Abstract
Background and Aim:
The emergence of drug-resistant strains of Eimeria spp. calls for the development of novel anticoccidial drugs. Plant extracts provide a possible natural source for such drugs. This study aimed to investigate the in vitro anticoccidial activity of encapsulated bromelain (EB) in chitosan nanocarriers on Eimeria spp. oocysts isolated from goats kept by farmers in Kenya.
Materials and Methods:
Bromelain was extracted from the peel of ripe pineapples using standard methods. Eimeria spp. oocysts were isolated from the feces of goats using a flotation method. The inhibition of sporulation was assayed after exposing the oocysts to solutions of EB, non-EB (NEB), and diclazuril (positive control) at concentrations between 4 mg/mL and 0.125 mg/mL for 48 h. The oocysts were examined under a microscope (40x) to determine the effects of the drugs on the sporulation process. The percentage of sporulation inhibition was calculated after 48 h and the inhibition concentration 50% (IC50) was determined by probit analysis.
Results:
Bromelain manifested anticoccidial activity through the inhibition of the sporulation of coccidia oocysts. EB achieved inhibition with a lower dose compared with NEB. The IC50 values of diclazuril, EB, and NEB were 0.078 mg/mL, 0.225 mg/mL, and 0.575 mg/mL, respectively. There were significant differences (p<0.01) between the IC50 of EB and NEB compared with the standard treatment drug.
Conclusion:
This preliminary study showed that EB has anticoccidial activity supporting further evaluation at an in vivo level to develop a novel drug for the management of coccidiosis in goats.
Keywords: anticoccidial activity, bromelain, chitosan, coccidia, goat, nanoencapsulation
Introduction
Worldwide, intestinal coccidiosis is one of the most important parasitic diseases of small ruminants [1]. The disease is caused by protozoan parasites belonging to the genera Eimeria spp. and Isospora spp. that develop in the small and large intestines and have devastating effects on younger animals [2,3]. The most common Eimeria species in goats in Kenya and Africa in general are Eimeria ninakohlyakimovae, Eimeria hirci, Eimeria caprina, Eimeria christenseni, Eimeria jolchijevi, Eimeria apsheronica, and Eimeria arloingi [4-7]. Coccidiosis losses in small ruminants are due to clinical and subclinical infections often associated with poor weight gain, reduced production, and increased mortality in younger stock [1,8]. In studies conducted in East Africa, coccidiosis appears to be a leading cause of mortality among small ruminants. It can compound the occurrence of other parasitic and infectious diseases, such as pneumonia and helminthosis [5,7,9].
Coccidiosis is mainly managed through anticoccidials and anticoccidiostats; these can be either administered orally or through feed and water. The current anticoccidial drugs include toltrazuril, diclazuril, decoquinate, amprolium, and sulfonamide [10-12]. However, the overuse and misuse of these drugs have led to the emergence of drug-resistant strains of Eimeria spp. [13]. This has stimulated the development of novel drugs with plant extracts being considered as potential sustainable alternatives. Herbal extracts, such as Curcuma longa, Artemisia absinthium, Saussurea lappa, Ageratum conyzoides, Olea europaea, Ruta pinnata, and Trachyspermum ammi, have been shown to have antiparasitic activity and to enhance the immune system and growth performance, thereby helping the host to overcome coccidiosis infection [14,15].
One plant that has been shown to exert appreciable levels of antiparasitic activity is the pineapple (Ananas comosus). The pineapple is a common tropical fruit grown in various countries, including Kenya [16]. Bromelain is the main compound extracted from pineapples and has been characterized as a mixture of cysteine proteases found in the tissues of plants from the Bromeliaceae family. The commercially available proteolytic enzymes extracted from pineapple are fruit and stem bromelain [17]. Besides its anthelmintic properties, bromelain possesses a wide range of therapeutic properties, such as antibacterial and anti-inflammatory effects and the ability to enhance drug absorption [18-20]. However, its effects against intestinal protozoan infection, such as coccidia, have not been investigated. One of the main challenges of bromelain is to maintain stability within the gastrointestinal system of animals. Recent studies have shown that the encapsulation of bromelain by chitosan can stabilize and maintain the activity of bromelain throughout the gut [16].
