Abstract
Introduction
In Japan, epidemiological studies on type 1 diabetes (T1D) have mainly focused on the disease in childhood. Meanwhile, limited information is available regarding the clinical features of adolescent and adult T1D. Therefore, we aimed to investigate their current clinical state in Saitama prefecture near Tokyo, Japan.
Materials and methods
We conducted a cross-sectional, hospital-based, multicenter study. Eight institutions participated in the study, all of which treated relatively large numbers of T1D patients. We identified 1241 T1D patients aged 16 or over: 814 with acute-onset T1D (AT1D), 362 with slowly progressive insulin-dependent diabetes mellitus (SPIDDM), and 65 with fulminant T1D (FT1D). Based on the patient’s medical records, various clinical parameters and complications were investigated.
Results
Of 1241 patients, 739 (59.5%) were females. Among all patients, the median age, onset age, and disease duration were 51, 38, and 13 years, respectively. The patients had a median BMI of 22.6 kg/m2, and 26.1% were obese, corroborating previous nationwide surveys. Moreover, the median HbA1c was 7.8%, similar to previous nationwide surveys. Among patients with AT1D, SPIDDM, and FT1D, 85.6%, 72.1%, and 81.5% carried out multiple daily insulin injection, respectively, while 10.3%, 2.2%, and 18.5% were subject to continuous subcutaneous insulin infusion. The proportions of retinopathy, nephropathy, and neuropathy were 26.3%, 20.8%, and 21.5%.
Conclusions
The glycemic control in T1D patients in Saitama was equivalent to that observed in previous nationwide surveys. Moreover, approximately one-quarter of T1D patients had obesity. Future studies should address whether our findings reflect those throughout Japan.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13340-021-00557-8.
Keywords: Body mass index, Clinical picture, Complications, HbA1c, Saitama prefecture, Type 1 diabetes
Introduction
Most type 1 diabetes (T1D) is caused by anti-islet autoimmunity and subsequent β-cell destruction, leading to insulin dependency [1]. Thus, patients with T1D usually require lifelong insulin therapy to prevent complications. Based on the onset pattern of hyperglycemia, T1D can be classified into three subtypes: acute-onset T1D (AT1D), slowly progressive insulin-dependent (type 1) diabetes (SPIDDM), and fulminant T1D (FT1D) [2, 3].
Epidemiological studies on T1D, including pediatric chronic disease treatment research projects [4] and the National Database [5], have provided some information on the estimated annual incidence and prevalence of T1D in Japan. However, limited information is available regarding the current clinical state of patients with T1D, especially adults. In particular, data are lacking regarding glycemic control, therapeutic regimen, and micro-/macrovascular complications. For example, one retrospective observational study from the Japan Diabetes Clinical Data Management (JDDM) study group has annually presented the longitudinal change in status of glycemic control and body mass index (BMI) in patients with T1D throughout Japan for the past 20 years, providing a standard, representative, and up-to-date clinical profile of patients with T1D in Japan [6]. A recent JDDM survey demonstrated that, since the start of the survey in 2002, patients with T1D have shown an improving trend in HbA1c levels and an increasing BMI [6]. However, no information is available regarding the subtypes of T1D, therapeutic regimens, diabetes-related complications, etc.
The Japan Diabetes Complication and its Prevention prospective (JDCP) study is a large-scale, prospective, observational study designed to elucidate the current state of management and treatment among patients with type 1 or 2 diabetes, as well as to clarify the progression of diabetic complications in Japan. As such, the study reported the baseline clinical characteristics of patients with T1D [7]. However, the sample size of patients with T1D was relatively small (n = 355), and data on the subsets of T1D were insufficient. Thus, a detailed clinical picture of the T1D subtypes remains to be elucidated.
Therefore, in this study, we conducted a questionnaire survey to clarify the current clinical profiles of T1D subtypes; we focused on HbA1c, BMI, therapeutic regimens, and micro-/macrovascular complications among adolescent and adult patients with T1D in Saitama prefecture, located just north of Tokyo. We also compared the clinical state of T1D between our patients and those of the previous nationwide surveys, including JDDM and JDCP studies.
Materials and methods
Research design and participating institutions
This research project was conducted as a cross-sectional, hospital-based, multicenter study. Eight institutions participated in the study: Dokkyo Medical University Saitama Medical Center, JCHO Saitama Medical Center, Jichi Medical University Saitama Medical Center, Saitama Red Cross Hospital, Saiseikai Kawaguchi General Hospital, Saitama Medical University Hospital, Saitama Medical University Saitama Medical Center, and Todachuo General Hospital. All these institutions employed resident diabetologists and prescribe insulin products to more than 100 patients in Saitama prefecture.
Inclusion and exclusion criteria
We enrolled all patients with T1D aged 16 or above who had visited the diabetes division of the department of internal medicine of participating institutions between October 2018 and March 2019 (study period). The onset patterns of T1D were classified into the following three subtypes: AT1D, SPIDDM, or FT1D, according to the diagnostic criteria proposed by the Committee of the Japan Diabetes Society (JDS) [8–10]. As for SPIDDM, the age at onset was defined as the age at which a patient was first diagnosed as having diabetes mellitus, but not as the age at which a patient was determined to be positive for glutamic acid decarboxylase antibody (GADA) during the clinical course of diabetes. Patients with T1D who met any of the following criteria were excluded from the study: unspecified onset pattern of T1D, interferon therapy-related T1D, and immune checkpoint inhibitor-related T1D.
Examinations and measurements
We conducted a survey by mail. The questionnaire form was sent to the leaders of each diabetes division of the participating institutions (Supplementary Fig. 1). They were asked to provide anonymized information about the clinical characteristics of cases satisfying the above selection criteria. In the questionnaire, information regarding the clinical characteristics and laboratory data of the patients during the study period were recorded, including gender, age, age of T1D onset, BMI, T1D subtype, and HbA1c level. Also noted were information regarding the categories of insulin regimen and treatment with oral hypoglycemic agents at the time of the investigation. Moreover, we obtained information on both microvascular (retinopathy, nephropathy, and neuropathy) and macrovascular complications (ischemic heart disease, cerebrovascular disease, and peripheral artery disease) from the patients’ medical records. However, no information was retrieved regarding the detailed stages of retinopathy/nephropathy or the type and severity of neuropathy. We optionally obtained the latest ad lib serum C-peptide levels and the latest results of GADA and insulinoma-associated protein-2 (IA-2) antibody tests, irrespective of the study period. The results of the anti-islet-associated antibody tests were determined based on the cut-off levels indicated by the manufacturer of each test. Obesity was defined as a BMI ≥ 25 kg/m2 [11].
