Abstract
There is a pressing need for drugs effective against the opportunistic protozoan pathogen Cryptosporidium parvum. Folate metabolic enzymes and enzymes of the thymidylate cycle, particularly dihydrofolate reductase (DHFR), have been widely exploited as chemotherapeutic targets. Although many DHFR inhibitors have been synthesized, only a few have been tested against C. parvum. To expedite and facilitate the discovery of effective anti-Cryptosporidium antifolates, we have developed a rapid and facile method to screen potential inhibitors of C. parvum DHFR using the model eukaryote, Saccharomyces cerevisiae. We expressed the DHFR genes of C. parvum, Plasmodium falciparum, Toxoplasma gondii, Pneumocystis carinii, and humans in the same DHFR-deficient yeast strain and observed that each heterologous enzyme complemented the yeast DHFR deficiency. In this work we describe our use of the complementation system to screen known DHFR inhibitors and our discovery of several compounds that inhibited the growth of yeast reliant on the C. parvum enzyme. These same compounds were also potent or selective inhibitors of the purified recombinant C. parvum DHFR enzyme. Six novel lipophilic DHFR inhibitors potently inhibited the growth of yeast expressing C. parvum DHFR. However, the inhibition was nonselective, as these compounds also strongly inhibited the growth of yeast dependent on the human enzyme. Conversely, the antibacterial DHFR inhibitor trimethoprim and two close structural analogs were highly selective, but weak, inhibitors of yeast complemented by the C. parvum enzyme. Future chemical refinement of the potent and selective lead compounds identified in this study may allow the design of an efficacious antifolate drug for the treatment of cryptosporidiosis.
The protozoan parasite Cryptosporidium parvum infects epithelial cells lining the small intestines of humans and a wide variety of mammals, causing diarrheal disease. The infectious life cycle stage, the oocyst, is present in both urban and rural environments and is a frequent contaminant of drinking and recreational water, through which the disease is spread. Oocysts are environmentally robust and are incompletely removed by current water treatment methods (18, 43). To add to the difficulty of control, the oocysts are also resistant to sterilization by chlorination: either ozonation or filtration is required for their removal. Cryptosporidiosis is usually self-limiting in immunocompetent individuals but often becomes chronic and may be lethal in immunodeficient hosts (11, 18, 21). No specific chemotherapy is yet available for cryptosporidiosis, and efficacious drugs are desperately needed.
C. parvum is difficult to maintain in the laboratory. With an asexual cycle time of ∼18 to 24 h in vitro, only a brief window is available for screening antiproliferative drugs in culture. Unless the drugs kill the parasites rapidly, it is unlikely that they would be identified in cell culture-based screens. Although the parasite can be grown and passaged more successfully in animals, the animals must either be neonates or severely immunocompromised (30), which makes animal-based drug screening both difficult and expensive. Screening can, of course, also be carried out in vitro using crude or purified native or recombinant enzyme. For Cryptosporidium, where large numbers of intracellular parasites are difficult to obtain, it is not feasible to use native enzyme for such studies. Direct enzymological screening mandates application of a molecular-biology-based approach to clone the targeted gene and express sufficient recombinant enzyme for biochemical assay. The study of C. parvum is at a very early stage, however. Each of these difficulties has hindered progress in identifying effective chemotherapy for C. parvum.
We have devised a complementary approach to anti-Cryptosporidium drug discovery that is independent of parasite culture or enzyme purification and exploits instead the well-developed classical and molecular genetics of the yeast Saccharomyces cerevisiae. S. cerevisiae is easy and inexpensive to grow in the laboratory, myriad techniques have been developed for its genetic manipulation (19, 22, 24, 36, 58), and the complete sequence of its genome was recently reported (15). We used a strain of S. cerevisiae engineered to be dependent for its growth on the functional expression of an exogenous gene encoding the essential enzyme dihydrofolate reductase (5,6,7,8-tetrahydrofolate NADP+ oxidoreductase; EC 1.5.1.3) (DHFR) (23). DHFR catalyzes the oxidation of NADPH and reduction of dihydrofolate to NADP and tetrahydrofolate, respectively (3), and its activity is required to replenish the reduced folate pool depleted by thymidylate biosynthesis during DNA replication. Subtle differences in the active sites of human and pathogen DHFR enzymes have made it possible to identify efficacious drugs that are potent and selective inhibitors of some pathogen DHFR enzymes without strongly affecting the human enzyme, thereby providing a high therapeutic index (3, 5, 14, 16, 27, 38, 63).
We previously introduced the Plasmodium falciparum DHFR gene into a mutant yeast strain (23) lacking endogenous DHFR function and showed that the heterologous DHFR gene and enzyme complemented the deficiency. Unlike the dfr1 strain, the complemented yeast strain was sensitive to antimalarial DHFR inhibitors like pyrimethamine (64). Here we report the development of similar yeast complementation systems using several heterologous DHFR genes and our application of the system to identify antifolate compounds that potently and/or selectively inhibit the growth of yeast carrying the C. parvum gene.
In C. parvum, other protozoa, and plants, DHFR forms the N-terminal domain of a bifunctional enzyme that also includes thymidylate synthase (TS; EC 2.1.1.45) (61). Two subspecies or variants of C. parvum have been identified. Although they both infect humans, the two subspecies have separate transmission cycles (4, 8, 35, 37, 60, 61). One subspecies (type I) has been isolated only from human hosts and appears to be anthroponotically transmitted, whereas the other (type II) has been isolated from both human and animal hosts and can be zoonotically transmitted. The coding sequences of the DHFR-TS alleles from the type I and type II subspecies differ by 39 to 40 single-nucleotide polymorphisms, which predict enzymes differing by 10 amino acids; 9 of these differences occur in the DHFR domain (61). Since humans can be infected by either subspecies, an effective drug must be able to inhibit the DHFR enzymes from both (21, 25, 31, 43). Thus, we screened potential antifolate drugs against the enzymes encoded by both C. parvum DHFR-TS alleles in the yeast complementation system. Potency and selectivity controls included the dfr1 yeast host complemented by the S. cerevisiae DHFR and heterologous DHFRs expressed from the human DHFR gene, the Toxoplasma gondii DHFR-TS gene (42), the DHFR domain of the P. falciparum DHFR-TS gene (6), and the Pneumocystis carinii DHFR gene (12).
MATERIALS AND METHODS
Strains and plasmids.
Escherichia coli strain DH5α was used for propagation and preparation of yeast-E. coli shuttle plasmids and the dhfr mutant E. coli strain PA414 (F− his-4 thr-1 leu-6 thi-1 lacY1 galK2 ara-1 xyl-5 mtl-1 proA2 argE3 rps-31 tsx-38 supE44 λ− thyA fol::kan) was used for the expression and preparation of recombinant DHFR and DHFR-TS enzymes. The E. coli expression plasmid pTrc99A was purchased from Pharmacia Biotech. S. cerevisiae strains TH1 (Mata ura3-52 leu2-3,112 trp1 tup1) and TH5 (Mata ura3-52 leu2-3,112 trp1 tup1 dfr1::URA3) were kindly provided by Tun Huang (23); TH5 must be maintained in the presence of adenine, histidine, methionine, and dTMP. The plasmid pGN-PC-dhfr, containing the P. carinii DHFR coding region (GenBank accession no. M26495 and M26496), was obtained through the DAIDS-NIAID-NIH Research and Reference Reagent Program. The T. gondii DHFR cDNA (42) was kindly provided by Mary Reynolds and David Roos (University of Pennsylvania). The heterologous DHFR genes were expressed in yeast from a shuttle plasmid derived from pRS314 (58, 64), and the plasmid constructs were transformed into TH5 to generate the haploid yeast strains listed in Table 1.
TABLE 1.
Yeast strains
| Strain | DHFR origin | Promoter (bp) | Source |
|---|---|---|---|
| Sc-yeast | S. cerevisiae | 600 | 64 |
| Pf-yeastS | P. falciparum; Pyr sensitive | 600 | 64 |
| Pf-yeastR | P. falciparum; Pyr resistant | 600 | 64 |
| Hu-yeast | Human | 450 | This work |
| Cp-yeast type I | C. parvum (human isolate); DHFR-TS | 450 | This work |
| Cp-yeast type II | C. parvum (bovine isolate); DHFR-TS | 450 | This work |
| Tg-yeast | T. gondii | 600 | Mary Reynolds |
| Pc-yeast | P. carinii | 450 | This work |
Compounds.
The names of the compounds screened are listed in Table 2. Trimetrexate and the National Cancer Institute (NCI) compounds were provided by Mohamed Nasr at the National Institute of Allergy and Infectious Diseases. The trimethoprim analogs were provided by William Ellis, Division of Experimental Therapeutics, Walter Reed Army Institute of Research (WRAIR). The synthesis of compounds in the PY series has been described in the references cited in Table 2 (44–47, 50, 51, 53, 56; A. Rosowsky, A. T. Papoulis, R. A. Forsch, and S. F. Queener, presented at the 10th NCI-EORTC Symposium on New Drugs in Cancer Therapy, Amsterdam, The Netherlands, 1998). Sulfanilamide was purchased from Sigma (St. Louis, Mo.). All compounds were dissolved in dimethyl sulfoxide (DMSO) (Sigma), and the concentration of DMSO in the yeast assays was less than 1% to avoid interference of the solvent with cell growth.
TABLE 2.
