Abstract
The intrapulmonary pharmacokinetics of rifapentine were studied in 30 volunteers who received a single, oral dose of rifapentine (600 mg). Subgroups of five subjects each underwent bronchoscopy and bronchoalveolar lavage (BAL) at timed intervals following drug administration. Drug concentrations, including the concentration of the primary metabolite 25-desacetyl rifapentine, were determined in plasma, BAL fluid, and alveolar cells (AC) by high-pressure liquid chromatography. The concentrations in epithelial lining fluid (ELF) were calculated by the urea diffusion method. The concentration-time data were fit to two-compartment (plasma) or one-compartment (AC and ELF) models. The peak concentrations in plasma, ELF, and AC, 26.2, 3.7, and 5.3 μg/ml, respectively, occurred at 5, 5, and 7 h after drug administration, respectively. The half-lives and areas under the curve for plasma, ELF, and AC were 18.3 h and 520 μg · h/ml, 20.8 h and 111 μg · h/ml, and 13.0 h and 133 μg · h/ml, respectively. Although the intrapulmonary rifapentine concentrations were less than the plasma rifapentine concentrations at all time periods, they remained above the proposed breakpoint for M. tuberculosis (0.5 μg/ml) for the 48-h observation period. These data provide a pharmacokinetic rationale for extended-interval dosing. The optimum dosing regimen for rifapentine will have to be determined by controlled clinical trials.
Rifapentine is an orally administered rifamycin derivative that has antituberculous activity and that is similar to rifampin (11, 12, 16, 27). The MICs for sensitive strains are usually in the range of 0.03 to 0.12 mg/liter, and MICs for resistant strains are ≥8 mg/liter (15). Cross-resistance between rifapentine and rifampin is virtually complete (15). Cross-resistance between rifapentine and rifampin is virtually complete (15). Rifapentine has a longer elimination half-life than rifampin, allowing the possibility of less frequent (twice- or once-weekly) administration (17).
There have been no previous reports of the intrapulmonary concentrations or pharmacokinetics of rifapentine in humans. The purpose of this study was to compare the concentrations of rifapentine in plasma, alveolar cells (ACs), and epithelial lining fluid (ELF) of normal volunteers and to compare the drug's pharmacokinetics in these three compartments.
MATERIALS AND METHODS
Subjects.
The protocol was approved by the Human Research Committee of the University of California, San Francisco. Written informed consent was obtained from each subject by an experienced research nurse. Subjects were required to be 18 to 45 years of age. If the subjects were female, they were required to be nonlactating and not pregnant and to agree to use adequate contraception (e.g., barrier methods or abstinence) during the study and for 2 weeks following completion of the study. Women using oral contraceptives were required to agree to use a barrier method in addition for 1 month following the study. Subjects who were lactating or pregnant, had a history of intolerance to rifamycin drugs or topical lidocaine, had clinically significant organ dysfunction, or were required to take chronic medications other than self-prescribed vitamins, birth control pills, or thyroid replacement therapy or who were smoking on a regular basis within 6 months of the study were excluded. Subjects who reported drug or alcohol dependence or who had psychiatric problems that would interfere with participation in the study were also excluded. Twenty subjects were women, and 10 subjects were men. The subjects' age, height, and weight (mean ± standard deviation [SD]) were 29.7 ± 6.2 years, 169 ± 9 cm, and 66.7 ± 13.8 kg, respectively. All subjects had normal renal function as measured by determination of the serum creatinine level (0.8 ± 0.1 mg/dl).
Study design.
The investigation was prospective but not randomized or blinded. Thirty normal volunteers divided into six subgroups of five subjects each were assigned to undergo standardized bronchoscopy and bronchoalveolar lavage (BAL) (7, 10) at specified time intervals from 4 to 48 h following oral administration of a single dose of rifapentine. Rifapentine (600 mg) was administered by a research nurse in the Endoscopy Unit, followed by a timed bronchoscopy, as indicated in Table 1. All patients were observed for at least 30 min after taking the antibiotic for any signs of an allergic reaction. Bronchoscopy and BAL were performed at 4, 5, 7, 12, 24, and 48 h. Baseline data included the results of a medical history and physical examination; blood tests including a complete blood count with differential and a platelet count and alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, total bilirubin, total protein, albumin, blood urea nitrogen, serum creatinine, and electrolyte level determinations; and urinalysis, including specific gravity, pH, albumin, glucose, ketone, and bilirubin level determinations, and microscopic examination of spun sediment. The postbronchoscopy assessment included a medical history and physical examination, a review of voluntarily described and observed adverse experiences, collection of a sample for antibiotic concentration determination, and a repeat of the laboratory testing of blood and urine. Major adverse reactions were defined as those that were fatal or life-threatening, resulted in permanent disability, required hospitalization, were the result of drug overdose, or suggested significant hazard to the subject.
TABLE 1.