Statistical analysis
We confirmed that all continuous variables were not normally distributed using the Shapiro–Wilk test. Thus, continuous variables are presented as median (minimum–maximum) or median (interquartile range [IQR, i.e., 25th–75th percentiles]). Comparisons of median values and proportions between the two groups were performed using the Mann–Whitney U-test and Chi-square test/Fisher's exact tests, respectively. Comparisons of median values among three groups were performed using the Kruskal–Wallis test, followed by a post hoc test using the Mann–Whitney U-test with a Bonferroni correction to determine which pairwise groups were significantly different. Comparisons of proportions among the three groups were performed using Chi-square/Fisher's exact tests, followed by a post hoc test using Chi-square/Fisher's exact tests with a Bonferroni correction to determine which pairwise group proportions were significantly different. A P-value < 0.05 was considered significant. However, in the post hoc tests using a Bonferroni correction, a P-value < 0.0167 (= 0.05/3) was considered significant. Statistical analysis was performed with the SPSS statistics software package.
Ethical considerations
This study was conducted in accordance with the Declaration of Helsinki. This study protocol was approved by the Institutional Review Boards of Saitama Medical University Hospital (No. 18119.02, dated 7 January 2019), Dokkyo Medical University Saitama Medical Center (No. 1955, dated 4 September 2019), Jichi Medical University Saitama Medical Center (No. S18-171, dated 18 April 2019), JCHO Saitama Medical Center (No. E19-9, dated 7 May 2019), Saitama Red Cross Hospital (No. 19-P, dated 22 July 2019), Todachuo General Hospital (No. 0434, dated 29 August 2019), and Saiseikai Kawaguchi General Hospital (No. 2019-23, dated 17 October 2019). All institutes provided patients with an opportunity to opt out, except for Saitama Medical University Saitama Medical Center, which participated as a cooperative research institute and provided anonymized data.
Results
Patients
Using the questionnaire survey, we obtained the information of 1323 patients with T1D from the eight hospitals. The patients comprised 814 patients with AT1D (61.5%), 362 with SPIDDM (27.4%), 65 with FT1D (4.9%), and 82 with other T1D subtypes (6.2%), including 76 with an unspecified onset pattern due to a lack of details in the medical records, four with interferon therapy-related T1D, and two with immune checkpoint inhibitor-related T1D. These 82 cases were excluded, and we analyzed the remaining 1241 patients (Supplementary Table 1 and Supplementary Fig. 2). All patients with T1D who visited the eight hospitals during the study period participated in this study. Thus, we believe that the results presented here are not affected by selection bias. There were no patients who underwent pancreas or islet transplantation.
According to the 2017 annual report of the Health Labour Sciences Research (Ministry of Health, Labour and Welfare of Japan), the prevalence of patients with T1D is estimated to be approximately 0.10% (0.09–0.11%) in Japan [12]. Given that Saitama prefecture had a population of approximately 7 million in 2020 [13], the total number of patients with T1D in the prefecture is estimated to be 7000. Thus, this study population possibly accounts for only < 20% of the estimated total number of patients with T1D in Saitama prefecture.
Clinical profiles of patients according to the subtypes of T1D
The clinical profiles of the 1241 patients are summarized in Table 1. Of the patients, 739 were females (59.5%). The proportions of females were 60.0%, 59.9%, and 52.3% in the AT1D, SPIDDM, and FT1D subtypes, respectively. There was no significant difference in gender proportions among the three T1D subtypes. The median (minimum–maximum) age among all patients was 51 years (16–93 years). The SPIDDM subgroup had the highest median age (63 years), followed by FT1D (53 years) and then by AT1D (46 years; P < 0.0167 by Mann–Whitney U-test with a Bonferroni correction). The median (minimum–maximum) disease duration was 13 years (0–52 years), and it was significantly shorter among patients with FT1D (6 years) than among those with AT1D or SPIDDM (13 and 12 years, respectively; P < 0.0167 by Mann–Whitney U-test with a Bonferroni correction). Incidentally, 5.7% (71/1241) of patients had a diabetes duration of less than 2 years, suggesting that the results of the analysis presented in this study reflect the current clinical state mostly in the chronic phase of T1D.
Table 1.
Clinical profiles of patients with type 1 diabetes
| Overall | n | AT1D | N | SPIDDM | n | FT1D | n | P-value$ | |
|---|---|---|---|---|---|---|---|---|---|
| Female/male (Female, %) | 739/502 (59.5%) | 1241 | 488/326 (60.0%) | 814 | 217/145 (59.9%) | 362 | 34/31 (52.3%) | 65 | N.S.* |
| Age (years) | 51 (16–93) | 1241 | 46 (16–93)a | 814 | 63 (20–92)a | 362 | 53 (21–83)a | 65 | < 0.01# |
| Onset age (years) | 38 (1–91) | 1206 | 32 (1–83)b | 794 | 48 (7–91) | 347 | 42 (14–82) | 65 | < 0.01# |
| Diabetes duration (years) | 13 (0–52) | 1206 | 13 (0–52) | 794 | 12 (0–45) | 347 | 6 (0–32)c | 65 | < 0.01# |
| BMI (kg/m2) | 22.6 (13.2–41.1) | 1188 | 22.4 (14.7–37.6) | 777 | 23.1 (13.2–41.1)d | 351 | 22.3 (18.0–30.3) | 60 | < 0.01# |
| Obesity (yes/no) (Obesity, %) | 310/878 (26.1%) | 1188 | 186/591 (23.9%) | 777 | 114/237e (32.5%) | 351 | 10/50 (16.7%) | 60 | < 0.01* |
