Abstract
The effectiveness of ziracin (SCH27899), a novel everninomicin, was at first investigated against lethal pneumonia caused by a penicillin-susceptible Streptococcus pneumoniae strain. A single intravenous injection of ziracin at a dose of 60 mg/kg of body weight given at 18 h postinfection protected 100% mice and led to the complete clearance of bacteria from their lungs. The activity of ziracin was observed to be the same as that of ceftriaxone: the 50% protective doses (PD50s) of ziracin and ceftriaxone were 24.8 and 24.6 mg/kg, respectively. Evaluation of this therapy with leukopenic mice showed that a single injection of ziracin protected 75% of these mice. A delay in therapy with ziracin, which was initiated at 48 h postinfection with 30 mg/kg given once daily for 3 days, resulted in an 83% survival rate of immunocompetent mice. The efficacy of ziracin was further compared to that of vancomycin against lethal pneumonia caused by a penicillin-resistant S. pneumoniae strain in leukopenic mice. The PD50s of ziracin and vancomycin were 40.5 and 44.2 mg/kg, respectively. Treatment with ziracin at 30 mg/kg once daily for 2 days (initiated 18 h postinfection) yielded an 83% survival rate and achieved complete eradication of the bacteria. The results were the same as those obtained with vancomycin administered at 15 mg/kg twice daily for 2 days. It is notable that the high survival rates for mice treated with ziracin were associated with effective eradication of the bacteria and rapid recovery of pulmonary tissues from pneumonia. The pharmacokinetic properties of ziracin, ceftriaxone, and vancomycin were estimated following intravenous administration of a single dose of 30 mg/kg to immunocompetent mice. The half-life of ziracin was observed to be longer than those of ceftriaxone and vancomycin (2.3 h versus 1.0 and 0.36 h in the bloodstream and 3 h versus 1.9 and 0.45 h in lung tissues). The areas under the concentration-time curves (AUCs) in lung tissue for ziracin versus those for ceftriaxone and vancomycin were 36 μg · h/g versus 20 and 9.5 μg · h/g. The prolonged half-life and high AUC for ziracin in tissue contributed to its excellent in vivo activities.
Streptococcus pneumoniae remains the most common pathogen responsible for community-acquired pneumonia throughout the world. Moreover, over the past decade there has been a dramatic increase in the frequency of multiple-antibiotic resistance among pneumococci in most parts of the world (2, 3, 9). The emergence of increasing numbers of pathogens resistant to the widely used antibiotics seriously threatens the effectiveness of these agents as therapy for infections caused by pneumococci, and this problem requires a renewed effort to develop new antibacterial agents effective against bacterial pathogens resistant to current antibiotics. Ziracin (SCH27899), an everninomicin derivative produced from Micromonospora carbonacea, is a new oligosaccharide antibiotic (15). Ziracin has been demonstrated to inhibit the growth of most gram-positive bacteria including emerging problematic bacteria such as penicillin-resistant streptococci, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant enterococci (13, 15, 16). Ziracin may provide an alternative treatment for infections caused by gram-positive bacteria.
To date, the in vivo activities and pharmacokinetics of ziracin have not been reported in any of the published literature. The purposes of the present study were to investigate the pharmacokinetics of ziracin in mice, to evaluate the in vivo activity of ziracin against penicillin-susceptible Streptococcus pneumoniae (PSSP)-induced pneumonia in mice (and to compare its activity to that of ceftriaxone), and finally, to evaluate the activity of ziracin against penicillin-resistant S. pneumoniae (PRSP)-induced pneumonia (and to compare its activity to that of vancomycin).
(This work was presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy [E. Wang, Y. Bergeron, M. Simard, M. Côté-Richer, and M. G. Bergeron, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-40, p. 56, 1998].)
MATERIALS AND METHODS
Animals and antibiotics.
Female CD1 Swiss mice (weight, 18 to 20 g; Charles River, St-Constant, Quebec, Canada) were used throughout the study. The animals were adapted to standardized environmental conditions (21 to 23°C) for 1 week before the beginning of the experiments. The powder of ziracin (Schering-Plough Research Institute, Kenilworth, N.J.), vancomycin (Eli Lilly & Company, Indianapolis, Ind.), or ceftriaxone (Hoffmann-La Roche Limited, Mississauga, Ontario, Canada) was dissolved just before use. The agents were injected intravenously in a volume of 0.1 ml of diluent into the tail vein of the animals. Infected control animals received the same volume of saline.
MICs and MBCs.
The MICs and minimum bactericidal concentrations (MBCs) of ziracin, ceftriaxone, and vancomycin for clinical isolates of PSSP (type 3) and PRSP were determined in Mueller-Hinton broth with 5% sheep blood by a tube dilution technique. Each tube contained one of twofold dilutions of an antibiotic from 16 to 0.016 μg/ml and a final bacterial density of 5 × 105 CFU/ml. The MIC was defined as the lowest concentration of an antibiotic that resulted in no visual turbidity to the naked eye after aerobic incubation for 18 h at 37°C. The MBC was determined by plating 0.01-ml aliquots from tubes with no visible growth onto blood agar. The MBC was defined as the lowest antibiotic concentration that resulted in no colony growth after 24 h of incubation in 5% CO2–air at 37°C.
Pneumonia model.
As described by Bergeron et al. (1), bacteria were grown on blood agar for 18 h, and then freshly grown colonies were suspended in brain heart infusion broth supplemented with 5% horse serum overnight. Mice were inoculated by intranasal instillation of a 50-μl suspension containing 107 CFU of the PSSP or PRSP strain. Animals were held in a vertical position for 5 min to facilitate distal migration of the bacteria to the alveoli by gravity. The size of the inoculum was confirmed by serial dilution and quantitative subculture. Leukopenia in mice was induced by intraperitoneal injections of 150 mg of cyclophosphamide (Charte-Horner Inc., Mississauga, Ontario, Canada) per kg of body weight 3 consecutive days before and 1 day after bacterial challenge. Circulating leukocyte counts in the mice were reduced from 7,000 to about 1,000/mm3 of blood on the day of infection. PSSP pneumonia was studied in immunocompetent mice as well as in leukopenic mice. Since the PRSP strain is less pathogenic than the PSSP strain for the induction of pneumonia in immunocompetent mice and pneumonia induced by the PRSP strain can be established in leukopenic animals (11), the efficacy of ziracin against the PRSP strain was studied only with leukopenic mice.
