Abstract
Resuscitative endovascular balloon occlusion of the aorta is a tool that can play an important role for the modern-day Trauma Surgeon. Although the concept of aortic balloon occlusion is not new, its use as a rescue device for managing life-threatening traumatic hemorrhage has increased dramatically. The ideal role for resuscitative endovascular balloon occlusion of the aorta continues to evolve. In situations of noncompressible truncal hemorrhage, its use can temporize bleeding while other means of hemorrhage control, including those discussed elsewhere in this supplement, are used. However, it is a tool with potentially significant complications and consequences. Studies examining resuscitative endovascular balloon occlusion of the aorta are ongoing as, despite its ever-increasing adoption, quality evidence to support its clinical use is lacking.
Resuscitative endovascular balloon occlusion of the aorta (REBOA) is a tool that can play an important role for the modern-day Trauma Surgeon. Although the concept of aortic balloon occlusion (ABO) is not new, its use as a rescue device for managing life-threatening traumatic hemorrhage has increased dramatically [[1], [2], [3], [4], [5], [6]]. The ideal role for REBOA continues to evolve. In situations of noncompressible truncal hemorrhage, its use can temporize bleeding while other means of hemorrhage control, including those discussed elsewhere in this supplement, are used [7]. However, it is a tool with potentially significant complications and consequences [[8], [9], [10]]. Studies examining REBOA are ongoing as, despite its ever-increasing adoption, quality evidence to support its clinical use is lacking [5,[11], [12], [13]].
THE END USER
REBOA itself is not a solution for hemorrhage but is simply a bridge that provides extra time for the implementation of definitive bleeding control techniques. As a stop gap, its use is not indefinite. Beyond 30 minutes for abdominal hemorrhage and beyond 60 minutes for pelvic hemorrhage, REBOA may, in fact, contribute to patient complications and demise [14]. As a result, REBOA should be used only by those technically skilled in its application and experienced in decision making surrounding its use [15]. Although a discussion of whether it should be the vascular surgeon, interventional radiologist, trauma/acute care surgeon, and/or emergency room physician who use REBOA is beyond the scope of this chapter, it is clear that it should not be used unless the patient is in a location where definitive hemorrhage control be rapidly provided [14]. Ultimately, this means a hospital setting in which a trauma or vascular surgeon and an operating room are immediately available. Such a facility must also have organizational familiarity with REBOA, with clear protocols in place for its use, so that decision making and care flow are streamlined and efficient [7,13,16]. Emergency physicians, trauma surgeons, anesthesiologists, interventional radiologists, critical care physicians, and nurses involved in trauma care must be familiar with REBOA and aware of its strengths, limitations, and potential complications before its use is adopted. The American College of Surgeons Committee on Trauma and the American College of Emergency Physicians have released 2 important joint statements in this regard on the clinical use of REBOA that are important to review [7,14].
DEFINING AORTIC ZONES
When discussing REBOA, 3 aortic zones are used: Zone 1 extends from the left subclavian artery down to the level of the celiac artery ostium, Zone 2 extends from the celiac artery to below the take-off of the renal arteries, and Zone 3 extends from below the origin of the renal arteries to the aortic bifurcation [17]. Using external landmarks, Zone 1 is roughly at the level of the xiphoid process, whereas Zone 3 is roughly at the level of the umbilicus. It is important to note that these aortic zones are different than the zones (Zones 0–11) that are used in the fields of cardiac and vascular surgery [18]. They do not correlate at all and must not be confused.
INDICATIONS
Although ABO is not a new concept, its use for resuscitation in trauma is fairly novel and continues to be questioned, studied, modified, and refined. As a result, clear indications and contraindications for its role have not been fully established. High-quality evidence regarding its ability to improve survival and long-term outcomes compared to contemporary trauma care is lacking [5,[11], [12], [13]]. Expert consensus suggests that its primary role is to act as a bridge to definitive hemorrhage control in situations of refractory hypovolemic shock secondary to acute subdiaphragmatic bleeding [7,14]. In select situations of cardiac arrest from noncompressible torso hemorrhage, many consider REBOA an alternate to the highly morbid resuscitative thoracotomy.
At its simplest level, ABO performs 2 basic tasks: (1) deceasing distal hemorrhage by minimizing arterial flow below the level of the inflated balloon and (2) improving central perfusion pressure by increasing afterload proximal to the inflated balloon. When inflated in Zone 3, REBOA limits hemorrhage from both pelvic fractures and vascular injuries of the iliac arteries, lower extremities, and the junctional region of the groin. Placed more proximally, in Zone 1, REBOA can also decrease bleeding from visceral injuries, including those to the liver, spleen, kidneys, and retroperitoneum. REBOA has also been used in cases of severe obstetrical bleeding, including placenta accreta spectrum [19,20]. In both zones, the elevation in central perfusion pressure provided by REBOA increases flow to the coronary vessels and to the brain. This provides the resuscitation team a short window of time to (A) rapidly increase the volume of a smaller, more critical, fluid circuit; (B) attempt to achieve return to spontaneous circulation in the setting of a cardiac arrest by increasing coronary blood flow; and (C) identify the hemorrhage source and determine the ideal location and strategy for its definitive management.
CONTRAINDICATIONS
REBOA has several established contraindications. It does not replace the resuscitative thoracotomy when refractory hypotension or cardiac arrest is secondary to obstructive shock. In cases of tension pneumothorax or cardiac tamponade, urgent decompression is essential and REBOA use may only delay a life-saving intervention. Although ABO replaces the physical aortic cross-clamp used in a resuscitative thoracotomy, it is of no assistance in treating hemorrhage above the diaphragm. Cardiac injuries, axillary junctional injuries, and injuries to the aorta, pulmonary vessels, or arch vessels including the innominate, carotid, or subclavian arteries are not treated or temporized by REBOA placement [[2], [3], [4], [5], [6],21,22]. In fact, in these situations, ABO may increase hemorrhage by raising the perfusion pressure to the site of the bleed. As a result, prior to REBOA use, a chest radiograph should be obtained to rule out a significant thoracic injury.
For similar reasons, REBOA use is contraindicated in patients with suspected traumatic brain injury. Increasing the perfusion pressure to the brain by increasing cardiac afterload may worsen intracranial hemorrhage.
REBOA is further contraindicated when a subdiaphragmatic aortic injury or a traumatic dissection is suspected, as there is a risk of the catheter or balloon worsening the injury [2]. It should also be used with an abundance of caution in patients with a significant burden of atherosclerotic disease, known aneurysmal disease, or known previous peripheral or abdominal vascular surgery interventions.