| Laboratory data | |||||||||
| Plasma glucose (mg/dL) | 153 (27–1436) | 1169 | 158 (27–782) | 762 | 148.5 (31–1436) | 346 | 158 (29–510) | 61 | N.S.# |
| HbA1c (%) | 7.8 (4.6–18.0) | 1221 | 7.9 (4.6–18.0)b | 800 | 7.6 (4.6–14.5) | 358 | 7.5 (5.5–10.5) | 63 | < 0.01# |
| HbA1c < 7.0% (yes/no) (HbA1c < 7.0%, %) | 281/940 (23.0%) | 1221 | 161/639 (20.1%) | 800 | 101/257f (28.2%) | 358 | 19/44 (30.2%) | 63 | < 0.01* |
| Serum C-peptide (ng/mL) | 0.09 (0.00–8.68) | 1085 | 0.04 (0.00–8.36)a | 708 | 0.70 (0.00–8.68)a | 321 | 0.03 (0.00–0.42)a | 56 | < 0.01# |
| Anti-GAD antibody (pos./neg.) (Positive, %) | 735/276 (72.7%) | 1011 | 445/196 g (69.4%) | 641 | 282/39 g (87.9%) | 321 | 8/41 g (16.3%) | 49 | < 0.01* |
| Anti-IA-2 antibody (pos./neg.) (Positive, %) | 66/94 (41.3%) | 160 | 50/54 (48.1%) | 104 | 12/21 (36.4%) | 33 | 4/19f (17.4%) | 23 | < 0.05* |
| Microangiopathy | |||||||||
| Retinopathy (yes/no) (Retinopathy, %) | 299/837 (26.3%) | 1136 | 190/560 (25.3%) | 750 | 104/223 (31.8%) | 327 | 5/54 h (8.5%) | 59 | < 0.01* |
| Nephropathy (yes/no) (Nephropathy, %) | 250/953 (20.8%) | 1203 | 141/648 (17.9%) | 789 | 100/251f (28.5%) | 351 | 9/54 (14.3%) | 63 | < 0.01* |
| Neuropathy (yes/no) (Neuropathy, %) | 199/728 (21.5%) | 927 | 115/514 (18.3%) | 629 | 77/170e (31.2%) | 247 | 7/44 (13.7%) | 51 | < 0.01* |
| Macroangiopathy | |||||||||
| Ischemic heart disease (yes/no) (Ischemic heart disease, %) | 77/1107 (6.5%) | 1184 | 34/742 (4.4%) | 776 | 40/304f (11.6%) | 344 | 3/61 (4.7%) | 64 | < 0.01* |
| Cerebrovascular disease (yes/no) (Cerebrovascular disease, %) | 38/1150 (3.2%) | 1188 | 19/757 (2.4%) | 776 | 19/329f (5.5%) | 348 | 0/64 (0.0%) | 64 | < 0.01* |
| Peripheral artery disease (yes/no) (Peripheral artery disease, %) | 31/1150 (2.6%) | 1181 | 16/758 (2.1%) | 774 | 12/331 (3.5%) | 343 | 3/61 (4.7%) | 64 | N.S.* |
Data of continuous variables are presented as the median (minimum–maximum)
AT1D acute-onset type 1 diabetes, BMI body mass index, FT1D fulminant type 1 diabetes, GAD glutamic acid decarboxylase, HbA1c glycosylated hemoglobin, IA-2 insulinoma-associated protein-2, neg. negative, N.S. not significant, pos. positive, SPIDDM slowly progressive insulin-dependent (type 1) diabetes mellitus, CVD cerebrovascular disease, IHD ischemic heart disease, PAD peripheral artery disease
$Statistical analysis of patients with AT1D, SPIDDM, or FT1D
#Kruskal–Wallis test
*Chi-square test
aP < 0.0167 for each pair of groups by Mann–Whitney U-test with Bonferroni correction
bP < 0.0167 vs. patients with SPIDDM or FT1D by Mann–Whitney U-test with Bonferroni correction
cP < 0.0167 vs. patients with AT1D and SPIDDM by Mann–Whitney U-test with Bonferroni correction
dP < 0.0167 vs. patients with AT1D by Mann–Whitney U-test with Bonferroni correction
eP < 0.0167 vs. patients with AT1D and FT1D by Chi-square test with Bonferroni correction
fP < 0.0167 vs. patients with AT1D by Chi-square test with Bonferroni correction
gP < 0.0167 for each pair of groups by Chi-square test with Bonferroni correction
hP < 0.0167 vs. patients with AT1D and SPIDDM by Chi-square test with Bonferroni correction
The median (minimum–maximum) onset age was 38 years (1–91 years), and it was significantly lower in patients with AT1D (32 years) than in those with SPIDDM or FT1D (48 and 42 years, respectively; P < 0.0167 by Mann–Whitney U-test with a Bonferroni correction; Table 1 and Fig. 1). The median onset age was significantly higher in females than in male patients with SPIDDM, and lower in female than in male patients with FT1D (P < 0.01 by Mann–Whitney U-test in both cases) (Fig. 1). This study included three patients with pregnancy-associated T1D, all of whom had FT1D; their median onset ages were 23, 31, and 33 years. Even excluding these three patients, the median onset age was significantly lower in female than in male patients with FT1D (P < 0.05 by Mann–Whitney U-test). Meanwhile, there was no significant difference in median onset age between female and male patients with AT1D.
Fig. 1.
Histograms of onset age by gender and subtype of type 1 diabetes. The distribution of onset age is demonstrated in all patients and in gender-segregated patients with AT1D, SPIDDM, or FT1D. a–c All patients with AT1D, SPIDDM, or FT1D (n = 794, 347, and 65, respectively). d–f Female patients with AT1D, SPIDDM, or FT1D (n = 477, 213, and 34, respectively). g–i Male patients with AT1D, SPIDDM, or FT1D (n = 317, 134, and 31, respectively). Onset age is presented as median (IQR). AT1D acute-onset type 1 diabetes, FT1D fulminant type 1 diabetes, IQR interquartile range, SPIDDM slowly progressive insulin-dependent (type 1) diabetes mellitus. *P < 0.0167 vs. all patients with AT1D by Mann–Whitney U-test with Bonferroni correction. **P < 0.05 vs. male patients with SPIDDM by Mann–Whitney U-test. #P < 0.05 vs. male patients with FT1D by Mann–Whitney U-test
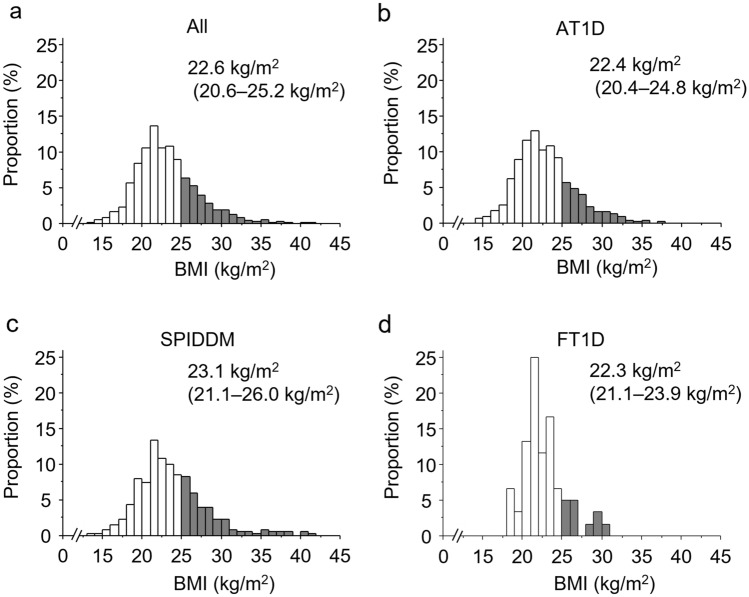
The median (minimum–maximum) BMI among all patients was 22.6 kg/m2 (13.2–41.1 kg/m2), and the proportion of obesity was 26.1% among all patients with T1D. The median BMI was significantly higher in patients with SPIDDM than in those with AT1D. The proportions of obesity were 23.9%, 32.5%, and 16.7% among patients with AT1D, SPIDDM, and FT1D, respectively, and it was significantly higher in patients with SPIDDM than in those with AT1D or FT1D (Table 1; Fig. 2).
