Abstract
BACKGROUND
Obesity is a major public health concern even in sub-Saharan Africa. In this part of the world, characterized by limited technical platform and resuscitation facilities, sleeve gastrectomy in the surgical management of obesity is a quite new procedure. We aimed to assess intraoperative complications and 30-day postoperative morbidity and mortality of this procedure in our setting.
METHODS
This study was conducted in the digestive and laparoscopic surgery unit of the National Insurance Fund Health Centre of Essos (Cameroon, Central Africa region). Retrospectively, we reviewed the medical reports of all patients who had undergone a bariatric surgery through a sleeve gastrectomy from January 2016 to December 2020. The 3 end points were intraoperative complications, postoperative 30-day morbidity, and postoperative 30-day mortality.
RESULTS
We included 21 patients among whom 19 were female (90.5%). Their mean age and body mass index were 40.3 ± 10.8 years and 44.9 ± 7.4 kg/m2, respectively. All of them presented with at least 1 comorbidity. All procedures were totally completed laparoscopically with 3 cases of intraoperative complications (14.3%) consisting on bleeding in all of them. The mean operative time was 192.2 ± 52.8 minutes, and the mean hospital stay was 4.7 ± 1.1 days. Eight patients (38.1%) presented a total of twelve 30-day postoperative complications, all of them classified as minor according to the Clavien–Dindo method. The main postoperative morbidity was represented by nausea and vomiting (n = 3, 14.3%). No 30-day readmission was recorded, and the 30-day mortality was nil.
CONCLUSION
Sleeve gastrectomy in the management of obesity is a safe procedure even in a limited setting like our own.
HIGHLIGHTS
-
•
Sleeve gastrectomy is a safe procedure in a sub-Saharan African low setting.
-
•
Bleeding is the only intraoperative complication encountered in 14.3% of cases.
-
•
The prevalence of 30-day postoperative morbidity of sleeve gastrectomy is 38.1%.
-
•
Postoperative 30-day complications after sleeve gastrectomy are minor.
-
•
The 30-day postoperative mortality after sleeve gastrectomy is nil.
INTRODUCTION
With the westernization of lifestyles in the last decades, the African continent has seen a rapid rise in obesity prevalence as well as associated comorbidities. The overall prevalence of overweight and obesity in sub-Saharan Africa is 31% and 34%, respectively [1]. In South Africa, obesity prevalence is 27.2% [2]. In our country, Cameroon (central Africa region), the prevalence of obesity is 15.1% in adults [3] and 12.5% in children aged 3 to 13 years [4]. In many black African countries, an increased level of body fat is associated with beauty, prosperity, health, and prestige, whereas, in contrast, thinness is perceived to be a sign of illness or poverty [5]. However, obesity is associated with cardiovascular, metabolic, and psychological complications and risk of premature mortality [[6], [7], [8]].
In the management of obese patients, the surgical treatment should be reserved for patients with a body mass index (BMI) > 40 kg/m2 or with BMI > 35 kg/m2 and 1 or more significant comorbid conditions when less invasive methods of weight loss have failed and the patient is at high risk for obesity-associated morbidity and mortality [9]. This surgical intervention can achieve long-term sustained weight loss and often induces resolution of comorbidities such as diabetes, sleep apnea, hyperlipidemia, and hypertension [10,11]. Thus, bariatric surgery is recommended as a first-line treatment for diabetes in morbidly obese patients (BMI ≥ 40 kg/m2) and in stage II obese patients (BMI between 35 and 39.9 kg/m2) with poor control glycemic.
Besides these many advantages, bariatric surgery may lead to many complications. In developed countries, morbidity rates after bariatric operations have progressively fallen from 10.5% in 1993 to 7.6% in 2006, with the majority of complications now being minor [12,13]. However, some of these complications like pulmonary embolism and gastrointestinal leakage can be severe and associated with a high risk of mortality [14]. The mortality rate within the 30 days following the surgery is 0.08% in these countries [15].
