Abstract
Nanotechnology is a novel approach to dermatologic treatment. Nanomaterials are materials typically defined as less than 100 nm in size. As this size approaches molecular dimensions, the chemical and physical properties vastly change due to a relative increase in surface area to volume ratio. Unique and altered properties ensue, such as carbon becoming an electrical conductant in the nano form, and glass becoming a liquid. The interaction of nanoparticles with biota likewise changes. Novel therapeutics may be possible with the use of nanomaterials. Advantages of nanoparticles include the ability to overcome microbial resistance and potentially induce immunomodulatory effects. Engineered nanomaterials or the development of nano-therapeutics with photo-induced antibacterial propensity and immunomodulatory activities has the potential to open new prospects for the treatment of ubiquitous cutaneous diseases, such as acne vulgaris.
Keywords: Acne vulgaris, Propionibacterium acnes, Nanodermatology, Nanotechnology, Phototherapy
Introduction
The noteworthy progress in nanotechnology has established its vital role in sustainable development [1, 2]. Nanomaterial synthesis and its composites are possible through various biological and physicochemical methods. They find applications in diverse biotechnological fields [3–8]. Nanomaterial's physical and chemical properties highly influence their applications due to their biocompatibility and composition [9–11]. The nanomaterials generated through bioprocesses have proved more beneficial as antimicrobials, and nanocatalysts [12–14]. Management of microbial pathogens and the infectious diseases caused by them significantly affect human health, management burden, and economy [15–18].
Acne vulgaris is a chronic inflammatory disease of the pilosebaceous unit (PSU) affecting ~ 85% of young adults worldwide [19]. This skin condition manifests itself with white and black comedones(closed and/or open), and inflammatory lesions; like papules, pustules, and cystic nodules [20]. The pathogenesis of acne has been historically considered to involve follicular hyperplasia and hyper-keratinization, leading to obstruction and inflammation of the pilosebaceous unit (PSU) [21]. The pathogenesis of acne is affected by androgens and their receptors. The accumulation of necrotic keratinocytes and fatty acids contribute to the proliferation of Propionibacterium acnes (P.acnes) [22, 23]. There is an inflammatory response involving the innate skin immune system. P. acnes, a Gram-positive aero-tolerant anaerobic bacillus, is a commensal skin organism that is purported to have a pathogenic contribution to acne development.
Treatment of acne often involves the oral and topical treatment of antibiotics (Table 1). Bacterial resistance to antibiotics hampers the treatment and also affects commensal and pathogenic microbiome organisms Staphylococcus aureus and Streptococcus pneumonia.
Table 1.
Different treatment options for acne vulgaris
| Administration option | Medication | Mechanism of action | References |
|---|---|---|---|
| Topical treatment | Retinoids:Adapalene,Isotretinoin,Tazarotene,Metretinide,Retinoyl-β-glucuronide, Tretinoin | Regulation of hyper proliferation of follicular epithelial cells and reduction in follicular plugging. Targets the micro comedones, inflammatory and non-inflammatory lesion of acne | [19, 38, 39] |
| Antibiotics: Azithromycin, Clindamycin, Clarithromycin,Erythromycin |
Inhibition of growth of P.acnes Reduction in inflammation |
[23, 32, 44] | |
|
New chemical agents: Benzoyl peroxide, Dapsone, Hydrogen peroxide, Niacinamide, New herbal agents: Tea tree oil. Green tea extract |
Combats development of resistance against P.acnes. Keratolytic and comedolytic Anti-microbial, anti-oxidant, anti-inflammatory |
[24, 45] | |
| Systemic treatment | Retinoids: Isotretinoin | Keratolytic, Regulation of hyperproliferation in follicular epithelial cells | [38, 39, 45] |
| Hormonal: Contraceptives |
Suppressing effects of androgens on sebaceous gland and keratinocytes Decreasing the levels of circulatory androgens mediated via inhibition of Luteinizing hormones(LH) and Follicle stimulating hormone(FSH) |
[25, 26] | |
| Antibiotics: Tetracycline, Azithromycin, Minocyclin, Doxycyclin, Erythromycin, Levofloxacin |
Inhibition of growth of P.acnes Mainly targets moderate and severe inflammatory acne lesions |
[23] | |
| Others:corticosteroids, ibuprofen, zinc sulfate | |||
| Surgical treatment | Cryolush therapy, comedone extraction, optical treatment, Laser treatment, intralesional corticosteroids | Targeted spot treatment | [34–36] |
| Alternative treatments | Tea-tree oil, Green tea,lime peel oil, Antimicrobial peptides, Taurine bromamine, Seaweed, Basil oil, neem oil, Resveratrol | Anti-microbial, anti-oxidant, anti-inflammatory | [33, 41, 42] |
Resistance is documented in erythromycin, azithromycin, clarithromycin, and clindamycin [20, 22, 24, 25]. Concerns exist that using antibiotics in acne may promote resistant pathogenic organisms and that the antibiotics used will temporarily, at least, not enable them to be used for other treatment purposes in that patient or their close contacts that become colonized with resistant organisms. Hormonal therapy may be administered to female patients with severe seborrhea, acne-tarda (late-onset acne), with proven ovarian or adrenal hyperandrogenism. The currently FDA-approved agents are norgestimate with Ethinyl estradiol and norethindrone acetate with Ethinyl estradiol [26, 27].