Volume of ELF and AC counts at six time periods following drug administration
| Characteristic | 4 h | 5 h | 7 h | 12 h | 24 h | 48 h |
|---|---|---|---|---|---|---|
| Total cell count in BAL fluida (cells/liter) | 1.31 × 108 ± 7.57 × 107 | 1.72 × 108 ± 1.18 × 108 | 1.41 × 108 ± 1.71 × 108 | 9.94 × 107 ± 1.60 × 107 | 8.52 × 107 ± 2.67 × 107 | 9.30 × 107 ± 6.62 × 107 |
| Neutrophilsa (%) | 1.0 ± 1.2 | 1.6 ± 0.9 | 0.8 ± 0.8 | 0.8 ± 0.8 | 1.0 ± 1.0 | 1.4 ± 1.1 |
| Lymphocytes (%) | 11.2 ± 8.6 | 11.4 ± 6.8 | 22.6 ± 10.8 | 8.8 ± 5.7 | 9.0 ± 8.6 | 18.4 ± 10.3 |
| Monocytes/macrophages (%) | 86.0 ± 11.8 | 84.6 ± 7.6 | 76.2 ± 11.7 | 84.8 ± 9.5 | 89.6 ± 9.0 | 70.8 ± 21.7 |
| Eosinophils (%) | 0.8 ± 1.8 | 1.4 ± 2.2 | 0.4 ± 0.9 | 2.0 ± 4.5 | 0.4 ± 0.5 | 0.4 ± 0.5 |
| Degenerated cells (%) | 1.0 ± 2.2 | 1.0 ± 2.2 | 0.0 ± 0.0 | 3.6 ± 8.0 | 0.0 ± 0.0 | 9.0 ± 11.6 |
| ELF vola (ml) | 0.82 ± 0.63 | 1.00 ± 0.35 | 1.21 ± 0.57 | 0.98 ± 0.39 | 1.13 ± 1.19 | 0.81 ± 0.48 |
| BAL volb (ml) | 71 ± 8.2 | 79.8 ± 7.4 | 77 ± 7.2 | 77.6 ± 3.0 | 78.2 ± 11.7 | 79.8 ± 7.5 |
The differences in AC count, differential cell count, or ELF volume among the six groups were not significant (P > 0.05).
Mean ± SD for pooled volume of BAL fluid recovered.
Bronchoscopy and BAL.
Bronchoscopy and BAL were performed in the Endoscopy Unit of the Moffitt-Long Hospitals. The blood pressure, respiratory rate, and heart rate of each subject were recorded before, at the completion of, and at approximately 1 h after bronchoscopy. Oxygenation was monitored by fingertip oximetry throughout the procedure. A 4% topical lidocaine gargle followed by a 4% topical lidocaine spray was used to prepare the subjects for the procedure. Four percent topical lidocaine was then applied to each side of the posterior pharynx, followed by the application of topical 1% lidocaine more distally. Systemic sedation was not used. A fiberoptic bronchoscope (Pentax FB-19H) was inserted into the right middle lobe. The instrument was in place for an average of 4.8 min (range, 3 to 9 min). Four 50-ml aliquots of normal saline were instilled into the right middle lobe, and each was immediately aspirated into a trap.
Specimen handling.
All specimens were kept in ice until they were frozen. The blood was centrifuged, and the plasma was separated and then frozen until it was assayed.
Since the BAL fluid aspirated from the first instillation contains significant contamination with cells from the proximal airways (3) it was discarded. The aspirates from the second, third, and fourth instillations were pooled. The volume of the pooled aspirates from the instillations was measured and recorded. Two-milliliter aliquots drawn from the pooled specimens were sent to the clinical laboratory for cell count and differential. Measured volumes were placed in 30-ml polypropylene tubes, and the tubes were immediately spun in a refrigerated centrifuge at 400 × g for 5 min. After separation of the supernatant from the cells, the supernatant was stored at −80°C until assay. The cells were immediately resuspended into 2 ml of acetonitrile with 200 μg of ascorbic acid per ml (acetonitrile/aa) and frozen at −80°C until they were assayed. A small aliquot of each supernatant was frozen separately for urea concentration determination.
Assay for rifapentine concentrations.
The rifapentine concentration in plasma was measured by the laboratory at Hoechst Marion Roussel, Inc. (Kansas City, Mo.) by a high-pressure liquid chromatography method that was reported previously (18). Briefly, plasma proteins were precipitated with methanol containing internal standard (25-O-desacetyl rifampin). After centrifugation the supernatant was injected onto a 5-μm Primesphere C18-HC column (2.0 by 150 mm; Phenomenex, Inc., Torrance, Calif.) preceded by a matching guard column. Analytes were eluted with a mobile phase consisting of 40% methanol, 25% acetonitrile, and 35% water containing 0.5% acetic acid. Drug peaks were detected at 480 nm. The validation statement provided by personal communication from an internal research and development report of 5 January 1996 (Marion Merrell Dow Inc., Kansas City, Mo.) stated the following: the assay was validated over the concentration range of 0.5 to 60 μg/ml. Two replicates of seven freshly prepared calibration standards (0, 0.5, 1.0, 15, 30, 45, and 60 μg/ml) and 30 replicates of four validation quality control sample pools (0.5, 1.0, 30, and 60 μg/ml) were assayed in each of three batches run over a period of 2 days. The intraday coefficients of variation ranged from 2.8 to 6.2% for rifapentine and 2.7 to 5.8% for 25-desacetyl rifapentine. The interday coefficients of variation ranged from 0.8 to 4.4% for rifapentine and 0.8 to 5.7% for 25-desacetyl rifapentine. The linearity of the assay (R2) ranged from 0.9993 to 0.9998.