Survival studies and determination of the PD50.
Cumulative survival rates (12 mice per group) were recorded daily over a 14-day period. The 50% protective dose (PD50) was defined as the single dose (from 10 to 70 mg per kg of body weight) given at 18 h postinfection that protected 50% of the mice.
Therapeutic regimens.
Four therapeutic regimens were compared: (i) a single injection of ziracin against PSSP pneumonia in immunocompetent mice that was given at 18 h postinfection at doses of either 30 or 60 mg/kg and that was compared with ceftriaxone at a dose of 60 mg/kg; (ii) a delayed treatment with ziracin against PSSP pneumonia in immunocompetent mice, starting at 48 h postinfection, as a single daily dose (q.d.) of either 10 or 30 mg/kg for 3 consecutive days; (iii) a single injection of ziracin against PSSP pneumonia in leukopenic mice that was initiated at 18 h postinfection at a dose of 60 mg/kg; and finally, (iv) a treatment with ziracin against PRSP pneumonia in leukopenic mice that began at 18 h postinfection with 30 mg/kg q.d. for 2 days and that was compared with vancomycin given at doses of 15 mg/kg twice daily (b.i.d.) for 2 days.
Assessment of efficacy in surviving mice.
The efficacy of ziracin in infected mice was assessed by determination of the level of bacterial clearance and histopathologic examination of the lungs. Six mice per group were killed with CO2 and were immediately exsanguinated by intracardiac puncture at each of the following time points: immediately before the initiation of therapy, as well as on days 3, 6, 8, 13, and 20 postinfection. Blood was used for bacterial cultures. Following blood collection, the lungs and heart of each animal were removed and weighed together. Blood was removed from the lungs through the infusion of 20 ml of sterile saline into the right ventricle until the effluent became clear. The heart was removed and weighed alone to calculate the exact weight of the lungs. The lungs were homogenized with a Potter-Elvehjem homogenizer in 2 ml of potassium phosphate buffer (50 mM pH 7.0) at 4°C. Among six mice in each group, five mice were used for the measurement of the bacterial clearance from lung tissues and blood. Values were expressed as the means ± standard deviations (SDs) for five mice. One mouse was used for histological examination. All results were compared to those for infected control mice until all control animals died.
Eradication of bacteria from blood and tissues.
Blood cultures were made by plating 10 μl of uncentrifuged blood onto blood agar. Negative blood cultures were defined as no colony growth after 18 h of incubation in 5% CO2–air at 37°C. To determine the bacterial counts in the lungs, serial 10-fold dilutions of uncentrifuged lung homogenates were plated onto blood agar. The plates were incubated for 18 h in 5% CO2–air at 37°C. The limit of detection for bacterial counts in lungs was 2 log10 CFU per lung.
Histopathologic examination.
The lungs were perfused with saline, fixed in formaldehyde, and embedded in paraffin. Paraffin sections were stained with hematoxylin and eosin. Micrographs were taken at a magnification of ×100.
Pharmacokinetic study.
The pharmacokinetics of ziracin, vancomycin, and ceftriaxone were investigated in immunocompetent mice following a single injection of the agents at 30 mg/kg of body weight. The pharmacokinetics of ziracin were studied simultaneously in normal and infected mice, with the latter receiving ziracin 18 h after the PSSP challenge. The pharmacokinetics of ceftriaxone and vancomycin were studied in normal mice only. At each time point of 5, 10, 30, and 45 min and 1, 2, 4, 8, and 24 h postdosing, four mice per group were exposed to CO2 and were immediately exsanguinated by cardiac puncture. Blood samples were centrifuged at 15,000 × g for 10 min, and the sera were frozen for further determination of drug levels. Lung tissues were obtained as described above. The lungs were homogenized with a Potter-Elvehjem homogenizer in 1 ml of potassium phosphate buffer (50 mM; pH 7.0) at 4°C. Tissues were centrifuged at 3,000 × g for 30 min, and the supernatants were used for the assay. Antibiotic concentrations were determined by an agar well bioassay with antibiotic medium no. 1 (Becton Dickinson) and by using S. aureus ATCC 6538P, Escherichia coli ATCC 39188, and Bacillus subtilis ATCC 6633 as the test organisms for ziracin, ceftriaxone, and vancomycin, respectively. For ziracin, the limit of detection of the bioassay was 0.025 μg/ml, the intraday coefficient of variation for the standard concentrations tested was <7.5%, and the interday coefficient of variation was <8.8%. The respective values for ceftriaxone were 0.05 μg/ml, <6.2%, and <6.7%. For vancomycin, these values were 0.25 μg/ml, <4.6%, and <8.9%, respectively. Each sample was assayed in triplicate. Results were expressed as arithmetical means of micrograms per milliliter of blood or per gram of lung tissue.
Pharmacokinetic analysis.
One- and two-compartment open models were examined by use of Akaike's information criterion to describe the serum concentration-time profiles of the agents studied (17). The elimination rate constant (k) was first estimated from the slope obtained by least-squares regression analysis for the apparently linear portion of the log concentration-versus-time curve. The elimination half-life was then calculated according to the formula ln 2/k. The area under the concentration-time curve (AUC) from time zero to 24 h was calculated by the trapezoidal rule. The penetration of the studied agents into lung tissue was determined as follows: (AUC for lung/AUC for serum). The total mean residence time was calculated as AUMC/AUC, where AUMC is the area under the first moment of the concentration-time curve.
Statistical analyses.
