Abstract
Background
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disorder of the central nervous system. It is an autoimmune disease of multifactorial etiology, linked to a variety of genetic and well-defined environmental factors. It typically affects more women than men and more frequently affects adults aged 20–45 years. Besides, vitamin B12 deficiency and obesity are associated with exacerbating central nervous system inflammation and a higher clinical disability.
Objective
The study aims to determine the association of the vitamin B12 serum concentration with the Body Mass Index BMI, thyroid-stimulating hormone serum levels and MS clinical features in Saudi MS patients.
Methods and results
This is a retrospective cohort study, and data were collected from the MS database at the King Fahad Medical City Multiple Sclerosis Clinic, from December 2015 to December 2019. Data were entered and analyzed using the Statistical Package for Social Sciences (SPSS ver. 20, Chicago, IL, USA). Cobalamin, also known as vitamin B12, has a reference concentration that ranges from 138 to 652 Pmol/L in adults. The patient's BMI was calculated by dividing the weight (in kilograms) by the square of the height (in square meters), expressed in kg/m2.
Data for 169 MS subjects were collected. A total 83 of them, with a mean age of 36.2 ± 9.57 years, had vitamin B12 results. Of all patients, 16.6% had vitamin B12 deficiency (<138 pmol/L) and 9.52% of them were overweight, BMI kg/m2 = (25–29.9). The mean vitamin B12 level in all MS subjects was 240 ± 117 pmol/L. Moreover, 58.33% of the MS patients had high BMIs (BMI >25). However, no significant correlation was found between vitamin B12 deficiency neither with the BMI nor TSH concentration in MS cases (r = 0.03, p = 0.64), (r = 0.00, P = 0.9) respectively.
Conclusion
These findings revealed no association between serum vitamin B12 concentration and TSH, BMI in MS clinical parameters, however, further studies are required to validate these results.
Keywords: Multiple sclerosis, Vitamin B12, Body mass index
1. Introduction
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disorder of the central nervous system (CNS). MS is the most common cause of disability among young people [1,2]. The prevalence and incidence of this pathology are increasing in both developing and developed countries [3]. MS is an autoimmune disease of multifactorial etiology linked to genetic and environmental factors like specific ultraviolet B (UVB) exposure, obesity, smoking, vitamin D deficiency, and Epstein–Barr virus (EBV) infection [4] (see Table 3, Table 4).
Table 3.
Comparison of the parameters of MS patients according to their B12 level.
| Parameters | Mean ± SD Non-deficient (N = 69) | Mean ± SD Deficient (N = 14) | P-Value |
|---|---|---|---|
| Age | 36.6 ± 9.7 | 33.3 ± 8.4 | 0.24 |
| Age at onset | 28.7 ± 9 | 25.7 ± 7.6 | 0.26 |
| Duration | 7.9 ± 5.2 | 7.57 ± 3.9 | 0.79 |
| BMI (kg/m2) | 27.9 ± 8.5 | 29.2 ± 8.28 | 0.59 |
| EDSS | 1.73 ± 1.1 | 1.64 ± 1.15 | 0.77 |
| TSH mlU/L | 1.23 ± 0.6 | 1 ± 0 | 0.16 |
Table 4.
Correlation analysis of the parameters and b12 level and linear regression analysis of MS patients.
| p-value | Pearson Correlation | Regression analysis | p-value | |
|---|---|---|---|---|
| Age | 0.7 | 0.04 | 0.002 | 0.7 |
| Age at onset | 0.24 | 0.13 | 0.17 | 0.24 |
| Duration | 0.168 | −0.15 | 0.023 | 0.16 |
| BMI (kg/m2) | 0.64 | −0.05 | 0.03 | 0.6 |
| EDSS | 0.68 | 0.45 | 0.002 | 0.68 |
| TSH mlU/L | 0.9 | 0.013 | 0.00 | 0.9 |
Vitamin D insufficiency is linked to the risk of several autoimmune disorders and gastrointestinal diseases. It has been reported that serum vitamin D is significantly decreased in chronic autoimmune atrophic gastritis (CAAG) patients [4]. In addition, a significantly lower concentration of vitamin D is observed in the serum of alcoholic cirrhotic patients compared with non-alcoholic cirrhotic patients, suggesting that vitamin D deficiency may be linked to an alcohol use state [5]. However, the relation between low vitamin D levels and multiple sclerosis is not fully elucidated.
