Abstract
Purpose
Intracranial infection after neurosurgery is one of the most serious complications, especially extensively drug-resistant (XDR) Acinetobacter baumannii (A. baumannii) seriously affects the prognosis of patients. At present, there is little experience in the treatment of this infection and limited effective treatment options, like tigecycline or polymyxin B. Therefore, this report aims to describe the efficacy of tigecycline combined with polymyxin B by intrathecal (ITH) injection in the treatment of XDR intracranial infection with A. baumannii.
Methods
We report a case of intracranial infection with XDR A. baumannii after ventricular drainage, treated by daily ITH and intravenous (IV) tigecycline, combined with polymyxin B ITH route. Moreover, tigecycline and polymyxin B treatments for XDR intracranial infection with A. baumannii that were reported in the literature were also reviewed and summarized.
Results
The white blood cells (WBCs) of the patient’s cerebrospinal fluid dropped to normal, and the symptoms of intracranial infection disappeared. The patient finally obtained good clinical results and transferred to the local hospital.
Conclusion
The polymyxin B ITH route is an ideal treatment strategy for XDR A. baumannii. The IV plus ITH tigecycline may be an effective treatment option. However, more researches should be conducted to confirm our observation.
Keywords: intracranial infection, Acinetobacter baumannii, intrathecal injection of polymyxin B, tigecycline
Background
Intracranial infection is a common serious complication after neurosurgery. The infection rate is about 1.50% to 6.6%, and the fatality rate is 3.8% to 30%.1,2 Clinical studies have shown that ventricular drainage is one of the important factors leading to intracranial infection after neurosurgery, and other risk factors include trauma, craniotomy and cerebrospinal fluid (CSF) leakage.3 Gram-positive bacteria and gram-negative bacteria are the main bacteria that cause intracranial infection. Among them, Acinetobacter baumannii (A. baumannii) intracranial infections are on the rise, which represent 3.6% to 11.2% of nosocomial intracranial infection cases.4 The mortality rate of intracranial infection with A. baumannii is reaching up to 71%, which suggests a poor prognosis.5 In China Antimicrobial Surveillance Network (CHINET) data, the meropenem-resistant rate of A. baumannii increased from 39.0% in 2005 to 73.4% in 2020.6 Faced with such a high mortality rate and drug resistant rate, it is urgent to treat intracranial infections caused by A. baumannii. Using intravenous (IV) anti-infective drugs alone cannot effectively treat intracranial infections because most drugs cannot enter the central nervous system (CNS). According to recent reports on the treatment of CNS infections, intraventricular (IVT)/intrathecal (ITH) antibiotics showed a good efficacy of extensively drug-resistant (XDR) A. baumannii. Current reports on IVT/ITH drugs are mainly colistin and tigecycline.4,5,7–9 Karaiskos et al reported the incidence of neurotoxicity for colistin was 11%, with the most significant adverse effect being chemical ventriculitis and meningitis, which provoked a serious dilemma for the clinician.4 However, the studies showed that the incidence of neurotoxicity for polymyxin B was 7%, which is even lower.10,11 Tigecycline is the first clinically available drug among glycylcycline class of antibiotics. Studies have shown that it has a good antibacterial effect on A. baumannii and Klebsiella pneumoniae.12,13 With a poor penetration through the blood brain barrier, the serum concentration of tigecycline in the CSF is only 11%.14 Therefore, IV tigecycline is not recommended. ITH tigecycline may be a viable alternative for the treatment of intracranial infections because of its low incidence of CNS adverse reactions.15 At present, the relevant reports are all small-sample studies or case reports. In this case report, we report a patient with intracranial infection after ruptured hemorrhage of an intracranial aneurysm. The continuous ITH tigecycline and polymyxin B was successful. This report shares the experience.
In addition, the intracranial infection in this report should meet the following criteria: positive CSF culture, fever>38°C, increased white blood cells (WBC) >10×106/L, and increased protein and/or decreased glucose levels in CSF.
Case Presentation