All statistical analyses were performed with StatView SE + Graphics (Abaccus Concepts Inc., Berkeley, Calif.). Statistical analysis of the difference between groups was performed by analysis of variance by a least-squares method. If the F test indicated a difference within groups (P < 0.05), group comparisons were performed by Fisher's protected least-significant-difference test, and a P value of <0.05 was considered significant. All data are presented as means ± SDs. The PD50 was estimated by a probit method.
RESULTS
In vitro activities.
The MICs and MBCs of ziracin, ceftriaxone, and vancomycin for the PSSP and PRSP strains are shown in Table 1. The MIC of ziracin was ≤0.016 μg/ml for both the PSSP and the PRSP strains. The MIC of ceftriaxone was the same as that of ziracin for the PSSP strain. The in vitro activity of ziracin was at least 32 times greater than that of vancomycin against the PRSP strain.
TABLE 1.
In vitro activities of the studied antibiotics against S. pneumoniae strains
| Antibiotic | MIC/MBC (μg/ml)
|
|
|---|---|---|
| Penicillin-susceptible strain | Penicillin-resistant strain | |
| Penicillin | 0.03/0.03 | ≥16/≥16 |
| Ceftriaxone | ≤0.016/0.03 | 0.12/0.12 |
| Vancomycin | 0.5/0.5 | 0.5/1 |
| Ziracin | ≤0.016/0.03 | ≤0.016/0.12 |
Efficacy of a single injection of ziracin in comparison with that of ceftriaxone against PSSP-induced pneumonia in immunocompetent mice.
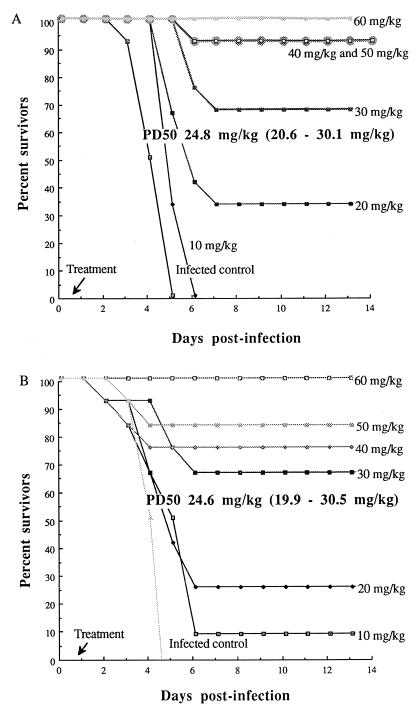
All infected mice exhibited signs of sickness: lethargy, decreased food intake, ruffled fur, and hunched appearance by 18 h postinfection. By day 2 postinfection, all of them developed progressive respiratory distress and septicemia. All infected control animals died by day 5 postinfection. Figure 1 shows the percent survival rates of mice treated with a single injection of ziracin or ceftriaxone given at various doses at 18 h postinfection. Ziracin at doses of 10, 20, 30, 40, 50, and 60 mg/kg protected 0, 33, 67, 92, 92, and 100% of the challenged mice, respectively, while ceftriaxone given at the corresponding doses protected 8, 25, 67, 75, 83, and 100% of the infected mice, respectively. The PD50s were identical for both ziracin and ceftriaxone (24.8 and 24.6 mg/kg, respectively). A clear dose-response relationship for ziracin could be observed at doses that varied from 20 to 60 mg/kg: higher doses of ziracin produced higher survival rates among infected mice.
FIG. 1.
Cumulative survival rates of immunocompetent mice infected with the PSSP strain (12 mice per group) after a single injection of ziracin (A) or ceftriaxone (B) initiated at 18 h postinfection.
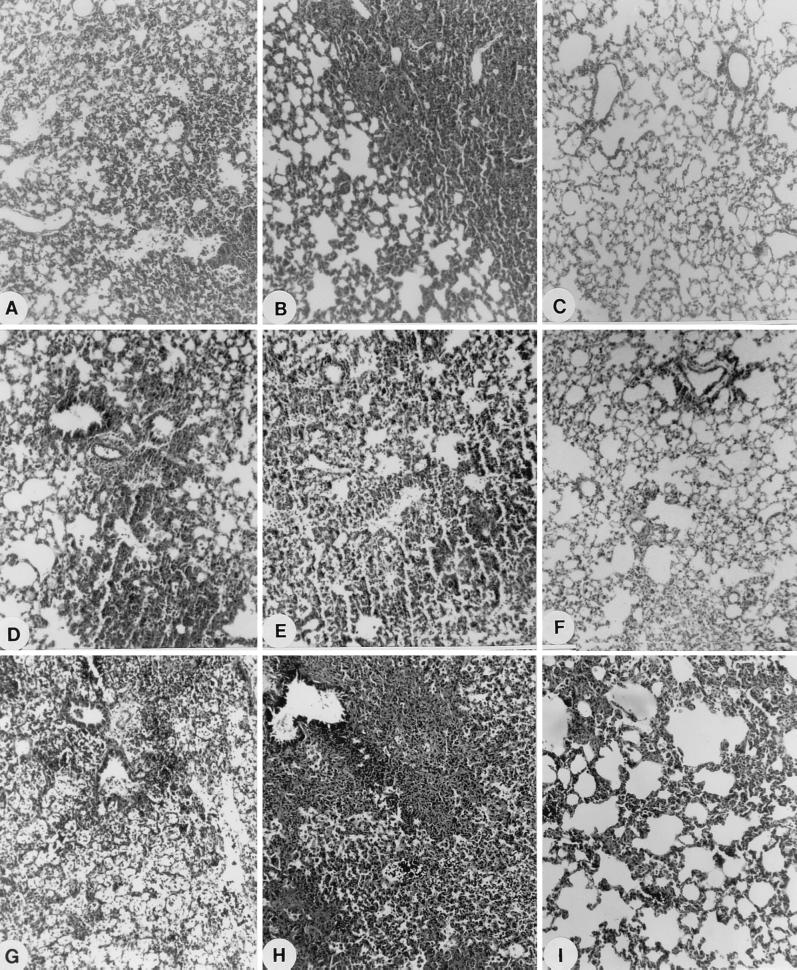
There was a gradual increase in bacterial counts for infected control animals up to 7 log10 CFU/lungs over time until the animals died (Table 2). Over 8 log CFU/lungs was always found in dead animals in the absence of treatment (data not shown). Ziracin injected at a single dose of 60 mg/kg led to the complete clearance of bacteria from the lungs by day 3 postinfection. Ceftriaxone given at a dose of 60 mg/kg had a similar effect. Therefore, the high survival rates were clearly due to the effective eradication of bacteria from tissues. (By contrast, we have observed at up to days 6 and 8 postinfection bacteria in lungs and septicemia in some mice treated with ziracin at a dose of 30 mg/kg.) Histopathological examination of animals that received either ziracin or ceftriaxone at a dose of 60 mg/kg showed that the infiltration by inflammatory cells, lung edema, intra-alveolar hemorrhage, and loss of alveolar structural integrity induced by bacteria were still present at days 3 and 6 postinfection (Fig. 2). However, there was little or no evidence of pathogenic signs of pneumonia in these mice by day 8 postinfection.