BAL fluid supernatants and ACs were analyzed in the Infectious Diseases Research Laboratory at the University of California, San Francisco. The BAL fluid supernatant was extracted through a Varian (Harbor City, Calif.) Bond Elut C8 solid-phase extraction column. Diazepam, (Sigma Chemical Co., St. Louis, Mo.) was added to the eluant as an internal standard, and then the eluant was evaporated and resuspended in 200 μl of methanol containing 200 μg of ascorbic acid per ml (methanol/aa). This preparation was injected onto a 5-μm Beckman Ultrasphere octyl column (4.6 mm [inner diameter] by 15 cm). The column was connected to the detector by tubing (polytetrafluoroethylene Teflon; 0.5 mm [inner diameter]) wrapped around a UVP (San Gabriel, Calif.) shortwave Pen-Ray lamp to irradiate the sample. Analytes were detected by measuring the fluorescence at an excitation wavelength of 290 nm and an emission wavelength of 520 nm. The mobile phase consisted of 40% acetonitrile in water, 0.2% phosphoric acid, and 0.5% hydrogen peroxide adjusted to pH 4.5 with sodium hydroxide. Calibration curves and controls were prepared at the same time as the samples.
AC suspensions were prepared as follows. Diazepam was added to the cells suspended in acetonitrile/aa as the internal standard. After centrifugation, the solvent was evaporated and resuspended in 200 μl of methanol/aa. The AC suspensions were then injected onto the system described above. Calibration curves and controls were prepared in acetonitrile on the same day as the samples.
The mean ± SD coefficients of variation for intraday and interday determinations together for rifapentine in BAL fluid supernatants and ACs were 4.91% ± 0.87% and 4.03% ± 1.33%, respectively. For 25-desacetyl rifapentine, they were 7.27% ± 3.40% and 5.48% ± 5.21%, respectively. The linearity of the assay (R2) for rifapentine and 25-desacetyl rifapentine in the BAL fluid supernatants ranged from 0.9950 to 0.9996 and 0.9958 to 0.9994, respectively. For ACs, linearity (R2) ranged from 0.9948 to 0.9998 for rifapentine and from 0.9948 to 0.9994 for 25-desacetyl rifapentine.
Determination of ELF volume and concentration of antibiotics in ELF and ACs.
The ELF volume was determined by the urea dilution method (29). The concentration of urea in serum was analyzed by the clinical laboratory at the University of California, San Francisco, by a coupled urease-glutamate dehydrogenase enzymatic method (32) modified by Boehringer Mannheim Corporation (Indianapolis, Ind.). Measurements were made at a fixed time interval that permitted automated analysis with a BM 747 analyzer (Boehringer Mannheim). The urea concentration in the BAL fluid supernatant was measured by a modified enzymatic assay (BUN kit UV-66; Sigma), and the results were read on a Spectronic 20D spectrophotometer (Milton Roy, Rochester, N.Y.). The proportions of the reagent to the specimen were changed from 3.0 ml/0.005 ml, as recommended by the manufacturer, to 2.5 ml/0.5 ml, as reported by Rennard et al. (29). Standard curves prepared in normal saline with concentrations ranging from 0.047 to 0.750 mg/dl were linear (r2 ≥ 0.99). Controls with concentrations of 0.094 and 0.375 mg/dl were run with every standard curve used to assay the BAL fluid samples. If the values for the controls were not within 10% of the known value, the standard curve, controls, and BAL fluid sample assays were repeated.
The volume of ELF in BAL fluid was derived from the following relationships: VELF = VBAL × (UREABAL/UREASER),where VELF is volume of ELF sampled by BAL, VBAL is volume of aspirated BAL fluid; UREABAL is the concentration of urea in BAL fluid, and UREASER is the concentration of urea in serum. The volume of ACs collected in the pellet suspension was determined from the cell count in the BAL fluid. The cells were counted in a hemocytometer which has a lower detection limit of 1.0 × 106/liter. The number of cells from which drug was extracted was calculated by multiplying the number of cells per milliliter in BAL fluid times the volume (in milliliters) of BAL fluid that was centrifuged to produce the pellet. It has been noted, however, that centrifugation causes an average loss of 21% of the cells (33), so that the actual number of cells recovered postcentrifugation may be less than the number counted, and the actual antibiotic concentration may be proportionately more than we report here. The volume of ACs in the pellet suspension was calculated by using a mean macrophage cell volume of 2.42 μl/106 cells (2). The concentration of antibiotic in alveolar cells, ABXAC, was calculated from the following relationship (7, 10): ABXAC = (ABXPELLET/VAC) where ABXPELLET is the antibiotic concentration in the 1-ml cell suspension, and VAC is the volume of ACs in the 1-ml cell suspension.
Differential cell counting was performed after the specimen was spun in a cytocentrifuge (33).
