Abstract
The environmental contamination by methylmercury (MeHg) is a major concern for public health. The effects of MeHg in the central nervous system (CNS) of adult animals have been extensively investigated; however, little is known about the effects of MeHg exposure during intrauterine and lactation periods on motor and cognitive functions of adolescent rats. Therefore, this study aimed to investigate the effect of MeHg exposure during intrauterine life and lactation on both motor and cognitive functions of offspring rats. Ten female Wistar rats were exposed to 40 μg/kg/day of MeHg through cookie treats from the first day of pregnancy until the last day of breastfeeding. Both motor and cognitive functions of offspring male rats were assessed by open field, rotarod, and step-down inhibitory avoidance tests. Forty-one days after birth, the hippocampus and cerebellum were collected to determine total Hg content, antioxidant capacity against peroxyl radicals (ACAP), reduced glutathione (GSH) levels, lipid peroxidation (LPO), and nitrite levels. MeHg exposure during CNS development increased Hg levels in both hippocampal and cerebellar parenchymas, triggered oxidative stress throughout ACAP and GSH decrease, increased LPO and nitrite levels. These alterations resulted in reduced spontaneous and stimulated locomotion and short- and long-term memory deficits. Therefore, damages triggered by MeHg exposure during intrauterine life and lactation had detrimental effects on oxidative biochemistry and motor and cognitive functions of offspring rats.
Keywords: Methylmercury, Neurotoxicology, Central nervous system, Cerebellum, Hippocampus
Highlights
-
•
The MeHg exposure during CNS development increased mercury levels in hippocampal and cerebellar parenchyma.
-
•
The MeHg intoxication during pregnancy and lactation impairs the redox status of hippocampus and cerebellum of the offspring.
-
•
MeHg exposure causes behavioral effects in motor ability and cognition of offspring rats.
1. Introduction
The environmental disposal of pollutants is one of the most dangerous anthropogenic actions. Mercury (Hg) is a harmful substance still used in illegal gold mining in developing countries [1], [2], [3], [4] that remains a major concern for public health [5].
The methylmercury (MeHg) is the major source of organic Hg exposure in humans and is environmentally produced through biomethylation of the inorganic Hg found in aquatic sediments. MeHg is accumulated into the marine food chain, which is the major source of human contamination [6].
Human Hg poisoning leads to multiple systemic impairments and the central nervous system (CNS) is one of the main targets due to its high metabolism [7], [8]. In the 1950 s, large amounts of Hg-containing industrial waste were dumped into a Japanese bay, poisoned people that consumed local seafood [9], and resulted in peripheral vision loss, dysarthria, and cerebellar ataxia [10]. Since the so-called Minamata disease, the detrimental effects of Hg have been investigated in humans [11], [12], [13], in vitro, and in vivo experimental models [14], [15], [16], [17].
The intake of a MeHg dose that simulates human environmental exposure can cause cognitive and motor damages in adult rats [14], [15], [16], [17]. Moreover, detrimental effects of MeHg have been observed during periods of critical neurodevelopment such as adolescence [18], [19], [20], [21], [22]. In humans, significant correlations have been already detected between chronic exposure to MeHg during development and delayed milestone achievements (for example, age of first walking and talking), language issues and low mental and psychomotor indices (reviewed by [8]), demonstrating the urgent need of better understanding the cellular and molecular changes underlying neural damage.
Brain development is a highly organized and dynamic multistep process that occurs in precisely timed stages including neurogenesis, neuronal migration and differentiation. These events occur during distinct time windows that span from the early embryonic stages to adulthood [25]. This development is not uniform, the timing of brain development differs from one region to another, as it also differs between substrates, neurotransmitter systems, and central endocrine circuitries [26]. In this context, the excessive generation of reactive oxygen species, such as in the exposure to mercury, causes a damage of biomolecules such as lipids, proteins and nucleic acids resulting in neurotoxicity damage, may compromise neurodevelopment [27], [28].
To the best of the author’s knowledge, no study has demonstrated both Hg in two neurodevelopmental regions (hippocampus and cerebellum) by using a comprehensive exposure model. Therefore, this study aimed to investigate the effect of MeHg exposure during intrauterine life and lactation on both motor and cognitive functions of offspring rats.
2. Methods
2.1. Animals and experimental design
Ten female Wistar rats (Rattus norvegicus) (90 days-old, 175 ± 25 g) were monitored at the Federal University of Para (UFPA) maintenance room under a 14:10 h light and dark cycle (lights on 7:00 AM) and a climate-controlled room (25 ± 2 °C). The experimental protocol started on the next day (D1) after the pregnancy was diagnosed. The pregnancy was tested after observing a genital plug (D0). The pregnant rats were kept in cages with food and water ad libitum associated with the experimental protocol.
All the experiments followed the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (see Table S1) and the Guide for the Care and Use of Laboratory Animals [29]. The study was reviewed and approved by the Ethics committee on animal experimentation of UFPA (protocol number 8613011217/CEUA-UFPA). All efforts were made to avoid suffering and distress and to reduce the number of animals used.
2.2. MeHg exposure protocol
The progenitor animals were equally and randomly distributed into two groups based on the experimental protocol: MeHg group (n = 5), that received MeHg (40 μg/kg body weight/day) according to the weekly weighing of female rats for dose adjustment; and the control group (n = 5), that received the vehicle in the same scheme of treatment. Thus, in a preclinical perspective, the elected MeHg dose mimics the environmental exposure faced by human populations in endemic areas of mercurial exposure, especially those dependent on fish consumption. This long-term and low-dose exposure were previously proposed by Kong et al. [30] as not capable of causing visible neurological effects common to high doses of intoxication in adult animals and deeply investigated by our group in the past four years [18], [31], [32], [33], [34], [22]. This evidence showed a reasonable proportionality between the values found in rats and humans.
The MeHg was orally and daily administered by cookie treats (Teddy Grahams, Nabisco, Canada). Each cookie was offered once a day to each pregnant animal during gestation. Prior to offer the cookie to the animal, the MeHg or only ethanol was incorporated into the treats, depending on the exposure group. The administration of the metal was carried out after dissolving the MeHg chloride (CH3HgCl; Sigma-Aldrich, Milwaukee, WI, USA) in ethanol and, later, incorporated into the cookies (Teddy Grahams, Nabisco, Canada). In this model, the cookies were air-dried for 12 h under controlled humidity to allow the ethanol to evaporate. The cookies in the control group were treated only with ethanol ([16], [35]; Nascimento et al. [36]). The MeHg was administered during the gestational (20–21 days) and lactation (20–21 days) periods only for the progenitor animal, once a day. In the lactation period, the pups were apart allocated in a suitable cage for 2 min. The cookies were immediately eaten by progenitor animals, as confirmed by a visual inspection ([36]; Nascimento et al. [37]). For every experimental group, 10 male offspring were used. At the end of MeHg administration, the offspring animals were divided by sex. The male pulps were maintained in collective cages (4 animals each) with food and water ad libitum and maintained in a climate-controlled room with a light/dark cycle appropriate until 41 days old when they were submitted to behavioral tests.
2.3. Behavior tests
The tests were conducted in a sound-attenuated room under low-intensity light (12 lux) and performed between 11:00 AM and 6:00 PM. Besides, the animals were habituated for at least two hours before beginning the assays.
2.3.1. Open Field
The spontaneous exploratory activity was analyzed by open field assay [38]. In this test, each animal was placed in the center of the apparatus (100 ×100 x 40 cm) to explore it for 5 min. The rat’s activity was monitored by a camera localized above the arena. The apparatus was virtually divided by Any-maze software (version 4.99; Stoelting Co., Wood Dale, IL, USA) into 25 equal quadrants (20 cm2) of which sixteen (equivalent to 64%) and nine (equivalent to 36%) quadrants were considered peripheral and central areas, respectively. The results of the peripheral and total distance traveled, in meters, assessed locomotion and time spent in central area in seconds as anxiety-like behavior.
2.3.2. Rotarod test
Motor coordination and balance were evaluated through forced tasks. The animals were trained to remain on the equipment's rotating axis for 2 min at 8 rpm. After training, the rats were subjected to the test. The animals were submitted to the apparatus in five stages of 120 s each, with continuous exposure at 16 rpm, with 120 s between exposures. Each time a rat fell off the rotating bar, the same rat was put back on it. It was counted the animal's first fall time (latency) from the cylinder's scroll bar, in the five phases (in seconds), and the number (n) of falls (adapted from Teixeira et al. [39].
2.3.3. Step-down inhibitory avoidance test
The step-down inhibitory avoidance test was performed to test the memory through an aversive stimulus and its behavioral response [40]. The inhibitory avoidance device (EP104R, Insight, Brazil) consists of a poly (methyl methacrylate) box (50 × 25 × 25 cm) with parallel stainless-steel bars (1 mm diameter, each pair spaced 1 cm apart) on the floor connected to an electrical stimulator. The equipment has a secure platform (7 cm wide × 2.5 cm high) located against the left wall. The animals were individually placed on the platform and freely explored the device for 3 min in a habituation session.
Thirty minutes later, the animals were reintroduced to the apparatus. In the exact moment in which they positioned the four paws in the metallic grid, we applied a shock of 0.4 mA for 1 s. Afterward, the animals were returned to their cages. The short-term memory assessment was performed 1.5 h after the shock by putting the animals once again in the safe platform and counting the time it took the animal to put its four paws on the grid (step-down latency) without any shock induction. The same procedures were repeated 24 h after the shock induction to evaluate the long-term memory [41], [42].
After the last behavioral test, the animals were deeply anesthetized with a mixture of ketamine hydrochloride (180 mg/kg) and xylazine hydrochloride (30 mg/kg) and euthanized by cervical dislocation. The brain was removed from skull, and the cerebellum was separated from telencephalon, from where we collected the hippocampus, and brainstem. The samples were gently washed in cold saline buffer, divided into two hemispheres, frozen in liquid nitrogen, and stored at − 80 °C until further analyses.
2.4. Mercury levels
Total mercury (Hg) present in the samples was estimated by using an atomic absorption spectrometry with cold vapor (CVAAS) (Semiautomatic Mercury Analyzer, model 201-Hg, Sanso Seisakusho Co. Ltd, Tokyo, Japan). Each sample of hippocampus and cerebellum were weighted (weighting 0.5 g maximum), which consists in wet digestion of samples through homogenizing a wet sample; further, 1 mL of distilled water, 2 mL of nitric acid-perchloric acid 1:1 (v:v) (HNO3-HClO4), and 5 mL of sulfuric acid (H2SO4) were sequentially added, which were maintained on a heat plate (200–230 °C) for 30 min. All procedures were based on protocol, following previously published studies by our research group [21], [22], [32], [33], [43], with a limit of detection of values until 0.001 mg/kg Hg. The results were expressed in mg/kg of total Hg levels.