Although REBOA use is considered contraindicated in cases of thoracic, cervical, and intracranial trauma, some groups are exploring its use in patients with these injuries [23]. Some argue that REBOA use could, in fact, reduce secondary brain injury by improving cerebral perfusion in the hypotensive patient. These areas of REBOA use remain extremely controversial. The use of REBOA in other settings, including in pediatrics, the prehospital setting, and for gastrointestinal bleeds, also remains controversial.
Outside of its use for hemorrhage control, REBOA has been proposed as an adjunct for resuscitation in nontraumatic cardiac arrest [24]. In this setting, its use to increase afterload and to maximize perfusion to the heart and the brain during cardiopulmonary resuscitation (CPR) has been proposed. As a minimally invasive aortic "cross-clamp" in this setting, REBOA creates a smaller central fluid circuit that may maximize the central concentration of vasoactive Advanced Cardiac Life Support drugs. REBOA use in this setting is controversial.
ACCESS
The first step in REBOA use is to gain access to the femoral artery. For the most part, this is done via the right groin for ease of use. A left-handed practitioner may choose to access the left femoral artery instead. However, patient factors including scar tissue in the groin suggestive of previous intervention, a junctional injury, or an injury pattern suggesting a side to a pelvic or extremity vascular injury should influence the decision on which side is accessed.
The common femoral artery (CFA) is located just below the inguinal ligament one third of the distance from the pubic tubercle to the anterior superior iliac spine. It sits just anterior to the femoral head between the femoral nerve and vein and is 4 cm in length, on average, before it divides into the superficial femoral and profunda femoris arteries [25]. Access to the CFA is essential to minimize the risk of a life-threatening retroperitoneal hemorrhage from an external iliac artery puncture (access that is too high) and to minimize the risk of distal arterial occlusion and limb ischemia from a superficial femoral artery puncture (access that is too low) [8,9].
Although rapid needle access to the CFA is vital to deploy REBOA, it is commonly the most rate-limiting step to ABO use and significant complications can occur [8,9,26]. Viable options to assist with access include ultrasound (U/S)-guided percutaneous puncture or an open arterial cutdown (either vertical or oblique). The surgeon should perform whichever approach they have the most experience with to access the CFA. Many use the U/S-guided percutaneous approach in a severely hypotensive patient but turn to a vertical femoral artery cutdown in a patient undergoing CPR because visualizing and successfully puncturing the CFA under U/S in a pulseless patient receiving chest compressions is extremely difficult.
When using the U/S-guided percutaneous approach, the operator should identify the CFA as a noncompressible, thick-walled, lateral vessel as compared to the compressible, thin-walled, medial femoral vein. The femoral artery may also be visually pulsatile depending on the clinical circumstance. Proximally, the inguinal ligament can be visualized, and at this point, the CFA dives deep to become the external iliac artery. The vessel should not be accessed this high. Distally, the CFA splits into the superficial femoral artery and the profunda femoris. If the 3 vessels of similar caliber are visualized, this is too low and the puncture site should be moved proximally.
When using a cutdown, the most reliable anatomical landmark is the inguinal ligament. The surgeon should identify this landmark first and then move both more distal and deeper. The femoral sheath emerges just below the medial third of the inguinal ligament and should be opened to identify the CFA laterally and femoral vein medially. The artery can be readily identified from the vein by the vasa vasorum found on its adventitial layer. The artery should be exposed 2–3 cm distally and punctured on its branchless anterior boarder.
Blind percutaneous access using external anatomic landmarks is difficult and can lead to significant access complications. In a severely hypotensive or pulseless patient, the femoral pulse will be nonpalpable and blood from the artery may be difficult to differentiate from that of the vein because the color and pressure may be indistinguishable. The blind approach is not ideal.
Most commonly, arterial access is obtained with a micropuncture needle (4F), the needle from a femoral arterial line kit, or a Seldinger needle.
INSERTION AND CONFIRMATION
The steps required for insertion and positioning of the aortic balloon depend upon the specific REBOA catheter that is used. In general, the Seldinger technique is used in which a guidewire is inserted through the access needle and the puncture site is then dilated up until an introducer sheath of appropriate size to accommodate the REBOA catheter is in position (Fig 1). The introducer sheath's dilator is then removed. The balloon is then flushed and inserted through the sheath and advanced to the appropriate position (either Zone 1 or Zone 3). Although the ideal is to insert and advance the balloon under live fluoroscopy with either a mobile c-arm or built-in fluoroscopy unit, this is a tool that is not available in most trauma bays. As a result, advancement is usually done blindly and should have minimal resistance. If resistance is encountered, the device should not be forced forward and troubleshooting must be undertaken.
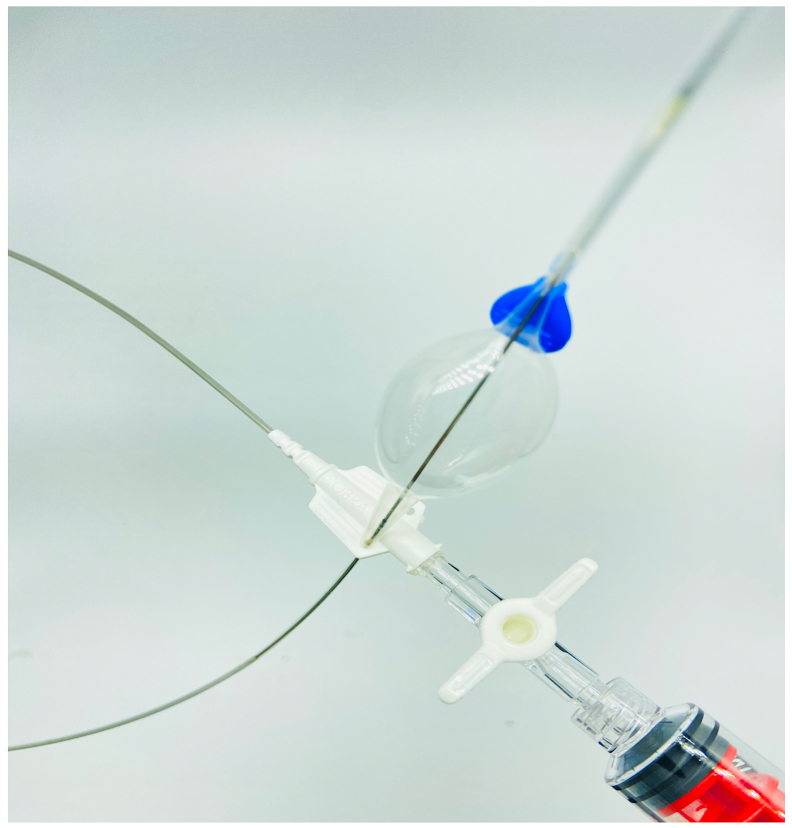
Fig 1.
