Abstract
Objectives
To describe the epidemiology, clinical, biochemical, immunological and radiological aspects of youth with type 2 diabetes.
Methods
Patients under 18 year of age with type 2 diabetes were recruited from 2018 to 2020, clinical data collected, autoantibodies (GAD65, IAA, IA2 and ZnT8), insulin, ALT and c-peptide were measured. Hepatic ultrasound was performed for assessment of non-alcoholic fatty liver disease (NAFLD).
Results
104 patients were identified. The incidence in 2020 and prevalence per 100,000 was 2.51 and 23.7, respectively. The age of onset was between 8.5 and 18 years with 74% of the patients being of Qatari nationality. Males were more affected than females (1.5/1). Overweight/obesity was present in 98% of all the patients, a positive family history (either both parents or a single parent) in 71% and maternal gestational diabetes mellitus (GDM) in 60% of patients. More than 90% of the patients had acanthosis nigricans. 5 patients had 1 autoantibody positivity and hepatic ultrasound detected evidence of NAFLD in majority of patients.
Conclusion
Obesity, maternal GDM and family history of diabetes were the key risk factors for the development of type 2 diabetes. Autoantibody positivity may be present in youth type 2 diabetes. As youth type 2 diabetes is associated with early onset microvascular and macrovascular complications, these findings have important social and health budget implications for Qatar. Tackling the burden of maternal GDM and childhood obesity and building programmes for early detection and intervention, are therefore, essential to reduce the risk of future complications.
Keywords: Type 2 diabetes, Paediatric diabetes, Epidemiology, Obesity
Introduction
Type 1 diabetes is the most common form of diabetes in the childhood period. However, over the last 2 decades type 2 diabetes in children has been increasing at an alarming rate. The prevalence of type 2 diabetes in children and adolescents in the USA between 2001 and 2009 increased by 30.5% [1], thus illustrating the magnitude of the problem. Type 2 diabetes is a complex, chronic metabolic disease, with a heterogeneous aetiology, with social, behavioural, environmental risk factors as well as genetic risk susceptibility [2]. In the childhood period type 2 diabetes is associated with certain risk factors including obesity, sedentary lifestyle, family history of type 2 diabetes, maternal gestational diabetes mellitus (GDM), low birth weight and ethnicity [3].
Biochemically type 2 diabetes is characterised by hyperglycemia and insulin resistance but also relative impairment in insulin secretion [4]. The presence of insulin resistance explains the strong clinical association of type 2 diabetes with obesity and other insulin-resistant states. Impaired insulin secretion has been demonstrated in a variety of studies that show that both adolescents and adults usually have lost approximately 80% of their pancreatic beta-cell function before the diagnosis of type 2 diabetes [4]. In most cases, the pancreatic beta-cell dysfunction does not appear to be mediated by antibodies against the pancreatic islet cells.
Childhood obesity is the primary cause of type 2 diabetes at a young age. The increased prevalence of obesity in the Arab world over the last two decades has increased the number of children and adolescents who have type 2 diabetes [5]. In the Arab world, it is estimated that the number of people with diabetes is projected to increase by 96.2% by 2035 mostly driven by the increase in type 2 diabetes [6]. Genetic risk factors may play a crucial role in this uncontrolled rise in the prevalence of type 2 diabetes in children in the Middle Eastern region. Genome wide association studies undertaken on mostly European and Asian cohort of adult patients have identified > 80 common variants for type 2 diabetes with small effect sizes (risk of type 2 diabetes increased by 5–40%) [7]. Interestingly a recent study has shown that SNPs associated with type 2 diabetes in Europeans and Asians do not contribute significantly to the high prevalence of type 2 diabetes in the Qatari population, suggesting that the genetic risks for type 2 diabetes are likely different in Qataris compared to other populations [8].
