Abstract
Background
With long-term metabolic malfunction, diabetes can cause serious damage to whole-body tissue and organs, resulting in a variety of complications. Therefore, it is particularly important to further explore the pathogenesis of diabetes complications and develop drugs for prevention and treatment. In recent years, different from apoptosis and necrosis, ferroptosis has been recognized as a new regulatory mode of cell death and involves the regulation of nuclear receptor coactivator 4 (NCOA4)-mediated ferritinophagy. Evidence shows that ferroptosis and ferritinophagy play a significant role in the occurrence and development of diabetes complications.
Scope of review
we systematically review the current understanding of ferroptosis and ferritinophagy, focusing on their potential mechanisms, connection, and regulation, discuss their involvement in diabetes complications, and consider emerging therapeutic opportunities and the associated challenges with future prospects.
Major conclusions
In summary, ferroptosis and ferritinophagy are worthy targets for the treatment of diabetes complications, but their complete molecular mechanism and pathophysiological process still require further study.
Keywords: Ferroptosis, Ferritinophagy, Mitochondria, Diabetes complications
Abbreviations: ACSL4, acyl CoA synthase long-chain family member 4; DCM, Diabetic cardiomyopathy; DF, Diabetic foot; DMT1, divalent metal transporter 1; DN, Diabetic nephropathy; DPN, Diabetic peripheral neuropathy; DR, Diabetic retinopathy; Fer-1, ferrostatin-1; FPN1, ferroportin 1; FTH1, ferritin heavy chain-1; GDM, gestational diabetes mellitus; GPX4, glutathione peroxidase 4; GSH, glutathione; HO-1, heme oxygenase-1; Keap1, Kelch-like ECH-associated protein 1; lamp2a, lysosome-associated membrane protein 2a; LIP, labile iron pool; LPCAT3, lysophosphatidylcholine acyltransferase 3; NCOA4, Nuclear receptor coactivator 4; NRF2, nuclear factor erythroid 2-related factor 2; PUFAs, polyunsaturated fatty acids; SGLT2Is, sodium-glucose cotransporter type2 inhibitors; SLC3A2, solute carrier family 3 member 2; SLC7A11, solute carrier family 7 members 11; TFR1, transferrin receptor 1
1. Introduction
Diabetes mellitus, a group of lifelong metabolic diseases with multiple etiologies, is characterized by chronic hyperglycemia [1]. Long-term metabolic disorder and continuous hyperglycemia can gradually aggravate the damage of the patient's nervous system and cardiovascular, kidney, and other systemic tissues and organs, resulting in various complications [2]. According to the 2019 International Diabetes Federation statistics, the morbidity and mortality rates of diabetes increase every year, threatening people's physical and mental health [3,4]. Therefore, a deep exploration of the pathogenesis of diabetes complications and emerging treatment opportunities are global public health goals.
Recent studies suggest that the development of diabetes complications is closely related to a cell death pattern called ferroptosis. Ferroptosis is a new form of iron-dependent regulatory death, which is morphologically, biochemically, and genetically distinct from apoptosis, necrosis, and autophagy [5]. The most marked peculiarities of ferroptosis are iron homeostasis imbalance and excessive lipid peroxidation [5]. Studies have shown that the regulatory effect of ferritinophagy on ferroptosis involves its over-activation-induced intracellular iron overload and that excessive iron is the basis of inducing ferroptosis [6].
Ferroptosis is associated with varieties of cellular metabolic pathways, including redox homeostasis [7], iron metabolism [5,8], mitochondrial activity [9], and various disease-related signal pathways [[10], [11], [12]]. Recently, it was shown that due to the disorder of cell metabolic pathways, ferroptosis and ferritinophagy exist in diabetes complications [9,13] to include iron overload caused by iron metabolism disorder, a link to promoting ferroptosis, which is closely related to the occurrence and development of diabetes complications [[13], [14], [15]]. In addition to the disorder of iron metabolism, the imbalance of redox homeostasis also leads to the accumulation of reactive oxygen species (ROS) and eventually promotes the occurrence of ferroptosis [9,[16], [17], [18]]. Understanding the potential mechanisms of ferroptosis and ferritinophagy and related regulatory networks may provide effective strategies for the treatment of diabetes complications.
Many studies have demonstrated the ability to signal molecules or drugs to regulate ferroptosis and ferritinophagy in the prevention and treatment of diabetes complications, for example, the application of some ferroptosis inducers or inhibitors [19] and the regulation of iron metabolism-related gene expression [20]. Consequently, ferroptosis and ferritinophagy are expected to become targets in the study of diabetes complications.
We review the recent progress in understanding ferroptosis and ferritinophagy and their potential mechanisms in diabetes complications, and we consider emerging therapeutic opportunities and the associated challenges.
2. Mechanism of ferroptosis and ferritinophagy
2.1. Function and mechanism of ferroptosis
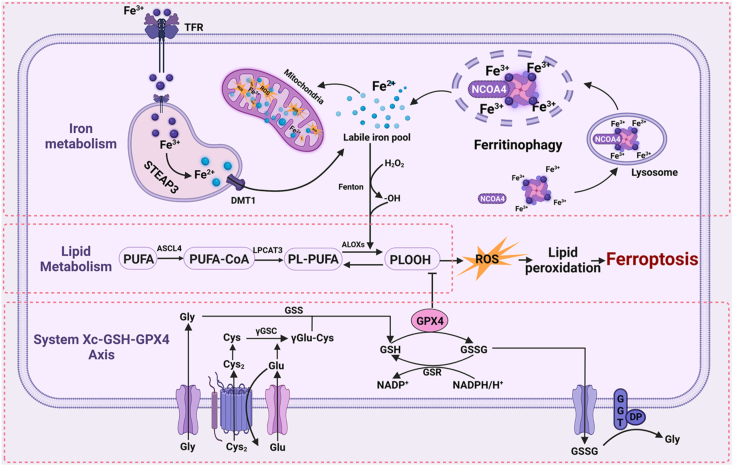
Cell death is a universal life phenomenon in the biological world. It is an irreversible and important link under certain physiological and pathological conditions [21]. There are a variety of death modes. Traditionally, cell death is divided into two main types: apoptosis and necrosis [22]. However, in recent years, studies have proved that ferroptosis, pyroptosis, parthanatos, entotic cell death, and others are also involved in cell death under many pathophysiological conditions [23]. Among these, ferroptosis has recently been described as a form of programmed cell death. Unlike the others, in ferroptosis no chromatin condensation is observed in apoptosis and no loss of plasma membrane integrity is observed in necrosis [24]. In 2003, a selective, lethal, small molecule, first labeled erastin, can induce cell death in rat sarcoma (RAS)-expressing tumorigenic cells, which is an activator of this phenomenon [25]. In 2012, Dixon et al. named this death mode of iron-dependent, non-apoptotic cell necrosis “ferroptosis” [5]. It is caused by the imbalance between the generation and degradation of intracellular lipid ROS. This process is inseparable from iron ion concentration, and the mechanism is shown in Figure 1. The regulatory mechanisms of iron death are mainly iron metabolism, system Xc-, glutathione (GSH) and glutathione peroxidase 4 (GPX4) regulation, and lipid metabolism.
Figure 1.
Three mechanisms of ferroptosis and the relationship between ferroptosis and ferritinophagy. The regulation mechanism of ferroptosis is mainly related to the regulation of iron metabolism, GSH and GPX4, and peroxide. The association between ferritinophagy and ferroptosis is mediated by NCOA4. Intracellular ferritin is transported to autophagy lysosomes for degradation and release of free iron, which eventually leads to ferroptosis. TFR, transferrin receptor; STEAP3, the six-transmembrane epithelial antigen of the prostate 3; DMT1, divalent metal transporter 1; ROS, reactive oxygen species; NCOA4, nuclear receptor co-activator 4; PUFA, polyunsaturated fatty acids; PUFA-CoA, polyunsaturated fatty acyl CoA; PL-PUFA, phospholipid-bound polyunsaturated fatty acids; PLOOH, phospholipid hydroperoxides; ACSL4, acyl-CoA synthetase long-chain family member 4; LPCAT3, lysophosphatidylcholine acyltransferase 3; ALOXs, arachidonic acid lipoxygenases; Gly, glycine; Cys2, cysteine residues; Cys, cysteine; GSS, glutathione synthetase; γGSC, γ-glutamylcysteinyl synthetase; γGlu-Cys, γglutamyl-cysteine; GSH, glutathione; GPX4, glutathione peroxidase 4; GSSG, glutathione oxidized; GSR, glutathione-disulfide reductase; NADPH, nicotinamide adenine dinucleotide phosphate; NADP+, the oxidized form of NADPH; GGT, Glutamyl transpeptidase.
2.1.1. Iron metabolism
Iron is one of the essential nutritional elements of organisms. It mainly exists in the form of Fe3+ in food and is absorbed in the intestinal epithelial cell [26]. In circulation, iron binds with transferrin (TF) in the form of Fe3+ and is transported through transferrin receptor 1 (TFR1) on the surface of the cell membrane. The conjugate enters the cell through an endocytosis manner [27]. In the acidic endosomal environment, Fe3+ is released from the conjugate, reduced to Fe2+ by six transmembrane epithelial antigens of prostate 3 (STEAP3), and then enters the cytoplasm from the endosome through divalent metal transporter 1 (DMT1) [28]. Excess iron is mainly stored in ferritin in the form of redox inactivity. A small amount of Fe2+ forms the labile iron pool (LIP) [29]. Fe2+ is transported to the outside of cells under the action of membrane iron transporter 1 (FPN1). Generally, the balance of iron in cells is reflected in the balance among iron absorption, output, utilization, and storage [30]. When intracellular iron is overloaded, on the one hand, the highly oxidizing free Fe2+ can easily undergo Fenton reaction with lipid peroxide, produce hydroxyl radical, arouse strong oxidative stress response, produce a large number of ROS and induce ferroptosis [31]. On the other hand, Fe2+ is a cofactor that enhances the activities of various metabolic enzymes, promotes the production of lipid ROS, and then promotes ferroptosis [28]. In conclusion, abnormal iron metabolism may increase intracellular iron, cause iron-related ROS deposition, and induce ferroptosis. Iron is an essential element of ferroptosis, and iron metabolism is a necessary process of ferroptosis.
2.1.2. System Xc-GSH-GPX4 axis
System Xc-is the cystine/glutamate antiporter system that is composed of two independent proteins; one is called solute carrier family 7 members 11 (SLC7A11), and the other is solute carrier family 3 member 2 (SLC3A2). The System Xc-exchanges glutamate and cystine inside and outside of cells in a ratio of 1:1 [32]. Cystine transported into cells is reduced to cysteine for the synthesis of GSH. Glutathione is a necessary cofactor of GPX4. GPX4 can simultaneously convert reduced GSH into oxidized GSH and reduce lipid peroxide, so as to reduce oxidative stress injury [33]. This step of uptake of cystine by System Xc-is the rate-limiting step of cysteine synthesis. Blocking or inhibiting this step can lead to the reduction of intracellular cysteine, inhibit the lipid repair function of GPX4, and promote ferroptosis by reducing the antioxidant capacity of cells [34].