2.5. Oxidative biochemistry assays
Prior to the biochemical analyses, the samples were thawed and homogenized in Tris buffer (20 mM HCl), pH 7.4, at 4 °C, by sonic disintegration (approximate concentration of 1 g/mL), and stored in individual microtubes at − 80º C until further analyses.
In the moment of the assays, from the crude homogenate, an aliquot was immediately taken to determinate protein concentration to normalize the parameters. The remaining homogenate was separated into three different aliquots and centrifuged at the specific rotation of each assay described below.
2.5.1. Antioxidant capacity against peroxyl radicals (ACAP)
Initially, the samples were centrifuged at 9700 rpm at 4 °C for 20 min. The supernatant was exposed in triplicates in a transparent 96-well microplate to a peroxyl radical generator, 2,2'-azobis (2 methylpropionamidine) dihydrochloride (ABAP, 4 mM; Sigma Company, St Louis, MO, USA) and, in another triplicate, they were exposed just to ultrapure water.
For fluorescence reading, 10 µL of 2′,7′ dichlorofluorescein diacetate (H2DCF-DA) was added to all samples. After that, data were collected every 5 min per 1 h in an opaque Microplate Reader (Victor 2, PerkinElmer Inc., Llantrisant, UK) at 35 °C. All steps of this assay followed the Amado et al. [44] protocol. For data analysis, the difference in fluorescence area in the same sample, with and without the addition of ABAP, was considered. The relative area was calculated, and the results expressed as an inverse of the relative area in the control percentage.
2.5.2. Reduced glutathione (GSH) levels
This analysis was performed according to Ellmans’ protocol [45]. The technique is based on GSH ability to reduce 5,5-dithiobis-2nitrobenzoic acid (DTNB) to 5-thio-2-nitrobenzoic acid (TNB), which was read by 412 nm wavelength. The samples were deproteinized with 2% trichloroacetic acid and the supernatant collected for analysis after centrifugation at 3000 rpm for 5 min. A 20 µL aliquot of the sample was added to a test tube containing 20 µL distilled water and 3 mL PBS/EDTA buffer to record the first reading of the sample (T0), and 100 µL DTNB was added and after 3 min the second sample reading (T3) was taken to determine the GSH concentration, which was expressed in μg/mL.
2.5.3. Lipid peroxidation (LPO)
The level of lipid peroxidation was based on the reaction of the polyunsaturated fatty acid metabolites, malonaldehyde (MDA), and 4-hydroxyalkenes (4HDA), with N-methyl-2-phenylindol (NMFI). In the presence of methanesulfonic acid, this reaction produces a stable chromophore. The color produced in the process is proportional to the concentration of oxidized lipids, measured by spectrophotometry. Thus, the LPO was determined using the method proposed by Esterbauer and Cheeseman [46].
For this purpose, the lysates were centrifuged at 5600 rpm for 10 min at 4 °C, and the supernatant was separated into aliquots to determine lipid peroxidation and protein concentration [47]. To 325 µL of 10.3 mM NMFI diluted in methanol (1:3) and 75 µL of methanesulfonic acid, 100 µL of the standard MDA solutions or samples were added. This mixture was then heated to 45 °C for 40 min. After this period, the reading was performed by 570 ηm wavelength, and the results were expressed as MDA nanomolar (nM) per microgram of protein by % of control.
2.5.4. Nitrite levels
To measure Nitrite levels, the lysate was centrifuged at 14,000 rpm for 10 min at 4 °C. The supernatant was aliquoted to determine the nitrites. The concentration of nitrites was determined based on their reaction with the Griess reagent (Nafty-ethylene-diamine 0.1% and Sulfanilamide 1% in phosphoric acid 5% −1:1). This reaction forms azoic compounds, which give a characteristic bluish color. The blue intensity is proportional to the chromogen's concentration, verified by spectrophotometric reading [48]. One hundred microliters of the supernatant were added to 100 µL of the Griess reagent and incubated for 20 min at room temperature. Then, the reading was performed (λ = 550 ηm), and the results were expressed as micromolar (µM) of nitrites for each microgram of protein by % of control. All the experimental steps and analyzes performed are summarized in Fig. 1.
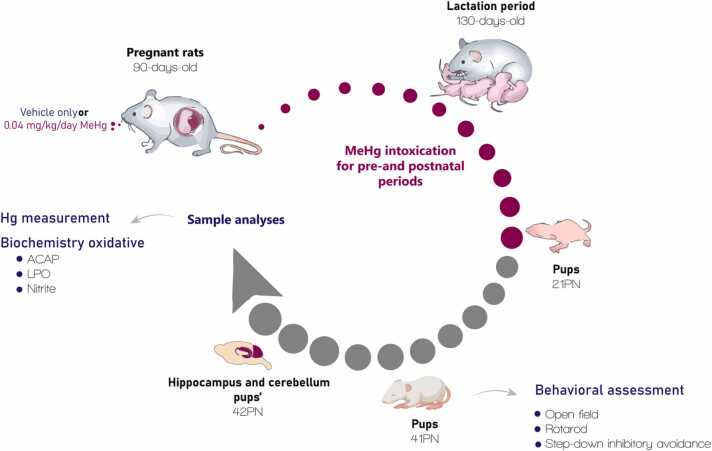
Fig. 1.
Sample description and experimental steps. Ten pregnant Wistar rats exposed to MeHg (0.04 mg/kg/day) or vehicle only (control group) during pregnancy and lactation periods (40-day exposure). At 41st postnatal day (PN), ten male pups per group were evaluated by behavioral assays: open field, rotarod, and step-down inhibitory avoidance tests. Then, after the last behavioral test, the animals were euthanized, and the hippocampus and cerebellum were collected for analyses of mercury (Hg) measurement, oxidative biochemistry thought antioxidant capacity against peroxyl radicals (ACAP); lipid peroxidation (LPO) and nitrite levels.
2.6. Statistical analyses
All data were tabulated and analyzed using the GraphPad Prism 8.2.0 software (GraphPad Software Inc., La Jolla, CA, USA). The normal distribution was tested by the Shapiro-Wilk method. The results of body mass and rotarod test were compared by one-way repeated measures ANOVA followed the Tukey post-hoc test. The Student’s independent t-test analyzed the data resulting from the other tests. All results were expressed in mean ± standard error of the mean (SEM), and values of p < 0.05 were considered statistically significant.
3. Results
3.1. Bodyweight and mercury concentration in the hippocampus and cerebellum after pre- and postnatal exposure to MeHg
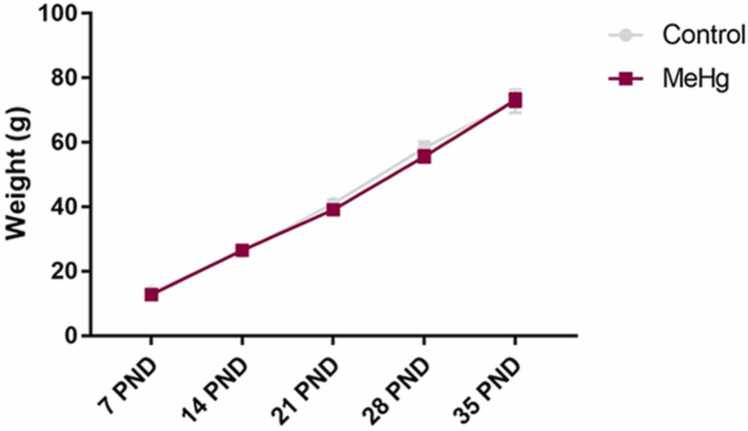
During the experimental period, there was no difference in body weight between progenitor animals in both control and MeHg groups (p > 0.05), as well as with offspring animals (Fig. 2, p > 0.05). All the animals displayed a gradual weight gain (Fig. 2, p < 0.05).
Fig. 2.
Effects of gestational and postnatal exposure to methylmercury on body weight gain (g) of the offspring rats (n = 10 per group) from 5 (per group) progenitor females. Results are expressed as mean ± standard error of mean. Two-way ANOVA and Tukey’s post-hoc test, p < 0.05.
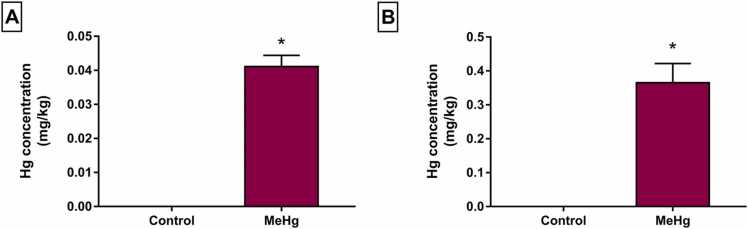
When the Hg levels in the hippocampus (Fig. 3A) and cerebellum (Fig. 3B) were analyzed, it was observed deposit of Hg in these offspring tissues, after the protocol of indirect exposure, compared with control animals (Fig. 3, p < 0.05). The cerebellum significantly accumulated more Hg than the hippocampus, presenting approximately ten times higher in this tissue.
Fig. 3.
Effects of gestational and postnatal exposure to methylmercury on Wistar offspring rats (n = 20). Mercury (Hg) total measurement in the hippocampus (A) and cerebellum (B). Results are expressed as mean ± standard error of the mean of Hg levels (µg/kg). *Student’s t-test, p < 0.05.