An image of the access kit that accompanies the COBRA-OS (FrontLine Medical Technologies Inc) showing the arterial micropuncture needle, the guidewire, and the 4F sheath and inner cannula. Copyright FrontLine Medical Technologies Inc. Reprinted with permission.
Once the device has been advanced into the aorta, its position in either Zone 1 or 3 should be confirmed before the balloon is inflated. As fluoroscopy is not generally available, other methods to verify position are often used, with radiography being the most common (Fig 2). Many REBOA catheters and aortic balloons have radio-opaque markers that identify the proximal and distal edges of the balloon. For those that do not, the balloon can be inflated with a mixture of saline and contrast to identify the balloon position (Fig 3).
Fig 2.
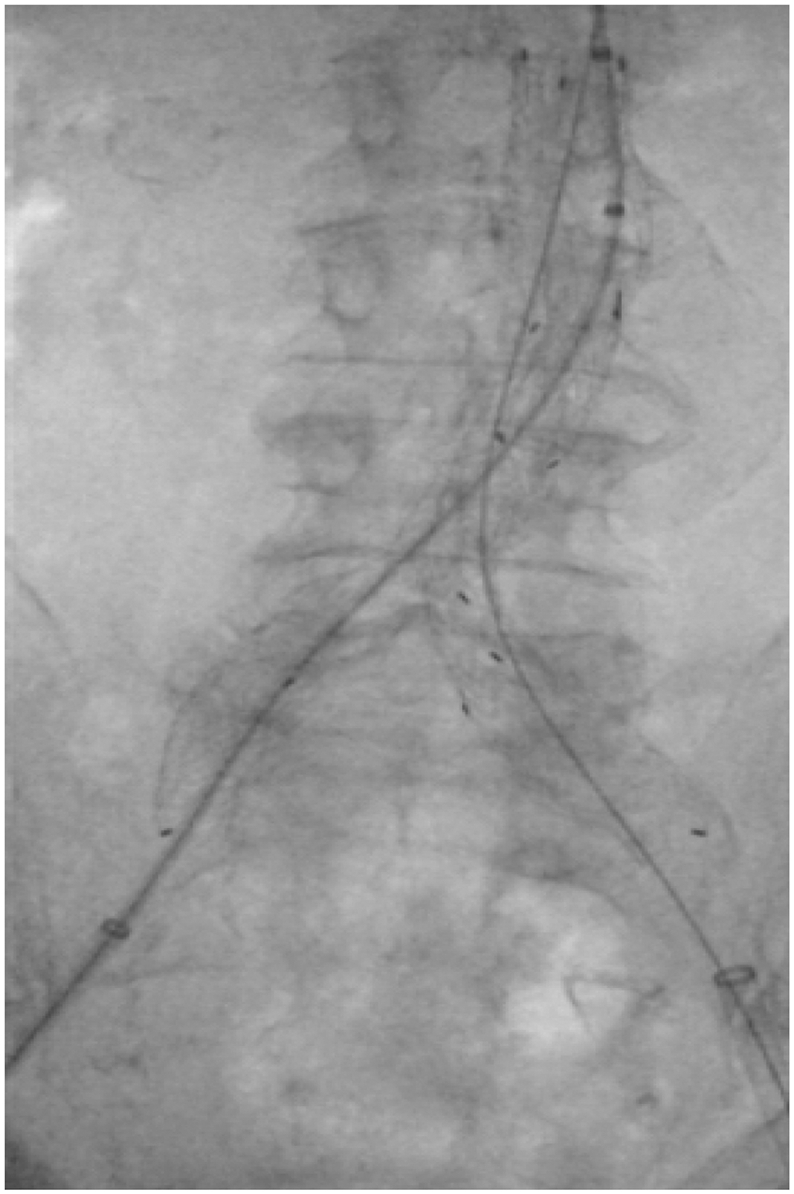
An image of an aortic balloon with its proximal and distal markers positioned in Zone 3 prior to inflation. In this case, in the setting of an elective endovascular abdominal aortic aneurysm repair via the right common femoral artery. A REBOA balloon positioned in Zone 3 would have a similar appearance on radiography or under fluoroscopy.
Fig 3.
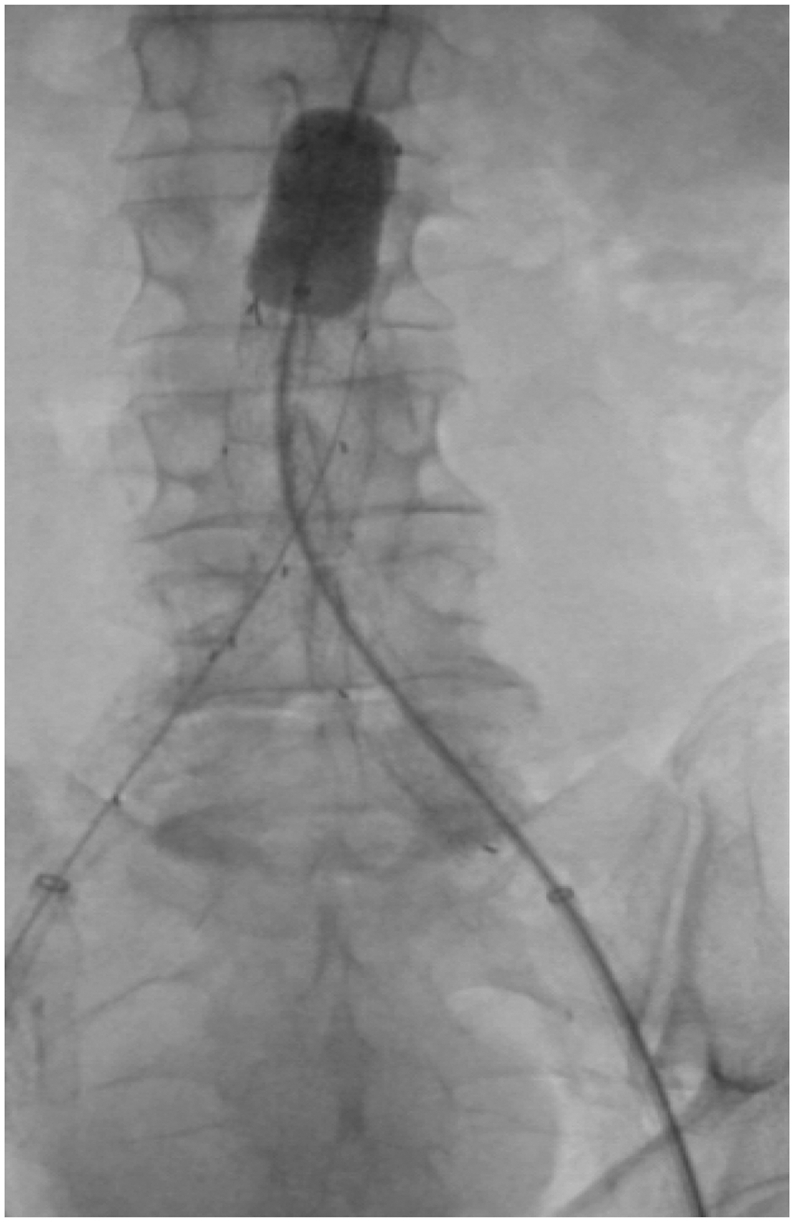
An image of an aortic balloon inflated in Zone 3 using a mix of contrast and saline. In this case, in the setting of an elective endovascular abdominal aortic aneurysm repair via the left common femoral artery. A REBOA balloon inflated with contrast would have a similar appearance on radiography or under fluoroscopy.