Nanodermatology utilizes nano-diagnostic devices for non-invasive dermoscopy and for improving transdermal or dermal drug delivery (TDDD/DDD). Nanomaterials have very unique and versatile physical and chemical attributes due to their high surface area to volume ratio as opposed to their non-nano counterparts [28]. Nanomaterials differ in their interactions with the host due to the altered physical and chemical characteristics [29]. The engineering of the nanoparticle-assisted transdermal or/and dermal drug delivery system is based on the interactions and long-term and short-term toxicity studies between the skin and the developed nanocarriers. The interaction of the nanoparticles with the skin largely depends upon (i) size and charge (ii) drug loading capability and (iii) means of administration. When treating acne, the hair follicle is the target site of action. Nanomaterial systems are synthesized for follicular and trans-follicular drug delivery as depicted in Fig. 1. Nanomaterial’s loaded with anti-acne agents enhances the dermal localization with the capability to direct the nanocarrier formulation to the site of action.
Fig. 1.

Nanomaterial based anti-acne formulations are synthesized for follicular and trans-follicular drug delivery
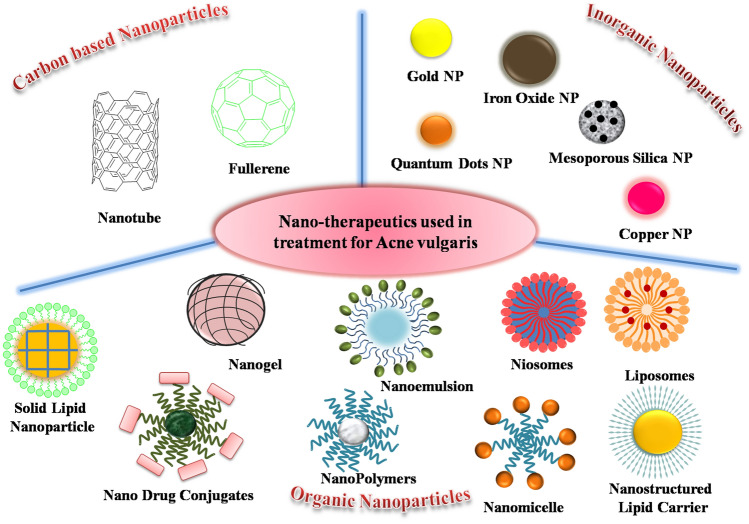
Nanomaterials are widely used for a variety of biotechnological applications (Table 2) due to their unique well-regulated size, composition, and properties [30–43]. Also, nanoparticles can counter bacterial resistance in treating acne [44]. Nanoparticles, including liposomes and micelles, can enhance drug bioavailability, control delivery,and act in an antibacterial manner. Metallic nanoparticles have diverse activities against multi-drug-resistant pathogens. While silver and gold are most widely studied, nanoparticles of oxides of copper, zinc, titanium, magnesium, calcium, manganese and iron have also shown antibacterial properties. Nanoparticles can also be combined with antibiotics and retinoids for improved antibacterial activity. Nanostructured lipid carriers (NLCs) are second-generation novel formulations composed of physiological and biocompatible lipids and surfactants [45]. Organic polymeric nanomaterials can be utilized for photo-thermal therapy.Nanocarriers protect the drug from (i) chemical degradation, (ii) release the drug in a controlled manner, and in many cases (iii) control the water loss from the skin. Figure 2 is a schematic representation of different nanotherapeutics used to treat acne vulgaris.
Table 2.