3.2. MeHg impairs the oxidative status of hippocampus and cerebellum of rats exposed for pre- and postnatal periods
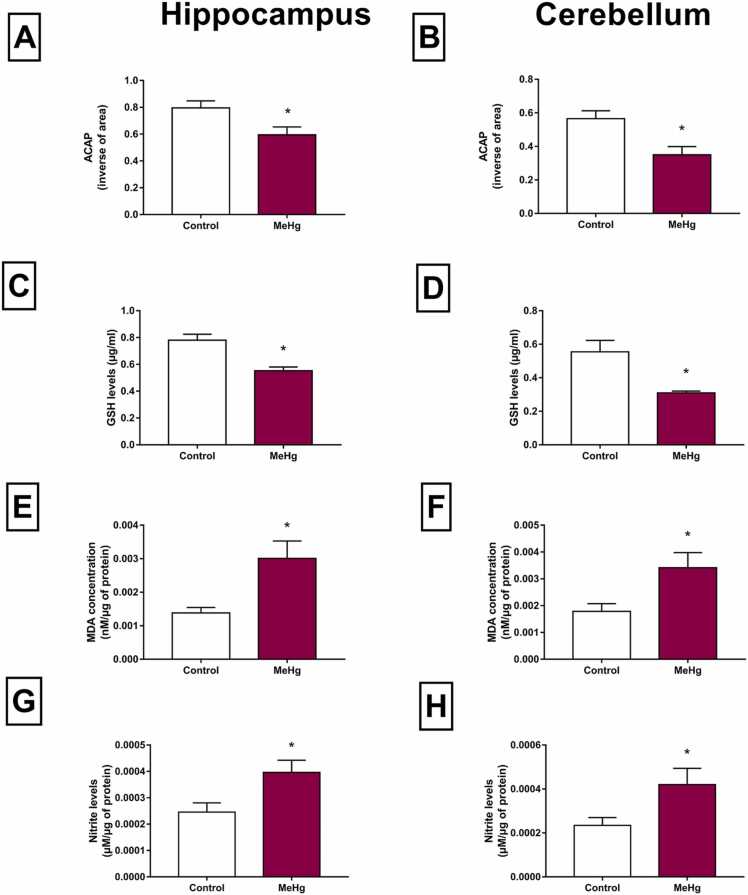
In oxidative biochemistry parameters, a reduction in ACAP levels was observed in the hippocampus (Table S2, p < 0.01, Fig. 4A) and cerebellum (Table S2, p = 0.01, Fig. 4B) of exposed rats when the MeHg and control groups were compared. Moreover, the GSH content decreased significantly in both regions (p < 0.01, Figs. 4C and 4D).
Fig. 4.
Effects of gestational and postnatal exposure to methylmercury on oxidative balance in the hippocampus and cerebellum of the Wistar offspring rats (n = 20). A-B: Antioxidant capacity against peroxyl radicals (ACAP) in inverse of area; C-D: Reduced Glutathione (GSH) levels expressed as µg/mL; E-F: MDA concentration in nanomolar per microgram of protein (LPO); G-H: nitrite levels in micromolar per microgram of protein. Results are expressed as mean ± SEM. *Student’s t-test, p < 0.05.
The levels of pro-oxidant parameters were increased, as evidenced by the increase in levels of MDA found in the hippocampus (Table S2, p < 0.01, Fig. 4E) and in the cerebellum (Table S2, p < 0.01, Fig. 4F) of exposed animals, compared with the control group. Furthermore, it was observed an increase in the levels of nitrite in the hippocampus (Table S2, p < 0.01, Fig. 4G) and cerebellum (Table S2, p < 0.01, Fig. 4H) in the exposed offspring rats to MeHg for intrauterine and neonatal periods, when analyzed with the control group. When the effects of the metal were compared between the two areas, some interesting differences were detected. Although the extension of lipid peroxidation was proportionally similar in both tissues at the time of death (Fig. 4, E and F), the cerebellum showed significantly higher levels of nitrites and lower ACAP levels, pointing to higher production of free radicals and lower antioxidant defenses, than those in the hippocampus.
3.3. MeHg-induced triggers motor and cognitive impairments on rats after exposure in intrauterine and neonatal periods
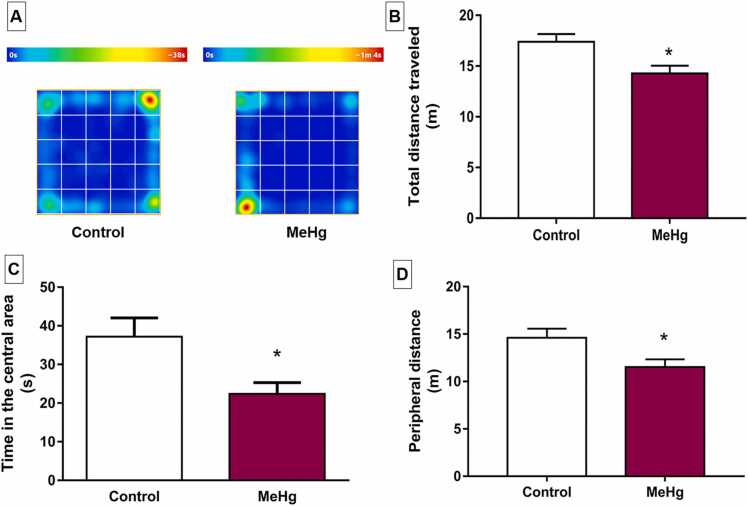
In the open field test, the group exposed to MeHg showed a reduction in the total distance traveled (Table S2, p < 0.01, Fig. 5B). There was also a decrease in the peripheral distance by the MeHg group (Table S2, p < 0.05, Fig. 5D).
Fig. 5.
Effects of gestational and postnatal exposure to methylmercury on spontaneous locomotor activity of the Wistar offspring rats (n = 20) in the open field test. In A, representative heat map of control and MeHg-exposed groups performances in open field. Results are expressed as mean ± SEM of B: total distance traveled (m); C: total distance traveled in the central area (s); and D: peripheral distance, both in meters (m). *Student’s t-test, p < 0.05.
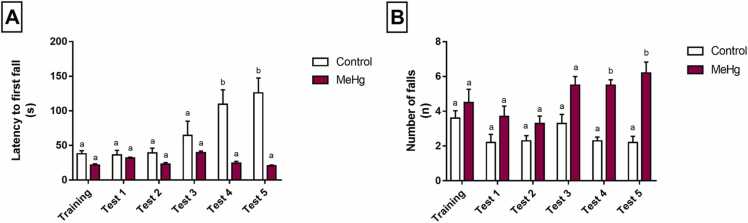
The rotarod test showed that MeHg intoxication led to a reduction in latency time, mainly in the 4th and 5th exposures to the apparatus (Table S2, adjusted p value <0.0001, Fig. 6A), which demonstrate motor-learning deficit. The number of falls was altered, with the MeHg exposed animals increasing such parameters in the 4th (adjusted p value = 0.0021) and 5th (adjusted p value = 0.0103) sessions (Table S2), which suggests motor coordination impairment (Fig. 6B).
Fig. 6.
Effects of gestational and postnatal exposure to methylmercury on forced locomotion of the Wistar offspring rats (n = 20) in the rotarod test. Results are expressed as mean ± SEM of latency of the fast fall in seconds (s) and the number of falls (n). One-way repeated measures ANOVA, p < 0.05. Similar overwritten letters do not reveal statistically significant differences.
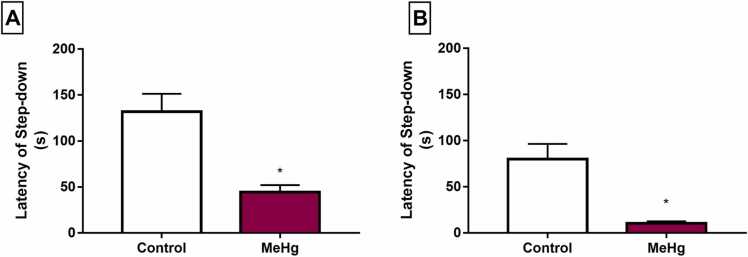
In the cognitive evaluation, the inhibitory avoidance test indicated a reduction in the step-down latency in short-term memory sessions (1.5 h after the training session) in animals exposed to MeHg (Table S2, p = 0.0021, Fig. 7A). This profile was also found in the long-term memory stage (24 h after the training session; p = 0.0002, Fig. 7B), which suggests cognitive impairment in short- and long-term memory after exposure to low doses of MeHg during the perinatal (prenatal and lactation) period.
Fig. 7.
Effects of gestational and postnatal exposure to methylmercury on the memory of the Wistar offspring rats (n = 20) in the step-down latency (s) of inhibitory avoidance test. Results are expressed as mean ± SEM of latency of step-down (s). In A, short-term memory sessions (1.5 h after the training session); in B, long-term memory stage (24 h after the training session) *Student’s t-test to control vs. MeHg group, p < 0.05.
4. Discussion
The present study investigated the effect of pre- and postnatal MeHg exposure on the motor and cognitive functions of offspring rats. Hg parameters were simultaneously investigated to demonstrate the relation between two relevant brain regions regarding motor and cognitive control. Therefore, MeHg exposure was shown associated with significant Hg levels in the hippocampus and cerebellum. In addition, the oxidative biochemistry analysis revealed that MeHg can trigger oxidative stress throughout the increase of pro-oxidant parameters (LPO and nitrite) and decrease of ACAP and GSH levels and lead to motor, short- and long-term memory deficits.
The MeHg dose (40 μg/kg) used in this comprehensive study aimed to mimic Hg levels detected in environmental chronic exposure of humans [30]. Moreover, the daily use of intragastric gavage in pregnant rats was replaced by a non-stressful administration through the spontaneous intake of MeHg-containing cookies [16], [35]; thus, this strategy better simulated the regular human consumption of Hg-contaminated food and avoided additional research bias.
In our model, we opted for the oral administration of MeHg, this route of administration increases the bioavailable in blood and tissues, with rapid distribution of organic Hg compounds in tissues, mainly liver, CNS and kidney, through the bloodstream (Bridges and Zalups [49], [50], [51]). MeHg is promptly balanced between blood and target organs due to its high affinity for thiols that enhances intracellular transport through cysteine or glutathione complexes [50], [51]. Urinary excretion only eliminates a small fraction of the MeHg dose.
Although the placental barrier separates maternal from fetal blood circulation, it allows the exchange of substances. The lipophilic MeHg is very efficiently transported throughout the human placenta and its accumulation affects cellular functions that result in detrimental consequences for the fetus developing, figuring MeHg as a considerable risk of tissue damage [52].
Moreover, Hg can be transferred to infants via breast milk [53] and even the small amount of MeHg that passes from maternal plasma into breast milk can become bioavailable in blood and tissues of the infant [54]. Since Hg remains deposited in the organism for long periods and its exposure during pregnancy would also cause effects during lactation, this study model investigated both periods. It is important to note that as Hg is deposited in the organism for long periods, therefore of this our study opted to consider the pregnancy and breastfeeding periods, since the exposure to MeHg only in pregnancy would also cause effects while breastfeeding occurred. In this way, due to the high absorption by the gastrointestinal tract, in our work we opted for an administration of MeHg incorporated into cookies that mimics the environmental exposure faced by populations in endemic areas of mercurial exposure. Thereby, our indirect prenatal and postnatal exposure model was able to observe the increase Hg levels content in cerebellum and hippocampus of the offspring.
