Abstract
Glucosinolate (GSL) is an important active substance in broccoli and can be hydrolyzed to isothiocyanates (ITCs) by endogenous myrosinase. The ITCs are well-known chemopreventive agents that have received significant attention across the nutrition and pharmaceutical industries due to their anticancer properties. Myrosinase activity decreases during the cooking of broccoli, thus it is essential to study the microbiota involved in GSL hydrolysis to maximize their health benefits. In this study, two strains (Enterococcus gallinarum HG001 and Escherichia coli HG002) isolated from the gut microbiota of C57BL/6 mice were identified through 16 S rRNA gene sequence and characteristic analyses. The maximum GSL hydrolysis activity of 12 strains was observed using the cyclocondensation method. Their growth curves, GSL-hydrolysis curves, ITC generation curves and GSL-hydrolysis products were analyzed. The En. gallisepticum HG001 hydrolyzed GSL to a greater level than the E. coli HG002. It was observed that they could hydrolyze GSL to produce erucin nitrile and 4-methylsulfanylbutyro nitrile through gas chromatography-mass spectrometer analysis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12088-022-01006-z.
Keywords: Enterococcus gallisepticum HG001, Escherichia coli HG002, Glucosinolate, Isothiocyanate, Hydrolysis
Introduction
Cruciferous vegetables have high nutritional value, and hence daily consumption of these vegetables is advocated worldwide. Common cruciferous vegetables in China include broccoli, radish, mustard, kale, cauliflower, cabbage, etc. Especially, broccoli has the maximum nutritional value among cruciferous vegetables and is known as the “vegetable crown” [1]. Epidemiological studies have shown that the consumption of cruciferous plants reduces the risk of chronic diseases (e.g., obesity, cardiovascular disease and cancer) [2]. These effects are usually attributed to a kind of biologically active substance called isothiocyanates (ITCs), which are the degradation products of glucosinolates (GSLs). In intact cruciferous vegetables, GSLs and myrosinase are spatially isolated. GSLs are mainly found in sulfur-rich cells between the plant phloem and the endothelium, while myrosinase is found in the phloem parenchyma vacuoles of cells. When plant tissues are squeezed, crushed, cut, chewed or subjected to other external forces, the cells are damaged and release endogenous myrosinase, which catalyzes the hydrolysis of GSLs and produces ITCs, nitriles (NITs), oxazolidine-2-thione, etc. [3].
The function of cruciferous plants is mainly attributed to ITCs, which have direct anti-proliferative and apoptosis-inducing effects on various tumor cells (e.g., lung cancer, bladder cancer, breast cancer, colon cancer, prostate cancer, ovarian cancer and leukemia) [2]. During cooking, endogenous myrosinases of cruciferous vegetables lose activity by heat and cannot hydrolyze GSLs into ITCs, and hence GSLs always enter the intestine in their intact form. However, ITC had been detected in the blood plasma of humans and animals following cooked broccoli consumption. It was the result of GSLs being hydrolyzed by gut microbiota. Research on gut microbiota has gained interest because of their important roles in immunity, metabolism and biotransformation of bioactive compounds in foods. Especially, GSLs can be hydrolyzed by microbiota to ITCs and NITs in vivo or in vitro (e.g., sulforaphane, erucin, sulforaphane nitrile and erucin nitrile). Reports showed that gut microbiota hydrolyze GSLs in the absence of plant myrosinase [4]. For example, Bacteroides thetaiotaomicron and Escherichia coli 0157:H7 were shown to hydrolyze sinigrin into allylisothiocyanates. Enterococcus casseliflavus CP1 and E. coli VL8 were able to metabolize GSLs to produce ITCs and NITs [5, 6].
Our previous study found that broccoli ingestion profoundly affected the composition of the gut bacteria community through 16 S rRNA gene sequencing [4]. To know which kinds of microbiota could hydrolyze GSL to ITC and the contribution of gut microbiota in GSL metabolites, this study aimed to isolate microorganisms from mouse feces that can degrade GSL, and to analyze their GSL degradation ability. This study provided two GSL degradation strains, and provided a basis for the study of GSL-hydrolysis enzymes of the two strains in the future.
