Abstract
Deep eutectic solvents (DES) are a new class of green solvents that have shown unique properties in several process applications. This study evaluates nonionic DES containing phenolic alcohols as solvents for carbon dioxide (CO2) capture applications. Potential phenolic alcohols and the molar ratio between DES constituents were preselected for experimental investigations based on the conductor-like screening model for realistic solvation (COSMO-RS). CO2 solubility was experimentally determined in two different DES, namely, L-menthol/thymol in 1:2 molar ratio and thymol/2,6-xylenol in 1:1 molar ratio, at various temperatures and pressures. CO2 solubility in the studied systems was higher than that reported in the literature for ionic DES and ionic liquids. This study demonstrates that nonionic DES containing phenolic alcohols can be excellent, inexpensive, and simple solvents for CO2 capture.
Keywords: CO2 capture, COSMO-RS, ionic liquids, hydrophobic deep eutectic solvents, green solvents
Introduction
An increasing relevance has developed in removing carbon dioxide (CO2) from gas mixtures, such as flue gas or biogas. Absorption in liquid solvents, in addition to membrane and absorption-based processes, is a major technology in this field. For many years, aqueous solutions of amines have been used for CO2 absorption. However, they suffer from some drawbacks, such as high vapor pressure, which causes evaporation during solvent regeneration. Recently, ionic liquids (IL) have drawn attention for CO2 capture application because of their negligible vapor pressure (Albo et al., 2010; Shiflett et al., 2010). Furthermore, the properties of IL can be tailored to the requirements of the specific application by combining different cations and anions (Jork et al., 2005). Although the hygroscopicity of IL can be overcome by using polymerized IL to prepare membranes for CO2 capture applications (Kammakakam et al., 2020; Galiano et al., 2021), the issues of IL cost, instability, and purity remain (Sowmiah et al., 2009).
Nevertheless, owing to the aforementioned problems of IL, researchers have focused on an alternative solvent class that has some similarities to IL, while avoiding some of their drawbacks. Deep eutectic solvents (DES) are eutectic mixtures with a large depression in the eutectic temperature obtained by mixing a hydrogen bond acceptor and donor. DES are a new class of designer solvents, which can also be prepared by simple mixing of natural and nontoxic components, usually referred to as Natural DES (NADES) (Smith et al., 2014; van Osch et al., 2020). Similar to IL, physicochemical properties of DES can be tuned by selecting their constituents and additionally molar ratios of those. Moreover, DES are easier and less expensive to prepare compared to IL. Therefore, more attention is directed to their use in several process applications, for example, in liquid–liquid chromatography (Roehrer et al., 2016; Bezold and Minceva, 2019; Cai and Qiu, 2019), extraction of bioactive compounds (Kalhor and Ghandi, 2019; Makoś et al., 2020; Perna et al., 2020; Rodríguez-Llorente et al., 2020; Fernández et al., 2022), and crystallization (Emami and Shayanfar, 2020; Hall et al., 2020; Hamilton et al., 2020; Potticary et al., 2020).
CO2 capture is one application that can benefit from DES. Existing studies have demonstrated the potential of DES for CO2 capture (Krishnan et al., 2020; Song et al., 2020; Wazeer et al., 2021a; Wazeer et al., 2021b). However, most studies investigating CO2 capture in DES proposed using ionic DES, which has the same drawbacks as those related to IL, especially in terms of hygroscopicity. Recently, hydrophobic DES based on natural and inexpensive nonionic constituents has attracted much attention (van Osch et al., 2019). Hydrophobic DES containing L-menthol possess outstanding properties, such as low viscosity and eutectic temperatures, especially when L-menthol is mixed with phenolic alcohols, such as thymol or carvacrol (Alhadid et al., 2020a; Alhadid et al., 2020b; Alhadid et al., 2021a; Alhadid et al., 2021b). Phenolic IL are good solvents for CO2 capture applications (Vafaeezadeh et al., 2015). Therefore, hydrophobic DES containing phenolic alcohols are assumed to be promising candidates for CO2 capture applications.
Nevertheless, the large pool of substances that can form DES can make selecting the DES constituents challenging. Furthermore, the ratio between the constituents can be tuned, which is an additional degree of freedom during the selection of DES constituents. Therefore, a predictive screening method could noticeably assist in preselecting DES constituents for CO2 capture applications. The conductor-like screening model for realistic solvation (COSMO-RS) is a predictive thermodynamic model based on quantum mechanics and statistical thermodynamics (Klamt, 1995; Klamt et al., 1998; Eckert and Klamt, 2002). COSMO-RS successfully provides qualitative predictions for screening IL and ionic DES for gas capture applications (Völkl et al., 2012; Song et al., 2020; Liu et al., 2021; Qin et al., 2021).
This study investigates nonionic DES containing phenolic alcohols as potential solvents for CO2 capture. COSMO-RS was used to screen a list of possible DES containing L-menthol and phenolic alcohols to preselect those with the highest CO2 solubility. Further, CO2 solubility was experimentally investigated for selected DES systems at various temperatures and pressures using an isochoric method.
Materials and Methods
Eutectic Mixture Preparation
Pure components (L-menthol, purity ≥99%, Sigma Aldrich; thymol, purity ≥99%, Sigma Aldrich; 2,6-xylenol, purity 99%, Acros Organics), was mixed under continuous stirring and gentle heating until a clear homogenous liquid was formed. The water content of the prepared eutectic mixtures was measured in triplicate using Karl Fischer Coulometer (Hanna Instrument, United States), and the results are shown in Table 1.