TABLE 2.
Efficacies of studied antibiotics on eradication of S. pneumoniae from lungs and blood
| S. pneumoniae | Treatment group | Just before treatment
|
Day postinfection
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3
|
6
|
8
|
13
|
20
|
|||||||||
| Lunga | Bloodb | Lunga | Bloodb | Lunga | Bloodb | Lunga | Bloodb | Lunga | Bloodb | Lunga | Bloodb | ||
| Penicillin-susceptible strain | Single injection | ||||||||||||
| Infected control | 5.9 ± 0.3 | 0/5 | 7.5 ± 0.9 | 5/5 | NSg | NS | NS | NS | NS | NS | NS | NS | |
| 30 mg of ziracin per kg | 2.6 ± 2.4c | 0/5 | 3.8 ± 2.3 | 1/5 | 3.9 ± 3.1 | 1/5 | <2d | 0/5 | <2d | 0/5 | |||
| 60 mg of ziracin per kg | <2cd | 0/5 | <2de | 0/5 | <2de | 0/5 | <2d | 0/5 | <2d | 0/5 | |||
| 60 mg of ceftriaxone per kg | <2cd | 0/5 | <2de | 0/5 | <2de | 0/5 | <2d | 0/5 | <2d | 0/5 | |||
| Delayed therapy | |||||||||||||
| Infected control | 6.8 ± 0.8 | 5/5 | 7.4 ± 0.7 | 5/5 | NS | NS | NS | NS | NS | NS | NS | NS | |
| 10 mg of ziracin per kg (q.d.), 3 days | 4.7 ± 0.5c | 1/5 | 3.5 ± 2.0 | 0/5 | 3.2 ± 2.8 | 0/5 | 2.6 ± 2.4 | 0/5 | <2d | 0/5 | |||
| 30 mg of ziracin per kg (q.d.), 3 days | 4.7 ± 0.3c | 0/5 | <2df | 0/5 | <2d | 0/5 | <2d | 0/5 | <2d | 0/5 | |||
| Single injection in leukopenic mice | |||||||||||||
| Infected control | 6.9 ± 0.3 | 0/5 | 8.3 ± 0.5 | 4/5 | NS | NS | NS | NS | NS | NS | NS | NS | |
| 60 mg of ziracin per kg | 2.7 ± 1.7c | 0/5 | 4.0 ± 1.7 | 1/5 | <2d | 1/5 | <2d | 0/5 | <2d | 0/5 | |||
| Penicillin-resistant strain | Therapy in leukopenic mice | ||||||||||||
| Infected control | 6.8 ± 1.2 | 3/5 | 7.1 ± 1.4 | 5/5 | NS | NS | NS | NS | NS | NS | NS | NS | |
| 30 mg of ziracin per kg (q.d.), 2 days | <2cd | 0/5 | <2d | 0/5 | <2d | 0/5 | <2d | 0/5 | <2d | 0/5 | |||
| 15 mg of vancomycin per kg (b.i.d.), 2 days | <2cd | 0/5 | <2d | 0/5 | <2d | 0/5 | <2d | 0/5 | <2d | 0/5 | |||
Log10 CFU per lung (mean ± SD for five mice).
Number of animals with positive blood cultures/total number of animals.
Significantly lower values compared to those for infected control group (P < 0.05).
The lower limit of detection was 2 log10 CFU/lung.
Significantly lower values compared to those for the group treated with ziracin at 30 mg/kg (P < 0.05).
Value was significantly lower than that for the group treated with ziracin at 10 mg/kg per day for 3 days (P < 0.05).
NS, no survivor.
FIG. 2.
Light microscopy of the lungs from PSSP-infected immunocompetent mice receiving ziracin or ceftriaxone. (A to C) Animals received a single injection of ziracin and were killed on days 3 (A), 6 (B), and 8 (C) postinfection; (D to E) animals received a single injection of ceftriaxone and were killed on days 3 (D), 6 (E), and 8 (F) postinfection; (G to I) animals received delayed therapy with multiple injections of ziracin and were killed on days 3 (G), 6 (H), and 13 (I) postinfection. Magnifications, ×89.
The prophylactic efficacy of ziracin was also evaluated in our studies (data not shown). Ziracin given at a dose of 30 mg/kg 4 h before bacterial inoculation prevented the development of PSSP pneumonia in 100% of immunocompetent mice.
Efficacy of delayed therapy against PSSP pneumonia in immunocompetent mice.