2.1.3. Lipid metabolism
Lipid metabolomics showed that polyunsaturated fatty acids (PUFAs) such as arachidonoyl (AA) or adrenoyl (ADA) are the lipids most prone to oxidation during ferroptosis and that they are regulated by three synthases [35]. Among them, the acyl CoA synthase long-chain family member 4 (ACSL4) and the lysophosphatidylcholine acyltransferase 3 (LPCAT3) involved in the synthesis of PUFAs were found by Dixon to play an important role in the ferroptosis pathway by inserting a large number of mutations into haploid cells [36]. In addition, lipoxygenase (LOX) also mediates ferroptosis [35]. Some studies have shown that knockout or inhibition of the above three synthase can inhibit the occurrence and development of ferroptosis [37,38].
The above data confirm that the most important characteristics of ferroptosis are the increase of intracellular iron ion concentration and the abnormal accumulation of lipid reactive oxygen species. The three ferroptosis mechanism pathways and their interrelation are shown in Figure 1.
2.2. The connection between ferroptosis and ferritinophagy
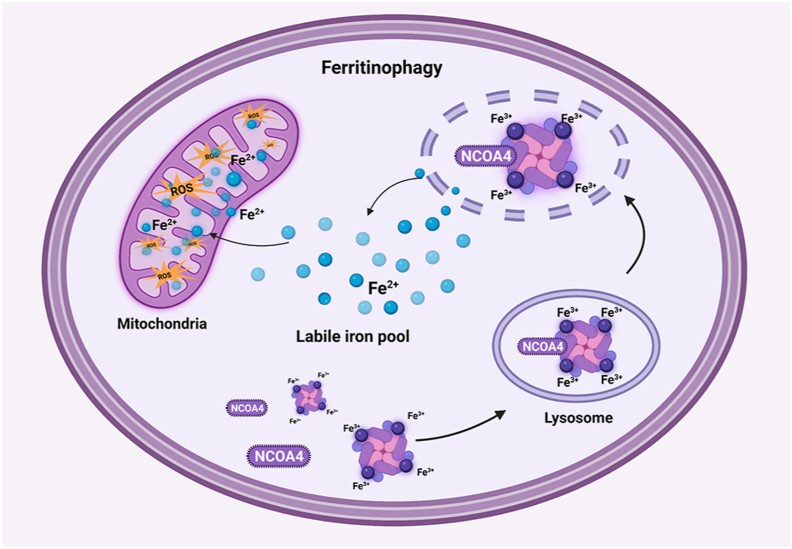
Nuclear receptor coactivator 4 (NCOA4)-mediated ferritinophagy to degrade ferritin is a feedback regulation mechanism of available iron in cells, and its activation will increase the content of available iron in cells. The process of ferritinophagy is shown in Figure 2. Mechanistically, NCOA4 acts as a selective autophagy receptor and binds to ferritin heavy chain-1 (FTH1) of ferritin to mediate the transport of intracellular ferritin to autophagy lysosomes and finally release free iron. This regulation is not unidirectional, and the intracellular iron level will also affect the ferritinophagy flux [39]. When iron is abundant, HERC2 (E3 ubiquitin ligase) mediates the degradation of NCOA4 in a ubiquitin-dependent manner to reduce ferritinophagy flux to balance iron levels [40]. Notably, the level of NCOA4 is the core factor in determining ferritinophagy flux.
Figure 2.
Mechanism of ferritinophagy. NCOA4 mediated ferritinophagy transports intracellular ferritin to autophagy lysosome for degradation and release free iron. By overloading intracellular iron, ROS production in the labile iron pool increases, so as to increase the sensitivity of cells to ferroptosis. NCOA4, nuclear receptor co-activator 4; ROS, reactive oxygen species.
Under physiological conditions, ferritinophagy can maintain intracellular iron balance, but its excessive activation will lead to intracellular iron overload [39]. Lipid peroxide deposition associated with iron overload is a necessary condition for ferroptosis. All these show that ferritinophagy is closely related to ferroptosis, as shown in Figure 1. Bellelli et al. confirmed that GPX4, an important antioxidant enzyme that inhibits ferroptosis, is highly expressed in NCOA4 knockout mice, which revealed the correlation between ferroptosis and ferritinophagy [41]. In addition, some studies have shown the promoting effect of ferritinophagy on ferroptosis, which is mainly due to iron overload caused by increased expression of NCOA4. Sorafenib, a ferroptosis inducer, whose potential mechanism is to increase the expression of NCOA4 to promote iron overload and the production of ROS [42]. In addition, it has been proved that the increase of iron level caused by overexpression of NCOA4 will increase the sensitivity to ferroptosis [41]. On the contrary, ferritinophagy has a protective effect on ferroptosis. When NCOA4 itself is missing or its binding to FTH1 is blocked, ferritinophagy is obstructed, which will reduce the sensitivity of cells to ferroptosis [43]. This confirms that synthesized compound 9a directly binds to NCOA4, blocking its binding to FTH1 and thereby reducing iron levels to block ferroptosis [44]. Moreover, knockout of the NCOA4 gene blocked sideroflexin 1 (SFXN1) mediated iron overload in mitochondria, which inhibited ferroptosis [45]. In conclusion, this data suggests that iron overload is the initiating link of ferritinophagy triggering ferroptosis. The key to regulating ferritinophagy is to affect its core substance, NCOA4, by regulating the level or hindering its binding to ferritin.
In summary, NCOA4-mediated ferritinophagy plays an important role in regulating ferroptosis, mainly by regulating intracellular iron balance and affecting the production of ROS. Future studies are necessary to explore ways that ferritinophagy regulates ferroptosis and to explore intervention drugs targeting ferritinophagy to improve the ferroptosis-related pathological process in various diseases.
2.3. Regulator of ferroptosis and ferritinophagy
2.3.1. Ferroptosis and ferritinophagy inducers
According to different mechanisms, ferroptosis and ferritinophagy inducers are divided into three types.
The first is System Xc-inhibitors, such as erastin, sorafenib, p53, and sulfasalazine. Erastin is a powerful inducer of ferroptosis. On the one hand, it directly inhibits system Xc-, blocks the uptake of cystine by cells, and reduces the content of GSH. It reduces GPX4 activity and weakens the anti-lipid oxidation ability of cells [5]. On the other hand, it also acts on the voltage-dependent, anion-selective channel protein 2 (VDAC2/3). Erastin inhibits its activity, leads to mitochondrial dysfunction, and aggravates cellular lipid peroxidation [46]. In addition, ferroptosis caused by erastin can increase the expression level of lysosome-associated membrane protein 2a (lamp2a) to promote chaperone-mediated autophagy and in turn promote the degradation of GPX4 [47]. Sorafenib can inhibit system Xc- and trigger ER stress and ferroptosis [48]. The mutation of the p53 DNA domain to p533KR can limit cystine uptake by inhibiting the transcription of SLC7A11, making cells more sensitive to ferroptosis induced by oxidative stress [49]. Sulfasalazine can induce ferroptosis in pancreatic cancer cells by inhibiting the System Xc-; such a method of killing cancer cells provides new ideas for the treatment of pancreatic cancer [50].
The second type is GSH up-regulators, including BSO, cysteinase, acetaminophen, and cisplatin. BSO inhibits the activity of the rate-limiting enzyme of GSH synthesis γ-Glutamyl cysteine synthase (γ-GCS) prevents the synthesis of dipeptides from glutamate and cysteine, which makes the reduced GSH unable to be synthesized, and finally leads to the lack of GSH [10]. Cysteinase can directly degrade cysteine and block the synthesis of GSH [51]. Ferroptosis is also associated with primary hepatocytes death induced by acetaminophen (APAP). The highly reactive metabolite of APAP is N-acetyl-p-benzoquinone imine (NAPQI). NAPQI in APAP reacts rapidly with GSH, resulting in a large consumption of GSH and eventually ferroptosis [52]. Cisplatin is a first-line broad-spectrum anticancer drug. Recent studies have shown that cisplatin synergizes with prlx93936 can induce ferroptosis in non-small-cell lung cancer (NSCLC). In the cytoplasm, the main part of cisplatin binds to GSH. Similar to erastin, GSH consumption and GPX4 inactivation are the basic mechanisms of cisplatin-induced ferroptosis [53].
The third type is GPX4 inhibitors causing ferroptosis, including RAS-selective lethal 3 (RSL3), diphenyleneiodonium7 (DPI7), diphenyleneiodonium10 (DPI10), FIN56, altretamine, and withaferin A. RSL3 can bind to GPX4 and inhibit its protein activity, resulting in excessive accumulation of intracellular lipid peroxide [24]. Similarly, DPI7 and DPI10 can induce ferroptosis by directly inhibiting GPX4 activity [10]. FIN56 is a specific ferroptosis inducer that can promote the degradation of GPX4. In addition, it also binds to and activates squalene synthase, resulting in the consumption of endogenous antioxidant coenzyme Q10. This process enhances the sensitivity of cells to ferroptosis caused by FIN56 [54]. Studies have shown that altretamine is a new GPX4 inhibitor and suggests a potential mechanism for its antineoplastic activity [55]. Withaferin A (WA) is a natural ferroptosis-inducing agent in neuroblastoma, which acts through a novel double-edged mechanism. One mechanism is that WA dose-dependently induces ferroptosis by activating the nuclear factor-like 2 pathway through targeting of Kelch-like ECH-associated protein 1 (Keap1). The other mechanism is to inactivate GPX4 and then induce ferroptosis [56].
Several inducers bring out ferroptosis in other ways. On the inhibition of nuclear factor erythroid 2-related factor 2 (NRF2) by trigonelline (fenugreen), the expression of MT-1G was blocked, which reduced the content of GSH and further caused ferroptosis [57]. Artesunate (Art) activates lysosomal function and promotes ferritin degradation. It forms free radicals through the Fenton reaction, resulting in lysosomal rupture and ferroptosis [58]. FINO can initiate ferroptosis through multiple mechanisms, including both indirectly inhibiting GPX4 enzymatic function and directly oxidizing iron, ultimately causing widespread lipid peroxidation [59]. AMID (AIF-homologous mitochondrion-associated inductor of death) was later renamed ferroptosis suppressor protein 1 (FSP1), which was initially described as a pro-apoptotic gene [60]. GPX4 gene-deleted cancer cells can be effectively cleared by FSP specific inhibitor iFSP1. In cancer cells expressing GPX4, iFSP1 can cooperate with RSL3 to induce ferroptosis of cancer cells [59]. In addition, ferric ammonium citrate leads to iron overload by up-regulating the level of intracellular free iron ions, and iron ions produce excessive free radicals through the Fenton reaction [61].
2.3.2. Ferroptosis and ferritinophagy inhibitors
Because the occurrence of ferroptosis is closely related to iron metabolism and lipid peroxidation, most inhibitors against ferroptosis belong to iron chelators or antioxidants. Iron chelators such as deferoxamine (DFO) can bind free iron ions, inhibit Fenton reaction, inhibit lipid peroxidation, and then inhibit the occurrence of ferroptosis [62]. Antioxidants such as ferrostatin-1 (Fer-1) and liproxstatins can inhibit lipid peroxidation and reduce the production of ROS [5,63]. In addition, lipid peroxidase inhibitors (such as vitamin E) can not only inhibit LOX activity, but also have weak chemical bonds to facilitate the transport of hydrogen atoms, which protect cells from ferroptosis and oxidative damage [64]. Recent studies have confirmed that transcription factor nuclear factor erythroid-2, like-1 (NFE2L1), maintains proteasome function, which reveals a new mechanism of anti-ferroptosis [65].
Many studies have reported the regulators of ferroptosis and ferritinophagy and their indications in different diabetes complications, as shown in Table 1.
Table 1.
