Abstract
The Wingless-types/beta-catenin (Wnt/β-catenin) signaling pathway plays an important role in embryonic development and affects the physiological development processes of feather follicles. To investigate the role of Wnt/β-catenin pathway in regulating feather follicles morphogenesis, in ovo injection of CHIR-99021, an activator of the Wnt/β-catenin signaling pathway, was conducted in chick embryo model. Initially, a total of 40 embryos were used to assess feather follicles morphogenesis and the expression of β-catenin (E9–E17). The histological results showed that feather follicle morphogenesis was mainly completed from E9 to E17. β-catenin was involved in the processing of the appearance of dermal cell condensation (E9) and the completion of the feather follicles morphogenesis (E17). Next, a total of 160 fertilized eggs were randomly divided into 8 groups for in ovo injection at E9, including a Normal Saline injected group (CON) and the 500, 1,000, 2,000, 5,000, 10,000, 50,000, and 100,000 ng CHIR-99021 groups. Dorsal skin tissue samples were collected at E17 for investigating feather follicles morphology and expressions of β-catenin and lymphoid enhancerbinding factor-1 (LEF1) at gene and protein levels. The results showed that feather follicle diameter in the injected groups were significantly (P < 0.05) increased with limit dose-independence compared to the CON group. CHIR-99021 significantly (P < 0.05) influenced the mRNA expressions of catenin beta-1 (CTNNB1) and downstream target LEF1. In ovo injection of CHIR-99021 caused that β-catenin and LEF1 were significantly (P < 0.05) increased followed the increased doses as determined by western blotting. The immunochemical results showed that β-catenin was detected in the dermal papilla of feather follicles. Given these results, this study suggests to developmental biology that in ovo injection of CHIR-99021 promoted feather follicles morphogenesis and development via activating Wnt/β-catenin signaling pathway and upregulating downstream target LEF1 during embryonic period in chick embryo model. Moreover, CHIR-99021 may be a strong candidate to promote the animal feather/hair industry, especially as a reference for bird feather production.
Key words: feather follicle, in ovo injection, Wnt/β-catenin, CHIR-99021, chick embryo
INTRODUCTION
Feather/hair is also one of the important productions in some animal husbandry, such as goose feather, duck feather, rabbit hair, and wool. However, development of hair/feather follicle undergoes complex morphological changes and physiological development processes and is influenced and regulated by various factors. In the genetic respect, the Wingless-types (Wnt) signal is the initial signal of hair follicles growth and participates in many stages of morphogenesis (Krause and Foitzik, 2006; Hardman et al., 2015; Wang et al., 2017). The Wnt signal also palys critical regulatory role in developmental physiological processes, such as the occurrence of dermal papilla, function of feather/hair follicles, cyclical changes of feather/hair follicles, and proliferation and differentiation of feather follicle stem cells (Ito, et al., 2007; Shao, et al., 2010; Rishikaysh, et al., 2014; Rognoni, et al., 2016). β-catenin, a molecular switch of the Wnt signal, is the core in the classical Wnt signaling pathway with responsibility of linking other development-related pathways, such as the transforming growth factor-beta (TGF-β) and bone morphogenetic proteins (BMPs) signaling pathways (Stenn and Paus, 2001; Telerman et al., 2017; Tripurani et al., 2018; Veltri et al., 2018).
Small molecules with membrane permeability have been applicated in maintenance and differentiation of stem cells (Wu et al. 2015). CHIR-99021 is a potent and selective GSK-3α/β inhibitor and the Wnt/β-catenin signaling pathway activator (Ring et al. 2003; Ye et al. 2012; Naujok et al. 2014), stabilizes and influences β-catenin in the Wnt/β-catenin pathway, regulates several other pluripotency-related pathways (such as TGF-β, Notch, MAPK, and BMP), and participates in embryonic development and differentiation (Wu et al. 2013). Advantageously, CHIR-99021 changes epigenetic expression regulation genes and long intergenic non-coding RNA (Wu et al. 2013), while it does not produce significant concomitant toxicity (Wu et al. 2013; Naujok et al. 2014). Moreover, in ovo injection is an efficient way to intervene embryonic development (Slawinska et al. 2016; Chen et al. 2020; Xie et al. 2020).
In this study, we used the chick embryo as a model system to observe the feather follicles morphogenesis during embryonic period. Then, different doses of CHIR-99021 were injected into the yolk sac to investigate the role of Wnt/β-catenin signaling pathway in the promotion effects on feather follicles morphogenesis and development.
MATERIALS AND METHODS
Experimental Animals
Fertilized eggs of the JingHong No.1 were obtained from Jilin Agricultural University in Jilin Province, Northeast of China, and incubated in an incubator according to the routine procedure. Animal experiment was approved by Animal Health Care Committee of Animal Science and Technology College of Jilin Agricultural University (Approval No. GR (J) 18-003).