This preclinical study design on the effects of MeHg in the CNS has applicability in human trials and is rarely found in the literature. The weekly acceptable MeHg intake recommended by the World Health Organization (WHO) results in Hg accumulation of approximately 2.3 and 0.046 ppm in hair and brain, respectively [7]. Moreover, chronically exposed populations such as the Amazon riverine usually present 6 ppm of Hg as mean value [55], [56], which is equivalent to approximately 0.12 ppm in the brain [7].
The effects of MeHg on CNS remain a major concern for public health due to Hg worldwide exposure [57]. Although it is well known that CNS is the main target for MeHg, the effects of low exposure levels in vulnerable individuals such as children and pregnant women remain unclear; thus, this original study was based on three key aspects: dose, exposure model, and offspring’s life stage. Detrimental effects have been already reported in adult animals chronically exposed to 40 μg/kg/day of MeHg and presented Hg levels between 0.04 and 0.08 ppm of Hg in the brain [18], [21], [22]. Although close to the WHO reference dose, those levels are lower than 0.12 ppm and support the comprehensiveness of this study.
Although the effects of MeHg on the nervous system have numerous publications, it remains a relevant public health issue due to the high number of exposed populations around the world [58], [7]. Moreover, though central nervous system (CNS) is knowingly the main target organ for MeHg, many questions remain open, especially those associated to the effects of relatively low levels of exposure in vulnerable individuals such as children and pregnant women. In this context, it is important to highlight that the originality of our study is based on three main points: dose, model of exposure, and the life stage of the offspring.
Our group has been investigating the effects of MeHg on different areas of the CNS, from molecular to behavioral aspects. Interestingly, our model used a low dose for long periods of exposure, and manages to trigger outcomes similar to those found in populations exposed to MeHg, for example: evidence of motor deficits and neuronal impairment was found [22] associated with motor cortex of rats, in addition to up-regulation of Apolipoprotein E in the hippocampus [18], which has been reported as an important biomarker of mercury exposure in riverine populations [55] associated with cognitive damage. Based on the reports in the literature that show perinatal and postnatal exposure to methylmercury and its consequences in the cerebellum and hippocampus, we know that the deleterious effects are caused by different doses of intoxication to MeHg and different protocols of exposure to the metal (Ghizoni et al. [59]; Cheng et al. [60]). Thus, the novelty proposed in this study is the exposure during the gestational and lactational period to a low dose (40 μg/kg/day) for a long period, in a model that allows animals to be exposed to MeHg in a way that simulates human exposure in the environment, representing the chronic exposure to MeHg of environmentally vulnerable populations with high mercury levels, such as those found in the Amazon ([56]; Berzas Nevado et al. [61]). In this study, we used a dose of 40 μg/kg body weight/day which is equivalent to the dose consumed by populations exposed to MeHg, showing the translational character of our investigation. Also, we managed a non-stressful administration to the female rats, without the stressful effects of orogastric gavage, nor the bias generated by spontaneous consumption, being representative to the human consumption of MeHg in contaminated foods. This care with a truly translational design is hardly found in the literature that analyzes the effects of MeHg on the CNS. This fact adds greater value to our results and conclusions.
Regarding the animal age, our analyses bring novelty once performed in 41 post-natal days the life stage when the CNS has established the neural maturation and synaptic refinement [62]. Thus, this makes our data representative, as it shows that maternal exposure during pregnancy and lactation can generate biochemical and functional damages to the nervous system of the offspring even after the established maturation of this system and persist significant damages after a breakdown period between administration and animal evaluation, providing evidence to the literature for new research in the literature of effects MeHg-induced beyond the teratogenic damage.
The MeHg in pregnant rats involved high absorption in the gastrointestinal tract and rapid distribution to all tissues [7]; however, its accumulation in the offspring brain areas is a result of placental and breast milk transfer, which confirms the key roles of both pathways.
Furthermore, Hg levels in the hippocampus of the offspring rats (Fig. 3A) were similar to those previously detected in brain areas of adult animals exposed to the same dose Hg was accumulated in the cerebellum almost ten times more than hippocampus (Fig. 3B); however, this phenomenon was not observed in adult rats [18], [22], [24]. This original data demonstrates the Hg accumulation in the offsprings that involves several mechanisms in different brain areas. This phenomenon must be further confirmed in adult animals through the analysis of these brain areas that present different tissue composition, vascularization, and biological barriers [63], [64].
Since the 40-day-old offsprings already presented neural maturation and synaptic refinement [63], [64], this study significantly demonstrates that maternal exposure to MeHg during pregnancy and lactation causes biochemical and functional damages in the CNS and provides novel evidence for future research. Although important, genetic and epigenetic changes are secondary issues in comparison to the need to confirm the effects of these new findings through classical methods. The methods used in this study answered the respective research questions in terms of biochemical and functional damage. The biochemical analysis was based on oxidative stress due to its classical mechanism Hg-induced damage, while the functional analysis was based on gold standard parameters of motor and cognitive disturbances [57].
In this context, MeHg can trigger molecular changes associated with CNS homeostasis such as the increase of ROS production at the expense of cellular antioxidant capacity [57]. This imbalance known as oxidative stress damages lipid membranes and oxidates biomolecules such as proteins, carbohydrates, and lipids [65], [66]. The ROS-induced damage can also reach the cell nucleus, affect DNA structure, replication and repair in humans [67], [68].
The offspring’s redox state showed an LPO increase in both cerebellum and hippocampus, which indicates ROS-induced cell membrane damage. LPO causes irreversible damage to membrane integrity that can lead to apoptosis through the mitochondrial oxidative chain [69]. In addition, the antioxidant capacity of the offspring rats was reduced, which suggests that cellular defense mechanisms were unable to impair excessive ROS production and triggered oxidative reactions in several cellular biomolecules. Despite differences in Hg accumulation, the proportional LPO increase (Fig. 4, C and D) indicates similar damage in both brain areas and may explain the behavioral alterations detected in both motor activity and memory processes (Fig. 6, Fig. 7). However, the highest Hg accumulation in the cerebellum was potentially responsible for the increased production of free radicals by nitrite and LPO levels and decreased antioxidant defense revealed by ACAP and GSH levels (Fig. 4, B and F), reinforcing that exposure to MeHg decreases antioxidant defenses and induces genotoxic damages, as observed in humans populations [67]. Future studies are needed to investigate whether this high pro-oxidant status detected in the cerebellum could explain long-term detrimental effects.
It is noteworthy that MeHg neurotoxicity can be expressed through oxidative stress mechanisms, genotoxicity, and Hg binding to cellular molecules in humans [68]. Therefore, MeHg can bind to sulfhydryl groups (-SH) of enzymatic and non-enzymatic molecules and impair several molecular pathways, which leads to a conformational change and alters their function [70]. The glutathione molecule binds to Hg and the GS-HgCH3 complex is excreted; thus, the concentration of this most abundant intracellular antioxidant system is reduced [71], [72], [73].
Since this study demonstrated that MeHg changes redox homeostasis, it is expected that other functional cell changes may occur (Fig. 4). Previous studies have shown that MeHg mainly affects astrocytes, which increases glutamate release, inhibits cystine and cysteine uptake, decreases glutathione synthesis and consequently reduces the defense against oxidative damage [74], [75], [76]. The great amount of glutamate in the synaptic cleft contributes to excitotoxicity associated with increased Ca+2 influx, mitochondrial dysfunction, and release of apoptotic factors [65].
These molecular changes affect synaptic connections and alter tissue functioning [77]. Therefore, this study observed MeHg-induced behavioral changes such as the reduction in spontaneous locomotor activity (both open field total distance traveled and peripheral exploration) (Fig. 5, A and B). The relationship among motor cortex, striatum, and cerebellum plays an important role in fine motor control, sensorimotor integration, and higher-level cognitive-motor tasks; thus, damages in these connections can impair spontaneous locomotion and incoordination in rats [39]. Freire et al. evaluated whether MeHg chronic exposure could aggravate tissue damage after induced stroke in the motor cortex of adult rats. Interestingly, long-term MeHg intoxication caused behavioral impairments and significant neuronal loss, which supports the great magnitude of the detrimental consequences of MeHg chronic exposure [21]. In this study, the fact that MeHg exposure during intrauterine life and lactation resulted in the reduction in spontaneous locomotion of offspring rats indicates that motor function impairment is related to oxidative stress-induced molecular changes [23].
The cerebellum coordinates a voluntary motor activity and balance [78], [79]. In the rotarod test, animals exposed to MeHg exhibited the highest number of falls, reduced balance on new tasks as well as reduced latency to fall from the rotating bar (Fig. 6, A and B). It is evident in our findings that animals intoxicated with MeHg, different from the control group, were not able to master the task of walking on the rotating cylinder at fixed speed rotarod protocol indicating motor learning deficit. Furthermore, the number of falls in rotarod is indicative of motor coordination, primarily at fixed speed rotarod protocol [21], [39], [80], [81], [82]. In our study, the animals MeHg-exposed had a greater number of falls from the rotating bar indicating motor coordination impairment. Furthermore, in order to avoid the fatigue of the animals, was selected a protocol of fixed speed with interval inter trials of 120 s each, time equal to the forced locomotion period Therefore, this finding confirmed that MeHg induced motor learning deficit.
Changes in the motor capacity of adult rats exposed to MeHg have been already observed in previous studies [19], [83], [84]]; however, this study model revealed significant motor damages during pre- and early postnatal periods, which are more prone to remain until adulthood [85].
The hippocampus is one of the most studied brain structures in neuroscience to better understand functions related to cognition, learning, and memory [86]. This structure is a critical participant in the bidirectional interaction of memory and exploration processes, which are interactively engaged throughout the learning course to build episodic memories [87]. Although the ventral hippocampus has been strongly associated with anxiety and interacts with the basolateral amygdala upon fear conditioning, the dorsal hippocampus is crucial for fear aversive memory consolidation and retrieval [88]; thus, elevated anxiety and aversive memory impairments can emerge from hippocampal disturbances or direct or indirect connections with the amygdala [89]. Hence, animals exposed to MeHg in this study presented cognitive deficits related to conditional fear memory and anxiety-like behavior (Fig. 5).
In fact, the step-down avoidance test has an intrinsic relationship with the amygdala due to emotional factors (aversive behavior); however, the hippocampus is necessary to memory consolidation [40]. The amygdala is an extension of the neural network since it has rich connections with the hippocampus. Comodulation between these subcortical structures allows the influence of the amygdala on the formation of hippocampally-mediated memory as well as the influence of the hippocampus on the amygdala’s response to emotional stimuli; thus, alterations in the amygdala induce adverse effects in the hippocampus [90], [91]. A recent study has shown that pre-existing amygdala reactivity can predict the association between the hippocampus and emotional stimuli (Admon et al. [92]) and suggests a specified temporal relationship between amygdala and hippocampus [93].