Fig. 2.
Histograms of BMI by subtype of type 1 diabetes. The distribution of BMI is demonstrated among all patients with type 1 diabetes (a, n = 1188), patients with AT1D (b, n = 777), those with SPIDDM (c, n = 351), and those with FT1D (d, n = 60). The gray bar indicates patients with a BMI of ≥ 25.0 kg/m2. BMI is presented as median (IQR). AT1D acute-onset type 1 diabetes, FT1D fulminant type 1 diabetes, IQR interquartile range, SPIDDM slowly progressive insulin-dependent (type 1) diabetes mellitus
Of 1,011 patients with T1D, 735 (72.7%) were positive for the GADA test: 69.4% of patients with AT1D, 87.9% of patients with SPIDDM, and 16.3% of patients with FT1D. There was a significant difference in GADA-positive rate between each pair of T1D groups. As for anti-IA-2 antibody, the positive rate was significantly lower in patients with FT1D than in those with AT1D, although the small number of patients may have prevented accurate analysis (Table 1).
There was a significant difference in serum C-peptide levels between each pair of T1D groups (Table 1).
Glycemic control and treatments
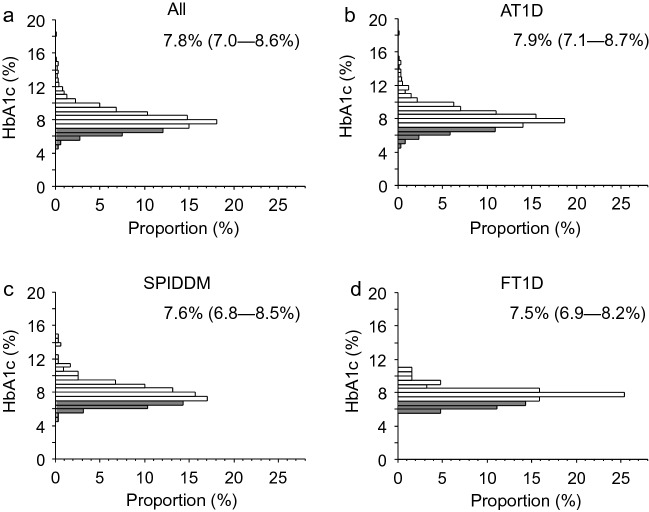
The median (minimum–maximum) HbA1c was 7.8% (4.6–18.0%). Median HbA1c was significantly higher in patients with AT1D than in those with SPIDDM or FT1D (7.9%, 7.6%, and 7.5%, respectively; P < 0.0167 by Mann–Whitney U-test with a Bonferroni correction), although the value did not significantly differ between patients with SPIDDM and those with FT1D. The proportion of patients with an HbA1c < 7.0% was 23.0% among all patients, while it was 20.1%, 28.2%, and 30.2% among patients with AT1D, SPIDDM, and FT1D, respectively. The proportion was significantly higher in patients with SPIDDM than in those with AT1D (P < 0.0167 by Mann–Whitney U-test with a Bonferroni correction) (Table 1; Fig. 3).
Fig. 3.
Histograms of HbA1c level by subtype of type 1 diabetes. The distribution of HbA1c level is demonstrated according among all patients with type 1 diabetes (a, n = 1221), patients with AT1D (b, n = 800), those with SPIDDM (c, n = 358), and those with FT1D (d, n = 63). The gray bar indicates patients with an HbA1c of < 7.0%. HbA1c level is presented as median (IQR). AT1D acute-onset type 1 diabetes, FT1D fulminant type 1 diabetes, IQR interquartile range, SPIDDM slowly progressive insulin-dependent (type 1) diabetes mellitus
Regarding therapeutic regimens, multiple daily insulin injection (MDI) was carried out by 85.6%, 72.1%, and 81.5% of patients with AT1D, SPIDDM, or FT1D, respectively, while continuous subcutaneous insulin infusion (CSII) was administered to 10.3%, 2.2%, and 18.5%. Combination therapy using basal insulin and oral hypoglycemic agents was prescribed to 2.5%, 12.2%, and 1.5% of patients, while other therapeutic regimens, such as diet therapy alone, glucagon-like peptide 1 (GLP-1) receptor agonists, and unknown medication, accounted for 1.1%, 11.3%, and 0.0% of patients (please be aware that GLP-1 receptor agonists are not currently approved for the treatment of T1D in Japan). Moreover, 0.4% of patients with AT1D and 1.7% of patients with SPIDDM, but no patients with FT1D, received once-daily or twice-daily insulin therapy using biphasic or premixed insulins (Fig. 4).
Fig. 4.