Obesity is another important risk factor for developing autoimmune disease [38], and it has also been linked to MS patients [39]. However, studies are not very consistent toward BMI and obesity with MS. For example, a remarkable reduction in the mean BMI was reported in the overall MS patients’ group during the MS course compared to the healthy group [6]. In contrast, a recent study found a contrary to what was described before, BMI does not show any significant difference between patients with MS and healthy controls [7].
MS is mainly characterized by multi-centric demyelination and inflammation; however, as the disease progresses, gliosis and axonal injury roles increase [8]. MS occurs mainly in adults aged 20–45 years, and females are more predisposed to the condition than men [9]. Initial clinical results in patients with MS are sensory abnormalities, and the most classic symptoms are paresthesia, dysesthesia, diplopia, ataxia, vertigo, and bladder disturbances such as urinary retention [10].
MS is classified based on the disease course into four main types; relapsing-remitting MS (RRMS), which is the most common type, accounts for about 85% of MS cases, and is characterized by exacerbations of symptoms, relapses, and periods of remission [10]. The second category is secondary progressive MS (SPMS), in which the disease course worsens with or without remission. The third one, primary progressive MS (PPMS), occurs in approximately 10% of the cases. It is symptoms consistently progress from the beginning; there may be occasional plateaus, but there are neither relapses nor remissions. The last one, which is rare, occurring in less than 5% of patients, is the progressive-relapsing MS (PRMS). It develops from the beginning, with neurological symptoms steadily worsening with the disease progression, without any remission [10].
Vitamin B12 is a water-soluble vitamin that acts as a cofactor in DNA synthesis, thus contributing to cell metabolism in the human body [11]. This vitamin is also essential for the normal function of the nervous system [12,13].
There are different reasons for vitamin B12 deficiency, such as inadequate intake of animal protein in vegetarians and malabsorption that results in inadequate extraction of cobalamin from food, insufficient generation of intrinsic factor generation such as in terminal ileum disorders, instances of pernicious anemia, and competition for cobalamins that could be caused by intestinal worms and blind loop syndrome [14].
The diagnosis of the deficiency is based on the serum concentration of vitamin B12 < 138 pmol/L (175 pg/mL) in adults [13]. Vitamin B12 is particularly essential in the normal function of the nervous system via it is role in the synthesis of myelin [13]. Its deficiency results in the dysfunctional production of myelin sheath due to the introduction of non-physiological fatty acids into neuronal lipids [15], and the defective methylation of myelin basic protein (MBP), a vital part of CNS myelin [16,17]. In addition, vitamin B12 deficiency is associated with increase tumor necrosis factor TNFa, modulates cytokines activity [18]. This might exacerbate current MS condition by worsening demyelination and the inflammatory processes and preventing remyelination [12] MS has recently been linked with obesity, as a study found that obesity is associated with exacerbation of central inflammation and higher clinical disability [19].
According to a study published in 2020, the TSH receptor plays an essential role in regulating body weight. The study findings suggest that elevation of TSH levels may be an indicator for obesity, particularly abdominal obesity during puberty [20]. Thus, elevated TSH levels that come from hormonal imbalances in the body can lead to many other weight-related disorders. Another study reported elevated TSH levels are linked with MS patients with myelitis [21], suggest that TSH level and obesity might contribute to the disease progression. Also previously reported that obese people are more risk for vitamin B12 deficiency [19].Therefore, the present study investigated the association between vitamin B12 level TSH and BMI in MS patients.
