Abstract
The P450 enzyme, CYP3A4, extensively metabolizes both amprenavir and clarithromycin. To determine if an interaction exists when these two drugs are coadministered, the pharmacokinetics of amprenavir and clarithromycin were investigated in healthy adult male volunteers. This was a Phase I, open-label, randomized, balanced, multiple-dose, three-period crossover study. Fourteen subjects received the following three regimens: amprenavir, 1,200 mg twice daily over 4 days (seven doses); clarithromycin, 500 mg twice daily over 4 days (seven doses); and the combination of the above regimens over 4 days (seven doses of each drug). Twelve subjects completed all treatments and the follow-up period. The erythromycin breath test (ERMBT) was administered at baseline, 2 h after the final dose of each of the three regimens and at the first follow-up visit. Coadministration of clarithromycin and amprenavir significantly increased the mean amprenavir AUCss, Cmax,ss, and Cmin,ss by 18, 15, and 39%, respectively. Amprenavir had no significant effect on the AUCss of clarithromycin, but the median Tmax,ssfor clarithromycin increased by 2.0 h, renal clearance increased by 34%, and the AUCss for 14-(R)-hydroxyclarithromycin decreased by 35% when it was given with amprenavir. Amprenavir and clarithromycin reduced the ERMBT result by 85 and 67%, respectively, and by 87% when the two drugs were coadministered. The baseline ERMBT value did not correlate with clearance of amprenavir or clarithromycin. A pharmacokinetic interaction occurs when amprenavir and clarithromycin are coadministered, but the effects are not likely to be clinically important, and coadministration does not require a dosage adjustment for either drug.
Amprenavir (Agenerase, USAN approved, VX-478, 141W94; Glaxo Wellcome Inc., Research Triangle Park, N.C.) is a new human immunodeficiency virus type 1 (HIV-1) protease inhibitor which has potent in vitro and in vivo activity (1, 14, 21). All of the currently available protease inhibitors are metabolized by the hepatic microsomal P450 enzyme, CYP3A4, which is the major isoform involved in the metabolism of many drugs (5). In vitro data indicate that amprenavir is also extensively metabolized by CYP3A4 (4, 20), and investigations in humans reveal that <2% of the administered dose appears in the urine as unchanged drug (27). Preclinical studies in rats in which amprenavir was administered in combination with ritonavir, a potent CYP3A4 inhibitor, resulted in an approximately eightfold increase in the area under the concentration-time curve from 0 to 8 h (AUC0–8) of amprenavir (11). In addition, human studies have demonstrated that the AUC of amprenavir is increased when it is administered with ketoconazole, another potent inhibitor of CYP3A4 (16).
Mycobacterium avium complex (MAC) disease is one of the most common opportunistic infections affecting AIDS patients at the terminal stage of illness, and the U.S. Public Health Service has recommended chemoprophylaxis when a patient's CD4+ cell count decreases to below 50 cells/μl (22). Clarithromycin is indicated for the chemoprophylaxis and treatment of disseminated MAC disease, and significant numbers of HIV-infected patients receiving amprenavir may also be treated with clarithromycin. Because clarithromycin is a well-known inhibitor of CYP3A4 and has been shown to result in a pharmacokinetic interaction when it is given with other protease inhibitors (5, 15; prescribing information for indinavir [Crixivan; Merck and Company Pharmaceuticals, West Point, Pa.], ritonavir [Norvir; Abbott Laboratories, Abbott Park, Ill.]), and saquinavir [Invirase; Roche Laboratories, Nutley, N.J.]), this study was undertaken to determine if a pharmacokinetic interaction occurs when amprenavir and clarithromycin are coadministered.
The erythromycin breath test (ERMBT) is a measure of hepatic CYP3A4 activity (26; ERMBT product information, Metabolic Solutions Inc., Nashua, N.H.) and has previously been used to measure the inhibition of hepatic CYP3A4 activity by drugs used in the treatment of HIV (2). The inclusion of the ERMBT in this study was intended to evaluate the following questions. (i) Is amprenavir an inhibitor of hepatic CYP3A4 in vivo? (ii) What is the relative potency of amprenavir as an inhibitor of hepatic CYP3A4, compared to clarithromycin? (iii) Do the results of the ERMBT help explain the pharmacokinetics of clarithromycin and amprenavir when administered alone and in combination?
(This work was presented at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., September 1998.)
MATERIALS AND METHODS
Subjects.
Fourteen healthy, nonsmoking men, aged 18 to 36 years, were enrolled in this study, which was approved by the Virginia Commonwealth University (VCU) Committee on the Conduct of Human Research. Subjects gave their written informed consent. A complete medical history, a physical examination, including vital signs, and routine laboratory tests that included a 13-test chemistry screen, complete blood count with differential, urinalysis, urine drug screen for illicit controlled substances, HIV test, and electrocardiogram were completed for each subject. Subjects were ineligible if they had a clinically significant abnormality at the screening evaluation, were currently participating in another research study or had participated in another research study within the past month, had donated >1 pint of whole blood within the past month, were receiving concurrent medication(s) which could not be withheld for the duration of their participation in the study, or had a prior adverse reaction to clarithromycin, erythromycin, or another macrolide antibiotic. Subjects were instructed to use a barrier method of contraception (i.e., condoms) while enrolled in the study and for a minimum of 1 month after administration of their last dose of study drug(s). Additionally, subjects abstained from taking concomitant medications and from consuming alcohol from 48 h before the first dose of study drug(s) until discharge from the study center following completion of the treatment phase. The same restrictions were placed on the consumption of grapefruit and grapefruit juice. Tea, coffee, chocolate, and other beverages and foods containing methylxanthines were prohibited on each blood sampling day for pharmacokinetic evaluation.
Experimental design and procedures.