A 31-year-old male patient was admitted to our hospital on July 10, 2021, for subarachnoid hemorrhage (1 week) and disturbances in consciousness lasting 9 hours. The patient received a head magnetic resonance imaging due to headache 1 week ago and found subarachnoid hemorrhage. He was conscious at the time and did not take special treatment. Before illness he had been healthy, but upon admission, the Glasgow Coma Scale (GCS) score was 5 points. Head computed tomography angiography (CTA) scan showed rupture and hemorrhage of the right anterior cerebral aneurysm, hemorrhage in the ventricle and hydrocephalus (Figure 1A). Urgent ventricular drainage was performed, and he was transferred to the Surgical Intensive Care Unit (SICU) after the operation. On the first day after the surgery, re-examination of the head CT showed that cerebral hemorrhage was less than before (Figure 1B). Chest CT showed lung infection (Figure 2A). Intracranial pressure was managed via dehydration, diuresis, and intracranial drainage. Cefoperazone-sulbactam 3g IV q12h was administered for infection. The anterior communicating artery aneurysm embolization was performed on July 12, and the patient’s temperature rose to 39.8°C and the GCS score was 7 points. The colour of CSF during lumbar puncture was light red. The CSF WBC count was 1160×106/ L, the red blood cell (RBC) count was 118,000×106/L, the red-to-white ratio was approximately 100:1, glucose was 1.75mmol/L, protein was 3.24g/L (July 13, 2021) (Table 1). Sputum culture reported Klebsiella pneumoniae, which was susceptible to all antibiotic drugs. Pneumonia was confirmed, but we also suspected intracranial infection. The antibiotic was changed to meropenem 1g IV q8h. On the second day after surgery, the patient’s limb response became worse, the GCS score was reduced to 4 points, CSF WBC increased to 1890×106/L, RBC was 48,000×106/L, glucose was 2.13mmol/L, and protein was 1.45g/L (July 14, 2021). Re-examination of chest CT: pneumonia improved significantly (Figure 2B). On the 4th day after the operation, the fever persisted. The CSF WBC was 1648×106/L, no significant decrease compared with before. The RBC was 51,000×106/L, the red-to-white ratio was approximately 30:1, we thought the drug efficacy is not ideal, so the antibiotic regimen was adjusted to meropenem 2g IV q8h and vancomycin 1g IV q12h. On the 7th day after surgery (July 19.2021), the patient’s sputum culture indicated XDR A. baumannii that was susceptible only to tigecycline. The CSF WBC was 12,834×106/L, which is significantly higher than before. The CSF exhibited a deep yellow color with turbidity. We consider that the intracranial infection is aggravated. Then we adjusted the antibiotic (meropenem 2g IV q8h, vancomycin 1g IV q12h, tigecycline 100mg IV q12h combined with tigecycline 5mg ITH qd). On July 20, a ventriculoscopic abscess removal operation was performed, and pus was visible in the ventricle, specimens were collected and submitted for examination. The re-examination of the head CT showed that the hydrocephalus was better than before on July 21 (Figure 1C). On July 23, the patient’s body temperature gradually dropped and he was in a shallow coma. He could open his eyes and move his limbs unconsciously, the GCS score was 9 points. But his CSF results are still not optimistic: WBC was 40,390×106/L, RBC was 13,000×106/L, red-to-white ratio was approximately 1:4. The patient’s CSF culture indicated XDR A. baumannii was susceptible only to tigecycline [(Minimum inhibitory concentration) MIC≤2μg/mL] (Table 2), though susceptibility to polymyxin B or colistin was not tested. It was not a routine test for polymyxin in our hospital. We added polymyxin B 50,000 IU ITH qd to the original antibiotics and tested susceptibility of polymyxin B at the same time. On July 25, the results of CSF were significantly improved: WBC was 7360×106/L, RBC was 5000×106/L, red-to-white ratio was approximately 1:1.5, glucose was 2.45mmol/L, protein was 8.87g/L. The CSF culture showed that the A. baumannii was susceptible to polymyxin B (MIC ≤0.5μg/mL). On July 29, the patient’s temperature returned to normal, the WBC of CSF dropped to 280×106/L, RBC was 1000×106/L, the red-to-white ratio was approximately 5:1. Ventricular drainage tubes were removed. The antibiotic regimen was adjusted to meropenem 1g IV q8h, linezolid 600mg IV q12h, tigecycline 100mg IV q12h and tigecycline 5mg ITH qd plus polymyxin B 50,000IU ITH qd. On August 4, the patient’s CSF cultures were negative for 3 consecutive tests, and the linezolid was discontinued. On August 7, the WBC in CSF decreased to 130×106/L, the RBC was 900×106/L, the red-to-white ratio was approximately 7:1, The antibiotic was adjusted to meropenem 1g IV q8h and tigecycline 100mg IV q12h combined with tigecycline 5mg ITH qd, and he was transferred to neurosurgery ward for specialized treatment. The head CT re-examined on August 13 showed increased hydrocephalus (Figure 1D), so lumbar cistern abdominal shunt was performed. On August 16, WBC of CSF dropped to 10×106/L, RBC dropped to 710×106/L, the red-to-white ratio was approximately 71:1. On August 20, the patient’s intracranial infection disappeared, and he returned to the local hospital to continue treatment.
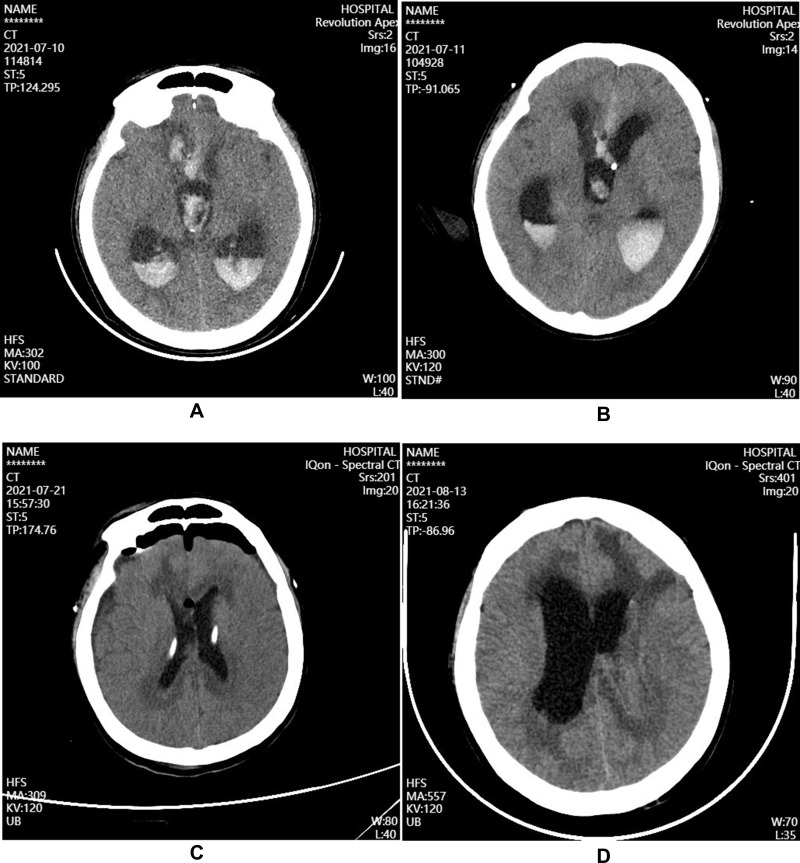
Figure 1.
Brain CT after admission in patient with intracranial A.baumannii infection. (A) Before the treatment on July 10, large high-density shadows can be seen on the right frontal lobe and lateral ventricle; (B) After the ventricular drainage on July 11, the right frontal lobe hemorrhage is slightly smaller than before; (C) During the treatment on July 21, ventricular hemorrhage and hydrocephalus was better than before; (D) During the treatment on August 13, bilateral lateral ventricle was dilated, and hydrocephalus progressed more than before.
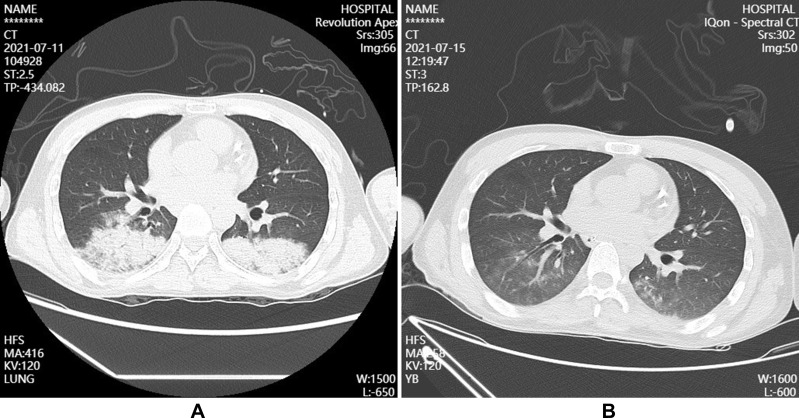
Figure 2.