2. Materials and methods
All are replacing remitting multiple sclerosis patients who attended the MS clinic from April 2016 to September 2019 were recruited retrospectively (n = 169). The inclusion criteria are; adult patients with (RRMS) multiple sclerosis; diagnosed according to McDonald criteria at a single center, King Fahad Medical City (KFMC), including both genders. The exclusion criteria are; patients with combined neurological disorders and lacking vitamin B12, the expanded disability status scale (EDSS), or thyroid stimulating hormone (TSH) concentration. Thus, 86 patients were excluded from the study who lacked these tests. In addition, patients' medical records were obtained, including; demographic data, age of onset, disease duration, height, weight, BMI, EDSS, and TSH levels.
This study was approved by the institutional review boards (IRBs) at King Fahad Medical City (KFMC). IRB number is 19-3560, IRB registration number with PHRP/NIH/USA = FWA00018774.
2.1. Vitamin B12 and serum thyroid stimulating hormone
Serum B12 and thyroid-stimulating hormone concentrations were determined by retrospective review of medical records. In cases where more than one concentration was available, the initial concentration was used. The standard reference range for B12 concentrations in our laboratory is 138–652 pg/mL. The standard reference range for thyroid-stimulating hormone concentrations in our laboratory is 0.35–4.5 mlU/L. The TSH and B12 levels were measured by electrochemiluminescent immunoassay (Roche Diagnostics, Indianapolis, IN, USA).
2.2. Statistical analysis
SPSS software was used to perform statistical analysis. According to the BMI, patients were classified as underweight when BMI <18.5 kg/m2, healthy weight when BMI ranged from 18.50 to 24.9 kg/m2, overweight when BMI ranged from 25 to 29.9 kg/m2, and obese when BMI ≥30 kg/m2. Vitamin B12 level was defined as deficient when its serum concertation was <138 pmol/L and as non-deficient when its serum concertation was ≥138 pmol/L. Continuous variables were presented using means and standard deviations (SD) and using percentages and frequencies for categorical variables. The Chi-Square test was used to determine the association between vitamin B12 levels, which was graded categorically (i.e., High, normal, low), and BMI. Pearson's correlation coefficients were used to measure the correlations,and stepwise linear multiple regression analyses were used to assess the relationship between vitamin B12 levels and independent variables. Values of P < 0.05 were considered statistically significant.
3. Results and discussion
3.1. Multiple sclerosis cases characteristics
Data from 83 relapsing-remitting MS patients (59 females and 27 males, aged from 18 to 61 years, mean 36.2 ± 9.57 years) were collected retrospectively from the KFMC medical records. The age of onset of the disease ranged from 19.4 to 36.9 years, with a mean value of 28.2 ± 8.76 years. The duration of the disease after the first diagnosis to the date data was obtained is 2.88–13 years, with a mean value of 8 ± 5.12 years. Information on the demographic data including age, disease duration, BMI, serum vitamin B12 concentration, and TSH levels are shown in (Table 1) (see Table 2).
Table 1.
Demographic characteristics of the patients.
| Total | |
|---|---|
| Number | 83 |
| Gender | 59 F/24 M (71%F/28.9% M) |
| Age (years) Mean, SD | 36.2 ± 9.57 |
| Disease duration (years) Mean, SD |
8 ± 5.12 |
| B12 status <138 pmol/L (%), Deficient | 14/83 (16.8) |
| Non-Deficient >138 | 69/83 (83.1%) |
| BMI (kg/m2) (%) Normal range |
32/83 (38.6%) |
| Overweight | 28/83 (33.7%) |
| Obese | 21/83 (25.3%) |
| TSH normal range (%) | 74/83 (89.2%) |
| Low level > 0.35 mlU/L (%) | 2/83 (2.4%) |
| High TSH < 4.9 mlU/L (%) | 7/83 (8.4%) |
| EDSS (Normal) | 54/83 (65.1%) |
| Minimal disability | 10/83 (11.9%) |
| Moderate disability | 7/83 (8.3%) |
| Sever disability | 12/83 (14.3%) |
Abbreviations: M, male; F, female, TSH, thyroid stimulating hormone, EDSS expanded disability status scale. Data are expressed as percentages and mean ± standard deviation.
Table 2.