Profile of therapeutic regimen by subtype of type 1 diabetes. The proportion of each indicated therapeutic regimen was demonstrated among patients with AT1D (n = 814), SPIDDM (n = 362), or FT1D (n = 65). AT1D acute-onset type 1 diabetes, CSII continuous subcutaneous insulin infusion, FT1D fulminant type 1 diabetes, MDI multiple daily insulin injection, OHA(s) oral hypoglycemic agent(s), SPIDDM slowly progressive insulin-dependent (type 1) diabetes mellitus
Next, we compared HbA1c levels among patients with AT1D, SPIDDM, or FT1D treated using MDI or CSII. There was no significant difference in HbA1c among the three groups using CSII. Meanwhile, HbA1c was significantly lower in MDI users with FT1D than in those with AT1D or SPIDDM (P < 0.0167 in both cases by Mann–Whitney U-test with a Bonferroni correction) (Supplementary Fig. 3).
Diabetes-related complications
Diabetes-related complications are summarized in Table 1 and Fig. 5. As for microvascular complications, the proportions of retinopathy, nephropathy, and neuropathy were 26.3%, 20.8% and 21.5%, respectively, among patients with AT1D, SPIDDM, or FT1D. The proportion of retinopathy was significantly lower in patients with FT1D than in those with AT1D or SPIDDM, perhaps because patients with FT1D had a significantly lower disease duration. The proportion of nephropathy was significantly higher in patients with SPIDDM than in those with AT1D. Moreover, the proportion of neuropathy was significantly higher in patients with SPIDDM than in those with AT1D or FT1D (Fig. 5). Similar findings were observed in patients with diabetes duration of ≥ 10 years who showed no significant difference in diabetes duration among the three subtypes of T1D (Supplementary Table 2b and Supplementary Fig. 4b).
Fig. 5.

Rate of diabetes complications by subtype of type 1 diabetes. The rates of diabetes-related complications were demonstrated among patients with AT1D, SPIDDM, or FT1D. Table 1 shows the number of patients with subtypes of type 1 diabetes according to each complication. AT1D acute-onset type 1 diabetes, CVD cerebrovascular disease, FT1D fulminant type 1 diabetes, IHD ischemic heart disease, PAD peripheral artery disease, SPIDDM slowly progressive insulin-dependent (type 1) diabetes mellitus. *P < 0.0167 by Chi-square test with a Bonferroni correction
Regarding macrovascular complications, the proportions of ischemic heart disease (IHD), cerebrovascular disease (CVD), and peripheral artery disease (PAD) were 6.5%, 3.2%, and 2.6%, respectively. The proportions of both IHD and CVD were significantly higher in patients with SPIDDM than in those with AT1D (Table 1; Fig. 5). Similar findings were observed in patients with diabetes duration of ≥ 10 years (Supplementary Table 2b and Supplementary Fig. 4b).
Meanwhile, when the diabetes duration was limited to < 10 years, the proportions of retinopathy, nephropathy, and IHD were significantly higher among patients with SPIDDM than among those with AT1D (Supplementary Table 2a and Supplementary Fig. 4a). However, there was a significant difference in diabetes duration among the three subtypes of T1D, possibly preventing an accurate analysis. Therefore, we selected patients with diabetes duration of < 5 years to adjust for the differences among the three subtypes of T1D and investigated the complications. As a result, the proportions of retinopathy, nephropathy, and IHD were again significantly higher among patients with SPIDDM than among those with AT1D (Supplementary Table 3 and Supplementary Fig. 5). Meanwhile, the proportions of retinopathy and nephropathy had a tendency to be higher among patients with FT1D than among those with AT1D, although without statistically significant difference, suggesting that FT1D is a high-risk subgroup for diabetic microangiopathy associated with the lack of endogenous insulin secretion at onset, as demonstrated in a previous nationwide survey in Japan [14].
Comparison of clinical profiles between childhood-onset and adult-onset type 1 diabetes
We investigated the clinical profiles of the patients according to childhood-onset T1D and adult-onset T1D (defined as the age at onset of < 15 years and ≥ 15 years, respectively). The study included 674 patients with adult-onset AT1D (54.3%), 340 with adult-onset SPIDDM (27.4%), 64 with adult-onset FT1D (5.2%), 120 with childhood-onset AT1D (9.7%), 7 with childhood-onset SPIDDM (0.006%), and one with childhood-onset FT1D (0.001%). Because the populations of childhood-onset SPIDDM and FT1D were very small, we only compared the clinical profiles between the patients with childhood-onset AT1D and those with adult-onset AT1D.
As shown in Supplementary Table 4 and Supplementary Fig. 6, the median (minimum–maximum) age was significantly lower in patients with childhood-onset AT1D than those with adult-onset AT1D (36 (20–57) vs. 49 (16–93) years, respectively; P < 0.01), whereas the median (minimum–maximum) diabetes duration was significantly longer in the former than the latter groups (26 (7–52) vs. 12 (0–46) years, respectively; P < 0.01). The proportion of HbA1c < 7.0% was significantly higher in patients with childhood-onset AT1D than in those with adult-onset AT1D (27.6% vs. 19.0%, respectively; P < 0.05), although the median HbA1c level was comparable between the two groups. In addition, the median BMI (minimum–maximum) was significantly higher in patients with childhood-onset AT1D than in those with adult-onset AT1D (23.1 (15.6–33.3) vs. 22.4 (14.7–37.1) kg/m2, respectively; P < 0.05), although the proportion of obesity was comparable between the two groups. These findings could be explained by an anabolic action of insulin with better glycemic control in patients with childhood-onset AT1D. Meanwhile, serum C-peptide level and positivity of GADA test were significantly lower in patients with childhood-onset AT1D than in those with adult-onset AT1D, which might be associated with the longer diabetes duration in patients with childhood-onset AT1D. As for diabetes-related complications, the proportion of retinopathy was significantly higher in patients with childhood-onset AT1D than in those with adult-onset AT1D (41.3% vs. 22.8%, respectively; P < 0.01), which might also be attributable to the longer diabetes duration in the former group, whereas there were no significant differences in the proportions of other complications between the two groups.
When the disease duration was limited to 15–25 years (median value of 19 years) to adjust a difference in the diabetes duration between the two ATID groups, there were no significant difference in all clinical profiles, excluding age and onset age, between the two groups (Supplementary Table 5). These findings suggest that the differences in some clinical profiles might be influenced by a difference in the diabetes duration between the two AT1D groups.
