Abstract
The landfill is an inexpensive way of municipal solid waste (MSW) management and contributes extensively to the total carbon budget and global climate change. Three landfills from two geographically distinct metro- cities of India were taken as model systems to create microcosms and study their physiochemistry, microbiology, and carbon emission. The microcosm experiments revealed that facultative anaerobic bacterial community showing the dominance in the beginning but with the progression of anoxia and anaerobic conditions, methanogenesis prevailed, resulting in a clear shift towards the abundance of methanogens especially the members of Methanosarcina, Methanocorpusculum, and Methanoculleus (70–90% of the total microbial population). Geochemical data showed a wide range of heterogeneity in landfills’ composition located even in the same city. In past, greenhouse gas emission from landfills is mainly estimated using different models which lack accuracy. As limited information is available as of now, this study can elicit researcher interest for in-depth characterization of microbial diversity and carbon emission from landfills. The microcosm model presented in the current study is a robust and straightforward method of accurate estimation of amounts of different types of gases release from landfill. It can also be extrapolate for estimation of different gases release from actual landfill sites by setting the on-site experiments.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12088-021-00995-7.
Keywords: Microcosm, Methanogenic archaea, Diversity, Metagenomics, Gas chromatography, Carbon release
Introduction
Municipal solid waste landfills are complex ecosystems rich in biotic and abiotic components [1, 2]. Biotic components mainly include a highly diverse spectrum of Bacteria, Archaea, and Fungi. They are primarily responsible for the degradation of organic wastes and the generation of gaseous elements (CO2, N2O, N2, CH4, H2S, etc.) [3, 4]. Significant contributors include Methanogenic archaea, which produce methane in anaerobic conditions via different pathways [5]. Denitrifying bacteria; degrade organic compounds by anaerobic respiration of nitrate, nitrite, or nitrous oxide to produce nitrogen, while fermentative microbes degrade organic substances and produce carbon dioxide and intermediates like short-chain fatty acids. These microbes have different metabolisms, but they grow in symphony with one another at landfill [6, 7]. Metagenomic studies reavealed that different layers of landfills comprise different groups of microorganisms [8], and they use multiple pathways of metabolism and co-metabolism to produce free gases, transform complex molecules to simpler ones, and mineralize inorganic molecules [6, 7, 9, 10].
Municipal solid waste handling and management is an essential part of a healthy economy. Landfilling is considered as one of the cheap and efficient methods of municipal solid waste (MSW) management [6, 7]. India, being a large economy with a vast population, is witnessing rapid urbanization, increasing the generation of MSW [11]. As per the data released by the Ministry of Housing and Urban Affairs in the Annual Report for the year 2020–21, it is estimated that the total generation of solid waste is approximately 1,50,000 MT/day. Out of the 90% collected waste, 20% is processed, and the remaining 80% is dumped in the dumpsites (http://mohua.gov.in/). It suggests that approximately 3.9 million metric tonnes of garbage are dumped annually at landfills. Overfilling such dumping grounds impacts health and sanitation of people residing in nearby areas. Previously, many case studies have been carried out on different landfill sites of India, which estimates their potential to produce GHGs by employing various methods [11]. However, it is also necessary to get a mechanistic understanding of the biological processes responsible for producing these gases for better management. Conversion of GHGs, especially CH4 released from landfills, into another physical form, are highly acclaimed to utilize them for social benefit, as CH4 directly can not be used due to its low density. Conversion of CH4 into methanol is being taken into consideration which has a density that is more than 400-times higher than that of CH4 [12–15]. Some methanotrophic bacteria can potentially metabolize CH4, through a complex pathway, for their growth and produce methanol and CO2 as the final product. Production of biofuels from biomass is an innovative approach to overcome the environmental problems associated with waste management [14, 16, 17]. In addition to biogas and methanol, landfill biomass can also be used to produce biohydrogen on the concept of waste-to-energy production [18–20]. In addition, landfill contribution to global climate change, landfills are an epicenter for the spread of chronic infections, antimicrobial resistance (AMR), and waterborne disease due to pathogenic microorganisms [1, 2].
In the present study, we have recruited three landfills belonging to the two states, Delhi and Maharashtra. Solid waste samples collected from landfills are used to set- up microcosms, which mimics the landfill conditions. The current study aims to estimate the total volume and types of gases produced from landfill sites due to microbial activities and waste degradation to get an idea about the contribution of Indian landfills to carbon release and global climate change. In addition, we also aimed to look at the microbial dynamics during the gas production to design a better strategy for its mitigation and management.
