Abstract
Background
There is a global call for more inclusive clinical research that is representative of all populations, particularly those historically under-represented or under-served. A lack of broad representation results in disproportionate health outcomes and limits the applicability and translation of research findings.
Aim
Identify and describe barriers to participation across the research lifecycle and consider the role of the Clinical Research Nurse (CRN) in promoting inclusivity, including for Aboriginal and Torres Strait Islander Peoples within Australia.
Discussion
Review of recent literature and best practice identified barriers to research participation across the research process; at system, participant and practitioner levels. This discussion paper explores the role of the CRN; acting as enablers, facilitators and navigators, to mitigate participation barriers.
Conclusion
With their comprehensive understanding of the research process, clinical care pathways, reflective practices and participant-centred approaches, CRNs are uniquely positioned to advocate for greater equity in access to clinical research and to motivate stakeholders across the research enterprise to embed inclusive approaches in the design, conduct and dissemination of research.
Implications for Practice
An in-depth understanding of the research process, self, and cultural norms of the populations they serve is essential for CRNs to effectively advocate for equity in access to research.
Keywords: Australia, community-based participatory research, cultural diversity, health equity, health services accessibility, nurses’ role
Background
Clinical research is a globally growing enterprise, with over 380,000 clinical research studies (interventional clinical trials and observational studies) from over 200 countries currently registered on ClinicalTrials.gov (ClinicalTrials.gov, 2021). COVID-19 studies alone currently represent over 4500 active clinical research studies worldwide (ClinicalTrials.gov, 2021). In Australia, 5.2 million Australians took part in over 10,000 clinical trials from 2006 to 2015 (Askie et al., 2017). Despite an increase in the number of studies being conducted, clinical research still proves inaccessible for many people and populations, particularly those historically under-represented and under-served.
The importance of engaging diverse populations in clinical research is driven by an ethical and moral responsibility of those conducting and delivering research to ensure that evidence-based care is representative of, and accessible to all populations. Meaningful translation of research findings into practice is only achievable through broad and inclusive representation of all populations, with this inclusivity embedded into the design, conduct and delivery of the research (Sardar et al., 2014; Treweek et al., 2020). This is particularly the case where the disease or condition studied has an increased prevalence within an under-represented group, illustrated most recently within the current COVID-19 pandemic, where clinical trials have failed to adequately include participants from populations most affected by the virus (Treweek et al., 2020, National Institute for Health Research (NIHR), 2020a).
The use of the terms ‘under-represented’ and ‘under-served’ in a clinical research setting are used varyingly across regions and within cultural and social contexts. ‘Under-represented’ often includes those from ethnically and culturally diverse backgrounds, women (including pregnant women), those with altered capacity to consent, children and older people (Australian Clinical Trials Alliance (ACTA); 2020b; Department of Health Medical Research Future Fund (MRFF), 2016; Herrera et al., 2010; National Institute for Health (NIH), 2017; U.S. Department of Health and Human Services Food and Drug Administration (FDA), 2020). The term ‘under-served’, whilst more commonly utilised in the UK in inclusivity discussions (National Institute for Health Research (NIHR), 2020b), within the Australian context is more closely linked to the availability and adequacy of services (including clinical research) for a particular group (Horsley, personal communication, 14 January 2021; Wagner, 2019). Under-served groups can include geographically isolated populations, rare disease patients, transient populations or people in detention (Forman et al., 2012; Thornicroft and Betts, 2002). Within this paper we use the term ‘under-represented’ to describe individuals or groups who are not proportionately represented in research (Wagner, 2019; Australian Clinical Trials Alliance (ACTA), 2020b; Horsley, personal communication, 14 January 2021).
Aboriginal and Torres Strait Islander peoples in Australia are an example of a group who are both under-represented and under-served in clinical research, despite the significant disparities in health outcomes, life expectancy and death rates for this population when compared to non-Indigenous Australians (AIHW, 2020).
Currently clinical research is optimised to serve an homogeneous participant group who are generally health literate, educated, financially stable and from a majority Caucasian background (Livaudais-Toman et al., 2014; National Institute for Health Research (NIHR), 2020b; U.S. Department of Health and Human Services Food and Drug Administration (FDA), 2019; U.S. Department of Health and Human Services Food and Drug Administration (FDA), 2020). Enhancing diversity in clinical trials is now a global priority and recognised as such by governments and patient advocacy groups internationally (National Health and Medical Research Council (NHMRC), 2020; National Institute for Health Research (NIHR), 2020b; U.S. Department of Health and Human Services Food and Drug Administration (FDA), 2020).
With their knowledge and influence across the research process, the CRN plays an important role in enabling and empowering access to clinical research for under-represented and under-served populations, using their position as patient/participant advocates to affect positive change to improve inclusivity of research.