Ultrasound can also be used to confirm balloon location [27]. A transhepatic view just below the ribs can often be used to visualize Zone 1 of the aorta. Body habitus and bowel gas can limit the utility of this technique, especially for Zone 3.
Finally, in some circumstances, such as when REBOA is inserted during CPR, blind placement may be considered. External anatomic landmarks are helpful in this scenario with the distance from the groin to the xiphoid being a rough guide for Zone 1 and that from the groin to the umbilicus being a rough guide for Zone 3. In an average-sized adult, Zone 1 is 46 cm from the groin, whereas Zone 3 is 28 cm from the groin [17,[28], [29], [30]]. If blind placement is used, confirmation of balloon location should be obtained as soon as is reasonably feasible. Of note, Zone 2 is most commonly located between T12 and L2. Balloon positioning between these vertebrae should be avoided.
INFLATION
Although saline or sterile water alone can be used, a mixture of contrast and saline for balloon inflation allows for easy visualization under radiography or fluoroscopy. Contrast alone is very viscous and can lead to difficulties with both balloon inflation and deflation. A 50%:50% or 33%:66% solution of contrast to saline minimizes this issue.
In general, less than 10 mL of solution is sufficient (depending on the catheter). Balloon overinflation can lead to balloon rupture, arterial dissection, or vessel rupture and should be avoided. Slow inflation with a few milliliters at a time is ideal. Inflation should be stopped when the pulse in the contralateral limb is no longer palpable, a measured distal arterial pressure diminishes, or the proximal arterial pressure increases to the desired level, depending on the form of access and monitoring that is available. Inflation should also be halted if any resistance is felt, even if these above goals are not met, to prevent a vascular injury. Once inflated, the stopcock on the catheter should be closed so that balloon inflation is maintained. The time of occlusion should be clearly documented, and ideally, a timer should be started so that the urgency of the situation is emphasized and maintained. With an increase in central pressure, the balloon may migrate distally. To minimize this, the system should be soundly secured, even manually if necessary.
PROXIMAL MONITORING
Measurement of perfusion pressure proximal to the REBOA balloon allows for the care team to evaluate and accurately achieve their resuscitation goals. Although a noninvasive blood pressure cuff is helpful, it does not provide a dynamic evaluation of the patient's central perfusion pressure. Direct intra-arterial pressure monitoring is preferred. Some REBOA catheters include a built-in lumen that extends proximal to the balloon and can be transduced to measure the central pressure. If this is not available, a proximal arterial line, most commonly placed in the radial artery, can monitor the central pressure. In a patient with an upper extremity injury, the location of proximal pressure monitoring needs to be considered. Most practitioners advocate for a proximal blood pressure of 80–90 mm Hg systolic after REBOA inflation or slightly higher if a traumatic brain injury has not been ruled out [31].
ZONE PLACEMENT
When placed in Zone 1, REBOA limits arterial flow to the visceral vessels, the pelvis, and the lower extremities. This significantly increases cardiac afterload. However, the duration of acceptable inflation in this region is limited [14]. Inflation beyond 30 minutes significantly increases the risk of visceral ischemia, which can be fatal in itself, and increases the risk of hypotension and circulatory collapse from the return of the anaerobic metabolites of ischemic reperfusion after balloon deflation. Zone 1 REBOA must be used with extreme caution. Absolute focus on hemorrhage control with the goal of balloon complete deflation, partial deflation, or repositioning in Zone 3 as soon as possible is essential to prevent organ ischemia or circulatory collapse during reperfusion.
Zone 3 deployment is generally better tolerated because visceral vessel perfusion is maintained and only pelvic and lower extremity perfusion is limited. These regions are able to better tolerate a longer ischemic time. Inflation times of 30–60 minutes are likely acceptable [14]. However, the risk of organ and limb ischemia and reperfusion injury still remains with deployment in Zone 3, and expedient hemorrhage control followed by balloon deflation remains the priority.
In cases of hemodynamic collapse, Zone 1 deployment can be considered even if the suspected source is, based on physical examination, focused assessment with sonography in trauma examination, or radiography, to be from a pelvic or lower extremity injury [2]. A Zone 1 balloon increases afterload and central pressure. Once proximal access and resuscitation are initiated and a reasonable proximal perfusion pressure is achieved, the balloon can be deflated, moved distally, and reinflated in Zone 3 prior to definitive hemorrhage control. To minimize the visceral ischemic time and the severity of the reperfusion injury, the transition from Zone 1 to Zone 3 should be as expeditious as possible. Moving the balloon distally requires deflation, partial withdrawal of the catheter, reconfirmation of the balloon position, and reinflation. A brief period of hypotension can be expected with this transition as (A) the proximal circulating volume increases by reintroducing the splanchnic circulation, (B) there is a reperfusion of the visceral organs leading to the release of anaerobic metabolites, and (C) distal hemorrhage may increase as the occlusion proximal to the site of injury is briefly removed.
Zone 2 deployment is contraindicated due to the risk of dissection and thrombosis of the visceral vessels.