Our study showed MeHg-induced neurotoxicity in the hippocampus of offspring rats and oxidative stress as one of the main toxicity mechanisms. A previous study from our group showed that MeHg exposure did not impair short-term memory; however, the animals were at the beginning of adolescence [23]. These findings suggest that the CNS is more vulnerable to toxicants during the perinatal period.
The behavioral impairment is a consequence of biochemical change as well as other mechanisms of brain area specifics that modulate behavioral impairment [23], [94]. MeHg-induced damage increases the pro-oxidative properties and contributes to behavioral impairments related to motor function [16], [28]; however, studies on these mechanisms are scarce.
These behavioral and biochemical alterations were detected during the early neurodevelopment of the offsprings, albeit the pregnant rats were exposed to MeHg levels close to the WHO reference dose; therefore, this study data indicate the need to revise these reference doses to improve the knowledge on potential detrimental consequences in the early childhood.
This model of pre- and postnatal MeHg exposure revealed that Hg accumulation causes simultaneous biochemical and behavioral effects in two different brain tissues of offspring rats. MeHg-induced changes can trigger a neurodegenerative process that reduces the density of neurons and astrocytes and leads to memory and learning deficits [18]. Therefore, MeHg can affect hippocampal neurogenesis and decrease the proliferation and differentiation of neural stem cells [95], [96].
An important issue concerning the employment of animal models of Hg intoxication is the attempts to comprehensively figure out the action of this metal on the nervous system, seeking for establishing a translational approach with the alterations observed in human beings. Overall, animal models using adult rats exposed to MeHg have shown brain alterations dose-dependent similar those observed in cases of human MeHg poisoning (National Research [97]). The same has been observed in rodent models evaluating nervous system alterations during neurodevelopment of offspring [98], [99] when compared with reports in humans [100], [101]. Recently, some factors have been highlighted as of fundamental importance for the translational meaning of the research using animal models of mercury intoxication [102]. As recommended, this study includes quantitation of mercury levels, analysis of different brain areas and different levels of analysis (behavioral and biochemical), among other characteristics. This profile guarantees the translational value of this work, standing out in the current literature as a more realistic approach when compared to other studies. For instance, cerebellar cortex is one of the most affected regions in humans chronically contaminated by Hg (Eto et al. [103]), a pattern also observed in rats submitted to a long-term intoxication (Bittencourt et al. [104]). Therefore, although it is important to bear in mind that the physiological particularities between humans and rats should be considered when evaluating the results of studies using animal models [105], this study took advantage of the improvement recommendations that were previously suggested to have a significant and differentiated translational value within the current literature.
5. Conclusions
Based on a model of pre- and postnatal exposure to MeHg, our study revealed that Hg deposits' presence causes biochemical and behavioral effects in two different tissues simultaneously of offspring. Thus, it was noticed an alteration in the oxidative homeostasis and damage in the offspring's motor and cognitive parameters. Therefore, our work indicates to more clinical scientific research that better elucidates this exposure model's effects after the organisms reach a more mature age.
Funding Statement
This work was supported by the Study funded by grants from Brazilian National Council for Scientific and Technological Development (CNPq, Brazil) and Fundação de Amparo a Pesquisa do Estado do Pará (FAPESPA, Brazil). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES, Brazil)—Finance Code 001. The APC was funded by Pró-reitoria de Pós-graduação and Pesquisa from Federal University of Pará.
Author contributions
Beatriz Helena Fernandes Fagundes: Methodology, Writing – original draft. Priscila Cunha Nascimento: Formal analysis, Methodology, Writing – original draft. Aragao Walessa Alana Bragança Aragão: Formal analysis. Victória Santos Chemelo: Formal analysis, Writing – original draft. Leonardo Oliveira Bittencourt: Formal analysis, Writing – original draft. Marcia Cristina Freitas Silva: Methodology. Marco Aurelio M. Freire: Methodology. Luanna Melo Pereira Fernandes: Methodology, Writing – review & editing. Cristiane do Socorro Ferraz Maia: Investigation, Supervision, Writing – review & editing. Maria Elena Crespo-Lopez: Conceptualization, Writing – review & editing. Rafael Rodrigues Lima: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing..
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Dr. Aristidis Tsatsakis
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2022.02.014.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
Data Availability
The quantitative analysis data used to support this study's findings are included within the article and available in the supplementary material.
References
- 1.Langeland A.L., Hardin R.D., Neitzel R.L. Mercury levels in human hair and farmed fish near artisanal and small-scale gold mining communities in the Madre de Dios River Basin, Peru. Int. J. Environ. Res. Public Health. 2018:14. doi: 10.3390/ijerph14030302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niane B., Guedron S., Moritz R., Cosio C., Ngom P.M., Deverajan N., Pfeifer H.R., Pote J. Human exposure to mercury in artisanal small-scale gold mining areas of Kedougou region, Senegal, as a function of occupational activity and fish consumption. Environ. Sci. Pollut. Res. Int. 2015;22:7101–7111. doi: 10.1007/s11356-014-3913-5. [DOI] [PubMed] [Google Scholar]
- 3.Vega C.M., Orellana J.D.Y., Oliveira M.W., Hacon S.S., Basta P.C. Human mercury exposure in Yanomami indigenous villages from the Brazilian Amazon. Int. J. Environ. Res. Public Health. 2018;15:1051. doi: 10.3390/ijerph15061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wickliffe J.K., Lichtveld M.Y., Zijlmans C.W., MacDonald-Ottevanger S., Shafer M., Dahman C., Ouboter P. Exposure to total and methylmercury among pregnant women in Suriname: sources and public health implications. J. Expo. Sci. Environ. Epidemiol. 2021;31(1):117–125. doi: 10.1038/s41370-020-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibb H., O’Leary K.G. Mercury exposure and health impacts among individuals in the artisanal and small-scale gold mining community: a comprehensive review. Environ. Health Perspect. 2014;122:667–672. doi: 10.1289/ehp.1307864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarkson T.W., Magos L., Myers G.J. The toxicology of mercury - current exposures and clinical manifestations. N. Engl. J. Med. 2003;349:1731–1737. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- 7.Crespo-Lopez M.E., Augusto-Oliveira M., Lopes-Araújo A., Santos-Sacramento L., Yuki Takeda P., Macchi B.M., do Nascimento J.L.M., Maia C.S.F., Lima R.R., Arrifano G.P. Environ Int. What can we learn from the Amazon?, Mercury. 2021;146 doi: 10.1016/j.envint.2020.106223. [DOI] [PubMed] [Google Scholar]
- 8.Santos-Sacramento L., Arrifano G.P., Lopes-Araújo A., Augusto-Oliveira M., Albuquerque-Santos R., Takeda P.Y., Souza-Monteiro J.R., Macchi B.M., do Nascimento J.L.M., Lima R.R., Crespo-Lopez M.E. Human neurotoxicity of mercury in the Amazon: a scoping review with insights and critical considerations. Ecotoxicol. Environ. Saf. 2021;208 doi: 10.1016/j.ecoenv.2020.111686. [DOI] [PubMed] [Google Scholar]
- 9.Nagashima K. A review of experimental methylmercury toxicity in rats: neuropathology and evidence for apoptosis. Toxicol. Pathol. 1997;25:624–631. doi: 10.1177/019262339702500613. [DOI] [PubMed] [Google Scholar]
- 10.Chang L.W. Neurotoxic effects of mercury - a review. Environ. Res. 1977;14:329–373. doi: 10.1016/0013-9351(77)90044-5. [DOI] [PubMed] [Google Scholar]
- 11.Davis L.E., Kornfeld M., Mooney H.S., Fiedler K.J., Haaland K.Y., Orrison W.W., Cernichiari E., Clarkson T.W. Methylmercury poisoning: long-term clinical, radiological, toxicological, and pathological studies of an affected family. Ann. Neurol. 1994;35:680–688. doi: 10.1002/ana.410350608. [DOI] [PubMed] [Google Scholar]
- 12.Ertas E., Aksoy A., Turla A., Karaarslan E.S., Karaarslan B., Aydin A., Eken A. Human brain mercury levels related to exposure to amalgam fillings. Hum. Exp. Toxicol. 2014;33:873–877. doi: 10.1177/0960327113509662. [DOI] [PubMed] [Google Scholar]
- 13.Greenwood M.R. Methylmercury poisoning in Iraq. An epidemiological study of the 1971-1972 outbreak. J. Appl. Toxicol. 1985;5:148–159. doi: 10.1002/jat.2550050305. [DOI] [PubMed] [Google Scholar]
- 14.Boomhower S.R., Newland M.C. Effects of adolescent exposure to methylmercury and d-amphetamine on reversal learning and an extradimensional shift in male mice. Exp. Clin. Psychopharmacol. 2017;25:64–73. doi: 10.1037/pha0000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freire M.A.M., Oliveira R.B., Picanço-Diniz C.W., Pereira A., Jr. Differential effects of methylmercury intoxication in the rat’s barrel field as evidenced by NADPH diaphorase histochemistry. Neurotoxicology. 2007;28:175–181. doi: 10.1016/j.neuro.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Kirkpatrick M., Benoit J., Everett W., Gibson J., Rist M., Fredette N. The effects of methylmercury exposure on behavior and biomarkers of oxidative stress in adult mice. Neurotoxicology. 2015;50:170–178. doi: 10.1016/j.neuro.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Nagashima K. A review of experimental methylmercury toxicity in rats: neuropathology and evidence for apoptosis. Toxicol. Pathol. 1997;25:624–631. doi: 10.1177/019262339702500613. [DOI] [PubMed] [Google Scholar]