This was a Phase I, open label, randomized, balanced, multiple-dose, three-period crossover study conducted at the School of Pharmacy Center for Drug Studies, Virginia Commonwealth University/Medical College of Virginia Campus. This study consisted of a screening evaluation (as noted above), three separate treatment periods, and a follow-up evaluation. The screening evaluation was scheduled 14 days before administration of the first dose of study drug(s). Subjects successfully completing the screening evaluation were randomized, based on two 3 by 3 Latin squares, to three treatments (below) in a balanced, crossover fashion. Specifically, two subjects were randomly assigned to each of six treatment sequences: 1/2/3, 1/3/2, 2/1/3, 2/3/1, 3/1/2, and 3/2/1, and two replacement subjects (below) were in treatment sequences 1/3/2 and 2/1/3, respectively.
Treatment 1 consisted of 1,200 mg of amprenavir twice daily over 4 days (seven doses); treatment 2, of 500 mg of clarithromycin twice daily over 4 days (seven doses); and treatment 3, of the combination of 1,200 mg of amprenavir and 500 mg of clarithromycin, twice daily over 4 days (seven doses).
To ensure compliance, subjects were required to complete a diary card recording the exact time of dosing, the number of capsules and/or tablets taken, and any missed doses while self-administering the study drug(s) at home or at work. When they were at the study center, dosing was performed under the supervision of staff.
Treatment period 1 included dosing days 1 to 4; treatment period 2 included dosing days 5 to 8; and treatment period 3 included dosing days 9 to 12. There was no washout period between treatments; subjects began dosing with the second and third treatments the morning after completing the preceding treatment. On the first dosing day of each treatment period, the subjects were discharged from the study center in the morning after receiving the first dose under the supervision of study center personnel. Subjects self-administered the second dose on the evening of the first dosing day, the third and fourth doses on the second dosing day, and the fifth dose on the morning of the third dosing day. Subjects were admitted to the study center and were administered the sixth dose on the evening of the third dosing day. The seventh and last dose of each treatment period was administered in the study center the morning of the fourth dosing day. Blood sampling for pharmacokinetic evaluations was performed on the fourth dosing day of each treatment period and on the morning of the next dosing day (prior to the administration of the first dose of the next treatment period).
The ERMBT was administered up to 1 week before the first treatment (to establish a baseline), on the fourth dosing day of each treatment period (i.e., days 4, 8, and 12), and at the follow-up evaluation. The ERMBT was performed according to the product information from Metabolic Solutions Inc. Each subject received an intravenous injection over 1 min of a trace amount of [N-methyl-14C]erythromycin (3 μCi in 0.5 ml of 100% ethanol, USP, diluted in 4.5 ml of 5% Dextrose Injection, USP). On days 4, 8, and 12, the injection was given 2 h after administration of the seventh dose of each treatment, immediately after collection of the 2-h postdosing pharmacokinetic blood sample(s). Twenty minutes after the injection, the subject exhaled through a plastic straw into 4 ml of benzethonium hydroxide-ethanol solution (a CO2-trapping agent) in 20-ml glass scintillation vials until the color changed from blue to clear, indicating that 2 mM CO2 had been trapped. The time required for the color change was approximately 1 min. Each sample was tightly capped and stored at 4°C until assayed.
If, following completion of all postdosing procedures for day 12, no clinical abnormalities were noted, then subjects were discharged from the study center with instructions to return 7 to 10 days later for the first follow-up visit. If there were significant elevations in liver function tests (LFTs), then subsequent follow-up visits to monitor LFTs were scheduled weekly until they resolved. If no significant LFT elevations were noted, subsequent follow-up visits were scheduled 1, 2, and 3 months after completion of the treatment phase. The occurrence of adverse effects was monitored throughout the treatment phase of the study and again at follow-up visits.
Pharmacokinetic samples.
On each of dosing days 4, 8, and 12, serial blood samples were drawn from each subject for evaluation of the plasma concentration-time profiles of amprenavir and/or clarithromycin or its 14-hydroxy metabolite [14-(R)-hydroxyclarithromycin]. Blood samples were collected by peripheral venous catheter at 5 min predosing to establish a baseline and thereafter at the following intervals: 0.25, 0.5, 0.75, 1.0, 1.50, 2.0, 2.5, 3.0, 4.0, 5.0, 6.0, 8.0, 10.0, 12.0, 16.0 and 24.0 h postdosing. Each blood sample for amprenavir analysis was collected in a prelabeled 4-ml lavender-stoppered VACUTAINER tube (containing freeze-dried K2EDTA). Each blood sample for the analysis of clarithromycin or 14-(R)-hydroxyclarithromycin was drawn into a 5-ml prelabeled green-stoppered VACUTAINER tube (containing sodium heparin). Each sample was centrifuged within 30 min of collection for 10 min in a refrigerated centrifuge at +4°C to separate the plasma.
Urine was collected predosing to establish a baseline and thereafter over the following intervals: 0 to 4, 4 to 8, 8 to 12, and 12 to 24 h postdosing on each of days 4, 8, and 12. For the predosing sample, subjects voided their bladders 15 min prior to dosing. For all postdosing collection intervals, subjects were allowed to void their bladders as needed during and at the end of the collection interval.
Individual plasma and urine samples were aliquoted into propylene storage tubes, labeled, and stored upright in a non-self-defrosting freezer (−20°C or lower) until they were shipped to Glaxo Wellcome, Inc., for analysis of amprenavir by International Bioanalysis (Glaxo Wellcome) or to BAS Analytics, West Lafayette, Ind., for analysis of clarithromycin or 14-(R)-hydroxyclarithromycin.
Plasma analytical methods.
