Abstract
Background and Aim:
Among several factors, the sperm quality of poultry is affected by the rooster’s body size and the availability of antioxidants like vitamin E. This study aimed to determine the effect of dietary vitamin E supplementation on rooster sperm quality through a meta-analysis.
Materials and Methods:
After verification and evaluation, a total of 19 articles were included in this study. Data, including dietary vitamin E, semen volume, concentration, total sperm cells, pH, motility, viability, percentage of dead and abnormal sperm, vitamin E sperm content, malondialdehyde (MDA) content, and testosterone levels, were tabulated in a database; these were subsequently analyzed using mixed modeling with vitamin E dose as a fixed effect and study identity as a random effect.
Results:
Dietary supplementation level of vitamin E significantly (p<0.001) affected sperm concentration, significantly affected motility (p<0.001), significantly affected sperm vitamin E (p<0.001), significantly affected viability (p<0.001), and significantly affected chicken sperm fertility (p=0.001). Vitamin E administration also significantly reduced the number of sperm cell deaths (p<0.001); however, increased dietary levels of vitamin E did not affect semen volume (p=0.853), pH (p=0.951), MDA (p=0.542), the percentage of abnormal sperm cells (p=0.343), nor testosterone levels (p=0.063).
Conclusion:
Dietary vitamin E supplementation is recommended for male chickens since it generally enhances the quality of their sperm.
Keywords: meta-analysis, rooster, sperm, vitamin E
Introduction
Low damage and death rates of sperm cells are important to ensuring the quality of healthy chicken sperm [1]. Cells typically have natural defense mechanisms to prevent free radical damage; however, if the concentration of antioxidants in seminal plasma decreases, the concentration of free-formed radical substances increases [2]. The chicken sperm cell membrane has a high concentration of polyunsaturated fatty acids, which increases cell activity through lipid peroxide processing to increase the reactive oxygen species (ROS) reaction [3]. Chicken sperm cells are also affected by low cytoplasm, which reduces the amount of natural antioxidants in the cells; if ROS increase, chicken sperm cells are more vulnerable to damage than those of other animals [4]. For decades, scientists have investigated the most effective methods of reducing harmful processes in both in vivo and in vitro chicken sperm [5]; one such method is to increase the amounts of antioxidants in in vivo sperm cells.
In chickens, increasing total antioxidants can improve sperm quality [6]. Since vitamin E was discovered in mice in the 1920s [7], poultry research has continued to progress with respect to investigating the effects of vitamin E as an antioxidant. On the cellular level, fat-soluble vitamin E protects cells from the damage caused by lipid peroxidase process ROS [8]. Although studies have demonstrated intracellular ROS reduction with vitamin E [9] in humans, this has never been done intracellularly in animal sperm, particularly poultry sperm. Vitamin E is an antioxidant with many protective functions, which have a positive effect on the improvement of rooster growth and resistance [10], and reproductive hormones [11] and in hens [12]. Meanwhile, introducing vitamin E to chicken sperm cells increases fertility to improve the reproductive performance of roosters [13].
Numerous studies of vitamin E supplementation in roosters have revealed several differentiating factors, including the chickens’ age and breed, as well as the vitamin E dose. Altogether, these three variables may play a role in determining the quality of chicken sperm [14-16]. As a result, a systematic review is required to determine the linearity of the effect of vitamin E on rooster sperm quality. Meta-analysis is a powerful technique for analyzing numerous studies conducted using consistent variables [17].
Therefore, the purpose of this study was to determine the effect of vitamin E supplementation on rooster sperm quality through a meta-analysis of previously published research.
Materials and Methods
Ethical approval
This is a meta-analysis, so ethical approval is not necessary for this study.
Database development
This meta-analysis study follows the method in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines reported by Selcuk [18]. All the data used in the present study were collected from published articles, which were then recorded in a database. Published articles were found using the keywords “vitamin E,” “ɑ-tocopherol,” “rooster sperm,” “cockerel sperm,” and/or “chicken sperm” to browse multiple search engines for scientific articles (i.e., Google Scholar, Scopus, Science Web, PubMed, and Mendeley).
Inclusion/exclusion criteria
The main inclusion criteria were: (1) An English language journal article published by a reputable publisher; (2) the experimental design must have complied with the correct statistical rules; (3) the amount of experimental and replicated material met the correct statistical standards; (4) animal experimental material was used specifically for chickens; (5) vitamin E dose was supplied in mg/kg or could be converted to mg/kg; and (6) the number of chickens used (n) must have met the correct statistical requirements.
Data extraction
From the selected articles, the following data were inputted: Author’s name(s), publication year, journal name, breedof chicken rooster, number of roosters, vitamin E treatment dose, observed parameters, recommended dose, source of vitamin E, units for each parameter, sampling technique, and parameter measurement technique.
Approximately 60 papers describing studies of vitamin E supplementation for roosters were initially retrieved, but only 40 of these papers had the potential to be included based on their title and abstract. The included parameters were semen volume, sperm concentration, total sperm, pH, motility, viability, percentage dead sperm, percentage abnormal sperm, fertility, sperm vitamin E, malondialdehyde (MDA) level, and testosterone level.