Aim
This discussion paper aims to describe common barriers to research participation across the research process as well as recognising challenges of engaging under-represented and under-served groups, such as Aboriginal and Torres Strait Islanders within Australia. The purpose of the paper is to offer perspectives on the role of the CRN in addressing these barriers, to empower research participation at system, practitioner and participant levels.
The authorship group comprises experienced CRNs who are passionate about embedding inclusive practices within research settings and have expertise in working with under-represented and under-served groups in Australia and the UK. The literature review that informed this discussion paper, alongside author group meetings, established the thematic sub-headings used within the discussion below, with an aim to aid readers in recognising key barriers to participation within their settings. Recognition of these barriers will support CRNs to plan and implement strategies to improve inclusivity.
Discussion
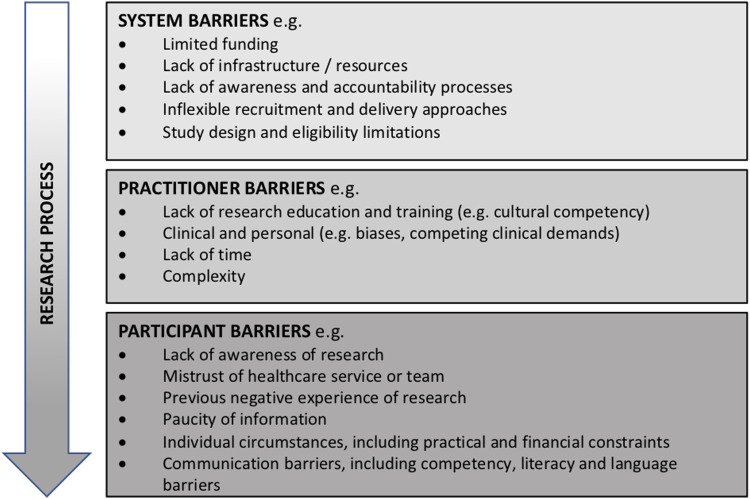
System barriers
At a system level, lack of accountability (by researchers and funding bodies), insufficient funding and inadequate resources all present significant challenges to research inclusivity (Redwood and Gill, 2013). Study design (and in particular overly rigorous eligibility criteria) can often present discriminatory research barriers impacting people from under-represented and under-served groups (Kim et al., 2017). For example, Low and colleagues (Low et al., 2019) conducted a search of the Australian New Zealand Clinical Trials Registry (ANZCTR) and ClinicalTrials.gov and found that 42 of 94 currently registered active dementia clinical trials in Australia (44.7%) excluded patients not fluent in English. Consideration of specific recruitment strategies and resources to support inclusion of under-represented and under-served groups is essential at the point of study design to avoid these populations succumbing to known barriers (Caren et al., 2014). The Australian Clinical Trials Alliance (ACTA), a national peak body (non-government umbrella organisation supporting and coordinating shared objectives of smaller organisations), recently commenced a programme of work to promote inclusive research, initially exploring this with and for people from Culturally and Linguistically Diverse (CALD) backgrounds, including an ‘Environmental Scan’ (Australian Clinical Trials Alliance (ACTA), 2020b) and Position Statement (Australian Clinical Trials Alliance (ACTA), 2020a) that captures the current landscape and barriers to research awareness, involvement and access for CALD populations within Australia.
For Aboriginal and Torres Strait Islander peoples in Australia, health research efforts, which are largely derived from Western knowledge systems, have largely failed to deliver on corresponding improvements in the health of Aboriginal and Torres Strait Island Peoples (Bainbridge et al., 2015), further compounding the enduring impacts of colonisation. Like many Indigenous populations globally, Aboriginal and Torres Strait Islanders have been over-researched – with research being conducted ‘on’ but not ‘with’ Aboriginal and Torres Strait Islander communities. Lack of recognition of Aboriginal and Torres Strait Islander sovereignty, leadership, values and partnership remains a significant system barrier to clinical research in Australia involving Aboriginal and Torres Strait Islanders. A recent publication exploring participation barriers to cancer trials for Aboriginal and Torres Strait Islanders by Cunningham and Garvey found that study eligibility criteria for these trials often disproportionately excluded Indigenous participants due to study design elements such as trial location, co-morbidities and also potential practitioner bias (Cunningham and Garvey, 2020).
System barriers are reasonably well understood and acknowledged – in the US and UK, government policies guide inclusion strategies and diversity targets (National Institute for Health Research (NIHR), 2020b; U.S. Department of Health and Human Services Food and Drug Administration (FDA), 2020). In the UK, the NIHR-funded INCLUDE Ethnicity Framework was recently developed to improve inclusion of under-served groups in research (Treweek et al., 2021). Within Australia, revisions have recently been drafted to the National Health and Medical Research Council’s (NHMRC) National Statement on Ethical Conduct in Human Research to support improved inclusion of vulnerable populations (National Health and Medical Research Council (NHMRC), 2020). These revisions have not yet been fully adopted, but do represent a long overdue focus on improvements to promote better inclusion of under-represented and under-served groups in research.