Different potential nanotherapeutic treatments used for various biotechnological applications
| Function | Type of Nanoparticles | Characteristics of Nanoparticles | References |
|---|---|---|---|
| Antibacterial agents | AgNPs | Inhibition of cell wall synthesis | [5, 6] |
| Cellular uptake of silver ions, | |||
| Generation of reactive oxygen species | |||
| Cascade of intracellular mechanism | |||
| Disrupting protein and nucleic acid synthesis | |||
| AuNPs | Efficient photothermal therapy with reactive oxygen species |
[3] [53] |
|
| Photoacoustic effect, membrane disintegrity, enzyme inhibition | |||
| CuNPs | Penetration of bacterial cell wall | [38] | |
| Release and accumulation of copper ions which subsequently bind with DNA and damage its helical structure | |||
| Antifungal agents | Chitosan NPs | Interaction with negatively charged groups of lipopolysccharides and proteins on the surface of microbial cells | [48] |
| Disintegration of cell membrane, inhibit mRNA and protein synthesis | |||
| Inhibition of sporulation and germination of spores | |||
| Interfering with activity of growth promoting hormones | |||
| ZnO NPs | Augmented activity against dermatophyte infections | [45] | |
| Synergistic antifungal effect in combination with other antifungal drugs | |||
| Lipid NPs | Less cytotoxicity and prolonged circulation time | [45] | |
| Improves drug concentration in epidermis and minimizes cutaneous irritation | |||
| Suncreen | TiO2NPs | Augmented UVB light absorbing capacity | [1] |
| ZnO NPs | Absorb and scatter visible light thus provides optimal transparency, broad spectrum activity against UVA and UVB | ||
| Ultrasomes | Endonuclease enzyme entrapped in specialized liposome, capable of detecting damaged DNA and initiates its removal | [1] | |
| Helps in stimulating production of melanin by melanocyte | |||
| Photosomes | Specialized liposomal structure encapsulating photolyase | [1] | |
| Releases photo-activated enzyme, capable of repairing skin’s DNA damaged due to UV exposure | |||
| Anti-ageing | Niosomes | Increased stability of entrapped drugs formulations improved bioavailability of poorly absorbed drugs and enhanced skin penetration | [60] |
| Ultrasomes | Specialized liposomal structure capable of boosting skin’s natural collagen production to retain elasticity, repair cellular damage | ||
| Nanoemulsion | Transport beneficial bioactive ingredients in high concentrations deep into the skin for pronounced effects | [61] | |
| Cancer Therapy and Diagnosis |
Superparamagnetic Iron oxide NPs |
Used in magenetic resonance imaging (MRI) with cancer cell lines with exceptionally | [41] |
| Quantum Dots | Emit fluorescence in near infra red region making it suitable for colorectal cancer and lymphoma | ||
| High tissue penetration depth and higher spatial and temporal resolution | |||
| AuNPs | Good contrast agent due to small size, good biocompatibility and high atomic number | [3] | |
| Carbon nanotubes | Non-invasive penetration of biofilms | [32] | |
| Delivery of various drug molecules into living cells | |||
| Polymeric nanoparticles | Favourable pharmacokinetic profile | [1] | |
| Well-tolerable toxicities | |||
| Cleansing agent | Nanoemulsions and Micelles | Preserves and protects skin barrier integrity due to reduction in trans epidermal water loss | [1] |
| Removes skin soil with high efficiency | |||
| Phototherapy | Fullerene | Increased anti-tumor effect in a dose dependent manner for C60 and high | [1] |
| fluence intensity | |||
| Reactive oxygen species generation with low level laser irradiation | |||
| Tolerable toxicities | |||
| Carbon nanotubes | Exceptional thermal behaviour on getting activated by suitable light source | [32] | |
| Prolong blood circulation time | |||
| Enhanced biocompatibility and less aggregation, thus a good ablation agent | |||
| Graphene nanoparticles | Strong optical absorption in near infrared spectrum | [33] | |
| High surface activity |
Fig. 2.
Schematic representation of various nano therapeutics applied in treatment for Acne vulgaris
Naturaceuticals Nanocarriers
Nicotinamide is an active form of vitamin B3 and is increasingly being investigated in the area of dermatology [46]. It exhibits anti-inflammatory properties and has been shown to reduce sebum production which makes this nutraceutical an ideal candidate for the treatment of acne [47]. Chitosan has the advantages of bio-adhesiveness and opening of inactive follicles [48]. Polysaccharide nicotinamide-loaded chitosan nanoparticles to treat patients suffering from acne vulgaris show clinical potential. Topical application in ex-vivo studies showed strong skin adhesion and about a total of ~ 68% nicotinamide build-up encompassing different layers of skin (stratum corneum, epidermis, and dermis). Clinical results showed a ~ 73% reduction in inflammatory acne lesions among patients using chitosan nanoparticles [48].
In another study,chitosan nanoparticles were used for the targeted delivery of clindamycin, with ~ 53% efficacy for topical treatment of acne vulgaris [49]. They repeated this study using hyaluronic acid nanoparticles loaded with ~ 77% of clindamycin and found that the drug-loaded nanoparticles reached the pilosebaceous unit. Subsequently, a cream-based formulation was developed for topical acne by encapsulating lime peel essential oil (Citrus hystrix) into chitosan nanoparticles via the ionotropic gelation technique [50]. Citrus hystrix(kaffir lime) consists of a tri-terpenoid that has shown antibacterial activity against P. acnes. It was demonstrated that essential oil encapsulated chitosan nanoparticles inhibited the growth of the bacteria.
