Case Presentation
A 58-year-old man presented to us with a 1-week history of high-grade fever and progressive dry cough. Four weeks before his presentation, he was diagnosed with COVID-19 infection and needed non-ICU hospital admission with no supplemental oxygen requirements for 6 days and was treated with a 5-day course of remdesivir and 3 weeks of dexamethasone. His steroid dose was commenced on dexamethasone 12 mg bid (four times the recommended dose) for 14 days and then gradually tapered over the remaining 7 days. His history was unremarkable, except for well-controlled asthma. He did not complain of any shortness of breath, weight loss, or loss of appetite. He was never a smoker and denied any alcohol use.
Physical Examination Findings
His vital signs on admission were as follows: temperature, 38.1°C; respiratory rate, 16 breaths/min; heart rate, 70 beats/min; BP, 124/68 mm Hg; and oxygen saturation by pulse oximetry, 98% on ambient air. The rest of the general and systemic examination was normal.
Diagnostic Studies
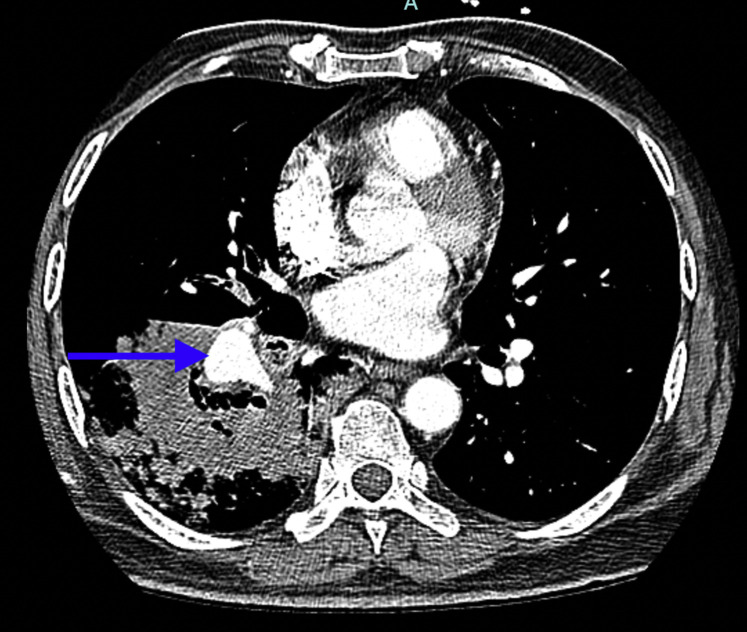
On admission, a chest radiograph showed a right lower zone cavitary lesion and a subsequent CT chest scan showed a cavitary lesion in the right lower lobe with surrounding ground-glass opacities (Fig 1 ). Although his CBC count and the basic metabolic panel were within normal ranges, his C-reactive protein was elevated at 24 international units/L (normal, <6 international units/L). His fasting and random blood sugar levels were normal on admission. Serum galactomannan and beta-d-glucan levels were within normal limits. He underwent a fiber optic bronchoscopy that showed occlusion of the apical segment of the right lower lobe. Endobronchial biopsy specimens were obtained, and BAL was collected from the right lower lobe. Initial cultures from the lavage were negative, and endobronchial biopsy specimens were nondiagnostic. He was begun on broad-spectrum antibiotics. On day 5 of his hospital admission, he had an episode of massive hemoptysis (approximately 250 mL) that led to hemodynamic instability. He underwent a CT scan that showed an increase in the size of the pulmonary cavity; a pulmonary angiogram showed the descending segmental branch of the right pulmonary artery traversing through the consolidative cavitating lesion with focal dilatation of the same measuring up to 1.5 × 1.9 cm (Fig 2 ).
Figure 1.
A, CT scan shows right lower lobe cavitary lesion with surround ground glass opacity. B, Chest radiograph shows right lower zone cavitary lesion.
Figure 2.
CT pulmonary angiogram shows pseudoaneurysm of descending branch of the right pulmonary artery (arrow).
What is the diagnosis?