In sub-Saharan Africa, the laparoscopic approach and bariatric surgery remain quite new. In Cameroon, our unit was the first one to routinely practice the laparoscopic approach in digestive surgery (since 2009) and is still the only one to practice bariatric surgery in the whole country to date. Our weight-loss surgery activity started in 2012 by placement of adjustable gastric band; our results were unsatisfactory with 38.89% of excess weight loss 1 year after surgery even if the postoperative 30-day morbidity was nil [16]. In 2016, we then initiated the practice of sleeve gastrectomy in our unit. In our environment, where the technical means (in particular, the placement of stent by endoscopic route) and the resuscitation capacities are limited, it is legitimate to pose the hypothesis of a greater morbidity and mortality after sleeve gastrectomy. This study aimed to verify this hypothesis in a cohort of obese patients operated by sleeve gastrectomy technique in a Cameroonian single institute.
METHODS
Study Setting
This study was conducted in a single institute, the digestive and laparoscopic surgery unit of the National Insurance Fund Health Centre of Essos. This hospital is a university hospital located in Yaoundé, the capital city of Cameroon (Central Africa region). It was the first digestive surgical unit of Cameroon that started to perform routinely laparoscopic approach (since 2010). We started to perform sleeve gastrectomy in January 2016 in this unit.
Study Design and Inclusion Criteria
This is a retrospective record review including operating reports of all patients who had undergone a bariatric surgery through a sleeve gastrectomy from January 2016 to December 2020. Information on sociodemographic parameters, comorbidities, BMI, intraoperative complications, operative time, conversion, and postoperative 30-day morbidity and mortality was collected. Unusable files and files of patients lost to follow-up before postoperative day 30 were excluded.
Statistical Analysis
Postoperative complications were collected according to Clavien–Dindo classification [17]. Data analysis was conducted using IBM SPSS software for Windows, version 23.0 (IBM Corp, Armonk, NY, USA). Means ± standard deviations were calculated for continuous variables, and categorical variables were reported using absolute values and percentages. The 3 end points were intraoperative complications, postoperative 30-day morbidity, and postoperative 30-day mortality.
Surgical Technique
All the procedures were performed by the same surgical team, 1 senior surgeon assisted by 2 junior surgeons. The patient, under general anesthesia, was inserted a 10-mm optic port in supra-umbilical region by "open celioscopy," and the pneumoperitoneum was achieved through this access. Four working ports were then inserted, among whom 2 are 10 mm (right pararectal and subxiphoid region) and 2 are 5 mm (both subcostal region). A bipolar thermofusion scalpel was used for freeing the greater gastric curvature from omentum (Fig 1). A 36F bougie was then inserted by the anesthesiologist along the lesser curvature for the calibration of the gastric tube. An endoscopic linear cutting stapler was used to serially staple and transect the stomach (Fig 2), applying 3–5 cartridges of stapler and staying just to the left and lateral to the bougie. The transected stomach (Fig 3) was removed from the peritoneal cavity through the supra-umbilical incision. Then, reinforcement with absorbable self-locking sutures was performed over the mechanical suture. In our study, we considered as a staple line bleeding an active spurting bleeding point anywhere along the staple line requiring a specific intervention such as compression or electrocautery before the staple line reinforcement.
Fig 1.
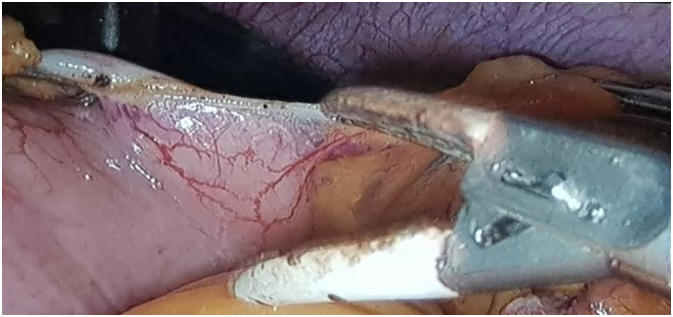
Gastro-omental detachment with a bipolar thermofusion device.
Fig 2.