TABLE 1.
Prepared eutectic mixtures, their molar ratio, and water content.
| Eutectic mixture | Mole ratio | Water content/ppm |
|---|---|---|
| L-menthol/thymol | 1:2 | 144.2 ± 2.1 |
| Thymol/2,6-xylenol | 1:1 | 108.4 ± 6.7 |
Carbon Dioxide Solubility Experiments
Before measurements, DES were degassed under vacuum for 48 h at a temperature of T = 413.15 K. After degassing, no mass loss was observed for the DES, indicating that there was no change in the stoichiometry between constituents and their negligible volatility under the experimental conditions. CO2 from Westfalen AG, Germany, with a purity of 99.995% (quality 4.5), was used without further purification. The experiments were performed using a pressure-drop isochoric method at various temperatures and pressures. The apparatus and operational procedures of solubility measurements are described in detail in previous studies (Safarov et al., 2013; Safarov et al., 2014). For the current measurements, the installation was used without modification. The temperature in the measuring cell was held constant (at T = 293.15–323.15 K) with an uncertainty of = 0.030 K. A pressure transducer with an accuracy of 0.1% was used to measure the pressure of CO2 filled in the gas reservoir. The temperature inside the gas reservoir was measured with an uncertainty of = 0.015 K. The initial amount of CO2 in the gas reservoir was determined from its pressure and temperature using the Span and Wagner equation of state (Span and Wagner, 1996).
To determine the concentration of CO2 in the solution, liquid and gas densities under experimental temperature and pressure were required. The density of DES was measured using a density meter (Density meter Easy D40, Mettler-Toledo GmbH, Germany), and the results are indicated in Table 2. The mass of CO2 in the gas phase was calculated using its density and volume. The gas volume in the cell was found by deducting the liquid volume from the total cell volume. The increase in the liquid volume because of dissolved CO2 was neglected (Safarov et al., 2013).
TABLE 2.
Density of eutectic mixtures measured in this work a .
| T/K | ρ/kg m−3 | |
|---|---|---|
| L-menthol/thymol (1:2) | Thymol/2,6-xylenol (1:1) | |
| 293.15 | 947.6 | 992.2 |
| 303.15 | 940.0 | 983.9 |
| 313.15 | 932.5 | 975.6 |
| 323.15 | 924.8 | 967.2 |
Standard uncertainty u(ρ) = 0.05 kg m−3
Correlation of the Gas Solubility
Henry’s law for a binary system (liquid + CO2) for a non-ideal gas phase can be given as (Prausnitz and Shair, 1961)
| (1) |
where and are the mole fraction of CO2 in the gas and liquid phases, respectively; is the fugacity coefficient of CO2 in the gas phase, and is Henry’s constant. Due to the negligible vapor pressure of the studied eutectic mixtures at studied temperatures (Xin et al., 2021) (see also Supplementary Table S1 in Supplementary Material for calculations), the gas phase can be assumed as pure CO2, i.e., (see also Supplementary Table S1 in Supplementary Material for calculations). Henry’s constant can be defined using the following equation.
| (2) |
COSMO-RS Calculations
The solubility of CO2 in eutectic mixtures was screened a priori by evaluating CO2 activity coefficients at infinite dilution. The activity coefficients of CO2 at infinite dilution in different pure constituents and eutectic mixtures were calculated using the COSMO-RS model (BIOVIA COSMOtherm X19, Dassault Systèmes) and BP_TZVP_19. ctd parameters. Molecular conformations of components were obtained using BIOVIA COSMOconf 17 (Dassault Systèmes). The geometry optimization and screening charge density were determined by density functional theory calculations using BP86 functional and def-TZVP basis set by Turbomole version 6.6 (TURBOMOLE GmbH).