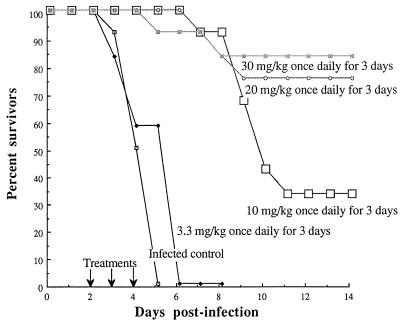
Figure 3 displays the percent survival rates for infected mice treated with multiple doses of ziracin initiated at 48 h postinfection. At that time all infected control animals had developed septicemia. The survival rates for infected mice treated with ziracin at 10, 20, and 30 mg/kg q.d. for 3 days were 33, 75, and 83%, respectively. Increasing the dose to over 30 mg/kg q.d. for 3 days did not increase the survival rate over 83%. The first injection of 30 mg/kg q.d. for 3 days rapidly eradicated the septicemia and significantly reduced the bacterial counts in the lungs compared with those in the lungs of infected controls. Three injections completely eradicated the bacteria from the lungs (Table 2), while the dosage of 10 mg/kg q.d. for 3 days was not sufficient for the clearance of bacteria from this site. Since no bacteria were found in dead mice that received ziracin at 30 mg/kg q.d. for 3 days, the deaths of these mice might have resulted from the lung injury caused by overwhelming inflammation. Histopathological examination of the animals receiving 30 mg/kg q.d. for 3 days showed intense infiltration by neutrophils and macrophages, severe edema, intra-alveolar hemorrhage, and loss of integrity of alveolar structure on days 3 and 6 postinfection (Fig. 2). However, histopathological examination on day 13 postinfection showed recovery of lung tissues from inflammatory injury.
FIG. 3.
Cumulative survival rates of immunocompetent mice infected with the PSSP strain (12 mice per group) following delayed therapy with multiple doses of ziracin. Treatments were given q.d. for 3 days and were initiated at 48 h postinfection.
Efficacy of a single injection of ziracin against PSSP pneumonia in leukopenic mice.
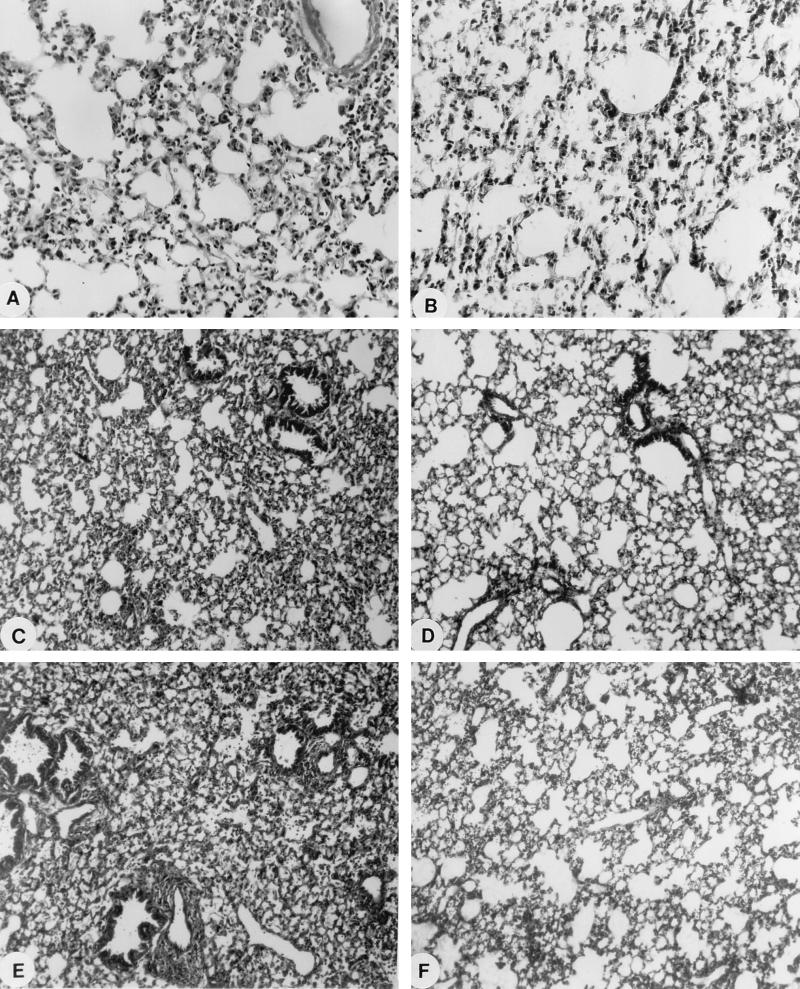
All infected leukopenic mice exhibited signs of more severe sickness than that in infected immunocompetent mice at 18 h postinfection. The bacterial count in the lungs of leukopenic mice was 1 log10 higher than that in immunocompetent mice at that time. Fifty and 100% of the infected untreated leukopenic mice died by days 3 and 5 postinfection, respectively. A single 60-mg/kg dose of ziracin given at 18 h postinfection yielded 92, 83, and 75% survival rates at days 4, 7, and 14 postinfection. Ziracin at this dosage significantly reduced the bacterial counts in lung tissues compared to those in the lungs of infected control mice (Table 2). The mean CFU reduction was 5.6 log units at day 3 postinfection. However, bacteria were still observed in the lung tissues or bloodstreams of some mice at days 6 and 8 postinfection. Micrographs from the histological examination of lung tissues are presented in Fig. 4. Histopathological analysis of the lungs of infected leukopenic mice showed less severe neutrophil infiltration and perivascular edema than those observed in infected immunocompetent mice. The loss of alveolar integrity, however, was still observed at day 3 postinfection. At the end of the observation period (day 20 postinfection), little or no evidence of structural destruction of the lungs was noted for mice treated with ziracin.
FIG. 4.
Light microscopy of the lungs from infected leukopenic mice receiving ziracin or vancomycin. Mice were examined at each time point following a single injection of ziracin at a dose of 60 mg/kg against PSSP pneumonia (days 3 [A] and 20 [B] postinfection), therapy with ziracin at 30 mg/kg q.d. for 2 days against PRSP pneumonia (days 3 [C] and 20 [D] postinfection), and therapy with vancomycin at 15 mg/kg b.i.d. for 2 days against PRSP pneumonia (days 3 [E] and 20 [F] postinfection). Magnifications, ×89.