Experimental Design and Treatments
The appropriate number of fertilized eggs was incubated in a fully automatic incubator with intermittent rotation. Candling procedure was conduct at the 6th day of incubation to identify and remove the unfertilized eggs and the mortality embryos. Succeeding, the number of 40 fertilized eggs was used to initially observe the morphogenesis and development of feather follicles (E9–E17). Then, a total of 160 fertilized eggs were divided into 8 groups of 20 eggs each group for further in ovo injection experiments. All in ovo injection solutions were freshly prepared (37.9℃) at the 9th day of incubation and the solutions were injected into the egg yolk sac. The 8 treatment groups consisted of
-
(1)
Injected group with 100 μL of 0.9% Normal Saline (NS) /egg (CON group)
-
(2)
Injected group with 100 μL of 0.9% NS /egg containing 500 ng CHIR-99021 /egg (500 ng CHIR-99021 group)
-
(3)
Injected group with 100 μL of 0.9% NS /egg containing 1,000 ng CHIR-99021 /egg (1,000 ng CHIR-99021 group)
-
(4)
Injected group with 100 μL of 0.9% NS /egg containing 2,000 ng CHIR-99021 /egg (2,000 ng CHIR-99021 group)
-
(5)
Injected group with 100 μL of 0.9% NS /egg containing 5,000 ng CHIR-99021 /egg (5,000 ng CHIR-99021 group)
-
(6)
Injected group with 100 μL of 0.9% NS /egg containing 10,000 ng CHIR-99021 /egg (10,000 ng CHIR-99021 group)
-
(7)
Injected group with 100 μL of 0.9% NS /egg containing 50,000 ng CHIR-99021 /egg (50,000 ng CHIR-99021 group)
-
(8)
Injected group with 100 μL of 0.9% NS /egg containing 100,000 ng CHIR-99021 /egg (100,000 ng CHIR-99021 group).
Preparation of CHIR-99021 Solutions and In Ovo Injection Procedure
CHIR-99021 (CHIR-99021 monohydrochloride, MedChemExpress, Shanghai, China) was dissolved in 0.9% NS. Beforehand, we injected ink to train the accuracy of the yolk sac injection and to determine a needle insertion distance. In the CHIR-99021 injected experiment, first, the center of large end of each egg was located under the egg candler. An alcohol swab was used to disinfect the target injection site, then a small hole was drilled at the injection site (Figure S1). The volume of 100 μL of the CHIR-99021 solution was rapidly injected into the egg yolk sac using a 0.8 mm × 38 mm needle. After paraffin sealing, the eggs were returned to the incubator. The injection operation process of each egg was controlled in about 15 min outside the incubator environment for standardization.
Sampling
Dorsal skin tissue samples were obtained at E17, and the survival rate was investigated using the percentage between surviving embryos and the total number of the eggs received injection. The cross region (about 1.5 cm2) of the midline of dorsal skin tissues were sampled. The central region of the tissue was fixed in 4% paraformaldehyde for 24 h for further histological processing and other tissues were stored at −80°C for further qRT-PCR and Western blotting assays.
RNA Extraction and qRT-PCR
The gene expressions of CTNNB1 (encording β-catenin) and LEF1 in dorsal skin tissues were determined by qRT-PCR. The primers sequence of CTNNB1, LEF1, and β-actin are listed in Table 1. The dorsal skin tissue total RNA of each sample was extracted using an RNAiso Plus Kit (TaKaRa Bio Inc., Shiga, Japan). Deoxyribonuclease I was used to treat total RNA samples against genomic DNA contamination and the quantity and quality of the harvested RNAs were measured using the Micro Drop Ultra-micro spectrophotometer (BIO-DL, Shanghai, China) and the 1.0 agarose gel electrophoresis, respectively. The total RNA of each sample was considered to be valid in the range of 1.9 < OD260/OD280 < 2.0 and 1.8 < 28S/18S < 2.0 simultaneously and the complementary DNA (cDNA) was synthesized for qRT-PCR using a PrimeScript RT Reagent Kit with gDNA Eraser (Morey Biosciences, Shanghai, China). However, qRT-PCR was performed using SYBR Green Kit (Morey Biosciences, Shanghai, China) in a CFX Connect Real-Time PCR Detection System (Bio-Rad, Hercules, CA). The qPCR reactions total volume of 20 μL comprising 2 μL of cDNA, 0.6 μL of both forward and reverse primers, 6.8 μL RNase-free H2O,10 μL SYBR qPCR mix. Amplification conditions were as follows: pre-denaturation at 95°C for 5 min, then 40 cycles of amplification (95°C for 15 s and 59°C for 60 s). The multicolored Real-Time PCR Detection System CFX manager (Bio-Rad) was used to calculate the relative expression levels of the candidate genes in the feather follicles based on the 2–ΔΔCt method. The β-actin gene was chosen as the control gene to normalize the mRNA expression levels of the target genes.
Table 1.
Primer sequences for qRT-PCR analysis.
| Gene | Primer sequences (5’-3’) | Accession No. |
|---|---|---|
| CTNNB1 | F: GTGTTGATCTTTGCAGTCGGC | XM_015281298.2 |
| R: AACTCCATCAAGTCAGCTTGGG | ||
| LEF1 | F: CCACCTCTTGGCTGGTTTTC | XM_015276137.1 |
| R: CTCACTGTCGTTGTGCGGAT | ||
| β-actin | F: ACACCCACACCCCTGTGATGAA | NM205518 |
| R TGCTGCTGACACCTTCACCATTC |
The candidate genes: CTNNB1: beta catenin; LEF1: lymphoid enhancer binding factor 1 and β-actin.
F denotes forward primers and R denotes reverse primers.
Histological Processing
The center of dorsal skin tissue of approximately 1.5 cm2 was collected and stored in 4% paraformaldehyde for 24 h. Then, the fixed tissues were dehydrated in different concentrations of ethanol (50, 75, 85, 90, 95, 100%) and were soaked twice in xylene for clearing. Tissues were embedded in paraffin according to conventional methods. In addition, 5-μm thick sections were cut onto gelatinized slides and stored at room temperature until they get ready for using.