Results and Discussion
Screening With COSMO-RS
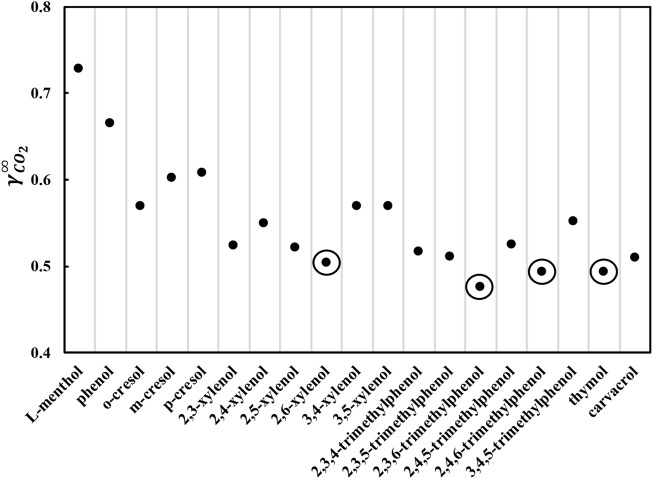
To enable the usage of a DES in CO2 capture applications, the DES should 1) be liquid in the appropriate temperature range, i.e., approximately room temperature; and 2) be a good solvent for CO2. A number of nonionic DES containing phenolic alcohols can satisfy these two criteria. The phenolic alcohols considered in this study are phenol, methylphenols (cresols), dimethylphenols (xylenols), trimethylphenols, and two natural phenolic terpenes, thymol (2-isopropyl-5-methylphenol) and carvacrol (5-isopropyl-2-methylphenol). Phenolic alcohols with higher molecular weights or dihydroxy benzenes were not considered because they are not expected to form liquid DES at room temperature because of their high melting temperature and enthalpy (Alhadid et al., 2019). When phenolic alcohols are mixed with L-menthol, a significant negative deviation from the ideal behavior is expected (Alhadid et al., 2020b; Alhadid et al., 2021a). The negative deviation from the ideal behavior results in the formation of DES with a sufficiently low melting temperature. Thus, it is desirable to have L-menthol as a DES constituent. First, the activity coefficients of CO2 at infinite dilution were calculated using COSMO-RS in pure constituents at 293.15 K because the physicochemical properties of pure constituents influence the physicochemical properties of DES (Alhadid et al., 2021b), and the results are shown in Figure 1. As COSMO-RS is based on quantum mechanical calculations, the calculated activity coefficients implicitly include the atomistic rationalization and quantify the intermolecular interactions between CO2 and the constituents in the liquid phase. The low CO2 activity coefficient values indicate strong intermolecular interactions between CO2 and the constituents and, accordingly, high CO2 solubility. The calculated CO2 activity coefficients in all phenolic alcohols are lower than in L-menthol (cyclohexyl alcohol) (Figure 1), proving that CO2 solubility is relatively high in phenolic alcohols.
FIGURE 1.
Activity coefficients at infinite dilution of carbon dioxide in pure L-menthol and various phenolic alcohols at 293.15 K calculated by COSMO-RS.
Figure 1 shows that the limiting activity coefficients decrease with the addition of methyl groups to phenolic alcohols. The general order of the CO2 activity coefficients in phenolic alcohols is phenol > methylphenol (cresol) > dimethylphenol (xylenol) > trimethylphenols. Furthermore, compared to thymol and carvacrol, substituting a methyl group with an isopropyl group, i.e., 2,5-xylenol, decreases CO2 limiting activity coefficients. By comparing different isomers, the limiting activity coefficients are lower in isomers with methyl groups at position 2, i.e., close to the hydroxyl group. Furthermore, the lowest values of CO2 activity coefficients are observed in dimethyl and trimethyl isomers with a methyl group on the two and six positions, respectively. The CO2 activity coefficient in thymol is lower than that in carvacrol, as the isopropyl group is nearer to the hydroxyl group in thymol than to carvacrol. Therefore, the structure of the phenolic alcohol influences CO2 solubility. The four marked phenolic alcohols with the lowest limiting activity coefficient values in CO2 were considered potential DES constituents for further screening.
Further, potential DES containing L-menthol with 2,6-xylenol, thymol, 2,3,6-trimethylphenol, and 2,4,6-trimethylphenol were screened using COSMO-RS. The calculated activity coefficients of CO2 at infinite dilution in five different DES and ratios between the constituents at 293.15 K are shown in Figure 2. CO2 activity coefficients in L-menthol-based DES at any molar ratio are in the order L-menthol:thymol (MTH) > L-menthol:2,6-xylenol (M26X) > L-menthol:2,4,6-trimethylphenol (M246) > L-menthol:2,3,6-trimethylphenol (M236) (Figure 2), which is consistent with the order of CO2 activity coefficients in the pure phenolic alcohols present in the DES (see Figure 1). Moreover, increasing the molar ratio of the phenolic alcohol to L-menthol decreases CO2 activity coefficients. Therefore, it is logical to select L-menthol-based DES containing trimethylphenols in a 1:2 ratio between the constituents as potential solvents for CO2 capture. However, melting properties of pure constituents influence the melting temperature of the DES (Alhadid et al., 2019). M236 and M246 in 1:2 ratio are solid at room temperature, which is attributed to the high melting temperature of 2,3,6- and 2,4,6- trimethylphenols (Tm = 331.2 and 342.15 K, respectively) (Verevkin, 1999). MTH and M26X in 1:2 ratio are liquid at room temperature (Alhadid et al., 2021a), which indicates that both can be considered for further experimental investigation. CO2 activity coefficients in MTH and M26X at 1:2 ratio are of similar values (Figure 2). However, MTH is considered a better option because of the low toxicity of thymol compared to 2,6-xylenol. Therefore, MTH was chosen for measurements.
FIGURE 2.
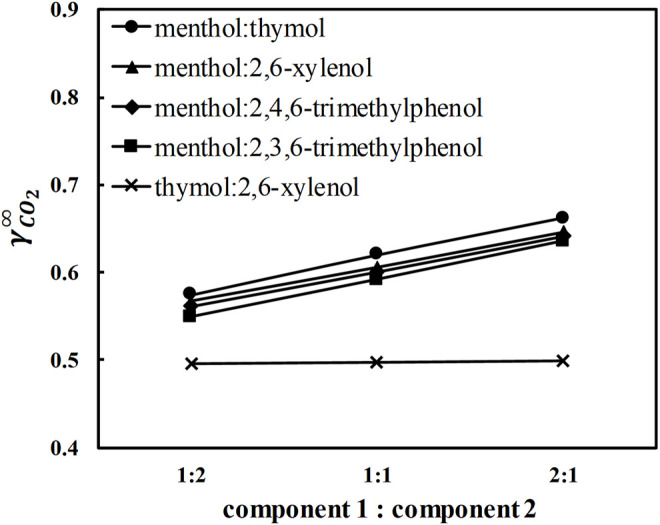
Activity coefficients at infinite dilution of carbon dioxide in selected eutectic mixtures at 293.15 K calculated by COSMO-RS.