Discussion
Previous JDS committee-driven nationwide surveys have revealed that the onset ages (mean ± standard deviation [SD]) of AT1D, SPIDDM, and FT1D are 30.1 ± 16.2, 51.1 ± 14.4, 39.1 ± 15.7 years in Japan [15, 16]. These values were somewhat similar to those in this study. With regard to gender, as in the previous JDS committee report [13], this study showed that the onset age of FT1D was significantly higher in males than in females. Similar findings were reported in a cross-sectional study in China [17], but the reason for the gender difference remains unknown. Conversely, we demonstrated that the onset age was significantly older in female than in male patients with SPIDDM. Similar findings have been demonstrated in other cross-sectional studies from India [18], as well as in a study from a single outpatient clinic in Japan [19]. Given that male patients with SPIDDM progress more rapidly to insulin dependency than female patients [20], sex hormones may have some effect on the onset age of SPIDDM; this warrants further investigation. Meanwhile, there was no significant difference in the onset age of AT1D between males and females in this study; a similar finding was reported in a cross-sectional study from Germany [21] and a single outpatient clinic in Japan [19].
Reportedly, the prevalence of T1D in adults is more than twice that in childhood-onset patients and that two-thirds of them have a SPIDDM in Japan [2]. However, this study revealed that the prevalence of AT1D was higher than that of SPIDDM. The reason might come from the possibility that more patients with SPIDDM especially in a non-insulin-dependent state, that is often easier than AT1D to manage, may be treated at surrounding hospitals or clinics, which warrants further investigations.
There is growing concern about the increase in obese patients with T1D. One recent JDDM survey demonstrated that BMI has been rising in patients with T1D since the survey began in 2002. The mean BMI values were 23.12 kg/m2 and 22.91 kg/m2 in the 2018 and 2019 surveys, respectively, while the mean (± SD) BMI was 22.1 (± 2.9) kg/m2 in the JDCP study, similar to this study [6, 7]. Meanwhile, this study revealed that approximately one-quarter of patients with T1D had obesity, which was comparable with the latest data from the 2020 survey of JDDM study, in which 24.7% of patients had obesity (Yagi et al., the 64th Annual Meeting of Japan Diabetes Society, Toyama, 2021). However, it was higher than the findings from the JDCP study, which reported that 14% of patients with T1D had obesity [7]. Relatedly, the evolution of insulin products, as well as advances in insulin pumps and continuous glucose monitoring, may lead to improvements in glycemic control in patients with T1D. This may in turn lead to increases in the proportion of patients with obesity, which is one disadvantage of insulin treatment.
In this study, 12.1% of patients with SPIDDM (39/321) showed a negative GADA test. However, because we collected the latest GADA values during the course of T1D, the presented values were not necessarily measured at the time of SPIDDM diagnosis. Thus, the negative GADA result might be due to the negative conversion of GADA during the course of SPIDDM. Meanwhile, since December 2015, an enzyme-linked immunosorbent assay (ELISA) has been used to measure GADA in Japan, whereas previously a radioimmunoassay (RIA) was used. Our earlier study demonstrated that the sera of 25.5% of patients with SPIDDM who were GADA positive by the RIA test were negative by the ELISA test [22]. Thus, the negative GADA results may have been due to a mismatch in results between the two assay kits.
Meanwhile, the positivity rates of the GADA and anti-IA-2 antibody tests in patients with FT1D in this study were higher than those in a previous nationwide study in Japan (16.3% vs. 4.8% for GADA, 17.4% vs. 0% for IA-2 antibody) [15] and those in a cross-sectional study from a single hospital (16.3% vs. 9.4% for GADA, 17.4% vs. 3.5% for IA-2 antibody) [23]. In the case of the latter study, this discrepancy may have arisen because the GADA and IA-2 antibody assay kits differed between studies [23], although the exact reason is unknown. Alternatively, some patients with AT1D may have been misdiagnosed as having FT1D in this study; the two subtypes are sometimes difficult to differentiate, which warrants further investigation.
The mean HbA1c levels in patients with T1D were 7.76% and 7.77%, respectively, in the 2018 and 2019 JDDM surveys [6], while the mean (± SD) HbA1c level was 7.8% (± 1.4%) in the JDCP study [7], similar to the finding of this study. Moreover, the proportion of patients with HbA1c < 7.0% was comparable between the present and the JDCP studies (23.0% vs. 25.3%, respectively) [7]. These findings suggest that patients with T1D in Saitama prefecture have stable glycemic control equivalent to that observed throughout Japan.
In this study, the median HbA1c levels tended to be lower in patients with FT1D than in those with AT1D and SPIDDM, probably because patients with FT1D had a shorter disease duration, and because MDI-treated patients with FT1D showed lower HbA1c levels than MDI-treated patients with AT1D or SPIDDM, while HbA1c levels in CSII users were comparable among the three T1D subtypes. Thus, MDI therapy seemed more successful in patients with FT1D than in those with AT1D or SPIDDM. However, it may be that the frequency of hypoglycemic events increases with a latency in MDI-treated patients with FT1D.
Regarding therapeutic regimens, MDI (defined as ≥ 3 insulin injections per day) and CSII were used to treat 86.9% and 2.8% of patients, respectively, in the JDCP study [7], suggesting that CSII was more proactively used to treat T1D in the eight participating institutions than in other regions throughout Japan.
The proportion of retinopathy among all patients with T1D was higher in this study than in the JDCP study (26.3% vs. 22.8%, respectively) [24], perhaps because the definition of retinopathy differed between the two studies; this study included all types of retinopathy, while JDCP study only evaluated non-proliferative retinopathy. Alternatively, the discrepancy may have occurred because the median disease duration differed between the two studies: 13 years in the JDCP study vs. 9 years in this study. Meanwhile, the proportion of nephropathy among all with T1D seemed comparable between this study and the JDCP study (20.8% vs. 18.3%, respectively) [25].
This study revealed that most complications were more common among patients with SPIDDM than among those with AT1D, even though the disease durations were comparable between the two. Unlike AT1D, SPIDDM usually has no hyperglycemic symptoms at onset, so physicians cannot usually identify the true time of onset. Thus, the true onset age might be younger than the reported onset age, and the actual disease duration might be longer than the presented data. This may be why most complications are more common in patients with SPIDDM than in those with AT1D, such as retinopathy and nephropathy in patients who have SPIDDM with a disease duration of < 5 years (Supplementary Table 3 and Supplementary Fig. 5). In addition, higher BMI or higher obesity frequency might be associated with higher proportions of nephropathy, IHD, and CVD in patients with SPIDDM than in those with AT1D (Table 1; Fig. 5). Meanwhile, an involvement of macrovascular risk factors other than obesity in higher proportions of IHD and CVD in patients with SPIDDM, i.e., hypertension and dyslipidemia, could not be evaluated because the relevant clinical information was not collected in this study, warranting further investigation.