Gender-wise comparison of all parameters of multiple sclerosis patients.
| Parameters | Mean ± SD Total (N = 83) | Mean ± SD Females (N = 59) | Mean ± SD Males (N = 24) | P-Value |
|---|---|---|---|---|
| Age | 36.2 ± 9.57 | 36.6 ± 10.66 | 34.87 ± 6 | 0.45 |
| Age at onset | 28.2 ± 8.76 | 28.59 ± 9.67 | 27.29 ± 6.3 | 0.54 |
| Duration | 8 ± 5.12 | 8 ± 5.27 | 7.58 ± 4.4 | 0.7 |
| BMI (kg/m2) | 26.7 ± 5.45 | 26.2 ± 5.12 | 27.95 ± 6.1 | 0.22 |
| B12 pmol/L | 240.28 ± 117 | 247.6 ± 128.7 | 222 ± 82.2 | 0.28 |
| EDSS | 1.89 ± 2.425 | 1.95 ± 2.3 | 1.75 ± 2.5 | 0.73 |
| TSH mlU/L | 2.19 ± 1.479 | 2.35 ± 1.6 | 1.79 ± 0.92 | 0.11 |
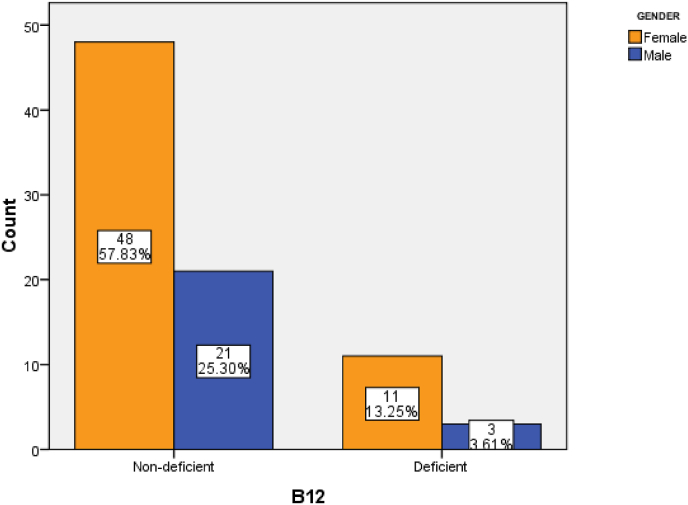
The serum vitamin B12 levels were normal in 83.1% of the patients and low (<138) in 16.6% of them. There was no significant difference in the vitamin B12 level between genders (P = 0.28) (Fig. 1). Furthermore, only 38.6% of the subjects had normal BM1, while 2.38% were underweight (BMI <18.5 kg/m2), 33.7% were overweight (BMI ranging from 25 to 29.9 kg/m2) and 25.3% were obese (BMI >30 kg/m2) (Fig. 2). However, there were no significant correlations between vitamin B12 levels and BMI in Saudi's MS patients (r = 0.03 p = 0.64).
Fig. 1.
BMI status among MS patients.
Fig. 2.
The Prevalence of vitamin 12 deficiency among MS patients.
Moreover, in this study, serum vitamin B12 level was found not to correlate significantly with serum thyroid stimulating hormone concentration TSH (r = 0.00, P = 0.9) with no significant difference in TSH levels between males and females (P = 0.11). In addition, it was found that 65.5% of MS patients had normal EDSS, with no significant correlation with vitamin B12 levels (r = 0.002, P = 0.68). Furthermore, no significant association was found between the serum vitamin B12 level and the age, at onset of the disease, disease duration, and gender (P > 0.05).
4. Discussion
Multiple sclerosis (MS) is one of the most common neurological diseases, usually characterized by the breakdown of myelin and cell death in the brain and spinal cord [2]. The exact etiology of MS is unknown; however, studies have presented several theories, including autoimmune responses, environmental toxins, infectious agents, genetic predispositions, nutritional deficiencies, or excesses [4,5].
The current study found that only 16.6% of MS patients have a vitamin B12 deficiency, and 84.3% are normal. These findings did not find any association between serum vitamin B12 concentration and MS patient's age, disease age of onset, duration, and EDSS.