Hematoxylin and Eosin Staining
The sections were stained using H&E and they were photographed using a microscope system (Olympus, Tokyo, Japan).
Immunohistochemical Observations
The slides with 5-μm thick paraffin-embedded dorsal skin tissues sections were baked at 65°C for 3 h, and finally dewaxed twice in xylene and hydrated in descending grades of alcohol. The slides were then succumbed to antigen retrieval by boiling in sodium citrate buffer (pH 6.0) for 20 min. In addition, these slides were rinsed with PBS solution for 5 min of 3 times. Following deparaffinization, rehydration, and enzyme digestion, they were blocked with 3% hydrogen peroxide for 20 min and with 3% goat serum (normal goat serum) at room temperature for 20 min, then incubated overnight with 50 μL of Rabbit anti-β-catenin antibody (Cat# bs-23663R, bioss, Beijing, China) at 4°C with 1:100 dilution, followed by operating according to UltraSensitive SP Rabbit IHC Kit (MXB, Fuzhou, Fujiang, China) instructions and DAB staining. The coversliping was then conducted with neutral resin. The slides were observed and photographed using a microscope system (Olympus).
Western Blotting Analysis
The total protein samples for electrophoresis were extracted using radio immunoprecipitation assay lysis buffer. The total protein concentration was determined by a BCA protein concentration detection kit (Beyotime, Shanghai, China). For each sample, the volume containing 10 μg total protein was pipetted onto a 10% SDS-PAGE. The transferred proteins were bound to the surface of the PVDF membrane. The membranes were blocked with 5% skim milk and incubated with primary antibodies (1:1,000 dilution) at 4°C overnight. The antibodies identifiers were as follows: a Rabbit anti-LEF1 Antibody (LifeSpan BioSciences Inc., Shanghai, China), an Rabbit anti-β-catenin Antibody (Cat# bs-23663R, Bioss, Beijing, China), and a GAPDH Rabbit Monoclonal Antibody (Cat# AT003, ABP Biosciences, Wuhan, Hubei, China). At the end, the membranes were incubated with a Goat Anti-Rabbit IgG (H+L)/HRP antibody secondary antibody (Biosynthesis Biotechnology, Beijing, China) for 1 h at room temperature. Finally, the membranes were visualized with an ECL Test Kit (Millopore, Darmstadt, Germany) under a Bio-Rad imaging system (Bio-Rad). The chemiluminescence of each protein band was quantified using the ImageJ software (NIH, Bethesda, MD), and protein levels were normalized by GAPDH as internal control.
Measurement of Feather Follicle Diameter
The distribution of feather follicles was observed and photographed from the longitudinal skin sections to measure feather follicle diameter. The number of 60 feather follicles from 6 biological samples of each group was measured using the microscope software (Olympus). The feather follicle diameter was measured from the outermost layer of the follicle, where the maximum diameter was observed. We also used the 3 criteria in our measurement process: 1) selecting the same tissue site for embed; 2) selecting completed feather follicles for measurement; 3) selecting the largest feather follicle width data from enormous amount of tissue sections for analysis.
Statistical Analyses
Statistical tests were performed with SPSS 23.0 software (IBM, Armonk, NY). The data was visualized using the GraphPad Prism 8 software (GraphPad, San Diego, CA). Next, layouts were conducted using Adobe Illustrator software (Adobe, San Jose, CA). Statistical significance was determined using one-way ANOVA by Duncan's multiple range test. The significant difference of the data was considered as P < 0.05. The results were expressed as mean + SEM.
RESULTS
Development of Feather Follicle During Chick Embryonic Period
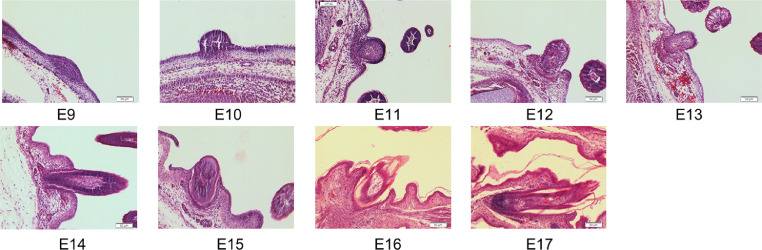
In order to identify the in ovo injection time and sampling time, histological observations of feather follicles were conducted at the different embryonic ages. The morphological results of embryonic dorsal skin tissues in the different stages (Figure 1) showed that the dorsal skin of E9–E10 chick embryo had grown obvious intra-buds. At E11 to E16, the follicles began and continued to form. By E17, the feather follicles were developed more fully and became more matureness. Therefore, we chose E9 for in ovo injection and E17 for sampling.
Figure 1.
Morphological observations of feather follicles at different embryonic ages (E9–E17) as indicated by H&E staining. Scale bars = 50 μm.