In the Arab region, factors such as obesity, rapid urbanisation and lack of exercise are other key determinants of this rapid increase in the rate of type 2 diabetes in children (especially those less than 18 years of age). The number of studies that focus primarily on type 2 diabetes in children from the Middle East is limited with most reporting only on prevalence and incidence rates [9–18]. Qatar is a small peninsula located in the Arabian Gulf region which has undergone rapid urbanisation and development over the last 10 years. As in other parts of the world, type 2 diabetes in adults in Qatar has become a major health concern [19]. A previous study assessing the incidence and prevalence of type 1 and type 2 diabetes in children in Qatar noted that there were no cases of childhood type 2 diabetes noted before 2008 [15]. However, in subsequent years the same study reported that the incidence increased from 1.82 per 100,000 in 2012 to 2.7 per 100,000 in 2016, with an incidence of type 2 diabetes equal to 2.9 per 100,000 per year [15]. No previous prospective studies on youth onset type 2 diabetes have been conducted in Qatar describing the detailed epidemiology, clinical, immunological, biochemical and radiological features of a large cohort of patients.
Research design and methods
Patient recruitment
In this prospective study every child with diabetes (aged 0–18 years), attending the diabetes clinics, or admitted as an inpatient in Sidra Medicine (which is the only paediatric diabetes centre in Qatar) were recruited. Clinical details about the birth history, gestational age, ethnicity, age of onset of type 2 diabetes, family history (gestational diabetes and obesity), BMI, weight, signs of insulin resistance (acanthosis nigricans) and other system involvement were collected and documented.
Peripheral blood samples were collected for complete antibody profiling—all 4 autoantibodies namely Glutamic Acid Decarboxylase 65 (GAD65), Insulin Autoantibody (IAA), Islet Antigen-2 Auto Antibody (IA-2A) and Zinc Transporter 8 (ZnT8A) were measured and titers recorded. Fasting blood glucose, serum insulin and c-peptide were measured and standard oral glucose tolerance tests were performed in some patients. Liver function tests, lipid profile and liver ultrasound investigations were conducted. The diagnosis of type 2 diabetes was made in subjects with hyperglycemia according to the ADA guidelines, with detectable c-peptide levels, signs of insulin resistance and type 1 diabetes autoantibody negativity. Patients with prediabetes range blood glucose levels and with associated obesity syndromes were excluded from the cohort.
Annual incidence for the year 2020 and prevalence of type 2 diabetes was calculated in patients aged 0–18 years. The population statistics in 2020 for children living in Qatar was obtained from the Planning and Statistics Authority in Qatar. For each age group, the prevalence per 100,000 is estimated with a 95% confidence interval. The method for confidence interval estimation is Clopper-Pearson Exact as it has good coverage guarantees. The epidemiology analysis is performed using epiR package (version 2.0.19) from R Statistics software (version 3.6.3).
Antibody assay methodology
Complete antibody profiling of every child with type 2 diabetes was performed. The tests were done at Mayo Clinic laboratories using standard methodology [20, 21].
Criteria for diagnosis of fatty liver and hepatomegaly on ultrasound
In our subjects fatty liver was diagnosed on ultrasound based on the increased echogenicity of liver parenchyma when compared to adjacent kidney, loss of appreciation of the intrahepatic vasculature and loss of appreciation of the deeper aspects of the liver. Hepatomegaly was diagnosed when liver span was greater than normal for age of the subject.
Results
The total cohort of patients (aged between 0 and 18 years) attending the diabetes clinic was 1325 of which 104 patients with type 2 diabetes were identified. The incidence of type 2 diabetes in 2020 was 2.51 (CI 1.25–4.49) per 100,000 and the prevalence was 23.7 (CI 19.36–28.71).
Clinical features
The clinical history of every patient was obtained. Table 1 summarises some of the clinical features observed. Most of the patients were products of term gestation (90%) with average birth weight (72%), large for gestational age (12%) and small for gestational age (6%). Evidence of clinical insulin resistance was observed in the form of acanthosis nigricans in 90% of the patients.
Table 1.
Summarises the clinical features observed
| Parameter | Value (most common) | Mean |
|---|---|---|
| Age of onset (years) | 13–15 | 13.87 (± 2.6 SD) |
| Male:female ratio | 56:44 | – |
| Gestational age (weeks) | 37–40 | 39 (± 0.98 SD) |
| Birth weight (kg) | 2.5–4 | 3.17 (± 0.55 SD) |
| Maternal GDM present | 60% | – |
| Mode of delivery—vaginal birth: caesarian section | 53:47 | – |
| Parents with diabetes | 71% | – |
| Siblings with diabetes | 28% | – |
| Body mass index SD | > + 2SD | + 2.43 (± 0.56 SD) |
| Nationality—Qatari:non-Qatari | 74:30 | – |
| Acanthosis Nigricans | 90% |
Laboratory investigations
Glycated hemoglobin (HbA1C) was in the pre-diabetes range (5.8–6.4%) in 8.4% of the patients at the time of diagnosis and before starting treatment, 22.9% of patients had HbA1C > 12% while the majority of patients had HbA1C in between 9 and 11%. Table 2 summarises the laboratory investigations conducted on youth with type 2 diabetes. Liver enzymes were measured at diagnosis, ALT was within the normal range in 65% of patients, within 3 times the upper limit in 30% and more than 3 times upper limit in 5% of patients. 5 patients had positive type 1 diabetes autoantibodies.