DEVICES
REBOA technology was adapted from endovascular surgery where balloons have been used for many years for "ironing" out the fabric of stent grafts used to treat aortic aneurysms. As a result, the endovascular balloons used by vascular surgeons (Cook Medical Technologies LLC Coda Balloon Catheter and Medtronic Vascular, Inc, Reliant Stent Graft Balloon Catheter) that were initially used by trauma surgeons for REBOA were done so off-label. These balloons require large access sheaths (minimum 12 F), and their positioning is dependent on long and cumbersome guidewires. This makes deployment in the trauma setting logistically challenging. More recently, many trauma-specific REBOA catheters have been developed, approved, and marketed (FrontLine Medical Technologies Inc COBRA-OS [Fig 4], Prytime Medical ER-REBOA PLUS [Fig 5], and Tokai Medical Products Rescue Balloon, among others). Their availability and approval are dependent on the jurisdiction, but they offer many advantages over the Coda and Reliant balloons that facilitate easier use and minimize potential complications. Depending on the device, these features include being significantly lower profile (as small as 4F), having an atraumatic tip, being wire free, allowing proximal blood pressure monitoring, and having built-in measurement markers and safety reservoirs. Familiarity with a device is vital to the minimize morbidity associated with REBOA.
Fig 4.
An image of the COBRA-OSTM (FrontLine Medical Technologies Inc) showing the traumatic flexible J-tip, the inflated compliant offset aortic balloon, the RO proximal and distal balloon markers, the J-tip straightener, the safety shoulder reservoir, the balloon inflation port, and the inflation syringe. Copyright FrontLine Medical Technologies Inc. Reprinted with permission.
Fig 5.
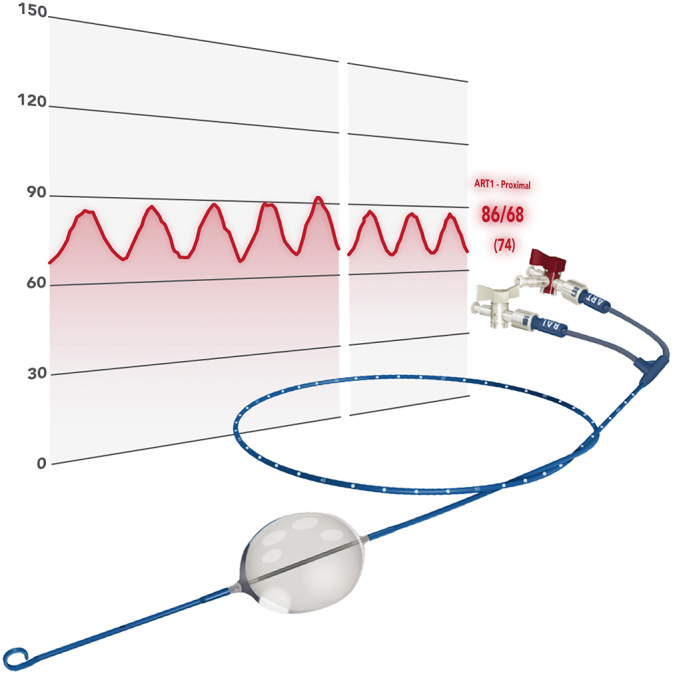
An image of the ER-REBOA PLUS Catheter (Prytime Medical Devices Inc) showing the atraumatic P-tip, the inflated aortic balloon, measurement markers along the catheter, the balloon inflation port, and the arterial port and integrated arterial line which allow monitoring of the arterial pressure above the inflated balloon as depicted in the background. Copyright Prytime Medical. Reprinted with permission.
WHERE NEXT?
When a REBOA balloon is inflated and the proximal blood pressure stabilizes to "normal," there is a tendency for urgency to be lost and time wasted. Unlike resuscitative thoracotomy, the patient's chest is not open with an obvious clamp in place. This normotensive inertia is a serious threat to the patient. An inflated REBOA balloon must be given the same respect as an aortic cross-clamp, and the patient must be taken emergently for definitive hemorrhage control. As a result, after a REBOA balloon has been inflated and resuscitation has been initiated, where to take the patient next is the immediate subsequent question. There are only 2 reasonable answers: the operating room or the angiography suite. Formal diagnostic imaging, including CT, should not be obtained, as spending the time required for such imaging rapidly narrows the brief time window available to obtain hemorrhage control. Delays that lead to longer REBOA inflation times contribute to visceral ischemia and greater reperfusion injury resulting in increased morbidity.
Taking a patient to the operating room allows for the patient to undergo ongoing balanced resuscitation by anesthesia simultaneously with procedures including laparotomy, pelvic packing, pelvic stabilization, junctional injury repair, or extremity vascular injury management for definitive hemorrhage control prior to balloon deflation.
Taking a patient to angiography may also be considered in select clinical situations because it allows for simultaneous diagnostic and therapeutic management with the ability to treat hemorrhage via angio-embolization. This is particularly useful in patients with significant pelvic hemorrhage but can also be of use in patients with other infradiaphragmatic bleeds, including splenic, hepatic, and lumbar vessel hemorrhage. In the angiography suite, contralateral femoral access can be obtained with the REBOA balloon still inflated, allowing the interventionalist to both potentially diagnose and treat the source of hemorrhage. Often, the balloon will have to be intermittently deflated for appropriate diagnostic imaging to be obtained in this scenario.
The ideal, of course, is a hybrid OR, where both open surgical and interventional techniques are available and can be completed either concurrently or sequentially without the patient having to be moved. Although these facilities are becoming more common, they are still not the reality in most institutions.
pREBOA/iREBOA
Although balloon deflation is a priority to minimize ischemic time and reperfusion injury, if hemorrhage control has not been accomplished, complete balloon deflation may rapidly result in a return to a state of hypovolemic shock. Two strategies, intermittent REBOA (iREBOA) and partial REBOA (pREBOA), have been proposed to manage this circumstance [[32], [33], [34]].
With iREBOA, the balloon is repeatedly inflated and deflated for short intervals of time to allow for periods of distal perfusion [32]. This theoretically minimizes the distal ischemic time and burden of reperfusion injury. This technique is also be used to locate a distal source of hemorrhage in a controlled manner, for example, during angiography, and may in fact expedite hemorrhage control through more rapid identification of the source [31]. The downside of this strategy is that it can be difficult to monitor and control. It tends to be reactive and may lead to wide swings in blood pressure that can complicate resuscitation.
With pREBOA, either the balloon is partially inflated initially, or after full inflation, the balloon is slowly deflated a few milliliters at a time, titrating to a targeted proximal systolic blood pressure [34]. As proximal venous access is obtained and balanced resuscitation is initiated, this partial balloon inflation permits some distal perfusion. As long as the resuscitation can keep up with the hemorrhagic loss below the balloon to maintain an acceptable central perfusion pressure, this strategy may extend the useful time of REBOA as it decreases the risk of a highly morbid reperfusion injury.