β-catenin Expression in Chick Embryonic Feather Follicles
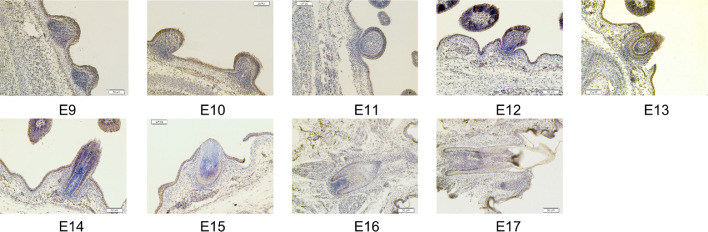
To justify the key role of β-catenin in feather follicles development, immunohistochemical observations were conducted. The results (Figure 2 and Figure S2) showed that β-catenin was expressing at E9 to E17 during feather follicles development. Notably, β-catenin was highly expressed at E9 and E10. These results also illustrated that the Wnt/β-catenin signaling pathway participated in feather follicles development during embryonic period.
Figure 2.
Immunohistochemical observations (β-catenin) of dorsal skin with feather follicles in chick embryos (E9–E17). Scale bars = 50 μm.
CHIR-99021 Promoted the Morphogenesis of Feather Follicle
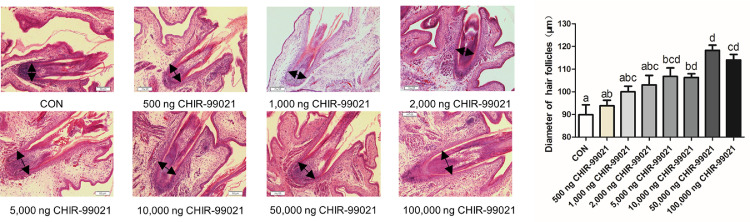
In the experiment of in ovo injection of CHIR-99021, H&E staining was performed to calculate the feather follicle diameter. The results (Figure 3) demonstrated that the in ovo injection of CHIR-99021 significantly increased the diameter of feather follicles at E17. In addition, the CON group and the injected groups have no significant effect on survivability (Table 2) and visual observation (Figure S3). The feather follicle diameter in embryos received injection was significantly (P < 0.05) increased at E17 compared with the CON group (Figure 3A). These findings suggested that in ovo injection of CHIR-99021 significantly promoted feather follicles development in chick embryos.
Figure 3.
In ovo injection of CHIR-99021 promotes dorsal feather follicles development in chick embryos. (A) Morphological photographs of longitudinal feather follicles between groups as indicated by H&E. The diameter of feather follicles is indicated by black arrows. (B) The statistical results of feather follicle diameter. Scale bars = 50 μm. Statistical significance was determined by Duncan's multiple range test. Values are presented as the mean + SEM. Different letters in a column indicate a significant difference (P < 0.05, n = 60 feather follicles from 6 biological samples).
Table 2.
Effect of in ovo injection of CHIR 99021 on survivability.
| Group | Survivability (%) |
|---|---|
| CON | 100 |
| 500 ng CHIR 99021 | 100 |
| 1,000 ng CHIR 99021 | 100 |
| 2,000 ng CHIR 99021 | 100 |
| 5,000 ng CHIR 99021 | 100 |
| 10,000 ng CHIR 99021 | 100 |
| 50,000 ng CHIR 99021 | 100 |
| 100,000 ng CHIR 99021 | 100 |
CHIR-99021 Influenced the mRNA Expression of the Wnt/β-catenin Signaling Pathway
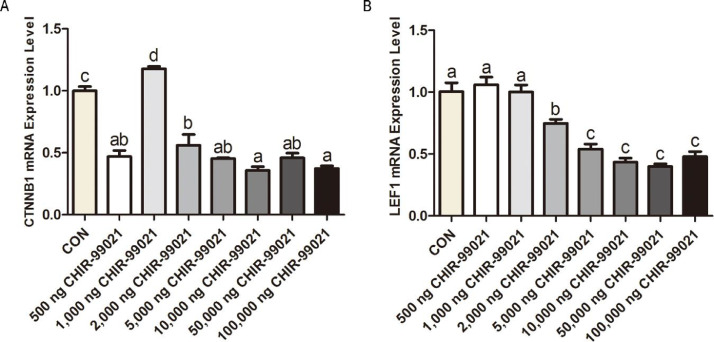
The expression of CTNNB1 in the injected groups except the 1,000 ng CHIR-99021 group was significantly (P < 0.05) downregulated compared to the CON group (Figure 4A). Also, the expression of LEF1 in the 2,000 to 100,000 ng CHIR-99021 groups were significantly (P < 0.05) downregulated compared to the CON group (Figure 4B). Therefore, in ovo injection of higher doses CHIR-99021 (2,000–100,000 ng) had more obvious ability to influence the mRNA expression of CTNNB1 and LEF1.
Figure 4.
The expression of CTNNB1 (A) and LEF1 (B) genes. Statistical significance was determined by Duncan's multiple range test. The results are expressed as the mean + SEM. Different letters in a column indicate a significant difference (P < 0.05, n = 6 biological samples).
CHIR-99021 Influenced the Protein Expression of the Wnt/β-catenin Signaling Pathway
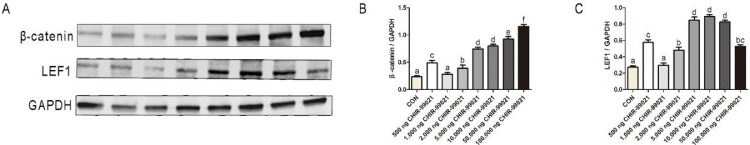
Western blotting was used to quantify the β-catenin and LEF1 expressions in dorsal skin samples between groups. The results (Figure 5) showed that injection of CHIR-00921 promoted the expressions of β-catenin and downstream target LEF1. Notably, the protein expression levels of β-catenin and LEF1 were increased following the increased doses of CHIR-99021, generally. Moreover, the immunohistochemical results under the cross and longitudinal sections (Figures S4 and S5) showed that the expression of β-catenin was determined in feather follicles of all groups. In concluding, CHIR-99021 promoted the activation of the Wnt/β-catenin pathway in dorsal skin with feather follicles.