Acquired and hereditary vitamin B12 deficiencies are well known to cause demyelination in the CNS [22,23], explaining the link between vitamin B12 and MS. In addition. It was also demonstrated in an experimental study on a wild-type male mouse exhibit that a decrease in dietary vitamin B12 causes neuronal neurodegeneration and dysfunction [24,25]. A previous study in Saudi also reported only a 30% decrease in vitamin B12 levels (<200 pg/mL)[26].
In contrast, a case-control study on MS patients reported a significant negative relationship between EDSS and serum vitamin B12 levels also, patients with a low vitamin B12 and abnormal MRI brain atrophic showed impaired cognitive functions of visuospatial abilities, language, and executive functions [27]. However, a study carried out in 2012 reported no relationship between serum vitamin B12 levels and EDSS scores and age of disease onset [28].
In addition, vitamin B12 deficiency is one of the causes of high serum homocysteine, which has been found to play a significant role in the pathogenesis of neurodegenerative diseases [29].
The associations between MS and homocysteine (Hcy), vitamin B12, and folate blood levels are increasingly controversial. Several meta-analysis studies suggested that Hcy might be linked to the pathogenesis of the disease. However, a study reported no significant differences between MS patients and healthy controls for vitamin B12 and folate [30,31]. A further study revealed that MS patients had lower vitamin B12 levels, while serum Hcy was elevated in MS patients [32]. A Chinese study reported increased Hcy levels in MS patients and decreased Vitamin B12 and Hcy during relapses which may be associated with disease pathogenesis [33]. Thus, the inconsistency of previous studies on the association between folate status, Vitamin B12, and Hcy with MS has remained unclear and needs further studies.
Many studies tested the efficiency of vitamin B12 as a treatment combined with lofepramine and l-phenylalanine on MS subjects. One of them was a randomized placebo-controlled exploratory study that showed a 2-point improvement on the disability scale [34]. Another randomized placebo-controlled double-blind study found that this combination effectively relieved MS symptoms [35]. On the other hand, a study on MS patients with the chronic progressive form of the disease reported that no significant change in disability status was demonstrated after starting a massive dose of vitamin B12 over six months [36].
Furthermore, the current study found that 58.33% of the MS patients had high BMIs (BMI >25). However, no significant correlation between vitamin B12 levels with the serum TSH levels and BMI were reported. Although the relationship between obesity and the risk of MS varies among different populations, some studies have reported that the mean BMI was reduced in the overall MS patients' group during the MS course compared to the healthy group [5]. In addition, a recent meta-analysis found that BMI did not show any significant differences between patients with MS and healthy controls [6]. It has been reported that obesity might deform absorption and indirectly cause genetic damage to vitamin B12 metabolism. Furthermore, dietary constituents have been shown to have multiple effects on the immune system, myelination, neuronal repair, and neuroprotection, which all together could affect MS risk and suggest pathophysiological pathways [6]. Thus, obesity and high BMI might be risk factors for vitamin B12 deficiency [7].
On the other hand, thyroid hormones (THs) were reported to play an essential role in the development of oligodendrocytes and are involved in the re-myelination process of EAE [37]. A study found that TSH levels were significantly lower in patients with MS. However, low TSH levels did not correlate with MS′ duration and EDSS. Another study reported that the TSH level was not significantly correlated with vitamin B12 in patients with MS [37].
There are several limitations to the current study. First, the study lacked a healthy control group to compare vitamin B12 and BMI variables. Another limitation to this study is that no folate, Hcy levels, and supplementation were reported. Hcy or folate measurement may have played a role in the results. Study limitations also included small size, varying treatments, and lack of MRI data of the patients, which might provide additional information. Therefore, further investigations are necessary to replicate this finding and explore the clinical factors underpinnings of the plausibility of vitamin B12 and the risk of MS.