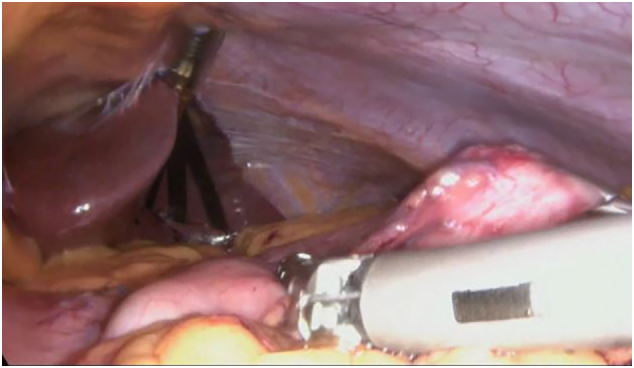
Longitudinal section of the stomach with an endoscopic linear cutting stapler.
Fig 3.
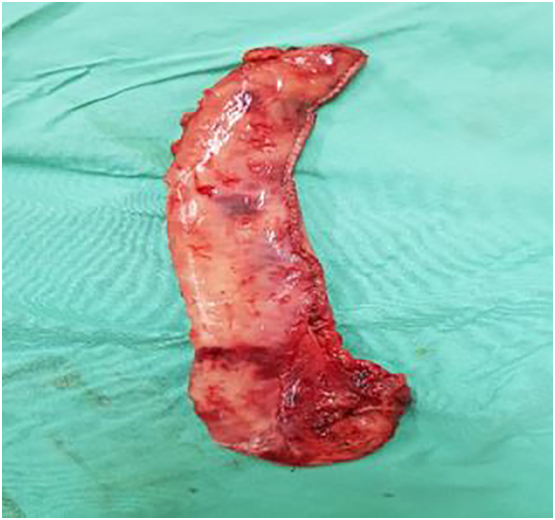
Operative specimen after a sleeve gastrectomy.
Postoperative 30-Day Follow-Up
At the end of the hospitalization period, each patient was received in consultation in our outpatient clinic once per week for a clinical evaluation. Patients started a sugar-free liquid diet on postoperative day 1 until the end of the postoperative first week; this included porridge and broths. During the second and the third week following surgery, diet was semi-liquid with mashed vegetables, mixed fish, or eggs combined with soups. During the postoperative fourth week, the diet included fruits, minced fish/meat, and vegetables. If a patient had vomiting or abdominal discomfort, he returned to the first week diet or stopped the diet for a few hours if he was at the postoperative first week.
RESULTS
During the study period, 21 patients had undergone a bariatric surgery through a sleeve gastrectomy. All of them met our inclusion criteria. Their mean BMI was 44.9 ± 7.4 kg/m2. Nineteen of them were female (90.5%). Their mean age was 40.3 ± 10.8 years. The most represented age group was 40–50 years with 10 cases (47.6%), and the majority of patients were married (n = 12, 57.1%). Twenty of these patients had a professional activity, mainly in the private sector (n = 13, 61.9%). The level of education was university in 18 patients (85.7%), and most patients were originated from the western region of the country (n = 13, 61.9%). Table 1 summarizes sociodemographic features of our patients.
Table 1.
Sociodemographic characteristics of patients
| Item | Number | Percentage (%) | |
|---|---|---|---|
| Sex | Male | 2 | 9.5 |
| Female | 19 | 90.5 | |
| Age group | 18–30 | 3 | 14.3 |
| 30–40 | 3 | 14.3 | |
| 40–50 | 13 | 61.9 | |
| 50–60 | 2 | 9.5 | |
| Profession | Unemployed | 0 | 0 |
| Student | 1 | 4.8 | |
| Official | 7 | 33.3 | |
| Private sector | 13 | 61.9 | |
| Level of education | No schooling | 0 | 0 |
| Primary | 0 | 0 | |
| Secondary | 3 | 14.3 | |
| University | 18 | 85.7 | |
| Region of origin in the country | Central | 5 | 23.7 |
| South | 0 | 0 | |
| Southwest | 0 | 0 | |
| Northwest | 0 | 0 | |
| West | 13 | 61.9 | |
| East | 1 | 4.8 | |
| Adamawa | 0 | 0 | |
| North | 0 | 0 | |
| Far north | 0 | 0 | |
| Littoral | 1 | 4.8 | |
| Expatriate | 1 | 4.8 | |
All these patients presented at least 1 comorbidity (Table 2), among which the commonest were osteoarthritis in 21 cases (100%), sleep apnea in 8 cases (38.1%), and hypertension in 4 cases (19.1%).