Regulators of ferroptosis and ferritinophagy as well as their indications in different diabetes complications.
| Regulators | Possible mechanisms | Induce or inhibit ferroptosis | Diseases | Reference |
|---|---|---|---|---|
| Erastin, RSL3 | by causing iron accumulation and high ACSL4 levels to sensitize cells to ferroptosis | inducer | DN | [19] |
| Erastin | by aggravating endoplasmic reticulum stress to promote ferroptosis injury | inducer | DMIRI | [66] |
| p53 | stimulation of ferroptosis by high glucose-induced activation of the p53-xCT-GSH axis | inducer | DED | [67] |
| Fer-1 | By improving high glucose and high fat-induced lipid peroxidation, and down regulating the production of ROS | inhibitor | DA | [68] |
RSL3, RAS-selective lethal 3; ACSL4, acyl CoA synthase long-chain family member 4; xCT, the substrate-specific subunit of system Xc-; GSH, glutathione; Fer-1, ferrostatin-1; ROS, reactive oxygen species; DN, diabetic nephropathy; DMIRI, diabetic myocardial ischemia-reperfusion injury; DED, diabetes-induced endothelial dysfunction; DA, diabetic atherosclerosis.
2.3.3. NRF2 signaling
The above elaborates some small molecule regulators of ferroptosis and ferritinophagy. At the same time, in specific cells, some macromolecular regulatory proteins and their related different signal pathways can also regulate ferroptosis. Among them, the most closely related to diabetes is the NRF2 signaling pathway. Therefore, we only explain the NRF2 signaling here and do not repeat the relationship between other pathways and ferroptosis.
As an important antioxidant stress transcription factor, NRF2 plays a key role in improving cell tolerance to oxidative stress. NRF2 binds to its activity inhibitor Keap1 and exists in the cytoplasm. When stimulated by oxidative damage, NRF2 separates from Keap1 and enters the nucleus to boost the expression of downstream target genes [69]. In terms of mechanism, NRF2 reduces the damage that oxidative stress causes by regulating antioxidant enzymes and iron metabolism [70]. Since the ferroptosis process is also dependent on the accumulation of lipid peroxide and iron, the inhibition of NRF2 may significantly increase the sensitivity to ferroptosis [70].
There also exists some evidence that NRF2 related pathways can regulate the occurrence of ferroptosis. After being treated with erastin and sorafenib, p62 inhibits the degradation of NRF2 and makes NRF2 aggregate in the nucleus through Keap1 inactivation, so as to regulate downstream gene transcription; in other words, the p62-Keap1-NRF2 pathway is activated to protect hepatocellular carcinoma cells from ferroptosis [71]. In addition, gastrodin (gas) increased the nuclear transport of NRF2 and up-regulated the expression of heme oxygenase-1 (HO-1) downstream protein of mice hippocampal neurons (HT-22) cells, that is, it protected HT-22 cells from glutamate-induced ferroptosis through NRF2/HO-1 signaling pathway [72]. In colorectal cancer cells, endoplasmic reticulum (ER) and oxidative stress induced by tagitin C activate protein kinase-like ER kinase (PERK), which can induce the uncoupling of NRF2 and Keap1 and promote the nuclear translocation of NRF2 [73]. As a downstream gene of NRF2, the expression of heme oxygenase-1 (HO-1) increased significantly. The up-regulation of HO-1 leads to the increase of unstable iron pool, which promotes lipid peroxidation and finally induces ferroptosis. That is, tagitin C induces ferroptosis through the activation of the PERK-NRF2-HO-1 signaling pathway mediated by ER [74]. In addition, there are other pathways related to NRF2 that regulate ferroptosis: GSK-3β/NRF2 signaling pathway [75], Akt/NRF2/HO-1 signaling pathway [76], Keap1/NRF2-ARE signaling pathway [77], and AMPK-NRF2 signaling pathway [78], all of which play a regulatory role on ferroptosis in specific cells.
3. Ferroptosis and ferritinophagy participate in the pathogenesis of diabetes complications
Many studies have shown that ferroptosis and ferritinophagy are involved in the development and progression of diabetes complications (some upstream core substances are involved in diabetes complications by regulating ferroptosis and ferritinophagy, as shown in Table 2). Studies have shown that iron overload, the key initiating factor of ferroptosis, will aggravate insulin resistance in the absence of inflammation in diabetic mice [79]. But there are limited reports of relevant mechanisms. Based on previous studies, as shown in Figure 3, we summarized the mechanisms involved in ferroptosis and ferritinophagy in several important diabetes complications and their cross mechanisms. Most literature shows that in some diabetes complications, high glucose promotes the change of the upstream core regulatory substances then makes iron overload and reduces the antioxidant capacity through different pathways [80,81]. The accumulation of lipid oxidants in cells is the common pathway and in the end results in ferroptosis. Notably, the NRF2 signaling pathway is inseparable from ferroptosis and ferritinophagy in the process of diabetes complications [80,82,83]. Because of the unclear mechanism of NRF2 and its downstream targets in some complications, there is no more NRF2 related content in Figure 3.
Table 2.
Upstream regulatory core substances participate in diabetes complications through the regulation of ferroptosis and ferritinophagy.
| Core substances regulated in upstream mechanisms | Possible mechanisms | Induce or inhibit ferroptosis | Diseases | Reference |
|---|---|---|---|---|
| HIF-1α | Through the enhancement of the HIF-1α/HO-1 pathway, heme decomposition increased, resulting in intracellular iron accumulation | induce | DN | [15] |
| HMGB1 | The NRF2 pathway includes its downstream targets HO-1, NQO-1, GCLC and GCLM | induce | DN | [80] |
| Sp1 | Sp1-mediated upregulation of Prdx6 expression | inhibit | DN | [94] |
| salusin-β | participate in NRF-2-dependent manner | induce | DN | [95] |
| TRIM46 | upregulate TRIM46, induce ubiquitination and accelerate clearance of GPX4 | induce | DR | [102] |
| HSF1 | maintain cellular iron homeostasis and GPX4 expression | inhibit | DCM | [20] |
| METTL3 | METTL3/ASK1-p38 signaling pathway is activated | induce | DO | [123] |
| NRF2 | Regulate iron metabolism homeostasis through NRF2/FPN1 pathway | inhibit | DMIRI | [124] |
HIF-1α, hypoxia-inducible factor-1α; HO-1,heme oxygenase-1; HMGB1, high-mobility group box-1; NRF2, nuclear factor E2-related factor2; NQO-1, oxidoreductase1, GCLC, glutathione cysteine ligase catalytic subunit; GCLM, glutathione cysteine ligase modulatory subunit; Sp1, specificity protein 1; Prdx6,peroxiredoxin 6; TRIM46, tripartite motif-containing 46;GPX4,glutathione peroxidase 4; HSF1,heat shock factor 1; METTL3, methyltransferase-like 3; ASK, Apoptosis signal-regulating kinase 1; p38, mitogen-activated protein kinase; FPN1, ferroportin1; DN, diabetic nephropathy; DR, diabetic retinopathy; DCM, diabetic cardiomyopathy; DO, diabetic osteoporosis; DMIRI, diabetic myocardial ischemia-reperfusion injury.
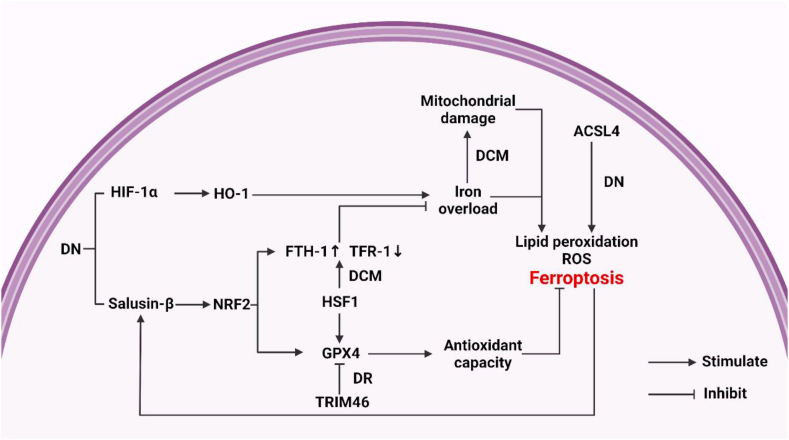
Figure 3.
Mechanism of ferroptosis and ferritinophagy involved in diabetes complications. HIF-1α, hypoxia-inducible factor-1α; HO-1, heme oxygenase-1; NRF2, nuclear factor E2-related factor 2; FTH-1,ferritin heavy chain-1; TFR-1, transferrin receptor-1; HSF1,heat shock factor 1; TRIM46, tripartite motif-containing 46; GPX4, glutathione peroxidase 4; ACSL4, acyl-CoA synthetase long-chain family member 4; DN, diabetic nephropathy; DR, diabetic retinopathy; DCM, diabetic cardiomyopathy.
3.1. Diabetic nephropathy
Diabetic nephropathy (DN) is the most common and frequent microvascular complication in the later stage of diabetes, and it is also a common cause of end-stage renal disease [81]. The pathogenesis of DN is complex, involving the disorder of glucose metabolism, oxidative stress, hemodynamic abnormalities, genes, and inflammation. The above are also important reasons for the progress of DN [84]. Although our understanding of the mechanism of DN has deepened, we are still unable to clarify the specific molecular mechanism and pathophysiological process of the occurrence and development of DN. New studies have shown that ferroptosis has been observed in renal diseases such as acute kidney failure [85], renal ischemia-reperfusion injury [86], and DN [19]. These findings show that ferroptosis plays a significant role in the occurrence and development of DN disease.
Many studies can reflect a conclusion: Ferroptosis exists in the occurrence and development of DN. One retrospective survey showed that compared with the healthy control group, the renal biopsy results of DN patients showed that renal tubular epithelial cells showed higher iron deposition and transferrin expression, and the key is that intracellular iron deposition is the significant factor inducing ferroptosis [87]. Moreover, by establishing the streptozotocin (STZ)-induced mouse type 1 diabetes mellitus model in vivo, Wang, Y et al. found for the first time that there were significant changes in markers related to ferroptosis in DN mice, such as the expression level of ACSL4, a key marker of lipid peroxide accumulation, increased and the expression level of GPX4, an important regulator of redox homeostasis, decreased [19]. The above studies show that there are typical characteristics of ferroptosis in DN: iron overload and lipid peroxide accumulation.
The application of iron death inducers and inhibitors in DN can also confirm the previous conclusion. It was found that for renal tubular cells 52E and HK-2 under a high glucose environment in vitro, the intracellular high iron and ACSL4 levels caused by ferroptosis inducers erastin and RSL3 may make the cells sensitive to ferroptosis [19]. In addition to renal tubular cells, studies have found that the use of ferroptosis inducer erastin on renal mesangial cells in vitro can also lead to ferroptosis [80]. On the contrary, it has been found that the use of ferroptosis inhibitor Fer-1 has a relieving effect on diabetic nephropathy induced by transforming growth factor-β1 (TGF-β1) [88].