Materials and Methods
Sampling
Sampling was carried out from two different landfill sites of Delhi (Ghazipur (28°37’49.5"N, 77°19’32.3"E) and Okhla (28°31’02.8"N, 77°16’54.3"E) and one landfill located in Pune (Moshi (18°39’31.0"N,73°50’27.5"E)) for comparative study. (Fig. S1). Ghazipur and Okhla landfills receives 2500- and 1200 -MT solid waste /day respectively. Moshi landfill is located in Pune and receives 700 MT solid waste /day. Sampling was carried during the winter season when the climatic condition was relatively arid, and humidity was in the range of 28-32%. One Kg sample was collected from the central upper layer of the landfill in sterile zip lock bags using a core- sampler from a depth of 0-15 cm. The samples were kept in an ice container and transported to the laboratory, where they were stored at 4 ºC until further processing.
Physicochemical Analysis
In the laboratory, sieved the samples to remove debris, plastic, and large stones and processed for different physicochemical analyses. pH, electric-conductivity (EC), total organic carbon (TOC), total organic matter, and sulfate were estimated by following the methodology previously described (C.A. Black, American Society of Agronomy, 5th edition, 65–15800). Prevalence and concentration of heavy metals were determined by the USEPA 3050B method. Sand, silt, and clay content were measured as per Indian Standards 2720-Methods of test for soils. Total soluble solids were estimated by the method described in Indian Standards methods of test for soil (PART XXI).
Microcosm Set-up
Collected samples were sieved through a 100 mesh sieve to remove debris, hard stones, and rocks. The moisture content of the samples was measured gravimetrically. After that, 200 g of each sample was taken and moisture content (30%) was adjusted by adding autoclaved distilled water. From the prepared mixture, 60 g was immediately stored at -80 ºC (Day 0 for metagenomics purpose), and the rest of 140 g was divided into 60 ml serum vials (70 g/vial) such that 2/3rd space is occupied and 1/3rd head space remain vacant. Serum vials with landfill samples were flushed with filter sterile inert gas (N2) for 10 min to create an anoxic condition and sealed using a blue butyl rubber septum. Finally, a 10 ml sterile syringe with a 23 g needle was pierced through the septum into the vial to capture the produced gas. Vials were then incubated at 30 ºC for 30 days. Gas being accumulated inside the syringe was measured at a regular interval of 4 days. At the end of incubation (t-final), the content of the vials was stored at -80 ºC until further processing for metagenomics purposes.
Gas Chromatography
A mixture of gases accumulated in the serum vials was analyzed using gas-chromatography (Gas Chromatograph GC 2010 Plus, SHIMADZU, Japan) equipped with Porapak-Q packed column (8ft long with 80-100 mesh size) and thermal conductivity detector (TCD).The minimum detection limit of TCD for other gases is 10 ppm except carrier gas. The maximum temperature for operation and the minimum detection limit of the column are 420 ºC ten ppm (all other components except than carrier gas) respectively. We were unable to detect the peak of methane in standard (74% V/V) if injected less than 50 ul with used GC-protocol. Inert argon (Ar) gas was used as carrier gas with a flow rate of 20 ml minute−1. The injector was on the splitless mode with a temperature 100 ºC, while the temperature of the column and detector was set to 90 ºC and 110 ºC, respectively. The run time of the analysis was kept at 10 min. Standards of methane, nitrogen, and carbon dioxide gases were run before the sample. Sample (500 µl) was taken in a gas-tight syringe and injected into the instrument. The peaks generated were compared with the standard for analysis. Quantification was done by generating the statndarad curve using the peak areas.
DNA Extraction, PCR Amplification, and Targeted Metagenomics
Total DNA from frozen samples was extracted using Qiagen DNeasy Powersoil Kit (Qiagen, USA) as per the manufacturer’s protocol and quantified using a NanoDrop-2000 spectrophotometer. PCR amplification of 400 bp V4 region of 16S rRNA gene was conducted using forward primer 515F (5’- GTGCCAGCMGCCGCGGTAA-3′) and reverse primer 806R (5’-GGACTACHVGGGTWTCTAAT-3′) [21, 22]. Amplified products were checked and visualized on 1% agarose gel. Next-generation-sequencing of the amplicons was done using Illumina MiSeq paired-end (2*250) chemistry. The sequences obtained were submitted to NCBI Sequence Read Archive (SRA) with the accession BioProjectID PRJNA626350.