Diagnosis: Massive hemoptysis secondary to pulmonary pseudoaneurysm caused by COVID-associated mucormycosis
Discussion
Mucormycosis is a fungal infection caused by members of the order Mucorales, which most commonly includes Rhizopus species, Mucor species, and Lichtheimia species. It can affect various organs that include the skin, paranasal sinus, orbit, lung, brain, and GI tract. It is associated with high morbidity and mortality rates; all-cause mortality rate for mucormycosis ranges from 40% to 80%, depending on the site of infection and underlying comorbid conditions.
Pulmonary mucormycosis (PM) is relatively rare as compared with other fungal infections of the lung. The first case of PM was described in 1876. Clinical symptoms are nonspecific with fever, weight loss, cough, and occasionally hemoptysis. Imaging shows the presence of consolidations, nodules, masses, and cavitary lesions. The presence of consolidation on the contralateral side is associated with a poorer prognosis. A reverse halo sign, defined as an area of ground glass lesion surrounded by a peripheral rim of consolidation, has been reported to be present in 19% to 94% of cases. Though not specific, it may serve as an important clue in a setting with clinical suspicion of PM, especially in an immunocompromised host.
Massive hemoptysis can occur rarely in PM, and pathophysiology includes direct invasion of the fungal hyphae into the vessel wall of large blood vessels leading to pseudoaneurysm formation. In this patient, pseudoaneurysm of the pulmonary artery (PAP) was the cause for massive hemoptysis.
PAP is a rare cause of hemoptysis with a wide array of causes. It arises because of inflammation of the pulmonary arterial wall that leads to gradual weakening of the vessel wall. Noninfective causes of PAP include Behcet’s syndrome, Takayasu arteritis, and malignancy. Infective causes include TB, syphilis, pyogenic bacteria (such as Staphylococcus aureus, Streptococcus pyogenes, Actinomyces, Klebsiella), and fungi (such as Mucor, Candida, and Aspergillus). CT pulmonary angiography remains the gold standard for diagnosis for PAP. Its diagnosis can be challenging and frequently can be missed on studies without IV contrast enhancement because they may appear as lung masses or endobronchial lesions. Endovascular treatment options for PAP include direct coil embolization, stent placement, and embolization of the feeding vessel. Direct intrasaccular embolization with coils has the advantage of preserving pulmonary arteries distal to the PAP, although it carries an increased risk of PAP rupture.
Mucormycosis is usually seen in people whose condition is immunocompromised. Patients with poorly controlled diabetes mellitus, after transplantation, and with hematologic malignancies are particularly at risk of contracting this disease. There has been a resurgence of mucormycosis after COVID-19 infection. India has reported more than 38 per million cases of COVID-19-associated mucormycosis (CAM) in 3 months (May to July 2021), with rhino orbital cerebral mucormycosis being seen most commonly in 60% of patients. Preexisting conditions, injudicious use of steroids, and antimicrobial use have contributed to the surge in these cases. Two large case series have shown that CAM was usually seen 2 to 3 weeks after COVID-19 infection diagnosis. Nearly 70% to 80% of these patients were treated with steroids; of these, 20% to 50% of patients received steroids in inappropriately high doses. However, the fact that a small proportion of patients without any traditional risk factors for invasive fungal infection also experienced CAM has postulated the immunomodulatory role of SARS-CoV-2 in the pathogenesis of CAM, which requires further research.
High clinical suspicion for PM, prompt diagnosis, and treatment initiation are essential because delayed treatment is associated with increased mortality rates. The mortality rate for PM remains high at 80%, despite aggressive treatment. Definitive diagnosis requires demonstration of the organism in tissue that often cannot be obtained easily. BAL can show the presence of broad-based nonseptate hyphae that are branched irregularly; however, the absence of septa makes the sample fragile, further decreasing the diagnostic yield. BAL has a diagnostic sensitivity of 50%.
Liposomal amphotericin-B (L-AMB) 5 to 10 mg/kg is the first-line treatment of choice, while isavuconazole and posaconazole have been used as salvage therapy. The duration of therapy necessary to treat mucormycosis is unknown; however, therapy is recommended to continue until the immune defect is resolved; generally, treatment is given for weeks to months. Surgical resection is essential in treatment of PM, mainly to overcome the serious disease burden of PM. In this patient, surgical resection was also imperative for control of hemoptysis.