Statistical analysis and modeling.
Statistical analysis and database management were performed by using Prophet, version 6.0 (AbTech Corp., Charlottesville, Va.). Modeling was performed by using Prophet, version 4.1 (BBN, Inc., Cambridge, Mass.), on a Sun 10 Sparc Station (Sun Microsystems, Milpitas, Calif.). The linear regression analysis used the method of least-squares estimation. A P value of <0.05 was regarded as statistically significant. Prior to comparison of the data sets, tests for normality (Wilk-Shapiro test) and equality of variances (Levene's test) were performed. Parametric and nonparametric comparisons were performed by the Neuman-Keuls (all pairwise) or Kruskal-Wallis (unblocked data) and Friedman (blocked data) tests, respectively (34). A P value of <0.05 was regarded as statistically significant.
The intracellular and ELF concentration-time data declined linearly on a logarithmic scale and were fit to a one-compartment model. The plasma concentration-time data were fit to a one-compartment model and a two-compartment model. The log likelihood of the fit, the Schwartz criterion (SC) (30), and the Leonard criterion (LC) (24) were calculated to assess the appropriateness of the two models that were used to fit the concentrations in plasma. SC was derived from the equation SC = log L − (K/2) ln N, where log L was the log likelihood of the fit, K was the number of parameters used in the model, and N was the number of observed concentrations. LC was derived from the equation LC = SC + ½ [K ln (2πN) + ln(DCM)], where DCM was the determinant of the covariant matrix. SC, LC, the log likelihood of the fit, and the correlation coefficient squared (R2) favored the two-compartment model for the concentrations in plasma. Therefore, this was the model that was used to derive the pharmacokinetic parameters.
The following parameters were estimated from the model: V1, the volume of distribution of the central compartment; LZ, the elimination rate constant; T1/2-LZ, the elimination half-life; Cmax, the maximum concentration in plasma; and Tmax, the time to Cmax. Fitting was performed by using a weighting function (1/Y2), where 1/Y is the reciprocal of the observed concentration. The area under the concentration-time curve (AUC) from time zero to infinity (AUC0–∞) was estimated by noncompartmental analysis by using the log-trapezoidal rule and was extrapolated to infinity by dividing the last predicted concentration by LZ.
RESULTS
None of the 30 subjects experienced a major adverse reaction. Systemic sedation was not required for any of the subjects. During the postbronchoscopy observation period, eight subjects (27%) experienced self-limited lightheadedness of unclear etiology, possibly a lidocaine effect. Transient shortness of breath, cough, fatigue, and headache were reported by one (3%), two (7%), four (13%), and one (3%) of the subjects, respectively. None of the subjects experienced postbronchoscopy fever. Fifteen of the 30 subjects (50%) experienced an elevation in the level of one of the liver enzymes, but the level returned to normal on retesting. Two of the subjects experienced borderline elevated (43 and 43 U/liter) concentrations of aspartate aminotransferase. One of the two subjects was retested, and the level returned to the normal range (<40 U/liter). Twelve of the subjects developed mildly abnormal total bilirubin concentrations (mean ± SD, 1.6 ± 0.3 mg/dl; range, 1.3 to 2.4 mg/dl). For all of these subjects the concentrations returned to normal on retesting.
Recovery of cells and ELF from BAL fluid.
The number of cells recovered ranged from 8.52 × 107 ± 2.67 × 107 to 1.72 × 108 ± 1.18 × 108 cells/liter (Table 1), and the number of cells recovered was not significantly different for the six groups (P > 0.05). The most predominant cell types were monocytes/macrophages (range, 70.8% ± 21.7% to 89.9% ± 9.0%). The percentages of each cell type were compared among the six groups, and none of the percentages were significantly different (P > 0.05). The volumes of ELF ranged from 0.81 ± 0.48 to 1.21 ± 0.57 ml (Table 1) and were not significantly different among the six groups (P > 0.05).
Plasma rifapentine and 25-desacetyl rifapentine concentrations at time of bronchoscopy.
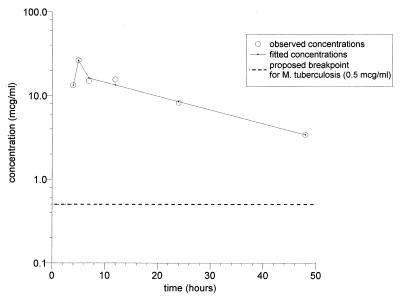
The plasma rifapentine concentrations ranged from 4.5 ± 1.7 to 13.9 ± 3.6 μg/ml at 2 h following drug administration and from 4.1 ± 2.6 to 10.7 ± 4.0 μg/ml at 20 to 24 h following drug administration. At the time of bronchoscopy, the concentrations in plasma ranged from a low of 3.4 ± 3.2 μg/ml at 48 h to a high of 26.2 ± 6.1 μg/ml at 5 h. The corresponding concentrations of 25-desacetyl rifapentine were less at all time periods (Table 2). The plasma rifapentine concentrations determined at the time of bronchoscopy (at 4, 5, 7, 12, 24, and 48 h) declined biexponentially (r2 = 0.98; log likelihood of the fit = 6.97; residual sum of squares = 3.59), with a t1/2 of 18.3 h and an AUC0–∞ of 520 mg · h ml−1 (Fig. 1 and Table 3).