Sequence Data Processing and Analysis
Biome files were generated in the FASTQ format. The quality of the sequences was checked using the FASTQC-tool. For the processing of data, DADA2 R-Package was used (23)[23]. The sequences were segregated as forward and reverse before initiating the task, and sequence quality retrieved from each sample was checked individually. On that basis, filtering and trimming were carried out, wherein the parameters used were as follows; forward reads were truncated at 240 bp, the reverse reads at 220 bp. The maximum number of unknown bp allowed was set to zero; the maximum number of errors allowed in forward and reverse reads were set to two; the truncQ parameter was also set to two. Phage sequences were removed, compression and multithreading were allowed. Filtered sequences were merged, and chimeras were removed using the consensus method (DADA2 tutorial). After that complete amplicon sequence variants (ASVs) table was generated eventually. Taxonomy was assigned up to species level using SILVA version 132 reference databases. Relative abundance plots and estimation of Beta Diversity (NMDS Plot) and Alpha-Diversity (Shannon and Simpson) were done using the Phyloseq package in R (24)[24].
Results
Physicochemical Properties and Measurement Produced Gases
Comparative data related to the physicochemical properties of all three sites are given in ( Supplementray Table 1). The gas produced in each of the serum vials during incubation was trapped in the pierced syringe (Fig. S1). Increase in the volume of produced gas from the microcosm of Ghazipur (GLF), Moshi (MLF), and Okhla (OLF) after 30 days of incubation was 2.75 ml, 9.25 ml, and 14.6 ml, respectively (Fig. 1 A). The bar plot depicts a gradual increase in gas production in all the vials. Gas production in OLF was highest, while in GLF it was lowest, and in MLF, it was intermediate. The fraction of gas analysed from headspace consisted of 4–8% CO2, 78-82% N2, along with other undetected gasese (Fig. 1B). No peak of methane was detected in the headspace gas mixture indicated that the level of CH4 was below the detection limit.
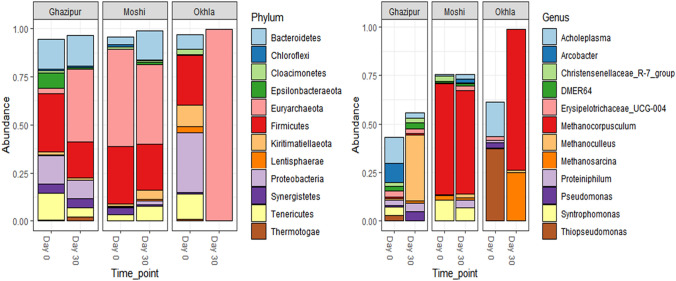
Fig. 2.
Relative abundance of bacterial diversity at Phylum, and Genus level on Day 0 and Day 30 at Ghazipur, Okhla, and Moshi samples
Fig. 1.
A Graphical representation of comparative gas production at Ghazipur (GLF), Okhla (OLF), and Moshi (MLF) samples. B Graphical representation of ratio of nitrogen and carbon dioxide detected in Ghazipur (GLF), Okhla (OLF), and Moshi (MLF) samples
Metagenomic Analysis
Sequencing through the Illumina MiSeq platform generated a total of 266,112 sequences, corresponding to six samples (Day-0 and Day-30). After filtering, trimming, merging, and chimera removal, good quality reads obtained were 27, 638, and average reads per sample were 4606. The total number of ASVs generated was 1763, while ASVs with more than two counts were 157. Phylum level data indicated that number of Euryarchaeota incresd while Proteobacteria, Epsilonbacteraeota decresed substantially while generic level analysis showed that number of Methanoculleus increased and Achloeplasma decresed substantially after 30days of incubation in microcosm conducted with Ghazipur landfill samples (Fig. 2). Like Gazipur same trend was observed in Okhla. Number of Euryarchaeota incresd while population of Proteobacteria and Firmicutes substantially reduced. Population of Methanocorpuscullum and Methanosarcina overhide all other taxa after 30-days in Okhla microcosms. Totally different trend was observed in sample collected from Pune. There was no drastic change observed in number of Euryarchaeota while population of Bacteroidates increased. At generic level number of syntrophomonas decresd after 30-days (Fig. 2 ).
This shift in diversity of microcosms is attributed to the metabolic activities of the earlier population, which created more favorable anoxic conditions and provided simplified metabolites. Complete anaerobic condition boosted the growth of Bacteroides and Euryarcheota, whereas discouraged the Firmicutes and Proteobacteria. At the genus level, mean relative abundance showed a remarkable shift from day-0 to day-30. The top twelve abundant genera are depicted in (Fig. 2). An ascending trend was observed in several genera of Methanogenic archaea in all three samples while denitrifiers decreased or increased.
