Abstract
Purpose
Nutritional problems after gastrectomy affect continuation of postoperative chemotherapy. There have been no studies limited to total gastrectomy, which is particularly prone to nutritional problems. In this study, we aimed to investigate the factors that predict the continuation of postoperative chemotherapy.
Materials and Methods
We included 101 patients who underwent curative total gastrectomy and postoperative chemotherapy at Hiroshima Memorial Hospital. The effects of 37 factors, including perioperative inflammatory, nutritional, and tumor status, on the persistence of postoperative chemotherapy were analyzed.
Results
In univariate analysis of preoperative factors, age, carbohydrate antigen 19-9, platelet-to-neutrophil ratio, Onodera's prognostic nutritional index (PNI), controlling nutritional status score, and nutritional risk screening (NRS-2002) score were significantly associated with the duration of postoperative chemotherapy. In multivariate analysis of preoperative factors, age (≥74 years) was an independent factor for a shorter duration of postoperative chemotherapy (hazard ratio [HR], 5.24; 95% confidence interval [CI], 2.19–12.96; P<0.01). In univariate analysis of factors before postoperative chemotherapy, intraoperative blood loss, perioperative weight loss rate, postoperative performance status, PNI, albumin-to-bilirubin index, and NRS-2002 score were significantly associated with the duration of postoperative chemotherapy. In multivariate analysis of factors before postoperative therapy, age (≥74 years) (HR, 5.75; 95% CI, 1.90–19.49; P<0.01) and PNI (<39) (HR, 3.29; 95% CI, 1.26–8.56; P=0.02) were independent factors for a shorter duration of postoperative chemotherapy.
Conclusions
Age and PNI are useful predictors of postoperative chemotherapy intolerance after total gastrectomy and may determine the treatment strategy and timing of chemotherapy initiation.
Keywords: Gastric cancer, Total gastrectomy, Postoperative chemotherapy, Nutritional assessment, Prognostic nutritional index
INTRODUCTION
Gastric cancer is the third leading cause of cancer-related deaths globally and the fifth most frequently diagnosed cancer [1]. The gold-standard treatment for gastric cancer is surgical resection, and perioperative chemotherapy is the standard treatment for locally advanced gastric cancer in the western hemisphere [2,3,4], while postoperative chemotherapy is the standard in East Asia. Postoperative adjuvant chemotherapy is either S1 (a combination of tegafur, gimeracil, and oteracil) monotherapy, capecitabine or S1 combined with oxaliplatin (L-OHP), or S1 combined with docetaxel (DTX) [5,6,7].
However, post-gastrectomy patients often have nutritional problems, leading to chemotherapy intolerance and a poor prognosis. Approximately 10% of patients are reported to have a postoperative weight loss of 15% or more, which is a risk factor for patient tolerance to postoperative chemotherapy with S1 and leads to a poor prognosis [8,9]. In particular, patients who undergo total gastrectomy are prone to malnutrition, with an average weight loss of 13.8% [10]. Hence, nutritional status must be properly assessed before the initiation of postoperative chemotherapy.
We hypothesized that indicators of nutritional status in patients with gastric cancer after total gastrectomy are needed, which could be used to improve chemotherapy tolerance. Therefore, in this study, we aimed to investigate methods of assessing nutritional status to predict the continuation of postoperative chemotherapy after total gastrectomy. To the best of our knowledge, this is the first study to assess nutritional status to predict tolerance to postoperative chemotherapy limited to total gastrectomy.
MATERIALS AND METHODS
Study design
Patients who underwent curative total gastrectomy followed by postoperative chemotherapy for gastric cancer at Hiroshima Memorial Hospital, Hiroshima, Japan, from April 2005 to September 2019, were eligible for this study. Patients who experienced relapse during postoperative chemotherapy and patients with remnant gastric cancer were excluded from this study. Patients with Union for International Cancer Control stage IV disease were included if they had positive cytology or curatively resected distant metastases. This study was conducted in compliance with the ethical principles of the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board of Hiroshima Memorial Hospital (approval number, 20100601). Informed consent was obtained from the patients in the form of an opt-out on the website of the institution.
