Abstract
Cocaine use disorder (CUD) is a major public health challenge for which there are no pharmacotherapeutics approved by the United States Food and Drug Administration (FDA). The propensity to relapse in CUD involves several vulnerability factors including sensitivity to cues associated with cocaine-taking. Serotonin (5-hydroxytryptamine, 5-HT) neurotransmission, particularly through the 5-HT2A receptor (5-HT2AR) and 5-HT2C receptor (5-HT2CR), is mechanistically linked to cocaine-seeking in preclinical models. In the present experiments, we employed self-administration assays in male rats to investigate whether acute and/or repeated administration of the FDA-approved selective 5-HT2AR antagonist/inverse agonist pimavanserin, selective 5-HT2CR agonist lorcaserin or their combination would alter cocaine intake and/or cocaine-seeking behavior. We found that acute administration of lorcaserin, but not pimavanserin, attenuated cocaine intake while pimavanserin plus lorcaserin did not impact cocaine self-administration. In contrast, 10-days of repeated administration of pimavanserin, lorcaserin, or pimavanserin plus lorcaserin during forced abstinence from cocaine self-administration, blunted cocaine-seeking, similar to the acute administration of each ligand. Taken together, these data reveal the efficacy of repeated treatment with pimavanserin plus lorcaserin to attenuate factors important to relapse-like behaviors in rodent models of CUD.
This article is part of the special issue entitled ‘Serotonin Research: Crossing Scales and Boundaries’.
Keywords: 5-HT2A receptor, 5-HT2C receptor, Cocaine, Drug-seeking behavior, Lorcaserin, Pimavanserin
1. Introduction
Rising numbers of cocaine overdose deaths are linked to the surge in cocaine availability and the ongoing opioid overdose crisis (McCall Jones et al., 2017), with the alarming news of a 61% increase in young adults (18–25 yo) initiating cocaine use in the United States (2013–2015) and the highest cocaine overdoses reported since 2006 (Hughes et al., 2016). In 2017, an estimated 2.2 million persons aged 12 or older were current users of cocaine with one million new initiates; ~966,000 were diagnosed with cocaine use disorder (CUD) (Center for Behavioral Health Statistics and Quality, 2018), a progressively debilitating acquired disorder characterized by disruption of drug and natural reward processing and higher order cognitive function (Koob and Volkow, 2016). Core deficits in the ability to inhibit behavior, whether a premorbid neurodevelopmental trait or an acquired consequence of chronic cocaine use, coupled with increased attentional bias toward drug reward stimuli factor into relapse vulnerability in CUD individuals (Anastasio et al., 2014a; Liu et al., 2012).
Cocaine generates its reinforcing effects via stimulation of dopamine (DA) mesolimbic efflux; DA and glutamate transmission have well-described roles in encoding drug reward and saliency for drug-associated cues at the level of mesocorticolimbic circuit (Koob and Volkow, 2016). In light of unsuccessful clinical trials for direct dopaminergic agents to reduce relapse (Indave et al., 2016; Kohut and Bergman, 2017; Minozzi et al., 2015), modulation of other neurotransmitters that may themselves regulate DA or otherwise regulate behavior are of interest in the development of pharmacotherapies for CUD. Importantly, preclinical studies implicate serotonin (5-HT) as an influential regulator of neural circuit dynamics involved in the substance use disorder (SUD) cycle, in part via direct and/or indirect interactions with DA systems (Lucki, 1998; Soubrié, 1986). The actions of 5-HT in neurons are transduced by 14 subtypes of 5-HT receptors (5-HTXRs) in which the 5-HT2AR and 5-HT2CR support a modulatory role in relapse-like behaviors in rodents (for reviews, (Cunningham and Anastasio, 2014; Howell and Cunningham, 2015). Selective inhibition of the 5-HT2AR (e.g., by M100907) or activation of the 5-HT2CR (e.g., by WAY163909), acutely, consistently suppresses both cue- and cocaine-evoked reinstatement (cocaine-seeking) after a period of cocaine self-administration and extinction (Cunningham et al., 2011; Fletcher et al., 2008; Neisewander and Acosta, 2007; Nic Dhonnchadha et al., 2009). Moreover, acute administration of M100907 plus WAY163909 curbs cocaine-induced hyperactivity (replicated by an independent laboratory) (Pockros et al., 2012), as well as cue-evoked and cocaine-primed reinstatement (Cunningham et al., 2013). These provocative outcomes prompted the hypothesis that 5-HT2AR and 5-HT2CR exhibit a special relationship, rooted in key nodes of SUD neurocircuitry, which control cocaine-associated behaviors (for reviews, (Cunningham and Anastasio, 2014; Howell and Cunningham, 2015).
The emergence of clinically available, selective 5-HT2AR and 5-HT2CR ligands presents new pharmacotherapeutic prospects for the treatment of CUD. Pimavanserin (Nuplazid®) is an FDA-approved, potent and selective 5-HT2AR antagonist (Vanover et al., 2006) approved for treatment of psychosis in Parkinson's disease (Sahli and Tarazi, 2018) and also displays inverse agonist activity in functional assays (Vanover et al., 2006), a property inherent in many such ligands that affect basal and constitutive activity of the 5-HT2AR (Aloyo et al., 2009). Pimavanserin exhibits robust activity in a variety of preclinical models of CNS disease (McFarland et al., 2011; Vanover et al., 2006, 2008) including cocaine associated relapse-like behaviors (Sholler et al., 2019). Lorcaserin (Belviq®) is a 5-HT2CR agonist that was until recently FDA-approved for the treatment of obesity (Gustafson et al., 2013). Lorcaserin suppresses cocaine self-administration (Collins et al., 2016) and reinstatement of drug-seeking for cocaine in preclinical models (Gerak et al., 2016, 2019; Harvey-Lewis et al., 2016), effects reversed by pretreatment with the selective 5-HT2CR antagonist SB242084 (Collins et al., 2016; Gerak et al., 2016, 2019; Harvey-Lewis et al., 2016). In the present study, we tested the hypothesis that pimavanserin, lorcaserin, or their combination would suppress cocaine self-administration and cocaine-seeking behavior in rats and coupled these analyses with investigation of potential pharmacokinetic interactions between pimavanserin and lorcaserin. In addition, we tested the hypothesis that repeated, daily treatment with combined administration of pimavanserin plus lorcaserin would blunt cocaine-seeking behavior similar to acute administration. Such findings would support the novel pharmacotherapeutic prospect that pimavanserin alone or in combination with lorcaserin may support behavioral recovery in CUD.
2. Materials and methods
2.1. Animals
Behaviorally and surgically-naïve male Sprague-Dawley rats (n = 156 total; Envigo, Indianapolis, IN) weighing 250–325 g at the start of experiments were acclimated for seven days in a colony room maintained at a constant temperature (21–23 °C) and humidity (45–50%) on a 12-h light-dark cycle (lights on 0600–1800 h). All rats for behavioral analyses were implanted with jugular catheters in our laboratory. For pharmacokinetic analyses, an additional cohort of male Sprague-Dawley rats (n = 63) were obtained with jugular vein catheters fitted with a PinPort™ (Instech Laboratory, Plymouth Meeting, PA) implanted by the supplier. Rats were housed two/cage, except for PinPort™ surgically prepared rats which were housed one/cage; all rats were handled daily throughout the study. Food and water were available ad libitum throughout all phases of the studies. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (2011) and with approval from the University of Texas Medical Branch Institutional Animal Care and Use Committee.
2.2. Drugs
(−)-Cocaine (National Institute on Drug Abuse, Research Triangle Park, NC) and lorcaserin hydrochloride (Trylead Chemical Technology Co., Ltd., Hangzhou, China) were dissolved in 0.9% NaCl (vehicle employed for comparison to cocaine and lorcaserin). Pimavanserin (Trylead Chemical Technology, Hangzhou, China) was dissolved in 0.9% NaCl with 1M HCl and brought to a pH of 5.5 using 1M NaOH (vehicle employed for comparison to pimavanserin). SB242084 [6-chloro-5-methyl-1-[[2-(2-methylpyrid-3-yloxy) pyrid-5-yl]carbamoyl] indolinedihydrochloride; Sigma Chemical Co., St. Louis, MO] was dissolved in 0.9% NaCl containing 10 mM citric acid (Sigma Chemical Co.) and 8% 2-hydroxypropyl-β-cyclodextrin (Trappsol® Hydroxpropyl Beta Cyclodextrin, Pharmaceutical Grade, Cyclodextrin Technologies Development Inc., High Springs, FL) with the final pH of the solution adjusted to 5.6.