Next, eutectic mixtures containing two phenolic alcohols were considered. These eutectic mixtures are expected to show ideal solution behavior with no significant decrease in the melting temperature of the mixture relative to pure constituents (Alhadid et al., 2019). For such mixtures, the melting temperature of the DES at any ratio between constituents can be obtained from the solid–liquid phase diagram based on the pure constituent melting properties. A brief explanation of solid–liquid equilibrium calculations is given in the Supplementary Material. Based on the ideal solution model calculations, thymol: 2,6-xylenol (T26X) eutectic system should form a liquid mixture at room temperature. The calculated eutectic composition and temperature for T26X are xe,thymol = 0.46, and Te = 292.8 K, respectively (see Supplementary Material for details about the calculations). Altering the ratio between constituents in T26X does not influence CO2 activity coefficients (Figure 2), in contrast to what is observed in L-menthol-based DES. Thus, the molar ratio close to the eutectic ratio of the T26X system (∼1:1 ratio) was selected to ensure that the mixture is liquid at room temperature. Eventually, the two DES, MTH in 1:2 ratio, and T26X in 1:1 ratio were selected for the solubilty measurements.
Experimental Solubility
CO2 solubility in the two DES was measured based on the pressure-drop isochoric method at four different pressures (∼4, 3, 2, and 1 MPa) and four different temperatures (293.15, 303.15, 313.15, and 323.15 K). The CO2 solubility in weight percentage and mole fraction , as well as its fugacity coefficient calculated using Span and Wagner equation of state for the MTH and T26X system are shown in Tables 3, 4, respectively. The values of Henry’s constant at different temperatures calculated using Eq. 2 are shown in Table 5. As one would expect, the solubility of CO2 in the two studied systems decreases as temperature increases.
TABLE 3.
Experimental carbon dioxide (CO2) solubility (both in weight percent and in mole fraction ) in the L-menthol/thymol (MTH) eutectic system and calculated CO2 fugacity coefficient at various temperatures T and pressures p. a
| T/K | p/MPa | % | /Mole fraction | b |
|---|---|---|---|---|
| 323.11 | 4.1939 | 11.410 | 0.3082 | 0.8453 |
| 313.12 | 4.1543 | 12.090 | 0.3224 | 0.8295 |
| 303.18 | 4.0967 | 13.409 | 0.3488 | 0.8121 |
| 293.15 | 4.0201 | 15.769 | 0.3931 | 0.7931 |
| 323.15 | 3.5059 | 9.290 | 0.2616 | 0.8701 |
| 313.16 | 3.4703 | 10.173 | 0.2815 | 0.8570 |
| 303.13 | 3.4247 | 11.780 | 0.3159 | 0.8426 |
| 293.13 | 3.3691 | 13.335 | 0.3474 | 0.8265 |
| 323.14 | 2.5790 | 7.090 | 0.2088 | 0.9039 |
| 313.18 | 2.5541 | 7.615 | 0.2219 | 0.8942 |
| 303.13 | 2.5229 | 8.347 | 0.2395 | 0.8835 |
| 293.14 | 2.4860 | 9.297 | 0.2617 | 0.8716 |
| 323.02 | 1.6819 | 4.644 | 0.1442 | 0.9369 |
| 313.18 | 1.6662 | 5.027 | 0.1547 | 0.9305 |
| 302.98 | 1.6478 | 5.436 | 0.1659 | 0.9235 |
| 293.15 | 1.6281 | 5.937 | 0.1792 | 0.9155 |
Standard uncertainties u are: u(T) = 0.030 K, u(p) = 0.1%, u(w) = 0.01 wt%, and u(x) = 0.0001 mol fraction.
Calculated using the Span and Wagner equation of state (Span and Wagner, 1996) implemented in ThermoFluids (Springer, V. 1.0).
TABLE 4.
Experimental carbon dioxide (CO2) solubility (both in weight percent and in mole fraction ) in the thymol/2,6-xylenol (T26X) eutectic system and CO2 calculated fugacity coefficient at various temperatures T and pressures p. a
| T/K | p/MPa | % | /Mole fraction | b |
|---|---|---|---|---|
| 322.90 | 4.0853 | 11.549 | 0.2878 | 0.8492 |
| 313.13 | 4.0441 | 12.563 | 0.3078 | 0.8339 |
| 303.17 | 3.9840 | 14.856 | 0.3506 | 0.8172 |
| 293.22 | 3.9057 | 17.093 | 0.3895 | 0.7990 |
| 323.14 | 3.3380 | 9.512 | 0.2455 | 0.8762 |
| 313.15 | 3.3029 | 10.417 | 0.2646 | 0.8638 |
| 303.13 | 3.2563 | 11.456 | 0.2859 | 0.8502 |
| 293.14 | 3.1988 | 13.073 | 0.3176 | 0.8352 |
| 323.13 | 2.6988 | 7.477 | 0.2000 | 0.8995 |
| 313.15 | 2.6696 | 8.022 | 0.2125 | 0.8895 |
| 303.16 | 2.6344 | 9.205 | 0.2388 | 0.8784 |
| 293.15 | 2.5932 | 10.284 | 0.2619 | 0.8661 |
| 323.14 | 1.6670 | 4.462 | 0.1263 | 0.9375 |
| 313.15 | 1.6500 | 4.909 | 0.1377 | 0.9313 |
| 303.16 | 1.6300 | 5.398 | 0.1501 | 0.9243 |
| 293.15 | 1.6070 | 6.057 | 0.1663 | 0.9166 |
Standard uncertainties u are: u(T) = 0.030 K, u(p) = 0.1%, u(w) = 0.01 wt%, and u(x) = 0.0001 mol fraction.