After a thorough assessment, 19 articles were selected for inclusion in the database (Table-1). The selected papers included eight studies with local breed roosters and 11 studies with broilers [13,19-36]. The roosters were between 18 and 65 weeks old. They were usually fed using factory produced feed with vitamin E supplementation at 0-13,400 mg/kg; whenever another dosing unit was used, it was converted to mg/kg for unit consistency. Vitamin E performance parameters included volume (mL), concentration (×109/mL), total sperm cell (×109/Ejac) pH, motility (%), viability (%), dead sperm (%), abnormal sperm (%), fertility (%), sperm vitamin E (ng/109 cell), MDA (nmol/mL), and testosterone (nmol/L). Dose and performance parameters for vitamin E were then entered into the database. For articles that used a different unit (e.g., ×109/mL or ×109/Ejac), data were transformed into a similar unit of measurement. Once all data regarding dietary vitamin E doses and semen performance were entered, the database was statistically analyzed. The article selection and evaluation process are visualized in Figure-1.
Table 1.
Studies included in the meta-analysis.
| S. No. | Reference | Chicken type | Age (week) | Number of animals | Parameters that were examined | Vitamin E dosage (mg/kg) | Dosage recommended (mg/kg) |
|---|---|---|---|---|---|---|---|
| 1. | [13] | Broiler roosters | 45 | 24 | Volume, concentration, viability, MDA, fertility, testosterone, | 30 and 200 | 200 |
| 2. | [19] | Broiler cockerels | 32, 42, and 52 | 48 | Volume, concentration, motility, sperm vitamin E | 30 and 200 | 200 |
| 3. | [20] | Ross broiler rooster | 30 | 24 | Motility, viability, and MDA | 30 and 200 | 200 |
| 4. | [21] | Mandarah native chickens | 32 | 45 | Volume, concentration, pH, motility, viability, fertility, total sperm cell | 20.5 and 150 | 150 |
| 5. | [22] | Broiler chicken | 22-52 | 32 | Volume, concentration, motility, abnormal sperm, total sperm cell | 100, 200, and 300 | 200 |
| 6. | [23] | Kadaknath native cockerels | 30 | 135 | Volume, concentration, motility, sperm vitamin E, viability, dead, abnormal, fertility | 10, 100, and 200 | 100 |
| 7. | [24] | Mandarah native roosters | 32-52 | 54 | Volume, concentration, pH, motility, viability, fertility, total cell sperm | 67 and 150 | 150 |
| 8. | [25] | Kampong rooster | 60-64 | 45 | Volume, concentration, motility, viability, dead, and abnormal cell sperm | 0, 134, and 268 | 268 |
| 9. | [26] | Taiwan native chicken | 23-52 | 90 | Concentration, viability, fertility | 0, 20, 40, 80, and 160 | 20-160 |
| 10. | [27] | Hubbard male broiler | 65 | 180 | Volume, concentration, motility, dead | 33.5 and 67 | 67 |
| 11. | [28] | White Leghorn broiler cockerels | 38-53 | 320 | Volume, motility | 20, 200, and 400 | 400 |
| 12. | [29] | Ros broiler rooster | 45 | 36 | Volume, concentration, motility, viability, MDA, fertility, testosterone, total cell sperm | 30 and 200 | 200 |
| 13. | [30] | Native cocks | 28 | 60 | Volume, pH, abnormal, total sperm cell | 0, 67, 670, 6700, and 13,400 | 67 |
| 14. | [31] | Rhode Island Red Broiler cockerels | 24 | 60 | Sperm vitamin E | 0, 20, 200, and 1000 | 200 |
| 15. | [32] | Native roosters | 45 | 120 | Motility, viability, MDA, testosterone | 0 and 200 | 200 |
| 16. | [33] | Mandarah native chickens | 40 | 36 | Volume, dead, total sperm cell | 0 and 200 | 200 |
| 17. | [34] | Broiler roosters | 45 | 30 | Volume, concentration, motility, viability, MDA | 0 and 200 | 200 |
| 18. | [35] | Broiler chickens | 18 | 50 | volume, concentration, motility, viability | 45, 145, and 245 | 245 |
| 19. | [36] | Broiler male | 22-54 | 64 | Fertility | 30 and 120 | 120 |
MDA=Malondialdehyde
Figure-1.
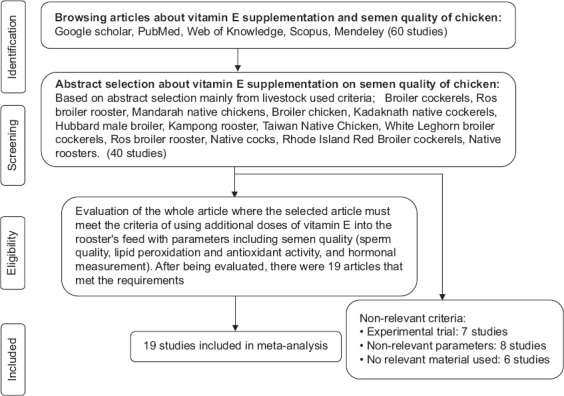
The selection of articles and evaluation process using PRISMA method. PRISMA=Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Statistical analysis
Data were processed using a mixed model procedure [37-39]. The analysis was performed using the PROC MIXED procedure in SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) [40]. The vitamin E dose was defined as a fixed effect, whereas different studies were determined as random effects (declared in the RANDOM statement). The following mathematical model was employed:

Where: (1) Linear mixed model (LMM) mathematical model in the 1st order, (2) LMM mathematical model in the 2nd order, β0+β1 Levelij (1st order) and β0+β1 Level ij+β2 Level ij (2nd order)=fixed effect, Experiment i+Experiment i Level ij (1st and 2nd order), β0 – overall intercept value across all experiments, β1 – linear regression coefficient of the 1st order, β2 – linear regression coefficient of the 2nd order, Level ij – additional level on the random effect, experiment – experiment i, and eij – unexplained residual errors. A linear regression model was applied when the respective quadratic regression model was not significant at p<0.05. Model statistics used were p-value and root mean square error. The significance of an effect was considered when p<0.05 [40].