Table 2.
Comorbidities associated with obesity
| Number | Percentage (%) | |
|---|---|---|
| Osteoarthritis | 21 | 100 |
| Sleep apnea | 8 | 38.1 |
| Hypertension | 4 | 19.1 |
| Dyslipidemia | 3 | 14.3 |
| Diabetes | 3 | 14.3 |
| Depression | 2 | 9.5 |
| Heart failure | 1 | 4.7 |
| Gout | 1 | 4.7 |
Intraoperative complications were reported in 3 cases (14.3%), represented by 2 cases (9.5%) of staple line bleeding and 1 case (4.7%) of liver bleeding in relation with a tear due to the liver retractor. Staple line bleeding stopped in all cases after the systematic suture over the mechanical suture. Liver bleeding stopped after compression. All procedures were completed laparoscopically without the need for conversion in any case. The mean operative time was 192.2 ± 52.8 minutes, and the mean hospital stay 4.7 ± 1.1 days.
No 30-day readmission was recorded. Eight patients (38.1%) presented a total of twelve 30-day postoperative complications (Table 3). All these complications were classified as minor according to Clavien–Dindo. The main postoperative morbidity was represented by nausea and vomiting (n = 3, 14.3%). No case of surgical site infection was recorded.
Table 3.
Postoperative 30-day morbidity
| Complications' stage according to Clavien–Dindo | Type of complications | Number | Percentage |
|---|---|---|---|
| 1 | Nausea and vomiting | 3 | 14.3 |
| Ileus | 2 | 9.5 | |
| Port site seroma | 2 | 9.5 | |
| Port site bleeding | 2 | 9.5 | |
| 2 | Melena | 2 | 9.5 |
| Minor pneumonia | 1 | 4.7 | |
| 3 | Nil | ||
| 4 | Nil | ||
| 5 | Nil |
No death was recorded during the 30 days following the surgery.
DISCUSSION
Laparoscopic sleeve gastrectomy (LSG) is the commonest primary performed bariatric procedure worldwide [18,19]. The aim of this study was to evaluate the safety of this procedure in a limited setting through the assessment of intraoperative and 30-day postoperative mortality and morbidity. With 14.3% of intraoperative complications without conversion, 38.1% of postoperative 30-day morbidity (all of them classified as minor according to Clavien–Dindo method), and zero postoperative mortality, LSG in the management of obesity is a safe procedure even in a limited setting like our own. African studies dedicated exclusively to sleeve gastrectomy are scarce and limited to Egypt and South Africa; in these reports, morbidity varies from 3.2% to 25% and mortality from 0 to 0.9 [[20], [21], [22], [23], [24]].
Postoperative nausea and vomiting (PONV) were the main postoperative complications in our patients. PONV are a well-known complication after bariatric surgery [25], and LSG is associated with an increased risk compared to other bariatric procedures [26]. Efficient use of antiemetic drugs is mandatory in such cases [27]. However, in our unit, the prevention of PONV was not systematically done and metopimazine was the commonest drug used when they happened. With this study, we started systematically the prevention of PONV with ondansetron. PONV in our patients can be related with our prolonged operative time associated with a longer general anesthesia [[27], [28], [29]]. Between the 1st and the 100th procedure of LSG, the mean operative time is 90 minutes (range, 70–120) [30], whereas it was of 122.2 minutes in our study. A surgeon is considered as an expert on LSG after 50–100 procedures, and the institutional learning process stabilization point for LSG in a newly established bariatric center is between the 100th and 200th operation [30,31]. The prolonged operative time in this report can then be related to our learning curve and our low-volume bariatric surgery activity.