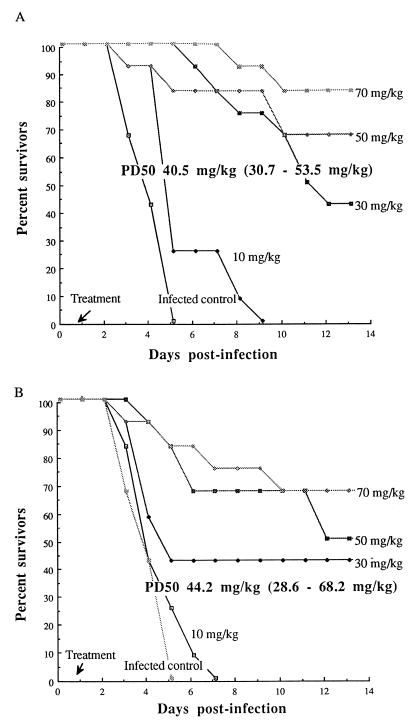
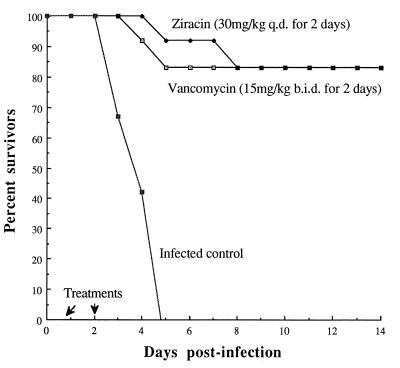
Comparative efficacy of ziracin and vancomycin against PRSP pneumonia in leukopenic mice.
The single injection of ziracin or vancomycin at various doses at 18 h postinfection resulted in PD50s of 40.5 and 44.2 mg/kg, respectively (Fig. 5). Moreover, ziracin given at a dose of 30 mg/kg q.d. for 2 days yielded an 83% survival rate, which was similar to that for vancomycin administered at 15 mg/kg b.i.d. for 2 days (Fig. 6). Bacterial clearance (Table 2) and the results of histopathological evaluation were also similar for both drugs. The disappearance or marked diminution of tissue damage was evident for mice treated with either ziracin or vancomycin on day 20 postinfection (Fig. 4). Moreover, the lung injuries induced by the PSSP strain and the PRSP strain were not different in leukopenic animals.
FIG. 5.
Cumulative survival rates of leukopenic mice infected with the PRSP strain (12 mice per group) after a single injection of ziracin (A) or vancomycin (B) initiated at 18 h postinfection.
FIG. 6.
Cumulative survival rates of leukopenic mice infected with the PRSP strain (12 mice per group) after injection of multiple doses of ziracin or vancomycin. Treatments were initiated at 18 h postinfection.
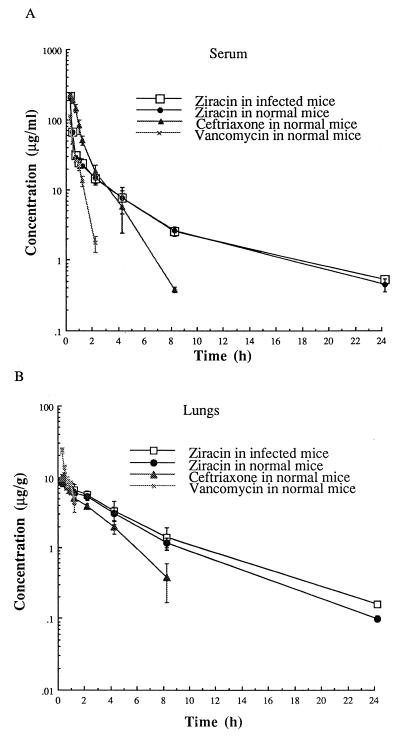
Pharmacokinetics for serum versus pulmonary tissue.
Figure 7 shows the time course of the concentrations in serum and lung tissues following the administration of a single 30-mg/kg dose of ziracin, ceftriaxone, or vancomycin. Ziracin achieved the same high peak concentration in serum as ceftriaxone and a much higher peak concentration than vancomycin. Compared with ceftriaxone and vancomycin, the concentration of ziracin in the serum and lungs decreased very slowly after the first hour. The pharmacokinetic profile of ziracin in the lungs was not altered by the presence of infection. The key pharmacokinetic parameters were calculated and are summarized in Table 3. The half-life of ziracin in serum and lungs was significantly longer than those of ceftriaxone and vancomycin. The AUC for ziracin in lung tissue was greater than those for ceftriaxone and vancomycin. The AUC for lung/AUC for serum ratio for ziracin was larger than that for ceftriaxone. The mean residence time was longer for ziracin than for ceftriaxone and vancomycin.
FIG. 7.
Concentrations (means ± SDs for four mice) of ziracin, ceftriaxone, and vancomycin in serum (A) and lung tissue (B) following a single injection of 30 mg/kg. Ziracin was measured in both normal and infected mice. Ziracin was injected in infected mice at 18 h postinfection.
TABLE 3.