Photo-Nanodermatology
Photothermal therapy (PTT) and photodynamic therapy (PDT) in conjunction with nanomaterials have been used to explore these strategies to treat recurrent acne. In a study by our research group, the antibacterial potency of chitosan-coated Prussian blue nanoparticles (CHPB) has been evaluated with mechanistic assays to study the nanoparticle-bacterial interface in depth. The particles exhibited dual modality of peroxidase-like ROS generation and photothermal effect upon irradiation with 635 nm laser light. On irradiation for about 10 min with the same light, local hyperthermia was observed with a rise in temperature of ~ 13.6 from room temperature which was found to be sufficient to make bacteria non-viable. These techniques for the elimination of bacteria do not fall in their armamentarium of natural defense thus avoiding the development of resistance [51].
Another study demonstrated the effectiveness of a transdermal drug delivery system comprising of liposomal gold nanoparticles encapsulating curcumin (Au Lipos Cur NPs) for dual-light activated therapy for treating acne. Iontophoresis was used to localize Au Lipos Cur NPs in the pilosebaceous unit attributed to the positive zeta potential of the nanoparticles [52]. The localized build-up of Au Lipos Cur NPsenabled local hyperthermia mediated destruction of the sebaceous glands when irradiated with NIR laser (808 nm, 650 mW). NIR irradiations not only caused photothermal effect but also triggered the release of curcumin from the liposomal structure exhibiting a bactericidal effect on irradiation with blue light(450 nm). In-vitro assessment of the nanoparticles with sequential irradiation with NIR light followed by blue light resulted in the inhibition of bacterial growth displaying the potential of the projected nano assembly for recalcitrant acne [52].
In a preliminary study conducted on Asian patients, 12 patients suffering from moderate to severe acne were given the treatment from a photo pneumatic device (Isolaz, 400 nm tip). After 10 min of sonophoresis with contents of gold nanoparticles onto the acne-infected area, more than 50% improvement in the condition of patients was observed. Histopathological studies showed a reduction in cell inflammation and dermal fibrotic changes. No major severe side effects were observed. Gold-mediated PTT, exhibiting light-mediated therapeutic effects, is also safe and rapid [53].
Immunomodulatory Nanoparticles
P.acnes induces an inflammatory response together with pro-inflammatory cytokines and chemokines. Pathogen-associated molecular pattern recognition Toll-like receptors2 and 6 (TLR2/6) are P.acnes-responsive receptors. In, inflamed acne lesions T helper type 1 cells recognize P.acnes antigens. Thus, immune-mediated reduction of inflammation could be a therapeutic approach while treating acne vulgaris. Following this strategy, Qin et al. [54] developed a formulation of nanoparticles capable of releasing/generating nitric oxide, a crucial biological messenger (NO-NP). Since NO exhibits broad-spectrum antimicrobial and immune-modulatory attributes.P.acnes was found to be sensitive to all concentrations of NO-NP, which also successfully suppressed interleukin-1β (IL-1β) secretion mediated via obstruction of caspase-1 and IL-1βgene expression. NO-NP was able to prevent the inflammation induced by P.acnes because of growth inhibition. It also restricted the microbial triggered innate immune response.
Similarly, Ridolfi et al. formulated solid lipid nanoparticles of myristyl-myristate in combination with a polysaccharide, chitosan, and tretinoin (all-trans-retinoic acid, ATRA). The projected formulation not only showed high inhibition of P.acnes and S.aureus but also exhibited cytocompatibility to keratinocytes and high physical stability which makes it possibly amenable for topical usage [55]. Encapsulation of tretinoin in nanostructured lipid carriers (NLC) can improve epidermal targeting, protect against oxidation, and reduce transepidermal water loss and the safety profile of Tretinoin [56]. A recent pilot study that evaluated the therapeutic effects of 0.1% adapalene-loaded nanostructured lipid carriers (NLC-ADA) showed safety, and a significant reduction in acne severity index, and the number of inflammatory and non-inflammatory lesions after 12 weeks of treatment [57].
Plant-Derived Nanoparticles
Several chemical treatments have been suggested for acne vulgaris, however, the safety of these treatments is not well understood. A recent study has reported a herbal topical gel formulation that has been used to treat acne. The gel was prepared using aloe gum and silver nanoparticles synthesized using an extract of Azadirachtaindica (neem) leaves [58]. The gel formulation of green silver nanoparticles was not only effective against P.acnes and S.epidermis but also maintained a pH between 6.6–6.9 very close to skin pH with ease of topical application. In another study silver nanoparticles have been synthesized using hydro-alcoholic extract of Curcuma caesiarhizome. The silver nanoparticle-loaded gel showed sustained drug release by Higuchi kinetic model and good antibacterial property against P.acnes [59].