Staple line leakage remains the major complication after LSG, registered in 1.1 to 4.7 of procedures [[32], [33], [34]]. Even if the impact of staple line reinforcement in the prevention of leakage in LSG still controversial [[35], [36], [37], [38]], we systematically performed it with an absorbable self-locking suture. Indeed, leak was found to be significantly associated with mortality after bariatric surgery [39], and its management in a limited setting would not be easy; no case of gastric leakage was noticed in this report.
Bleeding is a frequent intraoperative complication during LSG, occurring in 1.15% to 31.8% of cases [34,39,40]. We found 3 cases (14.3%) of intraoperative complications all represented by bleeding, the staple line being the commonest site involved as reported in the literature [34,39,40]. We believe that this result is an argument in favor of the systematic strengthening of the gastric section line with stitches during LSG. In 1 case, the bleeding site was the liver due to the static liver retractor. This uncommon site of bleeding during a bariatric surgery has been reported in the literature [41] and can be related to either a large fatty liver or an excessive pressure exerted on the liver with its retractor.
The conversion rate for LSG ranges from 1.05% to 1.85% [33]. In our study, all procedures were completed laparoscopically. No patients required a reintervention during the 30-day postoperative period. If some authors reported a reoperation rate of 7.4% [42], our small sample does not allow us to discuss furthermore this parameter.
The 30-day mortality was nil in this report. Mortality rate after LSG varies from 0% to 3.3% [42]. This result is satisfactory and in favor of the safety of LSG in our initial experience on bariatric surgery, irrespective of our limited setting.
The profile of the patient undergoing bariatric surgery in our context seems to be as follows: woman aged between 40 and 50 years, with a university education level, employed in the private sector, originated from the western region of the country, and in morbid obesity associated with osteoarthritis as the main comorbidity. Many African studies [[20], [21], [22], [23]] have found similar results with 57%–85.4% of female patients whose mean age and BMI ranged from 37 to 41.8 years and 41.5 to 49.38 kg/m2, respectively. In Cameroon, people originating from the western region of the country seem more affected than the rest of the population [43,44]. The hypothesis of a genetic disposition has been advocated in some African ethnic group in South Africa, Nigeria, and Ghana [45] and needs to be assessed in our setting.
The limitations of this study are related to the retrospective data collection, the single-institute design, and its small sample. Nevertheless, bariatric surgery records were properly kept and achieved since the beginning of this activity in our unit; this explains why all the operated patients could be included in this study. Despite a small sample size, this study is a report of an uncommon surgical activity to date in black Africa whose evaluation of surgery-related morbidity and mortality is important for its development. The procedures reported in this study were carried out by a single team with solid experience in laparoscopic surgery. We think that this experience may explain the low rate of major peri- and postoperative complications. Bariatric surgery should not be the first experience of laparoscopic surgery for a young African team. Long-term results on weight loss and comorbidities of LSG should be assessed in our setting on further studies. Because the prevalence of obesity is increasing in Africa and in Cameroon in particular, it is important to set up national policies against obesity that include weight loss surgery, which is not currently the case. This will in particular respond to a major limitation of the development of this surgery in our environment, namely, its high cost in a limited financial income context.
In conclusion, sleeve gastrectomy, even with a low volume activity, is a safe procedure in our limited setting with minor postoperative complications and a nil surgery-related death. It could then be the first step in the development of bariatric surgery activity in sub-Saharan African setting before the implementation of more invasive methods like gastric bypass.
Author Contribution
Guy Aristide Bang: Conceptualization, Data analysis, Writing – original draft. Blondel Nana Oumarou: Data collection, Data analysis, Writing – original draft. Eric Patrick Savom: Data collection, Writing – review & editing. Maurice Aurélien Sosso: Supervision, Writing –review & editing.
Conflict of Interest
None.
Funding Source
None.
Ethics Approval
The study was conducted after the approval of our institutional research and ethical committee.
Data Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Contributor Information
Guy Aristide Bang, Email: guyaristidebang@yahoo.fr.
Blondel Nana Oumarou, Email: nanablondel@yahoo.fr.
Eric Patrick Savom, Email: esavom@yahoo.fr.
Maurice Aurélien Sosso, Email: sossomaurice@yahoo.fr.