Practitioner barriers
From a practitioner perspective, inclusion of under-represented and under-served groups can be influenced by conscious and unconscious biases of clinical researchers (including CRNs) (Niranjan et al., 2020; Sheikh et al., 2009). Negative bias towards minority group participants can present a significant barrier to inclusion. For example, Niranjan et al. (2020) reported that clinical and health professionals perceived minority ethnic groups as challenging to recruit and potentially less compliant with the study protocol (Niranjan et al., 2020). Sheikh et al. (2009) described the ‘hassle’ factor reported by clinicians as a key barrier to recruitment of a South Asian population to an asthma study, despite this ethnic group being more than three times more likely to be hospitalised from asthma (Sheikh et al., 2009). Within a 2009 case study, Joseph and Dohan (2009) observed that practitioners within cancer clinics preferentially recruited ‘good study patients’, whom they assessed as more likely to adhere to protocol, with this recruitment bias driven by system pressures to deliver the trials in a ‘timely and efficient manner’ (Joseph and Dohan, 2009).
For Aboriginal and Torres Strait Islanders, barriers to research participation are complex and require cultural awareness, understanding and unique approaches to address (Aramoana et al., 2019; Cass, 2018; Marley et al., 2014). Indigenous self-determination, leadership, impact and value, sustainability and accountability are core ethical principles that underlie research done with and for Aboriginal and Torres Strait Islander populations in Australia (AIATSIS, 2020). An understanding and respect of these core principles, as well as Aboriginal and Torres Strait Islander peoples’ histories and culture, is crucial in enabling research participation for this population (Cass, 2018).
Participant barriers
For many under-represented and under-served populations, barriers to participation start with a lack of research awareness and poor health literacy levels, limiting the opportunity to consider participation (Christy et al., 2017; Livaudais-Toman et al., 2014). Research literacy as a component of health literacy may be low for many Australians. However, for people from under-represented groups this may be further exacerbated by language barriers, disparities in education and frequent transition between health systems (Christy et al., 2017, Australian Institute of Health and Welfare (AIHW), 2020).
Participant-specific barriers to research include cultural and language barriers, mistrust, economic factors, time constraints and mobility and health issues (Australian Clinical Trials Alliance (ACTA), 2020b; Clark et al., 2019; Hughson et al., 2016; National Institute for Health Research (NIHR), 2020b). These barriers are often compounded for people from lower socioeconomic groups, who arguably would benefit most from inclusion in and translational outcomes of clinical research. It is important that these populations are not excluded due to inherent social inequality and disadvantage.
Whilst not direct participant barriers but perhaps indirect consequences, practical and logistical barriers can also impact participation of under-represented and under-served groups. This includes the ‘9–5’ nature of some research, which can prevent those who are working, studying or caring/parenting full-time from taking part, and limitations such as transport or accessibility of the research setting (Kane et al., 2016). Within Australia, due to the vast geographical size of the country, the distribution of healthcare services also presents a challenging barrier to research participation. For example, 28% of Australia’s population are living in rural or remote areas (Australian Institute of Health and Welfare (AIHW), 2020), meaning that their access to clinical research opportunities and research-active health service providers is often severely limited. In their recent review of Indigenous participation in cancer trials, Cunningham and Garvey reported that while 39% of Aboriginal and Torres Strait Islander Peoples live outside metro/capital city areas, 89% of trials only had sites available with the metro/capital city areas (Cunningham and Garvey, 2020).
A summary of the multi-level barriers is presented in Figure 1.
Figure 1.
Barrier levels.
The CRN role in inclusivity
CRNs are centrally positioned within the healthcare system to recognise and act upon research participation barriers. CRNs, as specialised nurses with expertise in both clinical and research fields ((IACRN), 2012), play a critical role in enabling research participation for under-represented and under-served groups through their comprehensive understanding of the research process as well as clinical care pathways, and their involvement in all aspects of the research lifecycle, from study design and approvals through to recruitment and delivery (Sadler et al., 1999). CRNs work to balance competing demands in the delivery of high-quality research alongside healthcare, and bring knowledge of the patient journey as well as a nuanced understanding of barriers faced by participants (Pick et al., 2010). Identifying and addressing barriers to participation for under-represented and under-served groups is a key action area for CRNs, who as skilled and experienced patient and participant advocates, have the knowledge and influence to enable participation for these groups (Rubin, 2014; Sadler et al., 1999).