Ghazipur landfill microcosm showed the highest diversity on day-0 as compared to the other two landfills. It may be attributed to the age of the landfill, which has been functional for almost 35 years. Bacterial community from Ghazipur showed denitrifiers like Pseudomonas (3.7%) and Thiopseudomonas (7.8%) along with Arcobacter (36.1%), which is responsible for the fixation of nitrogen [25]. Day zero marked the presence of Syntrophomonas (9.3%), fatty acid-oxidizing bacteria that can produce hydrogen and acetate from butyrate and propionate, which can be utilized by hydrogenotrophic methanogens [26]. Thus, by day 30, complex substances were simplified, and anoxic conditions became more prevalent, due to which hydrogenotrophic methanogens, Methanosarcina (6.7%), Methanoculleus (78.0%), and Methanosphaera (23.0%) increased significantly. Okhla landfill microcosm showed the highest gas production and had the lowest diversity. High gas production is attributed mainly to Thiopseudomonas (89.8%), as well as Pseudomonas (16%) on day-0, which was completely replaced by day-30. Thiopseudomonas are facultative anaerobic denitrifiers, represented by a single species isolated from anaerobic activated sludge [27].
Unlike Ghazipur, there was a complete absence of nitrogen fixers like Arcobacter at Okhla . Proteiniphilum present on day 0 is responsible for producing acetate and propionate by fermentation [28] Syntrophomonas also accompanied it. The presence of these acetate-producing bacteria supported the growth of low abundant Methanogenic archaea, due to which by day 30, the diversity was completely replaced by a very high prevalence of Methanosarcina (205%), Methanocorpusculum (174.98%), and Methanoculleus (2.9%). Contrary to the other two sites, the Moshi landfill microcosm showed a less evident shift in diversity. Day- 0 depicted a higher abundance of Methanocorpusculum (105.5%) and Methanosarcina (15.6%), which later on day -30, was slightly shifted to Methanoculleus (4.6%). Syntrophomonas (29.4%) and Syntrophococcus (29%) were present on day- 0 but decreased by day- 30. Syntrophococcus, like Syntrophomonas, grows in consortia alongside methanogens and provides simplified substances for methanogenesis [29]. Along with the methanogens and denitrifiers, the landfill diversity also showed the presence of anaerobic Firmicutes like Erisipelotricaceae and Christensenellaceae, which are responsible for the conversion of complex organic substances by fermentation [8, 9]. Shannon and Simpson’s indices showed that a less significant diversity shift was observed in GLF and MLF while in OLF, the diversity was completely changed and also decreased. Beta diversity was plotted by the Bray NMDS method, which showed that the diversity at Okhla, Ghazipur, and Moshi was remarkably different at day- 0 as well as day- 30 (Fig. S2). Also, the diversity of the three sites was significantly different from one another. Despite in the same city the diversity of Okhla and Ghazipur is significantly different, which might be due to the variation in age and the nature of waste dumped at each site.
Discussion
Microbial Dynamics
To mimic the natural environmental conditions, we did not add any substrate inside the microcosms from outside. We just made microcosms anoxic by flushing with filter-sterilized inert gas and maintained the optimum moisture (30%) with sterile distilled water and incubated it at 30 ºC. It is speculated, during the first week of incubation, when the condition of the microcosm was anoxic, and redox potential was in the positive state, it favored denitrification and fermentation. This was evident by the accumulation of carbon dioxide in the headspace, and the presence of bacterial denitrifies in day-0 metagenomics data. With extended incubation, the condition of microcosms shifted towards strict anoxic due to the utilization of traces of oxygen by facultative anaerobes and alternative electron acceptors like nitrate by denitrifiers. Analysis of headspace accumulated gas showed a predominance of N2 followed by CO2 (Fig. 1B). Detection and prevelance of N2 in headspace is obvious because the microcosm was flushed by nitrogen to make it anoxic but denitrification could be one of the responsible factors in accumulation of headspace nitrogen which is supported by presence of denitrifying microbial community in all the three samples. Nitrous oxide, a potent greenhouse gas produced during denitrification plays a vital role in global warming and global climate change [30, 31]. Previously, nitrous oxide production has been reported from landfills [32], but we did not detect any residues of nitrous oxide in our microcosms. Certain groups of denitrifying bacteria have been reported to use nitrous oxide as a source of electron acceptor in the absence of oxygen [33]. Due to the closed environment of microcosms, accumulated N2O could be used by such bacteria as electron acceptors and further converted to free nitrogen (N2) [34]. The absence of nitrous oxide from headspace does not indicate that Indian landfills do not produce it. To confirm the presence and release of N2O, onsite collection and analysis of gas composition are essential.