Plasma concentrations of amprenavir were determined with a semi-automated solid-phase extraction method. A 0.5-ml portion of plasma was combined with 0.5 ml of internal standard solution (VB 11599, 5.0 μg/ml). Solid-phase extraction was performed with a Waters Millilab Workstation and C18 Sep-Pak cartridges. The samples were loaded onto Waters C18 Sep-Pak cartridges at room temperature. Extraction cartridges were primed with methanol, followed by water. After the calibration standard, the control or sample was loaded, and the cartridge was washed with water and methanol (65:35, vol/vol). The compound was eluted from the cartridges with 2.5 ml of acetonitrile. The volume of the eluate was reduced by evaporation under nitrogen at 37°C. A redissolved sample in the mobile phase was then loaded on the Waters Symmetry C18 column (3.9 by 150 mm) maintained at 40°C and eluted with a mobile phase consisting of acetonitrile-water in a 45:55 (vol/vol) ratio at a flow rate of 1.0 ml/min. Amprenavir was detected by fluorescence (λexcitation = 245 nm; λemission = 340 nm). The amprenavir calibration standard concentrations were linear from 10 to 1,000 ng/ml; the amprenavir plasma control concentrations were 30, 400, and 800 ng/ml. The clinical samples were diluted into the range of the calibration curve with blank human plasma and reassayed if they exceeded the upper limit of quantitation (1,000 ng/ml).
Upon validation of the amprenavir assay technique, the interassay precision, assessed from spiked validation control samples (n = 6) at four concentrations over four analytical runs with human plasma and expressed as percent coefficient of variation (CV), ranged from 1.8 to 4.7%; the intraassay precision ranged from 1.8 to 11.3%. The percent recovery of amprenavir was determined in human plasma at concentrations of 75, 400, and 800 ng/ml (n = 6 at each concentration) by injecting analytical standards (with internal standard) directly onto the column and comparing results to the nominal concentrations. Recovery from plasma ranged from 86 to 88% across the concentration range of 75 to 800 ng/ml.
Concentrations of clarithromycin and 14-(R)-hydroxyclarithromycin in plasma were determined by liquid chromatography-tandem mass spectroscopy (LC-MS-MS). Clarithromycin and 14-(R)-hydroxyclarithromycin were extracted from 1.0 ml of heparinized plasma by liquid-liquid extraction at an alkaline pH. Erythromycin B served as an internal standard. After the addition of carbonate solution and internal standard to the plasma, the macrolides were extracted into methyl-t-butyl ether. The ether layer was transferred to a clean tube and reconstituted with a pH 6 buffer-acetonitrile mixture. The reconstituted extract was washed with hexane and injected into an LC-MS-MS system with atmospheric pressure chemical ionization.
Clarithromycin and 14-(R)-hydroxyclarithromycin calibration standard concentrations ranged from 15.6 to 8,000 ng/ml, and the quality control concentrations were 40, 400, and 1,000 ng/ml in human plasma. For the clarithromycin calibration standards, the interday CV was ≤9.6%; the intraday CV ranged from 5.3 to 9.9%. For the 14-(R)-hydroxyclarithromycin calibration standards, the interday CV was ≤6.8%; the intraday CV ranged from 1.7 to 7.4%. Standard curve correlation coefficients for both compounds were ≥0.985.
ERMBT analytical procedures.
All ERMBT samples were assayed at the VCU School of Pharmacy Biopharmaceutical Analysis Laboratory. Liquid scintillation counting was used to measure exhaled 14CO2. Ten milliliters of Insta-Gel XF scintillation cocktail (Packard Instrument Co.) was added to decolorized samples in scintillation vials; samples were mixed well and left in the dark at room temperature for at least 16 h. The samples were counted on a Packard Model Tricarb 4530 for 14C using terminators of 1% standard deviation or 10 min, whichever came first. Generally, the samples were counted for 10 min. Counts per minute were converted to disintegrations per minute using a quench curve. Results of the ERMBT are expressed as percent erythromycin dose metabolized during the first hour postinjection and are calculated from disintegrations per minute as previously described (23). The reduction of isoenzyme activity due to the study drug(s) was calculated as 1 − (treatment period value/baseline value).
Pharmacokinetic analyses.
The observed peak plasma drug concentrations at steady state (Cmax,ss) and the time for each drug to reach peak concentrations (Tmax,ss) were obtained by inspection of the individual plasma concentration-time data. The minimum drug concentration at steady state (Cmin,ss) was calculated as (C0 + Ct)/2, where C0 is the plasma concentration before the last dose and Ct is the plasma concentration of the last sample of the steady-state dosing interval. The AUC at steady state (AUCss), from the time of the predosing sample to the last sample of the steady-state dosing interval was calculated for each volunteer using the linear trapezoidal rule. The apparent total clearance at steady-state (CL/F) was calculated as dose/AUCss. Similar formulae were used to determine 14-(R)-hydroxyclarithromycin pharmacokinetic parameters. The ratio of the metabolite AUC to the parent drug AUC (AUC14-OH-clar/AUCclar) was also calculated based on the AUCss.
Urine pharmacokinetic parameters were determined for clarithromycin and 14-(R)-hydroxyclarithromycin only. Renal clearance (CLR) was calculated as Aess/AUCss, where Aess is the amount of drug excreted in the urine over the dosing interval. The percentages of clarithromycin and its metabolite eliminated in the urine were calculated based on clarithromycin weight equivalents. The molecular sizes of clarithromycin and 14-(R)-hydroxyclarithromycin were 747.96 and 763.96 Da, respectively.
The pharmacokinetic profiles obtained when the two drugs were administered together were compared with the profiles obtained when the drugs were administered alone (i.e., amprenavir plus clarithromycin versus amprenavir alone; amprenavir plus clarithromycin versus clarithromycin alone).
Statistical analysis.
The primary analysis of pharmacokinetic parameters (other than Tmax,ss) was performed after loge transformation. Analyses of variance (ANOVA) considering sequence, period, and treatment as fixed effects and subject within sequence as the random effect, were performed using the Mixed Linear Models procedure (SAS PROC MIXED, version 6.12; SAS Institute, Cary, N.C.). The geometric least-squares mean and 90% confidence intervals (90% CI) were calculated for each pharmacokinetic parameter, along with their descriptive summary statistics. Two one-sided t tests (90% CI) were performed to compare the pharmacokinetic parameters obtained when the combination treatments were administered with those for drug given alone. The Tmax was analyzed on a pairwise basis using a Wilcoxon signed rank test ignoring periods. Estimations of the median difference between treatments and 90% CI were calculated. Pearson's correlation coefficient was calculated for potential linear relationships between continuous variables.