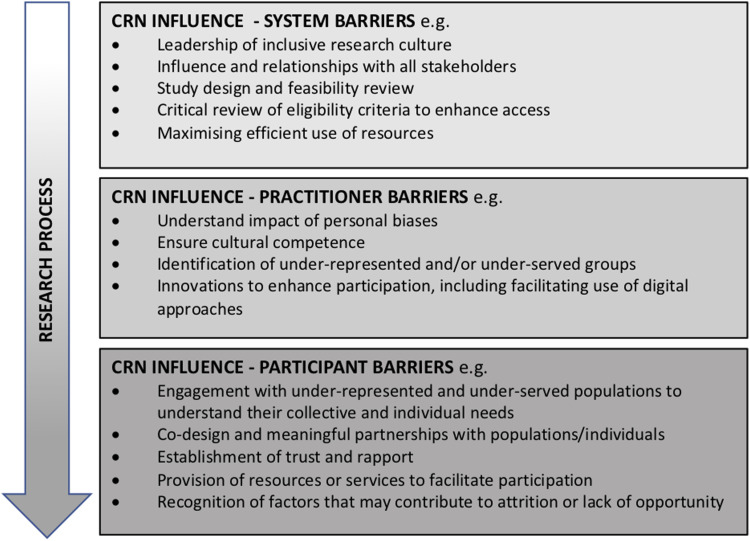
CRN as Enabler – Addressing system barriers
Affecting positive change and ensuring adoption of policies and guidelines that support greater inclusion of all populations requires influence across the research process. The CRN’s relationships with stakeholders, patient and community groups, and the inherent advocacy and care within their role (International Association of Clinical Research Nurses (IACRN), 2012) are key to the CRN’s ability to enable increased participation for under-represented and under-served populations.
The CRN has an important leadership role in building an inclusive research culture and enhancing research competence within their organisations to increase research activity and opportunity (Rubin, 2014). Alongside this, the CRN role often includes interaction with institutional research governance offices, which provides an opportunity to engage these stakeholders in initiatives to improve diversity, study design or to enhance the scope of research. The CRN’s involvement in feasibility and study design allows identification of areas for improved efficiency and streamlining of resources, and adjustments to protocol that can enhance inclusivity without overly impacting budget or timeline (Kane et al., 2016). Examples of CRN-led initiatives that have positively impacted participation barriers at a system level include a programme to provide 24h CRN cover for stroke trials recruitment (Maguire, 2017), a ‘Grounded Research’ initiative to take research into the community (Rotherham, 2021), and an organisational research nursing model that promotes participant-centric study design and delivery (Kane et al., 2016).
Clinical research studies involving Aboriginal and Torres Strait Islander peoples, including randomised controlled trials, have demonstrated higher success rates when there is evidence of commitment to co-design and the creation of meaningful partnerships to target areas of community-identified need (Cass, 2018). The CRN has a key role in advocating for the co-design of research with, and for, Aboriginal and Torres Strait Islander peoples, with participation increased where traditional trial designs have been adapted to suit the unique needs of Aboriginal and Torres Strait Islander populations and where local health service engagement and resourcing are compatible with the research being conducted (Liu et al., 2015).
CRN as Facilitator – Addressing practitioner barriers
Closely linked with an understanding of system barriers is the requirement for the CRN to consider, acknowledge and challenge their own inherent personal biases and the potential for these to impact engagement of under-represented and under-served groups (Niranjan et al., 2020). Differing norms and values can present a mismatch between how nurses deliver care to those from different cultural and social backgrounds, whose values and beliefs may be remote from their own (Centre for Epidemiology and Research, NSW Department of Health, 2003). Nurses, including CRNs, must have a robust understanding of culturally sensitive practice to deliver culturally appropriate healthcare, including within a research setting (Chenowethm et al., 2006; Jie-Lili, 2017). The capacity to acquire and then apply appropriate cultural knowledge about research participants from diverse cultural and ethnic backgrounds is essential to recruiting more representative populations (Wallington et al., 2016). Nursing practice, as part of culturally sensitive healthcare, involves a strong awareness of cultural considerations; therefore, CRNs are best placed to influence trial design to meet cultural and group needs, and empower inclusion of all populations. Culturally sensitive CRNs are also well equipped to pre-empt and address flaws in the design and conduct of research as it unfolds, and to promote factors that support inclusion, awareness and participation. This includes improved co-design and partnership models, appropriate adaptations in research design and advanced planning that embraces the unique needs of many different diverse populations.
In the Australian context, research involving Aboriginal and Torres Strait Island Peoples must be designed and conducted with respect for Indigenous authority as self-determining peoples (Australian Institute of Aboriginal and Torres Strait Islander Studies (AIATSIS), 2020). The CRN’s role is to advocate for recognition of Indigenous participants as rights holders, whose knowledge and contribution to research is respected and valued (Australian Institute of Aboriginal and Torres Strait Islander Studies (AIATSIS), 2020) and to be aware of the role of implicit bias (for themselves and recruiting investigators) in determining eligibility to participate in clinical research studies (Cunningham and Garvey, 2020).