Pulmonary CT after admission.(A) On July 11, infection occurred in the lower lobe of both lungs; (B) After the treatment on July 15, infection in the lower lobe of both lungs were better than before.
Table 1.
Based on the Timeline of the Patient’s Study Results
| Stay in ICU | In Neurosurgery Ward | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| July 10 | July 11 | July 13 | July 15 | July 16 | July 19 | July 21 | July 23 | July 25 | July 29 | August 4 | August 7 | August 14 | August 16 | August 20 | ||
| CSF | WBC (×106/L)/RBC (×106/L) | — | — | 1160/118,000 | 2072/90,000 | 1648/51,000 | 12,834/63,000 | 6147/29,000 | 40,390/13,000 | 7360/5000 | 280/1000 | 366/482 | 130/900 | 30/5000 | 10/710 | Cure |
| Glu (mmol/L)/Protein (g/L) | — | — | 1.75/3.24 | 2.3/1.78 | 2/2.12 | 0.26/9.69 | 1.39/8.64 | 1.58/8 | 2.45/8.87 | 3.73/10.29 | 3.64/5.25 | 4/3.99 | 2.63/2.87 | 1.64/1.56 | ||
| Invasive procedure | Ventricular drainage | Anterior communicating artery aneurysm embolization /ventricular drainage | Ventriculoscopic abscess removal/ventricular drainage | — | — | Lumbar cistern abdominal shunt | ||||||||||
| Antimicrobial susceptibilities | — | — | Sputum culture-Klebsiella pneumoniae, Susceptible to all | — | — | Sputum culture-XDR A. baumannii, Susceptible to TGC (MIC≤2μg/mL) | Sputum culture-XDR A. baumannii, Susceptible to TGC (MIC≤2μg/mL) | CSF culture-XDR A. baumannii, Susceptible to TGC (PMB was not tested) | CSF culture-XDR A. baumannii, Susceptible to PMB (MIC≤0.5μg/mL) | — | Sputum and CSF cultures were negative for 3 consecutive tests | — | — | — | ||
| Current antimicrobial regimens | — | CFP-S 3g IV q12h | MEM 1g IV q8h | MEM 2g IV q8h+VAN 1g IV q12h | MEM 2g IV q8h+VAN 1g IV q12h+TGC 100mg IV q12h + 5mg ITH q24h | MEM 2g IV q8h+VAN 1g IV q12h+TGC 100mg IV q12h+5mg ITH q24h+PMB 50,000IU ITH q24h | MEM 1g IV q8h+linezolid 600mg IV q12h+TGC 100mg IV q12h+5mg ITH q24h+PMB 50,000IU ITH q24h | MEM 1g IV q8h+TGC 100mg IV q12h+5mg ITH q24h+PMB 50,000IU ITH q24h. | MEM 1g IV q8h+TGC 100mg IV q12h+5mg ITH q24h | |||||||
| Head CT | Rupture and hemorrhage of the right anterior cerebral aneurysm, hemorrhage in the ventricle and hydrocephalus | Cerebral hemorrhage was less than before | — | — | — | — | Hydrocephalus was better than before | — | — | — | Hydrocephalus progressed slightly | — | Increased hydrocephalus | — | ||
| Chest CT | — | Lung infection | — | Infection in the lower lobe of both lungs were better than before | — | — | — | — | — | — | — | — | — | — | ||
Abbreviations: CSF, cerebrospinal fluid; XDR, extensively drug-resistant; TGC, tigecycline; MIC, minimum inhibitory concentration; PMB, polymyxin B; CFP-S, cefoperazone/sulbactam; IV, intravenous; q12h, every 12h; MEM, meropenem; q8h, every 8h; VAN, vancomycin; ITH, intrathecal; q24h, every 24h; CT, computed tomography.
Table 2.
Bacterial Culture of CSF in Patient with Intracranial Infection
| Antibiotics | MIC (μg/mL) | Drug Sensitivity |
|---|---|---|
| Cefotaxime | >32 | R |
| Ceftazidime | >16 | R |
| Ceftriaxone | >32 | R |
| Cefepime | >16 | R |
| Imipenem | 16 | R |
| Meropenem | >8 | R |
| Ampicillin/Sulbactam | >16/8 | R |
| Piperacillin/ Tazobactam | 128 | R |
| Ciprofloxacin | >2 | R |
| Levofloxacin | >4 | R |
| Tobramycin | >8 | R |
| Gentamicin | >8 | R |
| Amikacin | >32 | R |
| Tigecycline | ≤2 | S |
| Trimethoprim/Sulfa | >2/38 | R |
| Polymyxin B | ≤0.5 | S |
Abbreviations: MIC, minimum inhibitory concentration; R, resistant; S, susceptible.
Discussion
We discussed a patient with XDR A. baumannii after intracerebral hemorrhage who was treated by the ITH tigecycline and polymyxin B therapy. As far as we know, this treatment has not been reported before. ITH is a method of injecting drug directly into the subarachnoid space through lumbar puncture, allowing drug to infiltrate the CSF without passing through the blood-brain barrier and reaching its effective concentration quickly. Therefore, ITH antibiotics can achieve higher CSF concentration than IV antibiotics. In the published guidelines, it is recommended that if there is no response to IV antibiotics or the concentrations of CSF do not reach their respective MICs, IVT/ITH antibiotics should be considered, especially for multi-drug resistant bacteria.16 However, in our study, the patient’s intracranial infection was serious. Due to the poor effect of IV antibiotics, ITH antibiotics need to be added.