This study aimed to evaluate the in vitro anticoccidial activity of encapsulated bromelain (EB) against coccidia affecting goats and developing a novel drug for the management of coccidiosis in goats.
Materials and Methods
Ethical approval
Since no handling procedure was performed on the animals, approval from the Institutional Animal Ethics Committee to conduct the study was not required.
Study period and location
The study was conducted for a period of 5 months (February 2021 to June 2021). The study was carried out at Jomo Kenyatta University of Agriculture Technology, Juja, Kiambu County, Kenya located at latitude 1°05 S and longitude 37°00 E. It lies at an altitude of 1525 m above sea level and receives an annual rainfall of 850 mm with an average temperature of 18.7°C [16].
Extraction and chitosan encapsulation of bromelain
Bromelain was extracted from the stem and peels of pineapple (A. comosus) which was purchased from the local market in Juja Sub-County, Kenya. The enzyme was extracted using the procedure described by Hunduza et al. [20]. Briefly, fresh ripe pineapples were cut into small pieces and then ground in a blender in sodium acetate buffer, pH 7.4. The resultant crude extract was sieved and then precipitated by adding 40% ammonium sulfate salt. The extracted bromelain was purified using a 12 kDa dialysis membrane (Thermo Scientific, USA). The protein concentration was measured using a Nanodrop spectrophotometer (polymerase chain reaction max, Lambda, VacuTec, Germany). The ionic gelation method was used to encapsulate bromelain into chitosan by mixing equal volumes (30 mL each) of extracted bromelain (4 mg/mL), which was mixed with 1% sodium tripolyphosphate (STPP, Dentex Industries Ltd, Kenya) using a rotary mixer. Then, 12 mL of the bromelain-STPP mixture was added to 20 mL of 1% chitosan (Sigma-Aldrich, USA) under vigorous magnetic stirring and then sonicated for 45 min. The resultant suspension was centrifuged at 15,000 rpm for 45 min and the obtained pellet was washed with distilled water before freeze-drying. The aliquots of bromelain-loaded chitosan nanocarrier pellet were frozen at –60°C and dried in a freeze-dryer (MRC, Model FDL-10N-50-BA, Israel). The successful conjugation of bromelain to the chitosan nanoparticles was confirmed by Fourier-transform infrared spectroscopy (Shimadzu 8400, Japan) [20]. Protease activity was performed following the method described by Devakate et al. [21]. Briefly, 1 mL of bromelain was incubated with 5 mL of casein (as a substrate) for 10 min at 37°C. Trichloroacetic acid 98% extra pure (Loba Chimie Pvt. Ltd., India) was added and incubated for 30 min at 37°C. After the mixture was filtered through filter paper (Whatman No.1, GE Healthcare, Chicago, USA), 5 mL of sodium carbonate and 1 mL of Folin–Ciocalteu reagent (Loba Chimie Pvt. Ltd.) were added and incubated for 30 min at 37°C. The absorbance of the clear supernatant was measured at 660 nm.
Isolation and collection of Eimeria spp. oocysts
Oocyst samples of Eimeria spp. were isolated from fresh field fecal samples collected from goats kept by farmers in Juja Sub-County, Kenya. The harvesting of oocysts was a flotation technique using saturated salt (NaCl) solution [22,23]. Briefly, a 5 g sample of feces was weighed and mixed in a mortar. The mixture was dissolved in 50 mL of tap water and filtered through a sieve with a 105 μm aperture. The filtrate was centrifuged for 8 min at 1000 rpm. The supernatant was discarded and the sediment was suspended in a solution of 40% NaCl solution (specific gravity 1:2) and allowed to stand for 10 min to allow coarse fecal material to sink, minimizing the chance of trapping oocysts. The suspension was then centrifuged at 400 rpm for 6 min. Then, approximately 5 mL was aspirated from the top and the oocysts suspension was further washed by centrifugation (at 1000 rpm for 8 min) twice in distilled water. The supernatant was discarded and the oocysts were resuspended from the sediment in 2.5% (w/v) potassium dichromate [K2Cr2O7, (Bio-Chem, France)]. solution and then used directly to perform anticoccidial assays.