Descriptive statistics of ERMBT results at baseline, 2 h after dosing (days 4, 8, and 12) and at the first follow-up visit were summarized by calculation of the mean reduction in ERMBT compared with the baseline, and the respective 95% CI.
RESULTS
Study subjects.
A total of 14 HIV-seronegative, healthy males (12 Caucasian and 2 African-American) were enrolled in this study. Thirteen subjects received all three treatments, but only 12 subjects completed all phases of the study. One subject was withdrawn midway through his second treatment (amprenavir plus clarithromycin) after complaining of nausea and vomiting. The other subject withdrew during the third treatment (amprenavir plus clarithromycin) for personal reasons.
Adverse events.
There were no serious adverse events reported during this study, and all three treatments were generally well tolerated. The 14 subjects reported a total of 188 adverse events. The most common adverse events for amprenavir were mild gastrointestinal events (50%) and oral numbness (43%). Clarithromycin was most commonly associated with a bad taste (31%). Combination treatment with amprenavir plus clarithromycin resulted in greater subject intolerance than treatment with either drug alone, with any gastrointestinal events (71%) and oral numbness (50%) accounting for the majority of adverse effects. There was no apparent effect of the study drugs on hematology, clinical chemistry, or urinalysis laboratory values, nor any apparent changes in vital signs, physical examination findings, or electrocardiogram data from screening to follow-up.
Pharmacokinetics. (i) Amprenavir.
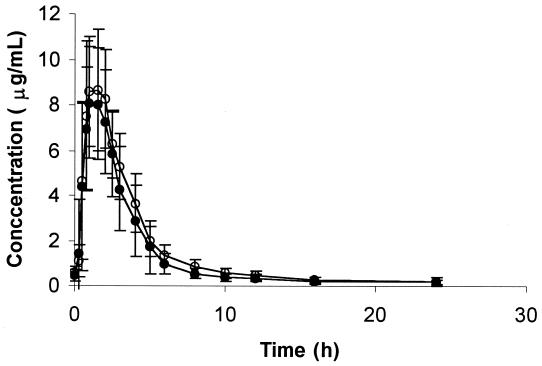
Concentrations of amprenavir immediately before the final dose (C0) were not different from concentrations 12 h after the final dose, indicating that steady state had been achieved. Figure 1 illustrates the effect of clarithromycin on mean plasma amprenavir concentrations. There were statistically significant increases in the amprenavir AUCss (18%), Cmax,ss (15%), and Cmin,ss, (39%), and a decrease in CL/F (15%), when amprenavir was administered with clarithromycin (Table 1). There was a nearly significant negative correlation between the baseline amprenavir AUC and the percent change in the amprenavir AUCss when amprenavir was given with clarithromycin (r2 = 0.30; P = 0.065). There was a significant negative correlation between the AUCss for clarithromycin and the magnitude of percent change from baseline in the amprenavir AUCss (r2 = 0.44; P = 0.02). There was no significant association between subject weight and the AUCss for amprenavir (r2 = 0.24; P = 0.10). The medians of Tmax,ss were not different between treatments.
FIG. 1.
Mean plasma amprenavir concentrations (± standard deviations) versus time (n = 12 subjects) when amprenavir was given alone (solid circles) or coadministered with clarithromycin (open circles).
TABLE 1.
Summary of results for amprenavir pharmacokinetic parameters
| Parameter | GLSM (arithmetic mean CVb) for:
|
Treatment 3 GLSM/treatment 1 GLSM ratio (90% CI) | |
|---|---|---|---|
| Treatment 1c | Treatment 3d | ||
| AUCss (h · μg/ml) | 27.40 (29.08, 26%) | 32.28 (32.98, 24%) | 1.18 (1.08–1.29) |
| Cmax,ss (μg/ml) | 8.42 (8.98, 26%) | 9.65 (10.10, 26%) | 1.15 (1.01–1.31) |
| Cmin,ss (μg/ml) | 0.38 (0.41, 45%) | 0.53 (0.53, 38%) | 1.39 (1.31–1.47) |
| CL/F (ml/min) | 730 (754, 38%) | 619 (649, 30%) | 0.85 (0.78–0.93) |
| Tmax,ss (h)e | 1.14 (1.25, 53%) | 1.38 (1.34, 38%) | 0.19 (0.00–0.39) |
GLSM, geometric least-squares mean.
CV, coefficient of variation.
Amprenavir, 1,200 mg twice a day.
Amprenavir, 1,200 mg twice a day, plus clarithromycin, 500 mg twice a day.
Median and median difference.
There were no significant period or sequence effects in any of the ANOVA comparisons.
(ii) Clarithromycin.
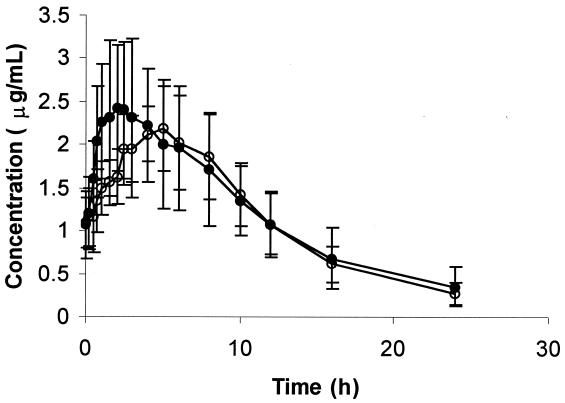
Concentrations of clarithromycin immediately before the final dose (C0) were not different from concentrations 12 h after the final dose, indicating that steady state had been reached. Amprenavir had no significant effect on the geometric least-squares means for the clarithromycin AUCss, Cmin,ss, and CL/F (Fig. 2; Table 2). The median Tmax,ss following administration of the combined treatment was 2.0 h later than that after the administration of clarithromycin alone (P < 0.05). There was a 34% increase in CLR with the combined treatment over that with clarithromycin alone (P < 0.05). There was no significant linear correlation between the baseline apparent oral clearances for clarithromycin and amprenavir (r2 = 0.22; P = 0.11). Weight was able to explain a significant amount of variability in the AUCss for clarithromycin (r2 = 0.34; P = 0.04); larger subjects had a lower AUCss.