Postoperative chemotherapy and follow-up
Postoperative chemotherapy after total gastrectomy was mainly S1 monotherapy (S1 80 mg/day for body surface area [BSA] <1.25 m2, 100 mg/day for BSA of 1.25–1.5 m2 and 120 mg/day for BSA >1.5 m2). After 4 weeks of S1 treatment, no chemotherapy was administered for the next 2 weeks. This 6-week cycle was repeated for 1 year after surgery [5,11]. Patients with stage III and well-tolerated therapy received platinum (cisplatin [CDDP] 60 mg/m2 BSA or L-OHP 100 mg/m2 BSA) or DTX 40 mg/m2 BSA every 3 weeks for 6 months, in combination with an S1 2-week dose, 1-week rest regimen [7,12,13]. S1 monotherapy was then administered for 6 months. The treatment was continued for 1 year, for as long as possible, with adverse events restricted within acceptable limits by reducing the dose or changing to other drugs. If the adverse events were not controlled, chemotherapy was interrupted. Patients with a high risk of recurrence, such as those with stage IV disease, received continued treatment beyond 1 year after surgery. Blood tests and clinical findings were assessed at least every 3–6 weeks during the postoperative chemotherapy. Tumor marker tests and computed tomography were performed at 3–6-month intervals for at least 5 years after surgery or until recurrence.
Study parameters
The factors assessed were obtained from the results of assessing patients’ physical condition and blood tests. In addition, the following inflammatory and nutritional markers were evaluated from the obtained data: the controlling nutritional status (CONUT score, calculated from the total lymphocyte count [TLC]; serum albumin level; cholesterol level), the nutritional risk screening 2002 (NRS-2002 score, calculated from general condition, body mass index [BMI], weight loss, food intake over the last week, the patient’s age), the Onodera’s prognostic nutritional index (PNI = 10 × albumin g/dL + 0.005 × TLC/mm3), the modified Glasgow prognostic score (calculated from serum C-reactive protein) level and serum albumin level), the lymphocyte-to-monocyte ratio (LMR = TLC/monocyte count), the neutrophil-to-lymphocyte ratio (neutrophil count/TLC), the platelet-to-lymphocyte ratio (TLC/platelet count × 100), the platelet-to-neutrophil ratio (PNR = neutrophil count/platelet count × 100), and the systemic inflammation score (calculated from serum albumin level and LMR) [14,15].
Statistical analysis
The association between the parameters and discontinuation of postoperative chemotherapy within 6 months was analyzed, and the cutoff values for continuous variables were calculated using receiver operating characteristic (ROC) curve analysis [16]. Univariate and multivariate analyses were performed using a Cox proportional hazards model to detect risk factors for a shorter duration of postoperative chemotherapy. The multivariate analysis was performed for the factors that were significantly different in the univariate analysis. Statistical analyses were performed using JMP statistical software (version 12; SAS Institute, Cary, NC, USA).
RESULTS
Patient demographics and tumor characteristics
Clinicopathological characteristics of the 101 patients included in this study are shown in Table 1. Fifteen patients (15%) were pathologically classified as stage IV: 10 (10%) were positive for ascites cytology, 6 (6%) were positive for peritoneal dissemination, and 1 (1%) was positive for liver metastasis. These patients underwent curative resection. Postoperative chemotherapy was initiated with S1 in 78 patients (77%), S1 + DTX in 9 patients (9%), S1 + L-OHP in 7 patients (7%), S1 + CDDP in 3 patients (3%), and combination of tegafur and uracil + leucovorin in 4 patients (4%). These treatments were switched to other agents if adverse events made it difficult to continue, with the aim of maintaining treatment for 1 year or as long as possible. The median follow-up period was 42 (range 7.6–174.2) months.
Table 1. Background characteristics of the patient (n=101).
| Variables | Value | |
|---|---|---|
| Age (yr) | 67 (33–88) | |
| Sex | ||
| Male | 68 (67) | |
| Female | 33 (33) | |
| Preoperative symptoms | ||
| Yes | 73 (72) | |
| No | 28 (28) | |
| Preoperative performance status | ||
| 0 | 53 (52) | |
| 1 | 48 (48) | |
| ASA physical status | ||
| 1 | 28 (28) | |
| 2 | 63 (62) | |
| 3 | 10 (10) | |
| Preoperative body mass index | 21.7±3.2 | |
| Preoperative chemotherapy | ||
| Yes | 12 (12) | |
| No | 89 (88) | |
| Tumor differentiation | ||
| Differentiated | 35 (35) | |
| Undifferentiated | 66 (65) | |
| Pathological T factor | ||
| T1 | 1 (1) | |
| T2 | 8 (8) | |
| T3 | 51 (50) | |
| T4a | 35 (35) | |
| T4b | 6 (6) | |
| Pathological N factor | ||
| N 0,1 | 43 (43) | |
| N 2,3 | 58 (57) | |
| Site of distant metastasis | ||
| Positive ascites cytology | 10 (10) | |
| Liver metastasis | 1 (1) | |
| Peritoneal dissemination | 6 (6) | |
| Pathological stage | ||
| I | 3 (3) | |
| II | 35 (35) | |
| III | 48 (47) | |
| IV | 15 (15) | |
| Postoperative complications (The Clavien-Dindo classification) | ||
| Grade II | 8 (8) | |
| Grade III | 17 (17) | |
| Initial selection of adjuvant chemotherapy | ||
| S1 | 78 (77) | |
| S1 + L-OHP | 7 (7) | |
| S1 + DTX | 9 (9) | |
| S1 + CDDP | 3 (3) | |
| UFT + LV | 4 (4) | |
| Duration of postoperative chemotherapy | ||
| <6 mon | 20 (20) | |
| ≥6 mon | 81 (80) | |
Values are presented as median (range), number (%) or mean ± standard deviation.