- 18.Bittencourt L.O., Dionizio A., Nascimento P.C., Puty B., Leao L.K.R., Luz D.A., Silva M.C.F., Amado L.L., Leite A., Buzalaf M.R., Crespo-Lopez M.E., Maia C.S.F., Lima R.R. Proteomic approach underlying the hippocampal neurodegeneration caused by low doses of methylmercury after long-term exposure in adult rats. Met. Integr. Biometal Sci. 2019;11:390–403. doi: 10.1039/c8mt00297e. [DOI] [PubMed] [Google Scholar]
- 19.Crespo-L´opez M.E., Soares E.S., Macchi B.M., Santos-Sacramento L., Takeda P.Y., Lopes-Araújo A., Paraense R.S.O., Souza-Monteiro J.R., Augusto-Oliveira M., Luz D.A., Maia C., Rogez H., Lima M.O., Pereira J.P., Oliveira D.C., Burbano R.R., Lima R.R., do Nascimento J.L.M., Arrifano G.P. Towards therapeutic alternatives for mercury neurotoxicity in the amazon: unraveling the pre-clinical effects of the superfruit Açaí (Euterpe oleracea, Mart.) as juice for human consumption. Nutrients. 2019:11. doi: 10.3390/nu11112585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eir´o L.G., Ferreira M.K.M., Bittencourt L.O., Arag˜ao W.A.B., Souza M.P.C., Silva M.C.F., Dionizio A., Buzalaf M.A.R., Crespo-L´opez M.E., Lima R.R. Chronic methylmercury exposure causes spinal cord impairment: Proteomic modulation and oxidative stress, Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020;146 doi: 10.1016/j.fct.2020.111772. [DOI] [PubMed] [Google Scholar]
- 21.Freire M.A.M., Santana L.N.S., Bittencourt L.O., Nascimento P.C., Fernandes R.M., Leao L.K.R., Fernandes L.M.P., Silva M.C.F., Amado L.L., Gomes-Leal W., Crespo-Lopez M.E., Maia C., Lima R.R. Methylmercury intoxication and cortical ischemia: pre-clinical study of their comorbidity. Ecotoxicol. Environ. Saf. 2019;174:557–565. doi: 10.1016/j.ecoenv.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Santana L., Bittencourt L.O., Nascimento P.C., Fernandes R.M., Teixeira F.B., Fernandes L.M.P., Freitas Silva M.C., Nogueira L.S., Amado L.L., Crespo-Lopez M.E., Maia C., Lima R.R. Low doses of methylmercury exposure during adulthood in rats display oxidative stress, neurodegeneration in the motor cortex and lead to impairment of motor skills. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. 2019;51:19–27. doi: 10.1016/j.jtemb.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Bel´em-Filho I.J.A., Ribera P.C., Nascimento A.L., Gomes A.R.Q., Lima R.R., Crespo-Lopez M.E., Monteiro M.C., Fontes-Júnior E.A., Lima M.O., Maia C.S.F. Low doses of methylmercury intoxication solely or associated to ethanol binge drinking induce psychiatric-like disorders in adolescent female rats. Environ. Toxicol. Pharmacol. 2018;60:184–194. doi: 10.1016/j.etap.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira A.N., Pinheiro A.M., Bel´em-Filho I.J.A., Fernandes L.M.P., Cart´agenes S.C., Ribera P.C., Fontes-Júnior E.A., Crespo-Lopez M.E., Monteiro M.C., Lima M.O., Maia C.S.F. Unravelling motor behaviour hallmarks in intoxicated adolescents: methylmercury subtoxic-dose exposure and binge ethanol intake paradigm in rats. Environ. Sci. Pollut. Res. Int. 2018;25:21937–21948. doi: 10.1007/s11356-018-2235-4. [DOI] [PubMed] [Google Scholar]
- 25.Saboory E., Ghasemi M., Mehranfard N. Norepinephrine, neurodevelopment and behavior. Neurochem. Int. 2020;135 doi: 10.1016/j.neuint.2020.104706. [DOI] [PubMed] [Google Scholar]
- 26.Benmhammed H., El Hayek S., Berkik I., Elmostafi H., Bousalham R., Mesfioui A., Ouichou A., El A. Hessni, Animal models of early-life adversity. Methods Mol. Biol. (Clifton, N. J.) 2019;2011:143–161. doi: 10.1007/978-1-4939-9554-7_10. [DOI] [PubMed] [Google Scholar]
- 27.Shi M.M., Fan K.M., Qiao Y.N., Xu J.H., Qiu L.J., Li X., Liu Y., Qian Z.Q., Wei C.L., Han J., Fan J., Tian Y.F., Ren W., Liu Z.Q. Hippocampal μ-opioid receptors on GABAergic neurons mediate stress-induced impairment of memory retrieval. Mol. Psychiatry. 2020;25(5):977–992. doi: 10.1038/s41380-019-0435-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farina M., Aschner M. Methylmercury-induced neurotoxicity: focus on pro- oxidative events and related consequences. Adv. Neurobiol. 2017;18:267–286. doi: 10.1007/978-3-319-60189-2_13. [DOI] [PubMed] [Google Scholar]
- 29.N.R. Council . Guide for the Care and Use of Laboratory Animals. Eighth ed. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 30.Kong H.K., Wong M.H., Chan H.M., Lo S.C. Chronic exposure of adult rats to low doses of methylmercury induced a state of metabolic deficit in the somatosensory cortex. J. Proteome Res. 2013;12:5233–5245. doi: 10.1021/pr400356v. [DOI] [PubMed] [Google Scholar]
- 31.Bittencourt L.O., Puty B., Charone S., Arag˜ao W.A.B., Farias-Junior P.M., Silva M.C.F., Crespo-Lopez M.E., Leite A.L., Buzalaf M.A.R., Lima R.R. Oxidative biochemistry disbalance and changes on proteomic profile in salivary glands of rats induced by chronic exposure to methylmercury. Oxid. Med. Cell. Longev. 2017;2017:5653291. doi: 10.1155/2017/5653291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farias-Junior P.M.A., Teixeira F.B., Fagundes N.C.F., Miranda G.H.N., Oliveira Bittencourt L., de Oliveira Paraense R.S., Silva M.C.F., Sagica F., de Oliveira E.H., Crespo-Lopez M.E., Lima R.R. Chronic intoxication by methylmercury leads to oxidative damage and cell death in salivary glands of rats. Met.: Integr. Biometal Sci. 2017;9:1778–1785. doi: 10.1039/c7mt00168a. [DOI] [PubMed] [Google Scholar]
- 33.Lima L.A.O., Bittencourt L.O., Puty B., Fernandes R.M., Nascimento P.C., Silva M.C.F., Alves-Junior S.M., Pinheiro J.J.V., Lima R.R. Methylmercury intoxication promotes metallothionein response and cell damage in salivary glands of rats. Biol. Trace Elem. Res. 2018;185:135–142. doi: 10.1007/s12011-017-1230-9. [DOI] [PubMed] [Google Scholar]
- 34.Freire M.A.M., Lima R.R., Nascimento P.C., Gomes-Leal W., Pereira A., Jr. Effects of methylmercury on the pattern of NADPH diaphorase expression and astrocytic activation in the rat. Ecotoxicol. Environ. Saf. 2020;201 doi: 10.1016/j.ecoenv.2020.110799. [DOI] [PubMed] [Google Scholar]
- 35.Liang J., Inskip M., Newhook D., Messier C. Neurobehavioral effect of chronic and bolus doses of methylmercury following prenatal exposure in C57BL/6 weanling mice. Neurotoxicol. Teratol. 2009;31:372–381. doi: 10.1016/j.ntt.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Nascimento P.C., Ferreira M.K.M., Balbinot K.M., Alves-Júnior S.M., Viana Pinheiro J.J., Silveira F.M., Martins M.D., Crespo-Lopez M.E., Lima R.R. Methylmercury-induced toxicopathologic findings in salivary glands of offspring rats after gestational and lactational exposure. Biol. Trace Elem. Res. 2021;199(8):2983–2991. doi: 10.1007/s12011-020-02409-z. [DOI] [PubMed] [Google Scholar]
- 37.Nascimento P.C., Arag˜ao W.A.B., Bittencourt L.O., Silva M.C.F., Crespo-Lopez M.E., Lima R.R. Salivary parameters alterations after early exposure to environmental methylmercury: a preclinical study in offspring rats. J. Trace Elem. Med. Biol. 2021;68 doi: 10.1016/j.jtemb.2021.126820. [DOI] [PubMed] [Google Scholar]
- 38.Walsh R.N., Cummins R.A. The open-field test: a critical review. Psychol. Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- 39.Teixeira F.B., Fernandes R.M., Farias-Junior P.M., Costa N.M., Fernandes L.M., Santana L.N., Silva-Junior A.F., Silva M.C., Maia C.S., Lima R.R. Evaluation of the effects of chronic intoxication with inorganic mercury on memory and motor control in rats. Int. J. Environ. Res. Public Health. 2014;11(9):9171–9185. doi: 10.3390/ijerph110909171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izquierdo J.H.Medina. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol. Learn. Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- 41.Alves Oliveira A.C., Dionizio A., Teixeira F.B., Bittencourt L.O., Nonato Miranda G.H., Oliveira Lopes G., Varela E.L.P., Nabiça M., Ribera P., Dantas K., Leite A., Buzalaf M.A.R., Monteiro M.C., Maia C.S.F., Lima R.R. Hippocampal impairment triggered by long-term lead exposure from adolescence to adulthood in rats: insights from molecular to functional levels. Int. J. Mol. Sci. 2020;21:6937. doi: 10.3390/ijms21186937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aragão W.A.B., Teixeira F.B., Fagundes N.C.F., Fernandes R.M., Fernandes L.M.P., da Silva M.C.F., Amado L.L., Sagica F.E.S., Oliveira E.H.C., Crespo-Lopez M.E., Maia C.S.F., Lima R.R. Hippocampal Dysfunction Provoked by Mercury Chloride Exposure: Evaluation of Cognitive Impairment, Oxidative Stress, Tissue Injury and Nature of Cell Death. Oxid Med Cell Longev. 2018;2018:7878050. doi: 10.1155/2018/7878050. Apr 10. PMID: 29849915; PMCID: PMC5914100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aragao W.A.B., da Costa N.M.M., Fagundes N.C.F., Silva M.C.F., Alves-Junior S.M., Pinheiro J.J.V., Amado L.L., Crespo-Lopez M.E., Maia C.S.F., Lima R.R. Chronic exposure to inorganic mercury induces biochemical and morphological changes in the salivary glands of rats. Met.: Integr. biometal Sci. 2017;9:1271–1278. doi: 10.1039/c7mt00123a. [DOI] [PubMed] [Google Scholar]
- 44.Amado L.L., Garcia M.L., Ramos P.B., Freitas R.F., Zafalon B., Ferreira J.L., Yunes J.S., Monserrat J.M. A method to measure total antioxidant capacity against peroxyl radicals in aquatic organisms: application to evaluate microcystins toxicity. Sci. Total Environ. 2009;407:2115–2123. doi: 10.1016/j.scitotenv.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 45.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 46.Esterbauer H., Cheeseman K.H. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 47.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 48.Green L.C., Ruiz de Luzuriaga K., Wagner D.A., Rand W., Istfan N., Young V.R., Tannenbaum S.R. Nitrate biosynthesis in man. Proc. Natl. Acad. Sci. USA. 1981;78:7764–7768. doi: 10.1073/pnas.78.12.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carocci A., Rovito N., Sinicropi M.S., Genchi G. Mercury toxicity and neurodegenerative effects. Rev Environ Contam Toxicol. 2014;229:1–18. doi: 10.1007/978-3-319-03777-6_1. [DOI] [PubMed] [Google Scholar]