In the closed environment of ICU, the emergence of drug-resistant bacteria is getting worse. Long-term ventricular drainage after intracranial hemorrhage, the use of broad-spectrum antibiotics and long-term mechanical ventilation are all high-risk factors for drug-resistant bacteria. The average infection time of A. baumannii was 12 days (range within 40 days).17 The emergence time of resistant bacteria ranges from 3 to 90 days.18 In the treatment of intracranial infections of gram-negative bacilli, carbapenems are usually the first choice. With the increasing resistance rate of carbapenems year by year, carbapenems may not be considered the treatment of choice in areas with high rates of carbapenem-resistant A. baumannii.19 Treatment has become increasingly challenging due to the growing number of XDR bacteria. In some cases, polymyxin B is the only available antimicrobial agent active against meningitis pathogens.20 In our study, CSF culture was resistant to carbapenems. The time for the development of XDR A. baumannii in sputum culture was 9 days. CSF culture found XDR A. baumannii for 13 days. The result was within the time range of the literature.17,18
Polymyxin has been used since the 1950s, but due to severe nephrotoxicity and neurotoxicity, such as chemical ventriculitis, chemical meningitis and seizures, its wide application has been limited. When carbapenem-resistant gram-negative bacteria appeared and spread in the 1990s, no antibiotics were available, leading to the re-entry of polymyxin into clinical use.21 So as to have a deeper understanding of the pharmacokinetics (PK), pharmacodynamics (PD) and clinical properties of colistin.22 In recent years, researchers have discovered that polymyxin has CNS permeability, is active against a variety of aerobic gram-negative bacteria, especially multi-drug resistant strains, including A. baumannii, Pseudomonas aeruginosa and Klebsiella pneumoniae.23 However, it has no activity against Gram-positive and anaerobic bacteria. At present, IVT/ITH colistin has been used to treat CNS infections caused by multidrug-resistant gram-negative bacteria, and it has been successful in some researched cases.4,8,24,25
In our study, the patient’s CSF samples were taken several times after admission, but no positive results were detected. The sputum culture showed sensitive Klebsiella pneumoniae and XDR A. baumannii. Combined with preoperative coma, high risk of aspiration and high fever, the patient was diagnosed pneumonia. The plan of antibiotic was adjusted to strengthen anti-infective, then the pneumonia improved significantly. The WBC of CSF gradually increased, the possibility of intracranial infection was considered. We used tigecycline 100mg IV q12h combined with tigecycline 5mg ITH qd to treat. A. baumannii was found in CSF culture of ventricle microscopy and it was susceptible only to tigecycline. It was not a routine for testing the susceptibility of polymyxin B or colistin in our hospital. After using tigecycline for 3 days, the effect was not significant. Then we communicated with the patient’s mother to ITH polymyxin B 50,000 IU qd and tested susceptibility of polymyxin B at the same time. The official instruction of polymyxin B clearly indicates that it can be used for IVT/ITH. In addition, compared with polymyxin E, polymyxin B shows superior PK characteristics in the human body. Polymyxin B is administered as its sulfate salt, that means the active antibacterial entity is directly administered to patients. Polymyxin B is eliminated mainly through non-renal clearance mechanisms, thereby reducing the possibility of nephrotoxicity.8 Current clinical PK data indicate that daily doses of polymyxin B should not be adjusted based on the kidney function of patients, even receiving renal replacement therapy. If unnecessary renal dose adjustments are made in patients, there is potential for drug underexposure.10,31 But colistin dose adjustments should be made in patients with renal insufficiency. The onset of polymyxin neurotoxicity usually occurs in intravenous infusion, which may be related to prolonged exposure and concentration.26 Polymyxin-induced neurotoxicity is generally reversible with discontinuation of the therapy. Our study only ITH polymyxin B 50,000 IU qd for 16 days. During this process, the patient’s renal function was closely monitored, and no nephrotoxic or neurotoxic side effects were observed.
According to the evidence provided and the treatment plan of our study, polymyxin B is an ideal drug. In the past 20 years, it has rarely caused significant neurotoxicity. The incidence rate of neurotoxicity associated with polymyxin B in patients is approximately 7% or less.26,27 However, studies have found that polymyxin has poor permeability to the blood-brain barrier.28–30 When patients with intracranial infection are only administered by IV, the level of polymyxin in the CSF is only 5–10% of the blood. The use of ITH administration can increase the level of the drug in the CSF to reach the concentration required to effectively kill bacteria.30 International Consensus Guidelines for the Optimal Use of the Polymyxins proposed in 2019 that the daily dose of polymyxin B administered by ITH/IVT is 50,000 IU (5 mg) per day with a mean duration of 18 days.31 The Infectious Diseases Society of America (IDSA) proposed in 2017 showed the treatment duration of intracranial infections caused by aerobic gram-negative bacilli should be 21 days, but we should individualize it based on clinical response until at least 3 consecutive CSF cultures produce negative results on different days.16 In many studies, the unit of measurement proposed as a therapeutic polymyxin level is milligrams (mg), while other studies use international units (IU).32,33 The dose of polymyxin B 10,000 IU equals 1 mg. According to the guidelines, the daily dose used in our study is 50,000 IU/d, which is 5 mg/d, and the therapeutic effect is satisfactory. Currently, there is limited experience with ITH/ITV polymyxin B. Pan et al reported that ITH/ITV polymyxin B has a good clinical effect on XDR A. baumannii intracranial infection, with a mortality rate of only 8.70%, and no cases of acute kidney injury or neurotoxicity were observed among the study participants.34 Therefore, it can be seen that polymyxin B is relatively safe. In addition, there are other small sample studies that have confirmed the same efficacy of polymyxin B35–40 (Table 3). In most cases, craniocerebral trauma and cerebral hemorrhage are common primary diseases. Infections are secondary to neurosurgery, in particular, the implantation of foreign bodies during neurosurgery. About 85% of patients were cured after IVT/ITH polymyxin B, and the curative effect was remarkable. Only one patient reported mild neurotoxicity.34–40 Therefore, the exact mechanism of polymyxin B nephrotoxicity and neurotoxicity are still under study. Therefore, close monitoring of renal function, more careful management of fluids and electrolytes, and avoiding the use of other drugs known to have nephrotoxicity may reduce the incidence of related side effects. This indicated that ITH polymyxin B was safe enough for the treatment of XDR A. baumannii intracranial infection. The CSF sterilization time in our study was 26 days (range 8–48 days), while the average CSF sterilization time in the literature was 4 days, which was much lower (range 1–18 days). This difference can be explained by the difference in the definition of CSF sterilization standards between us and most published cases. In addition, the patient in our study was infected with XDR bacteria, which made treatment more difficult. ITH polymyxin B is a good choice for intracranial infections caused by multi-drug resistant gram-negative bacteria. However, there is currently a lack of information on the PK of polymyxin B other than IV injection (such as inhalation, ITH /IVT).