TABLE 2.
Concentrations of rifapentine and its main metabolite (25-desacetyl rifapentine) in plasma
| BAL group and druga | Concn in plasma
|
||
|---|---|---|---|
| At 2 h postdosing | At time of BAL | At 20–24 h postdosing | |
| 4 h | |||
| RFP | 4.5 ± 1.7b | 13.3 ± 9.2 | 4.1 ± 2.6 |
| 25-dR | 0.0 ± 0.0c | 4.9 ± 3.5 | 4.8 ± 3.6 |
| 5 h | |||
| RFP | 13.2 ± 3.6 | 26.2 ± 6.1d | 7.6 ± 1.1 |
| 25-dR | 1.6 ± 0.4c | 8.2 ± 2.6 | 6.8 ± 2.6 |
| 7 h | |||
| RFP | 13.9 ± 3.6b | 14.9 ± 4.9 | 6.7 ± 1.7 |
| 25-dR | 1.5 ± 0.7 | 6.3 ± 2.1 | 5.0 ± 2.6 |
| 12 h | |||
| RFP | 9.7 ± 5.5 | 15.5 ± 4.4 | 10.7 ± 4.0 |
| 25-dR | 0.5 ± 0.5 | 7.6 ± 2.2 | 7.6 ± 3.1 |
| 24 h | |||
| RFP | 9.1 ± 4.7 | 8.2 ± 1.9d | 7.9 ± 2.9 |
| 25-dR | 0.6 ± 0.7 | 7.7 ± 4.7 | 5.8 ± 4.3 |
| 48 h | |||
| RFP | 9.7 ± 2.2 | 3.4 ± 3.2d | 9.8 ± 7.4 |
| 25-dR | 1.1 ± 0.5 | 5.1 ± 4.7 | 7.3 ± 5.3 |
RFP, rifapentine; 25-dR, 25-desacetyl rifapentine.
For 7-h rifapentine group versus 4-h rifapentine group, P < 0.05.
For 5-h 25-desacetyl rifapentine group versus 4-h 25-desacetyl rifapentine group, P < 0.05.
For 5-h rifapentine group versus 24-h and 48-h rifapentine groups, P < 0.05.
FIG. 1.
Plasma concentration-time curve derived from the means of the concentrations in the plasma of the six timed-bronchoscopy groups. See Table 2 for the SDs for each group.
TABLE 3.
Pharmacokinetic parameters for rifapentine in plasma, ELF, and ACs
| Parameter | Plasma | ELF | ACs |
|---|---|---|---|
| LZ (h−1) | 0.038 | 0.033 | 0.053 |
| t1/2-LZ (h) | 18.3 | 20.8 | 13.0 |
| AUC0–∞ (mg · h−1) | 520 | 111 | 133 |
| Cmax (mg) | 26.2 | 3.7 | 5.3 |
| Tmax (h) | 5 | 5 | 7 |
Intrapulmonary rifapentine concentrations.
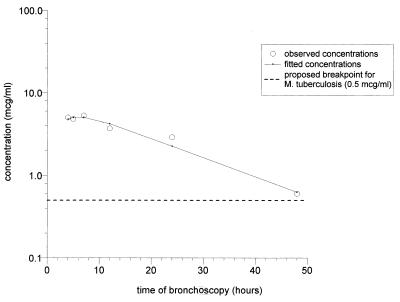
AC rifapentine concentrations (mean ± SD) ranged from a low of 0.6 ± 1.0 μg/ml at 48 h to a high of 5.3 ± 2.5 μg/ml at 7 h and were lower than the corresponding concentrations in plasma at all time periods (Table 4). Intracellular 25-desacetyl rifapentine was detectable at very low concentrations at all time periods except at 7 h, when it was not detectable. The concentrations in ACs declined monoexponentially (r2 = 0.95, log likelihood of the fit = −2.1, residual sum of squares = 0.71), with a t1/2 of 13.0 h and an AUC0–∞ of 133 mg · h ml−1 (Fig. 2 and Table 4). The AUC0–∞ for ACs/AUC0–∞ for plasma ratio was 0.26.
TABLE 4.
Rifapentine and main metabolite (25-desacetyl rifapentine) concentrations in ELF and ACs
| Drug | Mean ± SD concn (μg/ml) for the following group:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 h (n = 5)
|
5 h (n = 5)
|
7 h (n = 5)
|
12 (n = 5)
|
24 (n = 5)
|
48 (n = 5)
|
|||||||
| AC | ELF | AC | ELF | AC | ELF | AC | ELF | AC | ELF | AC | ELF | |
| Rifapentine | 5.0 ± 4.2 | 3.2 ± 2.1 | 4.8 ± 2.4 | 3.7 ± 1.4a | 5.3 ± 2.5b | 2.3 ± 1.1c | 3.7 ± 1.4 | 3.0 ± 1.5 | 2.9 ± 1.3 | 2.0 ± 1.2 | 0.6 ± 1.0b | 0.7 ± 0.7a |
| 25-Desacetyl rifapentine | 0.9 ± 1.1c | 0.1 ± 0.1d | 0.6 ± 0.8 | 0.5 ± 0.5 | 0 | 0.5 ± 0.3 | 0.6 ± 0.6 | 1.8 ± 1.0d | 1.5 ± 1.0 | 0.8 ± 1.0 | 0.6 ± 1.0 | 0.2 ± 0.5 |
For ELF rifapentine concentration in 5-h group versus ELF rifapentine concentration in 48-h group, P < 0.05; all other comparisons were not significant (P > 0.05).