In vitro anticoccidial tests
The in vitro sporulation inhibition assay was evaluated using the method described by Odden et al. [12]. Briefly, 15 mL Eppendorf tubes (Thermo Scientific™ Nunc™ 15mL) containing a total volume of 2 mL of each concentration of the encapsulated and non-EB (NEB) (4, 2, 1, 0.5, 0.25, and 0.125 mg/mL) were prepared. The tubes were then inoculated with an equal volume of unsporulated oocyst suspension in K2Cr2O7 and incubated at 28°C for 48 h. For comparison, diclazuril (Vetranal®, Sigma-Aldrich) was used as the positive control. A suspension of oocysts in K2Cr2O7 alone was used as the negative control. A serial dilution series of chitosan solution (4, 2, 1, 0.5, 0.25, and 0.125 mg/mL) was used as the control. At the end of incubation, the effect of the test EB on the oocysts sporulation was examined under a microscope at 40× at both 48 and 72 h. The numbers of sporulated and non-sporulated oocysts were counted and the percentage inhibition was estimated by counting the number of unsporulated oocysts from a total of 100 oocysts. Three replicates were measured for each concentration. The sporulation inhibition percentage was calculated as described by Cedric et al. [24];
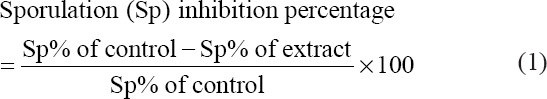
Statistical analysis
The obtained data were entered into and analyzed using the Statistical Package for the Social Sciences software version 28.0 (IBM Corp., NY, USA). The concentration at which inhibition concentration 50% (IC50) of sporulation occurred was determined using the regression line of probit in accordance with the log10 of the extract concentration. The mean percentages at different concentrations and ratios were compared using paired sample t-tests, with p<0.05 considered to indicate statistical significance.
Results
Bromelain concentration and activity
The protein content of crude and purified bromelain was 7.11 mg/mL and 2.80 mg/mL, respectively. The protease activity of purified bromelain was 0.0930 units/mL, whereas that of the EB was 0.0070 units/mL.
In vitro anticoccidial effect
The in vitro anticoccidial activity of the chitosan-EB against coccidial oocysts was examined after 48 h and 72 h which is summarized in Figures-1 and 2, respectively. The results showed that at all concentration levels, the EB inhibited sporulation of coccidia oocysts. The lowest concentration (0.125 mg/mL) inhibited sporulation of more than 50% of the oocysts after incubation for 48 h (Figure-1) and by 60% after 72 h. However, sporulation increased in the negative controls (Figure-2). The results also showed that chitosan alone did not affect sporulation inhibition, even at the highest concentration tested (4 mg/mL).
Figure-1.
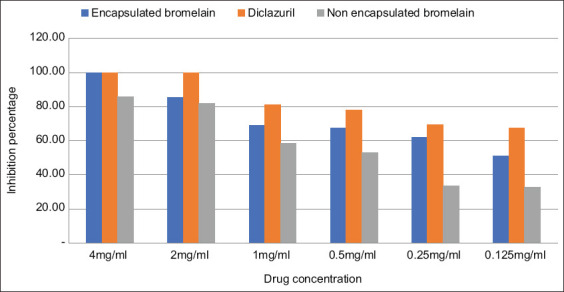
Effect of encapsulated bromelain, diclazuril and non-encapsulated bromelain on % sporulation inhibition after 48 h.
Figure-2.
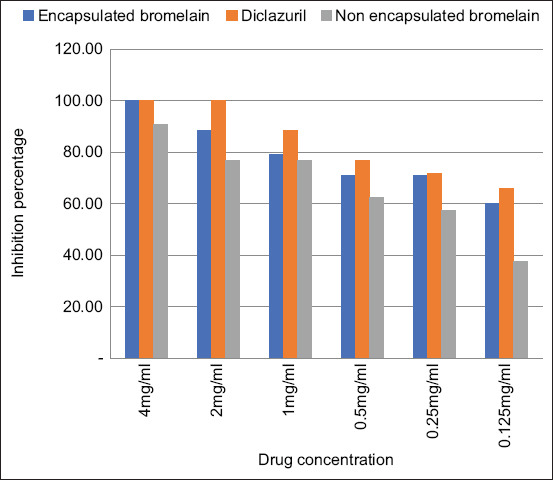
Effect of encapsulated bromelain, diclazuril, and non-encapsulated bromelain on % sporulation inhibition after 72 h.