Problem-solving is a fundamental part of the CRN role. An ability to recognise a problem, identify variables for influence and implement actions to affect change is an inherent part of CRN practice and is vitally important in enhancing inclusivity of clinical research. There is a growing appreciation amongst CRNs of their role in advocating for equity in research access, and recently this has become more apparent in professional networking and collaborative forums, such as conference discussions, Twectchats and in particular via the #WhyWeDoResearch community (Gibbs et al., 2015). These often also provide a glimpse at CRN-led initiatives, both small and large, that are influencing positive change. Within formally published literature, examples of CRN-led initiatives to improve research diversity can be difficult to find, although we can often identify CRN influence within organisational and policy strategies to enhance accessibility to research (Australian Clinical Trials Alliance (ACTA), 2020b). A challenge in identifying CRN-led strategies is that many of the actions and interventions undertaken by CRNs to support diverse participation are often intrinsic to the role, and viewed by many as ‘just part of the job’. Particularly within the COVID-19 pandemic, the role of the CRN has been placed into the fore, and the flexibility and adaptability of nurses has been utilised to support the embedding of research into routine care and the rapid transition to digital platforms for many research projects (Maxton et al., 2020, Iles-Smith and et al. on behalf of the Association of UK Lead Research Nurses, 2020).
CRN as Navigator – Addressing participant barriers
As key members of the study team involved in recruitment and conduct of clinical research, CRNs are well placed to identify populations that are under-represented or under-served within the studies or specialties that they support and to act on individual or group participant barriers. Nurses, and particularly CRNs, have in-depth knowledge on how to navigate complex health systems and are often privileged with high patient/participant contact time, and thus opportunities to foster meaningful relationships and build trust (Sadler et al., 1999). This relationship between the CRN and patient/participant can facilitate an understanding of a participant’s individual and group needs and how other determinants of health (i.e. psychological, economic, social, and cultural) may impact their consideration to participate in research. This knowledge allows the CRN to understand how research protocols may be amended or implemented to enable participation, and this individualised approach is particularly important to successfully engage people from under-represented and under-served groups (Kane et al., 2016). For example, CRNs have the ability to identify and advocate for language translations of research documents that would directly enhance access for people from different ethnic backgrounds populations within their health service area. For under-served populations of highly transient groups or those experiencing homelessness, the CRN can recognise the impact of frequent transitions in care at the point of study design to address known barriers and facilitate additional support to allow engagement of these populations in research. The CRN’s understanding of the research process and participant/patient journey also allows them to help to navigate research participation and anticipate ‘next steps’ in care pathways where supports may be needed to ensure continued research opportunity during care transitions.
Figure 2 summarises examples of the CRN’s ability to influence participation barriers across the research process.
Figure 2.
Examples of Clinical Research Nurse influence.
Conclusion
Barriers to research participation for under-served and under-represented research populations are complex and exist across system, practitioner and participant levels. Of optimal importance to any solution-driven approach is an understanding of the social and cultural systems and structures that underpin and support inclusion of these populations.
The CRN is able to appreciate barriers to participation at a group and individual level, and across system, practitioner and participant levels. The CRN’s close engagement with patient/participants allows a deeper understanding of the enablers and barriers to research participation and how the CRN can use their influence and involvement in the research to positively impact opportunity to participate.
A clinical research nursing workforce that is self-aware and culturally competent is fundamental in enabling CRNs to address and advocate for change in areas that limit participation of under-represented and under-served groups. Foundation level CRN education should include a compulsory requirement to undertake training in cultural safety, with a particular emphasis within Australia on the unique factors that both limit and support inclusion of Aboriginal and Torres Strait Islanders, to help to address the gaps in healthcare equity that exist for under-represented and under-served groups.
Effective engagement of under-represented and under-served populations requires an authentic communication with these populations, with an emphasis on partnership and solution co-design to enhance research participation. The CRN is perfectly positioned as a key leader, facilitator and influencer in the pursuit of inclusivity in the research arena.
Key points for policy, practice and/or research
• Clinical research is designed to preferentially include homogeneous participant groups that are not necessarily representative of the populations that the research endeavour seeks to serve.
• Barriers to research participation for under-represented and under-served populations are complex and exist across system, practitioner and participant levels.
• Working across the research process and with unique clinical and research expertise, CRNs are optimally placed to positively influence research participation for under-represented and under-served groups.
• CRNs must ensure that they are aware of their own implicit bias and the importance of cultural awareness.
• Within the Australian context, inclusion of Aboriginal and Torres Strait Islander peoples in clinical research is an important health priority, and requires CRNs to understand and advocate for study design that is inclusive and supportive for this group.
• To improve research inclusion, solution-driven approaches will require authentic partnerships, trust, co-design and understanding and acceptance of different cultural, social and linguistic backgrounds by the CRN and the broader research and healthcare sector.