The amount of total landfill gases produced in different microcosms is an indicator of the activity of landfill microbial communities. As a consequence of respiration and fermentation, active landfills produce more gases due to an active microbial community. Our data indicate that the Okhla landfill site harbors a more active community than Moshi and Ghazipur sites. It also suggests that the size of the landfill in terms of the total garbage dumped and the nature and composition of its microbial community decide how much and what type of gases it is likely to release in the environment.
Diversity Shift
This study’s most notable and interesting observation is the remarkable change in the microbial community from day-0 to the 30th day. Depression of the redox potential of microcosms and depletion of available alternative electron acceptors made favorable conditions for the growth of obligate anaerobes and archaea like methanogens. This succession is evident by our metagenomics data after 30-days (day-30) which showed the dominance of Methanogenic archaea in the community. The microbial community was shifted and to the methanogens belonging to the genera Methanocorpusculum, Methanosarcina and Methanoculleus. Similar to our results earlier study reported the dominance of hydrogenotrophic genera of methanogens like Methanocorpusculum, Methanosarcina, and Methanoculleus from landfill samples of China [28]. Thus, our study confirmed that the hydrogenotrophic mode of methanogenesis is predominated at the landfill sites. However, we were unable to detect the methane formation in the headspace of serum vials using the GC, which could be due to the low concentration of methane (below the detection limit) at the time of analysis.
Physicochemical Analysis
The higher electric conductivity of Delhi landfills than Pune’s indicates that Delhi’s landfills bear high salt concentrations. It could be due to the dumping of industrial waste and fertilizers and pesticide-rich agricultural waste at Delhi’s landfill sites from nearby areas. Detection of high arsenic concentration in Delhi landfills than Pune supports the previous reports concerning the surface and groundwater contamination with high proportions of arsenic in northern parts of India [35], which can be due to the mixing and percolation of arsenic from contaminated landfill leachate in the surface and subsurface waters bodies [36]. Our data Indicate that landfill leachate from highly polluted and contaminated sites of metro-cities could be one of the potent causes of heavy metal contamination of surface and subsurface water bodies and should be treated appropriately before discarding in natural and freshwater ecosystems. The presence of sulfate at Okhla and Ghaipur landfills and an abundance of Thiopseudomonas, (oxidize the sulfide with nitrate as electron source) indicate that the sulfate reduction process is also operating at landfill sites and sulfide generated during the sulfate reduction process triggered the growth of Thiopseudomonas. Mitigation of generated sulfide by inducing the population of Thiopseudomonas could be one of the self-cleaning mechanisms of the landfill because the production of sulfide from landfill sites possesses harmful health threats to the people residing nearby and is a matter of concern.
Although two research groups have estimated the emission of methane and other GHG from landfill sites of Delhi and the North-Eastern part of India using gas-chromatographic analysis and different models, respectively. They reported summer releases 7-10 times more GHG than winter [3, 4]. This observation is evident because GHG release from landfills is based on activities of microbes which are augmented by high temperature in summer and increase the rate of GHG emission. Two more studies have been conducted on the contamination potential of landfill leachate, and the isolation of methylotrophic bacteria from landfill sites [36, 37] but extensive data on microbiology and chemistry of Indian landfill sites are lacking. Methane and nitrous oxide are the prominent GHG and released under anaerobic conditions by methanogens and denitrifies, respectively. The microcosm’s model presented in this study is a straightforward and robust way to accurately estimate the amount and nature of gas released from a definite amount of waste. In addition, the effects of external factors on microbial community structure and functions and related gas release can also be accurately correlated using described microcosm model. Finally, we would like to advise here that utilization of CH4, generated from landfill sites as a promising feedstock to produce value-added products (methanol), is a good approach to reduce its adverse environmental effects and solve the emerging problem of energy crisis [38–40]. Instead of dumping at landfill sites, biowaste can also be used for the production of valuable products like hydrogen, methanol, and polyhydroxybutyrate using the dark fermentation processes. [41–45].