Compounds tested
| Origin | Identifier | Chemical name | Reference |
|---|---|---|---|
| NCI | TMX | 2,4-Diamino-5-methyl-6-(3,4,5-trimethoxyanilino)methylquinazoline | |
| 61670 | 2,4-Diamino-6-[2-(4-fluorophenyl)ethyl]pyrimidine | ||
| 104126 | 2,4-Diamino-5-[(4-nitrocinnamylidene)amino]-6-phenyl-pyrimidine | ||
| 106568 (TMP) | 2,4-Diamino-5-(3,4,5-trimethoxybenzyl)pyrimidine | ||
| 107242 | 2,4-Diamino-6-benzoyl-6,7-dihydro-7-methyl-5H-pyrrolo[3,4-d]pyrimidine | ||
| 112421 | N-[4-[N-(2,4-Diaminopyrimidin-5-yl)]methyl]amino]benzoyl-l-glutamic acid | ||
| 117288 | 2,4,6-Triamino-5-(3,4-dimethylphenylazo)pyrimidine | ||
| 121146 | N-[4-[N-(2,4-Diaminopyrimidin-5-yl)]methyl-N-methyl]amino]benzoyl-l-glutamic acid | ||
| 3081 | 2,4-Diamino-1-(4-chlorophenyl)-2-n-hexyl-1,2-dihydro-s-triazine; hydrochloride salt | ||
| 47532 | 2-Amino-4-chloro-5-ethyl-6-(4-bromoanilino)pyrimidine | ||
| 77028 | 4-[N-[3-(2-Amino-4(1H)-oxo-6-phenylpyrimidin-5-yl)propyl]amino]benzoyl-l-glutamic acid | ||
| 104133 | 2,4-Diamino-5-(4-chlorophenyl)-6-(4-nitrostyryl)pyrimidine | ||
| 114923 | 2,4,6-Triamino-5-[3-[2-chloro-4-(3-fluorosulfonylphenylcarbamoyl)-phenoxy]propyl]pyrimidine; ethanesulfonate salt | ||
| 127916 | Unavailable | ||
| Chlorasquin | |||
| Dana-Farber Cancer Institute | PY 359 | 2,4-Diamino-6-chloroquinazoline | 50 |
| PY 361 | 2,4-Diamino-5-methoxyquinazoline | 50 | |
| PY 365 | 2,4-Diamino-5-ethoxyquinazoline | 50 | |
| PY 460 | 2,4-Diamino-6,7-dimethoxy-9H-indeno-[2,3-d]pyrimidine | 47 | |
| PY 461 | 1,3-Diamino-7-methoxy-5,6-dihydrobenzo[f]quinazoline | 46 | |
| PY 468 | 1,3-Diamino-8-chloro-5,6-dihydrobenzo[f]quinazoline | 46 | |
| PY 488 | 1,3-Diamino-8-bromobenzo[f]quinazoline | 45 | |
| PY 489 | 1,3-Diamino-8-chlorobenzo[f]quinazoline | 45 | |
| PY 490 | 1,3-Diamino-9-chlorobenzo[f]quinazoline | 45 | |
| PY 497 | 1,3-Diamino-9-methoxybenzo[f]quinazoline | 45 | |
| PY 821 | 2,4-Diamino-5-methyl-6-(3,4-dichlorophenyl)thieno[2,3-d]pyrimidine | 44 | |
| PY 823 | 2,4-Diamino-5-methyl-6-(3,4-dichlorobenzyl)thieno[2,3-d]pyrimidine | 44 | |
| PY 826 | 2,4-Diamino-5-(4-chlorophenyl)-6-methylthienol[2,3-d]pyrimidine | 44 | |
| PY 833 | 2,4-Diamino-5-(4-chlorophenyl)-6-ethylthieno[2,3-d]pyrimidine | 44 | |
| PY 841 | 2,4-Diamino-6-(3,4,5-trimethoxyphenyl)-5-methylthieno[2,3-d]pyrimidine | 53 | |
| PY 842 | 2,4-Diamino-6-(3,4,5-trimethoxybenzyl)-5-methylthieno[2,3-d]pyrimidine | 53 | |
| PY 875 | 2,4-Diamino-6-(3,4,5-trimethoxyphenyl)-5,6,7,8-tetrahyrdopyrido[4,3-d]pyrimidine | 51 | |
| PY 888 | 2,4-Diamino-5-(3,4,5-trimethoxyanilino)methylthienol[2,3-d]pyrimidine | 57 | |
| PY 890 | 2,4-Diamino-5-(3,4,5-trimethoxy-N-methylanilino)methylthieno[2,3-d]pyrimidine | 57 | |
| PY 896 | (6R,6S)-2,4-Diamino-6-(3,4-dimethoxybenzyl)-5,6,7,8-tetrahydroquinazoline | 55 | |
| PY 898 | (6R,6S)-2,4-Diamino-6-benzyl-5,6,7,8-tetrahydroquinazoline | 55 | |
| PY 899 | (6R,6S)-2,4-Diamino-6-(3,4,5-trimethoxybenzyl)-5,6,7,8-tetrahydroquinazoline | 55 | |
| WRAIR | WR148858 | 6-Methyl analog of trimethoprim | |
| WR188744 | 4,6-Methyl-2-sulfhydryl-5-butyl pyrimidine | ||
| WR219121 | 2,4-Diamino-5-(3′,4′-dimethoxy-5′-(4-phenylbutoxy)-benzyl) pyrimidine | ||
| WR219123 | 2,4-Diamino-5-(3′,5′-dimethoxy-(4-phenylbutoxy)-benzyl) pyrimidine | ||
| WR219125 | 2,4-Diamino-5-(3′,5′-dimethoxy-5′-(4-isobutoxy)-benzyl) pyrimidine | ||
| WR219126 | 2,4-Diamino-5-(3′,4′,5′-triethoxybenzyl) pyrimidine |
Expression, purification, and enzymatic assay of recombinant DHFR and DHFR-TS enzymes.
The DHFR-TS allele characteristic of type I C. parvum subspecies, found exclusively in human hosts, was cloned from the C. parvum SFGH-1 AIDS isolate, while the allele characteristic of type II C. parvum subspecies, found in both human and animal hosts, was cloned from the C. parvum NINC-1 calf isolate (61). The NINC-1 DHFR-TS coding sequence was incomplete and lacked the 3′ terminus of the gene encoding the C-terminal 22 residues of TS (61). The C-terminal portion of the coding sequence was generated by substitution of a BclI restriction fragment from the 3′ half of the SFGH-1 TS gene. Thus, the bovine isolate C. parvum DHFR-TS expression construct is actually chimeric and encodes one amino acid residue not normally found in the DHFR-TS genes of animal isolates, an E518D substitution introduced by ligation of the BclI gene fragment. Given its location at the extreme C terminus of the TS domain, this substitution is not expected to alter the enzymatic activity or inhibitor susceptibility profile of the DHFR domain.
All enzymes were expressed in, and prepared from, the dhfr mutant E. coli strain PA414 (1). Recombinant human DHFR was expressed from plasmid pDFR (40). The human and bovine isolate C. parvum DHFR-TS genes were subcloned into expression plasmid pTrc99A and expressed under control of the IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible trc promoter. The recombinant DHFR and DHFR-TS enzymes were purified from clarified bacterial lysates by methotrexate affinity chromatography as described previously (1) and enzymatically assayed by following the decrease in A340 at room temperature in buffer containing 50 mM TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid] (pH 7.0), 1 mM EDTA, 75 mM β-mercaptoethanol, 1% bovine serum albumin, 20 μM dihydrofolate, and 100 μM NADPH using a Spectra Max 250 microtiter plate spectrophotometer. Enzyme concentrations were adjusted to give linear rates over the 5-min duration of the assay, and reactions were initiated by adding an equal volume of 40 μM dihydrofolate in assay buffer to microtiter plate wells containing 100 μl of the above assay buffer lacking dihydrofolate and containing various concentrations of the DHFR inhibitors. Each inhibitor was assayed in duplicate, and the mean DHFR activity was plotted against the concentration of the inhibitor; the 50% inhibitory concentration (IC50) was determined by inspection.
Transformations.
E. coli transformations were done by standard calcium chloride and heat shock protocols (2). Yeast transformations were done by a high-efficiency lithium acetate protocol (24) or with a yeast transformation kit (Zymo Research, Orange, Calif.). The transformed cells were plated on complete synthetic medium lacking tryptophan to select for the plasmid. In some cases, 100 μg of dTMP (Sigma)/ml was added to the plates to obviate the requirement for functional DHFR expression for cell viability.
Plasmid construction.
The parent expression vector, pEH2, is derived from pRS314 (58) and Pf-DHFR-D6 (64) and contains the P. falciparum DHFR domain coding sequence. The plasmid is a yeast shuttle vector and can be propagated in E. coli or S. cerevisiae. It has a yeast centromere and is maintained at approximately one copy per yeast cell. The pEH2 promoter is a 600-bp fragment from the region directly 5′ of the yeast DHFR gene, and the terminator is a 400-bp fragment from the region directly 3′ of the yeast DHFR gene. As opposed to the previous Pf-DHFR-D6 construct, the duplicate BamHI and EagI sites 3′ of the pEH2 terminator have been eliminated from the present construct for ease of cloning (E. Hankins, personal communication).
The C. parvum (GenBank accession no. U41365 and U41366) and human (GenBank accession no. J00140) DHFR sequences were amplified by PCR from the relevant plasmid templates and cloned directly into pEH2.