- 50.Cernichiari E., Myers G.J., Ballatori N., Zareba G., Vyas J., Clarkson T. The biological monitoring of prenatal exposure to methylmercury. Neurotoxicology. 2007;28(5):1015–1022. doi: 10.1016/j.neuro.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Pletz J., S´anchez-Bayo F., Tennekes H.A. Dose-response analysis indicating time- dependent neurotoxicity caused by organic and inorganic mercury-Implications for toxic effects in the developing brain. Toxicology. 2016:347–349. doi: 10.1016/j.tox.2016.02.006. 1–5. [DOI] [PubMed] [Google Scholar]
- 52.Granitzer S., Ellinger I., Khan R., Gelles K., Widhalm R., Hengstschl¨ager M., Zeisler H., Desoye G., Tupova L., Ceckova M., Salzer H., Gundacker C. In vitro function and in situ localization of multidrug resistance-associated protein (MRP)1 (ABCC1) suggest a protective role against methyl mercury-induced oxidative stress in the human placenta. Arch. Toxicol. 2020 doi: 10.1007/s00204-020-02900-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Saleh M., Abduljabbar R., Al-Rouqi R., Elkhatib A., Alshabbaheen N., Shinwari Mercury (Hg) exposure in breast-fed infants and their mothers and the evidence of oxidative stress. Biol. Trace Elem. Res. 2013;153:145–154. doi: 10.1007/s12011-013-9687-7. [DOI] [PubMed] [Google Scholar]
- 54.Bj¨ornberg K.A., Vahter M., Berglund B., Niklasson B., Blennow M. G. Sandborgh- Englund, Transport of methylmercury and inorganic mercury to the fetus and breast-fed infant. Environ. Health Perspect. 2005;113:1381–1385. doi: 10.1289/ehp.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arrifano G.P.F., Martín-Doimeadios R.C.R., Jim´enez-Moreno M., Fern´andez- Trujillo S., Augusto-Oliveira M., Souza-Monteiro J.R., Macchi B.M., Alvarez-Leite J.I., do Nascimento J.L.M., Amador M.T., Santos S., Ribeiro-Dos-Santos A., Silva- Pereira L.C., Ori´a R.B., Crespo-Lopez M.E. Genetic susceptibility to neurodegeneration in amazon: apolipoprotein e genotyping in vulnerable populations exposed to mercury. Front. Genet. 2018;9:285. doi: 10.3389/fgene.2018.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.(a) Arrifano G.P.F., Martín-Doimeadios R.C.R., Jim´enez-Moreno M., Ramírez- Mateos V., da Silva N.F.S., Souza-Monteiro J.R., Augusto-Oliveira M., Paraense R.S.O., Macchi B.M., do Nascimento J.L.M., Crespo-Lopez M.E. Large-scale projects in the amazon and human exposure to mercury: the case-study of the Tucuruí Dam. Ecotoxicol. Environ. Saf. 2018;147:299–305. doi: 10.1016/j.ecoenv.2017.08.048. [DOI] [PubMed] [Google Scholar]; (b) Santos Serr˜ao de Castro N., de Oliveira M. Lima, Hair as a biomarker of long term mercury exposure in brazilian amazon: a systematic review. Int. J. Environ. Res. Public Health. 2018:15. doi: 10.3390/ijerph15030500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reichmann D., Voth W., Jakob U. Maintaining a healthy proteome during oxidative stress. Mol. Cell. 2018;69:203–213. doi: 10.1016/j.molcel.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.UNEP . Program UNE. United Nations Environment Program; 2019. Global Mercury Assessment 2018. [Google Scholar]
- 59.Ghizoni H., Ventura M., Colle D., et al. Effects of perinatal exposure to n-3 polyunsaturated fatty acids and methylmercury on cerebellar and behavioral parameters in mice. Food Chem Toxicol. 2018;120:603–615. doi: 10.1016/j.fct.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 60.Cheng J., Fujimura M., Bo D. Assessing pre/post-weaning neurobehavioral development for perinatal exposure to low doses of methylmercury. J Environ Sci (China) 2015;38:36–41. doi: 10.1016/j.jes.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 61.Berzas Nevado J.J., Rodríguez Martín-Doimeadios R.C., Guzm´an F.J. Bernardo, Mercury in the Tapaj´os River basin, Brazilian Amazon: a review. Environ Int. 2010;36(6):593–608. doi: 10.1016/j.envint.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 62.Sabry F.M., Ibrahim M.K., Hamed M.R., Ahmed H. Neurobehavioral effects of alcohol in overcrowded male adolescent rats. Neurosci. Lett. 2020;731 doi: 10.1016/j.neulet.2020.135084. [DOI] [PubMed] [Google Scholar]
- 63.Austin S.A., Santhanam A.V., d’Uscio L.V., Katusic Z.S. Regional heterogeneity of cerebral microvessels and brain susceptibility to oxidative stress. PloS One. 2015;10 doi: 10.1371/journal.pone.0144062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu Y., Ruszn´ak Z., Herculano-Houzel S., Watson C., Paxinos G. Cellular composition characterizing postnatal development and maturation of the mouse brain and spinal cord. Brain Struct. Funct. 2013;218:1337–1354. doi: 10.1007/s00429-012-0462-x. [DOI] [PubMed] [Google Scholar]
- 65.do Nascimento J.L., Oliveira K.R., Crespo-Lopez M.E., Macchi B.M., Mau´es L.A., Pinheiro Mda C., Silveira L.C., Herculano A.M. Methylmercury neurotoxicity & antioxidant defenses, Indian. J. Med. Res. 2008;128:373–382. [PubMed] [Google Scholar]
- 66.Singh A., Kukreti R., Saso L., Kukreti S. Molecules. Basel; Switzerland: 2019. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases; p. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crespo-Lopez M.E., Costa-Malaquias A., Oliveira E.H., Miranda M.S., Arrifano G.P., Souza-Monteiro J.R., Sagica F.E., Fontes-Junior E.A., Maia C.S., Macchi B.M., do Nascimento J.L. Is low non-lethal concentration of methylmercury really safe? a report on genotoxicity with delayed cell proliferation. PloS One. 2016;11 doi: 10.1371/journal.pone.0162822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crespo-López M.E., Macêdo G.L., Pereira S.I., Arrifano G.P., Picanço-Diniz D.L., do Nascimento J.L., Herculano A.M. Mercury and human genotoxicity: critical considerations and possible molecular mechanisms. Pharmacological research. 2009;60:212–220. doi: 10.1016/j.phrs.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 69.Liu W., Yang T., Xu Z., Xu B., Deng Y. Methyl-mercury induces apoptosis through ROS-mediated endoplasmic reticulum stress and mitochondrial apoptosis pathways activation in rat cortical neurons. Free Radic. Res. 2019;53:26–44. doi: 10.1080/10715762.2018.1546852. [DOI] [PubMed] [Google Scholar]
- 70.Fujimura M., Usuki F. Methylmercury-mediated oxidative stress and activation of the cellular protective system. Antioxidants. 2020:9. doi: 10.3390/antiox9101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kudo H., Kokunai T., Kondoh T., Tamaki N., Matsumoto S. Quantitative analysis of glutathione in rat central nervous system: comparison of GSH in infant brain with that in adult brain. Brain Res. 1990;511:326–328. doi: 10.1016/0006-8993(90)90178-e. [DOI] [PubMed] [Google Scholar]
- 72.Ajsuvakova O.P., Tinkov A.A., Aschner M., Rocha J.B.T., Michalke B., Skalnaya M.G., Skalny A.V., Butnariu M., Dadar M., Sarac I., Aaseth J., Bjørklund G. Sulfhydryl groups as targets of mercury toxicity. Coord. Chem. Rev. 2020;417 doi: 10.1016/j.ccr.2020.213343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ajsuvakova O.P., Tinkov A.A., Aschner M., Rocha J.B.T., Michalke B., Skalnaya M.G., Skalny A.V., Butnariu M., Dadar M., Sarac I., Aaseth J., Bjørklund G. Sulfhydryl groups as targets of mercury toxicity. Coord. Chem. Rev. 2020;417 doi: 10.1016/j.ccr.2020.213343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Culbreth M., Aschner M. Dysregulation of glutamate cycling mediates methylmercury-induced neurotoxicity. Adv. Neurobiol. 2016;13:295–305. doi: 10.1007/978-3-319-45096-4_11. [DOI] [PubMed] [Google Scholar]
- 75.Liu W., Xu Z., Yang T., Deng Y., Xu B., Feng S. Tea polyphenols protect against methylmercury-induced cell injury in rat primary cultured astrocytes, involvement of oxidative stress and glutamate uptake/metabolism disorders. Mol. Neurobiol. 2016;53:2995–3009. doi: 10.1007/s12035-015-9161-y. [DOI] [PubMed] [Google Scholar]
- 76.Shanker G., Aschner M. Identification and characterization of uptake systems for cystine and cysteine in cultured astrocytes and neurons: evidence for methylmercury-targeted disruption of astrocyte transport. J. Neurosci. Res. 2001;66:998–1002. doi: 10.1002/jnr.10066. [DOI] [PubMed] [Google Scholar]
- 77.Todd A.C., Hardingham G.E. The regulation of astrocytic glutamate transporters in health and neurodegenerative diseases. Int. J. Mol. Sci. 2020:21. doi: 10.3390/ijms21249607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schmahmann J.D. The cerebellum and cognition. Neurosci. Lett. 2019;688:62–75. doi: 10.1016/j.neulet.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 79.Shevelkin A.V., Terrillion C.E., Abazyan B.N., Kajstura T.J., Jouroukhin Y.A., Rudow G.L., Troncoso J.C., Linden D.J., Pletnikov M.V. Expression of mutant DISC1 in Purkinje cells increases their spontaneous activity and impairs cognitive and social behaviors in mice. Neurobiol. Dis. 2017;103:144–153. doi: 10.1016/j.nbd.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fontes-Júnior E.A., Maia C.S., Fernandes L.M., Gomes-Leal W., Costa-Malaquias A., Lima R.R., Prediger R.D., Crespo-L´opez M.E. Chronic Alcohol Intoxication and Cortical Ischemia: Study of Their Comorbidity and the Protective Effects of Minocycline. Oxid. Med. Cell. Longev. 2016;2016:1341453. doi: 10.1155/2016/1341453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.da Silva F., Cunha P.A., Ribera P.C., Barros M.A., Cart´agenes S.C., Fernandes L., Teixeira F.B., Fontes-Júnior E.A., Prediger R.D., Lima R.R., Maia C. Heavy chronic ethanol exposure from adolescence to adulthood induces cerebellar neuronal loss and motor function damage in female rats. Front. Behav. Neurosci. 2018;12:88. doi: 10.3389/fnbeh.2018.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Valencia M., Illanes J., Santander O., Saavedra D., Adaros M., Ibarra A., Saavedra G., Pascual R. Environmental enrichment restores the reduced expression of cerebellar synaptophysin and the motor coordination impairment in rats prenatally treated with betamethasone. Physiol. Behav. 2019;209 doi: 10.1016/j.physbeh.2019.112590. [DOI] [PubMed] [Google Scholar]