Niosomes
Niosomes is vesicular drug delivery system with enhanced penetration of stratum corneum or intra-epidermal passages, sustained drug release profile, and long shelf-life. Nano-niosomes enjoy translation from lab-scale production to industrial scale because manufacturing is cost-effective, with no requirement of special handling methods or storage conditions. A recent study has used photodynamic therapy for the treatment of acne vulgaris using topical methylene blue (MB) niosomal hydrogel formulation [60]. After application of niosomal MB gel for 1 h, patients were subjected to intense pulsed light (IPL) treatment. Topical MB niosomal hydrogel treatment proved to be effective against acne lesions with minimum side effects in comparison to IPL treatment alone.The first clinical trial of niosomal formulation containing both clindamycin 1% and benzoyl peroxide1% against acne vulgaris has been reported. The double-blind clinical trial showed no adverse effects and was found to be more efficacious than niosomal clindamycin1% alone [61]. In another study, anti-acne combination treatment has been used consisting of niosomal formulation of tretinoin and antibacterial benzoyl peroxide for in-vitro, ex-vivo, and in-vivo evaluation. In vivo studies showed that niosomal gel was more effective than commercially available anti-acne cream as 4.16 fold lower concentration of benzoyl peroxide in the niosomal formulation achieved the same therapeutic efficacy juxtaposed to anti-acne cream [62].
Conclusion
Nanomaterials can modulate the body’s response to microbes by the innate or acquired immune system. Although the preponderance of nano-dermatology in the commercial market has started, the process is slow as the in-vitro results may not necessarily predict the in-vivo outcome. Another cause of concern is the potential toxicity of certain materials used to fabricate these nanostructures. Clinical translation from the bench needs a proper understanding of the nano-bio interface along with additional studies to augment knowledge about this field of nano-dermatology.
Acknowledgements
NC is thankful to CSIR for providing Senior Research Fellowship support.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Patel SKS, Kalia VC. Advancements in the nanobiotechnological applications. Indian J Microbiol. 2021;61:401–403. doi: 10.1007/s12088-021-00979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel SKS, Lee JK, Kalia VC. Nanoparticles in biological hydrogen production: an overview. Indian J Microbiol. 2018;58:8–18. doi: 10.1007/s12088-017-0678-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otari SV, Kumar M, Anwar MZ, et al. Rapid synthesis and decoration of reduced graphene oxide with gold nanoparticles by thermostable peptides for memory device and photothermal applications. Sci Rep. 2017;7:10980. doi: 10.1038/s41598-017-10777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel SKS, Anwar MZ, Kumar A, et al. Fe2O3 yolk-shell particles-based laccase biosensor for efficient detection of 2,6-dimethoxyphenol. BiochemEng. 2018;J132:1–8. doi: 10.1016/j.bej.2017.12.013. [DOI] [Google Scholar]
- 5.Otari SV, Pawar SH, Patel SKS, et al. Canna edulis leaf extract-mediated preparation of stabilized silver nanoparticles: characterization, antimicrobial activity, and toxicity studies. J Microbiol Biotechnol. 2017;27:731–738. doi: 10.4014/jmb.1610.10019. [DOI] [PubMed] [Google Scholar]
- 6.Otari SV, Shinde VV, Hui G, et al. Biomolecule-entrapped SiO2 nanoparticles for ultrafast green synthesis of silver nanoparticle-decorated hybrid nanostructures as effective catalysts. Ceram Int. 2019;45:5876–5882. doi: 10.1016/j.ceramint.2018.12.054. [DOI] [Google Scholar]
- 7.Kumar A, Kim I-W, Patel SKS, et al. Synthesis of protein-inorganic nanohybrids with improved catalytic properties using Co3(PO4)2. Indian J Microbiol. 2018;58:100–104. doi: 10.1007/s12088-017-0700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel SKS, Otari SV, Li J, et al. Synthesis of cross-linked protein-metal hybrid nanoflowers and its application in repeated batch decolorization of synthetic dyes. J Hazard Mater. 2018;347:442–450. doi: 10.1016/j.jhazmat.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Otari SV, Patel SKS, Kalia VC, et al. Antimicrobial activity of biosynthesized silver nanoparticles decorated silica nanoparticles. Indian J Microbiol. 2019;59:379–382. doi: 10.1007/s12088-019-00812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jha D, Pathak R, Thiruveedula PK, et al. Multifunctional biosynthesized silver nanoparticles exhibiting excellent antimicrobial potential against multi-drug resistant microbial populations along with remarkable anti-cancerous properties. Mater Sci Eng C. 2017;80:659–669. doi: 10.1016/j.msec.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Otari SV, Patel SKS, Kalia VC, et al. One-step hydrothermal synthesis of magnetic rice straw for effective lipase immobilization and its application in esterification reaction. BioresourTechnol. 2020;302:122887. doi: 10.1016/j.biortech.2020.122887. [DOI] [PubMed] [Google Scholar]