For significant parameters with quadratic regression, the optimum dose of vitamin E can be determined to achieve the maximum performance of these parameters. The optimum dose of vitamin E can be obtained using the differential method of the quadratic regression equation, with the formula below.
Y=aX2+bx+c
dy/dx=2ax+b=0
2ax+b=0
X=−b/2a
X=optimum dose of vitamin E
Results
Sperm quality
The relationship between vitamin E dose and the sperm quality parameters is presented in Table-2. Vitamin E dose had a significant positive relationship (p<0.001) with sperm concentration significantly affected motility (p<0.001), viability (p<0.001), and fertility (p<0.001). On the other hand, the vitamin E dose significantly (p<0.001) decreased the mortality percentage. please change the sentence to “Meanwhile, the dose of vitamin E did not affect volume (p=0.853), pH (p=0.951), and percentage abnormal sperm cells (p=0.343).
Table 2.
The effects of dosage vitamin E on semen quality of chicken.
| Parameter | Unit | Model | N | Intercept | SE intercept | Slope | SE slope | p-value | RMSE | AIC | Trend |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Volume | mL | L | 51 | 0.358 | 0.047 | −0.0000002 | 0.0000011 | 0.853 | 1.026 | −71.1 | Negative |
| pH | L | 10 | 7.42 | 0.055 | 0.0000005 | 0.0000078 | 0.951 | 0.847 | 20.3 | Positive | |
| Concentration | 109/mL | Q | 63 | 2.75 | 0.226 | 0.0013 | 0.00000023 | <0.001 | Positive | ||
| 0.0000015 | 0.00000001 | <0.001 | 1.67 | 156 | |||||||
| Motility | % | L | 54 | 60.9 | 5.05 | 0.0236 | 0.0042 | <0.001 | 1.11 | 418 | Positive |
| Viability | % | Q | 66 | 64.8 | 4.04 | 0.114 | 0.0289 | <0.001 | Positive | ||
| −0.00034 | 0.00011 | 0.004 | 1.17 | 570 | |||||||
| Dead | % | L | 16 | 22.1 | 3.45 | −0.0467 | 0.0027 | <0.001 | 0.992 | 120 | Negative |
| Abnormal | % | L | 20 | 7.70 | 1.33 | 0.00019 | 0.000197 | 0.343 | 0.963 | 119 | Positive |
| Fertility | % | L | 38 | 71.9 | 4.80 | 0.011 | 0.0031 | 0.001 | 1.26 | 262 | Positive |
| Sperm vitamin E | ng/109 cells | Q | 10 | 92.4 | 18.7 | 0.590 | 0.0063 | <0.001 | Positive | ||
| −0.00049 | 0.000006 | <0.001 | 0.786 | 104 | |||||||
| MDA | nmol/mL | L | 22 | 2.89 | 0.253 | 0.00031 | 0.00049 | 0.542 | 0.918 | 66.5 | Positive |
| Testosterone | nmol/L | L | 12 | 2.61 | 0.137 | −0.00058 | 0.00024 | 0.063 | 0.871 | 27.6 | Negative |
N=Sample size, RMSE=Root means square error, AIC=Akaike information criterion, MDA=Malondialdehyde,
SE=Standard error
Lipid peroxidation and antioxidant activity
The effect of the vitamin E dose on lipid peroxidation and antioxidant activity is presented in Table-2. The dose of vitamin E significantly (p<0.001) increased the concentration of vitamin E in sperm; however, it did not affect (p=0.542) the MDA content in semen.
Hormonal measurement
The effect of the vitamin E dose on testosterone level is shown in Table-2. The dose of vitamin E did not affect (p=0.063) the testosterone concentration.
Discussion
Cerolini et al. [22] noted that vitamin E is a natural antioxidant that can improve semen quality and fertility. Biswas et al. [41] added that vitamin E protects spermatozoa from free radicals and lipid peroxidation, thereby helping to maintain optimal fertilization ability. Furthermore, Tabatabaei et al. [42] showed that vitamin E is a natural antioxidant that can improve semen quality and fertilization ability in chickens. Vitamin E has been widely used in poultry feed to increase the production and reproductive performance of poultry species [43]. Bréque et al. [44] demonstrated that dietary vitamin E supplementation effectively inhibits lipid peroxidation of the plasma membrane of chicken spermatozoa. Moreover, Asrol and Baba [25] reported that the semen quality of roosters (i.e., motility, percentage of spermatozoa viability, and cement color) increases with the dietary supplementation of 400 IU vitamin E for 4 weeks. In addition, vitamin E can also be used as a source of antioxidants in both the feed and drinking water for roosters. In general, all parameters differ at least 2-fold with vitamin E supplementation. Variations in parameters such as viability, motility, and fertility can be caused by differences in the breed [15] and the rooster’s age [45]. The results of Mavi et al. [15] and Lagares et al. [45] study correspond to those of the current meta-analysis, which showed the effect of vitamin E supplementation on rooster local breed chickens and broiler chickens aged between 18 and 65 weeks.
Like in male mammals, the male avian group has almost no accessory glands; this leads poultry to have a small amount of seminal plasma, leading to a low semen volume [46]. A recent study found that the volume of chicken semen can only reach a maximum of 0.9 mL [47]. In contrast, the maximum noted volume of chicken semen was only 0.8 mL in studies conducted some decades ago [48]. The results of the study by Perry [48] correspond to the descriptive results of this study, which demonstrated a maximum chicken semen volume of 0.8 mL; this is also consistent with the results of the current meta-analysis, which demonstrated that vitamin E does not affect chicken semen volume (Table-2). Moreover, in the current meta-analysis, the pH of chicken semen was similar with and without vitamin E supplementation. The pH of semen can change from the male to the female reproductive organs [49], and semen pH affects chicken sperm speed and motility [50].