For AC rifapentine concentration in 7-h group versus AC rifapentine concentration in 48-h group, P < 0.05; all other comparisons were not significant (P > 0.05).
None of the differences in AC 25-desacetyl rifapentine concentrations were significant (P > 0.05).
For ELF 25-desacetyl rifapentine concentration in 12-h group versus 25-desacetyl rifapentine concentration in 4-h group, P < 0.05; all other comparisons were not significant.
FIG. 2.
AC concentration-time curve derived from the means of the concentrations in the ACs of the six timed-bronchoscopy groups. See Table 4 for the SDs for each group.
ELF rifapentine concentrations (mean ± SD) ranged from a low of 0.7 ± 0.7 μg/ml at 48 h to a high of 3.7 ± 1.4 μg/ml at 5 h. The concentrations of rifapentine in ELF were less than those in ACs at all time points except 48 h and were less than the corresponding concentrations in plasma. 25-Desacetyl rifapentine was detectable in ELF at low concentrations at all time periods. The concentrations in ELF declined monoexponentially (r2 = 0.80, log likelihood of the fit = −2.4, residual sum of squares = 0.78), with a t1/2 of 20.8 h and an AUC0–∞ of 111 mg · h ml−1 (Fig. 3 and Table 4). The AUC0–∞ for ELF/AUC0–∞ for plasma ratio was 0.21.
FIG. 3.
ELF concentration-time curve derived from the means of the concentrations in the ELF of the six timed-bronchoscopy groups. See Table 4 for the SDs for each group.
DISCUSSION
The observations presented here confirm our previous experiences (6, 7, 9, 10) and those of others (13, 14, 25, 26) that bronchoscopy and BAL for research purposes can safely be carried out with healthy volunteers.
The standardized technique that we use for bronchoscopy and BAL results in adequate and reliable recovery of ACs and ELF. The number of cells (approximately 100 × 106 to 150 × 106), the cell type (approximately 80 to 90% monocytes/macrophages), and the volume of ELF (approximately 1 ml) are similar to the values that we and others have reported previously (1, 2, 4, 7, 10, 26, 29, 33). Used with sensitive and specific drug assay techniques, this procedure permits an accurate estimation of the intrapulmonary drug concentrations in these compartments.
It is noteworthy that the Cmax (26.2 μg/ml), Tmax (5 h), t1/2-LZ (18.3 h), and AUC (520 mg · h ml−1) values derived from this study were very similar to those reported in earlier studies with normal volunteers (19–21).
These data confirm that the long plasma t1/2 of rifapentine also appears to result in a long intrapulmonary t1/2, i.e., 13.0 h in ACs and 20.8 h in ELF. We believe that these estimates of t1/2 are reasonably accurate. They would be improved by additional data obtained at intervals of greater than 48 h. While the drug concentrations and AUC values for ACs and ELF are substantially less than those for plasma, inspection of Fig. 2 and 3 reveals that the intrapulmonary drug concentrations (and the plasma drug concentrations in Fig. 1) resulting from the administration of a single 600-mg dose remain above the MIC breakpoint for Mycobacterium tuberculosis for 48 h. The data suggest a pharmacologic rationale for extended-interval dosing. Whether these pharmacokinetic estimates would be materially affected by the presence of pulmonary tuberculosis is unknown. The optimum dosing interval and the effect of multiple-dose administration on the intrapulmonary pharmacokinetics of rifapentine have not been determined. The relationship between the MIC of rifapentine for M. tuberculosis, dosing interval, and drug effectiveness requires further investigation. The reversible abnormalities in liver function that we observed in this study have been reported previously (17).
We have recently completed a steady-state study of intrapulmonary pyrazinamide concentrations (8). The t1/2-LZ of pyrazinamide is also long and has been reported to be from 9 to 23 h (5, 22, 23, 28, 31). We found that pyrazinamide, unlike rifapentine, is highly concentrated in ELF. After the administration of five daily 1.0-g doses, the concentration in ELF/concentration in plasma ratio at 4 h was 22, whereas the concentration in ELF/concentration in plasma ratio was 0.2 for rifapentine in this study. The concentration in ACs/concentration in plasma ratio at 4 h was 0.6 for pyrazinamide, whereas it was 0.26 for rifapentine.