DEFLATION
Although balloon deflation is a priority after hemorrhage control is obtained, it should be done cautiously. The proximal blood pressure should be closely monitored and controlled. With deflation, a larger volume fluid circuit is created, and there is a return of the end products of anaerobic metabolism, including lactic acid and potassium, from the distal ischemic tissues [35]. A flood of these metabolites can lead to hypotension and circulatory collapse. By slowly deflating the balloon, the return of these metabolites can be controlled and effectively managed. Prior to balloon deflation, the patient should be well resuscitated, ideally with balanced blood products, and the need for further fluids should be expected as the perfused circuit increases in volume. Vasopressor use may be necessary. Withdrawing 1–2 mL of fluid every 30 seconds is advised [31]. If a patient develops significant hypotension during deflation, the balloon may have to be reinflated to provide afterload. Further resuscitation will be necessary, and other sources of hemorrhage should be ruled out prior to further deflation attempts.
Once the balloon is completely deflated and is no longer required, it should be removed as rapidly as possible. The presence of an uneven foreign object, such as a deflated balloon, leads to turbulent blood flow acts as a nidus for thrombus development that can lead to complications including local occlusion or distal embolization. The introducer sheath is smoother, is less thrombogenic, and can be flushed intermittently or continuously with saline or heparin–saline, thereby minimizing, but not removing, its risk of leading to thrombus formation.
VASCULAR MONITORING
While the introducer sheath is in place, the vascular status of the ipsilateral limb should be closely monitored. This includes frequent checks (ideally every hour) of the patient's pedal arteries (both posterior tibial and dorsalis pedis) via either palpation or Doppler monitoring [31]. The sheath should be flushed continuously to minimize the risk of thrombus formation and possible local occlusion or distal embolization. Although heparinized saline (1000 U heparin in 500 mL 0.9% normal saline) would ideally be used, normal saline is often preferred in the post hemorrhage trauma patient. Any signs of distal ischemia should be investigated and managed urgently.
SHEATH REMOVAL
A critical final step when REBOA is used is the safe removal of the introducer sheath. Familiarity with the French size of the introducer sheath is essential. Establishing a close relationship with local interventional radiologists and vascular surgeons should be part of all REBOA programs [16]. The expertise of these services is necessary to assist with the removal of larger introducers sheaths, to perform vessel repair and soft tissues closure when appropriate, and to assist with the management of any access site complications including vessel occlusion, distal embolization, pseudoaneurysm formation, and hemorrhage. Integrating these services into a REBOA program minimizes potential harm.
Timely introducer sheath removal minimizes the risk of local thrombosis and distal embolization. The larger is the sheath being used, the greater is the risk that it is limiting flow and causing ischemia to an extremity. However, prior to sheath removal, a patient's coagulation status must be optimized to minimize the risk of hemorrhage and pseudoaneurysm formation. Patients requiring REBOA often present with, or develop, trauma-induced coagulopathy. Whether using conventional coagulation tests or thromboelastography, coagulation markers should be normalized prior to sheath removal. The exception to this rule is when an experienced interventional radiologist or vascular surgeon decides to use vascular closure device or the sheath is removed and vessel repaired in an open fashion in the operating room. Sheaths that are 7F or smaller can be removed at the bedside and the arterial puncture managed with direct manual pressure (with 3 minutes per French size as a good rule of thumb) as long as the patient's coagulation markers have normalized. If there are any concerns about removing a sheath that is 7F or smaller at the bedside, interventional radiology or vascular surgery should be consulted BEFORE the sheath is removed. A sheath larger than 7F should be removed by an interventional radiologist or vascular surgeon with the use of a closure device or via an open surgical arterial repair in the operating room.
Distal perfusion after sheath removal should be checked immediately. Manual palpation of pedal pulses, performance of an ankle brachial index with a normal result, and obtaining a distal angiogram are all means by which this can be accomplished (Fig 6). Once the sheath is removed, the patient should be kept on strict bedrest and completely supine with no hip flexion for 6 hours. Imaging of the groin 24–48 hours, usually via ultrasound, after sheath removal should be obtained to rule out any access site complications, including pseudoaneurysm formation.
Fig 6.
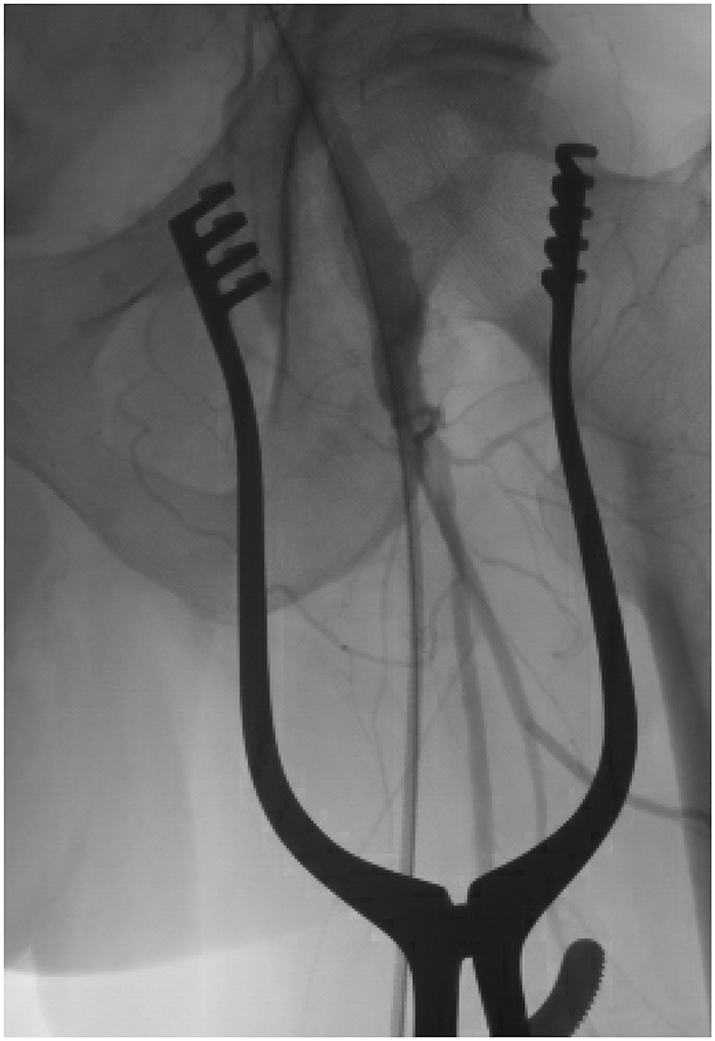
An angiographic image of the left common femoral artery, superficial femoral artery, and profunda femoris artery confirming flow into each of these vessels after an open femoral cutdown. If there is any doubt about distal vascular embolization or occlusion, these images should be obtained prior to sheath removal.