Further research has shown that, in quail semen, acidic conditions (pH<6) cause sperm flagellum to become inactive [51]. Moreover, bull sperm cells move at maximum motility at pH 7.5, compared to pH 7 and 8 immediately following ejaculation [52]. Altogether, these results demonstrate that vitamin E supplementation in chickens can aid in maintaining a stable semen pH, such that sperm motility can increase or be preserved.
The success of the spermatogenesis process strongly influences sperm density during each ejaculation. The older the chicken, the lower the sperm organ’s production performance, which reduces the sperm cell density [45]. Cells in the testes, including Sertoli cells, Leydig cells, spermatogonia, and spermatids, may experience cell programmed death, such that cell organelles like the endoplasmatic reticulum may also be destroyed [53-55]. External (e.g., heat stress) and internal factors (e.g., sperm cell lipid content) may be responsible for a reduction in testicular weight and chicken spermatids due to ROS [3,56,57]. Vitamin E eliminates the effects of heat stress on chickentestes and sperm [58], preventing excess sperm cell death, and improving testicular volume density [6,14]. The testes have Sertoli cells, which maintain the cells until spermatogenesis is complete [59]. Vitamin E has an important part in the spermatogenesis process of chicken sperm cells, even at older ages [26]; this could have had a significant effect on the concentrations of chicken sperm noted in this meta-analysis due to the administration of vitamin E (Table-2).
Since it can improve chicken sperm concentration, vitamin E positively correlates with semen microscopic qualities, such as increasing motility and the viability percentage and suppressing the sperm death percentage. The increased concentration of ROS due to increased sperm cell metabolism induces adenosine triphosphate (ATP) production to inhibit sperm cell motility [60,61]. ROS also causes damage to the deoxyribonucleic acid (DNA) of sperm cell organelles and decreases sperm motility [62], and leads to sperm cell death after ejaculation [63].
The number of dead sperm significantly affected the sperm viability percentage. This meta-analysis illustrated the significant impact of vitamin E dose on motility, viability, and reducing sperm mortality (Table-2). According to our calculation, the optimal dose of vitamin E in terms of maximum viability was 166 mg/kg. Non-enzymatic antioxidant classified vitamin E breaks the chain in peroxidation reactions [64]. Several recent studies have illustrated a correlation between higher sperm motility and feasibility at lower ROS levels across different antioxidant types [65-67]. Moreover, improving the microscopic quality of sperm cells through vitamin E supplementation has an impact on fertilization. The vitamin E level significantly impacted the fertility of roosters through the high number of normal and mobile sperm. This result is in line with those of a study conducted by Cerolini et al. [22], who showed that vitamin E could prevent a decrease in infertility when subjected to thermal stress.
This meta-analysis revealed that the dose of vitamin E did not significantly affect MDA concentration (Table-2). According to our calculations, the optimum vitamin E dose to achieve the best results with respect to MDA was 101 mg/kg. This illustrates that the concentration of MDA is not the main cause of damage to sperm cells, which can decrease mobility percentage and viability and increase the rate of cell death. The previous studies stated that MDA is not a radical component and is not fully responsible for declines in avian sperm motility and fertility [31]. Moreover, recent study has shown that hydroxyl radicals and hydrogen peroxide hold most of the responsibility for decreased mitochondrial activity, DNA damage, increased lipid peroxide, and acrosomal, and plasma membrane disorders, by causing damage to the acrosome, DNA, plasma membrane, and mitochondria [68].
Nonetheless, generally, research on the impact of vitamin E on poultry semen has only investigated its effect on MDA. Furthermore, one study [68] showed that the concentration of MDA also increased due to the influence of the hydroxyl radical itself; this may explain why vitamin E does not directly reduce the concentration of MDA. Although further evidence is needed, the results of this meta-analysis suggest that improvement in the microscopic quality of chicken semen due to vitamin E supplementation suppresses the concentration of other radicals.
The supplementation of vitamin E to roosters was associated with an increase in the vitamin E concentration in sperm cells (Table-2). According to our calculations, the optimal dose for maximizing sperm vitamin E was 599 mg/kg. Adequate concentrations of vitamin E can increase vitamin E levels in all parts of the body [10], including sperm cells. With increased vitamin E concentrations in sperm cells, the cells in chicken semen do not experience oxidative stress [16], even after cryopreservation [69]; this causes the concentration of MDA and other radical components to rise, while the motility and viability percentage do not decrease and the mortality rate in sperm cells does not increase (Table-2). Increased vitamin E levels can also numerically control the number of abnormal sperm cells, although this was not a significant effect (Table-2).
Vitamin E can also play a role in preventing damage to Leydig cells. Under cultivation media, the administration of vitamin E to horse Leydig cells increases testosterone production [70]. Conversely, a study examining the effect of vitamin E in vivo in rats found no effect on testosterone levels [71]. Similarly, this meta-analysis, focusing on the effect of in vivo vitamin E in chickens, did not demonstrate an impact of vitamin E on testosterone levels (Table-2). Chen et al. [71] noted that this may be possible if the vitamin E concentration is not great enough to reach the steroidogenesis cells; however, this meta-analysis linked vitamin E dose to increased vitamin E levels in sperm cells, refuting this theory (Table-2). The high vitamin E levels in chicken sperm cells suggest that, since the spermatogenesis process is high, vitamin E may reach Leydig cells. Nonetheless, the reason for vitamin E has no effect on testosterone levels in vivo remains unknown.