2.3. Assessment of cocaine self-administration and cocaine-seeking behavior
2.3.1. Surgery
Rats (n = 156) were implanted with intravenous catheters with back mounts under anesthesia with a cocktail containing 8.6 mg/kg of xylazine, 1.5 mg/kg of acepromazine, and 43 mg/kg of ketamine in bacteriostatic saline and allowed to recover for 5–7 days before initiation of self-administration training (Anastasio et al., 2014a, 2014b, 2013; Neelakantan et al., 2017; Sholler et al., 2019). Catheter patency was maintained by daily flushes with a solution of 0.1 ml bacteriostatic saline containing heparin sodium (10 U/ml; American Pharmaceutical Partners, East Schaumburg, IL), streptokinase (0.67 mg/ml; Sigma Chemical), and ticarcillin disodium (66.67 mg/ml; Research Products International, Mt. Prospect, IL) immediately following daily cocaine self-administration sessions.
2.3.2. Self-administration training
Standard operant conditioning chambers (Med-Associates, Inc., St. Albans, VT) housed in ventilated, sound-attenuating cubicles with fans (Med-Associates, Inc.) were utilized for cocaine self-administration studies. Each chamber was equipped with a pellet receptacle flanked by two retractable response levers, a stimulus light above each response lever, and a house light opposite the levers. Cocaine infusions were delivered via syringes attached to infusion pumps (Med-Associates, Inc.) located outside the cubicles. The infusion pumps were connected to liquid swivels (Instech, Plymouth Meeting, PA) fastened to the catheters via polyethylene 20 tubing encased inside a metal spring leash (Plastics One, Roanoke, VA).
Freely-fed rats were trained to lever press for cocaine infusions using established methods (Anastasio et al., 2014a, 2014b, 2013; Neelakantan et al., 2017; Sholler et al., 2019). Cocaine self-administration training consisted of daily 180-min sessions during which rats were trained to press the active lever to obtain an infusion on a fixed ratio (FR) 1 schedule of reinforcement before progressing to a final FR 5 schedule of reinforcement. Schedule completions on the active lever resulted in delivery of a cocaine infusion over a 6-sec period paired simultaneously with illumination of the house and stimulus lights and activation of the infusion pump (discrete cue complex); inactive lever presses produced no scheduled consequences. Following reinforcer delivery, the stimulus light and infusion pump were inactivated; the house light remained on for an additional 20 s to indicate a timeout period during which lever presses had no scheduled consequences.
2.3.3. Assessment of pimavanserin, lorcaserin, or the combination on cocaine self-administration
The efficacy of pimavanserin, lorcaserin, or their combination to alter cocaine intake was assessed in rats (n = 48) trained to self-administer cocaine (0.75 mg/kg/0.1 ml infusion) to stability. Once acquired, rats were transitioned to a lower dose of cocaine (0.25 mg/kg/ 0.1 ml infusion) and maintained on this dose throughout testing. Rats readily acquired cocaine self-administration in daily 180 min sessions to stability and displayed <10% variation in the number of infusions received (i.e., cocaine intake) (data not shown) per our previous publications (Anastasio et al., 2014a, 2014b, 2013; Sholler et al., 2019). Tests were administered in a pseudorandomized order with a minimum of two intervening sessions of cocaine self-administration to assure stability of baseline responding. Fourteen rats failed to meet acquisition/maintenance criteria or lost catheter patency and were excluded from subsequent analyses (pimavanserin efficacy: n = 9/12 analyzed; lorcaserin efficacy: n = 13–14/24 analyzed; pimavanserin plus lorcaserin efficacy: n = 12/12 analyzed).
Pretreatment with the 5-HT2CR antagonist SB242084 was employed to confirm that the effect of lorcaserin on cocaine self-administration was 5-HT2CR specific. Pimavanserin and lorcaserin were administered s.c. and SB242084 was administered i.p.; vehicles were dosed at 1 ml/ kg. Pretreatment times were consistent with numerous rat cocaine self-administration and reinstatement studies employing a 15 min pretreatment for lorcaserin and other 5-HT2CR agonists (Cunningham et al., 2011, 2013; Fletcher et al., 2008; Neisewander and Acosta, 2007) and 30 min pretreatment for pimavanserin and other 5-HT2AR antagonists/inverse agonists (Cunningham et al., 2013; Nic Dhonnchadha et al., 2009; Sholler et al., 2019).
Rats were injected with vehicle or pimavanserin (0.3–3 mg/kg; 30 min) prior to the start of the cocaine self-administration session in a within-subjects design.
Rats were injected with vehicle, lorcaserin (0.25–1 mg/kg; 15 min), or the 5-HT2CR antagonist SB242084 (0.5 mg/kg; 30 min) plus lorcaserin (1 mg/kg; 15 min) prior to the start of the cocaine self-administration session in a within-subjects design.
Rats were injected with vehicle, pimavanserin (0.5 mg/kg; 30 min), lorcaserin (0.5 mg/kg; 15 min) or the combination of pimavanserin plus lorcaserin prior to the start of the cocaine self-administration session in a within-subjects design.
2.3.4. Assessment of pimavanserin, lorcaserin, or the combination on cocaine-seeking behavior
The efficacy of acute or repeated pimavanserin, lorcaserin, or their combination to alter cocaine-seeking behavior during a period of forced abstinence (FA) was assessed in a separate cohort of rats (n = 108) trained to self-administer cocaine (0.75 mg/kg/0.1 ml infusion) to stability (as described above) prior to initiation of FA and similar to previous publications (Anastasio et al., 2014a, 2014b, 2013; Sholler et al., 2019). We assessed cocaine-seeking behavior in an abstinence model; in brief, lever presses during the test sessions which occurred during abstinence from cocaine self-administration were reinforced with the discrete cue complex which had previously been paired with cocaine delivery. Once the criterion for stable cocaine self-administration was attained and maintained, rats were subjected to FA from cocaine self-administration for 10 days. During the FA period, rats were returned to their home cages, weighed, and handled daily. Following the assigned FA period, rats were evaluated in a cocaine-seeking behavior test session (60 min) in which lever presses were reinforced by delivery of the discrete cue complex (stimulus light illuminated, infusion pump activated) on a FR1 schedule. Inactive lever presses were recorded but had no scheduled consequences. Seventeen rats failed to meet acquisition/maintenance criteria or lost catheter patency during the course of the study, and were excluded from subsequent analyses (vehicle: n = 13/15 analyzed; pimavanserin efficacy: n = 26/27 analyzed; lorcaserin efficacy: n = 25/25 analyzed; pimavanserin plus lorcaserin efficacy: n = 24/24 analyzed). Route of administration and pretreatment times were as described above.
For acute treatment efficacy experiments, rats were injected with vehicle, pimavanserin (3 mg/kg), lorcaserin (1 mg/kg) or the combination of pimavanserin (0.5 mg/kg) plus lorcaserin (0.5 mg/ kg) prior to the 60 min drug-seeking test session on FA day 10 in a between-subjects design.
For repeated treatment efficacy experiments, rats were injected with vehicle, pimavanserin (3 mg/kg), lorcaserin (1 mg/kg) or the combination of pimavanserin (0.5 mg/kg) plus lorcaserin (0.5 mg/ kg) twice daily for 10 days during abstinence in a between-subjects design.