5. Conclusions
Although the association between vitamin B12 deficiency and MS had been found in several previous articles, this study has shown that the majority of patients with MS had normal levels of vitamin B12. Additionally, vitamin B12 showed no correlations with the MS clinical features such as disease duration, age of onset, and EDSS, neither BMI nor TSH. Therefore, further studies with more sensitive measures such as total homocysteine or folate concentrations are needed to confirm the results.
Funding statement
The research did not receive funding from any sources.
Data availability
The data used to support the findings of this study are included within the article.
CRediT authorship contribution statement
N. Alsomali: Conceptualization, Methodology, Data curation, Writing – review & editing, Project administration. R. Alsharif: Validation, Investigation, Supervision, Resources, Project administration. B. Albalawi: Investigation, Writing – original draft, Resources. R. Alharthi: Investigation, Writing – original draft, Resources. Fatimah Alqubaydhi Fatimah Alqubaydhi: Investigation, Writing – original draft, Resources. Raghad Shiraz: Investigation, Writing - Original Draft, Resources. W. Junaidallah: Investigation, Writing – original draft, Resources. S. Alshammari: Investigation, Writing – original draft, Resources. F. Alhawiti: Investigation, Writing – original draft, Resources. A. Alenezi: Investigation, Writing – original draft, Resources. R. Alarieh: Investigation, Supervision, Resources. W. Alsaeed: Formal analysis, Supervision, Resources. G. AlTowaijri: Investigation, Supervision, Resources.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
We thank the Research Center of the King Fahad Medical City for their support.
References
- 1.Dutta R., Trapp B.D. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68(22 suppl 3):S22–S31. doi: 10.1212/01.wnl.0000275229.13012.32. [] [DOI] [PubMed] [Google Scholar]
- 2.Kobelt G., Thompson A., Berg J., Gannedahl M., Eriksson J., MSCOI Study Group & European Multiple Sclerosis Platform New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler. 2017;23(8):1123–1136. doi: 10.1177/1352458517694432. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browne P., Chandraratna D., Angood C., Tremlett H., Baker C., Taylor B.V., Thompson A.J. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology. 2014;83(11):1022–1024. doi: 10.1212/WNL.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massironi S., Cavalcoli F., Zilli A., Del Gobbo A., Ciafardini C., Bernasconi S., Peracchi M. Relevance of vitamin D deficiency in patients with chronic autoimmune atrophic gastritis: a prospective study. BMC Gastroenterol. 2018;18(1):1–8. doi: 10.1186/s12876-018-0901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anty R., Tonohouan M., Ferrari-Panaia P., Piche T., Pariente A., Anstee Q.M., Tran A. Low levels of 25-hydroxy vitamin D are independently associated with the risk of bacterial infection in cirrhotic patients. Clin Transl Gastroenterol. 2014;5(5):e56. doi: 10.1038/ctg.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dardiotis E., Tsouris Z., Aslanidou P., Aloizou A.M., Sokratous M., Provatas A., Hadjigeorgiou G.M. Body mass index in patients with Multiple Sclerosis: a meta-analysis. Neurol Res. 2019;41(9):836–846. doi: 10.1080/01616412.2019.1622873. [DOI] [PubMed] [Google Scholar]
- 7.Castro L.J.J.O., Pereira A., Ribeiro N. The Body mass index in patients with Multiple Sclerosis: a meta-analysis. J Stat Health Decis. 2021;3(1):99–104. [Google Scholar]
- 8.Trapp B.D., Peterson J., Ransohoff R.M., Rudick R., Mörk S., Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338(5):278–285. doi: 10.1056/NEJM199801293380502. [] [DOI] [PubMed] [Google Scholar]