Materials and Methods
Reagents and Chemicals
Feces were taken from C57BL/6 mice obtained from Beijing Biocytogen Co., Ltd (Beijing, China). Sinigrin was obtained from Sigma Chemicals (UK). Broccoli seeds were purchased from Qingfengyingke Seed Co., Ltd (Guangdong, China). Petroleum ether, ethyl acetate, methanol, anhydrous sodium sulfate, sodium dihydrogen phosphate, disodium hydrogen phosphate, and sodium chloride were purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd (Shanghai, China). HP-20 resin was purchased from Anhui Samsung Resin Technology Co., Ltd (Anhui, China). Glucoraphanin with 98% purity was purchased from J&K Chemical Ltd (Shanghai, China). 1,2-Benzenedithiol and sinigrin were purchased from Sigma-Aldrich (MO, USA).
Sample Collection and Preparation
Male C57BL/6 mice (aged about 3 weeks) were kept in a pathogen-free animal room at Zhejiang Chinese Medical University. The mice were housed under controlled environmental conditions (temperature 22–24 °C; humidity 40–60%; 12 h light/dark cycle) to adapt for 2 weeks. The commercial mouse diet (Zhejiang Chinese Medical University, Hangzhou, China) and water were given ad libitum. The procedures used in this study were authorized by the Animal Ethics and Welfare Committee of ZCMU, China (SYXK No. 2018-0012). The protocols were carried out in accordance with the European Community guidelines (Directive 2010/63/EU) for the care and use of mice. According to the method of Xiang et al. (2021) with some modifications, briefly, the fecal samples were collected using the disposable aseptic sampler and mixed thoroughly. Then, the fecal samples were immediately transferred to the anaerobic incubator to prevent anaerobic bacteria from touching the air and dying. In this anaerobic incubator, the fecal samples were added to sterile phosphate buffered saline (PBS, pH 7.0) and mixed thoroughly with a vortex mixer and used in subsequent experiments [7].
Isolation and Identification of GSL-Hydrolysis Bacteria
The aforementioned fecal mixture (100 µL) was then mixed with 900 µL of medium (containing 1 mg sinigrin). Two different media without glucose were used: Wilkins Chalgren (WC) and De Man, Rogosa, Sharpeand (MRS) according to the method of Luang-In et al. [8] with some modifications. The strains were cultured in an anaerobic incubator at 37 °C (5% CO2, 10% H2 and 85% N2). After every 2 days, the fecal mixture was sampled and added to a new medium in the ratio of 1:10. On the 16th day (8 generations), 100 µL of the microbial mixture in each medium was plated on the corresponding solid medium (containing 2% agar and 1 mM sinigrin) and cultivated anaerobically at 37 °C until obvious colonies appeared. Colonies of different morphologies were inoculated into the corresponding medium containing 1 mM sinigrin and cultured overnight. The total amount of ITCs in each bacterial supernatant was analyzed to screen colonies with GSL-hydrolysis capability. The culture broth without inoculated microorganisms was used as the control. Bacteria showing a greater capability of GSL hydrolysis were maintained at − 80 °C in 20% (v/v) glycerin. After isolating strains with higher GSL hydrolysis activity, their 16 S rRNA genes were sequenced according to the method of Tuo et al. (2020). The genomic DNA of strains were extracted and used as templates for amplification of the 16 S rRNA genes using polymerase chain reaction with the primers 27 F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′). The values of 16 S rRNA gene sequence similarity between isolated strains and the related species were analyzed using the GenBank database in the Basic Local Alignment Search Tool program (NCBI) and the EzBioCloud database. Multiple alignments with corresponding sequences of representatives of the genus were carried out using the BioEdit program. Evolutionary distances were calculated according to the algorithm of the Kimura two-parameter model. Phylogenetic trees were reconstructed using the neighbour-joining (NJ), maximum-likelihood (ML) and maximum-parsimony (MP) methods via the MEGA 7.0 software. Tree topology was examined by the bootstrap method of resampling using 1000 bootstraps [9].
Total ITC Quantification
The total ITC content was quantified by the cyclocondensation method with some modifications [10]. The sample was mixed with 20 mM potassium phosphate buffer (pH 8.5) and 10 mM 1,4-benzenedithiol and incubated at 65 °C for 2 h. After the reaction, it was cooled to room temperature and centrifuged at 16,000 g for 5 min. The supernatant was filtered through a 0.22 μm microfiltration membrane. The cyclocondensation product in the supernatant was analyzed using a Waters e2695 HPLC system (MA, USA) equipped with a Waters 2489 detector [4]. The conditions for the liquid-phase analysis were as follows: fluidity, 80% methanol and 20% water; flow rate, 1.0 mL/min; injection volume, 10 µL; detection wavelength, 365 nm. The chromatographic separation was performed using a Wonda Cract ODS-2 column (4.6 × 250 mm i.d., 5 μm) (Shimadzu, Japan). The standard curve was established using various concentrations of sulforaphane to quantify the ITC concentration of the samples.
