells. Oncotarget. 2014;5:2678–87. doi: 10.18632/oncotarget.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson N, Johnson SF, Yao W, Li YC, Choi YE, Bernhardy AJ, et al. Stabilization of mutant BRCA1 protein confers PARP inhibitor and platinum resistance. Proc Natl Acad Sci USA. 2013;110:17041–6. doi: 10.1073/pnas.1305170110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Do K, Speranza G, Chang LC, Polley EC, Bishop R, Zhu W, et al. Phase I study of the heat shock protein 90 (Hsp90) inhibitor onalespib (AT13387) administered on a daily for 2 consecutive days per week dosing schedule in patients with advanced solid tumors. Invest N Drugs. 2015;33:921–30. doi: 10.1007/s10637-015-0255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lomeli N, Bota DA. Targeting HSP90 in malignant gliomas: onalespib as a potential therapeutic. Transl Cancer Res. 2018;7:6215–26. doi: 10.21037/tcr.2018.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canella A, Welker AM, Yoo JY, Xu J, Abas FS, Kesanakurti D, et al. Efficacy of onalespib, a long-acting second-generation HSP90 inhibitor, as a single agent and in combination with temozolomide against malignant gliomas. Clin Cancer Res. 2017;23:6215–26. doi: 10.1158/1078-0432.CCR-16-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slovin S, Hussain S, Saad F, Garcia J, Picus J, Ferraldeschi R, et al. Pharmacodynamic and clinical results from a phase I/II study of the HSP90 inhibitor onalespib in combination with abiraterone acetate in prostate cancer. Clin Cancer Res. 2019;25:4624–33. doi: 10.1158/1078-0432.CCR-18-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu JF, Palakurthi S, Zeng Q, Zhou S, Ivanova E, Huang W, et al. Establishment of patient-derived tumor xenograft models of epithelial ovarian cancer for pre-clinical evaluation of novel therapeutics. Clin Cancer Res. 2016; 10.1158/1078-0432.CCR-16-1237. [DOI] [PMC free article] [PubMed]
- 17.Kochupurakkal BS, Parmar K, Lazaro J-B, Unitt C, Zeng Q, Reavis H, et al. Abstract 2796: development of a RAD51-based assay for determining homologous recombination proficiency and PARP inhibitor sensitivity. Cancer Res. 2017;77:2796–2796. [Google Scholar]
- 18.Parmar K, Kochupurakkal BS, Lazaro JB, Wang ZC, Palakurthi S, Kirschmeier PT, et al. The CHK1 inhibitor prexasertib exhibits monotherapy activity in high-grade serous ovarian cancer models and sensitizes to PARP inhibition. Clin Cancer Res. 2019;25:6127–40. doi: 10.1158/1078-0432.CCR-19-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu JF, Palakurthi S, Zeng Q, Zhou S, Ivanova E, Huang W, et al. Establishment of patient-derived tumor xenograft models of epithelial ovarian cancer for preclinical evaluation of novel therapeutics. Clin Cancer Res. 2017;23:1263–73. doi: 10.1158/1078-0432.CCR-16-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro GI, Kwak E, Dezube BJ, Yule M, Ayrton J, Lyons J, et al. First-in-human phase I dose escalation study of a second-generation non-ansamycin HSP90 inhibitor, AT13387, in patients with advanced solid tumors. Clin Cancer Res. 2015;21:87–97. doi: 10.1158/1078-0432.CCR-14-0979. [DOI] [PubMed] [Google Scholar]
- 21.Curry J, Fazal L, Graham B, Harada I, Lyons J, Reule M, et al. Significance of long term pharmacodynamic actions of the HSP90 inhibitor AT13387. Cancer Res. 2009;69:1856–1856. [Google Scholar]
- 22.Shimamura T, Perera SA, Foley KP, Sang J, Rodig SJ, Inoue T, et al. Ganetespib (STA-9090), a nongeldanamycin HSP90 inhibitor, has potent antitumor activity in in vitro and in vivo models of non-small cell lung cancer. Clin Cancer Res. 2012;18:4973–85. doi: 10.1158/1078-0432.CCR-11-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sholl LM, Do K, Shivdasani P, Cerami E, Dubuc AM, Kuo FC, et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI insight. 2016;1:e87062. doi: 10.1172/jci.insight.87062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagle N, Berger MF, Davis MJ, Blumenstiel B, Defelice M, Pochanard P, et al. High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov. 2012;2:82–93. doi: 10.1158/2159-8290.CD-11-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia EP, Minkovsky A, Jia Y, Ducar MD, Shivdasani P, Gong X, et al. Validation of OncoPanel: a targeted next-generation sequencing assay for the detection of somatic variants in cancer. Arch Pathol Lab Med. 2017;141:751–8. doi: 10.5858/arpa.2016-0527-OA. [DOI] [PubMed] [Google Scholar]