Calculated using the Span and Wagner equation of state (Span and Wagner, 1996) implemented in ThermoFluids (Springer, V. 1.0).
TABLE 5.
Calculated carbon dioxide Henry’s constant in the studied systems.
| T/K | /MPa | |
|---|---|---|
| L-menthol/thymol (1:2) | Thymol/2,6-xylenol (1:1) | |
| 293.15 | 8.52 ± 0.13 | 9.45 ± 0.32 |
| 303.15 | 8.98 ± 0.13 | 10.45 ± 0.23 |
| 313.15 | 9.61 ± 0.25 | 11.46 ± 0.15 |
| 323.15 | 10.51 ± 0.30 | 12.52 ± 0.17 |
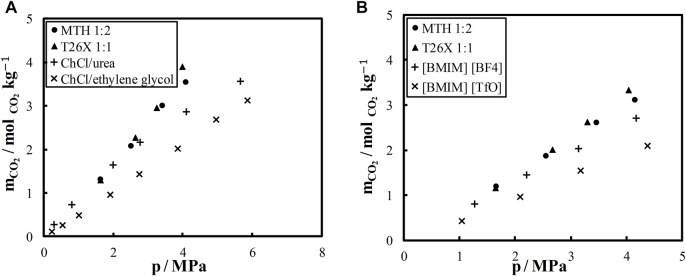
Further, the CO2 solubility in the studied DES was compared with that in some ionic DES and IL reported in the literature. The comparison was made in terms of molality, i.e., moles of CO2 absorbed per mass of solvent. The results are shown in Figure 3. CO2 solubility at 303.15 K in two ionic DES, namely, choline chloride (ChCl)/urea and ChCl/ethylene glycol in 1:2 molar ratio, is compared with the two DES from this study in Figure 3A. As seen, CO2 solubility is significantly higher in nonionic DES than in ionic DES, especially at high pressures (Figure 3A). The CO2 solubility in MTH and T26X is also higher than in the two IL (BMIM) (BF4) and (BMIM) (TfO), as shown in Figure 3B. In addition to good CO2 solubility, nonionic DES are more stable, less hygroscopic, and less expensive than IL and ionic DES.
FIGURE 3.
Carbon dioxide solubility (A) in L-menthol/thymol (MTH), thymol/2,6-xylenol (T26X), choline chloride (ChCl)/urea (Leron et al., 2013), and ChCl/ethylene glycol (Leron and Li, 2013) at 303.15 K (B) and in MTH, T26X (BIMI) (BF4), and (BMIM) (TfO) (Aki et al., 2004) at 313.15 K.
The solvent capacity to absorb CO2 is not the only selection criterion to consider when selecting a solvent for CO2 capture applications. The temperature dependence of CO2 solubility is critical as well because CO2 should be absorbed with high solubility and desorbed from the solvent at a higher temperature for solvent regeneration. Thus, a strong temperature dependence is required for CO2 solubility to reduce the energy demand for desorption. The temperature dependence of CO2 solubility in the two DES being studied is shown in Figure 4. The T26X system shows a slightly higher CO2 solubility than the MTH system. Furthermore, the T26X system has a stronger temperature dependence. Therefore, the T26X system can be identified as a very promising candidate for CO2 capture applications.
FIGURE 4.
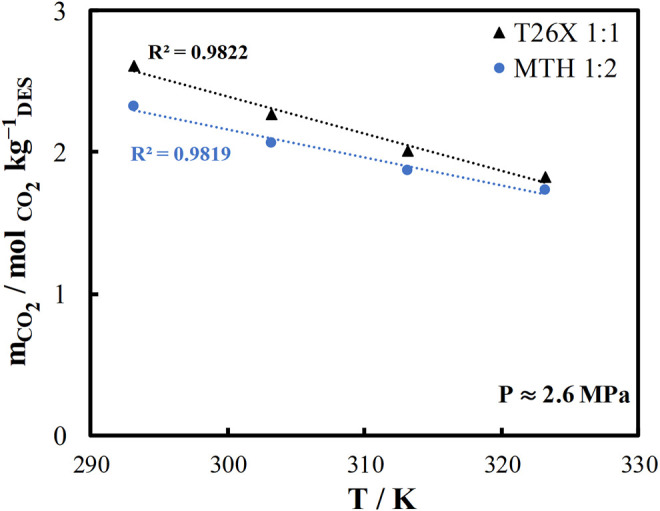
Temperature dependence of carbon dioxide solubility at medium pressure ∼2.6 MPa in L-menthol/thymol (MTH) and thymol/2,6-xylenol (T26X).