- 9.Brust J.C. McGraw-Hill Medical; New York: 2007. Current diagnosis and treatment in neurology. [Google Scholar]
- 10.Fauci A., Braunwald E., Kasper D., Hauser S. seventeenth ed. McGraw-Hill Medical; New York: 2008. Harrisons Principles of internal medicine. [Google Scholar]
- 11.Yamada K. vol. 13. Springer; Dordrecht: 2013. Cobalt: its role in health and disease. (Metal ions in lifesciences). 295-320. [DOI] [PubMed] [Google Scholar]
- 12.Miller A., Korem M., Almog R., Galboiz Y. Vitamin B12, demyelination, remyelination and repair in multiple sclerosis. J Neurol Sci. 2005;233(1–2):93–97. doi: 10.1016/j.jns.2005.03.009. . [DOI] [PubMed] [Google Scholar]
- 13.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes . National Academies Press (US); 1998. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, Pantothenic Acid, biotin, and choline. [PubMed] [Google Scholar]
- 14.Braunwald E., Fauci A., Kasper D., Hauser S., Longo D., Jameson J. fifteenth ed. McGraw-Hill; New York: 2001. Harrison's principles of internal medicine. [Google Scholar]
- 15.Victor M., Ropper A.H., Adams R.D. Adams and Victor's principles of neurology. . 2001 [Google Scholar]
- 16.Shevell M.I., Rosenblatt D.S. The neurology of cobalamin. Can J Neurol Sci. 1992;19(4):472–486. [] [PubMed] [Google Scholar]
- 17.Allen R.H., Stabler S.P., Savage D.G., Lindenbaum J. Metabolic abnormalities in cobalamin (vitamin B12) and folate deficiency. Faseb J. 1993;7(14):1344–1353. doi: 10.1096/fasebj.7.14.7901104. . [DOI] [PubMed] [Google Scholar]
- 18.Peracchi M., Bamonti Catena F., Pomati M., De Franceschi M., Scalabrino G. Human cobalamin deficiency: alterations in serum tumour necrosis factor‐α and epidermal growth factor. Eur J Haematol. 2001;67(2):123–127. doi: 10.1034/j.1600-0609.2001.t01-1-00507.x. . [DOI] [PubMed] [Google Scholar]
- 19.Stampanoni Bassi M., Iezzi E., Buttari F., Gilio L., Simonelli I., Carbone F., Finardi A. Obesity worsens central inflammation and disability in multiple sclerosis. Mult Scler J. 2019 doi: 10.1177/1352458519853473. 1352458519853473.. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Dong X., Fu C., Su M., Jiang F., Xu D., Jiang Q. Thyroid stimulating hormone (TSH) is associated with general and abdominal obesity: a cohort study in school-aged girls during puberty in East China. Front Endocrinol. 2020;620 doi: 10.3389/fendo.2020.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long Y., Zheng Y., Chen M., Zhang B., Gao C., Shan F., Fan Y. Serum thyroid-stimulating hormone and anti-thyroglobulin antibody are independently associated with lesions in spinal cord in central nervous system demyelinating diseases. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0100672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmel R., Watkins D., Goodman S.I., Rosenblatt D.S. Hereditary defect of cobalamin metabolism (cblG mutation) presenting as a neurologic disorder in adulthood. N Engl J Med. 1988;318(26):1738–1741. doi: 10.1056/NEJM198806303182607. [] [DOI] [PubMed] [Google Scholar]
- 23.Surtees R., Leonard J., Austin S. Association of demyelination with deficiency of cerebrospinal fluid S-adenosylmethionine in inborn errors of methyl-transfer pathway. Lancet. 1991;338(8782–8783):1550–1554. doi: 10.1016/0140-6736(91)92373-a. [] [DOI] [PubMed] [Google Scholar]
- 24.Chen H., Liu S., Ji L., Wu T., Ma F., et al. Associations between Alzheimer's disease and blood homocysteine, vitamin B12, and folate: a case-control study. Curr Alzheimer Res. 2015;12(1):88–94. doi: 10.2174/1567205012666141218144035. [DOI] [PubMed] [Google Scholar]