- 26.Lindemann K, Kristensen G, Mirza MR, Davies L, Hilpert F, Romero I, et al. Poor concordance between CA-125 and RECIST at the time of disease progression in patients with platinum-resistant ovarian cancer: analysis of the AURELIA trial. Ann Oncol. 2016;27:1505–10. doi: 10.1093/annonc/mdw238. [DOI] [PubMed] [Google Scholar]
- 27.Dean E, Middleton MR, Pwint T, Swaisland H, Carmichael J, Goodege-Kunwar P, et al. Phase I study to assess the safety and tolerability of olaparib in combination with bevacizumab in patients with advanced solid tumours. Br J Cancer. 2012;106:468–74. doi: 10.1038/bjc.2011.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancer Genome Atlas Research, N. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorski JW, Ueland FR, Kolesar JM. CCNE1 Amplification as a predictive biomarker of chemotherapy resistance in epithelial ovarian cancer. Diagnostics. 2020;10:279. [DOI] [PMC free article] [PubMed]
- 30.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 31.Bedin M, Gaben AM, Saucier C, Mester J. Geldanamycin, an inhibitor of the chaperone activity of HSP90, induces MAPK-independent cell cycle arrest. Int J Cancer. 2004;109:643–52. doi: 10.1002/ijc.20010. [DOI] [PubMed] [Google Scholar]
- 32.Burrows F, Zhang H, Kamal A. Hsp90 activation and cell cycle regulation. Cell Cycle. 2004;3:1530–6. doi: 10.4161/cc.3.12.1277. [DOI] [PubMed] [Google Scholar]
- 33.Etemadmoghadam D, Weir BA, Au-Yeung G, Alsop K, Mitchell G, George J, et al. Synthetic lethality between CCNE1 amplification and loss of BRCA1. Proc Natl Acad Sci USA. 2013;110:19489–94. doi: 10.1073/pnas.1314302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558–72. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- 35.Hallett ST, Pastok MW, Morgan RML, Wittner A, Blundell K, Felletar I, et al. Differential regulation of G1 CDK complexes by the Hsp90-Cdc37 chaperone system. Cell Rep. 2017;21:1386–98. doi: 10.1016/j.celrep.2017.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamphere L, Fiore F, Xu X, Brizuela L, Keezer S, Sardet C, et al. Interaction between Cdc37 and Cdk4 in human cells. Oncogene. 1997;14:1999–2004. doi: 10.1038/sj.onc.1201036. [DOI] [PubMed] [Google Scholar]
- 37.Vaughan CK, Gohlke U, Sobott F, Good VM, Ali MM, Prodromou C, et al. Structure of an Hsp90-Cdc37-Cdk4 complex. Mol Cell. 2006;23:697–707. doi: 10.1016/j.molcel.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garsed DW, Alsop K, Fereday S, Emmanuel C, Kennedy CJ, Etemadmoghadam D, et al. Homologous recombination DNA repair pathway disruption and retinoblastoma protein loss are associated with exceptional survival in high-grade serous ovarian cancer. Clin Cancer Res. 2018;24:569–80. doi: 10.1158/1078-0432.CCR-17-1621. [DOI] [PubMed] [Google Scholar]
- 39.Ohkubo S, Kodama Y, Muraoka H, Hitotsumachi H, Yoshimura C, Kitade M, et al. TAS-116, a highly selective inhibitor of heat shock protein 90alpha and beta, demonstrates potent antitumor activity and minimal ocular toxicity in preclinical models. Mol Cancer Ther. 2015;14:14–22. doi: 10.1158/1535-7163.MCT-14-0219. [DOI] [PubMed] [Google Scholar]
- 40.Polier S, Samant RS, Clarke PA, Workman P, Prodromou C, Pearl LH. ATP-competitive inhibitors block protein kinase recruitment to the Hsp90-Cdc37 system. Nat Chem Biol. 2013;9:307–12. doi: 10.1038/nchembio.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siddiqui FA, Parkkola H, Manoharan GB, Abankwa D. Medium-throughput detection of Hsp90/Cdc37 protein-protein interaction inhibitors using a split Renilla luciferase-based assay. SLAS Discov. 2020;25:195–206. doi: 10.1177/2472555219884033. [DOI] [PubMed] [Google Scholar]
- 42.Siddiqui FA, Parkkola H, Vukic V, Oetken-Lindholm C, Jaiswal A, Kiriazis A, et al. Novel small molecule Hsp90/Cdc37 interface inhibitors indirectly target K-Ras-signaling. Cancers. 2021;13:927. [DOI] [PMC free article] [PubMed]
- 43.Zhang Q, Wu X, Zhou J, Zhang L, Xu X, Zhang L, et al. Design, synthesis and bioevaluation of inhibitors targeting HSP90-CDC37 protein-protein interaction based on a hydrophobic core. Eur J Med Chem. 2021;210:112959. doi: 10.1016/j.ejmech.2020.112959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.