2.4. Assessment of pharmacokinetics (PK) of pimavanserin, lorcaserin, or the combination
Blood samples were collected from the jugular vein for determination of the plasma concentrations following administration of pimavanserin, lorcaserin, or the combination of pimavanserin plus lorcaserin. Upon receipt at the animal facility at UTMB, the jugular vein catheter implanted in each animal was checked for patency. Once blood was withdrawn through the PinPort™, heparinized 0.9% NaCl (10 IU heparin/ml diluted in saline) was infused into the lumen to clear the line. On the morning of dosing and 30 min prior to drug administration, the patency of each jugular vein catheter was again confirmed. Pre-dose blood samples (~0.25 ml) were collected at this point. Following dosing, blood (~0.25 ml) was collected from each animal in each treatment group via the PinPort™ at each of the following timepoints: 0, 15, 30, 60, 120, 180, 240, 360 min. Treatment groups included pimavanserin (0.5–10 mg/kg; s.c.), lorcaserin (0.5–3 mg/kg; s.c.) or pimavanserin plus lorcaserin (0.5 mg/kg + 0.5 mg/kg; 1 mg/kg + 1 mg/kg; s.c.). After each blood collection, ~0.25 ml of heparinized saline (10 IU/ml) was flushed through each rat via the catheter. Each blood sample (~0.25 ml) was collected into a heparinized test tube. Blood samples were centrifuged at approximately 1,800×g for 5 min. The plasma was transferred to new tubes and stored frozen at −20 °C until analysis. The PK analyses were performed by SRI International (Menlo Park, CA) under contract to the National Institute on Drug Abuse.
2.5. Statistical analyses
The data from the cocaine self-administration and cocaine-seeking experiments were analyzed separately. A one-way analysis of variance (ANOVA) for a within-subjects design was employed to assess (a) cocaine infusions, (b) active lever presses, and (c) inactive lever responses during self-administration. A one-way ANOVA for a between-subjects design was employed to assess (a) cue-reinforced lever presses, (b) inactive lever presses, and (c) first response latency on the cue-reinforced lever during cocaine-seeking test sessions. A priori comparisons were defined prior to the start of experimentation and conducted by Dunnett's procedure or Bonferroni's method as appropriate (Keppel, 1973). Investigators who performed ligand administration and endpoint analyses were blinded to group assignments. All statistical analyses were conducted with GraphPad Prism (version 7.05) with an experiment-wise error rate set at α = 0.05.
3. Results
3.1. Lorcaserin, but not pimavanserin, suppresses cocaine self-administration
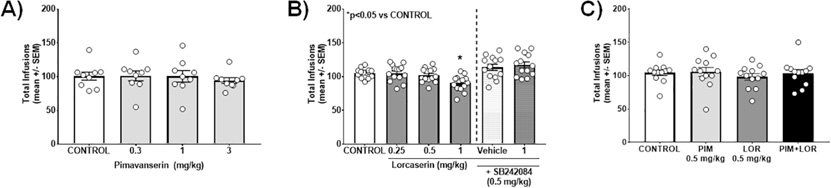
Acute administration of lorcaserin and investigatory 5-HT2CR agonists, but not selective 5-HT2AR antagonists (like M100907), dose-dependently decrease cocaine intake (for reviews, Cunningham and Anastasio, 2014; Higgins et al., 2020). Here we assessed acute administration of doses of pimavanserin and lorcaserin to block intake of a relatively low dose (0.25 mg/kg/inf) cocaine self-administration to establish doses for combination testing. No main effect of pimavanserin treatment on infusions (F(3,32) = 0.2315, p = 0.8738; Fig. 1A) active lever presses (F(3,32) = 0.2039, p = 0.8929; Table 1) or inactive lever presses (F(3,32) = 0.5564, p = 0.6476; Table 1) was observed. These are the first data to suggest that pimavanserin at these doses lacks efficacy to alter cocaine self-administration, similar to previous studies with M100907.
Fig. 1. Acute administration of pimavanserin plus lorcaserin does not suppress cocaine self-administration.
(A) Effects of acute pimavanserin (0.3, 1, 3 mg/ kg) on total cocaine infusions (mean ± SEM) are presented (n.s. vs. control). (B) Effects of acute lorcaserin (0.25, 0.5, 1 mg/kg) to alter total cocaine infusions (mean ± SEM) are presented. Lorcaserin (1.0 mg/kg) suppresses cocaine intake relative to control (*p < 0.05 vs. control; left panel); this effect is prevented by the selective 5-HT2CR antagonist SB242084 (right panel). (C) Effects of subthreshold doses of pimavanserin (PIM; 0.5 mg/kg) plus lorcaserin (LOR; 0.5 mg/kg) on total cocaine infusions (mean ± SEM) are presented (n.s. vs. control).
Table 1.
Effects of pimavanserin, lorcaserin, or their combination on active and inactive lever presses during cocaine self-administration (mean ± SEM).
| Active Lever Presses | Inactive Lever Presses | |
|---|---|---|
|
| ||
| Pimavanserin (mg/kg) | ||
| CONTROL | 564.94 ± 62.31 | 2.87 ± 0.74 |
| 0.3 | 587.11 ± 72.80 | 3.67 ± 2.11 |
| 1 | 540.33 ± 57.42 | 1.22 ± 0.86 |
| 3 | 523.00 ± 54.57 | 3.44 ± 1.74 |
| Lorcaserin (mg/kg) | ||
| CONTROL | 575.33 ± 11.89 | 3.10 ± 0.98 |
| 0.25 | 547.46 ± 16.57 | 1.77 ± 0.43 |
| 0.5 | 536.23 ± 16.03 | 2.08 ± 0.40 |
| 1 | 492.46 ± 18.99 | 3.00 ± 0.91 |
| Lorcaserin (mg/kg) + SB242084 (mg/kg) | ||
| CONTROL+ 0.5 | 663.92 ± 49.87 | 7.54 ± 3.59 |
| 1 + 0.5 | 636.15 ± 27.34 | 1.62 ± 0.49 |
| Pimavanserin (mg/kg) + Lorcaserin (mg/kg) | ||
| CONTROL | 558.63 ± 12.56 | 2.67 ± 0.75 |
| 0.5 +CONTROL | 562.60 ± 17.06 | 2.00 ± 0.65 |
| CONTROL + 0.5 | 562.50 ± 18.89 | 2.40 ± 0.65 |
| 0.5 + 0.5 | 550.80 ± 16.13 | 3.60 ± 1.72 |
A main effect of lorcaserin treatment on infusions (F(5,76) = 7.539, p < 0.0001; Fig. 1B) was observed; a priori comparisons indicated lorcaserin (1 mg/kg) suppressed cocaine intake vs. control (p < 0.05). The 5-HT2CR antagonist SB242084 prevented this lorcaserin-evoked suppression of cocaine intake (p < 0.05; Fig. 1B). A main effect of lorcaserin treatment on active lever presses [F(5,72) = 5.826, p = 0.0001], but not inactive lever presses [F(5,72) = 1.943, p = 0.0977], was observed (Table 1). These data replicate previous observations that 5-HT2CR agonists exhibit efficacy to suppress cocaine intake in the rat self-administration model (Burbassi and Cervo, 2008; Cunningham et al., 2011; Fletcher et al., 2008; Grottick et al., 2000; Higgins et al., 2020; Neisewander and Acosta, 2007).
3.1.1. Pimavanserin plus lorcaserin does not suppress cocaine self-administration
No main effect of combination treatment on infusions [F (3,36) = 1.221, p = 0.3162; Fig. 1C], active lever presses [F (3,36) = 0.1151, p = 0.9507; Table 1] or inactive lever presses [F (3,36) = 0.4227, p = 0.7379; Table 1] was observed. These data indicate that subthreshold doses of pimavanserin plus lorcaserin do not alter cocaine intake.
3.2. Acute and repeated pimavanserin or lorcaserin suppress cocaine-seeking behavior
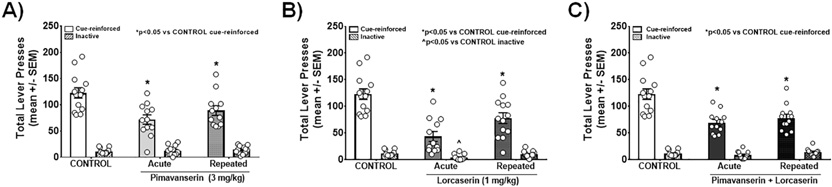
A main effect of pimavanserin treatment on cue-reinforced lever presses during a cocaine-seeking test on FA day 10 was observed (F (2,34) = 7.708, p = 0.0017; Fig. 2A); a priori comparisons demonstrated that both acute (~41.4% decrease, p < 0.05) and repeated pimavanserin (~27.1% decrease, p < 0.05) suppressed cue-reinforced lever presses vs. control. No main effect of pimavanserin treatment on inactive lever presses was observed (F(2,34) = 0.4201, p = 0.6603; Fig. 2A). A main effect of pimavanserin treatment on latency to the first response [F(2,34) = 3.91, p = 0.0296] was observed; a priori comparisons demonstrated that repeated (35.25 ± 7.81 s; ~2.5-fold increase, p < 0.05), but not acute (29.83 ± 5.43 s; n.s.), pimavanserin treatment increased latency to the first response vs. control (14.39 ± 2.41 s). These data suggest that acute and repeated treatment with pimavanserin alone attenuates cocaine-seeking behavior on FA day 10, with minor behavioral inhibition initially.