The EB had significantly higher (p<0.05) anticoccidial activity than unencapsulated bromelain. In contrast, diclazuril had significantly higher (p<0.05) anticoccidial activity than EB. The IC50 values for diclazuril, EB, and plain bromelain were 0.078, 0.225, and 0.575 mg/mL, respectively (Table-1).
Table 1.
IC50 values of encapsulated bromelain, diclazuril, and non-encapsulated bromelain on oocysts sporulation (with 95% confidence limits for concentration) (mg/mL).
| Drug | Lower boundary (mg/mL) | Upper boundary (mg/mL) | Average (mg/mL) |
|---|---|---|---|
| Encapsulated bromelain | 0.193 | 0.257 | 0.225a |
| Diclazuril | 0.001 | 0.211 | 0.078b |
| Non-encapsulated bromelain | 0.478 | 0.673 | 0.575c |
For the same column, values carrying the same superscript letter are not significantly different at p≥0.05 (t-test), IC50=Inhibition concentration 50%
Oocyst damage caused by EB
After coccidia were exposed to EB, the changes observed included weakening of the shell, bursting of the oocyst at the weakest point, and the destruction of the central cytoplasmic mass oocyst (Figure-3). Normal sporulation was observed in oocysts exposed to chitosan and water as negative controls.
Figure-3.
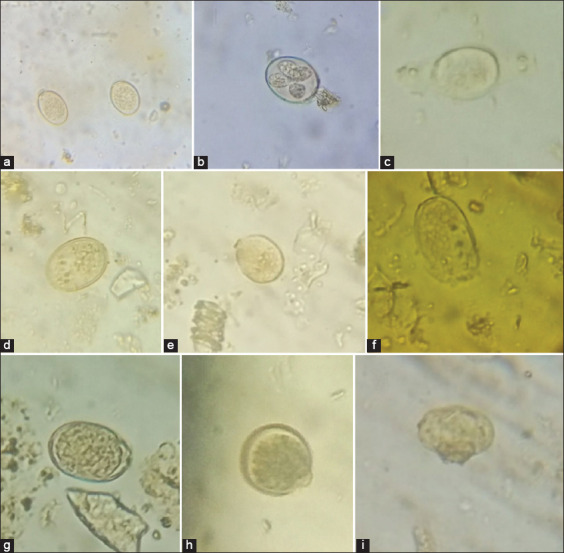
Changes observed after exposure of Eimeria oocysts to encapsulated bromelain (under the microscope 40×): (a) Normal coccidian oocysts; (b) normal sporulation from control group; (c-i) abnormal and unsporulated oocysts in EB.
Discussion
The use of plant extracts to develop new drugs against coccidia is the subject of a number of studies. This is because the current conventional drugs face problems, such as the emergence of drug-resistant coccidial species and consumer concern regarding the presence of drug residues in milk and meat [25,26]. The present study aimed to test the anticoccidial efficacy of bromelain nanoencapsulated by chitosan on Eimeria spp. oocysts isolated from the feces of goats kept by farmers in Kenya. As reported in the previous studies, the inhibition of sporulation and the damage to oocysts were used as the criteria for assessing anticoccidial properties [24,27].
Our study showed that the encapsulation of low concentrations (0.125 mg/mL) of bromelain inhibited sporulation of more than 50% of Eimeria spp. oocysts. The IC50 for EB was higher than that of pure bromelain, showing that the encapsulation process could increase the inhibition activity of bromelain. This confirms that EB has anticoccidial activity and can be developed further as drugs for coccidiosis. The previous studies have shown that extracts from plants, including A. conyzoides (Asteraceae), olive pulp (O. europaea L. var. Chemlal), and Canary rue (R. pinnata), have activity against Eimeria spp. oocyst sporulation [15,28,29]. Molan et al. [30] also observed the inhibition of sporulation in vitro when avian Eimeria tenella, Eimeria maxima, and Eimeria acervulina were exposed to aqueous extracts of pine bark (Pinus radiata). The sporulation inhibition was 28-84% by 500 μg/mL of P. radiata extract [30], whereas our study recorded more than 50% sporulation inhibition with 125 μg/mL bromelain. In addition, Hur et al. [31] studied the effects of condensed tannin-containing plants on natural coccidian infection in goats. They observed that goats fed pine needles and oak leaves in combination with Lucerne chaff resulted in a reduction in oocysts.