Conclusions
In conclusion, we conducted an anoxic microcosm experiment on the sample collected from three different landfill sites. Our physicochemical data indicated that they are different and showed the potential to release CO2, N2, and other gases in the environment. The amount and nature of gas generated by landfills depend upon its active microbial community. Even small landfills with very active microbial communities can release immense carbon and other toxic gases into the environment. In addition, we also concluded that denitrification and fermentation might be responsible for the degradation of organic carbon and available nitrate in anoxic or microoxic conditions. Finally, we reported a shift of the prevalent bacterial community to methanogenic archaea and substantiates the previous finding that the hydrogenotrophic mode of methanogenesis dominates at landfill sites. Finally, detection of heavy metals like arsenic and lead, presence of harmful microbes suggests that along with contribution to global climate change, landfills are the potential source of chemical and biological contamination and must be maintained appropriately. Finally, it is advisable here that no system in isolation can make a better prediction of GHG release from landfills. Therefore, a combination of microcosm and modeling should be used for the accurate prediction of gas release from landfill sites. Microcosm will also assist in the study of the effects of external factors those trigger or mitigate the process of GHG release from landfill sites and help in better management of the landfill. Presented microcosm model can be use to set future on-site experiment for acuurate prediction of greenhose gas release from different landfills.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
Research is carried out as a part of the sanctioned project (BT/13969/BCE/8/1142/2015) at the National Centre for Microbial Resource, National Centre for Cell Science under the funds granted by the Department of Biotechnology (DBT), Government of India.
Authors’ Contributions
OP, DD, YS, and DR, conceived and designed the experiments. IS and YN performed the experiments, collected and analyzed the data. IS and OP wrote the article, and SD and OP revised the paper. All authors read and approved the final manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Swati, Thakur IS, Vijay VK, et al. et al. Scenario of Landfilling in India: Problems, Challenges, and Recommendations. In: Hussain C, et al.et al., editors. Handbook of Environmental Materials Management. Cham: Springer; 2018. [Google Scholar]
- 2.Meyer-Dombard DR, Bogner JE, Malas J. A Review of Landfill Microbiology and Ecology: A Call for Modernization With ‘Next Generation’ Technology. Front Microbiol. 2020;11:1127. doi: 10.3389/fmicb.2020.01127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawat M, Singh UK, Mishra AK, et al. Methane emission and heavy metals quantification from selected landfill areas in India. Environ Monit Assess. 2008;137:67–74. doi: 10.1007/s10661-007-9729-8. [DOI] [PubMed] [Google Scholar]
- 4.Gollapalli M, Kota SH. Methane emissions from a landfill in north-east India: Performance of various landfill gas emission models. Environ Pollut. 2018;234:174–180. doi: 10.1016/j.envpol.2017.11.064. [DOI] [PubMed] [Google Scholar]
- 5.Yadav S, Kundu S, Ghosh SK, et al. Molecular Analysis of Methanogen Richness in Landfill and Marshland Targeting 16S rDNA Sequences. Archaea. 2015 doi: 10.1155/2015/563414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sekhohola-Dlamini L, Tekere M. Microbiology of municipal solid waste landfills: a review of microbial dynamics and ecological influences in waste bioprocessing. Biodegradation. 2020;31:1–21. doi: 10.1007/s10532-019-09890-x. [DOI] [PubMed] [Google Scholar]
- 7.Sekhohola-Dlamini L, Selvarajan R, Ogola HJO, et al. Community diversity metrics, interactions, and metabolic functions of bacteria associated with municipal solid waste landfills at different maturation stages. Microbiologyopen. 2021;10:e1118. doi: 10.1002/mbo3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Cao A, Zhao G, et al. Microbial community structure and diversity in a municipal solid waste landfill. Waste Manag. 2017;66:79–87. doi: 10.1016/j.wasman.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Zhao R, Liu J, Feng J, Li X, et al. Microbial community composition and metabolic functions in landfill leachate from different landfills of China. Sci Total Environ. 2021;767:144861. doi: 10.1016/j.scitotenv.2020.144861. [DOI] [PubMed] [Google Scholar]