To clone the various DHFR and DHFR-TS coding regions into the pEH2 vector, the P. falciparum DHFR insert was removed by BamHI and EagI restriction enzyme digestion, and BamHI and EagI sites were engineered into the ends of each of the DHFR and DHFR-TS coding regions by PCR using the primers (Life Technologies, Grand Island, N.Y.) shown in Table 3 and the respective DHFR and DHFR-TS plasmid templates. Amplifications were performed on a DNA Engine PTC-200 (MJ Research, Watertown, Mass.) using Replitherm polymerase (Epicentre Technologies, Madison, Wis.) and the following cycling conditions: 30 cycles of 94°C for 30 s, 52°C for 30 s, and 72°C for 30 s, and a final extension of 72°C for 2.5 min. The PCR amplification products were digested with BamHI and EagI, purified using a Wizard PCR column (Promega Corp., Madison, Wis.), and ligated to BamHI-EagI-cut pEH2 overnight at 16°C. The ligation products were transformed into E. coli DH5α, and the identities of the transformants were verified by restriction enzyme analysis of DNA minipreps.
TABLE 3.
PCR primers
| Specificitya | Primer sequenceb |
|---|---|
| 5′ C.p. DHFR-TS type II | AGGAGAGGATCCCATGAGTAAAAAGAACGTTT |
| 3′ C.p. DHFR-TS type II | GCCGGCCGTGGATAATTTAAAAGTAATAG |
| 5′ C.p. DHFR-TS type I | AGGAGAGGATCCCATGAGTGAAAAGAACGTTT |
| 3′ C.p. DHFR-TS type I | GATTATACCGGCCGGTCCATTTTATACCGCCATG |
| 5′ Human DHFR | CTATATGGATCCCATGGTTGGTTCGCTAAACTGC |
| 3′ Human DHFR | CGGGCCACGGCCGTATTAATCATTCTTCTCATATAC |
| 5′ P.c. DHFR | CGGCGCGGATCCGATGAATCAGCAAAAGTCTTTAAC |
| 3′ P.c. DHFR | CGCGTGCGGCCGTTATAAATCTCTTGTCCACATTTC |
| 5′ Yeast promoter | GAGGTACCCTATAGGAATCGTCACTC |
| 3′ Yeast Promoter | AGCGGATCCGCTCGTAGTTCGTTGCGCTCTTCA |
C.p., C. parvum; P.c., P. carinii.
The restriction enzyme sites added for cloning purposes are underlined.
Promoter mutagenesis.
Preliminary experiments revealed that the level of expression of C. parvum and human DHFRs in the yeast host was too high to allow easy screening of the antifolate inhibitors. In order to decrease the expression from the heterologous DHFR and DHFR-TS genes, we subjected the wild-type yeast DHFR promoter driving expression in the pEH2 constructs to mutagenesis by error-prone PCR (66). The promoter region from the pEH2-human DHFR expression plasmid constructed as described above was amplified in a PCR mutagenesis buffer containing 10 mM Tris-HCl, 50 mM KCl, 7 mM MgCl2, 0.5 mM MnCl2, 0.2 mM dATP and dGTP, and 1 mM dCTP and dTTP with 5 U of Taq polymerase (30 cycles of 94°C for 1 min, 45°C for 1 min, and 72°C for 1 min).
The pEH2-human DHFR plasmid and the mutagenized PCR products were digested with BamHI and KpnI (restriction sites flanking the promoter region), purified, and ligated as described above, directly transformed into TH5 yeast, and selected on defined medium lacking tryptophan. The colonies were patched onto rich medium and tested for drug sensitivity by replica plating them onto rich-medium plates containing 10 μM trimetrexate. The drug-sensitive transformants were further characterized by spoke assay and liquid IC50 (see below), and a strain exhibiting suitable antifolate sensitivity was identified. The plasmid was sequenced, revealing a truncation of 143 bp from the 3′ end of the promoter. The C. parvum and P. carinii coding sequences were transferred to the plasmid with the mutated promoter by digestion with BamHI and EagI to remove the existing human DHFR coding sequence and by ligation of other coding sequences in its place. The pEH2–T. gondii–DHFR-TS expression plasmid retained the original yeast DHFR promoter.
DNA sequence analysis of yeast expression constructs.
The mutagenized promoter and DHFR coding regions were sequenced on both strands using the Dye Terminator kit or BigDye kit (Perkin-Elmer, Foster City, Calif.) according to the manufacturer's instructions. The DNA sequence of the human DHFR construct matched the published sequence (40). The sequence of the P. carinii DHFR-pEH2 construct differed from the published P. carinii cDNA sequence (12) at five nucleotide positions. Two of these produce synonymous codons, while the three others predict amino acid differences: T57C (Ile19Thr), T125C (Phe42Leu), and T127G (Val43Ala). DHFR protein sequences vary widely among species, making precise alignment difficult. However, according to one alignment (61), the Ile19Thr change occurs in a relatively conserved residue whereas the Phe42Leu and Val43Ala alterations occur in poorly conserved residues. P. carinii DHFR complements the yeast deficiency efficiently, however, so these changes have apparently not compromised enzyme function. It seems unlikely that five mutations were introduced into a 550-bp sequence by PCR. There is known to be considerable polymorphism among P. carinii strains (29), suggesting that the clone obtained from the AIDS Research and Reference Reagent Program may derive from a source different from the original isolate sequenced.
The DNA sequence of the pEH2-SFGH-1 C. parvum DHFR-TS expression construct differed from the published sequence at three positions, producing one synonymous and two nonsynonymous codons: Glu3Lys and Ile198Val. The first of the nonsynonymous changes predicts a Lys residue at position 3 of the DHFR domain, which is the same as the naturally occurring third residue in the bovine isolate. The second nonsynonymous change occurs in the junction peptide connecting the DHFR and TS domains and is not expected to alter DHFR enzyme function. The DNA sequence of the pEH2–NINC-1 C. parvum DHFR-TS construct was identical to the published NINC-1 sequence (61).
Plasmid recovery.
Yeast genomic DNA (Zymoprep; Zymo Research, or Hoffman and Winston [22]) was transformed into E. coli DH5α to isolate the plasmid from the yeast.
Complementation tests.
Yeast transformants expressing heterologous DHFR genes were patched onto rich-medium plates (yeast extract-peptone-dextrose [YEPD]) plus 100 μg of dTMP/ml, grown for 3 days, and double replica plated (57) onto rich-medium plates without dTMP and synthetic medium plates lacking adenine, histidine, or methionine. Growth on all of these plates indicated that the heterologous DHFR was complementing the dfr1 disruption. In the same experiment, transformants were also replica plated onto synthetic medium lacking tryptophan, leucine, or uracil to verify the identity of the yeast host strain. All of the DHFR sequences tested complemented the yeast dfr1 disruption.
Spoke assay.
The strains listed in Table 1 were analyzed by spoke assay as previously described (57). In the screening experiments, the plates contained 1 mM sulfanilamide in addition to the antifolate drug. Each drug was tested three times, and the distances from the concentrated drug in the center of the plate to the beginning of yeast growth (“kill zone”) were averaged.
IC50 assays were also conducted as previously described (57). Log-phase yeast cells were diluted to 3.5 × 104/ml in medium containing 1 mM sulfanilamide (final concentration), and 0.8-ml aliquots were distributed in the 2-ml wells of a deep-well 96 microtiter plate. Drug dilutions were made in YEPD medium, and 200 μl of each dilution was added to the wells to generate the final concentrations described in the results. Yeast growth in control wells lacking the drug (containing 1 mM sulfanilamide and DMSO) was scored as 100% growth, and the optical density t 650 nm (OD650) values of the cells grown in various drug dilutions were divided by this control to determine the percentage of growth at each drug concentration. The IC50 was calculated using the two values that flanked the 50% mark and the following formula: y = mx + b, where m and b are the slope and the y intercept is calculated using the two flanking drug concentrations. The solution for x at y = 50% yields the IC50.
RESULTS
C. parvum and human DHFR enzymes complement dfr1 yeast.
Our goal was to develop an experimental system to rapidly and economically screen collections of drugs that are known inhibitors of some DHFR enzymes to identify potential inhibitors of the Cryptosporidium DHFR. To assess drug potency and selectivity, we constructed isogenic yeast strains whose growth and viability depend on genetic complementation by plasmid-borne C. parvum or human DHFR genes. The C. parvum DHFR-TS and the human DHFR coding regions were cloned into the pEH2 yeast expression plasmid, in which transcription initiation and termination are driven by sequences derived from those flanking the wild-type S. cerevisiae DHFR gene (28). The plasmid contains a yeast centromere sequence and is maintained at a single-copy level. Both the C. parvum DHFR-TS and the human DHFR expression constructs complemented the DHFR deficiency in the dfr1 yeast mutant, demonstrating that the heterologous genes are functionally expressed in S. cerevisiae.
P. falciparum, T. gondii, and P. carinii DHFR complement dfr1 yeast.
To develop a panel of isogenic control strains with diverse antifolate susceptibility profiles for the drug-screening experiments, we also transformed the dfr1 yeast mutant with pEH2 DHFR expression plasmids containing DHFR coding sequences from S. cerevisiae (28) and P. carinii (12) and the DHFR domains of the DHFR-TS coding sequences of T. gondii (42) and pyrimethamine-sensitive and -resistant P. falciparum (64). Each of these constructs complemented the DHFR deficiency of the host dfr1 yeast mutant. We refer to the members of this collection of isogenic dfr1 yeasts as Cp-yeast, Hu-yeast, Sc-yeast, Pc-yeast, Tg-yeast, and Pf-yeast to indicate the origin of the complementing DHFR gene (C. parvum, human, S. cerevisiae, P. carinii, T. gondii, and P. falciparum, respectively).