Fig. 2. Acute and repeated administration of pimavanserin plus lorcaserin suppress cocaine-seeking behavior during abstinence.
(A) Effects of acute and repeated pimavanserin (3 mg/kg) on cue-reinforced or inactive lever presses (mean ± SEM) on FA 10 are presented (*p < 0.05 vs. control). (B) Effects of acute and repeated lorcaserin (1 mg/kg) on cue-reinforced or inactive lever presses (mean ± SEM) are presented (*p < 0.05 vs. control cue-reinforced; ^p < 0.05 vs. control inactive). (C) Effects of acute and repeated combination of pimavanserin (0.5 mg/kg) plus lorcaserin (0.5 mg/kg) on cue-reinforced or inactive lever presses (mean ± SEM) on FA 10 are presented (*p < 0.05 vs. control cue-reinforced).
A main effect of lorcaserin treatment on cue-reinforced lever presses during a cocaine-seeking test on FA day 10 was observed (F (2,35) = 16.02, p < 0.0001; Fig. 2B); a priori comparisons demonstrate acute (~64.4% decrease, p < 0.05) and repeated lorcaserin (~36.4% decrease, p < 0.05) suppressed cue-reinforced lever presses vs. control. A main effect of lorcaserin treatment on inactive lever presses (F (2,35) = 5.673, p = 0.0073; Fig. 2B) was observed; a priori comparisons demonstrate acute (~64.6% suppression; p < 0.05), but not repeated, lorcaserin (n.s.) suppressed inactive lever presses vs. control. A main effect of lorcaserin treatment on latency to the first response [F (2,35) = 4.615, p = 0.0166] was observed; a priori comparisons demonstrate acute (95.83 ± 35.78 s; ~6.7-fold increase; p < 0.05), but not repeated (30.15 ± 5.52 s; n.s.), lorcaserin treatment increased latency to the first response vs. control (14.39 ± 2.41 s). These data suggest that acute and repeated treatment with lorcaserin blunts cocaine-seeking behavior on FA day 10, with a moderate impact on the latency to first response only following acute administration.
3.2.1. Acute and repeated pimavanserin plus lorcaserin suppresses cocaine-seeking behavior
A main effect of pimavanserin plus lorcaserin treatment on cue-reinforced lever presses was observed [F(2,34) = 13.02, p < 0.0001; Fig. 2C]; a priori comparisons demonstrate acute (~56.7% decrease, p < 0.05) and repeated pimavanserin plus lorcaserin (~63.7% decrease, p < 0.05) suppressed cue-reinforced lever presses vs. control. Multiple comparisons assessed using Bonferroni's method revealed no difference in cue-reinforced lever presses between rats receiving acute pimavanserin vs. acute pimavanserin plus lorcaserin (n.s.), acute lorcaserin vs. acute pimavanserin plus lorcaserin (n.s.), repeated pimavanserin vs. repeated pimavanserin plus lorcaserin (n.s.), or repeated lorcaserin vs. repeated pimavanserin plus lorcaserin (n.s.). No main effect of pimavanserin plus lorcaserin treatment on inactive lever presses was observed [F(2,34) = 2.154, p = 0.1316I; Fig. 2C]. A main effect of pimavanserin plus lorcaserin treatment on latency to the first response was observed [F(2,34) = 6.131, p = 0.0053]; a priori comparisons demonstrate acute (40.17 ± 4.20 s; ~2.8-fold increase; p < 0.05) and repeated pimavanserin plus lorcaserin (37.33 ± 9.04 s; ~2.5-fold increase; p < 0.05) increased latency to the first response vs. control (14.39 ± 2.41 s). These data suggest that acute and repeated treatment with pimavanserin plus lorcaserin attenuates cocaine-seeking behavior on FA day 10, with a small impact on the latency to first response.
3.3. Pharmacokinetic analyses of pimavanserin, lorcaserin or the combination
We assessed in vivo PK interactions for subcutaneously administered pimavanserin, lorcaserin or pimavanserin plus lorcaserin in naïve rats (Tables 2 and 3). Mean PK profiles after a single dose of pimavanserin indicated CMAX increased from ~12 to 213 ng/ml, TMAX ranged from ~15 to 60 min and AUC0-inf increased from ~15 to 1290 ng h/ml post-dosing that was dose-related (Table 2). The clearance (Cl/F) and half-life (t1/2) of pimavanserin were dose-related; t1/2 of pimavanserin ranged between ~53 and 218 min (Table 2). All doses of lorcaserin presented the relatively same metabolic profile (Table 2). Mean PK profiles after a single dose of lorcaserin indicated CMAX increased from ~15 to 106 ng/ml, TMAX ranged from ~15 to 25 min and AUC0-inf increased from ~33 to 199 ng h/ml post-dosing (Table 2). The clearance and t1/2 of lorcaserin were not dose-related; t1/2 of lorcaserin ranged between ~108 and 143 min (Table 2). Mean PK profiles after single dosing of pimavanserin plus lorcaserin demonstrated no pharmacological interaction relative to single dosing of pimavanserin or lorcaserin alone (Table 2). Plasma concentrations of pimavanserin and lorcaserin at various timepoints are provided; there was no interactive effect of lorcaserin plus pimavanserin on metabolism of the medications (Table 3).
Table 2.
Pharmacokinetic parameters of pimavanserin or lorcaserin following a single dose of each medication alone or in combination (mean ± SEM).
| CMAX (ng/ml) | TMAX (hr) | AUC0-t(ng-hr/ml) | AUC0-inf(ng-hr/ml) | AUCExtrap(%) | λ(1/hr) | Half-life, t1/2(hr) | Cl/F(L/hr/kg) | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Pimavanserin | ||||||||
| Pimavanserin(mg/kg; s.c.) | ||||||||
| 0.5 | 12.17 ± 0.38 | 0.29 ± 0.04 | 14.40 ± 0.31 | 15.02 ± 0.32 | 3.89 ± 1.42 | 0.76 ± 0.14 | 1.29 ± 0.45 | 33.40 ± 0.73 |
| 1 | 30.13 ± 2.17 | 0.25 ± 0.00 | 41.27 ± 2.64 | 41.72 ± 2.7 | 1.09 ± 0.28 | 0.79 ± 0.04 | 0.89 ± 0.05 | 24.55 ± 1.81 |
| 3 | 79.52 ± 3.76 | 0.38 ± 0.06 | 189.33 ± 7.75 | 197.5 ± 8.33 | 4.26 ± 0.68 | 0.59 ± 0.03 | 1.18 ± 0.05 | 15.28 ± 0.65 |
| 10 | 213.17 ± 15.86 | 0.96 ± 0.25 | 857.50 ± 54.72 | 1290.17 ± 112.09 | 32.22 ± 4.18 | 0.21 ± 0.02 | 3.63 ± 0.54 | 8.10 ± 0.83 |
| Pim + Lora(mg/kg; s.c.) | ||||||||
| 0.5 + 0.5 | 12.03 ± 1.97 | 0.25 ± 0.00 | 13.88 ± 3.32 | 14.34 ± 3.47 | 2.90 ± 0.69 | 0.80 ± 0.09 | 0.93 ± 0.12 | 41.15 ± 5.30 |
| 1 + 1 | 26.87 ± 5.04 | 0.25 ± 0.00 | 38.18 ± 11.19 | 39.05 ± 11.56 | 1.90 ± 0.44 | 0.68 ± 0.05 | 1.05 ± 0.08 | 28.00 ± 7.26 |
| Lorcaserin | ||||||||
| Lorcaserin(mg/kg; s.c.) | ||||||||
| 0.5 | 15.63 ± 0.74 | 0.29 ± 0.04 | 29.03 ± 0.53 | 33.43 ± 1.31 | 12.68 ± 2.45 | 0.31 ± 0.03 | 2.39 ± 0.33 | 15.07 ± 0.56 |
| 1 | 34.35 ± 3.19 | 0.25 ± 0.00 | 58.52 ± 3.41 | 63.70 ± 3.91 | 8.06 ± 0.47 | 0.39 ± 0.02 | 1.80 ± 0.09 | 15.97 ± 0.85 |
| 3 | 106.33 ± 14.74 | 0.42 ± 0.05 | 183.50 ± 7.71 | 199.17 ± 8.24 | 7.97 ± 0.50 | 0.38 ± 0.01 | 1.83 ± 0.07 | 15.18 ± 0.66 |
| Pim + Lora(mg/kg; s.c.) | ||||||||
| 0.5 + 0.5 | 14.74 ± 0.71 | 0.30 ± 0.05 | 26.46 ± 1.80 | 28.48 ± 1.88 | 7.14 ± 0.75 | 0.40 ± 0.02 | 1.74 ± 0.11 | 17.9 ± 1.29 |
| 1 + 1 | 26.62 ± 1.69 | 0.35 ± 0.06 | 48.88 ± 2.71 | 52.82 ± 2.84 | 7.55 ± 0.52 | 0.41 ± 0.02 | 1.71 ± 0.07 | 19.12 ± 0.99 |
Pimavanserin administered 15 min prior to lorcaserin; CMAX, maximum serum concentration; TMAX, time to reach CMAX; AUC0-t, area under plasma drug concentration-time curve (AUC) up to time ‘t’; AUC0-inf, extrapolated AUC to infinity; AUCExtrap, effect of extrapolated AUC on AUC0-inf estimation; λ, individual estimate of terminal elimination rate constant; t1/2, elimination half-life; Cl/F, apparent total body clearance.