Figure 5.
In ovo injection of CHIR-99021 promoted the expression of β-catenin. (A)Western blotting images of β-catenin, LEF1 in dorsal skin tissues with feather follicles. GAPDH was used as a control. The statistical results are presented in (B) and (C). Statistical significance was determined by Duncan's multiple range test. The results are expressed as the mean + SEM, and different letters in a column indicate a significant difference (P < 0.05).
DISCUSSION
It has been widely reported that the Wnt signaling pathway as an evolutionarily conserved cell-cell communication system is closely associated with differentiation and development (McLin et al., 2007). Notably, the classical Wnt signaling pathway (Wnt/β-catenin) plays a critical role in hair/feather follicles morphogenesis (Rishikaysh et al., 2014; Rognoni et al., 2016; Tripurani et al., 2018; Veltri et al., 2018). The expression of β-catenin is dynamic in the feather follicles morphogenesis during embryonic period (Gong et al., 2018; Majidinia et al., 2018), which has confirmed that the Wnt/β-catenin pathway joins and regulates the physiological processing of feather follicles morphogenesis. In the first section of this study, we investigated the feather follicles morphology and β-catenin distribution in the feather follicles during chick embryonic period (E9–E17) using histological observations to verify the times for further in ovo injection. The results (Figure 1) showed that a condensation of dermal cells appeared above epidermal placode at E9 where it would develop into feather follicles. Then, there emerged morphological changes in skin at E17, buds transforming into feather follicles structure. By E17, it was observed that feather follicles morphogenesis was mainly completed with feather fiber elongation, and then feather fiber continuously differentiated and matured into feather follicles (Gong et al., 2018). Moreover, we found that β-catenin distributed in the processing of feather follicles morphogenesis at all stages (Figure 2), which was similar to previous studies (Sun et al., 2020). Therefore, the time nodes, in ovo injection of CHIR-99021 at E9 and observations at E17, were chosen to further understand the role of the Wnt/β-catenin in the feather follicles morphogenesis during chick embryonic period.
CHIR-99021 is a potent selective GSK-3α/β inhibitor and the Wnt/β-catenin signaling pathway activator. It enhances the self-renewal ability of mouse and human embryonic stem cells and induces autophagy (Ring et al., 2003; Ye et al., 2012; Naujok et al., 2014). Furthermore, CHIR-99021 stimulates human dermal papilla spheroids contribute to hair follicle formation and production of reconstituted follicle enriched human skin (Yoshida et al., 2019). Zhou et al. (2018) reported that activation of the Wnt/β-catenin signaling pathway regulates hair growth and cycle transition in mouse model. While injecting Dickkopf-related protein 1 (DKK1), a secreted antagonist of the canonical Wnt signaling pathway, into chick embryos at E9 inhibits feather follicles development and feather growth via inhibiting the Wnt/β-catenin signaling pathway (Xie et al., 2020).
In this work, we assessed survivability of embryos injected with CHIR-99021. All survive of embryos given injection (Table 2) confirmed technical feasibility of our in ovo injection. The feather follicle diameter in all CHIR-99021 injected groups were significantly increased (P < 0.05) compared to the CON group (Figure 3). Thus, these findings suggested that injection of CHIR-99021 conducted at initial stage of embryonic feather follicles morphogenesis (E9) promoted feather follicles development.
Moreover, we found that CHIR-99021 injection obviously changed the expression of β-catenin at gene and protein levels. The protein expression of β-catenin showed an increased tendency with increase of injected doses except 1,000 ng CHIR-99021 group (Figure 5B). However, the level of gene expression of CTNNB1 emerged a downregulated tendency was inconsistent with the protein level (Figure 4A). Similarly, inconsistent expression tendencies of LEF1 between gene and protein levels were also found (Figures 4B and 5C). The inconsistency might be explained by post-transcriptional regulation. As a result, CHIR-99021 upregulates the processing of related-genes transcription and translation, which leads to rapid translation of mRNA and increasing protein expression (Hu et al., 2016; Münch and Harper, 2016; Golan-Lavi et al., 2017; Welles et al., 2021). The results in the 1,000 ng CHIR-99021 with upregulated CTNNB1 gene and increased β-catenin protein also support this explanation. Although inconsistent tendencies between gene and protein expression levels, protein as the terminal molecule performing biological functions should be the standard (de Sousa Abreu et al., 2009; Napierala et al., 2017). Here, this study suggested that CHIR-99021 injection has ability to increase the expression of β-catenin protein.
LEF1 is an important downstream target regulating transcription and translation of the Wnt signaling pathway. In addition, the transcription factor LEF1 is a member of the Lef/Tcf family that is necessary for the differentiation of Hair Follicle Stem Cells (HFSCs), and the formation of Inner Root Sheath in feather follicles (Brunner et al., 2017; Sun et al., 2020). Thus, we also investigated the expression of LEF1 at gene and protein levels. Our data, based on the results at protein level (Figure 5C), suggested that downstream target LEF1 was activated by increased β-catenin after in ovo injection of CHIR-99021. Therefore, we suggested that injection of CHIR-99021 promoted feather follicles development via activating Wnt/β-catenin upregulating downstream target LEF1.