Addition of sulfanilamide enhances the sensitivity of the antifolate-screening system.
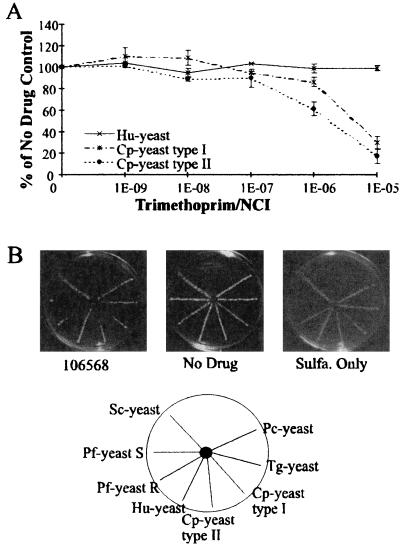
To use the complementation system to screen antifolate drugs, it was important that the heterologous DHFRs be expressed at an extremely low level so that inhibition of the enzyme would be reflected as a proportional decrease in yeast growth. Preliminary work (64) had shown that the two Pf-yeast strains expressed the Plasmodium DHFR domains at very low levels and that the growth of these strains was strongly inhibited by the potent lipophilic DHFR inhibitor trimetrexate. Unexpectedly, however, even high levels of trimetrexate did not inhibit the growth of any of the other complemented strains. For example, Hu-yeast grew normally in trimetrexate concentrations as high as 1 μM even though the IC50 of the drug for the purified human DHFR enzyme is about 1 nM. As trimetrexate binds essentially stoichiometrically to DHFR (9, 32; J. R. Bertino and W. L. Sawicki, Abstract, Proc. Am. Assoc. Cancer Res., 18:168, 1977), the amount of drug needed to inhibit the growth of yeast dependent on heterologous DHFR expression is proportional to the cellular DHFR concentration. We reasoned that the insensitivity of many of the complemented yeast strains to trimetrexate resulted from high DHFR levels and that it would be necessary to reduce DHFR expression to enhance the sensitivity of the system. To accomplish this, we mutagenized the pEH2 promoter using error-prone PCR (66), cloned the PCR products 5′ to the human DHFR gene in pEH2, and screened the transformants for increased sensitivity to trimetrexate. A truncated promoter was identified that resulted in a trimetrexate IC50 of approximately 3 μM for the Hu-yeast. The truncated promoter was also cloned 5′ of the C. parvum and P. carinii DHFR sequences and likewise increased the susceptibility of Cp-yeast and Pc-yeast to trimetrexate.
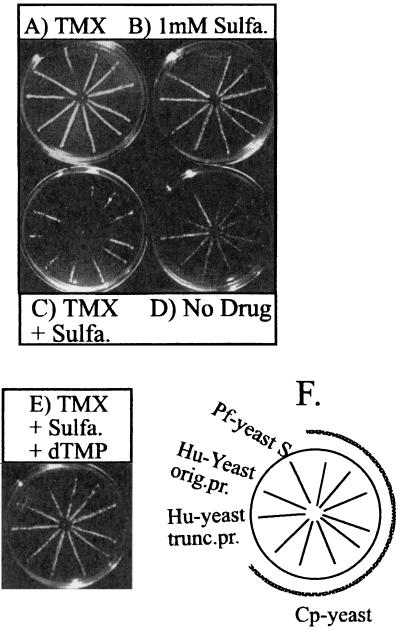
To further increase the sensitivity of the system, we exploited the profound synergy known to occur when sulfonamide or sulfone inhibitors of dihydropteroate synthase are used in combination with DHFR inhibitors (38, 39, 59). Sulfanilamide had been used previously to inhibit yeast dihydropteroate synthase (26, 33, 34), and we included this drug at 1 mM in our DHFR inhibitor assays. A higher concentration of a drug is required to completely inhibit the growth of the yeast, so in the spoke assay, 10 μl of a 5-μg/ml solution of trimetrexate alone inhibited the growth of only the Pf-yeast strain (Fig. 1A), while sulfanilamide alone failed to completely inhibit the growth of any of the strains (Fig. 1B). The growth of the Pf-yeast is slowed somewhat by sulfanilamide. In Fig. 1B, the Pf-yeasts were growing to the center of the plate but are not visible in the photograph. However, the combination of sulfanilamide and trimetrexate significantly inhibited the growth of all of the strains except Sc-yeast (Fig. 1C; see also Fig. 3B). Furthermore, inhibition was reversed by addition of dTMP (Fig. 1E), demonstrating that the drugs acted specifically on DHFR and the thymidylate cycle pathway. Clearly, the addition of sulfanilamide to the assay dramatically enhanced the susceptibilities of the complemented yeast strains to DHFR inhibitors and provided a sensitive screening system to identify potent and selective inhibitors.
FIG. 1.
Synergism between trimetrexate and sulfanilamide. (A) Ten microliters of a 5-μg/ml solution of trimetrexate was placed in the center of each plate, and the plates were allowed to grow for 2 days. (B) Sulfanilamide (sulfa.) was spread evenly on the plate to generate a final concentration of 1 mM. (C) Both trimetrexate (TMX) and sulfanilamide were used on the plate as described above. (D) No drug was used on the plate. (E) Both trimetrexate and sulfanilamide were used. In addition, dTMP was spread evenly on the plate to a final concentration of 100 μg/ml. (F) Strain legend. The spoke arrangements on all plates are identical and are as shown. orig. pr., original 600-bp promoter; trunc. pr., truncated 450-bp promoter. Cp-yeast refers to several clones expressing the human isolate of C. parvum DHFR-TS.
FIG. 3.
Sensitivity to trimethoprim (NCI106568). (A) IC50 assay with yeast expressing human or C. parvum DHFR. See the legend to Fig. 2 for details. (B) Spoke assay showing selective inhibition of C. parvum DHFR-TS but not human DHFR-expressing yeast. Plates with no drug or with sulfanilamide (sulfa.) alone are shown as controls. The plate diagram shows the location of each strain of yeast on each plate.
Known DHFR inhibitors were active in the yeast system.
To determine whether compounds known to inhibit particular DHFR enzymes in vitro would also inhibit the growth of the yeast strains complemented by these enzymes, we obtained 13 DHFR inhibitors from the NCI. Two of these, trimetrexate and chlorasquin, were known inhibitors of most DHFR enzymes, and the others were identified only by their code numbers. These compounds, in combination with 1 mM sulfanilamide, were assayed for the ability to inhibit the growth of the type I and type II Cp-yeast strains, Hu-yeast, and each of the control strains (Tg-yeast, Pc-yeast, Pf-yeast, and Sc-yeast) in the radial spoke assay. The resulting data shown in Table 4 indicate that 7 of the 13 compounds failed to inhibit the growth of either Cp-yeast strain or Hu-yeast. However, with the exception of 77028 and 47532, all the compounds clearly entered yeast cells, as each inhibited the growth of at least one of the control strains. Conversely, three compounds, 112421, 117288, and 121146, inhibited the growth of both Cp-yeast and Hu-yeast, although none was as potent as trimetrexate. One of these compounds, 117288, also inhibited the growth of all other yeast strains examined, including Sc-yeast. Moreover, the inhibition was not reversed by exogenous thymidylate, suggesting the compound was nonspecifically cytotoxic. Last and most importantly, we identified one highly selective inhibitory compound, 106568, that inhibited the growth of both Cp-yeast strains but had no effect on the growth of Hu-yeast.
TABLE 4.
Yeast strain sensitivity to NCI compounds
| Compound | Yeast inhibition (cm in spoke assay)a
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Cp-yeast type I | Cp-yeast type II | Hu-yeast | Pf-yeastS | Pf-yeastR | Tg-yeast | Pc-yeast | Sc-yeast | |
| Trimetrexate | 2.3 | 2.5 | 2.9 | >3.5 | 3 | 2.7 | 1.8 | −b |
| 61670 | − | − | − | >2 | − | − | − | − |
| 104126 | − | − | − | 0.9 | − | − | − | − |
| 106568 | 1.5 | 2 | − | >2.5 | 1.6 | − | − | − |
| 107242 | − | − | − | >1.7 | − | − | − | − |
| 112421 | 1.2 | 1.6 | 1.4 | >2.2 | − | − | − | − |
| 117288 | 0.6 | 0.6 | 0.7 | 1.8 | 0.9 | 0.9 | 0.6 | 0.7 |
| 121146 | 1.1 | 1.2 | 1.5 | >2.2 | − | − | − | − |
| 3081 | − | − | 1.8 | >2.3 | 2.8 | 2 | − | − |
| 47532 | − | − | − | − | − | − | − | − |
| 77028 | − | − | − | − | − | − | − | − |
| 104133 | − | − | 0.9 | >2.5 | 2.4 | 1.2 | − | − |
| 114923 | − | − | − | 0.9 | − | − | − | − |
| 127916 | − | − | − | >2.1 | − | − | − | − |
| Chlorasquin | 1.9 | 2.2 | 2.1 | >3 | 2.3 | 1.7 | 2 | − |
Numbers represent distance from the center of the plate to the beginning of yeast growth (kill zone) and are the averages of three independent experiments. The standard deviations were ≤0.5 cm. Pf-yeastS, pyrimethamine-sensitive Pf-yeast; Pf-yeastR, pyrimethamine-resistant Pf-yeast.
−, no inhibition detected.