TABLE 3.
Plasma concentration of pimavanserin or lorcaserin following a single dose of each medication alone or in combination (mean ± SEM).
| 0 h | 15 min | 30 min | 60 min | 120 min | 180 min | 240 min | 360 min | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Pimavanserin (ng/ml) | ||||||||
| Pimavanserin(mg/kg; s.c.) | ||||||||
| 0.5 | 0.08 ± 0.05 | 12.15 ± 0.39 | 9.93 ± 0.50 | 5.40 ± 0.23 | 2.09 ± 0.13 | 0.86 ± 0.11 | 0.45 ± 0.10 | 0.13 ± 0.06 |
| 1 | 0.05 ± 0.03 | 30.13 ± 2.17 | 24.50 ± 1.59 | 16.8 ± 1.02 | 6.34 ± 0.39 | 3.55 ± 0.81 | 1.12 ± 0.14 | 0.35 ± 0.08 |
| 3 | 0.02 ± 0.02 | 71.13 ± 5.89 | 74.48 ± 5.10 | 63.40 ± 3.59 | 39.83 ± 2.40 | 27.80 ± 2.37 | 15.66 ± 1.48 | 4.85 ± 0.62 |
| 10 | 0.09 ± 0.41 | 169.67 ± 18.84 | 190.50 ± 19.86 | 208.17 ± 15.62 | 179.17 ± 11.60 | 154.17 ± 15.09 | 116.15 ± 7.99 | 79.87 ± 6.24 |
| Pim + Lora(mg/kg; s.c.) | ||||||||
| 0.5 + 0.5 | 0.09 ± 0.06 | 12.03 ± 1.97 | 8.20 ± 1.99 | 5.49 ± 1.34 | 1.87 ± 0.52 | 0.91 ± 0.29 | 0.43 ± 0.16 | 0.28 ± 0.10 |
| 1 + 1 | 0.08 ± 0.04 | 26.87 ± 5.04 | 19.70 ± 4.29 | 14.27 ± 3.94 | 7.07 ± 2.74 | 3.19 ± 1.32 | 1.72 ± 0.78 | 0.53 ± 0.23 |
| Lorcaserin (ng/ml) | ||||||||
| Lorcaserin(mg/kg; s.c.) | ||||||||
| 0.5 | 0.00 ± 0.00 | 15.55 ± 0.76 | 13.97 ± 0.50 | 10.37 ± 0.26 | 4.83 ± 0.13 | 3.03 ± 0.18 | 2.05 ± 0.07 | 1.20 ± 0.12 |
| 1 | 0.00 ± 0.00 | 34.35 ± 3.19 | 27.52 ± 1.10 | 21.13 ± 1.06 | 9.88 ± 0.60 | 6.11 ± 0.48 | 3.86 ± 0.39 | 1.98 ± 0.16 |
| 3 | 0.00 ± 0.00 | 99.2 ± 15.97 | 97.47 ± 7.97 | 63.48 ± 4.38 | 32.35 ± 1.07 | 17.63 ± 0.77 | 12.35 ± 0.48 | 5.97 ± 0.28 |
| Pim + Lora(mg/kg; s.c.) | ||||||||
| 0.5 + 0.5 | 0.00 ± 0.00 | 14.68 ± 0.72 | 13.48 ± 0.92 | 9.49 ± 0.91 | 4.74 ± 0.34 | 2.58 ± 0.22 | 1.66 ± 0.10 | 0.80 ± 0.06 |
| 1 + 1 | 0.00 ± 0.00 | 25.46 ± 2.05 | 24.32 ± 2.34 | 17.14 ± 1.12 | 8.85 ± 0.80 | 5.08 ± 0.13 | 3.33 ± 0.17 | 1.60 ± 0.07 |
Pimavanserin administered 15 min prior to lorcaserin.
4. Discussion
We employed pimavanserin (0.3–3 mg/kg) (Hubbard et al., 2013; McFarland et al., 2011) and lorcaserin (0.25–1 mg/kg) (Higgins et al., 2012; Levin et al., 2011) at doses that do not alter spontaneous locomotor activity when administered alone or in combination, as reported previously. We demonstrate here that lorcaserin alone (1.0 mg/kg) given acutely suppresses cocaine intake in a self-administration model, while treatment with pimavanserin alone or the combination of lower, inactive doses of pimavanserin (0.5 mg/kg) plus lorcaserin (0.5 mg/kg) is insufficient to alter cocaine intake. The finding that the 5-HT2AR antagonist pimavanserin acutely does not alter cocaine intake is consistent with previous findings utilizing investigational 5-HT2AR antagonists in rats (e.g., M10907; (Filip, 2005; Fletcher et al., 2002; Nic Dhonnchadha et al., 2009). Conversely, we found that acute and repeated pimavanserin (3 mg/kg) or lorcaserin (1 mg/kg) each suppress cocaine-seeking after 10 days of forced abstinence without evidence for sensitization or tolerance upon repeated administration. Further, the combination of subthreshold doses of pimavanserin (0.5 mg/kg) plus lorcaserin (0.5 mg/kg) suppressed cocaine-seeking behavior on FA day 10. Finally, PK analyses indicate these medications may not share a common elimination mechanism. This series of findings suggests that this combination of 5-HT2R interventions might have greater utility for relapse-prevention than for cessation.
Plasma exposure of pimavanserin and lorcaserin alone or in combination following subcutaneous administration was similar to previous single dose studies in rodents (Higgins et al., 2017, 2020; Vanover et al., 2006). Pimavanserin (0.5–10 mg/kg) produced a dose-related increase in serum drug concentration, as did lorcaserin (0.5–1 mg/kg), which corresponded to effects on cocaine-taking and -seeking behaviors. The dose ranges employed for both pimavanserin and lorcaserin preclinically correlate to behavioral efficacy (Collins et al., 2016; Higgins et al., 2017; Price et al., 2018; Sholler et al., 2019; Vanover et al., 2007) as well as clinical steady-state plasma exposure levels (Higgins et al., 2020; Smith et al., 2008; Thomsen et al., 2008; Vanover et al., 2007). Of note, pimavanserin dose-dependently and non-competitively inhibited clearance, suggesting that repeated pimavanserin dosing may progressively reduce clearance, increasing plasma levels of pimavanserin, especially at higher doses (Vanover et al., 2007). Metabolism of pimavanserin occurs via cytochrome P450 enzymes, e.g., CYP3A4/A5 (major), CYP2J2 (minor), and CYP2D6 (minor) (Bozymski et al., 2017), while lorcaserin engages multiple cytochrome P450 enzymes at μM concentrations, e.g. CYP1A2 (minor), CYP2C8 (minor), CYP2C9 (minor), CYP2C19 (minor), CYP2D6 (major), and CYP3A4 (minor), with the major metabolite of lorcaserin, a sulfamate, produced independent of induction of the cytochrome P450 enzymes (Higgins et al., 2020). Thus, our findings of a lack of significant drug-drug interaction following pimavanserin plus lorcaserin is as expected (Bozymski et al., 2017; Gustafson et al., 2013). Taken together, these data support future studies for a combinatorial paradigm of pimavanserin plus lorcaserin in a therapeutic setting.