SUMMARY
Ultimately, REBOA is a powerful tool that is a modern adjunct in the armamentarium available to the trauma surgeon. Although it has many potential roles, including replacing the resuscitative thoracotomy in some situations, there are many nuances and subtleties to REBOA that must be understood and appreciated. If the trauma surgeon chooses to use this tool, it is imperative that the remainder of the team, from nursing to anesthesia to critical care, understands its function and role. Although REBOA can be lifesaving, it does not resolve hemorrhage but only temporizes it and, at its simplest, must be recognized as a device that can bridge a gap that may exist between hemodynamic collapse and hemorrhage control.
Author Contribution
Dr Cantle wrote and edited all components of this manuscript.
Conflict of Interest
Dr Cantle has no conflicts of interest to declare.
Funding Source
There was no funding for this article.
Ethics Approval
No patient data were included in this manuscript. No ethics approval was obtained.
References
- 1.Hughes C.W. Use of an intra-aortic balloon catheter tamponade for controlling intra- abdominal hemorrhage in man. Surgery. 1954;36:65–68. [PubMed] [Google Scholar]
- 2.Stannard A., Eliason J.L., Rasmussen T.E. Resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct for hemorrhagic shock. J Trauma. 2011;71:1869–1872. doi: 10.1097/TA.0b013e31823fe90c. [DOI] [PubMed] [Google Scholar]
- 3.Brenner M.L., Moore L.J., DuBose J.J., Tyson G.H., McNutt M.K., Albarado R.P., et al. A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. J Trauma Acute Care Surg. 2013;75:506–511. doi: 10.1097/TA.0b013e31829e5416. [DOI] [PubMed] [Google Scholar]
- 4.Moore L.J., Brenner M., Kozar R.A., Pasley J., Wade C.E., Baraniuk M.S., et al. Implementation of resuscitative endovascular balloon occlusion of the aorta as an alternative to resuscitative thoracotomy for noncompressible truncal hemorrhage. J Trauma Acute Care Surg. 2015;79:523–532. doi: 10.1097/TA.0000000000000809. [DOI] [PubMed] [Google Scholar]
- 5.Moore L.J., Martin C.D., Harvin J.A., Wade C.E., Holcomb J.B. Resuscitative endovascular balloon occlusion of the aorta for control of noncompressible truncal hemorrhage in the abdomen and pelvis. Am J Surg. 2016;212:1222–1230. doi: 10.1016/j.amjsurg.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Brenner M., Teeter W., Hoehn M., Pasley J., Hu P., Yang S., et al. Use of resuscitative endovascular balloon occlusion of the aorta for proximal aortic control in patients with severe hemorrhage and arrest. JAMA Surg. 2018;153:130–135. doi: 10.1001/jamasurg.2017.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner M., Bulger E.M., Perina D.G., Henry S., Kang C.S., Rotondo M.F., et al. Joint statement from the American College of Surgeons Committee on Trauma (ACS COT) and the American College of Emergency Physicians (ACEP) regarding the clinical use of resuscitative endovascular balloon occlusion of the aorta (REBOA) Trauma Surg Acute Care Open. 2018;3:1–3. doi: 10.1136/tsaco-2017-000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson A.J., Russo R.M., Reva V.A., Brenner M.L., Moore L.J., Ball C., et al. The pitfalls of REBOA: risk factors and mitigation strategies. J Trauma Acute Care Surg. 2017;84:192–202. doi: 10.1097/TA.0000000000001711. [DOI] [PubMed] [Google Scholar]
- 9.Ribeiro Junior M.A.F., Feng C.Y.D., Nguyen A.T.M., Rodrigues V.C., Bechara G.E.K., de-Moura R.R., et al. The complications associated with resuscitative endovascular balloon occlusion of the aorta (REBOA) World J Emerg Surg. 2018;13:20. doi: 10.1186/s13017-018-0181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manzano-Nunez R., Orlas C.P., Herrera-Escobar J.P., Galvagno S., DuBose J., Melendez J.J., et al. A meta-analysis of the incidence of complications associated with groin access after the use of resuscitative endovascular balloon occlusion of the aorta in trauma patients. J Trauma Acute Care Surg. 2018;85:626–634. doi: 10.1097/TA.0000000000001978. [DOI] [PubMed] [Google Scholar]
- 11.Bekdache O., Paradis T., Shen Y.B.H., Elbahrawy A., Grushka J., Deckelbaum D.L., et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA): a scoping review protocol concerning indications-advantages and challenges of implementation in traumatic non-compressible torso haemorrhage. BMJ Open. 2019;9(2) doi: 10.1136/bmjopen-2018-027572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joseph B., Zeeshan M., Sakran J.V., Hamidi M., Kulvatunyou N., Khan M., et al. Nationwide analysis of resuscitative endovascular balloon occlusion of the aorta in civilian trauma. JAMA Surg. 2019;154:500–508. doi: 10.1001/jamasurg.2019.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doucet J., Coimbra R. REBOA: is it ready for prime time? J Vasc Bras. 2017;16:1–3. doi: 10.1590/1677-5449.030317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bulger E.M., Perina D.G., Qasim Z., Beldowicz B., Brenner M., Guyette F., et al. Clinical use of resuscitative endovascular balloon occlusion of the aorta (REBOA) in civilian trauma systems in the USA, 2019: a joint statement from the American College of Surgeons Committee on Trauma, the American College of Emergency Physicians, the National Association of Emergency Medical Services Physicians and the National Association of Emergency Medical Technicians. Trauma Surg Acute Care Open. 2019;4 doi: 10.1136/tsaco-2019-000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner M., Hoehn M., Pasley J., Dubose J., Stein D., Scalea T. Basic endovascular skills for trauma course: bridging the gap between endovascular techniques and the acute care surgeon. J Trauma Acute Care Surg. 2014;77:286–291. doi: 10.1097/TA.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 16.Zakaluzny S.A., Beldowicz B.C., Salcedo E.S., DuBose J.J., Moore L.J., Brenner M. Guidelines for a system-wide multidisciplinary approach to institutional resuscitative endovascular balloon occlusion of the aorta implementation. J Trauma Acute Care Surg. 2019;86:337–343. doi: 10.1097/TA.0000000000002138. [DOI] [PubMed] [Google Scholar]