- 83.Macedo-Júnior S.J., Luiz-Cerutti M., Nascimento D.B., Farina M., Soares Santos A.R., de Azevedo Maia A.H. Methylmercury exposure for 14 days (short-term) produces behavioral and biochemical changes in mouse cerebellum, liver, and serum. J. Toxicol. Environ. Health Part A. 2017;80:1145–1155. doi: 10.1080/15287394.2017.1357324. [DOI] [PubMed] [Google Scholar]
- 84.Rodrigues K.E., de Oliveira F.R., Barbosa B.R.C., Paraense R.S.O., Bannwart C.M., Pinheiro B.G., Botelho A.S., Muto N.A., do Amarante C.B., Hamoy M., Macchi B.M., Maia C., do Prado A.F., do Nascimento J.L.M. Aqueous Coriandrum sativum L. extract promotes neuroprotection against motor changes and oxidative damage in rat progeny after maternal exposure to methylmercury, Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019;133 doi: 10.1016/j.fct.2019.110755. [DOI] [PubMed] [Google Scholar]
- 85.Sakamoto M., Kakita A., Sakai K., Kameo S., Yamamoto M., Nakamura M. Methylmercury exposure during the vulnerable window of the cerebrum in postnatal developing rats. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109776. [DOI] [PubMed] [Google Scholar]
- 86.Zeidman P., Maguire E.A. Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat. Rev. Neurosci. 2016;17:173–182. doi: 10.1038/nrn.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Voss J.L., Bridge D.J., Cohen N.J., Walker J.A. A closer look at the hippocampus and memory. Trends Cogn. Sci. 2017;21:577–588. doi: 10.1016/j.tics.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oliveira A.C., Pereira M.C., Santana L.N., Fernandes R.M., Teixeira F.B., Oliveira G.B., Fernandes L.M., Fontes-Júnior E.A., Prediger R.D., Crespo-L´opez M.E., Gomes-Leal W., Lima R.R., Maia C. Chronic ethanol exposure during adolescence through early adulthood in female rats induces emotional and memory deficits associated with morphological and molecular alterations in hippocampus. J. Psychopharmacol. 2015;29(6):712–724. doi: 10.1177/0269881115581960. [DOI] [PubMed] [Google Scholar]
- 89.Goosens K.A. Hippocampal regulation of aversive memories. Curr. Opin. Neurobiol. 2011;21(3):460–466. doi: 10.1016/j.conb.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang Y., Wang J.Z. From structure to behavior in basolateral amygdala- hippocampus circuits. Front. Neural Circuits. 2017;11:86. doi: 10.3389/fncir.2017.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chaaya N., Battle A.R., Johnson L.R. An update on contextual fear memory mechanisms: transition between amygdala and hippocampus. Neurosci. Biobehav. Rev. 2018;92:43–54. doi: 10.1016/j.neubiorev.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 92.Admon R., Lubin G., Stern O., et al. Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proc Natl Acad Sci U S A. 2009;106(33):14120–14125. doi: 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tottenham N., Sheridan M.A. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front. Hum. Neurosci. 2010;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Espitia-P´erez P., Albino S.M., Espitia-P´erez L., Brango H., da Rosa H., Kleber Silveira A., Moraes D.P., Cerveira C., Mingori M., Tiefensee Ribeiro C., Gelain D.P., Schnorr C.E., Fonseca Moreira J.C. Neurobehavioral and oxidative stress alterations following methylmercury and retinyl palmitate co-administration in pregnant and lactating rats and their offspring. Neurotoxicology. 2018;69:164–180. doi: 10.1016/j.neuro.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 95.Obiorah M., McCandlish E., Buckley B., DiCicco-Bloom E. Hippocampal developmental vulnerability to methylmercury extends into prepubescence. Front. Neurosci. 2015;9:150. doi: 10.3389/fnins.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tian J., Luo Y., Chen W., Yang S., Wang H., Cui J., Lu Z., Lin Y., Bi Y. MeHg suppressed neuronal potency of hippocampal NSCs contributing to the puberal spatial memory deficits. Biol. Trace Elem. Res. 2016;172:424–436. doi: 10.1007/s12011-015-0609-8. [DOI] [PubMed] [Google Scholar]
- 97.N.R. Council . Toxicological Effects of Methylmercury. The National Academies Press; Washington, DC: 2000. , Health Effects of Methylmercury. [Google Scholar]
- 98.Onishchenko N., Tamm C., Vahter M., Hokfelt T., Johnson J.A., Johnson D.A., Ceccatelli S. Developmental exposure to methylmercury alters learning and induces depression-like behavior in male mice. Toxicol. Sci. 2007;97:428–437. doi: 10.1093/toxsci/kfl199. [DOI] [PubMed] [Google Scholar]
- 99.Weiss B., Stern S., Cox C., Balys M. Perinatal and lifetime exposure to methylmercury in the mouse: behavioral effects. Neurotoxicology. 2005;26:675–690. doi: 10.1016/j.neuro.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 100.Myers G.J., Davidson P.W., Cox C., Shamlaye C.F., Palumbo D., Cernichiari E., Sloane-Reeves J., Wilding G.E., Kost J., Huang L.S., Clarkson T.W. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. Lancet. 2003;361:1686–1692. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- 101.Sandborgh-Englund G., Elinder C.G., Langworth S., Schütz A., Ekstrand J. Mercury in biological fluids after amalgam removal. J. Dent. Res. 1998;77:615–624. doi: 10.1177/00220345980770041501. [DOI] [PubMed] [Google Scholar]
- 102.Arrifano G.P., Augusto-Oliveira M., Souza-Monteiro J.R., Macchi B.M., Lima R.R., Su˜nol C., do Nascimento J., Crespo-Lopez M.E. Revisiting astrocytic roles in methylmercury intoxication. Mol. Neurobiol. 2021;58(9):4293–4308. doi: 10.1007/s12035-021-02420-y. [DOI] [PubMed] [Google Scholar]
- 103.Eto K., Marumoto M., Takeya M. The pathology of methylmercury poisoning (Minamata disease): The 50th Anniversary of Japanese Society of Neuropathology. Neuropathology. 2010;30(5):471–479. doi: 10.1111/j.1440-1789.2010.01119.x. [DOI] [PubMed] [Google Scholar]
- 104.Bittencourt L.O., Chemelo V.S., Arag˜ao W., Puty B., Dionizio A., Teixeira F.B., Fernandes M.S., Silva M., Fernandes L., Buzalaf M., Crespo-Lopez M.E., Maia C., Lima R.R. From Molecules to Behavior in Long-Term Inorganic Mercury Intoxication: Unraveling Proteomic Features in Cerebellar Neurodegeneration of Rats. Int. J. Mol. Sci. 2021;23(1):111. doi: 10.3390/ijms23010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jackson A. Chronic neurological disease due to methylmercury poisoning, Can. J. Neurol. Sci. 2018;45:620–623. doi: 10.1017/cjn.2018.323. [DOI] [PubMed] [Google Scholar]
Further reading
- 1.Abbott L.C., Nigussie F. Mercury toxicity and neurogenesis in the mammalian brain. Int. J. Mol. Sci. 2021;22(14):7520. doi: 10.3390/ijms22147520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trautwein S., Maurus P., Barisch-Fritz B., Hadzic A., Woll A. Recommended motor assessments based on psychometric properties in individuals with dementia: a systematic review. Eur. Rev. Aging Phys. Act. Off. J. Eur. Group Res. Elder. Phys. Act. 2019;16:20. doi: 10.1186/s11556-019-0228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drastichova Z., Rudajev V., Pallag G., Novotny J. Proteome profiling of different rat brain regions reveals the modulatory effect of prolonged maternal separation on proteins involved in cell death-related processes. Biol. Res. 2021;54(1):4. doi: 10.1186/s40659-021-00327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Paula Fonseca Arrifano G., Del Carmen Rodriguez Martin-Doimeadios R., Jiménez-Moreno M., Augusto-Oliveira M., Rogério Souza-Monteiro J., Paraense R., Rodrigues Machado C., Farina M., Macchi B., do Nascimento J.L.M., Crespo-Lopez M.E. Assessing mercury intoxication in isolated/remote populations: increased S100B mRNA in blood in exposed riverine inhabitants of the Amazon. Neurotoxicology. 2018;68:151–158. doi: 10.1016/j.neuro.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Eto K., Marumoto M., Takeya M. The pathology of methylmercury poisoning (Minamata disease) Neuropathology. 2020;30:471–479. doi: 10.1111/j.1440-1789.2010.01119.x. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes L., Cartágenes S.C., Barros M.A., Carvalheiro T., Castro N., Schamne M.G., Lima R.R., Prediger R.D., Monteiro M.C., Fontes-Júnior E.A., Cunha R.A., Maia C. Repeated cycles of binge-like ethanol exposure induce immediate and delayed neurobehavioral changes and hippocampal dysfunction in adolescent female rats. Behav. brain Res. 2018;350:99–108. doi: 10.1016/j.bbr.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira J.A., Foley A.M., Brown M. Sex hormones differentially influence voluntary running activity, food intake and body weight in aging female and male rats. Eur. J. Appl. Physiol. 2012;112(8):3007–3018. doi: 10.1007/s00421-011-2271-y. [DOI] [PubMed] [Google Scholar]
- 8.Fuentes-Abolafio I.J., Stubbs B., Pérez-Belmonte L.M., Bernal-López M.R., Gómez-Huelgas R., Cuesta-Vargas A. Vol. 50. 2021. Functional objective parameters which may discriminate patients with mild cognitive impairment from cognitively healthy individuals: a systematic review and meta-analysis using an instrumented kinematic assessment; pp. 380–393. (Age and ageing). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Data Availability Statement
The quantitative analysis data used to support this study's findings are included within the article and available in the supplementary material.