Conclusion
This meta-analysis demonstrated that vitamin E supplementation improves the quality of rooster sperm; thus, we recommend vitamin E supplementation in rooster feed as an important supplement. The recommended optimal dose of vitamin E supplementation in chicken feed is 599 mg/kg to optimize sperm vitamin E content, 166 mg/kg to maximize viability, and 101 mg/kg to optimize MDA content. The direction of future research development related to the use of vitamin E on reproductive performance in poultry should be directed at increasing the efficiency of the use of vitamin E doses through the application of nanotechnology, so that the dose of vitamin E can be further suppressed, and the effect can be further increased.
Authors’ Contributions
SYH and CH: Conceptualized, designed, and wrote the original draft. MMS: Analyzed the data. SYH, YW, MM, and NQ: Searched the literature and collected and processed the data. CH, AJ, MMS, and NQ: Supervised the research. CH, AJ, SR, YY, and YNA: Critical review of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors are thankful to Jambi Assessment Institute for Agricultural Technology, for providing the necessary facilities for the study. The authors did not receive any funds for this study.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Gonçalves F.M, Santos V.L, Farina G, Oliveira C.O, Anciuti M.A, Rutz F. Nutritional aspects and reproductive management of roosters in Brazil. Worlds Poult Sci J. 2015;71(3):515–522. [Google Scholar]
- 2.Fouad A.M, El-Senousey H.A.K, Ruan D, Xia W, Chen W, Wang S, Zheng C. Nutritional modulation of fertility in male poultry. Poult. Sci. 2020;99(11):5637–5646. doi: 10.1016/j.psj.2020.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heydari M.J, Mohammadzadeh S, Kheradmand A, Alirezaei M. Effect of dietary Satureja khuzistanica powder on semen characteristics and thiobarbituric acid reactive substances concentration in testicular tissue of Iranian native breeder rooster. Iran. J. Vet. Res. 2015;16(3):255–260. [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Sun Y, Ni A, Ni A, Shi L, Wang P, Isa A.M, Ge P, Jiang L, Fan J, Ma H, Yang G, Chen J. Seminal plasma proteome as an indicator of sperm dysfunction and low sperm motility in chickens. Mol. Cell. Proteomics. 2020;19(6):1035–1046. doi: 10.1074/mcp.RA120.002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan R.U. Antioxidants and poultry semen quality. Worlds Poult. Sci. J. 2011;67(2):1–12. [Google Scholar]
- 6.Al-hassani D.H, Al-Daraji H.J. Effect of Vitamin E on spermatogenesis and organs weights of male chickens. Int. J. Agric. Biol. 2014;4(5):1–8. [Google Scholar]
- 7.Evans H.M, Bishop K.S. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science. 1922;56(1458):650–651. doi: 10.1126/science.56.1458.650. [DOI] [PubMed] [Google Scholar]
- 8.Combs G. Assessment of Vitamin E status in animals and man. Nutr. Soc. 1981;40(2):187–194. doi: 10.1079/pns19810028. [DOI] [PubMed] [Google Scholar]
- 9.Yamadera S, Nakamura Y, Inagaki M, Ohsawa I, Gotoh H, Goto Y, Sato N, Oguchi T, Gomi Y, Tsuji M, Kiuchi Y, Iwai S. Vitamin E-coated dialyzer inhibits oxidative stress. Blood Purif. 2017;44(4):288–293. doi: 10.1159/000478971. [DOI] [PubMed] [Google Scholar]
- 10.Pompeu M.A, Cavalcanti L.F.L, Toral F.L.B. Effect of Vitamin E supplementation on growth performance, meat quality, and immune response of male broiler chickens:A meta-analysis. Livest. Sci. 2018;208:5–13. [Google Scholar]
- 11.Hezarjaribi A, Rezaeipour V, Abdollahpour R.S.C. Effects of intramuscular injections of Vitamin E-selenium and a gonadotropin-releasing hormone analogue (GnRHa) on reproductive performance and blood metabolites of post-molt male broiler breeders. Asian Pac. J. Reprod. 2016;5(2):156–160. [Google Scholar]
- 12.Yaripour M, Seidavi A, Dadashbeiki M, Laudadio V. Impact of dietary supra-nutritional levels of vitamins a and e on fertility traits of broiler breeder hens in late production phase. Agriculture. 2018;8(149):1–11. [Google Scholar]
- 13.Zanussi H.P, Shariatmadari F, Sharafi M, Ahmadi H. Dietary supplementation with flaxseed oil as a source of Omega-3 fatty acids improves seminal quality and reproductive performance in aged broiler breeder roosters. Theriogenology. 2019;130:41–48. doi: 10.1016/j.theriogenology.2019.02.030. [DOI] [PubMed] [Google Scholar]
- 14.Ye N, Lv Z, Dai H, Huang Z, Shi F. Dietary alpha-lipoic acid supplementation improves spermatogenesis and semen quality via antioxidant and anti-apoptotic effects in aged breeder roosters. Theriogenology. 2021;159:20–27. doi: 10.1016/j.theriogenology.2020.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Mavi G.K, Dubey P.P, Cheema R.S. Association of antioxidant defense system with semen attributes vis a vis fertility in exotic and indigenous chicken breeds. Theriogenology. 2020;144:158–163. doi: 10.1016/j.theriogenology.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Panda A.K, Cherian G. Role of Vitamin E in counteracting oxidative stress in poultry. J. Poult. Sci. 2014;51(2):109–117. [Google Scholar]