ASA = American Society of Anesthesiologists; L-OHP = oxaliplatin; DTX = docetaxel; CDDP = cisplatin; UFT = combination of tegafur and uracil; LV = leucovorin.
Compliance with postoperative chemotherapy
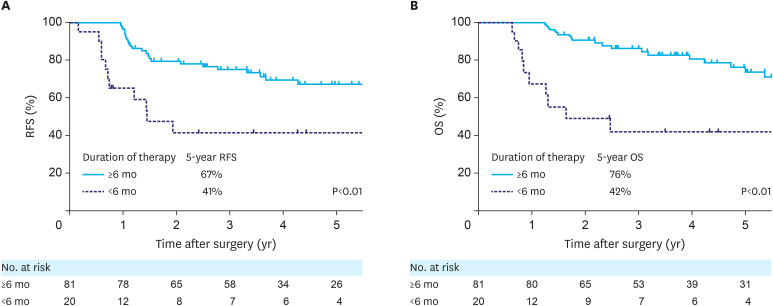
Of the 101 patients, 20 (20%) discontinued postoperative chemotherapy within 6 months. The reasons for discontinuation were anorexia in 17 patients (17%), diarrhea in 3 patients (3%), fatigue in 2 patients (2%), and neutropenia in 1 patient (1%). These patients were significantly associated with poor recurrence-free survival (RFS hazard ratio [HR], 3.09; 95% confidence interval [CI], 1.44–6.21; P<0.01, Fig. 1A) and overall survival (HR, 4.28; 95% CI, 1.88–9.25; P<0.01, Fig. 1B).
Fig. 1. Survival curves stratified by duration of postoperative chemotherapy: (A) RFS curves, (B) OS curves.
RFS = recurrence-free survival; OS = overall survival.
Analysis of risk factors for discontinuation of postoperative chemotherapy
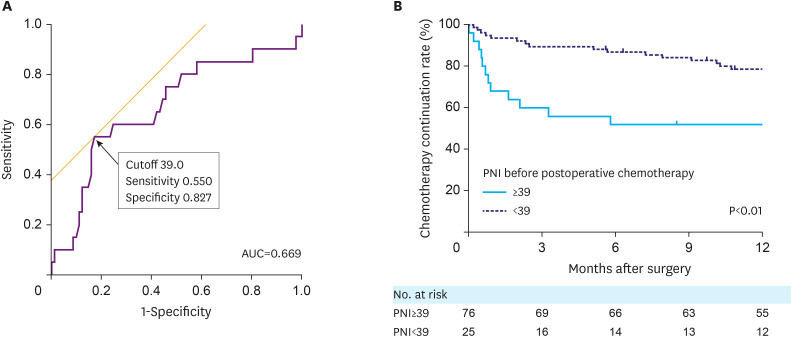
Cut-off values for each parameter were determined using ROC curve analysis, which analyzed the association of the parameters with the discontinuation of postoperative chemotherapy within a 6-month period. The relationship between each parameter and the duration of postoperative chemotherapy was analyzed using the Cox proportional hazards model. In univariate analysis of the preoperative factors, age (P<0.01), carbohydrate antigen 19-9 (P=0.02), CONUT score (P=0.01), NRS-2002 score (P=0.03), PNI (P=0.02), and PNR (P=0.02) were significantly associated with the duration of postoperative chemotherapy (Table 2). Multivariate analysis of the preoperative factors revealed that age was an independent predictor of shorter duration of postoperative chemotherapy (HR, 5.24; 95% CI, 2.19–12.96; P<0.01). Univariate and multivariate analyses of the factors before postoperative chemotherapy are shown in Table 3. In the univariate analysis, age (P<0.01), intraoperative bleeding (P=0.049), perioperative body weight loss (P=0.02), Eastern Cooperative Oncology Group performance status (P<0.01), albumin-to-bilirubin index (ALBI, P=0.03), NRS-2002 (P<0.01), and PNI (P<0.01) were significantly associated with the duration of postoperative chemotherapy. In the multivariate analysis, age (HR, 5.75; 95% CI, 1.90–19.49; P<0.01) and lower PNI (HR, 3.29; 95% CI, 1.26–8.56; P=0.02) were independent predictors of a shorter duration of postoperative chemotherapy (Table 3). The ROC curve analysis of the association between the PNI before postoperative chemotherapy and discontinuation of adjuvant chemotherapy within 6 months is shown in Fig. 2A. The Kaplan-Meier curves of the duration of postoperative chemotherapy by stratification of PNI before postoperative chemotherapy are shown in Fig. 2B.