References
- 1.Ajayi Ikeoluwapo O., Adebamowo C., Adami H0., Dalal S., Diamond M.B., Bajunirwe F., et al. Urban–rural and geographic differences in overweight and obesity in four sub-Saharan African adult populations: a multi-country cross-sectional study. BMC Public Health. 2016;16:1126. doi: 10.1186/s12889-016-3789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sartorius B., Veerman L.J., Manyema M., Chola L., Hofman K. Determinants of obesity and associated population attributability, South Africa: empirical evidence from a national panel survey, 2008-2012. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0130218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nansseu J.R., Noubiab J.J., Bigna J.J. Epidemiology of obesity and overweight in adults living in Cameroon: a systematic review and meta-analysis. Obesity. 2019;0:1–11. doi: 10.1002/oby.22566. [DOI] [PubMed] [Google Scholar]
- 4.Choukem S.P., Kamdeu-Chedeu J., Leary S.D., Mboue-Djieka Y., Nebongo D.N., Akazong C., et al. Overweight and obesity in children aged 3-13 years in urban Cameroon: a cross-sectional study of prevalence and association with socio-economic status. BMC Obes. 2017;4:7. doi: 10.1186/s40608-017-0146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muthuri S.K., Francis C.E., Wachira L.J.M., LeBlanc A.G., Sampson M., Onywera V.O., et al. Evidence of an overweight/obesity transition among school-aged children and youth in sub-Saharan Africa: a systematic review. PlosOne. 2014;9(3) doi: 10.1371/journal.pone.0092846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniels S.R. Complications of obesity in children and adolescents. Int J Obes. 2009;33:S60–S65. doi: 10.1038/ijo.2009.20. [DOI] [PubMed] [Google Scholar]
- 7.Reilly J.J., Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes. 2010;35(2):891–898. doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- 8.Pulgaron E.R. Childhood obesity: a review of increased risk for physicalandpsychological comorbidities. Clin ther. 2013;35(1):18–32. doi: 10.1016/j.clinthera.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pentin P.L., Nashelsky J. What are the indications for bariatric surgery? J Fam Pract. 2005;54(7):633–634. [PubMed] [Google Scholar]
- 10.Buchwald H., Estok R., Fahrbach K., Banel D., Sledge I. Trends in mortality in bariatric surgery: a systematic review and meta-analysis. Surgery. 2007;142(4):621–635. doi: 10.1016/j.surg.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Rubino F., Nathan D.M., Eckel R.H., Schauer P.R., Alberti K.G.M., Zimmet P.Z., et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Surg Obes Relat Dis. 2016;12(6):1144–1162. doi: 10.1016/j.soard.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Livingston E.H. The incidence of bariatric surgery has plateaued in the U.S. Am J Surg. 2010;200(3):378–385. doi: 10.1016/j.amjsurg.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longitudinal Assessment of Bariatric Surgery (LABS) Consortium, Flum D.R., Belle S.H., King W.C., Wahed A.S., Berk P., et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361(5):445–454. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potgieter A.J., Van der Merwe M.T. Metabolic surgery: a concise overview and understanding of potential complications. JEMDSA. 2011;16(3):138–144. [Google Scholar]
- 15.Chang S.H., Stoll C.R., Song J., Varela J.E., Eagon C.J., Colditz G.A. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149(3):275–287. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bang G.A., Nana Oumarou B., Savom E.P., Mbianda Nketcha J.J., Essomba A. L’Anneau Gastrique Ajustable dans la Prise en Charge Chirurgicale de l’Obésité : Bilan de 9 Années de Pratique au Centre Hospitalier d’Essos. Health Sci Dis. 2022;23(1):73–76. [Google Scholar]