- 12.Patel SKS, Kim JH, Kalia VC, et al. Antimicrobial activity of amino-derivatized cationic polysaccharides. Indian J Microbiol. 2019;59:96–99. doi: 10.1007/s12088-018-0764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel SKS, Das D, Kim SC, et al. Integrating strategies for sustainable conversion of waste biomass into dark-fermentative hydrogen and value-added products. Renew Sustain Energy Rev. 2021;150:111491. doi: 10.1016/j.rser.2021.111491. [DOI] [Google Scholar]
- 14.Patel SKS, Gupta RK, Kumar V, et al. Biomethanol production from methane by immobilized co-cultures of methanotrophs. Indian J Microbiol. 2020;60:318–324. doi: 10.1007/s12088-020-00883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalia VC, Patel SKS, Kang YC, et al. Quorum sensing inhibitors as antipathogens: biotechnological applications. Biotechnol Adv. 2019;37:68–90. doi: 10.1016/j.biotechadv.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Patel SKS, Lee J-K, Kalia VC. Deploying biomolecules as anti-COVID-19 agents. Indian J Microbiol. 2020;60:263–268. doi: 10.1007/s12088-020-00893-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rishi P, Thakur K, Vij S, et al. Diet, gut microbiota and COVID-19. Indian J Microbiol. 2020;60:420–429. doi: 10.1007/s12088-020-00908-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalia VC, Patel SKS, Cho B-K, et al. Emerging applications of bacteria as anti-tumor agents. Sem Cancer Biol. 2021 doi: 10.1016/j.semcancer.2021.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Mohiuddin AK (2019) A Comprehensive Review of Acne Vulgaris. Clin Res Dermatol Open Access, 6: 1–34. 10.15226/2378-1726/6/2/00186
- 20.Kumar B, Pathak R, Mary PB, et al. New insights into acne pathogenesis: Exploring the role of acne-associated microbial populations DermatologicaSinica New insights into acne pathogenesis: exploring the role of acne-associated microbial populations. Dermatologica Sin. 2016;34:67–73. doi: 10.1016/j.dsi.2015.12.004. [DOI] [Google Scholar]
- 21.Liu P-F, Hsieh YD, Lin YC, et al. Propionibacterium acnes in the Pathogenesis and Immunotherapy of Acne Vulgaris. Curr Drug Metab. 2015;16:245–254. doi: 10.2174/1389200216666150812124801. [DOI] [PubMed] [Google Scholar]
- 22.Alkhawaja E, Alkhawaja B, Hammadi S, et al. Antibiotic resistant Propionibacterium acnes among acne patients in Jordan: a cross-sectional study. BMC Dermatol. 2020;20:1–9. doi: 10.1186/s12895-020-00108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sardana K, Verma G. Propionibacterium acnes and the Th1/Th17 Axis, implications in acne pathogenesis and treatment. Indian J Dermatol. 2017;62:392–394. doi: 10.4103/ijd.IJD_483_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sardana K, Gupta T, Kumar B, et al. Cross-sectional pilot study of antibiotic resistance in propionibacterium acnes strains in Indian Acne patients using 16S-RNA polymerase chain reaction: a comparison among treatment modalities including Antibiotics, Benzoyl Peroxide, and Isotretinoin. Indian J Dermatol. 2016;61:45–52. doi: 10.4103/0019-5154.174025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta T, Sardana K, Kumar B, Gautam HK. Letter to the editor submitted in response to the extinction of topical erythromycin therapy for acne vulgaris and concern for the future of topical clindamycin. J Dermatolog Treat. 2018 doi: 10.1080/09546634.2017.1335852. [DOI] [PubMed] [Google Scholar]
- 26.Thorneycroft H, Gollnick H, Schellschmidt I. Superiority of a combined contraceptive containing drospirenone to a triphasic preparation containing norgestimate in acne treatment. Cutis. 2004;74:123–130. [PubMed] [Google Scholar]