Table 2. Univariate and multivariate Cox proportional hazards analysis of preoperative factors for duration of postoperative chemotherapy.
| Variables | Cutoff | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| Age | <74 | 1.00 | |||||
| ≥74 | 6.79 | 3.16–15.44 | <0.01 | 5.24 | 2.19–12.96 | <0.01 | |
| Sex | Male | 1.00 | |||||
| Female | 1.28 | 0.57–2.72 | 0.54 | ||||
| BMI | <20.8 | 1.29 | 0.59–2.72 | 0.51 | |||
| ≥20.8 | 1.00 | ||||||
| Preoperative symptoms | Yes | 1.02 | 0.47–2.48 | 0.95 | |||
| No | 1.00 | ||||||
| Diabetes mellitus | Yes | 1.00 | |||||
| No | 1.32 | 0.51–4.50 | 0.59 | ||||
| Cardiovascular comorbidities | Yes | 1.91 | 0.90–4.07 | 0.09 | |||
| No | 1.00 | ||||||
| Respiratory comorbidities | Yes | 1.87 | 0.78–4.10 | 0.15 | |||
| No | 1.00 | ||||||
| Cerebrovascular disease | Yes | 2.02 | 0.48–5.77 | 0.29 | |||
| No | 1.00 | ||||||
| PS | 0 | 1.00 | |||||
| 1 | 2.10 | 0.99–4.62 | 0.05 | ||||
| ASA physical status | 1 | 1.00 | |||||
| 2,3 | 1.53 | 0.66–4.15 | 0.34 | ||||
| Neoadjuvant chemotherapy | Yes | 1.00 | |||||
| No | 4.12 | 0.88–73.38 | 0.08 | ||||
| CEA | <4.7 | 3.02 | 0.90–18.76 | 0.08 | |||
| ≥4.7 | 1.00 | ||||||
| CA19-9 | <9.8 | 2.56 | 1.19–5.96 | 0.02 | 1.96 | 0.89–4.66 | 0.10 |
| ≥9.8 | 1.00 | ||||||
| Total protein | <6.2 | 1.33 | 0.45–3.24 | 0.57 | |||
| ≥6.2 | 1.00 | ||||||
| Albumin | <4.2 | 1.83 | 0.85–4.25 | 0.12 | |||
| ≥4.2 | 1.00 | ||||||
| Total bilirubin | <0.54 | 1.35 | 0.63–2.85 | 0.43 | |||
| ≥0.54 | 1.00 | ||||||
| ALBI | <−0.37 | 2.93 | 0.87–18.16 | 0.09 | |||
| ≥−0.37 | 1.00 | ||||||
| CONUT score | 0–2 | 1.00 | |||||
| 3–7 | 2.65 | 1.22–5.59 | 0.01 | 1.50 | 0.55–4.16 | 0.43 | |
| NRS-2002 | 2, 3 | 1.00 | |||||
| 4, 5, 6 | 2.31 | 1.10–4.95 | 0.03 | 1.15 | 0.49–2.73 | 0.74 | |
| Onodera’s PNI | <48.3 | 2.38 | 1.13–5.25 | 0.02 | 1.47 | 0.57–3.74 | 0.42 |
| ≥48.3 | 1.00 | ||||||
| LMR | <3.7 | 1.31 | 0.61–2.75 | 0.48 | |||
| ≥3.7 | 1.00 | ||||||
| NLR | <1.9 | 1.00 | |||||
| ≥1.9 | 2.02 | 0.87–5.49 | 0.10 | ||||
| PLR | <157 | 1.00 | |||||
| ≥157 | 2.02 | 0.94–4.69 | 0.07 | ||||
| PNR | <1.00 | 1.00 | |||||
| ≥1.00 | 3.97 | 1.19–24.63 | 0.02 | 2.76 | 0.77–17.60 | 0.13 | |
| SIS | 0, 1 | 1.00 | |||||
| 2 | 1.98 | 0.88–4.23 | 0.10 | ||||
HR = hazard ratio; CI = confidence interval; ASA = American Society of Anesthesiologists; BMI = body mass index; PS = performance status; CEA = carcinoembryonic antigen; CA19-9 = carbohydrate antigen 19-9; ALBI = albumin-to-bilirubin index; CONUT = controlling nutritional status; NRS = nutritional risk screening; PNI = prognostic nutritional index; LMR = lymphocyte-to-monocyte ratio; NLR = neutrophil-to-lymphocyte ratio; PLR = platelet-to-lymphocyte ratio; PNR = platelet-to-neutrophil ratio; SIS = systemic inflammation score.