As compared to the patients with adult-onset AT1D, those with childhood-onset AT1D in this study were found to be characterized by a longer diabetes duration despite younger age, possibly leading to a lower insulin secretion capacity and a lower positivity rate for the GADA test result. Meanwhile, given the fact that undetectable β-cell function is associated with the earlier occurrence of diabetic retinopathy via glycemic spikes in patients with T1D [26], more severe β-cell dysfunction might be associated with a higher proportion of retinopathy in addition to a longer diabetes duration in patients with childhood-onset AT1D in this study.
In this study, the median HbA1c level had a tendency to be lower, and the proportion of HbA1c < 7.0% was significantly higher in the patients with childhood-onset AT1D than those with adult-onset AT1D. It may be that a long experience of insulin therapy reflected by a longer diabetes duration contributes to a better glycemic control in the patients with childhood-onset AT1D. Alternatively, considering a higher proportion of retinopathy in the patients with childhood-onset AT1D, the frequency of hypoglycemic events along with glycemic fluctuation associated with a lower insulin secretion capacity might increase with a latency in the patients, leading to a higher proportion of HbA1c < 7.0%. In fact, when a difference in the diabetes duration was adjusted between the two ATID groups, serum C-peptide level was comparable between the two groups, resulting in no significant differences in both glycemic control and proportion of retinopathy between the two. Although the details must be elucidated in future studies.
There were several limitations in this study. First, many values of several clinical parameters were missing, which may have prevented accurate analyses. Second, because the study only targeted patients with T1D who visited the included eight institutions in Saitama prefecture, our findings do not necessarily reflect the clinical conditions of all patients with T1D throughout the prefecture. In particular, because diabetologists in the eight institutions treat especially large numbers of patients with T1D, our findings about the relatively small portion of patients with SPIDDM and the relatively large proportion of CSII users could have been influenced by selection bias associated with a hospital-based study. Moreover, we could not assess the difference in clinical progression or the severity of diabetic microangiopathy among patients with the three T1D subtypes because we lacked relevant detailed clinical information from medical records. This may have prevented accurate analysis of complications.
In conclusion, adolescent and adult patients with T1D treated at the eight main institutions in Saitama prefecture, which treated relatively large numbers of patients with T1D, showed glycemic control that was similar to that observed in nationwide surveys of T1D. However, approximately one-quarter of patients with T1D had obesity. To prevent obesity, clinicians should provide appropriate insulin treatment, as well as diet and exercise therapies, to patients with T1D. Randomized controlled trials using sodium-glucose cotransporter 2 inhibitors (SGLT2is), i.e., ipragliflozin [27] and dapagliflozin (DEPICT studies) [28], in addition to insulin therapy in Japanese adults with T1D have reported significant reduction in body weight. Thus, as necessary, using these SGLT2is may be helpful in preventing obesity in insulin-treated patients with T1D, although both patients and clinicians should pay attention to the development of diabetic ketoacidosis [29].
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are extremely grateful to the following doctors (affiliations at the time) for assisting with the present survey: M. Matsuda and Y. Izumida (Saitama Medical University Saitama Medical Center), T. Inukai (Seibu General Hospital), A. Haisa, S. Nakanishi, A. Satomura, and S. Suzuki (Saitama Medical University Hospital), A. Tada (JCHO Saitama Medical Center). In addition, we greatly appreciate H. Fujita (Saitama Medical University Hospital) for creating the database.
Funding
No specific funding or grant was received for this work.
Declarations
Conflict of interest
K.H. has received scholarship grants from Johnson & Johnson KK, Kyowa Kirin Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Ono Pharmaceutical Co., Ltd., and Terumo Corporation. K.N. has received lecture fees from Novo Nordisk Pharma Inc., Ono Pharmaceutical Co. Ltd., Sanofi KK, and Mitsubishi Tanabe Pharma Corporation. A.S. has received lecture fees from Astellas Pharm Inc., Eli Lilly Japan KK, Ono Pharmaceutical Co., Ltd., Terumo Corporation, and Sanofi KK, as well as research funding from Astellas Pharm Inc. and Mitsubishi Tanabe Pharma Corporation, and scholarship grants from Astellas Pharm Inc., Daiichi Sankyo Co., Ltd., Kyowa Kirin Co., Ltd., MSD KK, Novo Nordisk Pharma Ltd., and Ono Pharmaceutical Co., Ltd. S.T. has received lecture fees from Sanofi KK. The other authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314(21):1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 2.Kawasaki E, Matsuura N, Eguchi K. Type 1 diabetes in Japan. Diabetologia. 2006;49(5):828–836. doi: 10.1007/s00125-006-0213-8. [DOI] [PubMed] [Google Scholar]
- 3.Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y. A proposal of three distinct subtypes of type 1 diabetes mellitus based on clinical and pathological evidence. Ann Med. 2000;32(8):539–543. doi: 10.3109/07853890008998833. [DOI] [PubMed] [Google Scholar]
- 4.Onda Y, Sugihara S, Ogata T, et al. Incidence and prevalence of childhood-onset Type 1 diabetes in Japan: the T1D study. Diabet Med. 2017;34(7):909–915. doi: 10.1111/dme.13295. [DOI] [PubMed] [Google Scholar]
- 5.Nishioka Y, Noda T, Okada S, et al. Incidence and seasonality of type 1 diabetes: a population-based 3-year cohort study using the National Database in Japan. BMJ Open Diabetes Res Care. 2020;8(1):e001262. doi: 10.1136/bmjdrc-2020-001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Japan Diabetes Clinical Data Management Study Group. (n.d.) from http://jddm.jp. Accessed June 15, 2021.