Only a few studies have revealed the mechanism of ferroptosis in the development of DN. Feng's study revealed the internal mechanism, suggesting that ferroptosis may be through the hypoxia-inducible factor (HIF)-1α/HO-1 pathway that aggravates renal tubular injury and fibrosis in diabetic mice. The reason is that the further injury and fibrosis of renal tubules in DM mice will increase the levels of HIF-1α and HO-1. Increased heme decomposition leads to the accumulation of iron in mouse renal tubules, which results in increased production of ROS and accumulation of lipid peroxidation. The characteristics of ferroptosis are becoming more and more obvious [15]. However, it is worth noting that HO-1 seems to have a dual role in ferroptosis. Studies have shown that HO-1 plays an important intermediary role in the cause of ferroptosis and plays a pathogenic role in the development of several diseases [89,90]. Some studies have shown, though, that HO-1 plays an important protective role against oxidative stress in renal epithelial cells [91]. Nevertheless, ferroptosis is often accompanied by the reduced ability to resist oxidative stress which contradicts Feng's research conclusion. In addition, some studies have found that when ACSL4, a key marker of ferroptosis and lipid peroxidation accumulation, is inhibited by its inhibitor rosiglitazone (Rosi), it may inhibit the inflammatory response, then inhibit ferroptosis, and finally improve the damage of renal tubular cells in high glucose environment [19]. This suggests that ferroptosis may aggravate the further injury of renal tubules in DN through inflammatory reaction.
In DN, in addition to the injury mechanism of ferroptosis in renal tubular cells, studies have also explored the injury mechanism of ferroptosis in mesangial cells, podocytes, and other parts. High mobility group box-1 (HMGB1) is a transcription factor involved in DNA recombination and repair. It is abundant in the nucleus. Blocking the interaction between HMGB1 and its receptor has been proved effective in preventing DN [92]. Wu et al. found that HMGB1 regulates glucose-induced ferroptosis via the NRF2 pathway in mesangial cells including its downstream targets HO-1, NQO-1, GCLC, and GCLM [80]. In the clinic, podocyte loss and podocyte integrity damage occur in the early stage of diabetes mellitus. Podocyte injury is closely related to DN glomerular injury [93]. Q. Zhang et al. found that specific protein 1 (SP1)-mediated up-regulation of peroxiredoxin 6 (prdx6) expression can prevent DN podocyte injury through antioxidant stress and anti-cell ferroptosis [94].
It should be noted that there are contradictions about the role of the NRF2 pathway in DN. Different from the previously mentioned role of the NRF2 pathway, W. J. Wang et al. found salusin-β is involved in high glucose-induced ferroptosis of renal tubular cells HK-2 in an NRF2 dependent manner, indicating that salusin-β and ferroptosis form a positive feedback loop, which promotes high glucose-induced renal tubular cell injury [95]. However, this effect is only explored in cell experiments, and animal experiments are still needed for further research.
3.2. Diabetic retinopathy
Diabetic retinopathy (DR) is a common microvascular complication of diabetes, and it is also the main cause of blindness in the vision of 20–75-year-olds worldwide [96].
There is evidence that the pathogenesis of DR is associated with ferroptosis. The pathogenesis of DR is complex. Among them, oxidative stress also plays an important role in the occurrence and development of DR. It seems that the key mediator of oxidative stress can also activate the occurrence of ferroptosis. In hyperglycemia, the inhibition of thioredoxin-interacting protein (TXNIP), a mediator of oxidative stress on Trx may lead to cellular oxidative stress, mitochondrial dysfunction, lysosomal damage, and pro-inflammatory cell death in DR patients. The TXNIP-TRX-TrxR redox pathway may be involved in retinal pigment epithelium dysfunction of DR and other neurodegenerative diseases [97]. Moreover, studies have shown that interfering with the function of CISD 2 protein in human breast cancer cells can enhance the expression of tumor suppressor TXNIP, which is related to the activation of ferroptosis [98].
Many studies can reflect that there is a certain correlation between the mechanism of pathological changes in the occurrence and development of DR and ferroptosis. Among them, neuronal apoptosis and reactive glial degeneration have recently been considered to be the early pathological changes of DR [99]. Studies have shown that neuronal apoptosis and degenerative changes caused by abnormal high phosphorylation of tau-related proteins play a key role in the occurrence and development of DR [100]. Another study has shown that tau-related proteins can induce iron overload, lipid peroxidation, and inflammation, and all are related to ferroptosis [101]. This suggests that neuronal apoptosis and degenerative changes in DR seem to be associated with ferroptosis through tau-related proteins. In addition, the increased permeability of retinal capillary endothelial cells is another key feature of DR progress. This change is closely related to the occurrence of ferroptosis. Recent studies have shown that high glucose treatment induces ferroptosis in human retinal capillary endothelial cells (HRCECs) by up-regulating TRIM46, inducing ubiquitination, and accelerating GPX4 clearance [102].
3.3. Diabetic cardiomyopathy
Diabetic cardiomyopathy (DCM) is an independent, specific diabetic heart complication that does not depend on primary cases such as hypertension and other heart diseases in diabetes.
There is new evidence that the typical characteristics of ferroptosis exist in the occurrence and development of DCM, which indicates that there is an association between DCM and ferroptosis. It was found that there was iron deposition in the cells of DCM rats [103]. Moreover, iron deposition is one of the main causes of ferroptosis, and it is also a typical feature of ferroptosis. At the same time, intracellular iron deposition also promotes, to varying degrees, mitochondrial damage including abnormal mitochondrial structure (mitochondrial atrophy, rupture of the mitochondrial outer membrane, the disappearance of the mitochondrial ridge, etc.), burst of mitochondrial ROS, change of mitochondrial membrane potential, and accumulation of mitochondrial lipid peroxide, which are considered typical characteristics of cell ferroptosis [104]. In the heart of diabetic mice, the typical mitochondrial abnormalities of ferroptosis were also found. Wang's experiments showed that compared with non-diabetic mice, mitochondria in the heart of diabetic mice were a singularity, the crest was not clear, and the mitochondrial membrane potential decreased significantly. In addition, the expression of superoxide dismutase (SOD2) and glutathione peroxidase 1 (GPX1) in mitochondria was down-regulated, and the level of mitochondrial ROS was significantly high [105]. Under the condition of high glucose in the blood, oxidative stress and damage of the antioxidant system are the basis of DCM [106]. The above research evidence suggests that ferroptosis seems to lead to mitochondrial damage through iron overload so as to damage the antioxidant system and participate in the pathogenesis of DCM.
The application of some ferroptosis inhibitors also suggests the relationship between ferroptosis and DCM. Vitamin E not only acts as a ferroptosis inhibitor but also an antioxidant and an endogenous antioxidant defense factor, and the latter plays an important role in diabetes complications. Studies have shown that vitamin E can improve the antioxidant defense system by inhibiting lipid peroxidation and save diabetes-induced heart failure in rats [107]. The level of intracellular nutrient coenzyme Q10 (CoQ10) is closely related to the cellular antioxidant system and participates in the regulation of ferroptosis [108]. K. Huynh et al. found CoQ10 attenuated diabetes-induced diastolic dysfunction, cardiomyocyte hypertrophy, cardiac fibrosis, and cell death, and these can repair the heart damage caused by diabetes [109].
However, the internal mechanism of ferroptosis that leads to the development of DCM remains unclear. Zang briefly explained how ferroptosis led to the deterioration of DCM. Diabetes can lead to autophagy the deficiency. Autophagy deficiency will close the NRF2-mediated defense and initiate the NRF2-mediated pathological process of ferroptosis in cardiomyocytes, thus worsening the progression of DCM [83]. This indicates that the potential mechanism of ferroptosis leading to the development of DCM seems to be related to NRF2. HSF1, a stress-inducible transcription factor, is well known for its pivotal role in HSR through transcriptional activation of various HSPs. Recent studies have found that HSF1 may be a key defense substance to prevent palmitic acid (PA)-induced ferroptosis in cardiomyocytes by regulating the expression of iron metabolism-related genes and GPX4.
3.4. Diabetic foot
Diabetic foot (DF) is one of the serious complications of diabetes. The main reason is that the sustained hyperglycemia in the patient will cause damage to the endothelial cell membrane, and a large number of lipid deposits in the intima, increasing blood coagulation and hardening the arterial wall. DF microvascular and neurogenic lesions are vulnerable to infection and invasion, resulting in the diabetic foot [110]. Therefore, exploring the potential mechanism of DF is an effective way to determine its therapeutic targets and methods.
A few studies suggest that there is a certain relationship between ferroptosis and DF. For example, ferroptosis-related inhibitors play a protective role in DF. Studies have shown that polarized light (PL) combined with antioxidant water-soluble CoQ10, alpha-lipoic acid, and vitamin E is effective in controlling type 2 diabetes complications such as DF [111]. Diabetic foot ulcer (DFU) is a type of DF. Oral vitamin E and C can promote wound healing in patients with nonunion DFU by promoting the wound healing process and reducing oxidative stress [112]. In addition, studies have shown that paeoniflorin (PF) has the ability to promote DFU wound healing by activating NRF2 related ferroptosis pathway NRF2/HO-1 pathway [82].
3.5. Diabetic neuropathy
Diabetic neuropathy is one of the chronic complications of diabetes. This condition is relatively hidden and can involve any part of the whole nervous system, including a variety of neurological syndromes such as diabetic peripheral neuropathy (DPN), autonomic neuropathy, small fiber neuropathy, and multiple radiculopathies. Among them, DPN is one of the most common types [113].
There is a certain amount of evidence suggesting a link between ferroptosis and DN. CoQ10, as a ferroptosis inhibitor, plays an important protective and defensive role in DN. CoQ10 can not only prevent the development of DPN, resist nerve conduction injury, neuronal phenotypic changes, and prevent neuron loss [114], but also inhibit dorsal root ganglia (DRG) neuropathic pain [115]. Other studies have shown that CoQ10 combined with alpha-lipoic acid (ALA) can prevent apoptosis and degeneration of dorsal root ganglia [116].
In recent years, researchers have also actively explored the pathogenesis of DN, and many studies show an intersection between DPN and the potential pathological mechanism of ferroptosis. Endoplasmic reticulum (ER) stress is becoming an important mechanism of metabolic diseases including diabetes. Studies have shown that endoplasmic reticulum stress plays an important role in neuropathy of prediabetes [117]; similarly, endoplasmic reticulum stress-mediated autophagy activation is involved in cadmium-induced ferroptosis and ferritinophagy of renal tubular epithelial cells [118]. In addition, Ma J. et al. found that regulation of heat shock protein 70 (HSP70) offers an effective approach toward correcting sensory neuron bioenergetic deficits and DPN in both type 1 and type 2 diabetes [119]. There is new evidence that the activation of the HSP70/NQO1 axis will up-regulate the downstream NQO1, lead to the formation of H2O2 in cells, and promote the ferroptosis of Fe2+-mediated lipid peroxidation [120]. In recent years, studies have found that monocarboxylate transporter-1 (MCT1) plays an important role in the pathogenesis of DPN. In type 1 diabetes, the decrease of MCT1 aggravates DPN, but the mechanism is unclear [121]. One new study shows that blocking lactate uptake by inhibiting hydroxycarboxylic acid receptor 1 (hcar1)/MCT1 can activate AMPK to down-regulate steeloyl COA Desaturase-1 (SCD1) and promote ferroptosis [122]. The above evidence demonstrates the close relationship between DN and the pathological mechanism of ferroptosis, but the causal relationship between DN and ferroptosis needs further exploration.