- 17.Leandro G. Meta-analysis in Medical Research:The Handbook for the Understanding and Practice of Meta-analysis. Oxford: Blackwell Publishing; 2007. pp. 1–98. [Google Scholar]
- 18.Selcuk A.A. A guide for systematic reviews:PRISMA. Turk. Arch Otorhinolaryngol. 2019;57(1):57–58. doi: 10.5152/tao.2019.4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerolini S, Surai P.F, Speake B.K, Sparks N.H.C. Dietary fish and evening primrose oil with Vitamin E effects on semen variables in cockerels. Br. Poult. Sci. 2005;46(2):214–222. doi: 10.1080/00071660500065839. [DOI] [PubMed] [Google Scholar]
- 20.Asl R.S, Shariatmadari F, Sharafi M, Torshizi M.A.K, Shahverdi A. Dietary fish oil supplemented with Vitamin E improves quality indicators of rooster cold-stored semen through reducing lipid peroxidation. Cryobiology. 2018;84:15–19. doi: 10.1016/j.cryobiol.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Attia Y.A, Abou-Shehema B.M, Abdellah A.A, Aly O.M, El-Naggar A.S. Effect of ascorbic acid and/or alpha-tocopherol fortification on semen quality, metabolic profile, antioxidants status, and DNA of roosters exposed to heat stress. J. Anim. Plant Sci. 2020;30(2):325–335. [Google Scholar]
- 22.Cerolini S, Zaniboni L, Maldjian A, Gliozzi T. Effect of docosahexaenoic acid and a-tocopherol enrichment in chicken sperm on semen quality, sperm lipid composition and susceptibility to peroxidation. Theriogenology. 2006;66(4):877–886. doi: 10.1016/j.theriogenology.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Biswas A, Mohan J, Sastry K.V.H. Effect of higher dietary Vitamin E concentrations on physical and biochemical characteristics of semen in Kadaknath cockerels. Br. Poult. Sci. 2009;50(6):733–738. doi: 10.1080/00071660903264369. [DOI] [PubMed] [Google Scholar]
- 24.Attia Y.A, El-Naggar A.S, Abou-Shehema B.M, Abdella A.A. Effect of supplementation with trimethylglycine (Betaine) and/or vitamins on semen quality, fertility, antioxidant status, DNA repair and welfare of roosters exposed to chronic heat stress. Animals. 2019;9(8):547. doi: 10.3390/ani9080547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asrol M.K, Baba A.R. Effect of Vitamin E supplementation on semen quantity and quality of Local Kampong roosters. Malays. J. Anim. Sci. 2017;20(1):37–43. [Google Scholar]
- 26.Lin Y.F, Chang S.J, Yang J.R, Lee Y.P, Hsu A.L. Effects of supplemental Vitamin E during the mature period on the reproduction performance of Taiwan Native Chicken cockerels. Br. Poult. Sci. 2005;46(3):366–373. doi: 10.1080/00071660500098186. [DOI] [PubMed] [Google Scholar]
- 27.Khan R.U, Zia-Ur-Rahman Javed, I, Muhammad F. Effects of vitamins, probiotics, and protein level on semen traits and some seminal plasma macro- and microminerals of male broiler breeders after zinc-induced molting. Biol. Trace Elem. Res. 2012;148(1):44–52. doi: 10.1007/s12011-012-9341-9. [DOI] [PubMed] [Google Scholar]
- 28.Zanini S.F, Torres C.A.A, Bragagnolo N, Turatti J.M, Silva M.G, Zanini M.S. Evaluation of the ratio of w6:w3 fatty acids and Vitamin E levels in the diet on the reproductive performance of cockerels. Arch. Anim. Nutr. 2003;57(6):429–442. doi: 10.1080/0003942032000161072. [DOI] [PubMed] [Google Scholar]
- 29.Asl R.S, Shariatmadari F, Sharafi M, Amir M, Torshizi K, Shahverdi A. Improvements in semen quality, sperm fatty acids, and reproductive performance in aged Ross breeder roosters fed a diet supplemented with a moderate ratio of n-3:N-6 fatty acids. Poult. Sci. 2018;97(11):4113–4121. doi: 10.3382/ps/pey278. [DOI] [PubMed] [Google Scholar]
- 30.Danikowski S, Sallmann H.P, Halle I, Flachowsky G. Influence of high levels of Vitamin E on semen parameters of cocks. J. Anim. Physiol. Anim. Nutr. 2002;86(11-12):376–382. doi: 10.1046/j.1439-0396.2002.00396.x. [DOI] [PubMed] [Google Scholar]
- 31.Surai P.F, Kutz E, Wishart G.J, Noble R.C, Speake B.K. The relationship between the dietary provision of alpha-tocopherol and the concentration of this vitamin in the semen of chicken:Effects on lipid composition and susceptibility to peroxidation. Reproduction. 1997;110(1):47–51. doi: 10.1530/jrf.0.1100047. [DOI] [PubMed] [Google Scholar]
- 32.Min Y, Sun T, Niu Z, Liu F. Vitamin C and Vitamin E supplementation alleviates oxidative stress induced by dexamethasone and improves the fertility of breeder roosters. Anim. Reprod. Sci. 2016;171:1–6. doi: 10.1016/j.anireprosci.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Ebeid T.A. Vitamin E and organic selenium enhance the antioxidative status and quality of chicken semen under high ambient temperature. Br. Poult. Sci. 2012;53(5):708–714. doi: 10.1080/00071668.2012.722192. [DOI] [PubMed] [Google Scholar]