The most consistent evidence of a role for 5-HT receptors in cocaine relapse-like behaviors emerges from evaluation of 5-HT2AR and 5-HT2CR systemic manipulations. Pretreatment with 5-HT2AR antagonists, (e.g., selective 5-HT2AR antagonist/inverse agonists M100907 and pimavanserin; 5-HT2A/2CR antagonists/inverse agonists ketanserin and ritanserin), indicate that the 5-HT2AR does not evidently mediate intake of cocaine in the self-administration paradigm in rats (Fletcher et al., 2002; Lacosta and Roberts, 1993; Nic Dhonnchadha et al., 2009; Peltier and Schenk, 1993; Pockros et al., 2011) or rhesus monkeys (Murnane et al., 2013). Conversely, 5-HT2CR knockout mice exhibit heightened cocaine intake (Rocha et al., 2002), consistent with studies employing systemic administration of selective 5-HT2CR agonists (e.g., selective 5-HT2CR agonists WAY163909 and lorcaserin; 5-HT2B/2CR agonist Ro60–0175) in which the reinforcing effects of cocaine in animals is blunted (Collins and France, 2018; Collins et al., 2016; Cunningham et al., 2011; Fletcher et al., 2008; Grottick et al., 2000; Harvey-Lewis et al., 2016) and prevented by the 5-HT2CR antagonist SB242084 (present study) (Cunningham et al., 2011; Manvich et al., 2012). Future experiments are required to extend these observations to a broader dose range for pimavanserin and lorcaserin treatment as well as analysis of the effects of these ligands on intake following repeated administration.
Medications that attenuate sensitivity to cocaine-associated cues may afford CUD individuals heightened control over relapse-triggering stimuli. From a therapeutic perspective, recruitment of the 5-HT2AR and/or 5-HT2CR during and/or after abstinence from cocaine may differ relative to the goal to suppress ongoing intake. The control afforded by both acute and repeated administration of pimavanserin over cue-evoked cocaine-seeking, but not cocaine self-administration, supports other lines of evidence that the neural mechanisms underlying the incentive-motivational aspects of drugs and drug-associated cues are distinct (Everitt and Wolf, 2002; Kruzich et al., 2001; Robinson and Berridge, 2003). The findings presented here extend previous reports that M100907 suppresses cue-evoked reinstatement of cocaine seeking following extinction training from cocaine self-administration (Fletcher et al., 2002; Nic Dhonnchadha et al., 2009; Pockros et al., 2011). Furthermore, M100907 was most efficacious in suppressing cue-evoked reinstatement in those rats with the highest extinction responsiveness (Nic Dhonnchadha et al., 2009). Selective 5-HT2CR agonists (Ro60–0175, WAY163909, lorcaserin) not only reduce cocaine self-administration, but also cocaine-seeking behavior (Anastasio et al., 2014a; Burbassi and Cervo, 2008; Cunningham et al., 2011; Fletcher et al., 2008; Grottick et al., 2000; Higgins et al., 2020; Neisewander and Acosta, 2007; Swinford-Jackson et al., 2016). Higgins and Fletcher (Higgins et al., 2012) demonstrated that 1 mg/kg of lorcaserin did not impair measures of balance, coordination, or motor planning in the rotarod test. Rotarod performance is proposed as a stronger indicator of skilled performance (e.g., operant responding) than spontaneous locomotor activity (Stromberg, 1988). Taken together, the efficacy of 1 mg/ kg of lorcaserin to suppress cocaine intake and cocaine-seeking is not definitively attributable to a non-specific inhibition in motor behavior. Nonetheless, it is worth noting that a moderate impact on the latency to the first response was detected following lorcaserin treatment, which was dampened in the presence of pimavanserin, suggesting the potential for non-specific inhibition of spontaneous locomotor activity. Thus, once distinguished pharmacologically, the 5-HT2AR and 5-HT2CR play an excitatory and inhibitory role, respectively, over cocaine-seeking during abstinence. The added value of repeated administration of pimavanserin or lorcaserin to suppress the incentive motivational properties of these cocaine-paired cues, without evidence for the development of sensitization or tolerance to the medications, ascribes a further dimension of likely pharmacotherapeutic potential for CUD.
The possibility that the 5-HT2AR and 5-HT2CR may act in concert is suggested by the fact that both cue- and cocaine-primed drug-seeking were suppressed by the combination of low, singly ineffective doses of a selective 5-HT2AR antagonist (M100907, pimavanserin) plus a selective 5-HT2CR agonist (WAY163909, lorcaserin) (present study; (Cunningham et al., 2013). Further, growing evidence from biochemical, behavioral and pharmacological studies indicate that the 5-HT2AR and 5-HT2CR interact functionally at the cellular, brain region, and circuit level (Anastasio et al., 2015; Bazovkina et al., 2015; Burton et al., 2013; Cunningham et al., 2013; Moutkine et al., 2017; Pockros et al., 2012). Of note, the 5-HT2AR and 5-HT2CR are oppositional in some in vivo analyses (for review, Bubar and Cunningham, 2008) and therefore results observed following administration of nonselective 5-HT2A/2CR antagonists are difficult to interpret as simultaneous blockade of the 5-HT2CR would be expected to have opposing actions to 5-HT2AR antagonism. Thus, the ineffectiveness of non-selective 5-HT2A/2CR antagonists to attenuate cocaine-seeking in animals (Burmeister et al., 2004; Filip, 2005; Schenk, 2000) or humans (Ehrman et al., 1996) or influence self-reported euphoric effects of cocaine (Newton et al., 2001) or craving (De La Garza et al., 2005; Loebl et al., 2008) in clinical studies is not surprising. Nonetheless, our findings support the hypothesis that 5-HT neurotransmission can critically influence aspects of cocaine-mediated behaviors via the 5-HT2AR and 5-HT2CR systems and that these receptors act in concert to regulate the neural bases for behavior (for reviews, (Cunningham and Anastasio, 2014; Howell and Cunningham, 2015).
Preclinical models have greatly enhanced our understanding of the neurobiology of the rewarding and reinforcing properties of cocaine and cocaine-associated cues and have advanced the goal to identify medications that lack abuse liability, yet exhibit efficacy to enhance abstinence, reduce craving, and prevent relapse. The identification of efficacy following pimavanserin plus lorcaserin (but see) (Banks and Negus, 2017) indicate the promise of a combinational strategy of these two targeted 5-HT2R family medications to modulate relapse-related behaviors. While dosing and routes of administration could be further optimized, we propose strategies to couple 5-HT2AR antagonist and 5-HT2CR agonist pharmacotherapy (Booth et al., 2009; Chen et al., 2017; Gilbertson et al., 2018; Rowland et al., 2008; Shashack et al., 2011; Soto et al., 2018) or co-administration of otherwise ineffective doses of pimavanserin and lorcaserin presents the opportunity to yield therapeutic efficacy for each agent while reducing the potential for adverse effects (Morphy et al., 2004). In addition, our findings presented herein will guide future experiment to probe the neurobiology of the 5-HT2A and 5-HT2CR systems in cocaine relapse-related behaviors.
HIGHLIGHTS.
Lorcaserin, but not pimavanserin, suppresses cocaine self-administration in rats.
Repeated pimavanserin or lorcaserin sustains reduced cocaine-seeking behavior.
Low doses of pimavanserin plus lorcaserin reduce cocaine-seeking during abstinence.
Acknowledgements
We thank Drs. Jane Acri, Aiden Hampson, and Tanya Ramey (National Institute on Drug Abuse) as well as Ms. Amanda Price and Ms. Victoria Brehm for their thoughtful discussions of the findings and the manuscript.
Funding and disclosure
This work was supported by NIDA grant U54 DA038999 and by the Center for Addiction Research at the University of Texas Medical Branch. FGM has current research funding from Indivior Pharmaceuticals and Nektar Therapeutics for research unrelated to this study. All other authors declare no competing interests.