- 17.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varela J.E., Nguyen N.T. Laparoscopic sleeve gastrectomy leads the US utilization of bariatric surgery at academic medical centers. Surg Obes Relat Dis. 2015;11(5):987–990. doi: 10.1016/j.soard.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Ozsoy Z., Demir E. Which bariatric procedure is the most popular in the world? A bibliometric comparison. Obes Surg. 2018;28:2339–2352. doi: 10.1007/s11695-018-3163-6. [DOI] [PubMed] [Google Scholar]
- 20.ElGeidie A., ElHemaly M., Hamdy E., El Sorogy M., AbdelGawad M., GadElHak N. The effect of residual gastric antrum size on the outcome of laparoscopic sleeve gastrectomy: a prospective randomized trial. Surg Obes Relat Dis. 2015;11(5):997–1003. doi: 10.1016/j.soard.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 21.Hany M., Ibrahim M. Comparison between stable line reinforcement by barbed suture and non-reinforcement in sleeve gastrectomy: a randomized prospective controlled study. Obes Surg. 2018;28(8):2157–2164. doi: 10.1007/s11695-018-3175-2. [DOI] [PubMed] [Google Scholar]
- 22.Hussein A.H., Khaled I., Faisal M. The role of the surgical resection distance from the pylorus after laparoscopic sleeve gastrectomy: a prospective cohort study from an academic medical center in Egypt. Patient Saf Surg. 2020;14(1):42. doi: 10.1186/s13037-020-00270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sofianos C., Sofianos C. Outcomes of laparoscopic sleeve gastrectomy at a bariatric unit in South Africa. Ann Med Surg. 2016;12:37–42. doi: 10.1016/j.amsu.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Sayes I.A., Abdelbaki T.N., Elkeleny M.R., Sharaan M.A. Feasibility and efficacy of laparoscopic sleeve gastrectomy as a revisional procedure after failed gastric plication in morbidly obese patients. J Laparoendosc Adv Surg Tech A. 2021;31(3):284–289. doi: 10.1089/lap.2020.0432. [DOI] [PubMed] [Google Scholar]
- 25.Halliday T.A., Sundqvist J., Hultin M., Wallden J. Post-operative nausea and vomiting in bariatric surgery patients: an observational study. Acta Anaesthesiol Scand. 2017;61(5):471–479. doi: 10.1111/aas.12884. [DOI] [PubMed] [Google Scholar]
- 26.Kushner B.S., Freeman D., Sparkman J., Salles A., Eagon J.C., Eckhouse S.R. Assessment of postoperative nausea and vomiting after bariatric surgery using a validated questionnaire. Surg Obes Relat Dis. 2020;16(10):1505–1513. doi: 10.1016/j.soard.2020.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Groene P., Eisenlohr J., Zeuzem C., Dudok S., Karcz K., Hofmann-Kiefer K. Postoperative nausea and vomiting in bariatric surgery in comparison to non-bariatric gastric surgery. Wideochir Inne Tech Maloinwazyjne. 2019;14(1):90–95. doi: 10.5114/wiitm.2018.77629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong D., Peng X., Liu J., Qian H., Li J., Wu B. Morbid obesity alters both pharmacokinetics and pharmacodynamics of propofol: dosing recommendation for anesthesia induction. Drug Metabol Dispos. 2016;44:1579–1583. doi: 10.1124/dmd.116.071605. [DOI] [PubMed] [Google Scholar]
- 29.Li J.Y., Zou Q.R., Peng X.M. Pharmacokinetics of a cisatracurium dose according to fat-free mass for anesthesia induction in morbidly obese patients. J South Med Univ. 2016;36:1396–1400. [PubMed] [Google Scholar]
- 30.Major P., Wysocki M., Dworak J., Pędziwiatr M., Pisarska M., Wierdak M. Analysis of laparoscopic sleeve gastrectomy learning curve and its influence on procedure safety and perioperative complications. Obes Surg. 2018;28(6):1672–1680. doi: 10.1007/s11695-017-3075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Celio A.C., Kasten K.R., Brinkley J., Chung A.Y., Burruss M.B., Pories W.J., et al. Effect of surgeon volume on sleeve gastrectomy outcomes. Obes Surg. 2016;26(11):2700–2704. doi: 10.1007/s11695-016-2190-4. [DOI] [PubMed] [Google Scholar]