Table 2.
Summarises the laboratory investigations conducted on youth with type 2 diabetes
| Parameter | Value (most common) | Mean/median |
|---|---|---|
| Hba1C at diagnosis | 9–11% | Mean = 10.07 (± 2.35 SD) |
| Insulin at diagnosis (pmol/L) | 100–500 | Median = 202 (44, 360) |
| ALT (IU/L) | < 39 | Median = 30 (18.5, 41.85) |
| Triglycerides (mmol/L) | < 1.7 | Mean = 1.69 (± 1.54 SD) |
| GAD autoantibody positive | 4.8% | – |
| C-peptide (ng/ml) | 0.5–5.5 | Mean = 3.73 (± 3.43 SD) |
Radiology
Figure 1 shows the liver ultrasonography findings observed in the cohort.
Fig. 1.
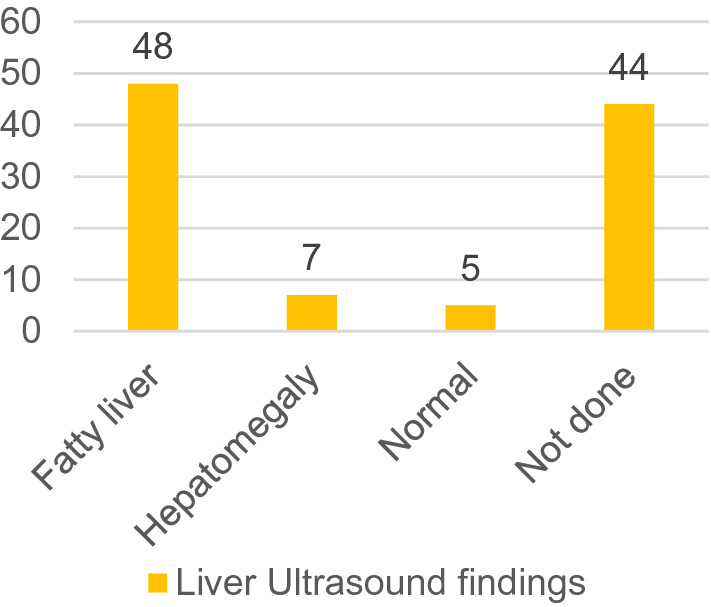
Shows the liver ultrasonography findings observed in the cohort
Conclusions
This is the first comprehensive prospective study of type 2 diabetes in children and adolescents from the Middle East region. In children and adolescents, obesity is the main risk factor for developing type 2 diabetes and this is now replicated in our study where we found that virtually all patients in our cohort were either overweight or obese with a large proportion being morbidly obese. Similar degrees of obesity in youth type 2 diabetes have been reported in other populations, for example in a young Pima Indian cohort with diabetes, 85% of the patients were found to be obese [22]. Obesity is strongly associated with insulin resistance and acanthosis nigricans is an established clinical marker of insulin resistance which is more prevalent in some ethnic groups (such as African–Americans and Mexican–Americans) than others (Japanese patients) More than 90% of our patient cohort had clinical evidence of clinical insulin resistance in the form of acanthosis nigricans suggesting that this is also an important clinical marker in our Arab population of youth with type 2 diabetes. The majority of patients also had biochemical evidence of insulin resistance as reflected in the high plasma insulin levels.