GSL Extraction
Crude GSLs were extracted according to the method of Sarvan et al. [11] with some modifications. The broccoli seeds (50 g) were baked in an oven at 100 °C for 5 h to inactivate myrosinase. The seeds waere then ground in a grinder to obtain a seed meal, which was mixed with 500 mL of hexane. After shaking the mixture for 3 h, the seed meal was filtered and dried in a fume hood overnight. The defatted seed meal was added to 350 mL of 70% methanol with glass beads, and GSL was extracted using a Soxhlet extractor. The crude GSL extract was concentrated and maintained at − 20 °C for subsequent experiments.
GSLs Purification
Resin-based column chromatography was performed on a low-pressure glass chromatographic column (35 × 150 mm) filled with HP-20 resins. The method of separation and purification was carried out according to Ji et al. [12] with some modifications. In brief, the resin-based column was pretreated before use. The resin was washed with 5% HCl and 4% NaOH solution, and then washed with deionized water. The sample solution (100 mL) was loaded in the pretreated column and adsorbed at a flow rate of 4.8 mL/min. After adsorption, the column was eluted with deionized water at the same flow rate. A fraction was collected and concentrated to 10 mL using a rotatory evaporator.
Growth Curves of the Bacteria
The growth curves of the bacteria were determined according to the method of Luang-In et al. [6] with some modifications. The preserved bacteria solution (50 uL) was inoculated into a centrifuge tube containing 1 mL of culture medium (three parallel for each kind of bacteria and six tubes in total) and incubated at 37 °C for 24 h in an anaerobic incubator to activate the bacteria. Then, the activated bacteria solution (1 mL) was injected into the 100 mL flasks containing 50 mL of the medium and cultured under the same conditions for 28 h. During this period, the bacteria solution was taken out every 2−4 h. The growth curve was determined by a Spectra Max microplate reader (Molecular Devices, USA) at 600 nm. The remaining samples were maintained at − 80 °C for the measurement of GSL-hydrolysis level.
Hydrolysis of GSLs by the Bacteria
The aforementioned remaining samples were used to analyze the GSL-hydrolysis level of bacteria according to the method of Gabrielle et al. [13] with some modifications using a Waters e2695 HPLC system (MA, USA) equipped with a Waters 2489 detector. The conditions for the liquid-phase analysis were as follows: the mobile phase was water (solvent A) and acetonitrile (solvent B), and the gradient mode was as follows: 2% B was maintained for 15 min, increased from 2 to 25% within 2 min, increased from 25 to 70% within 2 min, maintained for 2 min, then decreased from 75 to 2% within 2 min, maintained for 15 min. The temperature was 35 °C, the injection volume was 10 µL, the flow rate is 0.2 mL/min, and the detection wavelength was 229 nm. The chromatographic separation was performed using a Wonda Cract ODS-2 column (4.6 × 250 mm i.d., 5 μm) (Shimadzu, Japan). The concentration of GSL varied from 0 to 2000 nmol.
Analysis of GSL-Hydrolysis Product
The composition of the GSL-hydrolysis products was analyzed using gas chromatography-mass spectrometer (GC-MS) according to the method of Wu et al. (2018) with some modifications [10]. The chromatographic column was an ultra-insert capillary column of Hp-5MS (0.25 μm, 30 m × 0.25 i.d.). The injection volume was 1 µL, and the gasification chamber temperature was 300 °C. The column temperature program was set as follows: 50 °C maintained for 2 min, increased to 190 °C at 10 °C/min, then increased to 300 °C at 20 °C/min, and finally maintained for 5 min. The carrier gas was ultra-high purity grade helium with a split ratio of 10:1. The mass spectrometry conditions were as follows: the interface temperature 220 °C, the ionization method EI, the ionization energy 70 eV and the mass range 35−500 amu. Cyclohexanone was used as the internal standard.