Our study showed that bromelain nanoencapsulated by chitosan affected the coccidia sporulation process; the extract caused severe damage to the morphology of coccidian oocysts. As observed from their activity against the eggs of helminths, the bromelain extracts may inhibit sporulation by inactivating the endogenous enzymes responsible for the sporulation process [24,32]. Another study showed that the plant extracts could penetrate both layers of the oocyst shell and cause a loss of intracellular components, which would result in the destruction and softening of the central cytoplasmic mass [33]. In the present study, the EB would have affected the shell wall of oocysts, softening it and causing damage to the central cytoplasmic mass (sporont), as evidenced by the observation of abnormal sporocysts in oocysts exposed to higher concentrations.
This study has demonstrated the value of EB as an anticoccidial agent by the inhibition of sporulation of Eimeria spp. oocysts, even at the lowest concentration tested (0.124 mg/mL). This provides preliminary indicative data that the molecule may be suitable for development into a drug to control protozoal parasites.
Conclusion
EB has anticoccidial activity against Eimeria spp. oocysts isolated from goats (51% of sporulation inhibition at 0.125 mg/ml and 100% at 4 mg/ml). Further studies should be conducted to determine the in vivo efficacy of EB for the treatment of coccidiosis in goats. This will inform ongoing studies geared toward the development of EB as a novel drug that can be used to manage coccidial diseases affecting livestock.
Authors’ Contributions
ARD, JMK, MN, JK and NM: Involved in the conception of the research idea. ARD and JMK: Planned the study design. ARD, JK, and MN: Performed sample (feces) collection and laboratory work. ARD: Drafted the manuscript. JMK, MN, JK, and NM: Corrected the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This research was financially supported by Pan African University (Grant no. MB400-0002/19) and Japan International Cooperation Agency through the Africa-ai-Japan. The authors are grateful to Jomo Kenyatta University of Agriculture Technology for providing the technical and infrastructural support used during the project and acknowledge the technical assistance provided by Dr. Florence Ng’ong’a, Perminus Njururi, and Kipyegon Cheruiyot.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Khodakaram-Tafti A, Hashemnia M. An overview of intestinal coccidiosis in sheep and goats. Rev. Med. Vet. 2017;168(1-3):9–20. [Google Scholar]
- 2.Bakunzi F.R, Thwane S.N, Motsei L.E, Dzoma B.M. Diversity and seasonal occurrence of Eimeria species in a mixed flock of communally reared sheep and goats in Mafikeng in the North West Province, South Africa. J. S. Afr. Vet. Assoc. 2010;81(3):148–150. doi: 10.4102/jsava.v81i3.137. [DOI] [PubMed] [Google Scholar]
- 3.Dakpogan H.B, Mensah S, Attindehou S, Chysostome C, Aboh A, Naciri M, Salifou S, Mensah G.A. Anticoccidial activity of Carica papaya and Vernonia amygdalina extract. Int. J. Biol. Chem. Sci. 2019;12(5):2101–2108. [Google Scholar]
- 4.Maingi N, Munyua W.K. The prevalence and intensity of infection with Eimeria species in sheep in Nyandarua District of Kenya. Vet. Res. Commun. 1994;18(1):19–25. doi: 10.1007/BF01839257. [DOI] [PubMed] [Google Scholar]
- 5.Kusiluka L.J.M, Kambarage D.M, Harrison L.J.S, Daborn C.J, Matthewman R.W. Prevalence and seasonal patterns of coccidial infections in goats in two ecoclimatic areas in Morogoro, Tanzania. Small Rumin. Res. 1998;30(2):85–91. [Google Scholar]