Family history is another important risk factor for the development of youth onset type 2 diabetes. Among affected youth 45–80% have at least one parent with diabetes and 74–100% have a first or second‐degree relative with type 2 diabetes [23, 24]. Among Pima Indians, the cumulative incidence of type 2 diabetes was highest in offspring if both parents had diabetes [25]. In our study, the risk of developing type 2 diabetes was highest in those patients where both parents also had type 2 diabetes thus confirming the previous observations in other populations. Maternal history of type 2 diabetes was associated with twice the risk of developing diabetes as compared to a paternal history of diabetes. Studies on Pima Indian women and youth have shown that maternal GDM increases the risk of obesity and type 2 diabetes in youth [26, 27]. In our patient cohort, a history of maternal GDM significantly increased the risk of developing type 2 diabetes in the youth thus confirming that in some patients in-utero exposure to maternal glycaemia programmes the individual to obesity and type 2 diabetes later in life.
Type 2 diabetes in children and adolescents typically develops around the pubertal growth spurt but there have been reports of type 2 diabetes developing in children as young as 5 and 8 years [28, 29]. Most of our patients developed type 2 diabetes between the ages of 13–15 years of age but some of our patients are young as 8.5 years of age. Some previous studies [30] have reported that females are more likely to develop types 2 diabetes (ratio of 1.7/1) but in our study, we found that males were affected more than females. The reason for this different finding is unclear at the present.
In adults with type 2 diabetes ethnicity has been recognised as an important risk factor. There is evidence that the influence of ethnicity is even stronger for type 2 diabetes in youth as compared to adults [31]. Higher prevalences have been seen in Asians, Hispanics, indigenous people (USA, Canada, Australia), and African–Americans, with some of the highest rates in the world being observed among Pima Indians [23]. When we compared the ethnicity of our patients 74% were of Qatari nationality suggesting that type 2 diabetes is much more common in the obese Qatari youth compared to youth from other ethnic groups. These figures are similar to the prevalence of type 2 diabetes in the Pima Indian population.
The presence of beta-cell autoantibodies has been documented in a subset of youth with type 2 diabetes including anti-GAD, anti-IA-2, and anti-ICA [32–36]. One of these studies [33] did not find any difference between antibody positivity and negativity in relation to age, gender, weight status, lipids, blood pressure, C-peptide, glucose, and HbA1c at presentation of the patients. However another study [34] found that antibody positivity was significantly associated with race with positive subjects more likely to be white and male and BMI, C-peptide, HBA1c, triglycerides, HDL cholesterol, and blood pressure were significantly different by antibody status. In all these studies the degree of antibody positivity in all the studies varied from 10.8 to 70%. None of the previous studies measured ZnT8 autoantibody. In our study, 5 patients had GAD65 autoantibody positivity.
Nonalcoholic fatty liver disease (NAFLD) is commonly found in adults but adolescents with type 2 diabetes (T2DM) and NAFLD have also been reported [37]. In one study, nearly 30% of children with NAFLD also had type 2 diabetes or prediabetes [38]. The cause and effect relationship between insulin resistance, type 2 diabetes and NAFLD is unclear with each condition driving the other. Due to the low cost, safety profile and availability heptic ultrasound is the study of choice for detecting NAFLD [39]. In our study, the hepatic ultrasound showed evidence of a fatty liver in 55 patients but the hepatic ALT was only elevated in 35 patients. It is known that hepatic enzymes are not a sensitive marker of established NAFLD and in patients with type 2 diabetes and NAFLD abnormal hepatic enzymes may be seen in less than 20% of patients [40]. A limitation of our study is that 44 subjects did not agree to undergo a liver ultrasound who could, although unlikely, have normal findings.
In this study, we have summarised the epidemiological, clinical, biochemical, immunological and radiological aspects of a large well-defined group of Arab youth with type 2 diabetes. Youth of Qatari ethnicity are more severely affected than other ethnic groups and virtually all patients with type 2 diabetes are either overweight or obese. Interestingly males were more affected than females and the reasons for this are unclear and maternal GDM was a key association with type 2 diabetes in our patients. Some of the type 2 patients had antibody positivity and in our findings, a large proportion of the patients has a fatty liver on ultrasound.
As type 2 diabetes in the youth is associated with early onset microvascular and macrovascular complications, [41] these findings have important social and health budget implications for Qatar and possibly for the rest of the Middle East region. Tackling the burden of maternal GDM and childhood obesity and building programmes for early detection and intervention are therefore essential to reduce the risk of future complications.