- 7.Nishimura R, Izumi K, Hayashino Y, et al. A large-scale observational study to investigate the current status of diabetes complications and their prevention in Japan: research outline and baseline data for type 1 diabetes-JDCP study 2. Diabetol Int. 2016;7(1):4–11. doi: 10.1007/s13340-015-0248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawasaki E, Maruyama T, Imagawa A, et al. Diagnostic criteria for acute-onset type 1 diabetes mellitus (2012): report of the Committee of Japan Diabetes Society on the research of fulminant and acute-onset type 1 diabetes mellitus. J Diabetes Investig. 2014;5(1):115–118. doi: 10.1111/jdi.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka S, Ohmori M, Awata T, et al. Diagnostic criteria for slowly progressive insulin-dependent (type 1) diabetes mellitus (SPIDDM) (2012): report by the Committee on Slowly Progressive Insulin-Dependent (Type 1) Diabetes Mellitus of the Japan Diabetes Society. Diabetol Int. 2015;6(1):1–7. doi: 10.1007/s13340-014-0199-2. [DOI] [Google Scholar]
- 10.Imagawa A, Hanafusa T, Awata T, et al. Report of the Committee of the Japan Diabetes Society on the Research of Fulminant and Acute-onset Type 1 Diabetes Mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus (2012) J Diabetes Investig. 2012;3(6):536–539. doi: 10.1111/jdi.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Examination Committee of Criteria for “Obesity Disease” in Japan, Japan Society for the Study of Obesity New criteria for “obesity disease” in Japan. Circ J. 2002;66(11):987–992. doi: 10.1253/circj.66.987. [DOI] [PubMed] [Google Scholar]
- 12.Yokoyama T. Epidemiology of type 1 diabetes. The annual report 2017 from Health Labour Sciences Research. Ministry of Health, Labour and Welfare of Japan. https://mhlw-grants.niph.go.jp/system/files/2017/172031/201709008A_upload/201709008A0023.pdf. Accessed 12 Oct 2021. (Japanese).
- 13.Saitama prefectural government. Estimated population of Saitama prefecture. https://www.pref.saitama.lg.jp/a0206/news/page/news2021073001.html. Accessed 12 Oct 2021. (Japanese).
- 14.Murase Y, Imagawa A, Hanafusa T, et al. Fulminant type 1 diabetes as a high risk group for diabetic microangiopathy–a nationwide 5-year-study in Japan. Diabetologia. 2007;50(3):531–537. doi: 10.1007/s00125-006-0575-y. [DOI] [PubMed] [Google Scholar]
- 15.Imagawa A, Hanafusa T, Uchigata Y, et al. Fulminant type 1 diabetes: a nationwide survey in Japan. Diabetes Care. 2003;26(8):2345–2352. doi: 10.2337/diacare.26.8.2345. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka S, Awata T, Shimada A, et al. Clinical characteristics of slowly progressive insulin-dependent (type 1) diabetes mellitus (SPIDDM): 1st subcommittee report on SPIDDM, committee on type 1 diabetes, Japan Diabetes Society. J Japan Diab Soc. 2011;54(1):65–75. [Google Scholar]
- 17.Ying L, Ma X, Lu J, et al. Fulminant type 1 diabetes: the clinical and continuous glucose monitoring characteristics in Chinese patients. Clin Exp Pharmacol Physiol. 2019;46(9):806–812. doi: 10.1111/1440-1681.13099. [DOI] [PubMed] [Google Scholar]
- 18.Brahmkshatriya PP, Mehta AA, Saboo BD, Goyal RK. Characteristics and prevalence of latent autoimmune diabetes in adults (LADA) ISRN Pharmacol. 2012;2012:580202. doi: 10.5402/2012/580202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osaki Y, Kawai K, Motohashi S, et al. Type 1 diabetes mellitus and autoimmune thyroid disease in Japanese–prevalence and pattern of onset. J Japan Diab Soc. 2009;52(11):887–893. [Google Scholar]
- 20.Kobayashi T, Nakanishi K, Sugimoto T, et al. Maleness as risk factor for slowly progressive IDDM. Diabetes Care. 1989;12(1):7–11. doi: 10.2337/diacare.12.1.7. [DOI] [PubMed] [Google Scholar]
- 21.Leidig-Bruckner G, Grobholz S, Bruckner T, Scheidt-Nave C, Nawroth P, Schneider JG. Prevalence and determinants of osteoporosis in patients with type 1 and type 2 diabetes mellitus. BMC Endocr Disord. 2014;14:33. doi: 10.1186/1472-6823-14-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oikawa Y, Tanaka M, Horie I, et al. A study on the correlation between GAD antibody titers measured by ELISA kit and RIA Kit. Jpn J Med Pharm Sci. 2015;72:1551–1560. [Google Scholar]
- 23.Kawasaki E, Nakamura K, Kuriya G, et al. Differences in the humoral autoreactivity to zinc transporter 8 between childhood- and adult-onset type 1 diabetes in Japanese patients. Clin Immunol. 2011;138(2):146–153. doi: 10.1016/j.clim.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Kawasaki R, Kitano S, Sato Y, et al. Factors associated with non-proliferative diabetic retinopathy in patients with type 1 and type 2 diabetes: the Japan Diabetes Complication and its Prevention prospective study (JDCP study 4) Diabetol Int. 2019;10(1):3–11. doi: 10.1007/s13340-018-0357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shikata K, Kodera R, Utsunomiya K, et al. Prevalence of albuminuria and renal dysfunction, and related clinical factors in Japanese patients with diabetes: The Japan Diabetes Complication and its Prevention prospective study 5. J Diabetes Investig. 2020;11(2):325–332. doi: 10.1111/jdi.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakanishi K, Watanabe C. Rate of beta-cell destruction in type 1 diabetes influences the development of diabetic retinopathy: protective effect of residual beta-cell function for more than 10 years. J Clin Endocrinol Metab. 2008;93(12):4759–4766. doi: 10.1210/jc.2008-1209. [DOI] [PubMed] [Google Scholar]
- 27.Kaku K, Isaka H, Sakatani T, Toyoshima J. Long-term (52-week) efficacy and safety of ipragliflozin add-on therapy to insulin in Japanese patients with type 1 diabetes mellitus: an uncontrolled, open-label extension of a phase III study. J Diabetes Investig. 2020;11(3):662–671. doi: 10.1111/jdi.13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Araki E, Mathieu C, Shiraiwa T, et al. Long-term (52-week) efficacy and safety of dapagliflozin as an adjunct to insulin therapy in Japanese patients with type 1 diabetes: subgroup analysis of the DEPICT-2 study. Diabetes Obes Metab. 2021;23(7):1496–1504. doi: 10.1111/dom.14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horii T, Oikawa Y, Atsuda K, Shimada A. On-label use of sodium-glucose cotransporter 2 inhibitors might increase the risk of diabetic ketoacidosis in patients with type 1 diabetes. J Diabetes Investig. 2021;12(9):1586–1593. doi: 10.1111/jdi.13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.