We also assayed the 13 NCI compounds using the purified recombinant C. parvum DHFR-TS and human DHFR enzymes to compare the inhibitor susceptibility profiles obtained with the two assay systems (Table 5). In general, the drug concentration required to inhibit the enzymatic activity of the purified C. parvum and human enzymes by 50% in vitro (i.e., the IC50) correlated with the extent of drug inhibition of each Cp-yeast or Hu-yeast strain in the spoke assay. The majority of the compounds tested did not strongly inhibit the DHFR-TS enzymes from either type I or type II C. parvum isolates, either in vitro or when expressed heterologously in Cp-yeast.
TABLE 5.
Comparison of in vitro data and yeast sensitivity to NCI compounds
| Compound | IC50 (μM) for enzyme in vitro
|
Yeast inhibition (cm in spoke assay)a
|
||||
|---|---|---|---|---|---|---|
| Cp-yeast type I | Cp-yeast type II | Hu-yeast | Cp-yeast type I | Cp-yeast type II | Hu-yeast | |
| Trimetrexate | 0.004 | 0.001 | 0.001 | 2.3 | 2.5 | 2.9 |
| 61670 | >1,000 | NDb | 700 | − | − | − |
| 104126 | >100 | >100 | >100 | − | − | − |
| 106568 | 5 | 2 | 500 | 1.5 | 2 | − |
| 107242 | >100 | >100 | 300 | − | − | − |
| 112421 | 0.07 | 0.1 | 0.2 | 1.2 | 1.6 | 1.4 |
| 117288 | >1,000 | ND | >1,000 | 0.6 | 0.6 | 0.7 |
| 121146 | 0.006 | 0.01 | 0.1 | 1.1 | 1.2 | 1.5 |
| 3081 | 200 | 60 | 5 | − | − | 1.8 |
| 47532 | >200 | >10 | >100 | − | − | − |
| 77028 | >100 | >100 | 50 | − | − | − |
| 104133 | 80 | 30 | 2 | − | − | 0.9 |
| 114923 | 30 | 20 | 2 | − | − | − |
| 127916 | >100 | >100 | 20 | − | − | − |
| Chlorasquin | 0.001 | 0.0007 | 0.0009 | 1.9 | 2.2 | 2.1 |
Duplicates data in Table 4. −, no inhibition detected.
ND, not done.
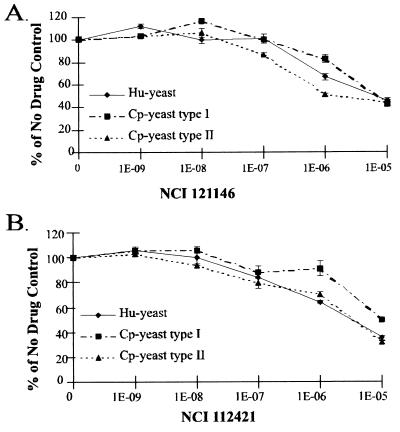
Four compounds, however, did inhibit the growth of the Cp-yeast strains. One of these was chlorasquin, a drug similar to trimetrexate in that it is a potent inhibitor of most if not all DHFR enzymes. Compounds 112421 and 121146 were potent inhibitors of both the type I and type II C. parvum DHFR-TS enzymes in vitro but showed little selectivity for the parasite over the human enzyme; both compounds inhibited the growth of Cp-yeast and Hu-yeast roughly equally in the spoke assay. To quantitate the sensitivities of Cp-yeast and Hu-yeast to these drugs, we measured the inhibition of yeast growth in liquid culture as a function of drug concentration; in this context, the IC50 is the drug concentration at which growth was inhibited by 50%. The results shown in Fig. 2 indicate that neither of these compounds selectively inhibited the growth of Cp-yeast compared to Hu-yeast, in agreement with the in vitro enzymatic data.
FIG. 2.
IC50 assays with NCI compounds 121146 and 112421. Liquid culture assays with Hu-yeast or Cp-yeast were conducted with varying concentrations of 121146 or 112421. Growth is measured by the optical density at 660 nm expressed as a percentage of the growth without drug. Each point on the graph represents the average of duplicate data points. The error bars indicate standard deviations.
The fourth inhibitory compound, 106568, inhibited the growth of Cp-yeast, but not Hu-yeast, in the spoke assay; thus, it appeared to be a selective inhibitor of the C. parvum enzyme (Fig. 3). This observation was consistent with in vitro enzyme assay data showing that the human DHFR was about 100-fold less sensitive to inhibition by 106568 than the two C. parvum DHFR-TS enzymes. The spoke assay, as well as experiments in liquid culture, revealed a clear and pronounced difference between the susceptibilities of Cp-yeast and Hu-yeast to 106568. In liquid culture, the 106568 IC50 was 6.8 μM for Cp-yeast (type I DHFR-TS allele) and 3.3 μM for Cp-yeast (type II allele) but >10 μM for Hu-yeast (Fig. 3A). When the numerical coding was broken, compound 106568 was identified as trimethoprim, a widely used antifolate antibiotic (13, 17, 20, 41, 62).
Some novel lipophilic compounds were potent inhibitors of the C. parvum DHFR.
Having validated the broad utility of this dfr1 yeast complementation system to correctly identify potent and/or selective inhibitors of the heterologous DHFR- or DHFR-TS-complementing enzymes, we proceeded to assay 22 novel lipophilic antifolates against each of the complemented yeast strains. These lipophilic DHFR inhibitors are assumed to cross the plasma membrane by passive or facilitated diffusion and, therefore, to obviate drug susceptibility and resistance problems associated with carrier- or receptor-mediated folate transport mechanisms (7). Many of these compounds have previously been assayed against P. carinii DHFR and T. gondii DHFR-TS in vitro (48, 49, 51–56). For example, the IC50 of PY875 against the P. carinii DHFR enzyme in vitro was ∼7 μM (51), suggesting that PY875 might also inhibit the growth of Pc-yeast; Table 6 shows this to indeed be the case.
TABLE 6.
Yeast strain sensitivity to PY compounds
| Compoundb | Yeast inhibition (cm in spoke assay)a
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Cp-yeast type I | Cp-yeast type II | Hu-yeast | Pf-yeastS | Pf-yeastR | Tg-yeast | Pc-yeast | Sc-yeast | |
| Trimetrexate | 2.6 | 2.7 | 3 | >3.5 | 3 | 2.5 | 2.1 | − |
| PY 359 | − | − | − | 3.6 | 0.8 | − | − | − |
| PY 361 | 2.5 | 2.9 | 3.4 | >3.5 | 2.8 | 1.9 | 1.9 | − |
| PY 365 | 2.4 | 2.7 | 2.8 | 3.4 | 1.3 | 1.9 | 1 | − |
| PY 460 | − | − | − | >3.5 | 1.4 | − | − | − |
| PY 461 | 1.4 | 1.7 | 2.8 | >3.5 | 2.9 | 1.7 | 1.5 | − |
| PY 468 | − | − | 0.9 | 3.4 | 2 | − | − | − |
| PY 488 | 1.5 | 1.7 | 2.6 | >3.5 | 2.8 | 1.7 | 1.8 | − |
| PY 489 | 1.8 | 1.8 | 2.5 | >3.5 | 3 | 1.7 | 1.9 | 0.9 |
| PY 490 | 2.7 | 2.9 | 3.1 | >3.5 | 2.7 | 2.2 | 1.2 | − |
| PY 497 | 1.8 | 2.8 | 3.1 | >3.5 | 3.1 | 2.1 | 1.3 | 0.7 |
| PY 821 | − | − | − | − | − | − | − | − |
| PY 823 | 0.8 | 0.8 | 0.8 | 1.7 | 1.4 | 0.8 | − | − |
| PY 826 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | − | − | − |
| PY 833 | − | − | − | − | − | − | − | − |
| PY 841 | − | − | − | 2.1 | 1.9 | − | − | − |
| PY 842 | − | − | − | 2.2 | − | − | − | − |
| PY 875 | 1.1 | 1.7 | 2 | >3.5 | 2.9 | 1.9 | 1.6 | − |
| PY 888 | − | − | − | >3.5 | 2.5 | − | 1 | − |
| PY 890 | − | − | − | >3.5 | − | − | − | − |
| PY 896 | 2.7 | 3.2 | 3.5 | >3.5 | >3.5 | 3.6 | 3.5 | 2.4 |
| PY 898 | 2.3 | 2.7 | 3.1 | >3.5 | >3.5 | 3 | 2.5 | − |
| PY 899 | 2.7 | 2.8 | 3.1 | >3.5 | 3.2 | 2.7 | 2.6 | − |
Numbers represent distance from the center of the plate to the beginning of yeast growth (kill zone) and are the averages of three independent experiments. The standard deviations were ≤0.5 cm. −, no inhibition detected. Pf-yeastS, pyrimethamine-sensitive Pf-yeast; Pf-yeastR, pyrimethamine-resistant Pf-yeast.
Boldface indicates compounds that were particularly effective against C. parvum DHFR-TS.