We also noticed that different injected doses of CHIR-99021 had different promotion effects on feather follicles development via activating the Wnt/β-catenin pathway and downstream target LEF1 expression in different degrees. Moreover, the effects of higher concentration of CHIR-99021 (5,000–100,000 ng/100 μL) on Wnt/β-catenin pathway were more stable than those of the lower concentration (500–2,000 ng/ 100 μL) in the present study. We also found that the irregular gene and protein expressions in the 500 ng group. To explain these issues, we investigated and found that treating organoid with the lower, higher and highest doses CHIR-9901 have the diametrically opposite effects. Lower dose promoted organoid size, while higher dose decreased growth, even highest dose arrested growth. These suggested that effects of CHIR 99021 were driven by dose, while mechanism is unclear (Delepine et al., 2021). Moreover, Wu et al. (2013) reported that the function of CHIR-99021 on pluripotency maintenance may occur at transcriptional and post-transcriptional levels. Wu et al. (2015) suggested that the responsiveness of downstream target genes is different during CHIR-99021 activating the Wnt signaling pathway. We thereby speculate that effects of 500 ng CHIR-99021 are unstable or even tiny. Theoretically, the initial target of CHIR-99021 is GSK-3β, which protects β-catenin from phosphorylation and upregulates β-catenin, further upregulates LEF1. The mRNA of CTNNB1 was depleted in translating, which caused the upregulated protein and downregulated gene results in the 500 ng group of this study. As for the different tendencies between LEF gene and protein, we speculate that low dose CHIR-99021 not enough to affect downstream target LEF1 mRNA, while the upregulated LEF protein was affected by the upregulated β-catenin protein. Although relative broad-range injection concentration of CHIR-99021 was conduct in this work, a lot of studies will be needed to screen the optimal concentration for applications.
The yolk sac is an extra-embryonic component surrounding the yolk and responses for nutrient absorption, digestion and transportation during incubation of the avian embryo, as well as during early term of mammalian embryonic development (Yadgary et al., 2014). Yolk sac plays an important role during embryonic development, yolk sac feeding, or injection is exhibiting positive effects on embryonic development modulation (Slawinska et al. 2016; Chen et al. 2020; Xie et al. 2020). Previous studies have illustrated that CHIR-99021 has ability to influence morphogenesis and development of various organs and tissues including fat (Chen et al., 2018), liver (Russell and Monga, 2018; Perugorria et al., 2019), tooth (Järvinen et al., 2018), lung (Stewart 2014), skeletal muscle (Girardi and Le Grand, 2018), and stomach (Barker et al., 2010) via regulating the Wnt signaling pathway, besides, transcription factors and enzymes have been identified as key mediators participating in the processing. In addition, this study has shown that in ovo injection of CHIR-99021 promoted feather follicles development via activating Wnt/β-catenin pathway and upregulating downstream target LEF1 during embryonic period in chick embryo model. Better understanding of the function of CHIR-99021 on development may be meaningful for optimizing the nutrient transfer efficiency and embryo development. In the future, it need be explored that how yolk sac injection of CHIR-99021 influence the overall development, as well as transmission mechanisms. And how the Wnt/β-catenin signaling pathway directly or indirectly regulates feather follicle development in chick embryo model.
In conclusion, this study suggests to developmental biology that in ovo injection of CHIR-99021 promoted feather follicles morphogenesis and development via activating Wnt/β-catenin signaling pathway and upregulating downstream target LEF1 during embryonic period in chick embryo model. Moreover, this study insinuates that CHIR-99021 may be a strong candidate to promote the animal feather/hair industry, especially as a reference for bird feather production.
ACKNOWLEDGMENTS
Authors appreciate the daily management of the goose farm and the operation of the hatching system of Jilin Agricultural University.
This work was supported by the Key Industrialization Project of the Education Department of Jilin Province: Expansion and Promotion of Jilin White Goose Germplasm Resources (JJKH20210376KJ), the Key R&D Projects Approved by Jilin Technology Department (20180201034NY), the Young and Middle-aged Technology Innovation Leading Talents and Team Projects Approved by Jilin Technology Department (20200301035RQ), the Livestock and Poultry Genetic Resources Development and Utilization Project Approved by Jilin Animal Husbandry Bureau (2021), and the Undergraduate Science and Technology Innovation Fund Project Approved by Jilin Agricultural University (2020)
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.101825.