Abbreviations
- GAD65
Glutamic acid decarboxylase 65
- IAA
Insulin autoantibody
- IA2
Islet antigen-2 auto antibody
- ZnT8
Zinc transporter 8
- NAFLD
Non-alcoholic fatty liver disease
- GDM
Gestational diabetes mellitus
- CI
Confidence interval
- TG
Triglyceride
Author contributions
SMA, BH, KH drafted the manuscript, SMA and BH recruited the patients, collected, analysed and interpreted data, KH designed the study, obtained funding, reviewed and edited the manuscript SS, AE, AK, NH, HA, TRA, HD, MA, MA, AS, FA, GP collected patient information, reviewed the manuscript. The guarantor for this project is Professor Khalid Hussain and takes responsibility for the integrity of the data presented.
Funding
This research was supported by the Qatar National Research Fund (QNRF-NPRP 10–6100017-AXX) awarded to Professor Khalid Hussain.
Data availability
Restrictions apply to the availability of some or all data generated or analysed during this study to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Declarations
Conflict of interest
All authors have no conflicts of interest to declare.
Human and animal rights
This study was approved by the Institutional Review Board (IRB) for the protection of human subjects in Sidra Medicine, Qatar. Approval number 1702007592, approved on 03–10-2017. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and/or with the Helsinki Declaration of 1964 and later versions.
Informed consent
Informed consent or substitute for it was obtained from all patients for being included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shayma M. Ahmed and Basma Haris are joint first authors.
References
- 1.Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn CR. Banting Lecture: Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes. 1994;43:1066–1084. doi: 10.2337/diab.43.8.1066. [DOI] [PubMed] [Google Scholar]
- 3.Nesmith JD. Type 2 diabetes mellitus in children and adolescents. Pediatr Rev. 2001;22(5):147–152. doi: 10.1542/pir.22-5-147. [DOI] [PubMed] [Google Scholar]
- 4.Gungor N, Arslanian S. Pathophysiology of type 2 diabetes mellitus in children and adolescents: treatment implications. Treat Endocrinol. 2002;1:359–371. doi: 10.2165/00024677-200201060-00002. [DOI] [PubMed] [Google Scholar]
- 5.Juárez-López C, Klünder-Klünder M, Medina-Bravo P, Madrigal-Azcárate A, Mass-Díaz E, Flores-Huerta S. Insulin resistance and its association with the components of the metabolic syndrome among obese children and adolescents. BMC Public Health. 2010;10:318. doi: 10.1186/1471-2458-10-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nahhas MA, Asamoah F, Mullen S, Nwaru BI, Nurmatov U. Epidemiology of overweight and obesity in early childhood in the Gulf Cooperation Council countries: a systematic review and meta-analysis protocol. BMJ Open. 2018;8(6):e019363. doi: 10.1136/bmjopen-2017-019363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stančáková A, Laakso M. Genetics of Type 2 diabetes. Endocr Dev. 2016;31:203–220. doi: 10.1159/000439418. [DOI] [PubMed] [Google Scholar]
- 8.O'Beirne SL, Salit J, Rodriguez-Flores JL, Staudt MR, Abi Khalil C, Fakhro KA, et al. Type 2 diabetes Risk Allele Loci in the Qatari Population. PLoS ONE. 2016;11(7):e0156834. doi: 10.1371/journal.pone.0156834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Punnose J, Agarwal MM, Bin-Uthman S. Type 2 diabetes mellitus among children and adolescents in Al-Ain: a case series. East Mediterr Health J. 2005;11:788–797. [PubMed] [Google Scholar]
- 10.Moussa MA, Alsaeid M, Abdella N, Refai TM, Al-Sheikh N, Gomez JE. Prevalence of type 2 diabetes mellitus among Kuwaiti children and adolescents. Med Princ Pract. 2008;17:270–275. doi: 10.1159/000129604. [DOI] [PubMed] [Google Scholar]