Clinical Course
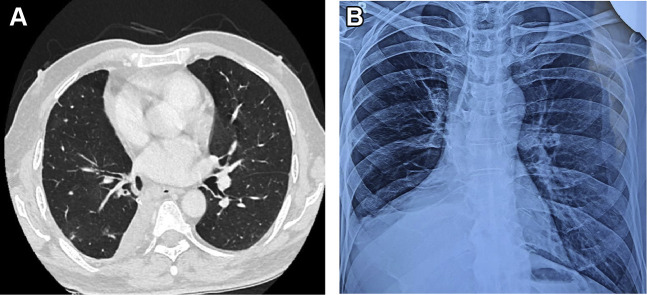
After massive hemoptysis, hemostasis was achieved after an interlock coiling was inserted in the pseudoaneurysm (Fig 3 ). His hemoptysis persisted despite embolization techniques; hence, he underwent video-assisted thoracic surgery for right lower lobectomy and stump ligation of the right lobar pulmonary artery. Lobectomy specimen on histopathologic evaluation revealed large areas of necrosis with aseptate fungal hyphae (Fig 4 ). The patient was started on antifungal treatment with L-AMB at 5 mg/kg and isavuconazole at 200 mg/d. Although there is no evidence for dual antifungal therapy in PM, this was initiated in the patient to maintain continuity of treatment because there was an acute shortage of L-AMB after a sudden surge in mucormycosis cases after COVID-19 infection in India. He completed 3 months of antifungal therapy; a repeat CT scan and chest radiograph shows complete resolution (Fig 5 ).
Figure 3.

Digital subtraction angiography of the descending branch of the right pulmonary artery after coil embolization.
Figure 4.
Hematoxylin and eosin stain of the right lower lobectomy sample shows broad aseptate hyphae (black arrows) with areas of necrosis that represent mucormycosis.
Figure 5.
Postlobectomy A, CT scan and B, chest radiograph show changes after 3 months of antifungal therapy.
Clinical Pearls
-
1.
PM is a very aggressive disease with high morbidity and mortality rates. Hence, a high index of clinical suspicion is needed in the presence of risk factors such as uncontrolled diabetes mellitus, malignancy, after transplantation, and after COVID-19 infection.
-
2.
Clinical symptoms may be nonspecific; imaging shows the presence of cavitary consolidation and ground-glass opacities. All attempts should be made to obtain tissue for histopathological diagnosis of PM.
-
3.
Mucormycosis is characterized by angioinvasion that results in vessel thrombosis and subsequent tissue thrombosis. Pseudoaneurysm formation rarely is seen in PM and can be associated with fatal hemoptysis.
-
4.
Treatment initiation should be early, and surgical resection should be offered, when possible, for disease control. An antifungal such as L-AMB is the drug of choice for treatment; isavuconazole and posaconazole may be used when L-AMB is contraindicated or not available.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Other contributions:CHEST worked with the authors to ensure that the Journal policies on patient consent to report information were met.
Suggested Readings
- Roden M.M., Zaoutis T.E., Buchanan W.L., et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- Chen Y., Gilman M.D., Humphrey K.L., et al. Pulmonary artery pseudoaneurysms: clinical features and CT findings. AJR Am J Roentgenol. 2017;208(1):84–91. doi: 10.2214/AJR.16.16312. [DOI] [PubMed] [Google Scholar]
- Lin E., Moua T., Limper A.H. Pulmonary mucormycosis: clinical features and outcomes. Infection. 2017;45(4):443–448. doi: 10.1007/s15010-017-0991-6. [DOI] [PubMed] [Google Scholar]
- Cornely O.A., Alastruey-Izquierdo A., Arenz D., et al. Mucormycosis ECMM MSG Global Guideline Writing Group. G: global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthu V., Rudramurthy S.M., Chakrabarti A., Agarwal R. Epidemiology and pathophysiology of COVID-19-associated mucormycosis: India versus the rest of the world. Mycopathologia. 2021;186(6):739–754. doi: 10.1007/s11046-021-00584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A., Agarwal R., Rudramurthy S., et al. Multicenter epidemiologic study of coronavirus disease-associated mucormycosis. India Emerg Infect Dis. 2021;27(9):2349–2359. doi: 10.3201/eid2709.210934. [DOI] [PMC free article] [PubMed] [Google Scholar]