4. Potential treatments targeting ferroptosis and ferritinophagy for diabetes complications
Previous studies have suggested that some hypoglycemic drugs can regulate iron homeostasis, thereby regulating ferroptosis, and play a role in the development of diabetes complications. Sodium-glucose cotransporter type2 inhibitors (SGLT2Is) are a new hypoglycemic drug. They inhibit glucose reabsorption by inhibiting the role of sodium-glucose cotransporter type2 (SGLT2) on the cell membrane of proximal renal tubules, and finally plays a hypoglycemic role [125]. One recent study shows that SGLT2Is play a role in restoring iron homeostasis, improving mitochondrial function, and antioxidant stress in diabetic cardiomyopathy. The recovery of iron homeostasis and mitochondrial function is inhibiting the occurrence of ferroptosis. This suggests that SGLT2Is may treat and prevent DCM by resisting ferroptosis [126]. In addition, rosiglitazone, a hypoglycemic drug, can not only reduce blood glucose but also be a targeted inhibitor of ACSL4. Some researchers have applied it to the diabetic nephropathy mouse model and found that rosiglitazone can reduce the content of lipid peroxidation products and iron, thereby blocking the ferroptosis of renal tubular cells to inhibit the production of proinflammatory cytokines and ultimately playing a role in improving diabetic nephropathy [19]. Liraglutide not only controls blood sugar but also has an excellent effect on neurodegenerative diseases [127]. The newly discovered liraglutide can improve the expression level of ferroptosis-related proteins (such as upregulation of FTH, GPX4, and SLC7A11 expression, down regulation of ACSL4 expression, etc.) to resist oxidative stress, lipid peroxidation, and iron overload in ferroptosis, all of which can reduce diabetic cognitive impairment [128]. Therefore, these studies indicate that ferroptosis can be used as a potentially effective target for alleviating diabetes complications. These results also provide an important theoretical basis for hypoglycemic drugs in the treatment of diabetes complications.
Ferroptosis-specific medications can play a role in regulating diabetes complications. Fenofibrate is a kind of blood lipid regulating drug of clofibric acid derivative. It is used in the treatment of hypertriglyceridemia because of its effect of improving blood lipids [129]. Li et al. expounded on the protective effect of fenofibrate on the diabetic kidney from a new perspective: it may inhibit ferroptosis by raising NRF2, so as to alleviate the progress of DN, rather than its traditional lipid-lowering effect [130]. Melatonin is a hormone secreted by the pineal gland in the human brain and has multiple pharmacological activities. Studies have shown that melatonin can inhibit the ferroptosis of osteoblasts by activating the NRF2/HO-1 signaling pathway in diabetic osteoporosis, thereby improving bone microstructure and bone mineral density and playing a role in the treatment of diabetic osteoporosis [131]. Adiponectin, as an adipocyte inflammatory factor, is beneficial to metabolic diseases such as diabetes [132]. Recent studies have shown that adiponectin inhibits the expression of carnitine palmityl transferase 1 (CPT1) and Glucose transporter 4 (GLUT4) in placenta tissue in order to resist the accumulation of lipid peroxides-induced ferroptosis, thereby improving placental injury in gestational diabetes mellitus (GDM) [133]. Moreover, the intervention effect of natural bioactive compounds is worthy of further exploration. Resveratrol, as a non-flavonoid polyphenol organic compound, is a natural antioxidant product. Resveratrol can reduce endoplasmic reticulum stress and upregulate the expression of PPARγ, thereby inhibiting acrolein (diabetes risk factor)-induced ferroptosis [134]. Baicalein, a natural flavonoid, not only reduces diabetes complications through activating the PERK/NRF2 signaling pathway [135] but also proves to be an effective inhibitor of ferroptosis [136]. Quercetin, one of the most widely distributed flavonoids in the plant kingdom, inhibits iron deposition in the pancreas to play a potentially beneficial role in diabetes mellitus [137]. In summary, natural compounds regulating ferroptosis in the treatment of diabetes complications can be expected in the future.
Long non-coding RNA (LncRNAs) can also contribute to controlling diabetes complications by regulating ferroptosis. Inhibition of the LncRNA zinc finger antisense 1 (ZFAS1) attenuates ferroptosis by sponging miR-150–5p and activates Cyclin D2 (CCND2) against diabetic cardiomyopathy [138]. Therefore, inhibition of lncRNA ZFAS1 may become a promising therapeutic target for the treatment and prevention of DCM. In addition, there is new evidence that LncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) regulates the antioxidant defense of DR through Keap1-NRF2. LncRNA MALAT1 promotes transcription by increasing the binding with the Keap1 promoter. Elevated levels of Keap1 prevent the master regulator NRF2 from moving inside the nucleus to initiate transcription of the antioxidant response genes, and the retina fails to defend itself from increased oxidative stress [139]. In summary, some LncRNA can be used as an intervention target for diabetes complications.
Gene interference technologies can also be applied to manage the occurrence and development of diabetes complications. Studies have found that knocking out the Heme oxygenase 1 (Hmox1) gene can reduce iron overload, reactive oxygen species, and lipid peroxidation and inhibit ferroptosis, in turn helping to resist diabetic atherosclerosis [68]. It has been demonstrated that inhibition of DNA (cytosine-5)-methyltransferase 1 (DNMT-1) can reduce ferroptosis in diabetes myocardial ischemia/reperfusion injury, probably due to the involvement of NCOA4-mediated ferritinophagy. However, the specificity of the DNMT-1 and NCOA4 promoter region needs to be further confirmed [140]. The above-mentioned drugs, some LncRNAs, genes, and gene-interference technologies for ferroptosis, ferritinophagy, and diabetes complications are summarized in Table 3.
Table 3.
Potential drugs, RNA, and genes that interfere with ferroptosis and ferritinophagy to affect diabetes complications.
| Potential drugs/genes that can interfere/RNA that can interfere | Possible mechanisms | Diseases | Reference |
|---|---|---|---|
| SGLT2Is | By restoring cardiac iron homeostasis, improving mitochondrial function and antioxidant stress to resist ferroptosis | DCM | [126] |
| Rosiglitazone | By reducing the content of lipid peroxidation products and iron, and then blocking the ferroptosis of renal tubular cells | DN | [19] |
| Liraglutide | By reducing oxidative stress, lipid peroxidation and iron overload | DCI | [128] |
| Fenofibrate | By raising NRF2 to inhibit ferroptosis | DN | [130] |
| Melatonin | By activating NRF2/HO-1 signaling pathway to inhibit ferroptosis of osteoblasts | T2DOP | [131] |
| Adiponectin | by restoring CPT-1 activity to resist fatty acid oxidation/peroxide imbalance-induced ferroptosis | Placental injury in GDM | [133] |
SGLT2Is, sodium-glucose cotransporter type2 inhibitors; NRF2, nuclear factor E2-related factor2; HO-1, heme oxygenase-1; CPT-1, carnitine palmityl transferase 1; DN, diabetic nephropathy; DCM, diabetic cardiomyopathy; DCI, diabetic cognitive impairment; T2DOP, T2DM-related osteoporosis; GDM, gestational diabetes mellitus.
5. Summary and prospect
Ferroptosis is a recently discovered regulatory cell death caused by iron-dependent lipid peroxidation. New evidence shows the role of ferroptosis and ferritinophagy in diabetes-related complications. In this review, we summarize three main regulatory mechanisms of ferroptosis and its inducers and inhibitors, explore the correlation among ferroptosis, ferritinophagy and the development of diabetes complications, and provide directions for future treatment and research of diabetes-related complications. Although many studies have shown that ferroptosis and ferritinophagy are closely related to the occurrence and development of diabetes complications, there are still many unsolved problems. The exact molecular mechanism and pathophysiology of drug-related ferroptosis need to be further studied, and some conflicting results need to be further clarified. For example, can NRF2 induce ferroptosis or protect cells from ferroptosis? Or does NRF2 have different effects on cells under different conditions? In any case, there is no doubt that ferroptosis and ferritinophagy may indicate a new era in the treatment of diabetes and its complications.
In summary, the ferroptosis process is a worthy target for the treatment of diabetes and its complications, but its complete molecular mechanism and pathophysiological process in diabetes and its complications still need further study.
Funding
This work was supported by the Natural Science Foundation in Jiangxi Province grant [grant numbers No.202002BAB216022 to J.Z., No.20192ACBL21037 and No.202004BCJL23049 to P.Y.]; the National Natural Science Foundation of China [grant number No. 82160371 to J.Z. and No. 82100869 to P.Y.].
Author contributions
Peng Yu and Jing Zhang: Conceptualization, Methodology, Funding acquisition. Jiahui He: Writing—Original draft preparation and Reviewing. Zhangwang Li: Visualization and Investigation. Panpan Xia: Software and Supervision. Ao Shi and Xinxi FuChen: Validation and Editing.
Acknowledgments
The graphical abstracts were created with BioRender software (BioRender.com).
Contributor Information
Jing Zhang, Email: zhangjing666doc@163.com.
Peng Yu, Email: yu8220182@163.com.
Conflict of interest
None declared.