Table 3.
Studies Regarding Intraventricular (IVT) or Intrathecal (ITH) Administration of Polymyxin B and Tigecycline in A. baumannii Meningitis/Ventriculitis
| Reference | Age | Underlying Disease | Invasive Procedure | Days From Admission to Positive CSF Culture | Antimicrobial Susceptibilities | Current Antimicrobial Regimens | IVT/ITH PMB | IV PMB (Duration in days) | IVT/ITH TGC | IV TGC (Duration in days) | Days to Sterilise CSF | Toxicity | Outcome/comments | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dosea | Duration (days) | Dose (mg) | Duration (days) | ||||||||||||
| [34] | 23 adult patients | Cerebral hemorrhage/Craniocerebral trauma/Benign intracranial tumor | LCD/VPS/LP | NR | Carbapenem-resistant, Susceptible to PMB | TGC plus CFP-S (39.13%), MEM/IPM plus TGC (17.39%), MEM/IPM (13.04%), CFP-S (17.39%). | IVT/ITH 50,000U q24h | NR | 450,000U q12h | None | None | NR | NR | None | 2 deaths, 21 cured |
| [35] | 14 years | Endoscopic endonasal resection of craniopharyngioma | LD | 27 | MDR, Susceptible to TGC, AMK, PMB | PMB, CFP-S | ITH 50,000U q24h for 4 days, then 50,000U q48h for 5 days | 9 | 450,000U q12h (19) | None | None | None | 42 | Hyperpigmentation, Dark red papule, Memory loss, Hypotonia | Cure,12-months follow-up |
| [36] | 33 years | Craniocerebral trauma | LCD/Ventricular borehole drainage | 28 | XDR, Susceptible to TGC (MIC≤1μg/mL), colistin (MIC ≤0.5μg/mL) | MEM, VAN, TGC, PMB | IVT 10mg q24h for 4days, then 5mg q48h for 14 days | 18 | 100mg q12h (18) | IVT 5mg q12h | 7 | 100mg q12h (7) | 24 | Hepatic damage | Cure |
| [37] | 57 years | Craniocerebral trauma | EVD | 5 | XDR, Susceptible to TGC (MIC≤1μg/mL), PMB (MIC=1μg/mL) | TGC, PMB | IVT 50,000U q24h for 5 days, then 25,000 q12h for 7 days | 12 | 450,000U q12h (5), followed 475,000U q12h (7), followed 50,0000U q12h (14) | None | None | 50mg q12h (26) | 5 | None | Cure,6-months follow-up |
| [38] | 14 Adult patients | Traumatic brain injury/ Intracerebral hemorrhage | EVD/LD | NR | MDR, XDR | NR | IVT 5mg q24h | 14.96±4.28b | 50 mg q12h | None | None | None | 8.23±4.02c | None | Cure (82.1%), death (17.6%) |
| [39] | 50 years | Cerebral hemorrhage | LD | 25 | Carbapenem-resistant | MEM, SUL, PMB | ITH 5mg q24h for 6 days, then 5mg q48h, then gradually extended | 64 | 100 mg 1st dose, followed 50mg q12h (4), followed 100 mg q12h (6), followed 50mg q12h (58) | None | None | None | 13 | None | Cure,6-months follow-up |
| [40] | 87month | Craniocerebral trauma | EVD/Ommaya reservoir | 30 | XDR, Susceptible to TGC, PMB | TGC, PMB, CFP-S | IVT (NR) | 21 | NR (33) | None | None | NR (8) | 22 | None | Cure,8-months follow-up |
| [5] | 22 years | Pituitary adenoma | EVD | NR | XDR, Susceptible to TGC (MIC=2μg/ mL) | MEM, VAN, TGC | None | None | None | IVT 2mg q24h for 10 days, then 2mg q12h | More than 2 months | None | None | None | Cure,12-months follow-up |
| [9] | 17 years | Tuberculous meningitis | Intracranial drainage | 36 | XDR, Susceptible to TGC (MIC=1μg/ mL) | TGC, CFP-S, FOS | None | None | None | IVT 4mg q12h for 9 days, then ITH 4mg q24h for 30 days | 39 | 47.5mg q12h (39) | 39 | None | Cure, 4-months follow-up |
| [42] | 56 years | Cerebral hemorrhage | EVD | 10 | MDR, Intermediate to TGC (MIC=4mg/ L) | MEM, TMS, TGC | None | None | None | IVT 2mg q12h for 3 days, then ITH 4mg q12h for 11 days | 14 | 200mg 1st dose, followed 100mg q12h (22) | 13 | None | Cure |
| [43] | 28 years | Intracranial tumor | VPS | 9 | XDR, Susceptible to TGC (MIC=2μg/mL), Colistin (MIC <0.5μg/mL) | TGC, CFP-S | None | None | None | ITH 5mg q24h | 9 | 100mg q12h (53) | 10 | Spinal arachnoiditis | Cure, 12-months follow-up |
| [44] | 70 years | Subarachnoid hemorrhage | Lumbar subarachnoid drainage/EVD | 22 | XDR, Susceptible to TGC (MIC≤1μg/ mL), CFP-S | TGC, CFP-S | None | None | None | IVT 2mg q12h | 10 | 100mg 1st dose, followed 50mg q12h (7) | 16 | None | Cure, 4-months follow-up |
| [45] | 68 years | Craniocerebral trauma | LCD/EVD | 26 | XDR, Susceptible to TGC | TGC | None | None | None | IVT 2mg q8h | 14 | 100mg 1st dose, followed 50mg q12h (17) | 7 | None | Cure,3-months follow-up |
| [46] | 50 years | Craniocerebral trauma | NR | 32 | XDR, Susceptible to TGC (MIC=2mg/ L) | TGC, CFP-S | None | None | None | IVT 3mg q24h for 6 days, then 4mg q12h for 6 days | 12 | 100mg q12h (17) | 15 | None | Cure,4-months follow-up |
| [47] | 45 years | Craniocerebral trauma | LP | 4 | XDR, Susceptible to TGC | TGC | None | None | None | ITH 10mg q12h | 6 | 100mg 1st dose, followed 50mg q12h (7) | 13 | None | Cure, 3-months follow-up |
Notes: aIn some cases, conversion of the dose from international units (IU) to milligrams (mg) was conducted for comparison reasons (1 mg PMB = 10,000 IU PMB). bThe mean period to IVT PMB of 28 post-neurosurgical Gram-negative meningitis cases and not days to IVT for each patient. cThe mean period to CSF sterilisation of 28 post-neurosurgical Gram-negative meningitis cases treated with IVT antibiotics is given and not days to sterilisation for each patient.