References
- Aloyo VJ, Berg KA, Spampinato U, Clarke WP, Harvey JA, 2009. Current status of inverse agonism at serotonin(2A) (5-HT(2A)) and 5-HT(2C) receptors. Pharmacol. Ther. 121, 160–173. [DOI] [PubMed] [Google Scholar]
- Anastasio NC, Liu S, Maili L, Swinford SE, Lane SD, Fox RG, Hamon SC, Nielsen DA, Cunningham KA, Moeller FG, 2014a. Variation within the serotonin (5-HT) 5-HT(2)C receptor system aligns with vulnerability to cocaine cue reactivity. Transl. Psychiatry 4, e369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Stutz SJ, Fink LH, Swinford-Jackson SE, Sears RM, DiLeone RJ, Rice KC, Moeller FG, Cunningham KA, 2015. Serotonin (5-HT) 5-HT2A receptor (5-HT2AR):5-HT2CR imbalance in medial prefrontal cortex associates with motor impulsivity. ACS Chem. Neurosci. 6, 1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Stutz SJ, Fox RG, Sears RM, Emeson RB, DiLeone RJ, O'Neil RT, Fink LH, Li D, Green TA, Moeller FG, Cunningham KA, 2014b. Functional status of the serotonin 5-HT2C receptor (5-HT2CR) drives interlocked phenotypes that precipitate relapse-like behaviors in cocaine dependence. Neuropsychopharmacology 39, 370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS, 2017. Repeated 7-day treatment with the 5-HT2C agonist lorcaserin or the 5-HT2A antagonist pimavanserin alone or in combination fails to reduce cocaine vs food choice in male rhesus monkeys. Neuropsychopharmacology 42, 1082–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazovkina DV, Kondaurova EM, Naumenko VS, Ponimaskin E, 2015. Genotype-Dependent difference in 5-HT2C receptor-induced hypolocomotion: comparison with 5-HT2A receptor functional activity. Neural Plast. 2015, 846589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth RG, Fang L, Huang Y, Wilczynski A, Sivendran S, 2009. (1R, 3S)-(-)-transPAT: a novel full-efficacy serotonin 5-HT2C receptor agonist with 5-HT2A and 5-HT2B receptor inverse agonist/antagonist activity. Eur. J. Pharmacol. 615, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozymski KM, Lowe DK, Pasternak KM, Gatesman TL, Crouse EL, 2017. Pimavanserin: a novel antipsychotic for Parkinson's disease psychosis. Ann. Pharmacother. 51, 479–487. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA, 2008. Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Prog. Brain Res. 172, 319–346. [DOI] [PubMed] [Google Scholar]
- Burbassi S, Cervo L, 2008. Stimulation of serotonin(2C) receptors influences cocaine-seeking behavior in response to drug-associated stimuli in rats. Psychopharmacology 196, 15–27. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Kirschner KF, Neisewander JL, 2004. Differential roles of 5-HT receptor subtypes in cue and cocaine reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology 29, 660–668. [DOI] [PubMed] [Google Scholar]
- Burton CL, Rizos Z, Diwan M, Nobrega JN, Fletcher PJ, 2013. Antagonizing 5-HT(2)A receptors with M100907 and stimulating 5-HT(2)C receptors with Ro60–0175 blocks cocaine-induced locomotion and zif268 mRNA expression in Sprague-Dawley rats. Behav. Brain Res. 240, 171–181. [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality, S.A.M.H.S.A, 2018. Key Substance Use and Mental Health Indicators in the United States: Results from the 2017 National Survey on Drug Use and Health. [Google Scholar]
- Chen YC, Hartley RM, Anastasio NC, Cunningham KA, Gilbertson SR, 2017. Synthesis and structure-activity relationships of tool compounds based on WAY163909, a 5-HT2C receptor agonist. ACS Chem. Neurosci. 8, 1004–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, France CP, 2018. Effects of lorcaserin and buspirone, administered alone and as a mixture, on cocaine self-administration in male and female rhesus monkeys. Exp. Clin. Psychopharmacol 26, 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Gerak LR, Javors MA, France CP, 2016. Lorcaserin reduces the discriminative stimulus and reinforcing effects of cocaine in rhesus monkeys. J. Pharmacol. Exp. Therapeut. 356, 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC, 2014. Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology 76 Pt B, 460–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC, Fox RG, Stutz SJ, Bubar MJ, Swinford SE, Watson CS, Gilbertson SR, Rice KC, Rosenzweig-Lipson S, Moeller FG, 2013. Synergism between a serotonin 5-HT2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS Chem. Neurosci. 4, 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Fox RG, Anastasio NC, Bubar MJ, Stutz SJ, Moeller FG, Gilbertson SR, Rosenzweig-Lipson S, 2011. Selective serotonin 5-HT2C receptor activation suppresses the reinforcing efficacy of cocaine and sucrose but differentially affects the incentive-salience value of cocaine- vs. sucrose-associated cues. Neuropharmacology 61, 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza R, Newton TF, Kalechstein AD, 2005. Risperidone diminishes cocaine-induced craving. Psychopharmacology 178, 347–350. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Cornish JW, Childress AR, O'Brien CP, 1996. Failure of ritanserin to block cocaine cue reactivity in humans. Drug Alcohol Depend. 42, 167–174. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME, 2002. Psychomotor stimulant addiction: a neural systems perspective. J. Neurosci. 22, 3312–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, 2005. Role of serotonin (5-HT)2 receptors in cocaine self-administration and seeking behavior in rats. Pharmacol. Rep. 57, 35–46. [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA, 2002. Differential effects of the 5-HT2A receptor antagonist M100,907 and the 5-HT2C receptor antagonist SB242,084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology 27, 576–586. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA, 2008. The 5-HT(2C) receptor agonist RO 60–0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine and contextual cues. Neuropsychopharmacology 33, 1402–1412. [DOI] [PubMed] [Google Scholar]
- Gerak LR, Collins GT, France CP, 2016. Effects of lorcaserin on cocaine and methamphetamine self-administration and reinstatement of responding previously maintained by cocaine in rhesus monkeys. J. Pharmacol. Exp. Therapeut. 359, 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Collins GT, Maguire DR, France CP, 2019. Effects of lorcaserin on reinstatement of responding previously maintained by cocaine or remifentanil in rhesus monkeys. Exp. Clin. Psychopharmacol 27, 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson SR, Chen YC, Soto CA, Yang Y, Rice KC, Cunningham KA, Anastasio NC, 2018. Synthesis and activity of functionalizable derivatives of the serotonin (5-HT) 5-HT2A receptor (5-HT2AR) antagonist M100907. Bioorg. Med. Chem. Lett 28, 1381–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottick AJ, Fletcher PJ, Higgins GA, 2000. Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior. J. Pharmacol. Exp. Therapeut. 295, 1183–1191. [PubMed] [Google Scholar]
- Gustafson A, King C, Rey JA, 2013. Lorcaserin (Belviq): a selective serotonin 5-HT2C agonist in the treatment of obesity. P T 38, 525–534. [PMC free article] [PubMed] [Google Scholar]
- Harvey-Lewis C, Li Z, Higgins GA, Fletcher PJ, 2016. The 5-HT2C receptor agonist lorcaserin reduces cocaine self-administration, reinstatement of cocaine-seeking and cocaine induced locomotor activity. Neuropharmacology 101, 237–245. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Fletcher PJ, Shanahan WR, 2020. Lorcaserin: a review of its preclinical and clinical pharmacology and therapeutic potential. Pharmacol. Ther. 205, 107417. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Patrick A, De Lannoy IA, Fletcher PJ, Parker LA, MacLusky NJ, Sullivan LC, Chavera TA, Berg KA, 2017. Studies to examine potential tolerability differences between the 5-HT2C receptor selective agonists lorcaserin and CP-809101. ACS Chem. Neurosci. 8, 1074–1084. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Rossmann A, Rizos Z, Noble K, Soko AD, Fletcher PJ, 2012. The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology 37, 1177–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Cunningham KA, 2015. Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol. Rev. 67, 176–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard D, Hacksell U, McFarland K, 2013. Behavioral effects of clozapine, pimavanserin, and quetiapine in rodent models of Parkinson's disease and Parkinson's disease psychosis: evaluation of therapeutic ratios. Behav. Pharmacol. 24, 628–632. [DOI] [PubMed] [Google Scholar]