FIG. 2.
Mean plasma clarithromycin concentrations (± standard deviations) versus time (n = 12 subjects) when clarithromycin was given alone (solid circles) or coadministered with amprenavir.
TABLE 2.
Summary of results for clarithromycin pharmacokinetic parameters
| Parameter | GLSM (arithmetic mean, CV)a for:
|
Treatment 3 GLSM/treatment 2 GLSM ratio (90% CI) | |
|---|---|---|---|
| Treatment 2b | Treatment 3c | ||
| AUCss (h · μg/ml) | 21.40 (22.31, 29%) | 20.51 (20.92 21%) | 0.96 (0.83–1.11) |
| Cmax,ss (μg/ml) | 2.57 (2.70, 29%) | 2.31 (2.36, 19%) | 0.90 (0.76–1.07) |
| Cmin,ss (μg/ml) | 1.03 (1.06, 33%) | 1.04 (1.09, 28%) | 1.02 (0.87–1.20) |
| CL/F (ml/min) | 390 (405, 30%) | 406 (417, 25%) | 1.04 (0.90–1.21) |
| Tmax,ss (h)d | 2.25 (2.46, 73%) | 4.99 (4.79, 29%) | 2.00 (0.75–3.38) |
| CLR (ml/min) | 114 (121, 32%) | 154 (159, 21%) | 1.34 (1.17–1.54) |
| % Dosee | 31.25 (31.62, 35%) | 38.82 (38.63, 19%) | 1.24 (1.03–1.45) |
GLSM, geometric least-squares mean; CV, coefficient of variation.
Consists of 500 mg of clarithromycin twice a day.
Consists of 1,200 mg of amprenavir twice a day plus 500 mg of clarithromycin twice a day.
Median and median difference.
Least-squares mean and least-squares mean ratio.
(iii) 14-(R)-Hydroxyclarithromycin.
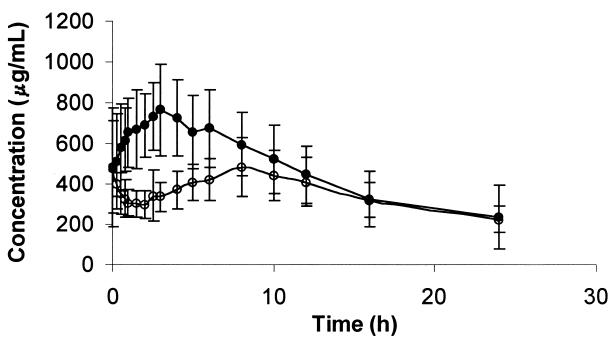
Figure 3 illustrates the effect of amprenavir on mean plasma 14-(R)-hydroxyclarithromycin concentrations. A summary of the results for 14-(R)-hydroxyclarithromycin parameters is presented in Table 3. Amprenavir clearly reduced the formation of the main metabolite for clarithromycin, resulting in statistically significant decreases in the 14-(R)-hydroxyclarithromycin AUCss (35%) and Cmax,ss (32%). There was a 37% decrease in the AUC14-OH-clar/AUCclar ratio. The median Tmax,ss following administration of the combined treatment was 2.0 h later than that following the administration of clarithromycin alone. The percentage of the dose excreted in the urine as 14-(R)-hydroxyclarithromycin was 16% lower with the combined treatment than with clarithromycin alone.
FIG. 3.
Mean plasma 14-(R)-hydroxyclarithromycin concentrations (± standard deviations) versus time (n = 12 subjects) when clarithromycin was administered alone (solid circles) or with amprenavir.
TABLE 3.
Geometric least-squares means for 14-(R)-hydroxyclarithromycin pharmacokinetic parameters
| Parameter | Geometric least-squares mean for:
|
Treatment 3/treatment 2 ratio (90% CI) | |
|---|---|---|---|
| Treatment 2a | Treatment 3b | ||
| AUCss (h · μg/ml) | 7.40 | 4.83 | 0.65 (0.59–0.72) |
| Cmax,ss (μg/ml) | 0.81 | 0.55 | 0.68 (0.59–0.79) |
| Cmin,ss (μg/ml) | 0.44 | 0.43 | 0.96 (0.78–1.17) |
| AUC14-OH/AUCCLARc | 0.38 | 0.24 | 0.63 (0.43–0.84) |
| Tmax,ss (h)d | 3.00 | 5.25 | 2.00 (0.25–4.25) |
| CLR (ml/min) | 134 | 171 | 1.28 (1.12–1.46) |
| % Dosec | 11.70 | 9.83 | 0.84 (0.76–0.92) |
Consists of 500 mg of clarithromycin twice a day.
Consists of 1,200 mg of amprenavir twice a day plus 500 mg of clarithromycin twice a day.
Least-squares mean and least-squares mean ratio.
Median and median difference.
The AUCss for amprenavir given alone did not predict the magnitude of the percent reduction in the baseline AUCss for 14-(R)-hydroxyclarithromycin (r = 0.17; P = 0.61).
ERMBT.
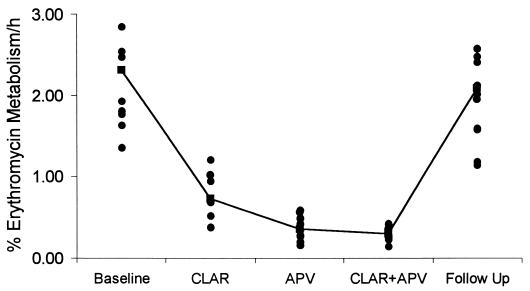
The mean reduction in the ERMBT result was 85% (95% CI, 78 to 92%) after the administration of amprenavir, 67% (95% CI, 59 to 74%) for clarithromycin, and 87% (95% CI, 79 to 94%) for both drugs administered concurrently (Fig. 4). These data are consistent with evidence that drug interactions between clarithromycin and CYP3A4 substrates are of a lower magnitude compared with the effects of HIV-1 protease inhibitors (5). There was no significant correlation between the baseline ERMBT result and the CL/F for amprenavir (r = 0.30; P = 0.35) or clarithromycin (r = 0.28; P = 0.38). There was a nearly significant negative correlation between the percent reduction in the ERMBT result following clarithromycin treatment and the percent reduction in the clearance of amprenavir (r2 = 0.34; P = 0.06). The mean ERMBT result at follow-up (2.08% ± 0.63% metabolized/h) was not significantly different from baseline (2.31% ± 0.68% metabolized/h; P = 0.107).