Determination of whether drugs such as pyrazinamide that achieve greater concentrations in the lungs and that therefore have greater inhibitory or killing ratios are more effective will require controlled trials. In general, high inhibitory or killing ratios are viewed as favorable in the treatment of infectious diseases. It is likely that the prolonged intrapulmonary rifapentine concentrations above the MIC for M. tuberculosis observed in these subjects are in part responsible for this drug's effectiveness for the treatment of pulmonary tuberculosis. The significance of the low (usually below 1.0 μg/ml) but detectable concentrations of rifapentine's main metabolite, 25-desacetyl rifapentine, in ACs and ELF is unknown. This was a single-dose study, and multiple doses may result in higher intrapulmonary concentrations than we observed in the subjects in the present study.
ACKNOWLEDGMENTS
This work was carried out with funds provided by Hoechst Marion Roussel, Inc.
We acknowledge Margareta Andersson for performing the assays and Eve Benton for manuscript preparation.
REFERENCES
- 1.Baldwin D R, Andrews J M, Wise R, Honeybourne D. Bronchoalveolar distribution of cefuroxime axetil and in-vitro efficacy of observed concentrations against respiratory pathogens. J Antimicrob Chemother. 1992;30:377–385. doi: 10.1093/jac/30.3.377. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin D R, Wise R, Andrews J M, Ashby J P, Honeybourne D. Azithromycin concentrations at the sites of pulmonary infection. Eur Respir J. 1990;3:886–890. [PubMed] [Google Scholar]
- 3.Baldwin D R, Wise R, Andrews J M, Gill M, Honeybourne D. Comparative bronchoalveolar concentrations of ciprofloxacin and lomefloxacin following oral administration. Respir Med. 1993;87:595–601. doi: 10.1016/s0954-6111(05)80262-8. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin D R, Wise R, Andrews J M, Honeybourne D. Microlavage: a technique for determining the volume of epithelial lining fluid. Thorax. 1991;46:658–662. doi: 10.1136/thx.46.9.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bareggi S R, Cerutti R, Pirola R, Riva R, Cisternino M. Clinical pharmacokinetics and metabolism of pyrazinamide in healthy volunteers. Arzneimittelforschung. 1987;37:849–854. [PubMed] [Google Scholar]
- 6.Conte, J. E., Jr., J. A. Golden, M. McQuitty, J. Kipps, E. T. Lin, and E. zurlinden. The effect of AIDS and gender on the steady state plasma and intrapulmonary concentrations of ethionamide. Antimicrob. Agents Chemother., in press. [DOI] [PMC free article] [PubMed]
- 7.Conte J E, Jr, Golden J, Duncan S, McKenna E, Lin E, Zurlinden E. Single-dose intrapulmonary pharmacokinetics of azithromycin, clarithromycin, ciprofloxacin, and cefuroxime in volunteer subjects. Antimicrob Agents Chemother. 1996;40:1617–1622. doi: 10.1128/aac.40.7.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conte J E, Jr, Golden J, Duncan S, McKenna E, Zurlinden E. Intrapulmonary concentrations of pyrazinamide. Antimicrob Agents Chemother. 1999;43:1329–1333. doi: 10.1128/aac.43.6.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conte J E, Jr, Golden J A. Intrapulmonary and systemic pharmacokinetics of aerosolized pentamidine used for prophylaxis of Pneumocystis carinii pneumonia in patients infected with the human immunodeficiency virus. J Clin Pharmacol. 1995;35:1166–1173. doi: 10.1002/j.1552-4604.1995.tb04042.x. [DOI] [PubMed] [Google Scholar]
- 10.Conte J E, Jr, Golden J A, Duncan S, McKenna E, Zurlinden E. Intrapulmonary pharmacokinetics of clarithromycin and of erythromycin. Antimicrob Agents Chemother. 1995;39:334–338. doi: 10.1128/aac.39.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhillon J, Dickinson J M, Guy J A, Ng T K, Mitchison D A. Activity of two long-acting rifamycins, rifapentine and FCE 22807, in experimental murine tuberculosis. Tuber Lung Dis. 1992;73:116–123. doi: 10.1016/0962-8479(92)90066-S. [DOI] [PubMed] [Google Scholar]
- 12.Dhillon J, Mitchison D A. Activity in vitro of rifabutin, FCE 22807, rifapentine, and rifampin against Mycobacterium microti and M. tuberculosis and their penetration into mouse peritoneal macrophages. Am Rev Respir Dis. 1992;145:212–214. doi: 10.1164/ajrccm/145.1.212. [DOI] [PubMed] [Google Scholar]
- 13.Ettensohn D B, Jankowski M J, Duncan P G, Lalor P A. Bronchoalveolar lavage in the normal volunteer subject. I. Technical aspects and intersubject variability. Chest. 1988;94:275–280. doi: 10.1378/chest.94.2.275. [DOI] [PubMed] [Google Scholar]