Abbreviations: CSF, cerebrospinal fluid; IVT, intraventricular; ITH, intrathecal; PMB, polymyxin B; IV, intravenous; TGC, tigecycline; LCD, lumbar cistern drainage; VPS, ventriculoperitoneal shunt; LP, lumbar puncture; NR, not reported; CFP-S, cefoperazone/sulbactam; MEM, meropenem; IPM, imipenem; q24h, every 24h; q12h, every 12h; LD, lumbar drainage; MDR, multidrug-resistant; AMK, amikacin; q48h, every 48h; XDR, extensively drug-resistant; MIC, minimum inhibitory concentration; VAN, vancomycin; EVD, external ventricular drain; FOS, fosfomycin; TMS, trimethoprim-sulfamethoxazole.
Tigecycline is a glycylcycline antibacterial drug. In vitro and in vivo tests have confirmed that tigecycline usually shows good antibacterial activity against drug-resistant gram-negative and gram-positive bacteria. Due to the lack of penetration into the CNS, the penetration rate of tigecycline in the CSF is only 11%, and IV injection has no significant effect on patients with intracranial infection. Therefore, conventional IV tigecycline treatment is not recommended.41 Therefore, we consider combining IV with ITH tigecycline, which may be a potential treatment option for patients. In 2016, the successful use of IVT tigecycline for the treatment of XDR A. baumannii intracranial infection was reported for the first time.5 The IVT dose was 2 to 4mg/d, while the IV tigecycline was continued. Antibiotic treatment was discontinued after 2 months of treatment. Deng9 reported the initial IVT injection of tigecycline at a dose of 4 mg q12h, and IV tigecycline 50 mg q12h at the same time. When the effect was not good after 9 days of treatment, the route of tigecycline was changed from IVT to ITH. After 1 month of treatment, the patient’s CSF cultures were negative for 3 consecutive times, and antibiotics were discontinued. When the IVT tigecycline fails, the ITH tigecycline should be considered instead of increasing the dose of IVT tigecycline. We use IV plus ITH tigecycline, which was initially ineffective. After discontinuation of polymyxin B, the patient has no signs of CSF infection using tigecycline. Thus, the efficacy of tigecycline is uncertain. IVT tigecycline seemed to be a safe treatment option. In recent years, some studies reported successful application of IVT/ITH tigecycline in patients36,42–47 (Table 3). IVT/ITH tigecycline was administered at doses of 3 to 20 mg per day, and one patient developed liver injury and another developed spinal arachnoiditis, which resolved after discontinuation of the antibiotic. All patients in these cases were cured. Tigecycline can be combined with polymyxin B or colistin to treat XDR bacteria. In vitro antibiotic studies showed that tigecycline combined with polymyxin B has a potential synergistic effect on carbapenem-resistant A. baumannii, and the strains with synergistic effect can be as high as 70%.48 But the antibacterial activity in vitro does not necessarily reflect activity in vivo. In clinical practice, the efficacy of TGC and polymyxin was uncertain, and more clinical studies are needed to prove the treatment of XDR A. baumannii.49
In this case, we only reported one patient and the sample size was too small, we could not predict the accuracy of the treatment and its side effects. There is no PK data in our study because our hospital currently cannot measure the concentration of tigecycline or polymyxin B in serum and CSF. Therefore, more research must be conducted to prove the therapeutic effect of this program. If it is proved to be safe and effective in the future, ITH tigecycline and polymyxin B can also be considered as a first-line treatment for XDR intracranial infections.
Conclusion
ITH tigecycline combined with polymyxin B is effective in the treatment of broadly drug-resistant intracranial infection of A. baumannii. ITH injection of polymyxin B is an ideal treatment strategy for XDR A. baumannii. The tigecycline IVT/ITH route also seems to be a safe treatment option. Currently, there is no large-sample clinical RCT study, and more studies should be conducted to confirm our observations. The effectiveness of ITH tigecycline still needs further research to prove the therapeutic effect.
Funding Statement
This work was supported by Guangdong Natural Science Foundation (2022A1515011919).
Abbreviations
A. baumannii, Acinetobacter baumannii; CNS, central nervous system; CSF, cerebrospinal fluid; CT, computed tomography; GCS, Glasgow Coma Scale; IV, intravenous; IVT, intraventricular; ITH, intrathecal administration; IU, international units; MIC, minimum inhibitory concentration; PD, pharmacodynamics; PK, pharmacokinetics; RBC, red blood cell; WBC, white blood cell; XDR, extensively drug-resistant.
Ethical Approval and Consent to Participate
The patient provided informed consent for publication of the case. No ethical committee approval was required for this study as the data had been analyzed in a retrospective manner.
Consent for Publication
Written informed consent for publication of the clinical details and clinical images was obtained from the patient.