FIG. 4.
Percent erythromycin metabolism per hour, as measured by the ERMBT at baseline, at the end of each dosing regimen, and at follow-up. The line connects the means. APV, 1,200 mg of amprenavir given orally twice a day; CLAR, 500 mg of clarithromycin given orally twice a day; CLAR+APV, concomitant amprenavir and clarithromycin.
DISCUSSION
The pharmacokinetics of amprenavir and clarithromycin when given alone are in agreement with the findings of previous investigations (3, 19). Clarithromycin given in combination with amprenavir resulted in statistically significant changes in selected pharmacokinetic parameters for both drugs. Clarithromycin increased the amprenavir AUCss, Cmax,ss, and Cmin,ss by 18, 15, and 39%, respectively, with an associated 15% decrease in CL/F. While this interaction is statistically significant, it is unlikely to be clinically important. An 18% increase in the AUC is within the intersubject variability normally seen when amprenavir, 1,200 mg every 12 h, is used clinically (19). In addition, the 39% mean increase in Cmin,ss is not likely to be a safety concern, since the absolute effect is small (mean increase from 0.38 to 0.53 μg/ml) and there is no known adverse event related to increased amprenavir trough concentrations.
Administration of amprenavir with clarithromycin had no statistically significant effect on the pharmacokinetic parameters AUCss, Cmax,ss, and Cmin,ss for clarithromycin. However, the AUCss and Cmax,ss of 14-(R)-hydroxyclarithromycin were decreased 35 and 32%, respectively by amprenavir; there was a 28% increase in CLR for this metabolite; and the CLR of clarithromycin increased by 34%. This reduced formation of 14-(R)-hydroxyclarithromycin appeared to be balanced by increased CLR of the parent drug, resulting in no net change in the AUCss for clarithromycin. The metabolism of clarithromycin to 14-(R)-hydroxyclarithromycin is mediated by CYP3A4 (18), and the decreases in the 14-(R)-hydroxyclarithromycin AUC and Cmax are consistent with inhibition of CYP3A4 by amprenavir. Ritonavir has a similar effect on the metabolism of clarithromycin, but of greater magnitude (15). The mechanism for increased CLR of clarithromycin is unclear but is unlikely to represent protein-binding displacement, since clarithromycin is approximately 70% bound to albumin, and binding would have to decrease to nearly zero to account for the increase in CLR. Furthermore, amprenavir has no known effects on renal function and should not alter renal secretion of clarithromycin. It is possible that the reduced formation of the metabolite may decrease competition with the parent compound for secretion, resulting in an increase in the CLR of clarithromycin, but this remains conjectural.
It is unlikely that the changes in clarithromycin and 14-(R)-hydroxyclarithromycin pharmacokinetics are clinically relevant 14-(R)-Hydroxyclarithromycin has in vitro activity against some bacterial pathogens and may contribute to the clinical efficacy of clarithromycin, especially for infections caused by Haemophilus influenzae (8), but is less important for MAC (13). While it is possible that the therapeutic efficacy of clarithromycin may be compromised as a result of this interaction, the effect of amprenavir is less than that of other protease inhibitors (Table 4). There are no published reports of therapeutic failure when clarithromycin has been used to treat bacterial infections in HIV-infected patients receiving protease inhibitors, and dosage adjustments are not recommended for patients receiving other protease inhibitors and clarithromycin.
TABLE 4.
Comparison of protease inhibitor effects on clarithromycin pharmacokineticsa
| Agent | % Clarithromycin increase (90% CI)
|
14-OH-CLARb decrease (90% CI)
|
||
|---|---|---|---|---|
| AUC | Cmax | AUC | Cmax | |
| Saquinavir (1,200 mg every 8 h)c | 45 (17–81) | 39 (10–76) | 24 (5–40) | 34 (14–50) |
| Ritonavir (200 mg every 8 h)d | 77 (56–103) | 31 (15–51) | 100 | 99 |
| Indinavir (800 mg every 8 h)e | 53 ± 36f | NSg | 52 | NS |
| Amprenavir (1,200 mg BID)h | No effect | −10 | 35 | 32 |
Results are expressed as percent change from values with clarithromycin alone.
14-OH-CLAR, 14-(R)-hydroxyclarithromycin.
Data are from saquinavir prescribing information (Roche Laboratories).
Data are from ritonavir prescribing information (Abbott Laboratories).
Data are from indinavir prescribing information (Merck and Company Pharmaceuticals).
Mean ± standard deviation.
NS, not stated.
Data are from this study. BID, twice a day.
We have attempted to determine a mechanism for these effects. Since erythromycin (in the ERMBT), clarithromycin, and amprenavir are at least partially metabolized by hepatic CYP3A4, we hypothesized that there would be significant correlations of metabolic parameters between these three drugs. However, the mechanism(s) of the interactions described above appears to be more complex than simple alterations in hepatic CYP3A4 metabolism, as suggested by a number of observations. First, a good correlation between the ERMBT result and clearance of a CYP3A substrate has been suggested as evidence that the substrate is largely metabolized by hepatic CYP3A (7). In contrast, we found that the ERMBT results at baseline did not predict clearance of either amprenavir or clarithromycin, which suggests that nonhepatic mechanisms are more relevant (below). Second, although both amprenavir and clarithromycin significantly reduce hepatic CYP3A4 activity as measured by the ERMBT, amprenavir caused significantly greater suppression than clarithromycin (Fig. 4). However, clarithromycin had a more pronounced effect on serum amprenavir concentrations than amprenavir had on serum clarithromycin concentrations, an effect opposite that which would be expected if hepatic metabolism were of central importance. Third, a number of correlation analyses are not consistent with a hepatic mechanism to explain the interaction. For example, there was a significant negative correlation between the AUCss of clarithromycin and the magnitude of percent increase in the amprenavir AUCss. Likewise, there was a near-significant (P = 0.06) negative correlation between the AUCss for amprenavir and the magnitude of reduction in the 14-(R)-hydroxyclarithromycin metabolite, an effect also opposite that predicted if impairment of hepatic metabolism was the main mechanism of interaction. Finally, the correlation between the clearance of amprenavir and the clearance of clarithromycin, two putative substrates of hepatic CYP3A4, was not significant.