- 11.Al-Rubeaan K. National surveillance for type 1, type 2 diabetes and prediabetes among children and adolescents: a population-based study (SAUDI-DM) J Epidemiol Community Health. 2015;69(11):1045–1051. doi: 10.1136/jech-2015-205710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Agha A, Ocheltree A, Shata N. Prevalence of hyperinsulinism, type 2 diabetes mellitus and metabolic syndrome among Saudi overweight and obese pediatric patients. Minerva Pediatr. 2012;64(6):623–631. [PubMed] [Google Scholar]
- 13.Mirbolouk M, Derakhshan A, Charkhchi P, Guity K, Azizi F, Hadaegh F. Incidence and predictors of early adulthood pre-diabetes/type 2 diabetes, among Iranian adolescents: the Tehran Lipid and Glucose Study. Pediatr Diabetes. 2016;17(8):608–616. doi: 10.1111/pedi.12343. [DOI] [PubMed] [Google Scholar]
- 14.Robert AA, Al Dawish MA, Braham R, Musallam MA, Al Hayek AA, Al Kahtany NH. Type 2 diabetes mellitus in saudi arabia: major challenges and possible solutions. Curr Diabetes Rev. 2017;13:59–64. doi: 10.2174/1573399812666160126142605. [DOI] [PubMed] [Google Scholar]
- 15.Alyafei F, Soliman A, Alkhalaf F, Sabt A, De Sanctis V, Waseef R, et al. Incidence of type 1 and type 2 diabetes, between 2012–2016, among children and adolescents in Qatar. Acta Biomed. 2018;89:7–10. doi: 10.23750/abm.v89i5.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Kandari H, Al-Abdulrazzaq D, Davidsson L, Sharma P, Al-Tararwa A, Mandani F, et al. Incidence of type 2 diabetes in kuwaiti children and adolescents: results from the childhood-onset diabetes electronic registry (CODeR) Front Endocrinol (Lausanne) 2019;10:836. doi: 10.3389/fendo.2019.00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osman HA, Elsadek N, Abdullah MA. Type 2 diabetes in Sudanese children and adolescents. Sudan J Paediatr. 2013;13(2):17–23. [PMC free article] [PubMed] [Google Scholar]
- 18.Moussa MA, Alsaeid M, Abdella N, Refai TM, Al-Sheikh N, Gomez JE. Prevalence of type 2 diabetes mellitus among Kuwaiti children and adolescents. Med Princ Pract. 2008;17(4):270–275. doi: 10.1159/000129604. [DOI] [PubMed] [Google Scholar]
- 19.Taheri S, Zaghloul H, Chagoury O, Elhadad S, Ahmed SH, El Khatib N, et al. Effect of intensive lifestyle intervention on bodyweight and glycaemia in early type 2 diabetes (DIADEM-I): an open-label, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2020;8(6):477–489. doi: 10.1016/S2213-8587(20)30117-0. [DOI] [PubMed] [Google Scholar]
- 20.Walikonis JE, Lennon VA. Radioimmunoassay for glutamic acid decarboxylase (GAD65) autoantibodies as a diagnostic aid for stiff-man syndrome and a correlate of susceptibility to type 1 diabetes mellitus. Mayo Clin Proc. 1998;73(12):1161–1166. doi: 10.4065/73.12.1161. [DOI] [PubMed] [Google Scholar]
- 21.Masuda M, Powell M, Chen S, Beer C, Fichna P, Rees Smith B, et al. Autoantibodies to IA-2 in insulin-dependent diabetes mellitus. Measurements with a new immunoprecipitation assay. Clin Chim Acta. 2000;291(1):53–66. doi: 10.1016/s0009-8981(99)00199-0. [DOI] [PubMed] [Google Scholar]
- 22.Dabelea D, Pettitt DJ, Hanson RL, Imperatore G, Bennett PH, Knowler WC. Birth weight, type 2 diabetes, and insulin resistance in Pima Indian children and young adults. Diabetes Care. 1999;22:944–950. doi: 10.2337/diacare.22.6.944. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association Type 2 diabetes in children and adolescents. Diabetes Care. 2000;23:381–389. doi: 10.2337/diacare.23.3.381. [DOI] [PubMed] [Google Scholar]
- 24.Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002;346:802–810. doi: 10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- 25.McCance DR, Pettitt DJ, Hanson RL, Jacobsson LT, Knowler WC, Bennett PH. Birth weight and non-insulin dependent diabetes: thrifty genotype, thrifty phenotype, or surviving small baby genotype? BMJ. 1994;308(6934):942–945. doi: 10.1136/bmj.308.6934.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pettitt DJ, Baird HR, Aleck KA, Bennett PH, Knowler WC. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med. 1983;308(5):242–245. doi: 10.1056/NEJM198302033080502. [DOI] [PubMed] [Google Scholar]