Table 3. Univariate and multivariate Cox proportional hazards analysis of factors before postoperative chemotherapy for duration of postoperative chemotherapy.
| Variables | Cutoff | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| Age | <74 | 1.00 | |||||
| ≥74 | 6.79 | 3.16–15.44 | <0.01 | 5.75 | 1.90–19.49 | <0.01 | |
| Sex | Male | 1.00 | |||||
| Female | 1.28 | 0.57–2.72 | 0.54 | ||||
| Intraoperative bleeding | <200 | 1.00 | |||||
| ≥200 | 2.74 | 1.01–9.59 | 0.04 | 1.61 | 0.51–6.21 | 0.43 | |
| Operative time | <186 | 1.87 | 0.63–4.55 | 0.23 | |||
| ≥186 | 1.00 | ||||||
| Splenectomy | Yes | 1.13 | 0.52–3.39 | 0.75 | |||
| No | 1.00 | ||||||
| Tumor differentiation | Differentiated | 1.00 | |||||
| Undifferentiated | 1.09 | 0.51–2.54 | 0.83 | ||||
| Pathological T factor | T1, T2 | 1.00 | |||||
| T3, T4 | 1.33 | 0.40–8.28 | 0.69 | ||||
| Pathological N factor | N0, N1 | 1.36 | 0.64–2.88 | 0.41 | |||
| N2, N3 | 1.00 | ||||||
| Pathological stage | I, II | 1.06 | 0.48–2.23 | 0.89 | |||
| III, IV | 1.00 | ||||||
| Postoperative complications (The Clavien-Dindo classification) | Grade 0–II | 1.00 | |||||
| Grade III | 1.34 | 0.49–3.11 | 0.53 | ||||
| BMI | <17.7 | 1.80 | 0.77–3.87 | 0.16 | |||
| ≥17.7 | 1.00 | ||||||
| Perioperative body weight loss | <9.6% | 1.00 | |||||
| ≥9.6% | 2.53 | 1.20–5.40 | 0.02 | 1.18 | 0.44–3.10 | 0.73 | |
| PS | 0, 1 | 1.00 | |||||
| 2 | 6.90 | 1.97–18.94 | <0.01 | 2.34 | 0.57–8.07 | 0.22 | |
| Total protein | <5.7 | 1.73 | 0.68–3.89 | 0.23 | |||
| ≥5.7 | 1.00 | ||||||
| Albumin | <3.3 | 1.87 | 0.86–3.93 | 0.11 | |||
| ≥3.3 | 1.00 | ||||||
| Total bilirubin | <0.46 | 1.07 | 0.50–2.42 | 0.86 | |||
| ≥0.46 | 1.00 | ||||||
| ALBI | <−0.67 | 1.00 | |||||
| ≥−0.67 | 3.24 | 1.14–13.60 | 0.03 | 3.24 | 0.93–15.4 | 0.07 | |
| CONUT score | 0–4 | 1.00 | |||||
| 5–10 | 1.79 | 0.77–3.85 | 0.17 | ||||
| NRS-2002 | 0–3 | 1.00 | |||||
| 4, 5 | 3.38 | 1.54–8.17 | <0.01 | 1.43 | 0.34–6.52 | 0.62 | |
| Onodera’s PNI | <39.0 | 3.06 | 1.41–6.45 | <0.01 | 3.29 | 1.26–8.56 | 0.02 |
| ≥39.0 | 1.00 | ||||||
| CRP | <0.67 | 1.00 | |||||
| ≥0.67 | 2.07 | 0.97–4.35 | 0.06 | ||||
| mGPS | 0 | 1.00 | |||||
| 1, 2 | 1.67 | 0.74–4.23 | 0.22 | ||||
| LMR | <2.6 | 1.98 | 0.82–4.34 | 0.12 | |||
| ≥2.6 | 1.00 | ||||||
| NLR | <1.95 | 1.00 | |||||
| ≥1.95 | 1.20 | 0.57–2.65 | 0.63 | ||||
| PLR | <169 | 1.00 | |||||
| ≥169 | 1.27 | 0.55–3.46 | 0.59 | ||||
| PNR | <0.88 | 1.00 | |||||
| ≥0.88 | 1.87 | 0.85–4.51 | 0.12 | ||||
| SIS | 0, 1 | 1.00 | |||||
| 2 | 1.02 | 0.49–2.21 | 0.95 | ||||
HR = hazard ratio; CI = confidence interval; BMI = body mass index; PS = performance status; ALBI = albumin-to-bilirubin index; CONUT = controlling nutritional status; NRS = nutritional risk screening; PNI = prognostic nutritional index; CRP = C-reactive protein; mGPS = modified Glasgow prognostic score; LMR = lymphocyte-to-monocyte ratio; NLR = neutrophil-to-lymphocyte ratio; PLR = platelet-to-lymphocyte ratio; PNR = platelet-to-neutrophil ratio; SIS = systemic inflammation score.