Author Contributions
ZYL made substantial contributions to the conception and design of the study; acquisition, analysis, and interpretation of the data; and drafting of the manuscript. LJL participated in the analysis and interpretation of the data. YLA participated in the acquisition and interpretation of the data, and revised the article. HMY made substantial contributions to the conception and design of the study; acquisition, analysis, and interpretation of the data; and critical revision of the manuscript for important intellectual content and was accountable for all aspects of the work to ensure that questions related to the accuracy and integrity of any part of the work were appropriately investigated and resolved. All authors made a significant contribution to the work reported, whether that is in the conception, study design, data collection, took part in drafting or revising the article; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
- 1.Korinek AM, Baugnon T, Golmard JL. Risk factors for adult nosocomial meningitis after craniotomy: role of antibiotic prophylaxis. Neurosurgery. 2008. [DOI] [PubMed] [Google Scholar]
- 2.Liu PF, Xu WH, Liu YL, et al. Analysis of 48 cases of intracranial infection after neurosurgery. J Binzhou Med Univ. 2011;34(3):3. [Google Scholar]
- 3.Kourbeti IS, Jacobs AV, Koslow M, et al. Risk factors associated with postcraniotomy meningitis. Neurosurgery. 2007;60(2):317. doi: 10.1227/01.NEU.0000249266.26322.25 [DOI] [PubMed] [Google Scholar]
- 4.Karaiskos I, Galani L, Baziaka F, et al. Intraventricular and intrathecal colistin as the last therapeutic resort for the treatment of multidrug-resistant and extensively drug-resistant Acinetobacter baumannii ventriculitis and meningitis: a literature review. Int J Antimicrob Agents. 2013;41(6):499–508. doi: 10.1016/j.ijantimicag.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 5.Lauretti L, D’Alessandris QG, Fantoni M, et al. First reported case of intraventricular tigecycline for meningitis from extremely drug-resistant Acinetobacter baumannii. J Neurosurg. 2016;127(2):1–4. doi: 10.3171/2016.4.JNS16169 [DOI] [PubMed] [Google Scholar]
- 6.CHINET. Institute of Antibiotics, Huashan Hospital, Fudan University; 2020. Available from: http://www.chinets.com/Data/GermYear. Accessed March 15, 2022.
- 7.De Bonis P, Lofrese G, Scoppettuolo G, et al. Intraventricular versus intravenous colistin for the treatment of extensively drug resistant Acinetobacter baumannii meningitis. Eur J Neurol. 2016;23(1):68–75. doi: 10.1111/ene.12789 [DOI] [PubMed] [Google Scholar]
- 8.Shahbazi F, Dashti-Khavidaki S. Colistin: efficacy and safety in different populations. Expert Rev Clin Pharmacol. 2015;8(4):423–448. [DOI] [PubMed] [Google Scholar]
- 9.Deng ZW, Wang J, Qiu CF, et al. A case report of intraventricular and intrathecal tigecycline infusions for an extensively drug-resistant intracranial Acinetobacter baumannii infection. Medicine. 2019;98(15):e15139. doi: 10.1097/MD.0000000000015139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai Y, Lee W, Kwa AL. Polymyxin B versus colistin: an update. Expert Rev Anti Infect Ther. 2015;13(12):1481–1497. doi: 10.1586/14787210.2015.1093933 [DOI] [PubMed] [Google Scholar]
- 11.Justo JA, Bosso JA. Adverse reactions associated with systemic polymyxin therapy. Pharmacotherapy. 2015;35(1):28–33. doi: 10.1002/phar.1493 [DOI] [PubMed] [Google Scholar]
- 12.Garnacho-Montero J, Dimopoulos G, Poulakou G, et al. Task force on management and prevention of Acinetobacter baumannii infections in the ICU. Intensive Care Med. 2015;41(12):2057–2075. doi: 10.1007/s00134-015-4079-4 [DOI] [PubMed] [Google Scholar]
- 13.Wu Y, Chen K, Zhao J, et al. Intraventricular administration of tigecycline for the treatment of multidrug-resistant bacterial meningitis after craniotomy: a case report. J Chemother. 2017;8:1. [DOI] [PubMed] [Google Scholar]
- 14.Rodvold KA, Gotfried MH, Cwik M, et al. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J Antimicrob Chemother. 2007;58(6):1221–1229. doi: 10.1093/jac/dkl403 [DOI] [PubMed] [Google Scholar]
- 15.Kadoyama K, Sakaeda T, Tamon A, et al. Adverse event profile of tigecycline: data mining of the public version of the U.S. Food and Drug Administration adverse event reporting system. Biol Pharm Bull. 2012;35(6):967. doi: 10.1248/bpb.35.967 [DOI] [PubMed] [Google Scholar]
- 16.Tunkel AR, Rodrigo H, Adarsh B, et al. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin Infect Dis. 2017;6:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim BN, Peleg AY, Lodise TP, et al. Management of meningitis due to antibiotic-resistant Acinetobacter species. Lancet Infect Dis. 2009;9(4):245–255. doi: 10.1016/S1473-3099(09)70055-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang CR, Chen SF, Lu CH, et al. Clinical characteristics and therapeutic outcomes of nosocomial super-infection in adult bacterial meningitis. BMC Infect Dis. 2011;11. doi: 10.1186/1471-2334-11-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garnacho-Montero J, Timsit JF. Managing Acinetobacter baumannii infections. Curr Opin Infect Dis. 2019;32(1):69–76. doi: 10.1097/QCO.0000000000000518 [DOI] [PubMed] [Google Scholar]
- 20.Karaiskos I, Giamarellou H. Multidrug-resistant and extensively drug-resistant Gram-negative pathogens: current and emerging therapeutic approaches. Expert Opin Pharmacother. 2014;15(10):1351–1370. doi: 10.1517/14656566.2014.914172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tullu MS, Dhariwal MS. Colistin: re-emergence of the “forgotten” antimicrobial agent. J Postgrad Med. 2013;59(3):208–215. doi: 10.4103/0022-3859.118040 [DOI] [PubMed] [Google Scholar]
- 22.Nation RL, Li J. Colistin in the 21st century. Curr Opin Infect Dis. 2009;22(6):535–543. doi: 10.1097/QCO.0b013e328332e672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velkov T, Dai C, Ciccotosto GD, et al. Polymyxins for CNS infections: pharmacology and neurotoxicity. Pharmacol Ther. 2018;181:85–90. doi: 10.1016/j.pharmthera.2017.07.012 [DOI] [PubMed] [Google Scholar]
- 24.Kim H-I, Kim S-W, Park G-Y, et al. The causes and treatment outcomes of 91 patients with adult nosocomial meningitis. Korean J Intern Med. 2012;27(2):171–179. doi: 10.3904/kjim.2012.27.2.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alfahad WA, Omrani AS. Update on colistin in clinical practice. Saudi Med J. 2014;35(1):9–19. [PubMed] [Google Scholar]