References
- 1.Alberti K., Zimmet P. Global burden of disease--where does diabetes mellitus fit in? Nature Reviews. Endocrinology. 2013;9(5):258–260. doi: 10.1038/nrendo.2013.54. [DOI] [PubMed] [Google Scholar]
- 2.Chowdhury T., Shaho S., Moolla A. Complications of diabetes: progress, but significant challenges ahead. Annals of Translational Medicine. 2014;2(12):120. doi: 10.3978/j.issn.2305-5839.2014.08.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas. Diabetes Research and Clinical Practice. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 4.Saeedi P., Salpea P., Karuranga S., Petersohn I., Malanda B., Gregg E.W., et al. Mortality attributable to diabetes in 20-79 years old adults, 2019 estimates: results from the international diabetes federation diabetes atlas. Diabetes Research and Clinical Practice. 2020;162:108086. doi: 10.1016/j.diabres.2020.108086. [DOI] [PubMed] [Google Scholar]
- 5.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santana-Codina N., Mancias J.D. The role of NCOA4-mediated ferritinophagy in health and disease. Pharmaceuticals. 2018;11(4) doi: 10.3390/ph11040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q., Han X., Lan X., Gao Y., Wan J., Durham F., et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight. 2017;2(7):e90777. doi: 10.1172/jci.insight.90777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao M., Yi J., Zhu J., Minikes A.M., Monian P., Thompson C.B., et al. Role of mitochondria in ferroptosis. Molecular Cell. 2019;73(2):354–363. doi: 10.1016/j.molcel.2018.10.042. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang W.S., SriRamaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1–2):317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y., Li N., Wang H., Wang N., Peng, H., Wang J., et al. Amentoflavone suppresses cell proliferation and induces cell death through triggering autophagy-dependent ferroptosis in human glioma. Life Sciences. 2020;247:117425. doi: 10.1016/j.lfs.2020.117425. [DOI] [PubMed] [Google Scholar]
- 12.Tian X., Li S., Ge G. Apatinib promotes ferroptosis in colorectal cancer cells by targeting ELOVL6/ACSL4 signaling. Cancer Management and Research. 2021;13:1333–1342. doi: 10.2147/CMAR.S274631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altamura S., Kopf S., Schmidt J., Müdder K., da Silva A.R., Nawroth P., et al. Uncoupled iron homeostasis in type 2 diabetes mellitus. Journal of molecular medicine (Berlin, Germany) 2017;95(12):1387–1398. doi: 10.1007/s00109-017-1596-3. [DOI] [PubMed] [Google Scholar]
- 14.Venkatesan P., Varghese J., Arthi T.S., James J.V., Anura A., Prasad J., et al. Evidence of dysregulated iron homeostasis in newly diagnosed diabetics, but not in pre-diabetics. Journal of Diabetes and Its Complications. 2021:107977. doi: 10.1016/j.jdiacomp.2021.107977. [DOI] [PubMed] [Google Scholar]
- 15.Feng X., Wang S., Sun Z., Dong H., Yu H., Huang M., et al. Ferroptosis enhanced diabetic renal tubular injury via HIF-1α/HO-1 pathway in db/db mice. Frontiers in Endocrinology. 2021;12:626390. doi: 10.3389/fendo.2021.626390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J., Wang H. vol. 2017. Oxid Med Cell Longev; 2017. p. 1930261. (Oxidative stress in pancreatic beta cell regeneration). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baseler W., Dabkowski E.R., Jagannathan R., Thapa D., Nichols C.E., Shepherd D.L., et al. Reversal of mitochondrial proteomic loss in Type 1 diabetic heart with overexpression of phospholipid hydroperoxide glutathione peroxidase. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2013;304(7):R553–R565. doi: 10.1152/ajpregu.00249.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu M., Li S., Yu X., Chen W., Ma H., Shao C., et al. Mitochondrial activity contributes to impaired renal metabolic homeostasis and renal pathology in STZ-induced diabetic mice. American Journal of Physiology. Renal Physiology. 2019;317(3):F593–F605. doi: 10.1152/ajprenal.00076.2019. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y., Bi R., Quan F., Cao Q., Lin Y., Yue C., et al. Ferroptosis involves in renal tubular cell death in diabetic nephropathy. European Journal of Pharmacology. 2020;888:173574. doi: 10.1016/j.ejphar.2020.173574. [DOI] [PubMed] [Google Scholar]
- 20.Wang N., Ma H., Li J., Meng C., Zou J., Wang H., et al. HSF1 functions as a key defender against palmitic acid-induced ferroptosis in cardiomyocytes. Journal of Molecular and Cellular Cardiology. 2021;150:65–76. doi: 10.1016/j.yjmcc.2020.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Bedoui S., Herold M., Strasser A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nature Reviews. Molecular Cell Biology. 2020;21(11):678–695. doi: 10.1038/s41580-020-0270-8. [DOI] [PubMed] [Google Scholar]
- 22.Walker N.I., Harmon B.V., Gobé G.C., Kerr J.F. Patterns of cell death. Methods and Achievements in Experimental Pathology. 1988;13:18–54. [PubMed] [Google Scholar]
- 23.Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death & Differentiation. 2018;25(3):486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang W., Stockwell B. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chemistry & Biology. 2008;15(3):234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolma S., Lessnick S.L., Hahn W.C., Stockwell B.R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3(3):285–296. doi: 10.1016/s1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 26.Das N.K., Schwartz A.J., Barthel G., Inohara N., Liu Q., Sankar A., et al. Microbial metabolite signaling is required for systemic iron homeostasis. Cell Metabolism. 2020;31(1):115–130.e6. doi: 10.1016/j.cmet.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng Y., Zak O., Aisen P., Harrison S.C., Walz T. Structure of the human transferrin receptor-transferrin complex. Cell. 2004;116(4):565–576. doi: 10.1016/s0092-8674(04)00130-8. [DOI] [PubMed] [Google Scholar]
- 28.Torti S., Torti F. Iron and cancer: more ore to be mined. Nature Reviews. Cancer. 2013;13(5):342–355. doi: 10.1038/nrc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanninelli G., Loréal O., Brissot P., Konijn A.M., Slotki I.N., Hider R.C., et al. The labile iron pool of hepatocytes in chronic and acute iron overload and chelator-induced iron deprivation. Journal of Hepatology. 2002;36(1):39–46. doi: 10.1016/s0168-8278(01)00222-7. [DOI] [PubMed] [Google Scholar]
- 30.Ganz T. Systemic iron homeostasis. Physiological Reviews. 2013;93(4):1721–1741. doi: 10.1152/physrev.00008.2013. [DOI] [PubMed] [Google Scholar]
- 31.Shen Z., Liu T., Li Y., Lau J., Yang Z., Fan W., et al. Fenton-reaction-acceleratable magnetic nanoparticles for ferroptosis therapy of orthotopic brain tumors. ACS Nano. 2018;12(11):11355–11365. doi: 10.1021/acsnano.8b06201. [DOI] [PubMed] [Google Scholar]
- 32.Bridges R., Lutgen V., Lobner D., Baker D.A. Thinking outside the cleft to understand synaptic activity: contribution of the cystine-glutamate antiporter (System xc-) to normal and pathological glutamatergic signaling. Pharmacological Reviews. 2012;64(3):780–802. doi: 10.1124/pr.110.003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seibt T., Proneth B., Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radical Biology & Medicine. 2019;133:144–152. doi: 10.1016/j.freeradbiomed.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Liu X., Zhang Y., Zhuang L., Olszewski K., Gan B. NADPH debt drives redox bankruptcy: SLC7A11/xCT-mediated cystine uptake as a double-edged sword in cellular redox regulation. Genes & diseases. 2021;8(6):731–745. doi: 10.1016/j.gendis.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kagan V., Mao G., Qu F., Angeli J.P., Doll S., Croix C.S., et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nature Chemical Biology. 2017;13(1):81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dixon S., Winter G.E., Musavi L.S., Lee E.D., Snijder B., Rebsamen M., et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chemical Biology. 2015;10(7):1604–1609. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doll S., Proneth B., Tyurina Y.Y., Panzilius E., Kobayashi S., Ingold I., et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nature Chemical Biology. 2017;13(1):91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shintoku R., Takigawa Y., Yamada K., Kubota C., Yoshimoto Y., Takeuchi T., et al. Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Science. 2017;108(11):2187–2194. doi: 10.1111/cas.13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mancias J.D., Wang X., Gygi S.P., Harper J.W., Kimmelman A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509(7498):105–109. doi: 10.1038/nature13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mancias J., Pontano Vaites L., Nissim S., Biancur D.E., Kim A.J., Wang X., et al. Ferritinophagy via NCOA4 is required for erythropoiesis and is regulated by iron dependent HERC2-mediated proteolysis. Elife. 2015;4 doi: 10.7554/eLife.10308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellelli R., Federico G., Matte A., Colecchia D., Iolascon A., Chiariello M., et al. NCOA4 deficiency impairs systemic iron homeostasis. Cell Reports. 2016;14(3):411–421. doi: 10.1016/j.celrep.2015.12.065. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z., Yao Z., Wang L., Ding H., Shao J., Chen A., et al. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy. 2018;14(12):2083–2103. doi: 10.1080/15548627.2018.1503146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hou W., Xie Y., Song X., Sun X., Lotze M.T., Zeh H.J., et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12(8):1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang Y., Chen X., Tan Q., Zhou H., Xu J., Gu Q., et al. Inhibiting ferroptosis through disrupting the NCOA4-FTH1 interaction: a new mechanism of action. ACS Central Science. 2021;7(6):980–989. doi: 10.1021/acscentsci.0c01592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang M., Huang Z., Luo X., Liu M., Wang L., Qi Z., et al. Ferritinophagy activation and sideroflexin1-dependent mitochondria iron overload is involved in apelin-13-induced cardiomyocytes hypertrophy. Free Radical Biology and Medicine. 2019;134:445–457. doi: 10.1016/j.freeradbiomed.2019.01.052. [DOI] [PubMed] [Google Scholar]
- 46.DeHart D., Fang D., Heslop K., Li L., Lemasters J.J., Maldonado E.N., et al. Opening of voltage dependent anion channels promotes reactive oxygen species generation, mitochondrial dysfunction and cell death in cancer cells. Biochemical Pharmacology. 2018;148:155–162. doi: 10.1016/j.bcp.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Z., Geng Y., Lu X., Shi Y., Wu G., Zhang M., et al. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(8):2996–3005. doi: 10.1073/pnas.1819728116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dixon S., Patel D.N., Welsch M., Skouta R., Lee E.D., Hayano M., et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang L., Kon N., Li T., Wang S.J., Su T., Hibshoosh H., et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lo M., Ling V., Low C., Wang Y.Z., Gout P.W. Potential use of the anti-inflammatory drug, sulfasalazine, for targeted therapy of pancreatic cancer. Current Oncology (Toronto, Ont.) 2010;17(3):9–16. doi: 10.3747/co.v17i3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cramer S., Saha A., Liu J., Tadi S., Tiziani S., Yan W., et al. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nature Medicine. 2017;23(1):120–127. doi: 10.1038/nm.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lőrincz T., Jemnitz K., Kardon T., Mandl J., Szarka A. Ferroptosis is involved in acetaminophen induced cell death. Pathology Oncology Research : POR. 2015;21(4):1115–1121. doi: 10.1007/s12253-015-9946-3. [DOI] [PubMed] [Google Scholar]
- 53.Liang Z., Zhao W., Li X., Wang L., Meng L., Yu R. Cisplatin synergizes with PRLX93936 to induce ferroptosis in non-small cell lung cancer cells. Biochemical and Biophysical Research Communications. 2021;569:79–85. doi: 10.1016/j.bbrc.2021.06.088. [DOI] [PubMed] [Google Scholar]