Statistical Analysis
The differences among groups were analyzed using ANOVA with Tukey’s analysis, and Pearson’s correlation coefficient was used to identify the correlation between the two groups. Analyses were performed using the SPSS software (version 19.0; SPSS Inc., IL USA). The data were represented as mean ± standard deviation (n = 3). Differences were considered significant at p < 0.05.
Results
Isolation and Identification of GSL-Hydrolysis Bacteria
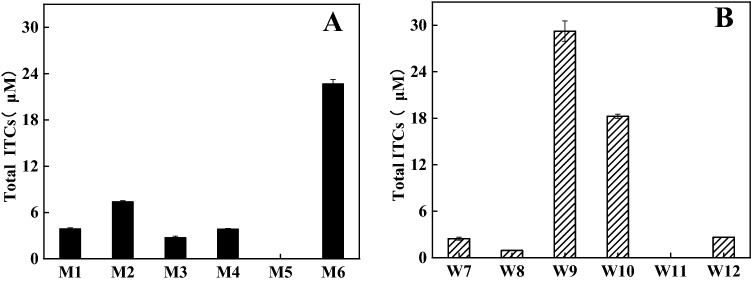
The GSL-hydrolysis levels for each strain were shown in Fig. 1. The strains M6, W9 and W10 could degrade GSL to generate more ITCs, and the GSL-hydrolysis level of W9 was higher than that of M6 and W10. The 16 S rRNA gene sequences of M6, W9 and W10 were obtained. W9 and M6 with the same 16 S rRNA gene sequence were taken for the same strain (data not shown). The nucleotide sequence of W9 showed the best match (99% homology) to those of Enterococcus gallinarum strain LMG 13,129T (NR_116240.1) and Enterococcus gallinarum strain NBRC 100675T (NR_113924.1). The nucleotide sequence of W10 showed the best match (99% homology) to those of Escherichia coli strain NT1N31T (CP075480.1), Escherichia coli strain NT1F25T (CP075472.1) and Escherichia coli strain CT02T (CP081698.1). On the phylogenetic tree based on 16 S rRNA gene sequences reconstructed by the NJ method (Fig. S1). W9 and W10 formed a distinct phyletic linegage within the genus Enterococcus and Escherichia with Enterococcus gallinarum strain LMG 13129T and Escherichia coli strain NT1N31T, respectively, being the strains showing the highest 16 S rRNA gene sequences similarity to W9 and W10. The W9 and W10 were phylogenetically affiliated to the genus Enterococcus and Escherichia, respectively. Combined with the morphological, physiological and biochemical characteristics of the strains, W9 and W10 were identified and defined as En. gallinarum HG001 and E. coli HG002, respectively. The 16 S rRNA gene sequences were submitted to the GenBank nucleotide sequence database under accession no. OL339429 (En. gallinarum HG001) and OL339479 (E. coli HG002).
Fig. 1.
The content of total ITC from GSL hydrolyzed by microorganisms from mice feces cultured in MRS (a) and WC media (b). The data are represented as mean ± SD (n = 3)
Growth Curves of En. gallinarum HG001 and E. coli HG002
In the WC medium without glucose at pH 6.5, the GSL extracted and purified from broccoli seeds was used as the carbon source. The strains were cultured in an anaerobic incubator at 37 °C for 28 h to determine the growth curves. According to the growth curve shown in Fig. 2, the exponential growth period of En. gallinarum HG001 was 2−14 h. It grew rapidly during 2−4 h and entered a stable phase at 14 h. The exponential growth period of E. coli HG002 was 2−14 h, grew rapidly during 2−8 h, and entered a stable phase at 10 h.
Fig. 2.