- 25.Nuru M., Muradashvili N., Kalani A., Lominadze D., Tyagi N. High methionine, low folate and low vitamin B6/B12 (HM-LF-LV) diet causes neurodegeneration and subsequent short-term memory loss. Metab Brain Dis. 2018;33(6):1923–1934. doi: 10.1007/s11011-018-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Khamis F.A. Serum vitamin B12 and thyroid hormone levels in Saudi patients with multiple sclerosis. J Fam Community Med. 2016;23(3):151–154. doi: 10.4103/2230-8229.189126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teunissen C.E., van Boxtel M.P., Jolles J., de Vente J., Vreeling F., et al. Homocysteine in relation to cognitive performance in pathological and non-pathological conditions. Clin Chem Lab Med. 2005;43(10):1089–1095. doi: 10.1515/CCLM.2005.190. . [DOI] [PubMed] [Google Scholar]
- 28.Li X., Yuan J., Han J., Hu W. Serum levels of homocysteine, vitamin B12 and folate in patients with multiple sclerosis: an updated meta-analysis. Int J Med Sci. 2020;17(6):751. doi: 10.7150/ijms.42058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroecksnadel K., Frick B., Wirleitner B., Winkler C., Schennach H., Fuchs D. Moderate hyperhomocysteinemia and immune activation. Curr Pharmaceut Biotechnol. 2004;5(1):107–118. doi: 10.2174/1389201043489657. [DOI] [PubMed] [Google Scholar]
- 30.Dardiotis E., Arseniou S., Sokratous M., Tsouris Z., Siokas V., et al. Vitamin B12, folate, and homocysteine levels and multiple sclerosis: a meta-analysis. Mult Scler Relat Disord. 2017;17:190–197. doi: 10.1016/j.msard.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Li X., Yuan J., Han J., Hu W. Serum levels of homocysteine, vitamin B12 and folate in patients with multiple sclerosis: an updated meta-analysis. Int J Med Sci. 2020;17(6):751. doi: 10.7150/ijms.42058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu Y., He Z.Y., Liu H.N. Meta-analysis of the relationship between homocysteine, vitamin B₁₂, folate, and multiple sclerosis. J Clin Neurosci: Off J Neurosurg Soc Australas. 2011;18(7):933–938. doi: 10.1016/j.jocn.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 33.Pan L., Yin Y., Chen J., Ma Z., Chen Y., Deng X.…Wu K. Homocysteine, vitamin B12, and folate levels in patients with multiple sclerosis in Chinese population: a case-control study and meta-analysis. Mult Scler R Relat Disord. 2019;36:101395. doi: 10.1016/j.msard.2019.101395. [DOI] [PubMed] [Google Scholar]
- 34.Wade D.T., Young C.A., Chaudhuri K.R., Davidson D.L. A randomised placebo controlled exploratory study of vitamin B-12, lofepramine, and L-phenylalanine (the “Cari Loder regime”) in the treatment of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2002;73(3):246–249. doi: 10.1136/jnnp.73.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loder C., Allawi J., Horrobin D.F. Treatment of multiple sclerosis with lofepramine, L-phenylalanine and vitamin B(12): mechanism of action and clinical importance: roles of the locus coeruleus and central noradrenergic systems. Med Hypotheses. 2002;59(5):594–602. doi: 10.1016/s0306-9877(02)00261-x. [DOI] [PubMed] [Google Scholar]
- 36.Kira J., Tobimatsu S., Goto I. Vitamin B12 metabolism and massive-dose methyl vitamin B12 therapy in Japanese patients with multiple sclerosis. Intern Med. 1994;33(2):82–86. doi: 10.2169/internalmedicine.33.82. [DOI] [PubMed] [Google Scholar]
- 37.Ghonimi N.A., Elsharkawy K.A., Abdel Ghani A.A., Khyal D. Zagazig university medical journal; 2020. Serum levels of thyroid hormones in relapsing remitting multiple sclerosis. [Google Scholar]
- 38.Tsigalou C., Vallianou N., Dalamaga M. Autoantibody production in obesity: is there evidence for a link between obesity and autoimmunity? Curr Obes Rep. 2020;9(3):245–254. doi: 10.1007/s13679-020-00397-8. [DOI] [PubMed] [Google Scholar]
- 39.Karampela I., Sakelliou A., Vallianou N., Christodoulatos G.S., Magkos F., Dalamaga M. Vitamin D and obesity: current evidence and controversies. Curr Obes Rep. 2021;10(2):162–180. doi: 10.1007/s13679-021-00433-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.