- 34.Lotfi S, Fakhraei J, Yarahmadi H.M. Dietary supplementation of pumpkin seed oil and sunflower oil along with Vitamin E Improves sperm characteristics and reproductive hormones in roosters. Poult. Sci. 2021;100(9):1–14. doi: 10.1016/j.psj.2021.101289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franchini A, Bergonzoni M.L, Melotti C, Minelli G. The effects of dietary supplementation with high doses of Vitamin E and C on the quality traits of chicken semen. Arch. Geflugelkunde. 2001;65(2):76–81. [Google Scholar]
- 36.Urso U.R.A, Dahlke F, Maiorka A, Bueno I.J.M, Schneider A.F, Surek D, Rocha C. Vitamin E and selenium in broiler breeder diets:Effect on live performance, hatching process, and chick quality. Poult. Sci. 2015;94(5):976–983. doi: 10.3382/ps/pev042. [DOI] [PubMed] [Google Scholar]
- 37.Hidayat C, Sumiati Jayanegara A, Wina E. Effect of zinc on the immune response and production performance of broilers:A meta-analysis. Asian Australas. J. Anim. Sci. 2020;33(3):465–479. doi: 10.5713/ajas.19.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanza Y.R, Szumacher-Strabel M, Jayanegara A, Kasenta A.M, Gao M, Huang H, Patra A.K, Warzych E, Cieślak A. The effects of dietary medium-chain fatty acids on ruminal methanogenesis and fermentation in vitro and in vivo:A meta-analysis. J. Anim. Physiol. Anim. Nutr. 2020;105(5):874–889. doi: 10.1111/jpn.13367. [DOI] [PubMed] [Google Scholar]
- 39.Hidayat C, Irawan A, Jayanegara A, Sholikin M.M, Prihambodo T.R, Yanza Y.R, Wina E, Sadarman S, Krisnan R, Isbandi I. Effect of dietary tannins on the performance, lymphoid organ weight, and amino acid ileal digestibility of broiler chickens:A meta-analysis. Vet. World. 2020;4(6):1405–1411. doi: 10.14202/vetworld.2021.1405-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.SAS Institute Inc. SAS/STAT Software Version 9.1. Cary, NC, USA: SAS Institute Inc; 2008. pp. 1–5136. [Google Scholar]
- 41.Biswas A, Mohan J, Sastry K.V.H, Tyagi J.S. Effect of dietary Vitamin E on the cloacal gland, foam and semen characteristics of male Japanese quail. Theriogenology. 2007;67(2):259–263. doi: 10.1016/j.theriogenology.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Tabatabaei S, Batavani R.A, Ayen E. Effects of Vitamin E addition to chicken semen on sperm quality during in vitro storage of semen. Vet. Res. Forum. 2011;2(2):103–111. [Google Scholar]
- 43.Nawab A, Tang S, Liu W, Jiang Wu, Ibtisham F, Kang K, Ghani M.W, Birmani M.W, Li G, Sun C, Zhao Y, Xiao M, An L. Vitamin E and fertility in the poultry birds;deficiency of Vitamin E and its hazardous effects. Appro Poult. Dairy Vet. Sci. 2018;6(1):2–7. [Google Scholar]
- 44.Bréque C, Surai P, Brillard J.P. Roles of antioxidants on prolonged storage of avian spermatozoa in vivo and in vitro. Mol. Reprod. Dev. 2003;66(3):314–323. doi: 10.1002/mrd.10347. [DOI] [PubMed] [Google Scholar]
- 45.Lagares M.A, Ecco R, Martins N.R.S, Lara L.J.C, Rocha J.S.R, Vilela D.A.R, Barbosa V.M, Mantovani P.F, Braga J.F.V, Preis I.S, Gheller V.A, Cardeal P.C, Baião N.C. Detecting reproductive system abnormalities of broiler breeder roosters at different ages. Reprod. Domest. Anim. 2017;52(1):67–75. doi: 10.1111/rda.12804. [DOI] [PubMed] [Google Scholar]
- 46.Santiago-Moreno J, Blesbois E. Functional aspects of seminal plasma in bird reproduction. Int. J. Mol. Sci. 2020;21(16):5664. doi: 10.3390/ijms21165664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohan J, Sharma S.K, Kolluri G, Dhama K. History of artificial insemination in poultry, its components and significance. Worlds Poult. Sci. J. 2018;74(3):475–488. [Google Scholar]
- 48.Perry E.J. The Artificial Insemination of Farm Animals. 4th ed. New Brunswick, NJ: Rutgers University Press; 1968. p. 258. [Google Scholar]
- 49.Mishra A.K, Kumar A, Swain D.K, Yadav S, Nigam R. Insights into pH regulatory mechanisms in mediating spermatozoa functions. Vet. World. 2018;11(6):852–858. doi: 10.14202/vetworld.2018.852-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holm L, Wishart G.J. The effect of pH on the motility of spermatozoa from chicken, Turkey and quail. Anim. Reprod. Sci. 1998;54(1):45–54. doi: 10.1016/s0378-4320(98)00142-0. [DOI] [PubMed] [Google Scholar]
- 51.Matsuzaki M, Mizushima S, Hiyama G, Hirohashi N, Shiba K. Lactic acid is a sperm motility inactivation factor in sperm storage tubules. Sci. Rep. 2015;5(1):17643. doi: 10.1038/srep17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Contri A, Gloria A, Robbe D, Valorz C, Wegher L, Carluccio A. Kinematic study on the effect of pH on bull sperm function. Anim. Reprod. Sci. 2013;136(4):252–259. doi: 10.1016/j.anireprosci.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 53.Nazarabadi R.B. Methodology (Metodologia) apoptotic factors in testicular cells of chickens Fatores apoptóticos nas células testiculares de frango. Acta Biol. Par. 2019;48(2-4):51–76. [Google Scholar]