Biography
Kelly Beer is the Clinical Research Manager for the Myositis Discovery Programme (Perth, WA). Kelly is an experienced Clinical Research Nurse, with a career interest in participant engagement and involvement.
Melanie Gentgall started her career as a CRN and has held various senior leadership roles across the Australian trials ecosystem. She is now Director of Clinical Trials at the South Australian Health and Medical Research Institute (SAHMRI).
Nicola Templeton is a Registered Nurse and clinical educator within refugee and asylum seeker health. Her career has developed around optimising engagement with vulnerable populations through valuable patient interactions.
Claire Whitehouse is Senior Nurse for Nursing, Midwifery and Allied Health Professions (NMAHP) Research at James Paget University Hospitals NHSFT and a Senior Research Leader with the NIHR 70@70 programme.
Nicola Straiton is a Registered Nurse, specialising in cardiovascular disease, and researcher at the University of Sydney. Nicola is the program and operations manager with the Australian Clinical Trials Alliance (ACTA).
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics: Ethical approval was not required for this discussion paper.
ORCID iDs
Kelly Beer https://orcid.org/0000-0003-0875-781X
Claire Whitehouse https://orcid.org/0000-0002-7038-6709
Nicola Straiton https://orcid.org/0000-0002-5013-0159
References
- Aramoana J, Koea J, Collaboration C. (2019) An integrative reivew of the barriers to indigenous peoples participation in biobanking and genomic research. Journal Of Global Oncology 5: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askie LM, Hunter KE, Berber S, et al. (2017) The Clinical Trials Landscape in Australia 2006–2015. Sydney, Australia: Australian New Zealand Clinical Trials Registry. [Google Scholar]
- Australian Clinical Trials Alliance (ACTA) (2020. a) Advancing Clinical Trial Awareness, Involvement and Access for People from Culturally and Linguistically Diverse (CALD) Backgrounds: Position Statement. South Melbourne, Australia: Australian Clinical Trials Alliance (ACTA). [Google Scholar]
- Australian Clinical Trials Alliance (ACTA) (2020. b) Clinical Trial Awareness and Access Amongst Culturally and Linguistically Diverse (CALD) Populations: Environmental Scan. South Melbourne, Australia: Australian Clinical Trials Alliance (ACTA). [Google Scholar]
- Australian Institute Of Aboriginal And Torres Strait Islander Studies (AIATSIS) (2020) AIATSIS Code of Ethics for Aboriginal and Torres Strait Islander Research. Canberra, Australia: Australian Institute Of Aboriginal And Torres Strait Islander Studies (AIATSIS). [Google Scholar]
- Australian Institute Of Health And Welfare (AIHW) (2020) Australia’s Health 2020. In: AIHW (ed) Australia’s Health Series Number 17. Canberra, Australia: Australian Institute Of Health And Welfare (AIHW). [Google Scholar]
- Bainbridge R, Tsey K, Mccalman J, et al. (2015) No one’s discussing the elephant in the room: contemplating questions of research impact and benefit in Aboriginal and Torres Strait Islander Australian health research. BMC Public Health 15: 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caren H, Balls-Berry JE, Dumbauld Nery J, et al. (2014) Strategies addressing barriers to clinical trial enrollment of underrepresented populations: a systematic review. Contemporary Clinical Trials 39: 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass A. (2018) Challenges and successes in clinical research with Aboriginal and Torres Strait Islander Australians [Conference presentation]. In: Australian Clinical Trials Alliance (ACTA) Summit 2018, Sydney, NSW, Australia. Available at: http://www.clinicaltrialsalliance.org.au/wp-content/uploads/2018/12/Alan-Cass.pdf. [Google Scholar]
- Chenowethm L, Jeon Y-H, Goff M, et al. (2006) Cultural competency and nursing care: an Australian perspective. International Nursing Review 53: 34–40. [DOI] [PubMed] [Google Scholar]
- Christy SM, Gwede CK, Sutton SK, et al. (2017) Health literacy among medically underserved: the role of demographic factors, social influence, and religious beliefs. Journal of Health Communication 22: 923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LT, Watkins L, Piña IL, et al. (2019) Increasing diversity in clinical trials: overcoming critical barriers. Current Problems in Cardiology 44: 148–172. [DOI] [PubMed] [Google Scholar]
- Clinicaltrials.gov . (2021). Covid-19 Studies From The World Health Organization Database [Online]. Available: Https://Clinicaltrials.Gov/Ct2/Who_Table (Accessed 19 February 2021). [Google Scholar]
- Cunningham J, Garvey G. (2020) Are there systematic barriers to participation in cancer treatment trials by Aboriginal and Torres Strait Islander cancer patients in Australia? Australia And New Zealand Journal Of Public Health 45: 39–45. [DOI] [PubMed] [Google Scholar]