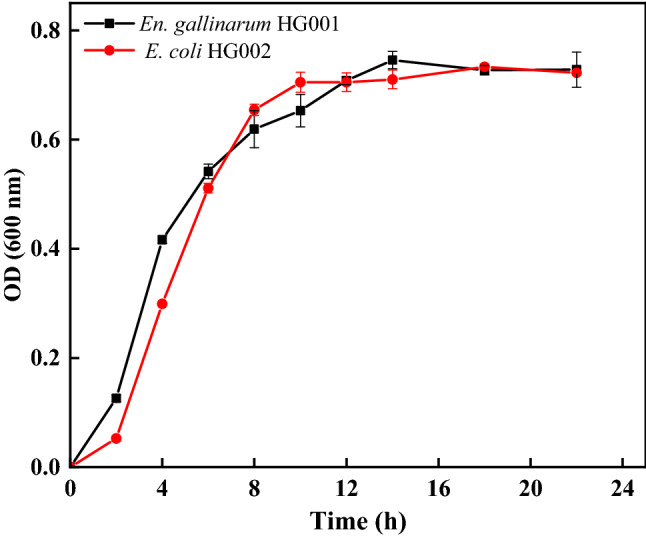
Growth of En. gallisepticum HG001 (a) and E. coli HG002 (b) within 24 h. The data are represented as mean ± SD (n = 3)
GSL Hydrolysis Ability of En. gallinarum HG001 and E. coli HG002
The time course curves of GSL hydrolysis and total ITC production were analyzed and are shown in Fig. 3. The GSL-hydrolysis ability of En. gallinarum HG001 was slightly better than that of E. coli HG002, and the hydrolysis rate was 39.54% and 29.17%, respectively. The ITC production was observed after GSL hydrolysis. Figure 3 shows that En. gallinarum HG001 entered the period of rapid GSL hydrolysis earlier (in 8 h) than E. coli HG002 (in 10 h). However, the GSL-hydrolysis ability of the two strains entered a stationary period after 22 h, and the content of ITCs did not increase. The content of ITCs generated by En. gallinarum HG001 was slightly higher than that generated by E. coli HG002.
Fig. 3.
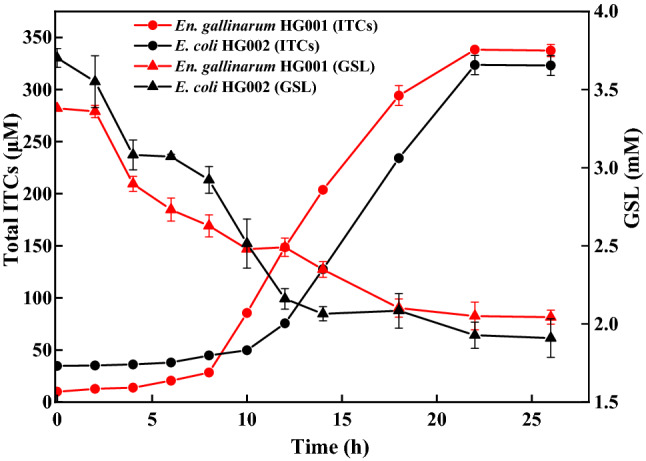
The content of GSL hydrolysis and total ITC caused by En. gallisepticum HG001 and E. coli HG002. The data are represented as mean ± SD (n = 3)
Analysis of the Production of GSLs Following Hydrolysis
The degradation products of GSL hydrolyzed by En. gallinarum HG001 and E. coli HG002 were analyzed by GC-MS and was shown in Fig. S2. When the two strains were cultured in the same medium (WC) containing GSL, their metabolites were mainly 4-methylsulfanylbutyro nitrile and erucin nitrile. The retention times of the two compounds were 8.012 and 9.729 min, respectively.
Discussion
Plant myrosinases are inactivated by cooking, thus the biotransformation of GSL is dependent on gut microbiota. Two strains (En. gallisepticum HG001 and E. coli HG002) were screened and exhibited greater GSL-hydrolysis ability. Studies reported that some bacteria could hydrolyze GSL (Table S1). However, this study was novel in reporting that En. gallisepticum could hydrolyze GSL. Luang-In et al. confirmed that human gut bacteria were diverse in their capacity of hydrolyzing the same GSLs to different products. In addition, different fermentation systems or different kinds of GSLs might lead to various times of reaching the maximum hydrolysis level. For example, sinigrin was used by Luang-In et al. (2016), while GSL in this study was extracted from broccoli seeds [6]. Among the GSL-hydrolysis products (ITC and NIT) of strains, the common ITC product is allyl isothiocyanate and the NIT products are allyl nitrile, sulforaphane nitrile and erucin nitrile (Table S1). In this study, the erucin nitrile from GSL hydrolyzed by En. gallisepticum HG001 and E. coli HG002 was similar to that by Lactobacillus plantarum KW30, En. casseliflavus CP1 and E. coli VL8. The inhibited effects of erucin nitrile against prostate, pancreas, lung, liver and colon cancers have been proved by several studies [14, 15]. Erucin nitrile inhibited the growth of harmful bacteria (e.g., Helicobacter pylori) and showed antihypertensive and neuroprotective activities [15]. In addition to the erucin nitrile, 4-methylsulfanylbutyro nitrile was a unique NIT product generated from GSL hydrolyzed by En. gallisepticum HG001 and E. coli HG002. The 4-methylsulfanylbutyro nitrile is generally used to make essential oil, which has antioxidant and anticancer activity. Moreover, it is similar to 5-methylthiopentyl isothiocyanate. According to Masuda et al. (2008), 5-methylthiopentyl isothiocyanate has a synergistic antibacterial effect on periodontal pathogens [16]. However, it has not been reported in GSL-hydrolysis products by gut microorganisms. In addition to ITC, Meng et al. and Elfoul et al. reported that some bacteria exhibited sulfatase activity, which desulfated intact GSL to produce desulfo-GSL and NITs [17, 18]. Luang-In et al. reported that E. coli VL8 could hydrolyze desulfo-glucoraphanin to produce erucin nitrile, which is the analog of sulforaphane nitrile. It was mainly caused by the sulfoxide reductase activity, which could reduce the sulfoxide group on desulfo-glucoraphanin. Then it underwent desulfation and hydrolysis to become erucin nitrile [6, 8].