- 6.Mohamaden W.I, Sallam N.H, Abouelhassan E.M. Prevalence of Eimeria species among sheep and goats in Suez Governorate, Egypt. Int. J. Vet. Sci. Med. 2018;6(1):65–72. doi: 10.1016/j.ijvsm.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Etsay K, Megbey S, Yohannes H. Prevalence of sheep and goat coccidiosis in different districts of Tigray region, Ethiopia. Niger. J. Anim. Sci. 2020;22(3):61–69. [Google Scholar]
- 8.De Macedo L.O, Santos M.A.B, da Silva N.M.M, Barros G.M.M, Alves L.C, Giannelli A, Ramos R.A.N, de Carvalho G.A. Morphological and epidemiological data on Eimeria species infecting small ruminants in Brazil. Small Rumin. Res. 2019;171(2):37–41. [Google Scholar]
- 9.Kanyari P.W.N. The relationship between coccidial and helminth infections in sheep and goats in Kenya. Vet. Parasitol. 1993;51(1-2):137–141. doi: 10.1016/0304-4017(93)90204-z. [DOI] [PubMed] [Google Scholar]
- 10.Iqbal A, Tariq K.A, Wazir V.S, Singh R. Antiparasitic efficacy of Artemisia absinthium, toltrazuril and amprolium against intestinal coccidiosis in goats. J. Parasit. Dis. 2013;37(1):88–93. doi: 10.1007/s12639-012-0137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noack S, Chapman H.D, Selzer P.M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 2019;118(7):2009–2026. doi: 10.1007/s00436-019-06343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odden A, Stuen S, Enemark H.L, Robertson L.J, Molina J.M, Ruiz A. Preliminary studies on in vitro methods for the evaluation of anticoccidial efficacy/resistance in ruminants. Exp. Parasitol. 2019;201:34–41. doi: 10.1016/j.exppara.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Hema S, Arun T, Senthilkumar B, Senbagam D, Sureshkumar M. In vivo anticoccidial effects of Azadirachta indica and Carica papaya L. with salinomycin drug as a dietary feed supplement in broiler chicks. Pak. J. Pharm. Sci. 2015;28(4):1409–1415. [PubMed] [Google Scholar]
- 14.Zaman M.A, Iqbal Z, Abbas R.Z, Ehtisham-Ul-Haque S. In vitro efficacy of herbal extracts against Eimeria tenella. Int. J. Agric. Biol. 2015;17(4):848–850. [Google Scholar]
- 15.Debbou-Iouknane N, Nerín C, Amrane M, Ghemghar M, Madani K, Ayad A. In vitro anticoccidial activity of olive pulp (Olea europaea L. var. Chemlal) extract against Eimeria oocysts in broiler chickens. Acta Parasitol. 2019;64(4):887–897. doi: 10.2478/s11686-019-00113-0. [DOI] [PubMed] [Google Scholar]
- 16.Wasso S, Maina N, Kagira J. Toxicity and anthelmintic efficacy of chitosan encapsulated bromelain against gastrointestinal strongyles in Small East African Goats in Kenya. Vet. World. 2020;13(1):177–83. doi: 10.14202/vetworld.2020.177-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Misran E, Idris A, Mat Sarip S.H, Ya'akob H. Properties of bromelain extract from different parts of the pineapple variety Morris. Biocatal. Agric. Biotechnol. 2019;18:101095. [Google Scholar]
- 18.Kahiro S, Kagira J, Maina N, Karanja S. Specific activity of pineapples bromelain extracts at different purification stages obtained from different agro-ecological zones of Thika region, Kenya. J. Appl. Life Sci. Int. 2018;17(2):1–8. [Google Scholar]
- 19.Mahlangu P, Maina N, Kagira J. Prevalence, risk factors, and antibiogram of bacteria isolated from milk of goats with subclinical mastitis in Thika East Sub-county, Kenya. J. Vet. Med. 2018;2018:1–8. doi: 10.1155/2018/3801479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunduza A, Kagira J, Maina N, andala D, Cheruiyot K, Kahiro S. In vitro anthelmintic activity of chitosan encapsulated bromelain against eggs, larval and adult stages of Haemonchus contortus. J. Appl. Life Sci. Int. 2020;23(3):28–38. [Google Scholar]