Conclusion
This study examines the use of nonionic DES for CO2 capture applications. DES were designed to contain phenolic alcohols for improving CO2 solubility and L-menthol to decrease the melting temperature of the DES. COSMO-RS was used to preselect the DES constituents from a pool of possible phenolic alcohols and to tune the molar ratio between the constituents. It was found that the structure of phenolic alcohols can influence CO2 solubility. Furthermore, increasing the phenolic alcohol molar content in the DES can enhance the CO2 solubility. However, the selection of the constituents and their molar ratio was restricted by the melting temperature of the DES. The COSMO-RS screening results identified two potential DES: MTH in 1:2 molar ratio and T26X in 1:1 molar ratio.
In the two preselected DES, the CO2 solubility was studied experimentally using a pressure-drop isochoric method at various temperatures and pressures. The high experimentally determined CO2 solubility in the two DES validated the COSMO-RS preselection, demonstrating the model’s advantage as a screening tool. CO2 solubility in the DES proposed in this study is significantly higher than in ionic DES and IL proposed in the literature. Furthermore, the temperature dependence of CO2 solubility in DES proves their suitability for CO2 capture applications. The high solubility of CO2 at lower temperatures and its high decrease at higher temperatures make the two DES promising candidates for CO2 capture. This study shows that simple and widely available organic substances can be used to form novel solvents with unique properties.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
Conceptualization: AA; Investigation: AA and JS; Formal Analysis: AA, JS, and LM; Writing–Original Draft: AA and JS; Writing–Review and Editing: LM, KM, and MM; Supervision: KM and MM.
Funding
This work was supported by the German Research Foundation (DFG) and the Technical University of Munich (TUM) in the framework of the Open Access Publishing Program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.864663/full#supplementary-material
References
- Aki S. N. V. K., Mellein B. R., Saurer E. M., Brennecke J. F. (2004). High-Pressure Phase Behavior of Carbon Dioxide with Imidazolium-Based Ionic Liquids. J. Phys. Chem. B. 108 (52), 20355-20365. 10.1021/jp046895+ [DOI] [Google Scholar]
- Albo J., Luis P., Irabien A. (2010). Carbon Dioxide Capture from Flue Gases Using a Cross-Flow Membrane Contactor and the Ionic Liquid 1-Ethyl-3-Methylimidazolium Ethylsulfate. Ind. Eng. Chem. Res. 49 (21), 11045–11051. 10.1021/ie1014266 [DOI] [Google Scholar]
- Alhadid A., Jandl C., Mokrushina L., Minceva M. (2021a). Experimental Investigation and Modeling of Cocrystal Formation in L-Menthol/Thymol Eutectic System. Cryst. Growth Des. 21 (11), 6083–6091. 10.1021/acs.cgd.1c00306 [DOI] [Google Scholar]
- Alhadid A., Mokrushina L., Minceva M. (2020a). Design of Deep Eutectic Systems: A Simple Approach for Preselecting Eutectic Mixture Constituents. Molecules 25 (5), 1077. 10.3390/molecules25051077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadid A., Mokrushina L., Minceva M. (2020b). Formation of Glassy Phases and Polymorphism in Deep Eutectic Solvents. J. Mol. Liquids 314, 113667. 10.1016/j.molliq.2020.113667 [DOI] [Google Scholar]
- Alhadid A., Mokrushina L., Minceva M. (2021b). Influence of the Molecular Structure of Constituents and Liquid Phase Non-ideality on the Viscosity of Deep Eutectic Solvents. Molecules 26 (14), 4208. 10.3390/molecules26144208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadid A., Mokrushina L., Minceva M. (2019). Modeling of Solid-Liquid Equilibria in Deep Eutectic Solvents: A Parameter Study. Molecules 24 (12), 2334. 10.3390/molecules24122334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezold F., Minceva M. (2019). A Water-free Solvent System Containing an L-Menthol-Based Deep Eutectic Solvent for Centrifugal Partition Chromatography Applications. J. Chromatogr. A 1587, 166–171. 10.1016/j.chroma.2018.11.083 [DOI] [PubMed] [Google Scholar]
- Cai T., Qiu H. (2019). Application of Deep Eutectic Solvents in Chromatography: A Review. Trac Trends Anal. Chem. 120, 115623. 10.1016/j.trac.2019.115623 [DOI] [Google Scholar]
- Eckert F., Klamt A. (2002). Fast Solvent Screening via Quantum Chemistry: COSMO-RS Approach. Aiche J. 48 (2), 369–385. 10.1002/aic.690480220 [DOI] [Google Scholar]