Although the bacteria played an important role in ITC production, NITs looked like the end product of GSL hydrolysis. The NITs are more stable than ITC. In addition, the NITs have detoxication and become the preferable GSL-hydrolysis products. This behavior of the bacteria is the same as that of some insects (e.g., cabbage white butterfly and Pieris rapae). They prevent the harm of toxic products by hydrolyzing GSL to produce more NITs [19]. It gives us a reasonable explanation for the absence of ITC detected by GC-MS. The GSL might be converted into NITs through the hydrolysis of bacteria to reduce toxicity [20]. However, the ITC produced by bacterial hydrolysis of GSL was observed by the cyclocondensation method in this study (Fig. 1). This method is a reaction that used the binding of thiol compound benzene dithiol and ITC or ITC metabolites, which is accurate and rapid for measuring the trace amount of ITC, however, it does not identify the kinds of ITCs. The GSL-hydrolysis products (ITC) in this study was trace amount. Therefore, it could be detected by the cyclocondensation method rather than the GC-MS method. Similar results were observed in our previous studies [4].
Some researchers reported genes or proteins related to GSL hydrolysis in bacteria. For example, Citrobacter WYE1 (family GH3) was identified from a Citrobacter strain isolated from soil. It was the first and complete gene sequence of bacterial myrosinase [21]. Cordeiro et al. reported that the genes bglA (family GH1), ascB (family GH1) and chbF (family 4) played an important role in β-glucoside hydrolysis. These genes enabled E. coli O157:H7 to hydrolyze sinigrin into allyl isothiocyanate [5]. It was possible that the enzymes related to GSL hydrolysis in the En. gallisepticum HG001 and E. coli HG002 also belonged to this family or were similar. Liou et al. found that the operon BT2159-BT2156 palyed an essential role in converting GSLs into ITCs in B. thetaiotaomicron through the genome-wide transposon insertion screen [22]. The discovery of microbial pathways that produce biologically active products (e.g., ITCs generated from GSL hydrolysis) combined with the latest developments in genetic tools for cell therapy can help design gut symbionts to obtain therapeutic benefits. GSL-hydrolysis enzymes and genes of the En. gallisepticum HG001 and E. coli HG002 should be studied in the future. It will provide the basics to analyze the mechanism of GSL-hydrolysis and maximize the health benefits of GSL hydrolysis products in the gut.
Conclusions
In this study, two strains (En. gallinarum HG001 and E. coli HG002) exhibiting great GSL-hydrolysis activity were isolated and identified from the gut microbiota of C57BL/6 mice. The hydrolytic ability of En. gallisepticum HG001 to GSLs was higher than that of E. coli HG002. They could hydrolyze GSL to produce ITC and NITs (erucin nitrile and 4-methylsulfanylbutyro nitrile). However, the kinds of NITs were different from those in other reports. The GSL-hydrolysis enzymes involved in the two strains may be different, which needs further verification. The results of this study revealed that GSL could be hydrolyzed by bacteria from animals without endogenous myrosinase. The myrosinase-like enzymes and genes of the two strains need to be identified in the future.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Figure S1. Phylogenetic tree based on 16S rRNA gene sequences of the isolated HG001 (A) and HG002 (B). GenBank accession numbers are given in parentheses. Numbers at nodes refer to bootstrap values (based on 1000 replicates). Only bootstrap values above 50% are shown. Figure S2. The products generated from GSL hydrolyzed by En. gallinarum HG001 and E. coli HG002.“1”: 4-methylsulfanylbutyro nitrile; “2”: erucin nitrile. (DOCX 609 kb)
Acknowledgements
This work was supported by Fundamental Research Funds for the Zhejiang University of Science and Technology (2019JL03), Fundamental Research Funds for the Zhejiang University of Science and Technology (2021JLZD006) and Scientific Research Foundation of Zhejiang University of Science and Technology (F70110K07).