- Centre for Epidemiology and Research, NSW Department of Health (2003) New South Wales Adult Health Survey 2002. NSW Public Health Bulletin 14: S–4. [Google Scholar]
- Department Of Health Medical Research Future Fund (MRFF) (2016) Australian Medical Research And Innovation Strategy 2016-2021. In: Australian Government, Department of Health. Canberra: Commonwealth of Australia. [Google Scholar]
- Forman J, Taruscio D, Llera VA, et al. (2012) The need for worldwide policy and action plans for rare diseases. Acta Paediatrica 101: 805–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs CL, Greaves A, Keeling M, et al. (2015) Clinical research benefits go viral via twitter. Nursing Times 111: 16–17. [PubMed] [Google Scholar]
- Herrera AP, Snipes SA, King DW, et al. (2010) Disparate inclusion of older adults in clinical trials: priorities and opportunities for policy and practice change. American Journal of Public Health 100 (Suppl 1): S105–S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughson J-A, Woodward-Kron R, Parker A, et al. (2016) A review of approaches to improve participation of culturally and linguistically diverse populations in clinical trials. Trials 17: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles-Smith H, et al On behalf of The Association of UK Lead Research Nurses (2020). How Research Nurses And Midwives Are Supporting Covid-19 Clinical Trials. Nursing Times [Online], 116, 20–22. [Google Scholar]
- International Association Of Clinical Research Nurses (IACRN) (2012) Enhancing Clinical Research Quality and Safety through Specialized Nursing Practice. Scope and Standards of Practice Committee Report. [Google Scholar]
- McCabe MA, Williams MJ, Browning SM, Vessey JA, Behrens L, Lyle S-A. (2016, March 21) The Clinical Research Nurse: A Global Perspective on Role, Value and Leadership [Conference presentation]. In: Sigma Theta Tau International, the Honor Society of Nursing, 43rd Biennial Convention, Las Vegas, Nevada, USA. Available at: https://sigma.nursingrepository.org/bitstream/handle/10755/603031/2_McCabe_M_p76946_1.pdf?sequence=1&isAllowed=y [Google Scholar]
- Li J-L. (2017) Cultural barriers lead to inequitable healthcare access for Aboriginal Australians and Torres Strait Islanders. Chinese Nursing Research 4: 207–210. [Google Scholar]
- Joseph G, Dohan D. (2009) Diversity of participants in clinical trials in an academic medical center: the role of the ‘good study patient? Cancer 115: 608–615. [DOI] [PubMed] [Google Scholar]
- Kane J, Graves B, Cook T, et al. (2016) Research model places volunteers at centre. Nursing Times (Online) 112: 1–4. [Google Scholar]
- Kim ES, Bruinooge SS, Roberts S, et al. (2017) Broadening eligibility criteria to make clinical trials more representative: American society of clinical oncology and friends of cancer research joint research statement. Journal Of Clinical Oncology 35: 3737–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Laba TL, Massi L, et al. (2015) Facilitators and barriers to implementation of a pragmatic clinical trial in Aboriginal health services. The Medical Journal of Australia 203: 24–27. [DOI] [PubMed] [Google Scholar]
- Livaudais-Toman J, Burke NJ, Napoles A, et al. (2014) Health literate organizations: are clinical trial sites equipped to recruit minority and limited health literacy patients? Journal Of Health Disparities Research And Practice 7: 1–13. [PMC free article] [PubMed] [Google Scholar]
- Low LF, Barcenilla‐Wong AL, Brijnath B. (2019) including ethnic and cultural diversity in dementia research. The Medical Journal Of Australia [Online] 211: 345–346. Available: Https://Www.Mja.Com.Au/Journal/2019/211/8/Including-Ethnic-And-Cultural-Diversity-Dementia-Research [DOI] [PubMed] [Google Scholar]
- Maguire H. (2017) Using on-call research nurses to boost recruitment to clinical trials. Nursing Times (Online) 113: 57. [Google Scholar]
- Marley JV, Kitaura T, Atkinson D, et al. (2014) Clinical trials in a remote Aboriginal setting: lessons from the boab smoking cessation study. BMC Public Health 14: 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxton F, Darbyshire P, Thompson DR. (2020) Research nurses rising to the challenges of covid-19. Journal Of Clinical Nursing 10: e13–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health And Medical Research Council (NHMRC) (2020) NHMRC Public Consultation | National Statement on Ethical Conduct in Human Research Sections 4 and 5. [Online]. Australia: National Health And Medical Research Council (NHMRC). Available: Https://Online.Nhmrc.Gov.Au/Public-Consultation/National-Statement-Ethical-Conduct-Human-Research-Sections-4-And-5 (Accessed 04 January 2021) [Google Scholar]