Appendix. Supplementary materials
REFERENCES
- Barker N., Huch M., Kujala P., van de Wetering M., Snippert H.J., van Es J.H., Sato T., Stange D.E., Begthel H., van den Born M., Danenberg E., van den Brink S., Korving J., Abo A., Peters P.J., Wright N., Poulsom R., Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Brunner M.A.T., Jagannathan V., Waluk D.P., Roosje P., Linek M., Panakova L., Leeb T., Wiener D.J., Welle M.M. Novel insights into the pathways regulating the canine hair cycle and their deregulation in alopecia X. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.J., Pan N.X., Xie W.Y., Wang X.Q., Yan H.C., Gao Q. Methionine improves feather follicle development in chick embryos by activating Wnt/beta-catenin signaling. Poult. Sci. 2020;99:4479–4487. doi: 10.1016/j.psj.2020.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Ayala I., Shannon C., Fourcaudot M., Acharya N.K., Jenkinson C.P., Heikkinen S., Norton L. The diabetes gene and Wnt pathway effector TCF7L2 regulates adipocyte development and function. Diabetes. 2018;67:554–568. doi: 10.2337/db17-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa Abreu R., Penalva L.O., Marcotte E.M., Vogel C. Global signatures of protein and mRNA expression levels. Mol. Biosyst. 2009;5:1512–1526. doi: 10.1039/b908315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delepine C., Pham V.A., Tsang H.W.S., Sur M. GSK3 ss inhibitor CHIR 99021 modulates cerebral organoid development through dose-dependent regulation of apoptosis, proliferation, differentiation and migration. Plos One. 2021;16:e0251173. doi: 10.1371/journal.pone.0251173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi F., Le Grand F. Wnt signaling in skeletal muscle development and regeneration. Prog. Mol. Biol. Transl. Sci. 2018;153:157–179. doi: 10.1016/bs.pmbts.2017.11.026. [DOI] [PubMed] [Google Scholar]
- Golan-Lavi R., Giacomelli C., Fuks G., Zeisel A., Sonntag J., Sinha S., Köstler W., Wiemann S., Korf U., Yarden Y., Domany E. Coordinated pulses of mRNA and of protein translation or degradation produce EGF-induced protein bursts. Cell Rep. 2017;18:3129–3142. doi: 10.1016/j.celrep.2017.03.014. [DOI] [PubMed] [Google Scholar]
- Gong H., Wang H., Wang Y., Bai X., Liu B., He J., Wu J., Qi W., Zhang W. Skin transcriptome reveals the dynamic changes in the Wnt pathway during integument morphogenesis of chick embryos. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman J.A., Haslam I.S., Farjo N., Farjo B., Paus R. Thyroxine differentially modulates the peripheral clock: lessons from the human hair follicle. PLoS One. 2015;10 doi: 10.1371/journal.pone.0121878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Zhao W., Zhan S., Xiao P., Zhou J., Wang L., Li L., Zhang H., Niu L., Zhong T. Delta-like 1 homolog in Capra hircus: molecular characteristics, expression pattern and phylogeny. Mol. Biol. Rep. 2016;43:563–571. doi: 10.1007/s11033-016-3989-8. [DOI] [PubMed] [Google Scholar]
- Ito M., Yang Z., Andl T., Cui C., Kim N., Millar S.E., Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Järvinen E., Shimomura-Kuroki J., Balic A., Jussila M., Thesleff I. Mesenchymal Wnt/β-catenin signaling limits tooth number. Development. 2018;145 doi: 10.1242/dev.158048. dev158048. [DOI] [PubMed] [Google Scholar]
- Krause K., Foitzik K. Biology of the hair follicle: the basics. Semin. Cutan. Med. Surg. 2006;25:2–10. doi: 10.1016/j.sder.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Majidinia M., Aghazadeh J., Jahanban-Esfahlani R., Yousefi B. The roles of Wnt/β-catenin pathway in tissue development and regenerative medicine. J. Cell Physiol. 2018;233:5598–5612. doi: 10.1002/jcp.26265. [DOI] [PubMed] [Google Scholar]
- McLin V.A., Rankin S.A., Zorn A.M. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–2217. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- Münch C., Harper J.W. Mitochondrial unfolded protein response controls matrix pre-RNA processing and translation. Nature. 2016;534:710–713. doi: 10.1038/nature18302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napierala J.S., Li Y., Lu Y., Lin K., Hauser L.A., Lynch D.R., Napierala M. Comprehensive analysis of gene expression patterns in Friedreich's ataxia fibroblasts by RNA sequencing reveals altered levels of protein synthesis factors and solute carriers. Dis. Model. Mech. 2017;10:1353–1369. doi: 10.1242/dmm.030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naujok O., Lentes J., Diekmann U., Davenport C., Lenzen S. Cytotoxicity and activation of the Wnt/beta-catenin pathway in mouse embryonic stem cells treated with four GSK3 inhibitors. BMC Res. Notes. 2014;7:273. doi: 10.1186/1756-0500-7-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perugorria M.J., Olaizola P., Labiano I., Esparza-Baquer A., Marzioni M., Marin J.J.G., Bujanda L., Banales J.M. Wnt-β-catenin signalling in liver development, health and disease. Nat. Rev. Gastroenterol. Hepatol. 2019;16:121–136. doi: 10.1038/s41575-018-0075-9. [DOI] [PubMed] [Google Scholar]
- Ring D.B., Johnson K.W., Henriksen E.J., Nuss J.M., Goff D., Kinnick T.R., Ma S.T., Reeder J.W., Samuels I., Slabiak T., Wagman A.S., Hammond M.E., Harrison S.D. Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo. Diabetes. 2003;52:588–595. doi: 10.2337/diabetes.52.3.588. [DOI] [PubMed] [Google Scholar]