Author Contributions
YZ: Performed the data analyses and wrote the manuscript. SSH: Performed the experiment. JS: Helped perform the analysis with constructive discussions. XJS: Contributed significantly to analysis and manuscript preparation. CMJ: Helped perform the experiment. YFW: Contributed to the conception of the study.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Favela-González KM, Hernández-Almanza AY, Fuente-Salcido NMDL. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. J Food Biochem. 2020;44:e13414. doi: 10.1111/jfbc.13414. [DOI] [PubMed] [Google Scholar]
- 2.Ilahy R, Tlili I, Pék Z, et al. Pre- and post-harvest factors affecting glucosinolate content in broccoli. Front Nutr. 2020;7:147. doi: 10.3389/fnut.2020.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouranis DL, Malagoli M, Avice JC, et al. Advances in plant sulfur research. Plants. 2020;9:256. doi: 10.3390/plants9020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu YF, Shen YK, Zhu Y, et al. Broccoli ingestion increases the glucosinolate hydrolysis activity of microbiota in the mouse gut. Int J Food Sci Nutr. 2019;70:585–594. doi: 10.1080/09637486.2018.1554624. [DOI] [PubMed] [Google Scholar]
- 5.Cordeiro RP, Doria JH, Zhanel GG, et al. Role of glycoside hydrolase genes in sinigrin degradation by E.coli O157:H7. Int J Food Microbiol. 2015;205:105–111. doi: 10.1016/j.ijfoodmicro.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Luang-In V, Albaser AA, Nueno-Palop C, et al. Glucosinolate and desulfo-glucosinolate metabolism by a selection of human gut bacteria. Curr Microbiol. 2016;73:442–451. doi: 10.1007/s00284-016-1079-8. [DOI] [PubMed] [Google Scholar]
- 7.Xiang SS, Ye K, Li M, et al. Xylitol enhances synthesis of propionate in the colon via cross-feeding of gut microbiota. Microbiome. 2021;9:62. doi: 10.1186/s40168-021-01029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luang-In V, Narbad A, Nueno-Palop C, et al. The metabolism of methylsulfinylalkyl- and methylthioalkyl‐glucosinolates by a selection of human gut bacteria. Mol Nutr Food Res. 2014;58:875–883. doi: 10.1002/mnfr.201300377. [DOI] [PubMed] [Google Scholar]
- 9.Tuo L, Liu F, Yan XR, et al. Bacillus taxi sp. nov. a novel endophytic bacterium isolated from root of taxus chinensis (pilger) rehd. Int J Syst Evol Microbiol. 2020;70:481–486. doi: 10.1099/ijsem.0.003776. [DOI] [PubMed] [Google Scholar]
- 10.Wu YF, Shen YK, Wu XP, et al. Hydrolysis before stir-frying increases the isothiocyanate content of broccoli. J Agric Food Chem. 2018;66:1509–1515. doi: 10.1021/acs.jafc.7b05913. [DOI] [PubMed] [Google Scholar]
- 11.Sarvan I, Kramer E, Bouwmeester H, et al. Sulforaphane formation and bioaccessibility are more affected by steaming time than meal composition during in vitro digestion of broccoli. Food Chem. 2017;214:580–586. doi: 10.1016/j.foodchem.2016.07.111. [DOI] [PubMed] [Google Scholar]
- 12.Ji W, Ao W, Sun M, et al. Separation and purification of horseradish peroxidase from horseradish roots using a novel integrated method. New J Chem. 2021 doi: 10.1039/D0NJ04614K. [DOI] [Google Scholar]
- 13.Gabrielle R, Sylvie R, Brian R, et al. Influence of plant and bacterial myrosinase activity on the metabolic fate of glucosinolates in gnotobiotic rats. Br J Nutr. 2003;90:395–404. doi: 10.1079/BJN2003900. [DOI] [PubMed] [Google Scholar]