- 32.Aurora A., Khaitan L., Saber A. Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surg Endosc. 2012;26(6):1509–1515. doi: 10.1007/s00464-011-2085-3. [DOI] [PubMed] [Google Scholar]
- 33.Rosenthal R.J., Diaz A.A., Arvidsson D., Baker R.S., Basso N., Bellanger D., et al. International Sleeve Gastrectomy Expert Panel consensus statement: best practice guidelines based on experience of > 12,000 cases. Surg Obes Relat Dis. 2012;8(1):8–19. doi: 10.1016/j.soard.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Sakran N., Goitein D., Raziel A., Keidar A., Beglaibter N., Grinbaum R., et al. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27(1):240–245. doi: 10.1007/s00464-012-2426-x. [DOI] [PubMed] [Google Scholar]
- 35.Baltasar A., Serra C., Pérez N., Bou R., Bengochea M., Ferri L. Laparoscopic sleeve gastrectomy: a multi-purpose bariatric operation. Obes Surg. 2005;15:1124–1128. doi: 10.1381/0960892055002248. [DOI] [PubMed] [Google Scholar]
- 36.Angrisani L., Cutolo P.P., Buchwald J.N., McGlennon T.W., Nosso G., Persico F., et al. Laparoscopic reinforced sleeve gastrectomy: early results and complications. Obes Surg. 2011;21:783–793. doi: 10.1007/s11695-011-0400-7. [DOI] [PubMed] [Google Scholar]
- 37.Kasalicky M., Michalsky D., Housova J., Haluzik M., Housa D., Haluzikova D., et al. Laparoscopic sleeve gastrectomy without an over-sewing of the staple line. Obes Surg. 2008;18:1257–1262. doi: 10.1007/s11695-008-9635-3. [DOI] [PubMed] [Google Scholar]
- 38.Parikh M., Issa R., McCrillis A., Saunders J.K., Ude-Welcome A., Gagner M. Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy: a systematic review and meta-analysis of 9991 cases. Ann Surg. 2013;257:231–237. doi: 10.1097/SLA.0b013e31826cc714. [DOI] [PubMed] [Google Scholar]
- 39.Chopra A., Chao E., Etkin Y., Merklinger L., Lieb J., Delany H. Laparoscopic sleeve gastrectomy for obesity: can it be considered a definitive procedure? Surg Endosc. 2012;26(3):831–837. doi: 10.1007/s00464-011-1960-2. [DOI] [PubMed] [Google Scholar]
- 40.Wahby M., Salama A.F., Elezaby A.F., Belgrami F., Abd Ellatif M.E., El-Kaffas H.F., et al. Is routine postoperative gastrografin study needed after laparoscopic sleeve gastrectomy? Experience of 712 cases. Obes Surg. 2013;23(11):1711–1717. doi: 10.1007/s11695-013-1013-0. [DOI] [PubMed] [Google Scholar]
- 41.Nowara H.A. Egyptian experience in laparoscopic adjustable gastric banding (technique, complications and immediate results) Obes Surg. 2001;11:70–75. doi: 10.1381/096089201321454141. [DOI] [PubMed] [Google Scholar]
- 42.Daskalakis M., Berdan Y., Theodoridou S., Weigand G., Weiner R.A. Impact of surgeon experience and buttress material on postoperative complications after laparoscopic sleeve gastrectomy. Surg Endosc. 2011;25(1):88–97. doi: 10.1007/s00464-010-1136-5. [DOI] [PubMed] [Google Scholar]
- 43.Cohen E., Amougou N., Ponty A., Loinger-Beck J., Nkuintchua T., Monteillet N., et al. Nutrition transition and biocultural determinants of obesity among Cameroonian migrants in urban Cameroon and France. Int J Environ Res Public Health. 2017;14(7):696. doi: 10.3390/ijerph14070696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen E., Boetsch G., Palstra F.P., Pasquet P. Social valorisation of stoutness as a determinant of obesity in the context of nutritional transition in Cameroon: the Bamiléké case. Soc Sci Med. 2013;96:24–32. doi: 10.1016/j.socscimed.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Yako Y.Y., Echouffo-Tcheugui J.B., Balti E.V., Matsha T.E., Sobngwi E., Erasmus R.T., et al. Genetic association studies of obesity in Africa: a systematic review. Obes Rev. 2015;16(3):259–272. doi: 10.1111/obr.12260. [DOI] [PubMed] [Google Scholar]