- 10.Chukwuma OB, Rafatullah M, Tajarudin HA, Ismail N. Bacterial Diversity and Community Structure of a Municipal Solid Waste Landfill: A Source of Lignocellulolytic Potential. Life (Basel). 2021;11:493. doi: 10.3390/life11060493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar A, Sharma MP. Estimation of GHG emission and energy recovery potential from MSW landfill sites. Sustain Energy Technol Assess. 2014;5:50–61. doi: 10.1016/j.seta.2013.11.004. [DOI] [Google Scholar]
- 12.Patel SKS, Kalia VC, Joo JB, et al. Biotransformation of methane into methanol by methanotrophs immobilized on coconut coir. Bioresour Technol. 2020;297:122433. doi: 10.1016/j.biortech.2019.122433. [DOI] [PubMed] [Google Scholar]
- 13.Patel SKS, Gupta RK, Kumar V, et al. Biomethanol production from methane by immobilized cocultures of methanotrophs. Indian J Microbiol. 2020;60:318–324. doi: 10.1007/s12088-020-00883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel SKS, Jeon MS, Gupta RK, et al. Hierarchical macro-porous particles for efficient whole-cell immobilization: application in bioconversion of greenhouse gases to methanol. ACS Appl Mater Interfaces. 2019;11:18968–18977. doi: 10.1021/acsami.9b03420. [DOI] [PubMed] [Google Scholar]
- 15.Patel SKS, Selvaraj C, Mardina P, et al. Enhancement of methanol production from synthetic gas mixture by Methylosinus sporium through covalent immobilization. Appl Energy. 2016;171:383–391. doi: 10.1016/j.apenergy.2016.03.022. [DOI] [Google Scholar]
- 16.Kondaveeti S, Patel SKS, Pagolu R, et al. Conversion of simulated biogas to electricity: sequential operation of methanotrophic reactor effluents in microbial fuel cell. Energy. 2019;189:116309. doi: 10.1016/j.energy.2019.116309. [DOI] [Google Scholar]
- 17.Patel SKS, Gupta RK, Kondaveeti S, et al. Conversion of biogas to methanol by methanotrophs immobilized on chemically modified chitosan. Bioresour Technol. 2020;315:123791. doi: 10.1016/j.biortech.2020.123791. [DOI] [PubMed] [Google Scholar]
- 18.Patel SKS, Ray S, Prakash J, et al. Co-digestion of biowastes to enhance biological hydrogen process by defined mixed bacterial cultures. Indian J Microbiol. 2019;59:154–160. doi: 10.1007/s12088-018-00777-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel SKS, Gupta RK, Das D, et al. Continuous biohydrogen production from poplar biomass hydrolysate by a defined bacterial mixture immobilized on lignocellulosic materials under non-sterile conditions. J Clean Prod. 2021;287:125037. doi: 10.1016/j.jclepro.2020.125037. [DOI] [Google Scholar]
- 20.Patel SKS, Kondaveeti S, Otari SV, et al. Repeated batch methanol production from a simulated biogas mixture using immobilized Methylocystis bryophila. Energy. 2018;145:477–485. doi: 10.1016/j.energy.2017.12.142. [DOI] [Google Scholar]
- 21.Caporaso JG, Lauber CL, Walters WA, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci. 2011;108:4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jani K, Dhotre D, Bandal J, et al. World’s Largest Mass Bathing Event Influences the Bacterial Communities of Godavari, a Holy River of India. Microb Ecol. 2018;76:706–718. doi: 10.1007/s00248-018-1169-1. [DOI] [PubMed] [Google Scholar]
- 23.Callahan BJ, McMurdie PJ, Rosen MJ, et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMurdie PJ, Holmes S (2013) phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. Watson M, editor. PLoS ONE. Apr 22:e61217. doi: 10.1371/journal.pone.0061217 [DOI] [PMC free article] [PubMed]
- 25.Evans MV, Panescu J, Hanson AJ, et al. Members of Marinobacter and Arcobacter Influence System Biogeochemistry During Early Production of Hydraulically Fractured Natural Gas Wells in the Appalachian Basin. Front Microbiol. 2018;9:2646. doi: 10.3389/fmicb.2018.02646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song L, Wang Y, Zhao H, et al. Composition of bacterial and archaeal communities during landfill refuse decomposition processes. Microbiol Res. 2015;181:105–111. doi: 10.1016/j.micres.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Tan W-B, Jiang Z, Chen C, et al. Thiopseudomonas denitrificans gen. nov., sp. nov., isolated from anaerobic activated sludge. Int J Syst Evol Microbiol Jan. 2015;65:225–229. doi: 10.1016/j.micres.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Hahnke S, Langer T, Koeck DE, et al. Description of Proteiniphilum saccharofermentans sp. nov., Petrimonas mucosa sp. nov. and Fermentimonas caenicola gen. nov., sp. nov., isolated from mesophilic laboratory-scale biogas reactors, and emended description of the genus Proteiniphilum. Int J Syst Evol Microbiol. 2016;66:1466–1475. doi: 10.1099/ijsem.0.000902. [DOI] [PubMed] [Google Scholar]