- 54.Xiong Y, Yin Q, Li J, He S. Oxidative stress and endoplasmic reticulum stress are involved in the protective effect of alpha-lipoic acid against heat damage in chicken testes. Animals. 2020;10(3):384. doi: 10.3390/ani10030384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Z, Wen D, Wang F, Wang C, Yang L. Curcumin protects against palmitic acid-induced apoptosis via the inhibition of endoplasmic reticulum stress in testicular Leydig cells. Reprod. Biol. Endocrinol. 2019;17(1):71. doi: 10.1186/s12958-019-0517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Faure M, Guibert E, Alves S, Pain B, Ramé C, Dupont J, Brillard J.P, Froment P. The insulin sensitiser metformin regulates chicken Sertoli and germ cell populations. Reproduction. 2016;151(5):527–538. doi: 10.1530/REP-15-0565. [DOI] [PubMed] [Google Scholar]
- 57.Harsha M, Swathi B, Neeradi R, Kannaki T.R, Rao V.R.S, Shanmugam M, Kumar P.K, Reddy V.A. Effects of heat stress on semen quality of Gramapriya male line roosters. Pharm. Innov. J. 2021;10(7):1413–1417. [Google Scholar]
- 58.Kapakin K.A.T, Imik H, Gumus R, Kapakin S, Saglam Y.S. Effect of vitamin E on the secretion of HSP-70 in testes of broilers exposed to heat stress. Kafkas Univ. Vet. Fakult. Derg. 2013;9(2):305–310. [Google Scholar]
- 59.Chen Z, Zhang J.R, Zhou Y.W, Liang C, Jiang Y.Y. Effect of heat stress on the pituitary and testicular development of Wenchang chicks. Arch. Tierzucht. 2015;58(2):373–378. [Google Scholar]
- 60.Partyka A, Łukaszewicz E, Nizański W. Lipid peroxidation and antioxidant enzymes activity in avian semen. Anim. Reprod. Sci. 2012;134(3-4):184–190. doi: 10.1016/j.anireprosci.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 61.Guthrie H.D, Welch G.R. Effects of reactive oxygen species on sperm function. Theriogenology. 2012;78(8):1700–1708. doi: 10.1016/j.theriogenology.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017;14(8):470–485. doi: 10.1038/nrurol.2017.69. [DOI] [PubMed] [Google Scholar]
- 63.Turk G, Simsek U.G, Ceribasi A.O, Ceribas S, Kaya S.O, Guvenç M, Ciftci M, Sonmez M, Yuce A, Bayrakdar A, Yaman M, Tonbak F. Effect of cinnamon (Cinnamomum zeylanicum) bark oil on heat stress-induced changes in sperm production, testicular lipid peroxidation, testicular apoptosis, and androgenic receptor density in developing Japanese quails. Theriogenology. 2015;84(3):365–376. doi: 10.1016/j.theriogenology.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 64.Freisleben H.J, Packer L. Free-radical scavenging activities, interactions and recycling of antioxidants. Biochem. Soc. Trans. 1993;21(2):325–330. doi: 10.1042/bst0210325. [DOI] [PubMed] [Google Scholar]
- 65.Mussa N.J, Ratchamak R, Ratsiri T, Chumchai R, Vongpralub T, Boonkum W, Semaming Y, Chantikisakul V. Lipid peroxidation and antioxidant enzyme activity in fresh rooster semen with high and low sperm motility. Vet. Integr. Sci. 2020;18(3):183–192. [Google Scholar]
- 66.Raei H, Torshizi K.M.A, Sharafi M, Ahmadi H. Improving seminal quality and reproductive performance in male broiler breeder by supplementation of camphor. Theriogenology. 2021;166:1–8. doi: 10.1016/j.theriogenology.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 67.Behnamifar A, Rahimi S, Torshizi K.M.A, Sharafi M, Grimes J.L. Effects of dietary alpha-lipoic acid supplementation on the seminal parameters and fertility potential in aging broiler breeder roosters. Poult. Sci. 2021;100(2):1221–1238. doi: 10.1016/j.psj.2020.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rui B.R, Shibuya F.Y, Kawaoku A.J.T, Losano J.D.A, Angrimani D.S.R, Dalmazzo A, Nichi M, Pereira R.J.G. Impact of induced levels of specific free radicals and malondialdehyde on chicken semen quality and fertility. Theriogenology. 2017;90:11–19. doi: 10.1016/j.theriogenology.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 69.Leao A.P.A, de Souza A.V, Mesquita N.F, Pereira L.J, Zangeronimo M.G. Antioxidant enrichment of rooster semen extenders a systematic review. Res. Vet. Sci. 2021;136:111–118. doi: 10.1016/j.rvsc.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 70.Mather J.P, Saez J.M, Dayt F, Haour F. Vitamin E prolongs survival and function of porcine Leydig cells in culture. Acta Endocrinol. 1983;102(3):470–475. doi: 10.1530/acta.0.1020470. [DOI] [PubMed] [Google Scholar]
- 71.Chen H, Liu J, Luo L, Baig M.U, Kim J.M, Zirkin B.R. Vitamin E, aging and Leydig cell steroidogenesis. Exp. Gerontol. 2005;40(8-9):728–736. doi: 10.1016/j.exger.2005.06.004. [DOI] [PubMed] [Google Scholar]