Pharmacokinetic parameters after a single injection of 30 mg/kg of the studied antibiotics in micea
| Antibiotic | Mouse status and compartment | Cmax.obs (μg/ml or μg/g) | t1/2 (h) | AUC0–24 (μg · h/ml or μg · h/g) | AUC for lung/AUC for serum (%) | MRT (h) | AUC/MIC (h) |
|---|---|---|---|---|---|---|---|
| Ziracin | |||||||
| Healthy | |||||||
| Serum | 186 ± 4 | 2.3 ± 0.1 | 128 ± 15 | 3.4 ± 0.2 | 8,016 ± 938 | ||
| Lung | 8.2 ± 0.2 | 3 ± 0.4 | 36 ± 2 | 28 ± 3 | 4.5 ± 0.1 | 2,259 ± 16 | |
| Infected | |||||||
| Serum | 191 ± 3 | 2.2 ± 0.2 | 130 ± 6 | 3.5 ± 0.1 | 8,145 ± 417 | ||
| Lung | 8.0 ± 0.3 | 3.2 ± 1 | 40 ± 4 | 31 ± 3 | 4.7 ± 0.3 | 2,538 ± 30 | |
| Ceftriaxone | |||||||
| Healthy | |||||||
| Serum | 202 ± 8 | 1.04 ± 0.2 | 181 ± 17 | 1.1 ± 0.1 | 11,350 ± 1,091 | ||
| Lung | 9.2 ± 2 | 1.87 ± 0.4 | 20 ± 0.3 | 11 ± 1 | 2.5 ± 0.4 | 1,272 ± 22 | |
| Vancomycin | |||||||
| Healthy | |||||||
| Serum | 93 ± 25 | 0.36 ± 0.05 | 39 ± 4 | 0.6 ± 0.08 | 78 ± 10 | ||
| Lung | 20.9 ± 4 | 0.45 ± 0.1 | 9.3 ± 1 | 24 ± 3 | 0.7 ± 0.1 | 18 ± 2 |
Abbreviations: Cmax.obs, observed maximum concentration; t1/2, elimination half-life; AUC0–24, area under concentration-time curve from time zero to 24 h; MRT, mean residence time.
DISCUSSION
As PRSP strains are developing rapidly throughout the world and a high percentage of them are also resistant to other β-lactams (including carbapenem antibiotics) and the macrolides (7, 10, 12), glycopeptides sometimes represent the last antibiotics that are effective against these strains (10). Therefore, new therapeutic alternatives must be elaborated. In the present study ziracin, a new everninomicin derivative, showed excellent in vitro activities against both PSSP and PRSP strains, with MICs of 0.016 μg/ml for both types of strains. Our results are consistent with those of other investigators: Hare et al. (R. S. Hare, F. J. Sabatelli, and the Ziracin Susceptibility Testing Group, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-119, p. 204, 1998) reported that ziracin is highly active against S. pneumoniae, with an MIC at which 90% of strains are inhibited of 0.064 μg/ml for 1,490 clinical isolates collected from North America, Europe, and South Africa. Among the 1,490 strains, 35, 24, 16, and 14% of them were resistant to penicillin, erythromycin, ceftriaxone, and clindamycin, respectively. Although the mechanism of action of ziracin is not entirely clear, ziracin appears to inhibit protein synthesis in gram-positive bacteria. However, the target and mechanism of action of ziracin might differ from those of other antibiotics that also inhibit bacterial protein synthesis. This likely explains why ziracin is still active against bacteria resistant to other antibiotics (P. V. Adrian and K. P. Klugman, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-110, p. 100, 1998; P. V. Adrian, C. Mendrick, D. Loebenberg, K. J. Shaw, K. P. Klugman, R. S. Hare, and T. A. Black, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 845, p. 117, 1999; T. A. Black, W. Zhao, K. J. Shaw, and R. S. Hare, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-106, p. 99, 1998).
In our study, there was a good correlation between the in vitro activity of ziracin against S. pneumoniae and its in vivo efficacy. Our data have shown that ziracin has a marked, rapid, and prolonged antibacterial effect against PSSP- and PRSP-induced pneumonia. Mortality studies, bacterial counts, and histopathological observations consistently confirmed the excellent in vivo efficacy of ziracin, whether therapy was initiated early with a single dose or was started at a time when the animals already experienced septicemia.
Ceftriaxone was reported to be the most potent of 14 cephalosporins studied in an experimental murine model of pneumococcal infection (8). Due to its potency and long half-life, ceftriaxone was frequently chosen as a comparative agent in the study of pneumococcal pneumonia. In the present study, ziracin was as effective as ceftriaxone in protecting the infected mice from death (PD50) and in clearing PSSP from tissues.
Pneumonia caused by S. pneumoniae in leukopenic patients is becoming more common and warrants intense investigations (4–6). The use of leukopenic mice in our pneumonia model provides an accurate evaluation of the antibacterial efficacies of antibiotics in the absence of any synergistic antibacterial effect from leukoocytes. This absence of an effect from leukoocytes in animal models may also resemble the situation for S. pneumoniae pneumonia in humans. In particular, infections caused by PRSP strains may frequently be seen in leukopenic patients (5). Here we show the excellent efficacy of ziracin for the treatment of leukopenic mice infected with PSSP or PRSP strains. Importantly, the efficacy of ziracin given q.d. against PRSP infection in leukopenic mice was as potent as that of vancomycin given b.i.d.
The penetration of antibiotics into lung tissue has long been a matter of interest in the study of pulmonary infections. The high level of accumulation of ziracin in lung tissues may have contributed to its efficacy for bacterial clearance. For most antibiotics, increased or decreased levels of drugs are found in infected foci as a result of altered vascular permeability in the lung, infiltration of leukocytes filled with the drug, or degradation of the antibiotic locally. In our study, the levels of ziracin in lung tissues were not altered by the presence of pulmonary infection and inflammation.
The high peak concentration and the long half-life of ziracin in serum contributed to the clearance of bacteria from the blood and the suppression of any subsequent bacteremia and septicemia. This is crucial, as we already showed a good correlation between septicemia and death (1). The half-life of ziracin in serum and lungs was longer than that of ceftriaxone in serum and lungs in our mouse model. Our results are consistent with results of the pharmacokinetics of ziracin in humans: the half-life of ziracin (9 h) in human serum (C. Banfield, S. Pai, S. Menon, L. Lambrecht, M. Laughlin, and M. Affrime, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-50, p. 16, 1998) was longer than that of ceftriaxone (7 h) (14). A long half-life is a benefit when establishing persistently effective concentrations in tissues. It may thus prove to be an important characteristic of this agent that would explain its efficacy when given as a large single dose or as small daily doses.