- 27.Barbieri JS, Mitra N, Margolis DJ, et al. Influence of Contraception Class on Incidence and Severity of Acne Vulgaris. Obstet Gynecol. 2020;135:1306–1312. doi: 10.1097/AOG.0000000000003880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonio JR, Antônio CR, Cardeal IL, et al. Nanotechnology in dermatology. An Bras Dermatol. 2014;89:126–136. doi: 10.1590/abd1806-4841.10.1590/abd1806-4841.20142228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta S, Bansal R, Gupta S, et al. Nanocarriers and nanoparticles for skin care and dermatological treatments. Indian Dermatol Online J. 2013;4:267–272. doi: 10.4103/2229-5178.120635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim TS, Patel SKS, Selvaraj C, et al. A highly efficient sorbitol dehydrogenase from Gluconobacteroxydans G624 and improvement of its stability through immobilization. Sci Rep. 2016;6:33438. doi: 10.1038/srep33438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel SKS, Choi SH, Kang YC, et al. Large-scale aerosol-assisted synthesis of biofriendly Fe2O3 yolk-shell particles: a promising support for enzyme immobilization. Nanoscale. 2016;8:6728–6738. doi: 10.1039/C6NR00346J. [DOI] [PubMed] [Google Scholar]
- 32.Anwar MZ, Kim DJ, Kumar A, et al. SnO2 hollow nanotubes: a novel and efficient support matrix for enzyme immobilization. Sci Rep. 2017;7:15333. doi: 10.1038/s41598-017-15550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel SKS, Choi SH, Kang YC, et al. Eco-friendly composite of Fe3O4-reduced graphene oxide particles for efficient enzyme immobilization. ACS Appl Mater Interfaces. 2017;9:2213–2222. doi: 10.1021/acsami.6b05165. [DOI] [PubMed] [Google Scholar]
- 34.Patel SKS, Otari SV, Kang YC, et al. Protein-inorganic hybrid system for efficient his-tagged enzymes immobilization and its application in L-xylulose production. RSC Adv. 2017;7:3488–3494. doi: 10.1039/c6ra24404a. [DOI] [Google Scholar]
- 35.Kumar A, Patel SKS, Madan B, et al. Immobilization of xylanase using a protein-inorganic hybrid system. J Microbiol Biotechnol. 2018;28:638–644. doi: 10.4014/jmb.1710.10037. [DOI] [PubMed] [Google Scholar]
- 36.Kumar A, Park GD, Patel SKS, et al. SiO2 microparticles with carbon nanotube-derived mesopores as an efficient support for enzyme immobilization. Chem Eng J. 2019;359:1252–1264. doi: 10.1016/j.cej.2018.11.052. [DOI] [Google Scholar]
- 37.Kumar V, Patel SKS, Gupta RK, et al. Enhanced saccharification and fermentation of rice straw by reducing the concentration of phenolic compounds using an immobilization enzyme cocktail. Biotechnol J. 2019;14:1800468. doi: 10.1002/biot.201800468. [DOI] [PubMed] [Google Scholar]
- 38.Otari SV, Patel SKS, Kim S-Y, et al. Copper ferrite magnetic nanoparticles for the immobilization of enzyme. Indian J Microbiol. 2019;59:105–108. doi: 10.1007/s12088-018-0768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel SKS, Choi H, Lee J-K. Multi-metal based inorganic–protein hybrid system for enzyme immobilization. ACS Sustain Chem Eng. 2019;7:13633–13638. doi: 10.1021/acssuschemeng.9b02583. [DOI] [Google Scholar]
- 40.Patel SKS, Gupta RK, Kumar V, et al. Influence of metal ions on the immobilization of β-glucosidase through protein-inorganic hybrids. Indian J Microbiol. 2019;59:370–374. doi: 10.1007/s12088-019-0796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel SKS, Jeon MS, Gupta RK, et al. Hierarchical macro-porous particles for efficient whole-cell immobilization: application in bioconversion of greenhouse gases to methanol. ACS Appl Mater Interfaces. 2019;11:18968–18977. doi: 10.1021/acsami.9b03420. [DOI] [PubMed] [Google Scholar]
- 42.Pagolu R, Singh R, Shanmugam R, et al. Site-directed lysine modification of xylanase for oriented immobilization onto silicon dioxide nanoparticles. Bioresour Technol. 2021;331:125063. doi: 10.1016/j.biortech.2021.125063. [DOI] [PubMed] [Google Scholar]
- 43.Patel SKS, Gupta RK, Kim S-Y, et al. Rhus vernicifera laccase immobilization on magnetic nanoparticles to improve stability and its potential application in bisphenol A degradation. Indian J Microbiol. 2021;61:45–54. doi: 10.1007/s12088-020-00912-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mba IE, Nweze EI. Nanoparticles as therapeutic options for treating multidrug-resistant bacteria: research progress, challenges, and prospects. World J MicrobiolBiotechnol. 2021;37:1–30. doi: 10.1007/S11274-021-03070-X/TABLES/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chauhan I, Yasir M, Verma M, Singh AP. Nanostructured lipid carriers: a groundbreaking approach for transdermal drug delivery. Adv Pharm Bull. 2020;10:150. doi: 10.34172/APB.2020.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forbat E, Al-Niaimi F, Ali FR. Use of nicotinamide in dermatology. Clin Exp Dermatol. 2017;42:137–144. doi: 10.1111/CED.13021. [DOI] [PubMed] [Google Scholar]