- Rishikaysh P., Dev K., Diaz D., Qureshi W.M., Filip S., Mokry J. Signaling involved in hair follicle morphogenesis and development. Int. J. Mol. Sci. 2014;15:1647–1670. doi: 10.3390/ijms15011647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognoni E., Gomez C., Pisco A.O., Rawlins E.L., Simons B.D., Watt F.M., Driskell R.R. Inhibition of β-catenin signalling in dermal fibroblasts enhances hair follicle regeneration during wound healing. Development. 2016;143:2522–2535. doi: 10.1242/dev.131797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J.O., Monga S.P. Wnt/β-catenin signaling in liver development, homeostasis, and pathobiology. Annu. Rev. Pathol. 2018;13:351–378. doi: 10.1146/annurev-pathol-020117-044010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y., Ni Z., Li Y. Wnt signal transduction pathways and hair follicle stem cells. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2010;27:945–948. [PubMed] [Google Scholar]
- Slawinska A., Plowiec A., Siwek M., Jaroszewski M., Bednarczyk M. Long-term transcriptomic effects of prebiotics and synbiotics delivered in ovo in broiler chickens. Plos One. 2016;11:e0168899. doi: 10.1371/journal.pone.0168899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenn K.S., Paus R. Controls of hair follicle cycling. Physiol. Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Stewart D.J. Wnt signaling pathway in non-small cell lung cancer. J. Natl. Cancer Inst. 2014;106:djt356. doi: 10.1093/jnci/djt356. [DOI] [PubMed] [Google Scholar]
- Sun Y., Zhou Y., Msuthwana P., Liu J., Liu C., Sello C.T., Song Y., Feng Z., Li S., Yang W., Xu Y., Yan X., Li C., Sui Y., Hu J., Sun Y. The role of CTNNB1 and LEF1 in feather follicles development of anser cygnoides and Anser anser. Genes Genomics. 2020;42:761–771. doi: 10.1007/s13258-020-00950-8. [DOI] [PubMed] [Google Scholar]
- Telerman S.B., Rognoni E., Sequeira I., Pisco A.O., Lichtenberger B.M., Culley O.J., Viswanathan P., Driskell R.R., Watt F.M. Dermal blimp1 acts downstream of epidermal TGFβ and Wnt/β-catenin to regulate hair follicle formation and growth. J. Invest. Dermatol. 2017;137:2270–2281. doi: 10.1016/j.jid.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripurani S.K., Wang Y., Fan Y.X., Rahimi M., Wong L., Lee M.H., Starost M.F., Rubin J.S., Johnson G.R. Suppression of Wnt/β-catenin signaling by EGF receptor is required for hair follicle development. Mol. Biol. Cell. 2018;29:2784–2799. doi: 10.1091/mbc.E18-08-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltri A., Lang C., Lien W.H. Concise review: wnt signaling pathways in skin development and epidermal stem cells. Stem Cells. 2018;36:22–35. doi: 10.1002/stem.2723. [DOI] [PubMed] [Google Scholar]
- Wang A.B., Zhang Y.V., Tumbar T. Gata6 promotes hair follicle progenitor cell renewal by genome maintenance during proliferation. Embo. J. 2017;36:61–78. doi: 10.15252/embj.201694572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welles J.E., Toro A.L., Sunilkumar S., Stevens S.A., Purnell C.J., Kimball S.R., Dennis M.D. Retinol-binding protein 4 mRNA translation in hepatocytes is enhanced by activation of mTORC1. Am. J. Physiol. Endocrinol. Metab. 2021;320:E306–E315. doi: 10.1152/ajpendo.00494.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Ai Z., Yao K., Cao L., Du J., Shi X., Guo Z., Zhang Y. CHIR99021 promotes self-renewal of mouse embryonic stem cells by modulation of protein-encoding gene and long intergenic non-coding RNA expression. Exp. Cell Res. 2013;319:2684–2699. doi: 10.1016/j.yexcr.2013.08.027. [DOI] [PubMed] [Google Scholar]
- Wu Y., Liu F., Liu Y., Liu X., Ai Z., Guo Z., Zhang Y. GSK3 inhibitors CHIR99021 and 6-bromoindirubin-3 ’-oxime inhibit microRNA maturation in mouse embryonic stem cells. Sci. Rep. 2015;5:8666. doi: 10.1038/srep08666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W.Y., Chen M.J., Jiang S.G., Yan H.C., Wang X.Q., Gao C.Q. The Wnt/β-catenin signaling pathway is involved in regulating feather growth of embryonic chicks. Poult. Sci. 2020;99:2315–2323. doi: 10.1016/j.psj.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadgary L., Wong E.A., Uni Z. Temporal transcriptome analysis of the chicken embryo yolk sac. BMC Genomics. 2014;15:690. doi: 10.1186/1471-2164-15-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S., Tan L., Yang R., Fang B., Qu S., Schulze E.N., Song H., Ying Q., Li P. Pleiotropy of glycogen synthase kinase-3 inhibition by CHIR99021 promotes self-renewal of embryonic stem cells from refractory mouse strains. PLoS One. 2012;7:e35892. doi: 10.1371/journal.pone.0035892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Soma T., Matsuzaki T., Kishimoto J. Wnt activator CHIR99021-stimulated human dermal papilla spheroids contribute to hair follicle formation and production of reconstituted follicle-enriched human skin. Biochem. Biophys. Res. Commun. 2019;516:599–605. doi: 10.1016/j.bbrc.2019.06.038. [DOI] [PubMed] [Google Scholar]
- Zhou L., Wang H., Jing J., Yu L., Wu X., Lu Z. Morroniside regulates hair growth and cycle transition via activation of the Wnt/β-catenin signaling pathway. Sci. Rep. 2018;8:13785. doi: 10.1038/s41598-018-32138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.