Fig. 2. (A) ROC curve analysis of the relationship between Onodera’s PNI level before postoperative chemotherapy and discontinuation of postoperative chemotherapy within 6 months. (B) The Kaplan-Meier curves of duration of postoperative chemotherapy stratified by PNI level before postoperative chemotherapy.
ROC = receiver operating characteristic; PNI = prognostic nutritional index; AUC = area under the receiver operating characteristic curve.
DISCUSSION
Many factors may affect a patient’s tolerability of chemotherapy, including physical strength, organ function, nutritional status, inflammatory status, mental status, allergic constitution, abnormalities in drug-metabolizing enzymes, genetic predisposition, and living environment. Among these factors, nutritional status has a significant impact on patients with gastric cancer after total gastrectomy. The current study investigated indicators of nutritional status as predictors of tolerability to postoperative chemotherapy following total gastrectomy in gastric cancer patients. To eliminate the effect of cancer recurrence on the tolerability of postoperative chemotherapy and to focus on the effect of nutritional impairment after total gastrectomy, this study included only patients who discontinued treatment owing to adverse events and excluded patients who discontinued owing to recurrence. Age and PNI before postoperative chemotherapy were independent predictors of chemotherapy duration.
Body weight loss after gastrectomy and PNI have been shown to affect patient adherence to postoperative chemotherapy [8,17]. However, no reports have shown a correlation between nutritional status and duration of chemotherapy. Age, postoperative infectious complications, preoperative ALBI, and TNM stage have been reported as risk factors for compliance with postoperative chemotherapy after gastric cancer surgery [16,18,19]. Iizuka et al. [15] reported a scoring scale to estimate the risk of postoperative chemotherapy discontinuation using age, preoperative urea nitrogen level, and ALBI. These reports did not show a predominant correlation between nutritional status and tolerability of postoperative chemotherapy. In the current study, only total gastrectomy, in which nutritional disturbance is more pronounced, was analyzed and it showed a significant difference. Moreover, the data were collected prior to postoperative chemotherapy, not gastrectomy, and this may have had more direct effects on chemotherapy tolerability. To the best of our knowledge, the current study is the first to analyze various nutritional indicators after total gastrectomy and identify the optimal indicators.
Onodera et al. [20] proposed the PNI as a predictor of complications after gastrointestinal cancer surgery. This index was easily obtained using the serum albumin level and lymphocyte count. Subsequently, correlations between PNI and postoperative prognosis, prognosis after chemotherapy, and toxicity of chemotherapy have been reported in cancers of various organs, indicating the necessity of appropriate nutritional management in different aspects of cancer treatment [21,22,23,24,25,26,27,28,29,30,31,32,33]. The present study also demonstrated the importance of nutritional management for continuation of chemotherapy after total gastrectomy.
This study had some limitations. First, it was a single-institutional retrospective cohort study. Second, the dataset included data on several postoperative chemotherapeutic agents. However, the study did not need to be limited to a single chemotherapy regimen. If adverse events make it difficult to continue chemotherapy, other drugs should be substituted to continue the treatment. We believe that it is important to continue chemotherapy for as long as possible, using drugs that have acceptably limited adverse effects for each patient. In the current study, 80% of patients were able to continue postoperative chemotherapy for more than 6 months, which is better than the results of previous clinical trials [5]. Further prospective studies with larger numbers of patients are required.