- 54.Shimada K., Skouta R., Kaplan A., Yang W.S., Hayano M., Dixon S.J., et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nature Chemical Biology. 2016;12(7):497–503. doi: 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woo J., Shimoni Y., Yang W.S., Subramaniam P., Iyer A., Nicoletti P., et al. Elucidating compound mechanism of action by network perturbation analysis. Cell. 2015;162(2):441–451. doi: 10.1016/j.cell.2015.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hassannia B., Wiernicki B., Ingold I., Qu F., Van Herck S., Tyurina Y.Y., et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. Journal of Clinical Investigation. 2018;128(8):3341–3355. doi: 10.1172/JCI99032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun X., Ou Z., Chen R., Niu X., Chen D., Kang R., et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63(1):173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eling N., Reuter L., Hazin J., Hamacher-Brady A., Brady N.R. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience. 2015;2(5):517–532. doi: 10.18632/oncoscience.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gaschler M.M., Andia A.A., Liu H., Csuka J.M., Hurlocker B., Vaiana C.A., et al. FINO(2) initiates ferroptosis through GPX4 inactivation and iron oxidation. Nature Chemical Biology. 2018;14(5):507–515. doi: 10.1038/s41589-018-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu M., Xu L.G., Li X., Zhai Z., Shu H.B. AMID, an apoptosis-inducing factor-homologous mitochondrion-associated protein, induces caspase-independent apoptosis. Journal of Biological Chemistry. 2002;277(28):25617–25623. doi: 10.1074/jbc.M202285200. [DOI] [PubMed] [Google Scholar]
- 61.Fang S., Yu X., Ding H., Han J., Feng J. Effects of intracellular iron overload on cell death and identification of potent cell death inhibitors. Biochemical and Biophysical Research Communications. 2018;503(1):297–303. doi: 10.1016/j.bbrc.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 62.Umemura M., Kim J.H., Aoyama H., Hoshino Y., Fukumura H., Nakakaji R., et al. The iron chelating agent, deferoxamine detoxifies Fe(Salen)-induced cytotoxicity. Journal of Pharmacological Sciences. 2017;134(4):203–210. doi: 10.1016/j.jphs.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 63.Hofmans S., Vanden Berghe T., Devisscher L., Hassannia B., Lyssens S., Joossens J., et al. Novel ferroptosis inhibitors with improved potency and ADME properties. Journal of Medicinal Chemistry. 2016;59(5):2041–2053. doi: 10.1021/acs.jmedchem.5b01641. [DOI] [PubMed] [Google Scholar]
- 64.Angeli J., Shah R., Pratt D.A., Conrad M. Ferroptosis inhibition: mechanisms and opportunities. Trends in Pharmacological Sciences. 2017;38(5):489–498. doi: 10.1016/j.tips.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Kotschi S., Jung A., Willemsen N., Ofoghi A., Proneth B., Conrad M., et al. NFE2L1-mediated proteasome function protects from ferroptosis. Molecular Metabolism. 2022;57:101436. doi: 10.1016/j.molmet.2022.101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li W., Li W., Leng Y., Xiong Y., Xia Z. Ferroptosis is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress. DNA and Cell Biology. 2020;39(2):210–225. doi: 10.1089/dna.2019.5097. [DOI] [PubMed] [Google Scholar]
- 67.Luo E., Li H.X., Qin Y.H., Qiao Y., Yan G.L., Yao Y.Y., et al. Role of ferroptosis in the process of diabetes-induced endothelial dysfunction. World Journal of Diabetes. 2021;12(2):124–137. doi: 10.4239/wjd.v12.i2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meng Z., Liang H., Zhao J., Gao J., Liu C., Ma X., et al. HMOX1 upregulation promotes ferroptosis in diabetic atherosclerosis. Life Sciences. 2021:119935. doi: 10.1016/j.lfs.2021.119935. [DOI] [PubMed] [Google Scholar]
- 69.Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cellular and Molecular Life Sciences. 2016;73(17):3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang L., Zhang J., Jin Y., Yao G., Zhao H., Qiao P., et al. Nrf2 is a potential modulator for orchestrating iron homeostasis and redox balance in cancer cells. Frontiers in Cell and Developmental Biology. 2021;9:728172. doi: 10.3389/fcell.2021.728172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun X., Ou Z., Chen R., Niu X., Chen D., Kang R., et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology (Baltimore, Md. 2016;63(1):173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang T., Cheng H., Su J., Wang X., Wang Q., Chu J., et al. Gastrodin protects against glutamate-induced ferroptosis in HT-22 cells through Nrf2/HO-1 signaling pathway. Toxicology in Vitro : An International Journal Published in Association with BIBRA. 2020;62:104715. doi: 10.1016/j.tiv.2019.104715. [DOI] [PubMed] [Google Scholar]
- 73.Lee E.J., Cárdenes N., Álvarez D., Sellarés J., Sembrat J., Aranda P., et al. Mesenchymal stem cells reduce ER stress via PERK-Nrf2 pathway in an aged mouse model. Respirology. 2020;25(4):417–426. doi: 10.1111/resp.13646. [DOI] [PubMed] [Google Scholar]
- 74.Wei R., Zhao Y., Wang J., Yang X., Li S., Wang Y., et al. Tagitinin C induces ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal cancer cells. International Journal of Biological Sciences. 2021;17(11):2703–2717. doi: 10.7150/ijbs.59404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu X., Liu C., Li Z., Gai C., Ding D., Chen W., et al. Regulation of GSK3β/Nrf2 signaling pathway modulated erastin-induced ferroptosis in breast cancer. Molecular and Cellular Biochemistry. 2020;473:217–228. doi: 10.1007/s11010-020-03821-8. [DOI] [PubMed] [Google Scholar]
- 76.Wei N., Lu T., Yang L., Dong Y., Liu X. Lipoxin A4 protects primary spinal cord neurons from Erastin-induced ferroptosis by activating the Akt/Nrf2/HO-1 signaling pathway. FEBS open bio. 2021;11:2118–2126. doi: 10.1002/2211-5463.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gao G., Xie Z., Li E.W., Yuan Y., Fu Y., Wang P., et al. Dehydroabietic acid improves nonalcoholic fatty liver disease through activating the Keap1/Nrf2-ARE signaling pathway to reduce ferroptosis. Journal of Natural Medicines. 2021;75(3):540–552. doi: 10.1007/s11418-021-01491-4. [DOI] [PubMed] [Google Scholar]
- 78.Wang S., Yi X., Wu Z., Guo S., Dai W., Wang H., et al. CAMKK2 defines ferroptosis sensitivity of melanoma cells by regulating AMPK‒NRF2 pathway. Journal of Investigative Dermatology. 2021;142:189–200.e8. doi: 10.1016/j.jid.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 79.Altamura S., Müdder K., Schlotterer A., Fleming T., Heidenreich E., Qiu R., et al. Iron aggravates hepatic insulin resistance in the absence of inflammation in a novel db/db mouse model with iron overload. Molecular Metabolism. 2021;51:101235. doi: 10.1016/j.molmet.2021.101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu Y., Zhao Y., Yang H.Z., Wang Y.J., Chen Y. HMGB1 regulates ferroptosis through Nrf2 pathway in mesangial cells in response to high glucose. Bioscience Reports. 2021;41(2) doi: 10.1042/BSR20202924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cundy T., Holden A., Stallworthy E. Early Worsening of Diabetic Nephropathy in Type 2 Diabetes After Rapid Improvement in Chronic Severe Hyperglycemia. Diabetes Care 2021. Diabetes Care. 2021;44(5):e112. doi: 10.2337/dc20-2646. e55-e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun X., Wang X., Zhao Z., Chen J., Li C., Zhao G. Paeoniflorin accelerates foot wound healing in diabetic rats though activating the Nrf2 pathway. Acta Histochemica. 2020;122(8):151649. doi: 10.1016/j.acthis.2020.151649. [DOI] [PubMed] [Google Scholar]
- 83.Zang H., Wu W., Qi L., Tan W., Nagarkatti P., Nagarkatti M., et al. Autophagy inhibition enables Nrf2 to exaggerate the progression of diabetic cardiomyopathy in mice. Diabetes. 2020;69(12):2720–2734. doi: 10.2337/db19-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun Y.M., Su Y., Li J., Wang L.F. Recent advances in understanding the biochemical and molecular mechanism of diabetic nephropathy. Biochemical and Biophysical Research Communications. 2013;433(4):359–361. doi: 10.1016/j.bbrc.2013.02.120. [DOI] [PubMed] [Google Scholar]
- 85.Müller T., Dewitz C., Schmitz J., Schröder A.S., Bräsen J.H., Stockwell B.R., et al. Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cellular and Molecular Life Sciences. 2017;74(19):3631–3645. doi: 10.1007/s00018-017-2547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee H., Zandkarimi F., Zhang Y., Meena J.K., Kim J., Zhuang L., et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nature Cell Biology. 2020;22(2):225–234. doi: 10.1038/s41556-020-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao L., Zou Y., Zhang J., Zhang R., Ren H., Li L., et al. Serum transferrin predicts end-stage renal disease in type 2 diabetes mellitus patients. International Journal of Medical Sciences. 2020;17(14):2113–2124. doi: 10.7150/ijms.46259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim S., Kang S.W., Joo J., Han S.H., Shin H., Nam B.Y., et al. Characterization of ferroptosis in kidney tubular cell death under diabetic conditions. Cell Death & Disease. 2021;12(2):160. doi: 10.1038/s41419-021-03452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chang L., Chiang S.K., Chen S.E., Yu Y.L., Chou R.H., Chang W.C., et al. Heme oxygenase-1 mediates BAY 11-7085 induced ferroptosis. Cancer Letters. 2018;416:124–137. doi: 10.1016/j.canlet.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 90.Menon A., Liu J., Tsai H.P., Zeng L., Yang S., Asnani A., et al. Excess heme upregulates heme oxygenase 1 and promotes cardiac ferroptosis in mice with sickle cell disease. Blood. 2021;139:936–941. doi: 10.1182/blood.2020008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adedoyin O., Boddu R., Traylor A., Lever J.M., Bolisetty S., George J.F., et al. Heme oxygenase-1 mitigates ferroptosis in renal proximal tubule cells. American Journal of Physiology. Renal Physiology. 2018;314(5):F702–F714. doi: 10.1152/ajprenal.00044.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen X., Ma J., Kwan T., Stribos E. G. D, Messchendorp A.L., Loh Y.W., et al. Blockade of HMGB1 attenuates diabetic nephropathy in mice. Scientific Reports. 2018;8(1):8319. doi: 10.1038/s41598-018-26637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu J., Chen P.P., Zhang J.X., Li X.Q., Wang G.H., Yuan B.Y., et al. GPR43 deficiency protects against podocyte insulin resistance in diabetic nephropathy through the restoration of AMPKα activity. Theranostics. 2021;11(10):4728–4742. doi: 10.7150/thno.56598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Q., Hu Y., Hu J.E., Ding Y., Shen Y., Xu H., et al. Sp1-mediated upregulation of Prdx6 expression prevents podocyte injury in diabetic nephropathy via mitigation of oxidative stress and ferroptosis. Life Sciences. 2021;278:119529. doi: 10.1016/j.lfs.2021.119529. [DOI] [PubMed] [Google Scholar]
- 95.Wang W.J., Jiang X., Gao C.C., Chen Z.W. Salusin-β participates in high glucose-induced HK-2 cell ferroptosis in a Nrf-2-dependent manner. Molecular Medicine Reports. 2021;24(3) doi: 10.3892/mmr.2021.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Romero-Aroca P., de la Riva-Fernandez S., Valls-Mateu A., Sagarra-Alamo R., Moreno-Ribas A., Soler N. Changes observed in diabetic retinopathy: eight-year follow-up of a Spanish population. British Journal of Ophthalmology. 2016;100(10):1366–1371. doi: 10.1136/bjophthalmol-2015-307689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yumnamcha T., Devi T.S., Singh L.P. Auranofin mediates mitochondrial dysregulation and inflammatory cell death in human retinal pigment epithelial cells: implications of retinal neurodegenerative diseases. Frontiers in Neuroscience. 2019;13:1065. doi: 10.3389/fnins.2019.01065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karmi O., Sohn Y.S., Zandalinas S.I., Rowland L., King S.D., Nechushtai R., et al. Disrupting CISD2 function in cancer cells primarily impacts mitochondrial labile iron levels and triggers TXNIP expression. Free Radical Biology and Medicine. 2021;176:92–104. doi: 10.1016/j.freeradbiomed.2021.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ola M., Alhomida A., LaNoue K. Gabapentin attenuates oxidative stress and apoptosis in the diabetic rat retina. Neurotoxicity Research. 2019;36(1):81–90. doi: 10.1007/s12640-019-00018-w. [DOI] [PubMed] [Google Scholar]