- Emami S., Shayanfar A. (2020). Deep Eutectic Solvents for Pharmaceutical Formulation and Drug Delivery Applications. Pharm. Develop. Techn. 25 (7), 779–796. 10.1080/10837450.2020.1735414 [DOI] [PubMed] [Google Scholar]
- Fernández A., Longo M. A., Deive F. J., Álvarez M. S., Rodríguez A. (2021). Effective Lipase Extraction: Designing a Natural Liquid Support for Immobilization. Separat. Purif. Techn. 278, 119601. 10.1016/j.seppur.2021.119601 [DOI] [Google Scholar]
- Galiano F., Mancuso R., Guazzelli L., Mauri M., Chiappe C., Simonutti R., et al. (2021). Phosphonium Ionic Liquid-Polyacrylate Copolymer Membranes for Improved CO2 Separations. J. Membr. Sci. 635, 119479. 10.1016/j.memsci.2021.119479 [DOI] [Google Scholar]
- Hall C. L., Potticary J., Hamilton V., Gaisford S., Buanz A., Hall S. R. (2020). Metastable Crystalline Phase Formation in Deep Eutectic Systems Revealed by Simultaneous Synchrotron XRD and DSC. Chem. Commun. 56 (73), 10726–10729. 10.1039/d0cc04696e [DOI] [PubMed] [Google Scholar]
- Hamilton V., Andrusenko I., Potticary J., Hall C., Stenner R., Mugnaioli E., et al. (2020). Racemic Conglomerate Formation via Crystallization of Metaxalone from Volatile Deep Eutectic Solvents. Cryst. Growth Des. 20 (7), 4731–4739. 10.1021/acs.cgd.0c00497 [DOI] [Google Scholar]
- Jork C., Kristen C., Pieraccini D., Stark A., Chiappe C., Beste Y. A., et al. (2005). Tailor-made Ionic Liquids. The J. Chem. Thermodynamics 37 (6), 537–558. 10.1016/j.jct.2005.04.013 [DOI] [Google Scholar]
- Kalhor P., Ghandi K. (2019). Deep Eutectic Solvents for Pretreatment, Extraction, and Catalysis of Biomass and Food Waste. Molecules 24 (22), 4012. 10.3390/molecules24224012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammakakam I., Bara J. E., Jackson E. M., Lertxundi J., Mecerreyes D., Tomé L. C. (2020). Tailored CO2-Philic Anionic Poly(ionic Liquid) Composite Membranes: Synthesis, Characterization, and Gas Transport Properties. ACS Sustain. Chem. Eng. 8 (15), 5954–5965. 10.1021/acssuschemeng.0c00327 [DOI] [Google Scholar]
- Klamt A. (1995). Conductor-like Screening Model for Real Solvents: A New Approach to the Quantitative Calculation of Solvation Phenomena. J. Phys. Chem. 99 (7), 2224–2235. 10.1021/j100007a062 [DOI] [Google Scholar]
- Klamt A., Jonas V., Bürger T., Lohrenz J. C. W. (1998). Refinement and Parametrization of COSMO-RS. J. Phys. Chem. A. 102 (26), 5074–5085. 10.1021/jp980017s [DOI] [Google Scholar]
- Krishnan A., Gopinath K. P., Vo D.-V. N., Malolan R., Nagarajan V. M., Arun J. (2020). Ionic Liquids, Deep Eutectic Solvents and Liquid Polymers as Green Solvents in Carbon Capture Technologies: A Review. Environ. Chem. Lett. 18 (6), 2031–2054. 10.1007/s10311-020-01057-y [DOI] [Google Scholar]
- Leron R. B., Li M. H. (2013). Solubility of carbon dioxide in a choline chloride–ethylene glycol based deep eutectic solvent. Thermochimica Acta 551, 14-19. 10.1016/j.tca.2012.09.041 [DOI] [Google Scholar]
- Leron R. B., Caparanga A., Li M. H. (2013). Carbon Dioxide Solubility in a Deep Eutectic Solvent Based on Choline Chloride and urea at T = 303.15–343.15K and Moderate Pressures. J. Taiwan Inst. Chem. Eng. 44 (6), 879–885. 10.1016/j.tca.2012.09.041 [DOI] [Google Scholar]
- Liu Y., Dai Z., Zhang Z., Zeng S., Li F., Zhang X., et al. (2021). Ionic Liquids/deep Eutectic Solvents for CO2 Capture: Reviewing and Evaluating. Green. Energ. Environ. 6 (3), 314–328. 10.1016/j.gee.2020.11.024 [DOI] [Google Scholar]
- Makoś P., Słupek E., Gębicki J. (2020). Hydrophobic Deep Eutectic Solvents in Microextraction Techniques-A Review. Microchemical J. 152, 104384. 10.1016/j.microc.2019.104384 [DOI] [Google Scholar]
- Perna F. M., Vitale P., Capriati V. (2020). Deep Eutectic Solvents and Their Applications as Green Solvents. Curr. Opin. Green Sustain. Chem. 21, 27–33. 10.1016/j.cogsc.2019.09.004 [DOI] [Google Scholar]
- Potticary J., Hall C., Hamilton V., McCabe J. F., Hall S. R. (2020). Crystallization from Volatile Deep Eutectic Solvents. Cryst. Growth Des. 20 (5), 2877–2884. 10.1021/acs.cgd.0c00399 [DOI] [Google Scholar]
- Prausnitz J. M., Shair F. H. (1961). A Thermodynamic Correlation of Gas Solubilities. Aiche J. 7 (4), 682–687. 10.1002/aic.690070430 [DOI] [Google Scholar]