- 14.Ettensohn D B, Jankowski M J, Redondo A A, Duncan P G. Bronchoalveolar lavage in the normal volunteer subject. 2. Safety and results of repeated BAL, and use in the assessment of intrasubject variability. Chest. 1988;94:281–285. doi: 10.1378/chest.94.2.281. [DOI] [PubMed] [Google Scholar]
- 15.Heifets L, Sanchez T, Vanderkolk J, Pham V. Development of rifapentine susceptibility tests for Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1999;43:25–28. doi: 10.1128/aac.43.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heifets L B, Lindholm-Levy P J, Flory M A. Bactericidal activity in vitro of various rifamycins against Mycobacterium avium and Mycobacterium tuberculosis. Am Rev Respir Dis. 1990;141:626–630. doi: 10.1164/ajrccm/141.3.626. [DOI] [PubMed] [Google Scholar]
- 17.Jarvis B, Lamb H M. Rifapentine. Drugs. 1998;56:607–616. doi: 10.2165/00003495-199856040-00008. [DOI] [PubMed] [Google Scholar]
- 18.Kenny M T, Reynolds D L, Brackman M A, Dulworth J K. Comparison of biological and chemical assays for the quantitation of rifapentine in human plasma. Diagn Microbiol Infect Dis. 1997;27:107–111. doi: 10.1016/s0732-8893(97)00027-8. [DOI] [PubMed] [Google Scholar]
- 19.Keung A C, Eller M G, Weir S J. Pharmacokinetics of rifapentine in patients with varying degrees of hepatic dysfunction. J Clin Pharmacol. 1998;38:517–524. doi: 10.1002/j.1552-4604.1998.tb05789.x. [DOI] [PubMed] [Google Scholar]
- 20.Keung A C, Eller M G, Weir S J. Single-dose pharmacokinetics of rifapentine in elderly men. Pharm Res. 1998;15:1286–1291. doi: 10.1023/a:1011960428896. [DOI] [PubMed] [Google Scholar]
- 21.Keung A C, Eller M G, Weir S J. Single-dose pharmacokinetics of rifapentine in women. J Pharmacokinet Biopharm. 1998;26:75–85. doi: 10.1023/a:1023276808298. [DOI] [PubMed] [Google Scholar]
- 22.Lacroix C, Hoang T P, Nouveau J, Guyonnaud C, Laine G, Duwoos H, Lafont O. Pharmacokinetics of pyrazinamide and its metabolites in healthy subjects. Eur J Clin Pharmacol. 1989;36:395–400. doi: 10.1007/BF00558302. [DOI] [PubMed] [Google Scholar]
- 23.Lacroix C, Tranvouez J L, Phan H T, Duwoos H, Lafont O. Pharmacokinetics of pyrazinamide and its metabolites in patients with hepatic cirrhotic insufficiency. Arzneimittelforschung. 1990;40:76–79. [PubMed] [Google Scholar]
- 24.Leonard T. Why do we need significance levels? MRC Technical Report. University of Wisconsin, Madison; 1979. [Google Scholar]
- 25.Limper A H, Specks U, Brutinel W M, Martin W J, Rohrbach M S. Interlobar variation in the recovery of bronchoalveolar lavage fluid, cell populations, and angiotensin-converting enzyme in normal volunteers. J Lab Clin Med. 1993;121:785–791. [PubMed] [Google Scholar]
- 26.Merchant R K, Schwartz D A, Helmers R A, Dayton C S, Hunninghake G W. Bronchoalveolar lavage cellularity. The distribution in normal volunteers. Am Rev Respir Dis. 1992;146:448–453. doi: 10.1164/ajrccm/146.2.448. [DOI] [PubMed] [Google Scholar]
- 27.Mor N, Simon B, Mezo N, Heifets L. Comparison of activities of rifapentine and rifampin against Mycobacterium tuberculosis residing in human macrophages. Antimicrob Agents Chemother. 1995;39:2073–2077. doi: 10.1128/aac.39.9.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peloquin C A, Bulpitt A E, Jaresko G S, Jelliffe R W, James G T, Nix D E. Pharmacokinetics of pyrazinamide under fasting conditions, with food, and with antacids. Pharmacotherapy. 1998;18:1205–1211. [PubMed] [Google Scholar]
- 29.Rennard S I, Basset G, Lecossier D, O'Donnell K M, Pinkston P, Martin P G, Crystal R G. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol. 1986;60:532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz G. Estimating the dimensions of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- 31.Stamatakis G, Montes C, Trouvin J H, Farinotti R, Fessi H, Kenouch S, Mery J P. Pyrazinamide and pyrazinoic acid pharmacokinetics in patients with chronic renal failure. Clin Nephrol. 1988;30:230–234. [PubMed] [Google Scholar]
- 32.Talke H S G E. Enzymatische Harnstoffbestimmung im Blut und Serum im optischem test nach Warburg. Klin Wochschr. 1965;43:174. doi: 10.1007/BF01484513. [DOI] [PubMed] [Google Scholar]
- 33.Willcox M, Kervitsky A, Watters L C, King T E J. Quantification of cells recovered by bronchoalveolar lavage. Comparison of cytocentrifuge preparations with the filter method. Am Rev Respir Dis. 1988;138:74–80. doi: 10.1164/ajrccm/138.1.74. [DOI] [PubMed] [Google Scholar]
- 34.Zar J H. Multisample hypotheses: the analysis of variance. Multiple comparisons. In: Zar J H, editor. Biostatistical analysis. Englewood Cliffs, N.J: Prentice-Hall, Inc.; 1984. pp. 162–205. [Google Scholar]