In conclusion, age and PNI were associated with the continuation of postoperative chemotherapy in patients with gastric cancer after total gastrectomy. Nutritional management during chemotherapy is important, and PNI can be used to assess nutritional status. Further follow-up studies are required to confirm this finding.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: T.K.
- Data curation: M.M., H.S., Y.S., K.H.
- Formal analysis: T.K.
- Investigation: T.K.
- Methodology: H.Y.
- Project administration: S.Y.
- Supervision: Y.Y., M.Y., M.K.
- Validation: Y.R., K.H.
- Visualization: T.K.
- Writing - original draft: Y.K.
- Writing - review & editing: T.K.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 3.Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210–5218. doi: 10.1200/JCO.2009.26.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 5.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 6.Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida K, Kodera Y, Kochi M, Ichikawa W, Kakeji Y, Sano T, et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. J Clin Oncol. 2019;37:1296–1304. doi: 10.1200/JCO.18.01138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoyama T, Yoshikawa T, Shirai J, Hayashi T, Yamada T, Tsuchida K, et al. Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2013;20:2000–2006. doi: 10.1245/s10434-012-2776-6. [DOI] [PubMed] [Google Scholar]
- 9.Aoyama T, Sato T, Maezawa Y, Kano K, Hayashi T, Yamada T, et al. Postoperative weight loss leads to poor survival through poor S-1 efficacy in patients with stage II/III gastric cancer. Int J Clin Oncol. 2017;22:476–483. doi: 10.1007/s10147-017-1089-y. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi M, Terashima M, Kawahira H, Nagai E, Uenosono Y, Kinami S, et al. Quality of life after total vs distal gastrectomy with Roux-en-Y reconstruction: use of the Postgastrectomy Syndrome Assessment Scale-45. World J Gastroenterol. 2017;23:2068–2076. doi: 10.3748/wjg.v23.i11.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–4393. doi: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]
- 12.Takahari D, Hamaguchi T, Yoshimura K, Katai H, Ito S, Fuse N, et al. Feasibility study of adjuvant chemotherapy with S-1 plus cisplatin for gastric cancer. Cancer Chemother Pharmacol. 2011;67:1423–1428. doi: 10.1007/s00280-010-1432-8. [DOI] [PubMed] [Google Scholar]
- 13.Shitara K, Chin K, Yoshikawa T, Katai H, Terashima M, Ito S, et al. Phase II study of adjuvant chemotherapy of S-1 plus oxaliplatin for patients with stage III gastric cancer after D2 gastrectomy. Gastric Cancer. 2017;20:175–181. doi: 10.1007/s10120-015-0581-1. [DOI] [PubMed] [Google Scholar]
- 14.Inaoka K, Kanda M, Uda H, Tanaka Y, Tanaka C, Kobayashi D, et al. Clinical utility of the platelet-lymphocyte ratio as a predictor of postoperative complications after radical gastrectomy for clinical T2-4 gastric cancer. World J Gastroenterol. 2017;23:2519–2526. doi: 10.3748/wjg.v23.i14.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iizuka A, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, et al. Proposal of a scoring scale to estimate risk of the discontinuation of S-1 adjuvant monotherapy in patients with stage II to III gastric cancer: a multi-institutional dataset analysis. World J Surg. 2019;43:2016–2024. doi: 10.1007/s00268-019-04942-y. [DOI] [PubMed] [Google Scholar]
- 16.Miwa T, Kanda M, Tanaka C, Kobayashi D, Hayashi M, Yamada S, et al. Albumin-bilirubin score predicts tolerability to adjuvant S-1 monotherapy after curative gastrectomy. J Gastric Cancer. 2019;19:183–192. doi: 10.5230/jgc.2019.19.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao H, Zhou H, Zhang P, Xiao H, Liu K, Chen X, et al. Association among the prognostic nutritional index, completion of adjuvant chemotherapy, and cancer-specific survival after curative resection of stage II/III gastric cancer. Eur J Clin Nutr. 2020;74:555–564. doi: 10.1038/s41430-019-0502-1. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita K, Kurokawa Y, Yamamoto K, Hirota M, Kawabata R, Mikami J, et al. Risk factors for poor compliance with adjuvant S-1 chemotherapy for gastric cancer: a multicenter retrospective study. Ann Surg Oncol. 2017;24:2639–2645. doi: 10.1245/s10434-017-5923-2. [DOI] [PubMed] [Google Scholar]