- 17.Stannard A., Morrison J.J., Sharon D.J., Eliason J.L., Rasmussen T.E. Morphometric analysis of torso arterial anatomy with implications for resuscitative aortic occlusion. J Trauma Acute Care Surg. 2013;75:S169–S172. doi: 10.1097/TA.0b013e31829a098d. [DOI] [PubMed] [Google Scholar]
- 18.Lombardi J.V., Hughes G.C., Appoo J.J., Bavaria J.E., Beck A.W., Cambria R.P., et al. Society for Vascular Surgery (SVS) and Society of Thoracic Surgeons (STS) reporting standards for type B aortic dissections. J Vasc Surg. 2020;71:723–747. doi: 10.1016/j.jvs.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Whittington J.R., Pagan M.E., Nevil B.D., Kalkwarf K.J., Sharawi N.E., Hughes D.S., et al. Risk of vascular complications in prophylactic compared to emergent resuscitative endovascular balloon occlusion of the aorta (REBOA) in the management of placenta accreta spectrum. J Matern Fetal Neonatal Med. 2020;11:1–4. doi: 10.1080/14767058.2020.1802717. [DOI] [PubMed] [Google Scholar]
- 20.Riazanova O.V., Reva V.A., Fox K.A., Romanova L.A., Kulemin E.S., Riazanov A.D., et al. Open versus endovascular REBOA control of blood loss during cesarean delivery in the placenta accreta spectrum: a single-center retrospective case control study. Eur J Obstet Gynecol Reprod Biol. 2021;258:23–28. doi: 10.1016/j.ejogrb.2020.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Norii T., Crandall C., Terasaka Y. Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score-adjusted untreated patients. J Trauma Acute Care Surg. 2015;78:721–728. doi: 10.1097/TA.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 22.Inoue J., Shiraishi A., Yoshiyuki A., Haruta K., Matsui H., Otomo Y. Resuscitative endovascular balloon occlusion of the aorta might be dangerous in patients with severe torso trauma: a propensity score analysis. J Trauma Acute Care Surg. 2016;80:559–567. doi: 10.1097/TA.0000000000000968. [DOI] [PubMed] [Google Scholar]
- 23.Johnson M.A., Williams T.K., Ferencz S.E., Davidson A.J., Russo R.M., O’Brien W.T., Sr., et al. The effect of resuscitative endovascular balloon occlusion of the aorta, partial aortic occlusion and aggressive blood transfusion on traumatic brain injury in a swine multiple injuries model. J Trauma Acute Care Surg. 2017;83:61–70. doi: 10.1097/TA.0000000000001518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daley J., Morrison J.J., Sather J., Hile L. The role of resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct to ACLS in non-traumatic cardiac arrest. Am J Emerg Med. 2017;35:731–736. doi: 10.1016/j.ajem.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Swift H., Bordoni B. StatPearls Publishing; Treasure Island (FL): 2019. Anatomy, bony pelvis and lower limb, femoral artery. StatPearls. [PubMed] [Google Scholar]
- 26.Romagnoli A., Teeter W., Pasley J., Hu P., Hoehn M., Stein D., et al. Time to aortic occlusion: It’s all about access. J Trauma Acute Care Surg. 2017;83:1161–1164. doi: 10.1097/TA.0000000000001665. [DOI] [PubMed] [Google Scholar]
- 27.Ball C.G., Wilson S.R., Cantle P. Ultrasonography for resuscitative endovascular balloon occlusion of the aorta: a practical leap forward using microbubble contrast agent. J Trauma Acute Care Surg. 2016;81:616–617. doi: 10.1097/TA.0000000000001130. [DOI] [PubMed] [Google Scholar]
- 28.Linnebur M., Inaba K., Haltmeier T., Rasmussen T.E., Smith J., Mendelsberg R., et al. Emergent non-image-guided resuscitative endovascular balloon occlusion of the aorta (REBOA) catheter placement: a cadaver-based study. J Trauma Acute Care Surg. 2016;81:453–457. doi: 10.1097/TA.0000000000001106. [DOI] [PubMed] [Google Scholar]
- 29.Morrison J.J., Stannard A., Midwinter M.J., Sharon D.J., Eliason J.L., Rasmussen T.E. Prospective evaluation of the correlation between torso height and aortic anatomy in respect of a fluoroscopy free aortic balloon occlusion system. Surgery. 2014;155:1044–1051. doi: 10.1016/j.surg.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 30.MacTaggart J.N., Poulson W.E., Akhter M., Seas A., Thorson K., Phillips N.Y., et al. Morphometric roadmaps to improve accurate device delivery for fluoroscopy-free resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg. 2016;80:941–946. doi: 10.1097/TA.0000000000001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubose J.J., Morrison J.J., Reva V.A., Matsumoto J., Matsumura Y., Falkenberg M., et al. Orebro University Hospital Publisher; Orebro (Sweden): 2017. Top stent—the art of endovascular hybrid trauma and bleeding management. [Google Scholar]
- 32.Kuckelman J.P., Barron M., Moe D., Derickson M., Phillips C., Kononchik J., et al. Extending the golden hour for zone 1 resuscitative endovascular balloon occlusion of the aorta: improved survival and reperfusion injury with intermittent versus continuous resuscitative endovascular balloon occlusion of the aorta of the aorta in a porcine severe truncal hemorrhage model. J Trauma Acute Care Surg. 2018;85:318–326. doi: 10.1097/TA.0000000000001964. [DOI] [PubMed] [Google Scholar]
- 33.Matsumura Y., Matsumoto J., Kondo H., Idoguchi K., Ishida T., Kon Y., et al. Fewer REBOA complications with smaller devices and partial occlusion: evidence from a multicentre registry in Japan. Emerg Med J. 2017;34:793–799. doi: 10.1136/emermed-2016-206383. [DOI] [PubMed] [Google Scholar]
- 34.Dubose J.J. How I do it: partial resuscitative endovascular balloon occlusion of the aorta (P-REBOA) J Trauma Acute Care Surg. 2017;83:197–199. doi: 10.1097/TA.0000000000001462. [DOI] [PubMed] [Google Scholar]
- 35.Qasim Z.A., Sikorski R.A. Physiologic considerations in trauma patients undergoing resuscitative endovascular balloon occlusion of the aorta. Anesth Analg. 2017;125:891–894. doi: 10.1213/ANE.0000000000002215. [DOI] [PubMed] [Google Scholar]