- 100.Oskarsson M.E., Paulsson J.F., Schultz S.W., Ingelsson M., Westermark P., Westermark G.T. In vivo seeding and cross-seeding of localized amyloidosis: a molecular link between type 2 diabetes and Alzheimer disease. American Journal Of Pathology. 2015;185(3):834–846. doi: 10.1016/j.ajpath.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 101.Zhang Y.H., Wang D.W., Xu S.F., Zhang S., Fan Y.G., Yang Y.Y., et al. α-Lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S Tau transgenic mice. Redox Biology. 2018;14:535–548. doi: 10.1016/j.redox.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang J., Qiu Q., Wang H., Chen C., Luo D. TRIM46 contributes to high glucose-induced ferroptosis and cell growth inhibition in human retinal capillary endothelial cells by facilitating GPX4 ubiquitination. Experimental Cell Research. 2021;407(2):112800. doi: 10.1016/j.yexcr.2021.112800. [DOI] [PubMed] [Google Scholar]
- 103.Zou C., Liu X., Xie R., Bao Y., Jin Q., Jia X., et al. Deferiprone attenuates inflammation and myocardial fibrosis in diabetic cardiomyopathy rats. Biochemical and Biophysical Research Communications. 2017;486(4):930–936. doi: 10.1016/j.bbrc.2017.03.127. [DOI] [PubMed] [Google Scholar]
- 104.Wu H., Wang F, Ta N, Zhang T, Gao W. The multifaceted regulation of mitochondria in ferroptosis. Life (Basel, Switzerland) 2021;11(3) doi: 10.3390/life11030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang S., Zhu S., Wu J., Zhang M., Xu Y., Xu W., et al. Exercise enhances cardiac function by improving mitochondrial dysfunction and maintaining energy homoeostasis in the development of diabetic cardiomyopathy. Journal of molecular medicine (Berlin, Germany) 2020;98(2):245–261. doi: 10.1007/s00109-019-01861-2. [DOI] [PubMed] [Google Scholar]
- 106.Shah M.S., Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circulation Research. 2016;118(11):1808–1829. doi: 10.1161/CIRCRESAHA.116.306923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shirpoor A., Salami S., Khadem-Ansari M.H., Ilkhanizadeh B., Pakdel F.G., Khademvatani K. Cardioprotective effect of vitamin E: rescues of diabetes-induced cardiac malfunction, oxidative stress, and apoptosis in rat. Journal of Diabetic Complications. 2009;23(5):310–316. doi: 10.1016/j.jdiacomp.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 108.Bersuker K., Hendricks J.M., Li Z., Magtanong L., Ford B., Tang P.H., et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575(7784):688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huynh K., Kiriazis H., Du X.J., Love J.E., Jandeleit-Dahm K.A., Forbes J.M., et al. Coenzyme Q10 attenuates diastolic dysfunction, cardiomyocyte hypertrophy and cardiac fibrosis in the db/db mouse model of type 2 diabetes. Diabetologia. 2012;55(5):1544–1553. doi: 10.1007/s00125-012-2495-3. [DOI] [PubMed] [Google Scholar]
- 110.Sloan G., Selvarajah D., Tesfaye S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nature Reviews. Endocrinology. 2021;17(7):400–420. doi: 10.1038/s41574-021-00496-z. [DOI] [PubMed] [Google Scholar]
- 111.Palacka P., Kucharska J., Murin J., Dostalova K., Okkelova A., Cizova M., et al. Complementary therapy in diabetic patients with chronic complications: a pilot study. Bratislavske Lekarske Listy. 2010;111(4):205–211. [PubMed] [Google Scholar]
- 112.Yarahmadi A., Saeed Modaghegh M.H., Mostafavi-Pour Z., Azarpira N., Mousavian A., Bonakdaran S., et al. The effect of platelet-rich plasma-fibrin glue dressing in combination with oral vitamin E and C for treatment of non-healing diabetic foot ulcers: a randomized, double-blind, parallel-group, clinical trial. Expert Opinion on Biological Therapy. 2021;21(5):687–696. doi: 10.1080/14712598.2021.1897100. [DOI] [PubMed] [Google Scholar]
- 113.Perez-Matos M., Morales-Alvarez M., Mendivil C. Lipids: a suitable therapeutic target in diabetic neuropathy? Journal of Diabetes Research. 2017;2017:6943851. doi: 10.1155/2017/6943851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shi T., Zhang M.D., Zeberg H., Nilsson J., Grünler J., Liu S.X., et al. Coenzyme Q10 prevents peripheral neuropathy and attenuates neuron loss in the db-/db- mouse, a type 2 diabetes model. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(2):690–695. doi: 10.1073/pnas.1220794110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Y., Song C.Y., Yuan Y., Eber A., Rodriguez Y., Levitt R.C., et al. Diabetic neuropathic pain development in type 2 diabetic mouse model and the prophylactic and therapeutic effects of coenzyme Q10. Neurobiology of Disease. 2013;58:169–178. doi: 10.1016/j.nbd.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 116.Sadeghiyan Galeshkalami N., Abdollahi M., Najafi R., Baeeri M., Jamshidzade A., Falak R., et al. Alpha-lipoic acid and coenzyme Q10 combination ameliorates experimental diabetic neuropathy by modulating oxidative stress and apoptosis. Life Sciences. 2019;216:101–110. doi: 10.1016/j.lfs.2018.10.055. [DOI] [PubMed] [Google Scholar]
- 117.Lupachyk S., Watcho P., Obrosov A.A., Stavniichuk R., Obrosova I.G. Endoplasmic reticulum stress contributes to prediabetic peripheral neuropathy. Experimental Neurology. 2013;247:342–348. doi: 10.1016/j.expneurol.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 118.Zhao C., Yu D., He Z., Bao L., Feng L., Chen L., et al. Free radical biology & medicine; 2021. Endoplasmic reticulum stress-mediated autophagy activation is involved in cadmium-induced ferroptosis of renal tubular epithelial cells. [DOI] [PubMed] [Google Scholar]
- 119.Ma J., Farmer K.L., Pan P., Urban M.J., Zhao H., Blagg B.S., et al. Heat shock protein 70 is necessary to improve mitochondrial bioenergetics and reverse diabetic sensory neuropathy following KU-32 therapy. Journal of Pharmacology and Experimental Therapeutics. 2014;348(2):281–292. doi: 10.1124/jpet.113.210435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xue C., Li M., Liu C., Li Y., Fei Y., Hu Y., et al. NIR-actuated remote activation of ferroptosis in target tumor cells through a photothermally responsive iron-chelated biopolymer nanoplatform. Angewandte Chemie. 2021;60(16):8938–8947. doi: 10.1002/anie.202016872. [DOI] [PubMed] [Google Scholar]
- 121.Jha M., Ament X.H., Yang F., Liu Y., Polydefkis M.J., Pellerin L., et al. Reducing monocarboxylate transporter MCT1 worsens experimental diabetic peripheral neuropathy. Experimental Neurology. 2020;333:113415. doi: 10.1016/j.expneurol.2020.113415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao Y., Li M., Yao X., Fei Y., Lin Z., Li Z., et al. HCAR1/MCT1 regulates tumor ferroptosis through the lactate-mediated AMPK-SCD1 activity and its therapeutic implications. Cell Reports. 2020;33(10):108487. doi: 10.1016/j.celrep.2020.108487. [DOI] [PubMed] [Google Scholar]
- 123.Lin Y., Shen X., Ke Y., Lan C., Chen X., Liang B., et al. Activation of osteoblast ferroptosis via the METTL3/ASK1-p38 signaling pathway in high glucose and high fat (HGHF)-induced diabetic bone loss. The FASEB Journal. 2022;36(3):e22147. doi: 10.1096/fj.202101610R. [DOI] [PubMed] [Google Scholar]
- 124.Tian H., Xiong Y., Zhang Y., Leng Y., Tao J., Li L., et al. Cell Stress Chaperones; 2022. Activation of NRF2/FPN1 pathway attenuates myocardial ischemia-reperfusion injury in diabetic rats by regulating iron homeostasis and ferroptosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Woo V., Connelly K., Lin P., McFarlane P. The role of sodium glucose cotransporter-2 (SGLT-2) inhibitors in heart failure and chronic kidney disease in type 2 diabetes. Current Medical Research and Opinion. 2019;35(7):1283–1295. doi: 10.1080/03007995.2019.1576479. [DOI] [PubMed] [Google Scholar]
- 126.Li N., Zhou H. SGLT2 inhibitors: a novel player in the treatment and prevention of diabetic cardiomyopathy. Drug Design, Development and Therapy. 2020;14:4775–4788. doi: 10.2147/DDDT.S269514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Talbot K., Wang H.Y. The nature, significance, and glucagon-like peptide-1 analog treatment of brain insulin resistance in Alzheimer's disease. Alzheimers Dement. 2014;10(1 Suppl):S12–S25. doi: 10.1016/j.jalz.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.An J.R., Su J.N., Sun G.Y., Wang Q.F., Fan Y.D., Jiang N., et al. Liraglutide alleviates cognitive deficit in db/db mice: involvement in oxidative stress, iron overload, and ferroptosis. Neurochemical Research. 2022;47(2):279–294. doi: 10.1007/s11064-021-03442-7. [DOI] [PubMed] [Google Scholar]
- 129.Tenenbaum A., Fisman E.Z. Fibrates are an essential part of modern anti-dyslipidemic arsenal: spotlight on atherogenic dyslipidemia and residual risk reduction. Cardiovascular Diabetology. 2012;11:125. doi: 10.1186/1475-2840-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li S., Zheng L., Zhang J., Liu X., Wu Z. Inhibition of ferroptosis by up-regulating Nrf2 delayed the progression of diabetic nephropathy. Free Radical Biology & Medicine. 2021;162:435–449. doi: 10.1016/j.freeradbiomed.2020.10.323. [DOI] [PubMed] [Google Scholar]
- 131.Ma H., Wang X., Zhang W., Li H., Zhao W., Sun J., et al. Melatonin suppresses ferroptosis induced by high glucose via activation of the Nrf2/HO-1 signaling pathway in type 2 diabetic osteoporosis. Oxidative Medicine and Cellular Longevity. 2020;2020:9067610. doi: 10.1155/2020/9067610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nauck M.A., Wefers J., Meier J.J. Treatment of type 2 diabetes: challenges, hopes, and anticipated successes. Lancet Diabetes & Endocrinology. 2021;9(8):525–544. doi: 10.1016/S2213-8587(21)00113-3. [DOI] [PubMed] [Google Scholar]
- 133.Zheng Y., Hu Q., Wu J. Adiponectin ameliorates placental injury in gestational diabetes mice by correcting fatty acid oxidation/peroxide imbalance-induced ferroptosis via restoration of CPT-1 activity. Endocrine. 2021;75:781–793. doi: 10.1007/s12020-021-02933-5. [DOI] [PubMed] [Google Scholar]
- 134.Zhang X., Jiang L., Chen H., Wei S., Yao K., Sun X., et al. Resveratrol protected acrolein-induced ferroptosis and insulin secretion dysfunction via ER-stress- related PERK pathway in MIN6 cells. Toxicology. 2022;465:153048. doi: 10.1016/j.tox.2021.153048. [DOI] [PubMed] [Google Scholar]
- 135.Dong Y., Xing Y., Sun J., Sun W., Xu Y., Quan C. Baicalein alleviates liver oxidative stress and apoptosis induced by high-level glucose through the activation of the PERK/Nrf2 signaling pathway. Molecules. 2020;25(3) doi: 10.3390/molecules25030599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xie Y., Song X., Sun X., Huang J., Zhong M., Lotze M.T., et al. Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochemical and Biophysical Research Communications. 2016;473(4):775–780. doi: 10.1016/j.bbrc.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 137.Li D., Jiang C., Mei G., Zhao Y., Chen L., Liu J., et al. Quercetin alleviates ferroptosis of pancreatic β cells in type 2 diabetes. Nutrients. 2020;12(10) doi: 10.3390/nu12102954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ni T., Huang X., Pan S., Lu Z. Inhibition of the long non-coding RNA ZFAS1 attenuates ferroptosis by sponging miR-150-5p and activates CCND2 against diabetic cardiomyopathy. Journal of Cellular and Molecular Medicine. 2021;25:9995–10007. doi: 10.1111/jcmm.16890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Radhakrishnan R., Kowluru R.A. Long noncoding RNA MALAT1 and regulation of the antioxidant defense system in diabetic retinopathy. Diabetes. 2021;70(1):227–239. doi: 10.2337/db20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li W., Li W., Wang Y., Leng Y., Xia Z. Inhibition of DNMT-1 alleviates ferroptosis through NCOA4 mediated ferritinophagy during diabetes myocardial ischemia/reperfusion injury. Cell Death & Disease. 2021;7(1):267. doi: 10.1038/s41420-021-00656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]