- 26.Nang SC, Azad MAK, Velkov T, et al. Rescuing the last-line polymyxins: achievements and challenges. Pharmacol Rev. 2021;73(2):679–728. doi: 10.1124/pharmrev.120.000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khawcharoenporn T, Apisarnthanarak A, Mundy LM. Intrathecal colistin for drug-resistant Acinetobacter baumannii central nervous system infection: a case series and systematic review. Clin Microbiol Infect. 2010;16(7):888–894. doi: 10.1111/j.1469-0691.2009.03019.x [DOI] [PubMed] [Google Scholar]
- 28.Bargiacchi O, Rossati A, Car P, et al. Intrathecal/intraventricular colistin in external ventricular device-related infections by multi-drug resistant Gram negative bacteria: case reports and review. Infection. 2014;42(5):801–809. doi: 10.1007/s15010-014-0618-0 [DOI] [PubMed] [Google Scholar]
- 29.Blount JP, Campbell JA, Haines SJ. Complications in Ventricular Cerebrospinal Fluid Shunting. Neurosurg Clin N Am. 1993;4(4):633–656. doi: 10.1016/S1042-3680(18)30556-4 [DOI] [PubMed] [Google Scholar]
- 30.Ziaka M, Markantonis SL, Fousteri M, et al. Combined intravenous and intraventricular administration of colistin methanesulfonate in critically ill patients with central nervous system infection. Antimicrob Agents Chemother. 2013;57(4):1938–1940. doi: 10.1128/AAC.01461-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuji BT, Pogue JM, Zavascki AP, et al. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy. 2019;39(1):10–39. doi: 10.1002/phar.2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falagas ME, Kasiakou SK. Use of international units when dosing colistin will help decrease confusion related to various formulations of the drug around the world. Antimicrob Agents Chemother. 2006;50(6):2274–2275. doi: 10.1128/AAC.00101-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nation RL, Li J, Cars O, et al. Consistent global approach on reporting of colistin doses to promote safe and effective use. Clin Infect Dis. 2014;58(1):139–141. doi: 10.1093/cid/cit680 [DOI] [PubMed] [Google Scholar]
- 34.Pan S, Huang X, Wang Y, et al. Correction to: efficacy of intravenous plus intrathecal/intracerebral ventricle injection of polymyxin B for post-neurosurgical intracranial infections due to MDR/XDR Acinectobacter baumannii: a retrospective cohort study. Antimicrob Resist Infect Control. 2019;8(1). doi: 10.1186/s13756-019-0462-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xing H, Cheng C, Zhang Y, et al. Successful treatment with intrathecal and intravenous Polymyxin B-Based combination against MDR Acinetobacter baumannii meningitis in pediatric patient: a Case Report. Front Pediatr. 2021;9:564991. doi: 10.3389/fped.2021.564991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong L, Shi XZ, Su L, et al. Sequential intraventricular injection of tigecycline and polymyxin B in the treatment of intracranial Acinetobacter baumannii infection after trauma: a case report and review of the literature. Military Med Res. 2020;7. doi: 10.1186/s40779-020-00253-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo W, Guo SC, Li M, et al. Successful treatment of extensively drug-resistant Acinetobacter baumannii ventriculitis with polymyxin B and tigecycline- A case report. Antimicrob Resist Infect Control. 2018;7:22. doi: 10.1186/s13756-018-0313-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen H, Guo X, Xie D, et al. A clinical study on the use of intraventricular Polymyxin B supplemented by continuous external ventricular drainage in the treatment of drug-resistant gram-negative bacilli intracranial infection. Infect Drug Resist. 2020;13:2963–2970. doi: 10.2147/IDR.S261510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun X, Zhang Y, Zhang L, et al. Long course of polymyxin B for the treatment of brain abscess caused by carbapenem resistant Acinetobacter baumannii: a case report. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021;33(11):1370–1372. doi: 10.3760/cma.j.cn121430-20210326-00452 [DOI] [PubMed] [Google Scholar]
- 40.Ye J, Tan LH, Shen ZP, et al. Polymyxin for the treatment of intracranial infections of extensively drug-resistant bacteria in children after neurosurgical operation. World J Pediatr. 2020;16(5):528–532. doi: 10.1007/s12519-020-00350-8 [DOI] [PubMed] [Google Scholar]
- 41.Pankey GA. Tigecycline. J Antimicrob Chemother. 2005;56(3):470–480. doi: 10.1093/jac/dki248 [DOI] [PubMed] [Google Scholar]
- 42.Abdallah M, Alsaleh H, Baradwan A, et al. Intraventricular tigecycline as a last resort therapy in a patient with difficult-to-treat healthcare-associated Acinetobacter baumannii ventriculitis: a Case Report. SN Compr Clin Med. 2020;2(9):1683–1687. doi: 10.1007/s42399-020-00433-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li LM, Zheng WJ, Shi SW. Spinal arachnoiditis followed by intrathecal tigecycline therapy for central nervous system infection by extremely drug-resistant Acinetobacter baumannii.. J Int Med Res. 2020;48(7):300060520920405. doi: 10.1177/0300060520920405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Pu Z, Zhao M. Case report of successful treatment of extensively drug-resistant Acinetobacter baumannii Ventriculitis with intravenous plus intraventricular tigecycline. Antimicrob Agents Chemother. 2018;62(11):e01625–18. doi: 10.1128/AAC.01625-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li W, Li DD, Yin B, et al. Successful treatment of pyogenic ventriculitis caused by extensively drug-resistant Acinetobacter baumannii with multi-route tigecycline: a case report. World J Clin Cases. 2021;9(3):651–658. doi: 10.12998/wjcc.v9.i3.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fang YQ, Zhan RC, Jia W, et al. A case report of intraventricular tigecycline therapy for intracranial infection with extremely drug resistant Acinetobacter baumannii. Medicine. 2017;96(31):e7703. doi: 10.1097/MD.0000000000007703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Zhang J, Yu X, et al. Intrathecal injection of tigecycline in treatment of multidrug-resistant Acinetobacter baumannii meningitis: a case report. Eur J Hosp Pharm. 2017;24(3):182–184. doi: 10.1136/ejhpharm-2016-000972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guangqiong YU, Zou Y, Liu H, et al. Synergistic effect of a combination of colistin and tigecycline against multidrug-resistant Acinetobacter baumannii. Drug Eval Res. 2018;54:e34. [Google Scholar]
- 49.Qu J, Yu R, Wang Q, et al. Synergistic antibacterial activity of combined antimicrobials and the clinical outcome of patients with carbapenemase-producing Acinetobacter baumannii infection. Front Microbiol. 2020;11:541423. doi: 10.3389/fmicb.2020.541423 [DOI] [PMC free article] [PubMed] [Google Scholar]