- 29.Oosterkamp MJ, Bauer S, Ibáñez AB, et al. Identification of methanogenesis and syntrophy as important microbial metabolic processes for optimal thermophilic anaerobic digestion of energy cane thin stillage. Bioresour Technol Rep. 2019;7:100254. doi: 10.1016/j.biteb.2019.100254. [DOI] [Google Scholar]
- 30.Jia M-S, Wang X-J, Chen S-H. [Nitrous oxide emissions from municipal solid waste landfills and its measuring methodology: a review] Ying Yong Sheng Tai Xue Bao J Appl Ecol. 2014;25:1815–1824. [PubMed] [Google Scholar]
- 31.Ishigaki T, Nakagawa M, Nagamori M, et al. Anaerobic generation and emission of nitrous oxide in waste landfills. Environ Earth Sci. 2016;75:750. doi: 10.1007/s12665-016-5543-3. [DOI] [Google Scholar]
- 32.Zhang C, Xu T, Feng H, et al. Greenhouse Gas Emissions from Landfills: A Review and Bibliometric Analysis. Sustainability. 2019;8:2282. doi: 10.3390/su11082282. [DOI] [Google Scholar]
- 33.Conthe M, Parchen C, Stouten G, et al. O2 versus N2O respiration in a continuous microbial enrichment. Appl Microbiol Biotechnol. 2018;102:8943–8950. doi: 10.1007/s00253-018-9247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Strous M. Denitrification and aerobic respiration, hybrid electron transport chains and co-evolution. Biochim Biophys Acta BBA-Bioenerg. 2013;1827:136–144. doi: 10.1016/j.bbabio.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Shrivastava A, Ghosh D, Dash A, et al. Arsenic Contamination in Soil and Sediment in India: Sources, Effects, and Remediation. Curr Pollut Rep. 2015;1:35–46. doi: 10.1007/s40726-015-0004-2. [DOI] [Google Scholar]
- 36.Naveen BP, Mahapatra DM, Sitharam TG, et al. Physico-chemical and biological characterization of urban municipal landfill leachate. Environ Pollut. 2017;220:1–12. doi: 10.1016/j.envpol.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Yadava S, Maitra SS. Molecular detection of methylotrophs from an Indian landfill site and their potential for biofuel production. Glob Nest J. 2017;19:533–539. doi: 10.30955/gnj.002380. [DOI] [Google Scholar]
- 38.Patel SKS, Gupta RK, Kalia VC, et al. Integrating anaerobic digestion of potato peels to methanol production by methanotrophs immobilized on banana leaves. Bioresour Technol. 2021;323:124550. doi: 10.1016/j.biortech.2020.124550. [DOI] [PubMed] [Google Scholar]
- 39.Patel SKS, Kumar V, Mardina P, et al. Methanol peoduction from simulated biogas mixtures by co-immobilized Methylomonas methanica and Methylocella tundrae. Bioresour Technol. 2018;263:25–32. doi: 10.1016/j.biortech.2018.04.096. [DOI] [PubMed] [Google Scholar]
- 40.Patel SKS, Shanmugam R, Kalia VC, et al. Methanol production by polymer-encapsulated methanotrophs from simulated biogas in the presence of methane vector. Bioresour Technol. 2020;304:123022. doi: 10.1016/j.biortech.2020.123022. [DOI] [PubMed] [Google Scholar]
- 41.Patel SKS, Das D, Kim SC, et al. Integrating strategies for sustainable conversion of waste biomass into dark-fermentative hydrogen and value-added products. Renew Sust Energ Rev. 2021;150:111491. doi: 10.1016/j.rser.2021.111491. [DOI] [Google Scholar]
- 42.Patel SKS, Kumar P, Singh S, et al. Integrative approach to produce hydrogen and polyhydroxybutyrate from biowaste using defined bacterial cultures. Bioresour Technol. 2015;176:136–141. doi: 10.1016/j.biortech.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 43.Patel SKS, Mardina P, Kim D, et al. Improvement in methanol production by regulating the composition of synthetic gas mixture and raw biogas. Bioresour Technol. 2016;218:202–208. doi: 10.1016/j.biortech.2016.06.065. [DOI] [PubMed] [Google Scholar]
- 44.Porwal S, Kumar T, Lal S, et al. Hydrogen and polyhydroxybutyrate producing abilities of microbes from diverse habitats by dark fermentative process. Bioresour Technol. 2008;99:5444–5451. doi: 10.1016/j.biortech.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 45.Patel SKS, Purohit HJ, Kalia VC. Dark fermentative hydrogen production by defined mixed microbial cultures immobilized on ligno-cellulosic waste materials. Int J Hydrogen Energy. 2010;35:10674–10681. doi: 10.1016/j.ijhydene.2010. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.