- Qin H., Wang Z., Zhou T., Song Z. (2021). Comprehensive Evaluation of COSMO-RS for Predicting Ternary and Binary Ionic Liquid-Containing Vapor-Liquid Equilibria. Ind. Eng. Chem. Res. 60 (48), 17761–17777. 10.1021/acs.iecr.1c03940 [DOI] [Google Scholar]
- Rodríguez-Llorente D., Cañada-Barcala A., Álvarez-Torrellas S., Águeda V. I., García J., Larriba M. (2020). A Review of the Use of Eutectic Solvents, Terpenes and Terpenoids in Liquid-Liquid Extraction Processes. Processes 8 (10), 1220. 10.3390/pr8101220 [DOI] [Google Scholar]
- Roehrer S., Bezold F., García E. M., Minceva M. (2016). Deep Eutectic Solvents in Countercurrent and Centrifugal Partition Chromatography. J. Chromatogr. A 1434, 102–110. 10.1016/j.chroma.2016.01.024 [DOI] [PubMed] [Google Scholar]
- Safarov J., Hamidova R., Stephan M., Kul I., Shahverdiyev A., Hassel E. (2014). Carbon Dioxide Solubility in 1-Hexyl-3-Methylimidazolium Bis(trifluormethylsulfonyl)imide in a Wide Range of Temperatures and Pressures. J. Phys. Chem. B 118 (24), 6829–6838. 10.1021/jp5012044 [DOI] [PubMed] [Google Scholar]
- Safarov J., Hamidova R., Stephan M., Schmotz N., Kul I., Shahverdiyev A., et al. (2013). Carbon Dioxide Solubility in 1-Butyl-3-Methylimidazolium-Bis(trifluormethylsulfonyl)imide over a Wide Range of Temperatures and Pressures. J. Chem. Thermodynamics 67, 181–189. 10.1016/j.jct.2013.08.008 [DOI] [PubMed] [Google Scholar]
- Shiflett M. B., Drew D. W., Cantini R. A., Yokozeki A. (2010). Carbon Dioxide Capture Using Ionic Liquid 1-Butyl-3-Methylimidazolium Acetate. Energy Fuels 24 (10), 5781–5789. 10.1021/ef100868a [DOI] [Google Scholar]
- Smith E. L., Abbott A. P., Ryder K. S. (2014). Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 114, 11060–11082. 10.1021/cr300162p [DOI] [PubMed] [Google Scholar]
- Song Z., Hu X., Wu H., Mei M., Linke S., Zhou T., et al. (2020). Systematic Screening of Deep Eutectic Solvents as Sustainable Separation Media Exemplified by the CO2 Capture Process. ACS Sustain. Chem. Eng. 8 (23), 8741–8751. 10.1021/acssuschemeng.0c02490 [DOI] [Google Scholar]
- Sowmiah S., Srinivasadesikan V., Tseng M.-C., Chu Y.-H. (2009). On the Chemical Stabilities of Ionic Liquids. Molecules 14, 3780–3813. 10.3390/molecules14093780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Span R., Wagner W. (1996). A New Equation of State for Carbon Dioxide Covering the Fluid Region from the Triple‐Point Temperature to 1100 K at Pressures up to 800 MPa. J. Phys. Chem. Reference Data 25 (6), 1509–1596. 10.1063/1.555991 [DOI] [Google Scholar]
- Vafaeezadeh M., Aboudi J., Hashemi M. M. (2015). A Novel Phenolic Ionic Liquid for 1.5 Molar CO2 Capture: Combined Experimental and DFT Studies. RSC Adv. 5 (71), 58005–58009. 10.1039/C5RA09845A [DOI] [Google Scholar]
- van Osch D. J. G. P., Dietz C. H. J. T., van Spronsen J., Kroon M. C., Gallucci F., van Sint Annaland M., et al. (2019). A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustain. Chem. Eng. 7 (3), 2933–2942. 10.1021/acssuschemeng.8b03520 [DOI] [Google Scholar]
- van Osch D. J. G. P., Dietz C. H. J. T., Warrag S. E. E., Kroon M. C. (2020). The Curious Case of Hydrophobic Deep Eutectic Solvents: A Story on the Discovery, Design, and Applications. ACS Sustain. Chem. Eng. 8 (29), 10591–10612. 10.1021/acssuschemeng.0c00559 [DOI] [Google Scholar]
- Verevkin S. P. (1999). Thermochemistry of Phenols: Buttress Effects in Sterically Hindered Phenols. J. Chem. Thermodynamics 31 (11), 1397–1416. 10.1006/jcht.1999.0466 [DOI] [Google Scholar]
- Völkl J., Müller K., Mokrushina L., Arlt W. (2012). A Priori Property Estimation of Physical and Reactive CO2 Absorbents. Chem. Eng. Technol. 35 (3), 579–583. 10.1002/ceat.201100319 [DOI] [Google Scholar]
- Wazeer I., AlNashef I. M., Al-Zahrani A. A., Hadj-Kali M. K. (2021a). The Subtle but Substantial Distinction between Ammonium- and Phosphonium-Based Deep Eutectic Solvents. J. Mol. Liquids 332, 115838. 10.1016/j.molliq.2021.115838 [DOI] [Google Scholar]
- Wazeer I., Hadj-Kali M. K., Al-Nashef I. M. (2021b). Utilization of Deep Eutectic Solvents to Reduce the Release of Hazardous Gases to the Atmosphere: A Critical Review. Molecules 26 (1), 75. 10.3390/molecules26010075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin K., Roghair I., Gallucci F., van Sint Annaland M. (2021). Total Vapor Pressure of Hydrophobic Deep Eutectic Solvents: Experiments and Modelling. J. Mol. Liquids 325, 115227. 10.1016/j.molliq.2020.115227 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.