Additional mechanisms that may explain the effects observed include alterations in CYP3A4-mediated gastrointestinal metabolism and alterations in P-glycoprotein (P-gp)-mediated gastrointestinal absorption (7). Clarithromycin has been shown to inhibit gastrointestinal CYP3A4 and thereby increase the absorption of midazolam, a substrate of CYP3A4 but not of P-gp (6). Clarithromycin has also been shown to increase the absorption of digoxin, a substrate of P-gp but not of CYP3A4 (24). Since all of the HIV-1 protease inhibitors are substrates of CYP3A4 (5) and are transported by P-gp (12, 17, 25), the increase in the AUC for amprenavir following clarithromycin pretreatment could be due to one or both of these mechanisms. There was a near-significant (P = 0.065) negative relationship between the baseline amprenavir AUC and the magnitude of the increase in the AUC following clarithromycin pretreatment. This suggests that those subjects with a low baseline amprenavir AUC, possibly resulting from greater first-pass clearance mediated by CYP3A4 and/or P-gp, have a larger interaction with clarithromycin, since it interferes with those processes that act to reduce absorption. Similar mechanisms explain the effects when two protease inhibitors are given together, as when ritonavir is given with either saquinavir (9) or indinavir (10). Modeling of these interactions suggests that the main effect of ritonavir on indinavir is a reduction in systemic clearance via inhibition of hepatic CYP3A4 metabolism (10), whereas the effect of ritonavir on saquinavir is mediated mainly through a reduction in first-pass gastrointestinal CYP3A4 metabolism (9). It is not yet possible to quantify the relative contribution of P-gp versus CYP3A4 to these interactions in vivo. Irrespective of the mechanisms for these interactions, these data indicate that clarithromycin and amprenavir can be given together with no need for dosage adjustment.
ACKNOWLEDGMENTS
This study was supported by a grant from Glaxo Wellcome, Inc.
Appreciation is expressed to Cindy Rawls (Glaxo Wellcome, Inc.), who performed the amprenavir assays; to Clark March (School of Pharmacy, VCU), who performed the ERMBT scintillation counts; and to the nurses and staff of the Center for Drug Studies at the Virginia Commonwealth University School of Pharmacy.
REFERENCES
- 1.Adkins J C, Faulds D. Amprenavir. Drugs. 1998;55:837–842. doi: 10.2165/00003495-199855060-00015. [DOI] [PubMed] [Google Scholar]
- 2.Cheng C L, Smith D E, Carver P L, Cox S R, Watkins P B, Blake D S, Kauffman C A, Meyer K M, Amidon G L, Stetson P L. Steady-state pharmacokinetics of delavirdine in HIV-positive patients: effect on erythromycin breath test. Clin Pharmacol Ther. 1997;61:531–543. doi: 10.1016/S0009-9236(97)90133-8. [DOI] [PubMed] [Google Scholar]
- 3.Chu S, Wilson D S, Deaton R L, Mackenthun A V, Eason C N, Cavanaugh J H. Single- and multiple-dose pharmacokinetics of clarithromycin, a new macrolide antimicrobial. J Clin Pharmacol. 1993;33:719–726. doi: 10.1002/j.1552-4604.1993.tb05613.x. [DOI] [PubMed] [Google Scholar]
- 4.Decker C J, Laitinen L M, Bridson G W, Raybuck S A, Tung R D, Chaturvedi P R. Metabolism of amprenavir in liver microsomes: role of CYP3A4 inhibition for drug interactions. J Pharm Sci. 1998;87:803–807. doi: 10.1021/js980029p. [DOI] [PubMed] [Google Scholar]
- 5.Flexner C. HIV-protease inhibitors. N Engl J Med. 1998;338:1281–1292. doi: 10.1056/NEJM199804303381808. [DOI] [PubMed] [Google Scholar]
- 6.Gorski J C, Jones D R, Haehner-Daniels B D, Hamman M A, O'Mara E M, Hall S D. The contribution of intestinal and hepatic CYP3A to the interaction between midazolam and clarithromycin. Clin Pharmacol Ther. 1998;64:133–143. doi: 10.1016/S0009-9236(98)90146-1. [DOI] [PubMed] [Google Scholar]
- 7.Hall S D, Thummel K E, Watkins P B, Lown K S, Benet L Z, Paine M F, Mayo R R, Turgeon D K, Bailey D G, Fontana R J, Wrighton S A. Molecular and physical mechanisms of first-pass extraction. Drug Metab Dispos. 1999;27:161–166. [PubMed] [Google Scholar]
- 8.Hoover W W, Barrett M S, Jones R N. Clarithromycin in vitro activity enhanced by its major metabolite, 14-hydroxyclarithromycin. Diagn Microbiol Infect Dis. 1992;15:259–266. doi: 10.1016/0732-8893(92)90122-a. [DOI] [PubMed] [Google Scholar]
- 9.Hsu A, Granneman G R, Cao G, Carothers L, El-Shourbagy T, Baroldi P, Erdman K, Brown F, Sun E, Leonard J. Pharmacokinetic interactions between two human immunodeficiency virus protease inhibitors, ritonavir and saquinavir. Clin Pharmacol Ther. 1998;63:453–464. doi: 10.1016/S0009-9236(98)90041-8. [DOI] [PubMed] [Google Scholar]