- National Institute For Health (NIH) (2017) NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects Clinical Research [Online]. National Institute For Health (NIH). Available: Https://Grants.Nih.Gov/Policy/Inclusion/Women-And-Minorities/Guidelines.Htm#:∼:Text=The%20nih%20revitalization%20act%20of,And%20minorities%20in%20clinical%20research.&Text=The%20statute%20includes%20a%20specific,And%2c%20in%20particular%20clinical%20trial (accessed 04 January 2021) [Google Scholar]
- National Institute For Health Research (NIHR) (2020. a) Ensuring Ethnic Diversity in Covid-19 Research. National Institute For Health Research (NIHR). Available: .Https://Www.Nihr.Ac.Uk/Blog/Ensuring-Ethnic-Diversity-In-Covid-19-Research/25160?Pr= (Accessed 04 January 2021) [Google Scholar]
- National Institute For Health Research (NIHR) . 2020. b. Improving Inclusion Of Under-Served Groups In Clinical Research: Guidance From Include Project. National Institute For Health Research (NIHR). Available: Https://Www.Nihr.Ac.Uk/Documents/Improving-Inclusion-Of-Under-Served-Groups-In-Clinical-Research-Guidance-From-Include-Project/25435?Pr= (Accessed 04 January 2021). [Google Scholar]
- Niranjan SJ, Martin MY, Fouad MN, et al. (2020) Bias and stereotyping among research and clinical professionals: perspectives on minority recruitment for oncology clinical trials. Cancer 126: 1958–1968. [DOI] [PubMed] [Google Scholar]
- Pick A, Liu A, Drew VL, et al. (2010) Getting started in clinical research: the role of the research nurse. Nursing Times [Online], 107 Online Edition. Available: Https://Www.Nursingtimes.Net/Roles/Nurse-Educators/The-Role-Of-The-Research-Nurse-26-04-2011/ [Google Scholar]
- Redwood S., Gill P. S. (2013) Under-Representation Of Minority Ethnic Groups In Research--Call For Action. The British Journal Of General Practice : The Journal Of The Royal College Of General Practitioners 63: 342–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotherham Doncaster and South Humber NHS Trust (RDaSH) (2021). Grounded Research. Available: Https://Www.Rdash.Nhs.Uk/About-Us/Grounded-Research/?Doing_Wp_Cron=1572518764.1655519008636474609375 (Accessed 04 January 2021). [Google Scholar]
- Rubin SL. (2014) The clinical trials nurse as subject advocate for minority and culturally diverse research subjects. Journal Of Transcultural Nursing 25: 383–387. [DOI] [PubMed] [Google Scholar]
- Sadler GR, Lantz JM, Fullerton JT, et al. (1999) Nurses’ unique roles in randomized clinical trials. Journal Of Professional Nursing 15: 106–115. [DOI] [PubMed] [Google Scholar]
- Sardar MR, Badri M, Prince CT, et al. (2014) Underrepresentation of women, elderly patients, and racial minorities in the randomized trials used for cardiovascular guidelines. Jama Intern Med 174: 1868–1870. [DOI] [PubMed] [Google Scholar]
- Sheikh A, Halani L, Bhopal R, et al. (2009) Facilitating the recruitment of minority ethnic people into research: qualitative case study of South Asians and asthma. Plos Medicine 6: e1000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornicroft G, Betts V. (2002) International Mid-term Review. Of The Second National Mental Health Plan For Australia [Online]. Canberra, Australia: Mental Health And Special Programs Branch, Department Of Health And Ageing. Available: Https://Www1.Health.Gov.Au/Internet/Publications/Publishing.Nsf/Content/Mental-Pubs-I-Midrev2-Toc (Accessed 2021) [Google Scholar]
- Treweek S, Banister K, Bower P, et al. (2021) Developing the include ethnicity framework—a tool to help trialists design trials that better reflect the communities they serve. Trials 22: 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treweek S, Forouhi NG, Narayan KMV, et al. (2020) Covid-19 and ethnicity: who will research results apply to? Lancet 395: 1955–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department Of Health And Human Services Food And Drug Administration (FDA) (2019) 2019 Drug Trials Snapshots Summary Report. Silver Spring, MD: U.S. Food And Drug Administration (FDA). [Google Scholar]
- U.S. Department Of Health And Human Services Food And Drug Administration (FDA) (2020) Enhancing the Diversity of Clinical Trial Populations — Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry. Silver Spring, MD: U.S. Department Of Health And Human Services Food And Drug Administration (FDA). [Google Scholar]
- Wagner JK. (2019) Ethical and legal considerations for the inclusion of underserved and underrepresented immigrant populations in precision health and genomic research in the United States. Ethnicity And Disease 29: 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallington SF, Dash C, Sheppard VB, et al. (2016) Enrolling minority and underserved populations in cancer clinical research. American Journal Of Preventative Medicine 50: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]