- 14.Citi V, Piragine E, Pagnotta E, et al. Anticancer properties of erucin, an H2S-releasing isothiocyanate, on human pancreatic adenocarcinoma cells (AsPC-1) Phytother Res. 2019;33:845–855. doi: 10.1002/ptr.6278. [DOI] [PubMed] [Google Scholar]
- 15.Morroni F, Sita G, Djemil A, et al. Comparison of adaptive neuroprotective mechanisms of sulforaphane and its interconversion product erucin in vitro and in vivo models of parkinson’s disease. J Agric Food Chem. 2018;64:856–865. doi: 10.1021/acs.jafc.7b04641. [DOI] [PubMed] [Google Scholar]
- 16.Masuda H, Hirooka S. Synergistic antibacterial effect of 5-Methylthiopentyl isothiocyanate and (-)-Limonene on periodontal pathogen. Acs Sym Ser. 2008;993:389–398. doi: 10.1021/bk-2008-0993.ch033. [DOI] [Google Scholar]
- 17.Meng L, Hashimoto K, Uda Y. Rat intestinal microbiota digest desulfosinigrin to form allyl cyanide and 1-cyano-2,3-epithiopropane. Food Res Int. 2011;44:1023–1028. doi: 10.1016/j.foodres.2011.03.001. [DOI] [Google Scholar]
- 18.Elfoul L, Rabot S, Khelifa N, et al. Formation of allyl isothiocyanate from sinigrin in the digestive tract of rats monoassociated with a human colonic strain of bacteroides thetaiotaomicron. FEMS Microbiol Lett. 2001;197:99–103. doi: 10.1016/S0378-1097(01)00093-3. [DOI] [PubMed] [Google Scholar]
- 19.Jeschke V, Gershenzon J, Vasso DG. Insect detoxification of glucosinolates and their hydrolysis products. Adv Bot Res. 2016;80:199. doi: 10.1016/bs.abr.2016.06.003. [DOI] [Google Scholar]
- 20.Mays JR, Roska R, Sarfaraz S, et al. Identification, synthesis, and enzymology of non-natural glucosinolate chemopreventive candidates. ChemBioChem. 2010;9:729–747. doi: 10.1002/cbic.200700586. [DOI] [PubMed] [Google Scholar]
- 21.Albaser A, Kazana E, Bennett MH, et al. Discovery of a bacterial glycoside hydrolase family 3 (GH3) β-glucosidase with myrosinase activity from a citrobacter strain isolated from soil. J Agric Food Chem. 2016;64:1520–1527. doi: 10.1021/acs.jafc.5b05381. [DOI] [PubMed] [Google Scholar]
- 22.Liou CS, Sirk SJ, Diaz C, et al. A metabolic pathway for activation of dietary glucosinolates by a human gut symbiont. Cell. 2020;180:717–728. doi: 10.1016/j.cell.2020.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narbad A, Mark HB, Rossiter JT, et al. Gut glucosinolate metabolism and isothiocyanate production. Mol Nutr Food Res. 2018;62:e1700991. doi: 10.1002/mnfr.201700991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mullaney JA, Kelly WJ, Mcghie TK, et al. Lactic acid bacteria convert glucosinolates to nitriles efficiently yet differently from enterobacteriaceae. J Agric Food Chem. 2013;61:3039–3046. doi: 10.1021/jf305442j. [DOI] [PubMed] [Google Scholar]
- 25.Lai RH, Miller M, Jeffery EH. Glucoraphanin is hydrolyzed by lactobacilli in vitro and rat cecal microbiota in vitro and in situ. Faseb J. 2009 doi: 10.1096/fasebj.23.1_supplement.561.4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Phylogenetic tree based on 16S rRNA gene sequences of the isolated HG001 (A) and HG002 (B). GenBank accession numbers are given in parentheses. Numbers at nodes refer to bootstrap values (based on 1000 replicates). Only bootstrap values above 50% are shown. Figure S2. The products generated from GSL hydrolyzed by En. gallinarum HG001 and E. coli HG002.“1”: 4-methylsulfanylbutyro nitrile; “2”: erucin nitrile. (DOCX 609 kb)