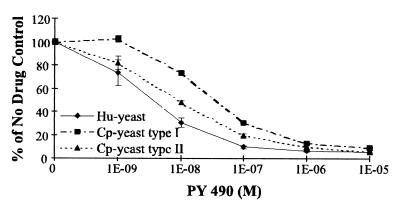
The compounds that were very potent inhibitors of the growth of both Cp-yeast and Hu-yeast are indicated in Table 6. For example in liquid culture the IC50 of PY 490 for Cp-yeast was 5.9 nM for the type I allele and 9.4 nM for the type II allele, while it was 6.0 nM for Hu-yeast (Fig. 4). Several of the compounds were potent and selective inhibitors of Pf-yeast strains complemented by the pyrimethamine-sensitive and -resistant P. falciparum DHFR domains and thus are potential lead compounds for the development of drugs against pyrimethamine-resistant malaria; these include PY 359, PY 460, PY 841, and PY 888. The lipophilic antifolate PY 896 potently inhibited the growth of all of the complemented yeast strains, including the homologous Sc-yeast control. Inhibition was reversed by inclusion of the folate coenzyme-requiring metabolic end products dTMP, methionine, histidine, and adenine in the media, indicating that PY896 may target folate-requiring enzymes from multiple species. In summary, although none of the lipophilic antifolates demonstrated selectivity for the C. parvum DHFR-TS enzymes, several were very potent inhibitors and may be good lead compounds for further development.
FIG. 4.
IC50 assays with compound PY 490. Liquid growth assays with Hu-yeast or Cp-yeast were conducted with varying concentrations of PY 490 as described in the legend to Fig. 2. Each point on the graph represents the average of duplicate data points. The error bars represent standard deviations.
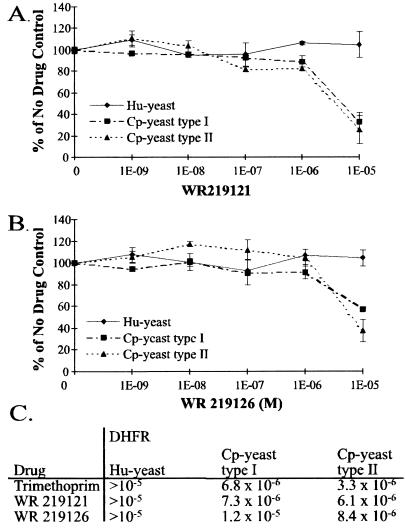
In light of our discovery that the common antibacterial antifolate trimethoprim was a highly selective inhibitor of the growth of Cp-yeast, as well as of the activity of the C. parvum DHFR-TS enzymes, we assayed six additional trimethoprim analogs against the type I and type II Cp-yeast and Hu-yeast strains. The six compounds were obtained from the WRAIR trimethoprim analog collection; and two of them, WR219121 and WR219126, selectively inhibited Cp-yeast in liquid culture with IC50s comparable to that of trimethoprim (Fig. 5), validating the potential of trimethoprim and trimethoprim analogs as lead compounds for development of new drugs against C. parvum.
FIG. 5.
Yeast strain sensitivity to the trimethoprim analogs from WRAIR. Liquid growth assays with Hu-yeast or Cp-yeast were conducted with varying concentrations of WR 219121 (A) or WR 219126 (B). Each point on the graph represents the average of duplicate data points. Both compounds show selectivities for C. parvum DHFR-TS similar to that of trimethoprim. (C) Comparison of the calculated IC50s for the two compounds and trimethoprim.
DISCUSSION
We targeted our anti-Cryptosporidium drug discovery research to the C. parvum DHFR enzyme for several reasons. First, DHFR inhibitor-based drugs are effective in the treatment of infections caused by the related apicomplexan parasites P. falciparum and T. gondii (38). Second, a large number of classical (folate analogs) and nonclassical DHFR inhibitors have been synthesized over the last 5 decades, so there are numerous libraries of inhibitors available for testing. Lastly, the potential drugs tested in this work were designed to specifically bind and inhibit DHFR. The yeast complementation assay measures the inhibitory potencies and selectivities of antifolate drugs for particular DHFR enzymes by measuring the drug's ability to inhibit the growth and multiplication of isogenic dfr yeast strains complemented by the corresponding DHFR-TS or DHFR genes. This yeast system provides both a qualitative spoke assay and a quantitative liquid growth assay to rapidly classify antifolate drugs that are effective and/or selective inhibitors of the DHFR enzymes of particular pathogens. It is a very useful preliminary screen to identify lead compounds that can then be more extensively examined and optimized using cell culture and enzymological assays.
The yeast assay has been validated by the congruence of the yeast results with those obtained using the purified recombinant C. parvum DHFR-TS enzymes. The assay has proved its usefulness by identifying trimethoprim and several trimethoprim analogs as highly selective, if not potent, inhibitors of C. parvum DHFR compared to the human host enzyme.
This complementation-based screening system depends on engineering a yeast strain that is absolutely dependent for its growth on a low level of the DHFR enzyme. The expression of DHFR must be sufficient to support cell growth, but inhibition of the enzyme must be reflected in a proportional decrease in cell growth. Achieving this level of expression required diminishing the activity of the promoter used to drive expression of the C. parvum and human genes. Adaptation of the assay to other systems would require similar adjustments to optimize expression for those situations. The wealth of molecular and genetic tools available for manipulations of S. cerevisiae make this kind of adjustment relatively straightforward.
The construct that contained the truncated promoter still produced somewhat more enzyme than was optimal for easy assay of many of the DHFR inhibitors. To increase the sensitivity, we added 1 mM sulfanilamide to the assay mixture. This improved the sensitivity markedly and allowed us to test these novel compounds at a low enough concentration to be practical. In clinical treatment of microbial infections, a DHFR inhibitor is almost always used in conjunction with a sulfa drug. Thus, addition of sulfanilamide to the assay does not detract from the goal of identifying DHFR inhibitors that could be effective in treatment of cryptosporidiosis.
Drugs differ markedly in their capacity to enter cells, either passively by diffusion through the membrane or by carrier-mediated transport. Comparison of the in vitro data with the yeast data reveals some differences that probably reflect this factor. For example, the in vitro IC50 for 112421 is 0.07 μM for the human isolate of C. parvum but for yeast it is 14 μM (this compound is a charged folate analog that may require a transporter to cross the yeast membrane). In contrast, the lipophilic compound trimethoprim (106568) has similar IC50s in both systems; in vitro the IC50 is 5 μM, and in yeast, the IC50 is 6.8 μM. Even though 112421 shows a greater efficacy in vitro than trimethoprim, in yeast the C. parvum enzymes are more sensitive to trimethoprim. This underlines the precision of the information gained in vitro: these values reflect a direct interaction between the drug and the enzyme. The discrepancy probably reflects differences in permeability or efflux of the two drugs in live cells. Although the biology of C. parvum and S. cerevisiae is most certainly not the same, permeability differences of this kind may provide valuable information. A particular compound such as 112421 that has difficulty entering yeast may also permeate poorly into mammalian and C. parvum cells. It is important to emphasize that permeability or transport of any particular drug will be the same in all of the yeast strains compared, because the constructs are expressed in isogenic strains. This means that comparison of the relative sensitivities of DHFR enzymes from different species to the same drug will still be valid.
To maximize the information on drug penetration, we included a large number of compounds whose activities were already known in all of our experiments. The human enzyme was a key control; the goal is to identify a drug that efficiently inhibits the C. parvum enzyme but not the human enzyme. We included the T. gondii, P. falciparum and P. carinii enzymes as additional controls. The activities of many of these drugs against these parasites or their purified DHFR enzymes was known already. For example, members of the PY series had been tested in vitro to measure inhibition of the P. carinii or T. gondii enzyme (48, 49, 52–54, 56). We knew at the outset which compounds were effective in vitro against the DHFRs of other pathogens, which allowed us to quickly identify drugs that did not effectively penetrate the yeast host. Then, differences in sensitivity between the strains could be examined in detail to determine whether the differences were caused by direct selective effects on the DHFR enzyme. It is an added benefit, of course, that in screening novel compounds we also identify those that are effective against these other pathogens. For example, several of the PY series, 359, 460, 841, and 888, showed excellent activity against pyrimethamine-sensitive and -resistant P. falciparum alleles and thus are potential leads for development of new antimalaria compounds.
The host dfr1 yeast strain complemented with the wild-type yeast DHFR gene served as a control for generally toxic compounds. This yeast strain is unaffected by most of the DHFR inhibitors tested, so when inhibition was observed, the specificity of the particular compound was retested as described in Fig. 1E. The drug screening was repeated in the presence of dTMP, methionine, adenine, and histidine, metabolic end products that remove the requirement for functional folate coenzymes and the associated folate biosynthetic and homeostatic pathways. If a compound still inhibited under these supplemented conditions, then it had to be acting on some other aspect of the cell, not by specific inhibition of DHFR. NCI compound 117288 is an example of this phenomenon. This represents one advantage of using live cells in the assay and allows some subtle distinctions to be made. For example, Pf-yeasts were inhibited somewhat more in the absence of dTMP than in its presence, suggesting that 117288 may inhibit both a folate-requiring enzyme and some other yeast function.
While we found no compounds that are useful clinically, we did identify several interesting leads. Trimethoprim (NCI 106568) is selective for both C. parvum DHFR enzymes over the human enzyme, a requirement for clinical use. However, the sensitivity of the C. parvum enzymes to this particular drug is not sufficiently high for it to be effective against cryptosporidiosis (10, 65). Hundreds of trimethoprim analogs have been synthesized, and this analog collection may contain compounds that have significantly greater inhibitory potency for the C. parvum enzymes but still maintain selectivity. Of the six trimethoprim analogs assayed using the Cp-yeast complementation system, two showed inhibition profiles similar to that of trimethoprim. Further screening may identify better inhibitors. Additionally, NCI compounds 112421 and 121146 and several PY compounds were very effective against the C. parvum enzymes. Using these data, it may be possible to rationally design better inhibitors based on the observed sensitivities of existing compounds. Combining the lessons from trimethoprim selectivity and the potency of some of the other lipophilic compounds we tested may lead to a clinically useful agent against C. parvum.