The comparison of the AUCs for ziracin in mice and humans shows that the AUC produced in mice after the administration of a dose of 30 mg/kg was equivalent to that produced in human serum after the administration of a dose of 3 mg/kg (Banfield et al., 38th ICAAC). Since the AUC above the MIC appeared to be an important parameter for prediction of the efficacy of ziracin and the protein binding of ziracin is similar in mouse and human sera (approximately 96% in both mouse and human sera) (G. L. Drusano, S. L. Preston, C. J. Hardalo, R. S. Hare, C. Banfield, and W. A. Craig, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1207, p. 37, 1999), it is therefore apparent that ziracin can be expected to have similar antibacterial effects in humans and in mice but at a considerably lower daily dose in humans.
In summary, ziracin exhibits reliable efficacy for the clearance of S. pneumoniae and ensures high survival rates in a mouse model of lethal pneumonia. Ziracin holds promise as an antibiotic that can be administered q.d. and that has activity against both PSSP and PRSP in immunocompetent and leukopenic hosts.
ACKNOWLEDGMENTS
This study was supported by a grant from Schering-Plough Research Institute, Kenilworth, N.J.
We thank Mélanie Côté-Richer for assistance as a summer student.
REFERENCES
- 1.Bergeron Y, Ouellet N, Deslauriers A M, Simard M, Olivier M, Bergeron M G. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect Immun. 1998;66:912–922. doi: 10.1128/iai.66.3.912-922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breiman R F, Butler J C, Tenover F C, Elliott J A, Facklam R R. Emergence of drug-resistant pneumococcal infections in the United States. JAMA. 1994;271:1831–1835. [PubMed] [Google Scholar]
- 3.Butler J C, Hofmann J, Cetron M S, Elliott J A, Facklam R R, Breiman R F. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention's Pneumococcal Sentinel Surveillance System. J Infect Dis. 1996;174:986–993. doi: 10.1093/infdis/174.5.986. [DOI] [PubMed] [Google Scholar]
- 4.Carratala J, Marron A, Fernandez-Sevilla A, Linares J, Gudiol F. Treatment of penicillin resistant pneumococcal bacteremia in neutropenic patients with cancer. Clin Infect Dis. 1997;24:148–152. doi: 10.1093/clinids/24.2.148. [DOI] [PubMed] [Google Scholar]
- 5.Carratala J, Roson B, Fernandez-Sevilla A, Alcaide F, Gudiol F. Bacteremic pneumonia in neutropenic patients with cancer: causes, empirical antibiotic therapy, and outcome. Arch Intern Med. 1998;158:868–872. doi: 10.1001/archinte.158.8.868. [DOI] [PubMed] [Google Scholar]
- 6.Collin B A, Ramphal R. Pneumonia in the compromised host including cancer patients and transplant patients. Infect Dis Clin N Am. 1998;12:781–805. doi: 10.1016/s0891-5520(05)70210-5. [DOI] [PubMed] [Google Scholar]
- 7.Doern G V, Brueggemann A, Holley H P, Jr, Rauch A M. Antimicrobial resistance of Streptococcus pneumoniae recovered from outpatients in the United States during the winter months of 1994 to 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother. 1996;40:1208–1213. doi: 10.1128/aac.40.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frimodt-Moller N, Bentzon M W, Thomsen V F. Experimental infection with Streptococcus pneumoniae in mice: correlation of in vitro activity and pharmacokinetic parameters with in vivo effect for 14 cephalosporins. J Infect Dis. 1986;154:511–517. doi: 10.1093/infdis/154.3.511. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein F W, Acar J F. Antimicrobial resistance among lower respiratory tract isolates of Streptococcus pneumoniae: results of a 1992–93 western Europe and USA collaborative surveillance study. The Alexander Project Collaborative Group. J Antimicrob Chemother. 1996;38(Suppl. A):71–84. doi: 10.1093/jac/38.suppl_a.71. [DOI] [PubMed] [Google Scholar]
- 10.McGowan J E, Jr, Metchock B G. Penicillin-resistant pneumococci—an emerging threat to successful therapy. J Hosp Infect. 1995;30(Suppl.):472–482. doi: 10.1016/0195-6701(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 11.Moine P, Vallee E, Azoulay-Dupuis E, Bourget P, Bedos J P, Bauchet J, Pocidalo J J. In vivo efficacy of a broad-spectrum cephalosporin, ceftriaxone, against penicillin-susceptible and -resistant strains of Streptococcus pneumoniae in a mouse pneumonia model. Antimicrob Agents Chemother. 1994;38:1953–1958. doi: 10.1128/aac.38.9.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreillon P, Wenger A. Antibiotic resistance in pneumococci. Schweiz Med Wochenschr. 1996;126:255–263. [PubMed] [Google Scholar]
- 13.Nakashio S, Iwaswa H, Don F Y, Kanemitso K, Shimada I. Everninomicin, a new oligosaccharide antibiotic: its antimicrobial activity, postantibiotic effect and synergistic bactericidal activity. Drugs Exp Clin Res. 1995;1:7–16. [PubMed] [Google Scholar]
- 14.Richards D M, Heel R C, Brogden R N, Speight T M, Avery G S. Ceftriaxone. A review of its antibacterial activity, pharmacological properties and therapeutic use. Drugs. 1984;27:469–527. doi: 10.2165/00003495-198427060-00001. [DOI] [PubMed] [Google Scholar]
- 15.Sanders W E, Jr, Sanders C C. Microbiological characterization of everninomicin B and D. Antimicrob Agents Chemother. 1974;6:232–238. doi: 10.1128/aac.6.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urban C, Mariano N, Mosinka-Snipas K, Wadee C, Chahrour T, Rahal J J. Comparative in vitro activity of SCH-27899, a novel everninomicin, and vancomycin. J Antimicrob Chemother. 1996;37:361–364. doi: 10.1093/jac/37.2.361. [DOI] [PubMed] [Google Scholar]
- 17.Yamaoka K, Nskagawa T, Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978;6:165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]