- 21.Devakate R.V, Patil V.V, Waje S.S, Thorat B.N. Purification and drying of bromelain. Sep. Purif. Technol. 2009;64(3):259–264. [Google Scholar]
- 22.Holdsworth P.A, Conway D.P, McKenzie M.E, Dayton A.D, Chapman H.D, Mathis G.F, Skinner J.T, Mundt H.C, Williams R.B. World association for the advancement of veterinary parasitology (WAAVP) guidelines for evaluating the efficacy of anticoccidial drugs in chickens and turkeys. Vet. Parasitol. 2004;121(3-4):189–212. doi: 10.1016/j.vetpar.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Joachim A, Altreuther G, Bangoura B, Charles S, Daugschies A, Hinney B, Lindsay D.S, Mundt H.C, Ocak M, Sotiraki S. WAAVP guideline for evaluating the efficacy of anticoccidials in mammals (pigs, dogs, cattle, sheep) Vet. Parasitol. 2018;253:102–119. doi: 10.1016/j.vetpar.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 24.Cedric Y, Payne V.K, Nadia N.A.C, Kodjio N, Kollins E, Megwi L, Kuiate J.R, Mbida M. In vitro anticoccidial, antioxidant activities and cytotoxicity of Psidium guajava extracts. Res. J. Parasitol. 2018;13(1):1–13. [Google Scholar]
- 25.Abbas R.Z, Iqbal Z, Blake D, Khan M.N, Saleemi M.K. Anticoccidial drug resistance in fowl coccidia:The state of play revisited. Worlds Poult. Sci. J. 2011;67(2):337–349. [Google Scholar]
- 26.Habibi H, Firouzi S, Nili H, Razavi M, Asadi S.L, Daneshi S. Anticoccidial effects of herbal extracts on Eimeria tenella infection in broiler chickens:In vitro and in vivo study. J. Parasit. Dis. 2016;40(2):401–407. doi: 10.1007/s12639-014-0517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbas A, Iqbal Z, Abbas R.Z, Khan M.K, Khan J.A. In vitro anticoccidial potential of Saccharum officinarum extract against Eimeria oocysts. Bol. Latinoam. Caribe Plantas Med. 2015;14(6):456–461. [Google Scholar]
- 28.Arlette N.T, Anangmo N, Nadia C, Gertrude M.T, Stephanie M.T, Pone W. The in vitro anticoccidial activity of aqueous and ethanolic extracts of Ageratum conyzoides and Vernonia amygdalina (Asteraceae) World J. Pharm. Pharm. Sci. 2019;8(3):38–49. [Google Scholar]
- 29.López A.M, Muñoz M.C, Molina J.M, Hermosilla C, Taubert A, Zárate R, Hildebrandt I, McNaughton-Smith G, Eiroa J.L, Ruiz A. Anticoccidial efficacy of Canary rue (Ruta pinnata) extracts against the caprine apicomplexan Eimeria ninakohlyakimovae. J. Anim. Sci. 2019;97(1):101–110. doi: 10.1093/jas/sky389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molan A.L, Liu Z, De S. Effect of pine bark (Pinus radiata) extracts on sporulation of coccidian oocysts. Folia Parasitol. 2009;56(1):1–5. doi: 10.14411/fp.2009.001. [DOI] [PubMed] [Google Scholar]
- 31.Hur S.N, Molan A.L, Cha J.O. Effects of feeding condensed tannin-containing plants on natural coccidian infection in goats. Asian-Australas. J. Anim. Sci. 2005;18(9):1262–1266. [Google Scholar]
- 32.Molan A.L, Meagher L.P, Spencer P.A, Sivakumaran S. Effect of flavan-3-ols on in vitro egg hatching, larval development and viability of infective larvae of Trichostrongylus colubriformis. Int. J. Parasitol. 2003;33(14):1691–1698. doi: 10.1016/s0020-7519(03)00207-8. [DOI] [PubMed] [Google Scholar]
- 33.Abbas A, Abbas R.Z, Raza M.A, Khan M.K, Saleemi M.K, Saeed Z. In vitro anticoccidial activity of Trachyspermum ammi (Ajwain) extract on oocysts of Eimeria species of chicken. Adv. Life Sci. 2019;7(1):44–47. [Google Scholar]