- 47.Bains P, Kaur M, Kaur J, Sharma S. Nicotinamide: mechanism of action and indications in dermatology. Indian J Dermatol VenereolLeprol. 2018;84:234–237. doi: 10.4103/ijdvl.IJDVL_286_17. [DOI] [PubMed] [Google Scholar]
- 48.Abd-allah H, Abdel-aziz RTA, Nasr M. Chitosan nanoparticles making their way to clinical practice: a feasibility study on their topical use for acne treatment. Int J BiolMacromol. 2020;156:262–270. doi: 10.1016/j.ijbiomac.2020.04.040. [DOI] [PubMed] [Google Scholar]
- 49.Tolentino S, Pereira MN, Cunha-filho M, et al. Targeted clindamycin delivery to pilosebaceous units by chitosan or hyaluronic acid nanoparticles for improved topical treatment of acne vulgaris. Carbohydr Polym. 2020 doi: 10.1016/j.carbpol.2020.117295. [DOI] [PubMed] [Google Scholar]
- 50.Wijayadi LJ, Rusliati T. Encapsulated lime peel essential oil ( Citrushystrix ) into chitosan nanoparticle: new entity to enhanced effectivity against propionilbacterium acne in vitro. IOP Conf Ser Mater Sci Eng. 2020;852:012016. doi: 10.1088/1757-899X/852/1/012016. [DOI] [Google Scholar]
- 51.Chakraborty N, Jha D, Gautam HK, Roy I. Peroxidase-like behavior and photothermal effect of chitosan-coated Prussian-blue nanoparticles: dual-modality antibacterial action with enhanced bioaffinity. Mater Adv. 2020;1:774–782. doi: 10.1039/d0ma00231c. [DOI] [Google Scholar]
- 52.Alvi SB, Rajalakshmi PS, Jogdand A, et al. Iontophoresis mediated localized delivery of liposomal gold nanoparticles for photothermal and photodynamic therapy of acne. BiomaterSci. 2021;9:1421–1430. doi: 10.1039/d0bm01712d. [DOI] [PubMed] [Google Scholar]
- 53.Suh DH, Park TJ, Jeong JY, et al. Photothermal therapy using gold nanoparticles for acne in Asian patients: a preliminary study. DermatolTher. 2021;34:e14918. doi: 10.1111/dth.14918. [DOI] [PubMed] [Google Scholar]
- 54.Qin M, Landriscina A, Rosen JM, et al. Nitric Oxide-Releasing Nanoparticles Prevent Propionibacteriumacnes-Induced Inflammation by Both Clearing the Organism and Inhibiting Microbial Stimulation of the Innate Immune Response. J Invest Dermatol. 2015;135:2723–2731. doi: 10.1038/jid.2015.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ridolfi DM, Marcato PD, Justo GZ, et al. Chitosan-solid lipid nanoparticles as carriers for topical delivery of tretinoin. Colloids Surf B Biointerfaces. 2012;93:36–40. doi: 10.1016/j.colsurfb.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 56.Lima FA, Vilela RVR, Oréfice RL, et al. Nanostructured lipid carriers enhances the safety profile of tretinoin: in vitro and healthy human volunteers’ studies. Nanomedicine (Lond) 2021;16:1391–1409. doi: 10.2217/NNM-2021-0031. [DOI] [PubMed] [Google Scholar]
- 57.Ahmad Nasrollahi S, Koohestani F, Naeimifar A, et al. Preparation and evaluation of adapalene nanostructured lipid carriers for targeted drug delivery in acne. DermatolTher. 2021;34:e14777. doi: 10.1111/DTH.14777. [DOI] [PubMed] [Google Scholar]
- 58.Baber MS, Ruhi S, Rajendran PS, et al. Development and evaluation of a nano particle aloe gel containing azadirachtaindica leaves extract for the treatment of acne vulgaris. Int J Med Toxicol Leg Med. 2020;23:47–58. doi: 10.5958/0974-4614.2020.00009.1. [DOI] [Google Scholar]
- 59.Rai N, Shukla TP, Loksh KR, Karole S. Synthesized silver nanoparticle loaded gel of Curcuma Caesia for effective treatment of acne. J Drug Deliv Ther. 2020;10:75–82. doi: 10.22270/jddt.v10i6-s.4453. [DOI] [Google Scholar]
- 60.El-Mahdy M, Mohamed E-El, Saddik M, et al. (2020) Formulation and clinical evaluation of niosomal methylene blue for successful treatment of acne. J Adv Biomed Pharm Sci 3:116-126. 10.21608/jabps.2020.25846.1079
- 61.Mohammadi S, Pardakhty A, Khalili M, et al. Niosomal benzoyl peroxide and clindamycin lotion versus niosomal clindamycin lotion in treatment of acne vulgaris: A randomized clinical trial. Adv Pharm Bull. 2019;9:578–583. doi: 10.15171/apb.2019.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gupta A, Singh S, Kotla NG, Webster TJ. Formulation and evaluation of a topical niosomal gel containing a combination of benzoyl peroxide and tretinoin for antiacne activity. Int J Nanomedicine. 2014;10:171–182. doi: 10.2147/IJN.S70449. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]