The genetic-complementation-based drug-screening system described here used dfr1 yeast strains expressing heterologous C. parvum DHFR-TS and human DHFR genes to identify compounds that are potent and selective inhibitors of the C. parvum enzyme. Like all assay systems, it has strengths and limitations. However, it provides a rapid and inexpensive initial screen to identify drugs that may be effective against C. parvum or the other pathogens included in the screening set. A drug identified in this way can then be intensively studied in vitro and in the C. parvum culture systems. This approach can provide an additional tool in the search for drugs effective against this important pathogen.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI42321 to C.H.S., AI29904 to A.R., and NIH NIAID U01 AI40319 to R.G.N. V.H.B. was a trainee under NIH grant 67-1123 awarded to the Department of Genetics.
We thank Mohamed Nasr of the NIAID and William Ellis of WRAIR for providing us with compounds for testing and for advice on this work, Clement Furlong for initial help with the spoke assay, and Mary Reynolds, David Roos, and Steven Meshnick for plasmids with cloned DHFR genes. Sarah Pownder and Jason Munning provided technical assistance with portions of this work.
REFERENCES
- 1.Ahrweiler P M, Frieden C. Construction of a fol mutant strain of Escherichia coli for use in dihydrofolate reductase mutagenesis experiments. J Bacteriol. 1988;170:3301–3304. doi: 10.1128/jb.170.7.3301-3304.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. 2. J. New York, N.Y: Wiley; 1991. [Google Scholar]
- 3.Blakley R L. Eukaryotic dihydrofolate reductase. Vol. 70. New York, N.Y: John Wiley & Sons; 1995. pp. 23–102. [DOI] [PubMed] [Google Scholar]
- 4.Bonnin A, Fourmaux M N, Dubremetz J F, Nelson R G, Gobet P, Harly G, Buisson M, Puygauthier-Toubas D, Gabriel-Pospisil G, Naciri M, Camerlynck P. Genotyping human and bovine isolates of Cryptosporidium parvum by polymerase chain reaction-restriction fragment length polymorphism analysis of a repetitive DNA sequence. FEMS Microbiol Lett. 1996;137:207–211. doi: 10.1111/j.1574-6968.1996.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 5.Bowden K, Harris N V, Watson C A. Structure-activity relationships of dihydrofolate reductase inhibitors. J Chemother. 1993;5:377–388. [PubMed] [Google Scholar]
- 6.Bzik D J, Li W B, Horii T, Inselburg J. Molecular cloning and sequence analysis of the Plasmodium falciparum dihydrofolate reductase-thymidylate synthase gene. Proc Natl Acad Sci USA. 1987;84:8360–8364. doi: 10.1073/pnas.84.23.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camenisch G, Folkers G, van de Waterbeemd H. Review of theoretical passive drug absorption models: historical background, recent developments and limitations. Pharm Acta Helv. 1996;71:309–327. doi: 10.1016/s0031-6865(96)00031-3. [DOI] [PubMed] [Google Scholar]
- 8.Carraway M, Tzipori S, Widmer G. Identification of genetic heterogeneity in the Cryptosporidium parvum ribosomal repeat. Appl Environ Microbiol. 1996;62:712–716. doi: 10.1128/aem.62.2.712-716.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen B K, Horvath C, Bertino J R. Multivariate analysis and quantitative structure-activity relationships. Inhibition of dihydrofolate reductase and thymidylate synthetase by quinazolines. J Med Chem. 1979;22:483–491. doi: 10.1021/jm00191a005. [DOI] [PubMed] [Google Scholar]
- 10.Current W L. Cryptosporidium spp. In: Walzer P D, Genta R M, editors. Parasitic infections in the compromised host. New York, N.Y: Marcel Dekker; 1989. pp. 281–341. [Google Scholar]
- 11.Current W L, Reese N C, Ernst J V, Bailey W S, Heyman M B, Weinstein W M. Human cryptosporidiosis in immunocompetent and immunodeficient persons. Studies of an outbreak and experimental transmission. N Engl J Med. 1983;308:1252–1257. doi: 10.1056/NEJM198305263082102. [DOI] [PubMed] [Google Scholar]
- 12.Edman J C, Edman U, Cao M, Lundgren B, Kovacs J A, Santi D V. Isolation and expression of the Pneumocystis carinii dihydrofolate reductase gene. Proc Natl Acad Sci USA. 1989;86:8625–8629. doi: 10.1073/pnas.86.22.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falco E A, Hitchings G H, Russell P B, Vander Werff H. Antimalarials as antagonists of purines and pteroylglutamic acid. Nature (London) 1949;164:107–108. doi: 10.1038/164107a0. [DOI] [PubMed] [Google Scholar]
- 14.Genther C S, Schoeny R S, Loper J C, Smith C C. Antifolate studies. Activities of 40 potential antimalarial compounds against sensitive and chlorguanide triazine resistant strains of folate-requiring bacteria and Escherichia coli. J Med Chem. 1977;20:237–243. doi: 10.1021/jm00212a010. [DOI] [PubMed] [Google Scholar]
- 15.Goffeau A, Barrell B G, Bussey H, Davis R W, Dujon B, Feldmann H, Galibert F, Hoheisel J D, Jacq C, Johnston M, Louis E J, Mewes H W, Murakami Y, Philippsen P, Tettelin H, Oliver S G. Life with 6000 genes. Science. 1996;274:546. doi: 10.1126/science.274.5287.546. , 563–567. [DOI] [PubMed] [Google Scholar]
- 16.Grindley G, Moran R, Werkheiser W C. Drug design. Vol. 5. New York, N.Y: Academic Press; 1975. [Google Scholar]
- 17.Grunberg E, DeLorenzo W F. Potentiation of sulfonamides and antibiotics by trimethoprim [2,4-diamino-5-(3,4,5-trimethoxybenzyl) pyrimidine] Antimicrob Agents Chemother. 1966;6:430–433. [PubMed] [Google Scholar]
- 18.Guerrant R L. Cryptosporidiosis: an emerging, highly infectious threat. Emerg Infect Dis. 1997;3:51–57. doi: 10.3201/eid0301.970106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guthrie C, Fink G R. Guide to yeast genetics and molecular biology. San Diego, Calif: Academic Press; 1991. [Google Scholar]
- 20.Hitchings G H, Elion G B, Slinger S. Chemistry and biology of Pteridines. Ciba Found Symp. 1954;1954:290. [Google Scholar]
- 21.Hoepelman I M. Human cryptosporidiosis. Int J STD Aids. 1996;7(Suppl. 1):28–33. doi: 10.1258/0956462961917285. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman C S, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 23.Huang T, Barclay B J, Kalman T I, von Borstel R C, Hastings P J. The phenotype of a dihydrofolate reductase mutant of Saccharomyces cerevisiae. Gene. 1992;121:167–171. doi: 10.1016/0378-1119(92)90177-q. [DOI] [PubMed] [Google Scholar]
- 24.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch K L, Phillips D J, Aber R C, Current W L. Cryptosporidiosis in hospital personnel. Evidence for person-to-person transmission. Ann Intern Med. 1985;102:593–596. doi: 10.7326/0003-4819-102-5-593. [DOI] [PubMed] [Google Scholar]
- 26.Kunz B A, Taylor G R, Haynes R H. Induction of intrachromosomal recombination in yeast by inhibition of thymidylate biosynthesis. Genetics. 1986;114:375–392. doi: 10.1093/genetics/114.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuyper L F, Baccanari D P, Jones M L, Hunter R N, Tansik R L, Joyner S S, Boytos C M, Rudolph S K, Knick V, Wilson H R, Caddell J M, Friedman H S, Comley J C, Stables J N. High-affinity inhibitors of dihydrofolate reductase: antimicrobial and anticancer activities of 7,8-dialkyl-1,3-diaminopyrrolo[3,2-f]quinazolines with small molecular size. J Med Chem. 1996;39:892–903. doi: 10.1021/jm9505122. [DOI] [PubMed] [Google Scholar]
- 28.Lagosky P A, Taylor G R, Haynes R H. Molecular characterization of the Saccharomyces cerevisiae dihydrofolate reductase gene (DFR1) Nucleic Acids Res. 1987;15:10355–10371. doi: 10.1093/nar/15.24.10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lane B R, Ast J C, Hossler P A, Mindell D P, Bartlett M S, Smith J W, Meshnick S R. Dihydropteroate synthase polymorphisms in Pneumocystis carinii. J Infect Dis. 1997;175:482–485. doi: 10.1093/infdis/175.2.482. [DOI] [PubMed] [Google Scholar]
- 30.Lemeteil D, Roussel F, Favennec L, Ballet J J, Brasseur P. Assessment of candidate anticryptosporidial agents in an immunosuppressed rat model. J Infect Dis. 1993;167:766–768. doi: 10.1093/infdis/167.3.766. [DOI] [PubMed] [Google Scholar]
- 31.Marshall M M, Naumovitz D, Ortega Y, Sterling C R. Waterborne protozoan pathogens. Clin Microbiol Rev. 1997;10:67–85. doi: 10.1128/cmr.10.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCormack J J. Structure-activity relationships among pteridine derivatives and related quinazolines as inhibitors of dihydrofolate reductase. In: Pfleiderer W, editor. Chemistry and biology of pteridines. New York, N.Y: de Gruyter; 1975. pp. 125–132. [Google Scholar]