- 10.Hsu A, Granneman G R, Cao G, Carothers L, Japour A, El-Shourbagy T, Dennis S, Berg J, Erdman K, Leonard J, Sun E. Pharmacokinetic interaction between ritonavir and indinavir in healthy volunteers. Antimicrob Agents Chemother. 1998;42:2784–2791. doi: 10.1128/aac.42.11.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kempf D J, Marsh K C, Kumar G, Rodrigues A D, Denissen J F, McDonald E, Kukulka M J, Hsu A, Granneman G R, Baroldi P A, Sun E, Pazzuti D, Plattner J J, Norbeck D W, Leonard J M. Pharmacokinetic enhancement of inhibitors of the human immunodeficiency virus protease by coadministration with ritonavir. Antimicrob Agents Chemother. 1997;41:654–660. doi: 10.1128/aac.41.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim R B, Fromm M F, Wandell C, Leake B, Wood A J, Roden D, Wilkinson G R. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Investig. 1998;101:289–294. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langtry H D, Brogden R N. Clarithromycin: a review of its efficacy in the treatment of respiratory tract infections in immunocompetent patients. Drugs. 1997;53:973–1004. doi: 10.2165/00003495-199753060-00006. [DOI] [PubMed] [Google Scholar]
- 14.Murphy R L, Gulick R M, DeGruttola V, D'Aquila R T, Eron J J, Sommadossi J P, Currier J S, Smeaton L, Frank I, Caliendo A M, Gerber J G, Tung R, Kuritzkes D R. Treatment with amprenavir alone or amprenavir with zidovudine and lamivudine in adults with human immunodeficiency virus infection. AIDS Clinical Trials Group 347 Study Team. J Infect Dis. 1999;179:808–816. doi: 10.1086/314668. [DOI] [PubMed] [Google Scholar]
- 15.Ouellet D, Shu A, Granneman G R, Carlson G, Cavanaugh J, Guenther H, Leonard J M. Pharmacokinetic interaction between ritonavir and clarithromycin. Clin Pharm Ther. 1998;64:355–362. doi: 10.1016/S0009-9236(98)90065-0. [DOI] [PubMed] [Google Scholar]
- 16.Polk R E, Crouch M, Israel D S, Pastor A, Sadler B M, Chittick G E, Symonds B T, Gouldin W, Lou Y. Pharmacokinetic interaction between ketoconazole and amprenavir after single dose administration to healthy male subjects. Pharmacotherapy. 1999;19:1378–1384. doi: 10.1592/phco.19.18.1378.30905. [DOI] [PubMed] [Google Scholar]
- 17.Polli J W, Jarrett J L, Studenberg S D, Humpreys J E, Dennis S W, Brouwer K R, Wooley J L. Role of P-glycoprotein in the CNS penetration of amprenavir (141W94), an HIV protease inhibitor. Pharm Res. 1999;16:1206–1212. doi: 10.1023/a:1018941328702. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues A D, Roberts E M, Mulford D J, Yao Y, Ouellet D. Oxidative metabolism of clarithromycin in the presence of human liver microsomes. Major role for the cytochrome P4503A subfamily. Drug Metab Dispos. 1997;25:623–630. [PubMed] [Google Scholar]
- 19.Sadler B M, Hanson C D, Chittick G E, Symonds W T, Roskell N S. Safety and pharmacokinetics of amprenavir (141W92), a human immunodeficiency virus (HIV) type 1 protease inhibitor, following oral administration of single doses to HIV-infected adults. Antimicrob Agents Chemother. 1999;43:1686–1692. doi: 10.1128/aac.43.7.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh R, Taylor L C, Chang S Y. In vitro metabolism of a potent HIV-protease inhibitor (141W94) using rat, monkey and human liver S9. Rapid Commun Mass Spectrom. 1996;10:1019–1026. doi: 10.1002/(SICI)1097-0231(19960715)10:9<1019::AID-RCM618>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 21.St. Clair M H, Millard J, Rooney J, Tisdale M, Perry N, Sadler B M, Blum M R, Painter G. In vitro antiviral activity of 141W94 (VX-478) in combination with other antiretroviral agents. Antiviral Res. 1996;29:53–56. doi: 10.1016/0166-3542(95)00916-7. [DOI] [PubMed] [Google Scholar]
- 22.U. S. Public Health Service (USPHS) and Infectious Diseases Society of America (IDSA) 1999 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. Morbid Mortal Weekly Rep. 1999;48:1–59. [PubMed] [Google Scholar]
- 23.Wagner D. CYP3A4 and the erythromycin breath test. Clin Pharmacol Ther. 1998;64:129. doi: 10.1016/S0009-9236(98)90031-5. [DOI] [PubMed] [Google Scholar]
- 24.Wakasugi H, Yano I, Ito T, Hashida T, Futami T, Nohara R, Sasayama S, Inui K. Effect of clarithromycin on renal excretion of digoxin: interaction with P-glycoprotein. Clin Pharmacol Ther. 1998;64:123–128. doi: 10.1016/S0009-9236(98)90030-3. [DOI] [PubMed] [Google Scholar]
- 25.Washington C B, Duran G E, Man M C, Sikic B I, Blaschke T. Interaction of anti-HIV protease inhibitors with the multidrug transporter P-glycoprotein (P-gp) in human cultured cells. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:203–209. doi: 10.1097/00042560-199811010-00001. [DOI] [PubMed] [Google Scholar]
- 26.Watkins P B. Non-invasive tests of CYP3A enzymes. Pharmacogenetics. 1994;4:171–184. doi: 10.1097/00008571-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Woolley J, Studenberg S, Sadler B M, Chittick G, Dahl R, Correa I, Bowers G, Castellino S, Kerkering T, Pastor A, March C, Polk R. The disposition of [14C]-amprenavir in human volunteers. Pharm Sci. 1998;1(Suppl.):2044. . (Abstract.) [Google Scholar]