- 27.Pettitt DJ, Nelson RG, Saad MF, Bennett PH, Knowler WC. Diabetes and obesity in the offspring of Pima Indian women with diabetes during pregnancy. Diabetes Care. 1993;16(1):310–314. doi: 10.2337/diacare.16.1.310. [DOI] [PubMed] [Google Scholar]
- 28.Hutchins J, Barajas RA, Hale D, Escaname E, Lynch J. Type 2 diabetes in a 5-year-old and single center experience of type 2 diabetes in youth under 10. Pediatr Diabetes. 2017;18(7):674–677. doi: 10.1111/pedi.12463. [DOI] [PubMed] [Google Scholar]
- 29.Scott CR, Smith JM, Cradock MM, Pihoker C. Characteristics of youth-onset noninsulin-dependent diabetes mellitus and insulin-dependent diabetes mellitus at diagnosis. Pediatrics. 1997;100(1):84–91. doi: 10.1542/peds.100.1.84. [DOI] [PubMed] [Google Scholar]
- 30.Rosenbloom A, Joe J, Young R, Winter W. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999;22:345–354. doi: 10.2337/diacare.22.2.345. [DOI] [PubMed] [Google Scholar]
- 31.Ramachandran A, Snehalatha C, Satyavani K, Sivasankari S, Vijay V. Type 2 diabetes in Asian-Indian urban children. Diabetes Care. 2003;26:1022–1025. doi: 10.2337/diacare.26.4.1022. [DOI] [PubMed] [Google Scholar]
- 32.Umpaichitra V, Banerji MA, Castells S. Autoantibodies in children with type 2 diabetes mellitus. J Pediatr Endocrinol Metab. 2002;15(Suppl. 1):525–530. [PubMed] [Google Scholar]
- 33.Reinehr T, Schober E, Wiegand S, Thon A, Holl RL. Beta-cell autoantibodies in children with type 2 diabetes mellitus: subgroup or misclassification? Arch Dis Child. 2006;91:473–477. doi: 10.1136/adc.2005.088229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klingensmith GJ, Pyle L, Arslanian S, Copeland KC, Cuttler L, Kaufman F, et al. The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype: results from the TODAY study. Diabetes Care. 2010;33(9):1970–1975. doi: 10.2337/dc10-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alyafei F, Soliman A, Alkhalaf F, Sabt A, De Sanctis V, Elsayed N, et al. Prevalence of β-cell antibodies and associated autoimmune diseases in children and adolescents with type 1 diabetes (T1DM) versus type 2 diabetes (T2DM) in Qatar. Acta Biomed. 2018;89(S5):32–39. doi: 10.23750/abm.v89i5.7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hathout EH, Thomas W, El-Shahawy M, Nahab F, Mace JW. Diabetic autoimmune markers in children and adolescents with type 2 diabetes. Pediatrics. 2001;107(6):E102. doi: 10.1542/peds.107.6.e102. [DOI] [PubMed] [Google Scholar]
- 37.Hecht L, Weiss R. Nonalcoholic fatty liver disease and type 2 diabetes in obese children. Curr Diab Rep. 2014;14(1):448. doi: 10.1007/s11892-013-0448-y. [DOI] [PubMed] [Google Scholar]
- 38.Newton KP, Hou J, Crimmins NA, Lavine JE, Barlow SE, Xanthakos SA, et al. Prevalence of prediabetes and type 2 diabetes in children with nonalcoholic fatty liver disease. JAMA Pediatr. 2016;170(10):e161971. doi: 10.1001/jamapediatrics.2016.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williamson RM, Perry E, Glancy S, Marshall I, Gray C, Nee LD, et al. The use of ultrasound to diagnose hepatic steatosis in type 2 diabetes: intra- and interobserver variability and comparison with magnetic resonance spectroscopy. Clin Radiol. 2011;66(5):434–439. doi: 10.1016/j.crad.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 40.Soares JB, Pimentel-Nunes P, Roncon-Albuquerque R, Leite-Moreira A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol Int. 2010;4(4):659–672. doi: 10.1007/s12072-010-9219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krakoff J, Lindsay RS, Looker HC, Nelson RG, Hanson RL, Knowler WC. Incidence of retinopathy and nephropathy in youth onset compared with adult-onset type 2 diabetes. Diabetes Care. 2003;26:76–81. doi: 10.2337/diacare.26.1.76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analysed during this study to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.