- 33.Miyajima A, Miyajima I, Arai K, Arai N. Expression of plasmid R388-encoded type II dihydrofolate reductase as a dominant selective marker in Saccharomyces cerevisiae. Mol Cell Biol. 1984;4:407–414. doi: 10.1128/mcb.4.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moir D T, Dorman T E, Smyth A P, Smith D R. A human genome YAC library in a selectable high-copy-number vector. Gene. 1993;125:229–232. doi: 10.1016/0378-1119(93)90334-y. [DOI] [PubMed] [Google Scholar]
- 35.Morgan U M, Constantine C C, O'Donoghue P, Meloni B P, O'Brien P A, Thompson R C. Molecular characterization of Cryptosporidium isolates from humans and other animals using random amplified polymorphic DNA analysis. Am J Trop Med Hyg. 1995;52:559–564. doi: 10.4269/ajtmh.1995.52.559. [DOI] [PubMed] [Google Scholar]
- 36.Mumberg D, Muller R, Funk M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene. 1995;156:119–122. doi: 10.1016/0378-1119(95)00037-7. [DOI] [PubMed] [Google Scholar]
- 37.Peng M M, Xiao L, Freeman A R, Arrowood M J, Escalante A A, Weltman A C, Ong C S, Mac Kenzie W R, Lal A A, Beard C B. Genetic polymorphism among Cryptosporidium parvum isolates: evidence of two distinct human transmission cycles. Emerg Infect Dis. 1997;3:567–573. doi: 10.3201/eid0304.970423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters W. Chemotherapy and drug resistance in malaria. 2nd ed. London, United Kingdom: Academic Press; 1987. [Google Scholar]
- 39.Poe M. Antibacterial synergism: a proposal for chemotherapeutic potentiation between trimethoprim and sulfamethoxazole. Science. 1976;194:533–535. doi: 10.1126/science.788154. [DOI] [PubMed] [Google Scholar]
- 40.Prendergast N J, Delcamp T J, Smith P L, Freisheim J H. Expression and site-directed mutagenesis of human dihydrofolate reductase. Biochemistry. 1988;27:3664–3671. doi: 10.1021/bi00410a022. [DOI] [PubMed] [Google Scholar]
- 41.Reisberg B, Herzog J, Weinstein L. In vitro antibacterial activity of trimethoprim alone and combined with sulfonamides. Antimicrob Agents Chemother. 1966;6:424–429. [PubMed] [Google Scholar]
- 42.Roos D S. Primary structure of the dihydrofolate reductase-thymidylate synthase gene from Toxoplasma gondii. J Biol Chem. 1993;268:6269–6280. [PubMed] [Google Scholar]
- 43.Rose J B. Environmental ecology of Cryptosporidium and public health implications. Annu Rev Public Health. 1997;18:135–161. doi: 10.1146/annurev.publhealth.18.1.135. [DOI] [PubMed] [Google Scholar]
- 44.Rosowsky A, Chen K K N, Lin M. 2,4-Diaminothieno[2,3-d]pyrimidines as antifolates and antimalarials. 3. Synthesis of 5,6-disubstituted derivatives and related tricyclic analogues. J Heterocycl Chem. 1972;9:775–782. doi: 10.1021/jm00261a004. [DOI] [PubMed] [Google Scholar]
- 45.Rosowsky A, Chen K K N, Nadel M E, Papathanasopoulos N, Modest E J. Quinazolines. VIII. Synthesis of 1,3-diaminobenzo[f]quinazolines. J Heterocycl Chem. 1972;9:275–283. [Google Scholar]
- 46.Rosowsky A, Chen K K N, Papathanasopoulos N, Modest E J. Quinazolines. VII. Synthesis of 1,3-diamino-5,6-dihydrobenzo[f]quinazolines. J Heterocycl Chem. 1972;9:263–273. [Google Scholar]
- 47.Rosowsky A, Dey A S, Battaglia J, Modest E J. Synthesis of 2,4-diamino-9H-indeno[2,1-d]pyrimidines. J Heterocycl Chem. 1969;6:613–622. [Google Scholar]
- 48.Rosowsky A, Forsch R A, Queener S F. 2,4-Diaminopyrido[3,2-d]pyrimidine inhibitors of dihydrofolate reductase from Pneumocystis carinii and Toxoplasma gondii. J Med Chem. 1995;38:2615–2620. doi: 10.1021/jm00014a014. [DOI] [PubMed] [Google Scholar]
- 49.Rosowsky A, Hynes J B, Queener S F. Structure-activity and structure-selectivity studies on diaminoquinazolines and other inhibitors of Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase. Antimicrob Agents Chemother. 1995;39:79–86. doi: 10.1128/aac.39.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosowsky A, Marini J L, Nadel M E, Modest E J. Quinazolines. VI. Synthesis of 2,4-diaminoquinazolines from anthranilonitriles. J Med Chem. 1970;13:882–886. doi: 10.1021/jm00299a021. [DOI] [PubMed] [Google Scholar]
- 51.Rosowsky A, Mota C E, Queener S F. Synthesis and antifolate activity of 2,4-diamino-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine analogues of trimetrexate and piritrexim. J Heterocycl Chem. 1995;32:335–340. [Google Scholar]
- 52.Rosowsky A, Mota C E, Queener S F, Waltham M, Ercikan Abali E, Bertino J R. 2,4-Diamino-5-substituted-quinazolines as inhibitors of a human dihydrofolate reductase with a site-directed mutation at position 22 and of the dihydrofolate reductases from Pneumocystis carinii and Toxoplasma gondii. J Med Chem. 1995;38:745–752. doi: 10.1021/jm00005a002. [DOI] [PubMed] [Google Scholar]
- 53.Rosowsky A, Mota C E, Wright J E, Freisheim J H, Heusner J J, McCormack J J, Queener S F. 2,4-Diaminothieno[2,3-d]pyrimidine analogues of trimetrexate and piritrexim as potential inhibitors of Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase. J Med Chem. 1993;36:3103–3112. doi: 10.1021/jm00073a009. [DOI] [PubMed] [Google Scholar]
- 54.Rosowsky A, Mota C E, Wright J E, Queener S F. 2,4-Diamino-5-chloroquinazoline analogues of trimetrexate and piritrexim: synthesis and antifolate activity. J Med Chem. 1994;37:4522–4528. doi: 10.1021/jm00052a011. [DOI] [PubMed] [Google Scholar]
- 55.Rosowsky A, Papoulis A T, Forsch R A, Queener S F. Synthesis and antiparasitic and antitumor activity of 2,4-diamino-6-(arylmethyl)-5,6,7,8-tetrahydroquinazoline analogues of piritrexim. J Med Chem. 1999;42:1007–1017. doi: 10.1021/jm980572i. [DOI] [PubMed] [Google Scholar]
- 56.Rosowsky A, Papoulis A T, Queener S F. 2,4-Diaminothieno[2,3-d]pyrimidine lipophilic antifolates as inhibitors of Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase. J Med Chem. 1997;40:3694–3699. doi: 10.1021/jm970399a. [DOI] [PubMed] [Google Scholar]
- 57.Sibley C H, Brophy V H, Cheesman S, Hamilton K L, Hankins E G, Wooden J M, Kilbey B. Yeast as a model system to study drugs effective against apicomplexan proteins. Methods. 1997;13:190–207. doi: 10.1006/meth.1997.0511. [DOI] [PubMed] [Google Scholar]
- 58.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sirotnak F M. Folate antagonists as therapeutic agents. New York, N.Y: Academic Press; 1984. [Google Scholar]
- 60.Spano F, Putignani L, McLauchlin J, Casemore D P, Crisanti A. PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) gene discriminates between C. wrairi and C. parvum, and between C. parvum isolates of human and animal origin. FEMS Microbiol Lett. 1997;150:209–217. doi: 10.1016/s0378-1097(97)00115-8. [DOI] [PubMed] [Google Scholar]
- 61.Vasquez J R, Gooz'e L, Kim K, Gut J, Petersen C, Nelson R G. Potential antifolate resistance determinants and genotypic variation in the bifunctional dihydrofolate reductase-thymidylate synthase gene from human and bovine isolates of Cryptosporidium parvum. Mol Biochem Parasitol. 1996;79:153–165. doi: 10.1016/0166-6851(96)02647-3. [DOI] [PubMed] [Google Scholar]
- 62.Warner P, Maniar A C. Combined action of sulfadiazine and trimethoprim. Appl Microbiol. 1966;14:299–300. doi: 10.1128/am.14.2.299-300.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Welch A D. Folic acid: discovery and the exciting first decade. Perspect Biol Med. 1983;27:64–75. doi: 10.1353/pbm.1983.0006. [DOI] [PubMed] [Google Scholar]
- 64.Wooden J M, Hartwell L H, Vasquez B, Sibley C H. Analysis in yeast of antimalaria drugs that target the dihydrofolate reductase of Plasmodium falciparum. Mol Biochem Parasitol. 1997;85:25–40. doi: 10.1016/s0166-6851(96)02808-3. [DOI] [PubMed] [Google Scholar]
- 65.Woods K M, Nesterenko M V, Upton S J. Efficacy of 101 antimicrobials and other agents on the development of Cryptosporidium parvum in vitro. Ann Trop Med Parasitol. 1996;90:603–615. doi: 10.1080/00034983.1996.11813090. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Y H, Zhang X P, Ebright R H. Random mutagenesis of gene-sized DNA molecules by use of PCR with Taq DNA polymerase. Nucleic Acids Res. 1991;19:6052. doi: 10.1093/nar/19.21.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]