- 19.Jang SH, Jung YJ, Kim MG, Kwon SJ. The prognostic significance of compliance with postoperative adjuvant chemotherapy in patients with stage III gastric cancer: an observational study. J Gastric Cancer. 2018;18:48–57. doi: 10.5230/jgc.2018.18.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nippon Geka Gakkai Zasshi. 1984;85:1001–1005. [PubMed] [Google Scholar]
- 21.Sakurai K, Ohira M, Tamura T, Toyokawa T, Amano R, Kubo N, et al. Predictive potential of preoperative nutritional status in long-term outcome projections for patients with gastric cancer. Ann Surg Oncol. 2016;23:525–533. doi: 10.1245/s10434-015-4814-7. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto H, Okamoto Y, Kawai A, Ueno D, Kubota H, Murakami H, et al. Prognosis prediction for postoperative esophageal cancer patients using Onodera’s prognostic nutritional index. Nutr Cancer. 2017;69:849–854. doi: 10.1080/01635581.2017.1339093. [DOI] [PubMed] [Google Scholar]
- 23.Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268–274. doi: 10.1002/bjs.7305. [DOI] [PubMed] [Google Scholar]
- 24.Tanemura A, Mizuno S, Hayasaki A, Gyoten K, Fujii T, Iizawa Y, et al. Onodera’s prognostic nutritional index is a strong prognostic indicator for patients with hepatocellular carcinoma after initial hepatectomy, especially patients with preserved liver function. BMC Surg. 2020;20:261. doi: 10.1186/s12893-020-00917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broggi MS, Patil D, Baum Y, Nieh PT, Alemozaffar M, Pattaras JG, et al. Onodera’s prognostic nutritional index as an independent prognostic factor in clear cell renal cell carcinoma. Urology. 2016;96:99–105. doi: 10.1016/j.urology.2016.05.064. [DOI] [PubMed] [Google Scholar]
- 26.Yenibertiz D, Ozyurek BA, Erdogan Y. Is Onodera’s prognostic nutritional index (OPNI) a prognostic factor in small cell lung cancer (SCLC)? Clin Respir J. 2020;14:689–694. doi: 10.1111/crj.13185. [DOI] [PubMed] [Google Scholar]
- 27.Ihara K, Yamaguchi S, Shida Y, Fujita J, Matsudera S, Kikuchi M, et al. Nutritional status predicts adjuvant chemotherapy outcomes for stage III colorectal cancer. J Anus Rectum Colon. 2019;3:78–83. doi: 10.23922/jarc.2018-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sagawa M, Yoshimatsu K, Yokomizo H, Osawa G, Matsumoto A, Yano Y, et al. Assessment of host status in patients treated with mFOLFOX6 adjuvant chemotherapy after colorectal cancer surgery. Gan To Kagaku Ryoho. 2013;40:1587–1589. [PubMed] [Google Scholar]
- 29.Ihara K, Yamaguchi S, Shida Y, Ogata H, Domeki Y, Okamoto K, et al. Poor nutritional status before and during chemotherapy leads to worse prognosis in unresectable advanced or recurrent colorectal cancer. Int Surg. 2015 doi: 10.9738/INTSURG-D-15-00079.1. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 30.Bozkaya Y, Köstek O, Sakin A, Özyükseler DT, Şakalar T, Çil İ. Is the prognostic nutritional index a prognostic and predictive factor in metastatic non-small cell lung cancer patients treated with first-line chemotherapy? Support Care Cancer. 2020;28:2273–2282. doi: 10.1007/s00520-019-05055-x. [DOI] [PubMed] [Google Scholar]
- 31.Go SI, Jeon H, Park SW, Kang MH, Kim HG, Lee GW. Low pre-treatment nutritional index is significantly related to poor outcomes in small cell lung cancer. Thorac Cancer. 2018;9:1483–1491. doi: 10.1111/1759-7714.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto Y, Zhou Q, Kamimura K, Moriyama M, Saijo Y. The prognostic nutrition index predicts the development of hematological toxicities in and the prognosis of esophageal cancer patients treated with cisplatin plus 5-fluorouracil chemotherapy. Nutr Cancer. 2018;70:447–452. doi: 10.1080/01635581.2018.1445765. [DOI] [PubMed] [Google Scholar]
- 33.Kono T, Sakamoto K, Shinden S, Ogawa K. Pre-therapeutic nutritional assessment for predicting severe adverse events in patients with head and neck cancer treated by radiotherapy. Clin Nutr. 2017;36:1681–1685. doi: 10.1016/j.clnu.2016.10.021. [DOI] [PubMed] [Google Scholar]