- Hughes A, Williams MR, Lipari RN, Van Horn S, 2016. State estimates of past year cocaine use among young adults: 2014 and 2015. Cent. Behav. Health. Qual. Rep December 20,2016. [PubMed] [Google Scholar]
- Indave BI, Minozzi S, Pani PP, Amato L, 2016. Antipsychotic medications for cocaine dependence. Cochrane Database Syst. Rev. 3, CD006306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G, 1973. Design and Analysis: A Researcher's Handbook. Prentice-Hall, Inc., Englewood Cliffs, NJ. [Google Scholar]
- Kohut SJ, Bergman J, 2017. Medication strategies for the management of cocaine use disorder. In: Preedy VR (Ed.), The Neuroscience of Cocaine: Mechanisms and Treatment. Elsevier/Academic Press, London, pp. 627–637. [Google Scholar]
- Koob GF, Volkow ND, 2016. Neurobiology of addiction: a neurocircuitry analysis. Lancet 3, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich PJ, Congleton KM, See RE, 2001. Conditioned reinstatement of drug-seeking behavior with a discrete compound stimulus classically conditioned with intravenous cocaine. Behav. Neurosci. 115, 1086–1092. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Roberts DC, 1993. MDL 72222, ketanserin, and methysergide pretreatments fail to alter breaking points on a progressive ratio schedule reinforced by intravenous cocaine. Pharmacol., Biochem. Behav. 44, 161–165. [DOI] [PubMed] [Google Scholar]
- Levin ED, Johnson JE, Slade S, Wells C, Cauley M, Petro A, Rose JE, 2011. Lorcaserin, a 5-HT2C agonist, decreases nicotine self-administration in female rats. J. Pharmacol. Exp. Therapeut. 338, 890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Lane SD, Schmitz JM, Green CE, Cunningham KA, Moeller FG, 2012. Increased intra-individual reaction time variability in cocaine-dependent subjects: role of cocaine-related cues. Addict. Behav. 37, 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebl T, Angarita GA, Pachas GN, Huang KL, Lee SH, Nino J, Logvinenko T, Culhane MA, Evins AE, 2008. A randomized, double-blind, placebo-controlled trial of long-acting risperidone in cocaine-dependent men. J.Clin.Psychiatry 69, 480–486. [DOI] [PubMed] [Google Scholar]
- Lucki I, 1998. The spectrum of behaviors influenced by serotonin. Biol. Psychiatr. 44, 151–162. [DOI] [PubMed] [Google Scholar]
- Manvich DF, Kimmel HL, Howell LL, 2012. Effects of serotonin 2C receptor agonists on the behavioral and neurochemical effects of cocaine in squirrel monkeys. J. Pharmacol. Exp. Therapeut. 341, 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall Jones C, Baldwin GT, Compton WM, 2017. Recent increases in cocaine-related overdose deaths and the role of opioids. Am. J. Publ. Health 107, 430–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Price DL, Bonhaus DW, 2011. Pimavanserin, a 5-HT2A inverse agonist, reverses psychosis-like behaviors in a rodent model of Parkinson's disease. Behav. Pharmacol. 22, 681–692. [DOI] [PubMed] [Google Scholar]
- Minozzi S, Amato L, Pani PP, Solimini R, Vecchi S, De Crescenzo F, Zuccaro P, Davoli M, 2015. Dopamine agonists for the treatment of cocaine dependence. Cochrane Database Syst. Rev., CD003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morphy R, Kay C, Rankovic Z, 2004. From magic bullets to designed multiple ligands. Drug Discov. Today 9, 641–651. [DOI] [PubMed] [Google Scholar]
- Moutkine I, Quentin E, Guiard BP, Maroteaux L, Doly S, 2017. Heterodimers of serotonin receptor subtypes 2 are driven by 5-HT2C protomers. J. Biol. Chem. 292, 6352–6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Winschel J, Schmidt KT, Stewart LM, Rose SJ, Cheng K, Rice KC, Howell LL, 2013. Serotonin 2A receptors differentially contribute to abuse-related effects of cocaine and cocaine-induced nigrostriatal and mesolimbic dopamine overflow in nonhuman primates. J. Neurosci. 33, 13367–13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakantan H, Holliday ED, Fox RG, Stutz SJ, Comer SD, Haney M, Anastasio NC, Moeller FG, Cunningham KA, 2017. Lorcaserin suppresses oxycodone self-administration and relapse vulnerability in rats. ACS Chem. Neurosci. 8, 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Acosta JI, 2007. Stimulation of 5-HT2C receptors attenuates cue and cocaine-primed reinstatement of cocaine-seeking behavior in rats. Behav. Pharmacol. 18, 791–800. [DOI] [PubMed] [Google Scholar]
- Newton TF, Ling W, Kalechstein AD, Uslaner J, Tervo K, 2001. Risperidone pretreatment reduces the euphoric effects of experimentally administered cocaine. Psychiatr. Res. 102, 227–233. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Fox RG, Stutz SJ, Rice KC, Cunningham KA, 2009. Blockade of the serotonin 5-HT2A receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior in a rat self-administration model. Behav. Neurosci. 123, 382–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier R, Schenk S, 1993. Effects of serotonergic manipulations on cocaine self-administration in rats. Psychopharmacology 110, 390–394. [DOI] [PubMed] [Google Scholar]
- Pockros LA, Pentkowski NS, Conway SM, Ullman TE, Zwick KR, Neisewander JL, 2012. 5-HT(2A) receptor blockade and 5-HT(2C) receptor activation interact to reduce cocaine hyperlocomotion and Fos protein expression in the caudate-putamen. Synapse 66, 989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockros LA, Pentkowski NS, Swinford SE, Neisewander JL, 2011. Blockade of 5-HT2A receptors in the medial prefrontal cortex attenuates reinstatement of cue-elicited cocaine-seeking behavior in rats. Psychopharmacology 213, 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AE, Brehm VD, Hommel JD, Anastasio NC, Cunningham KA, 2018. Pimavanserin and lorcaserin attenuate measures of binge eating in male Sprague-Dawley rats. Front. Pharmacol. 9, 1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC, 2003. Addiction. Annu. Rev. Psychol. 54, 25–53. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Goulding EH, O'Dell LE, Mead AN, Coufal NG, Parsons LH, Tecott LH, 2002. Enhanced locomotor, reinforcing, and neurochemical effects of cocaine in serotonin 5-hydroxytryptamine 2C receptor mutant mice. J. Neurosci. 22, 10039–10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland NE, Crump EM, Nguyen N, Robertson K, Sun Z, Booth RG, 2008. Effect of (-)-trans-PAT, a novel 5-HT2C receptor agonist, on intake of palatable food in mice. Pharmacol. Biochem. Behav. 91, 176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahli ZT, Tarazi FI, 2018. Pimavanserin: novel pharmacotherapy for Parkinson's disease psychosis. Expet Opin. Drug Discov. 13, 103–110. [DOI] [PubMed] [Google Scholar]
- Schenk S, 2000. Effects of the serotonin 5-HT(2) antagonist, ritanserin, and the serotonin 5-HT(1A) antagonist, WAY 100635, on cocaine-seeking in rats. Pharmacol. Biochem. Behav. 67, 363–369. [DOI] [PubMed] [Google Scholar]
- Shashack MJ, Cunningham KA, Seitz PK, McGinnis A, Smith T, Watson CS, Gilbertson SR, 2011. Synthesis and evaluation of dimeric derivatives of 5-HT(2A) receptor (5-HT(2A)R) antagonist M-100907. ACS Chem. Neurosci. 2, 640–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholler DJ, Stutz SJ, Fox RG, Boone EL, Wang Q, Rice KC, Moeller FG, Anastasio NC, Cunningham KA, 2019. The 5-HT2A receptor (5-HT2AR) regulates impulsive action and cocaine cue reactivity in male Sprague-Dawley Rats. J. Pharmacol. Exp. Therapeut. 368, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BM, Smith JM, Tsai JH, Schultz JA, Gilson CA, Estrada SA, Chen RR, Park DM, Prieto EB, Gallardo CS, Sengupta D, Dosa PI, Covel JA, Ren A, Webb RR, Beeley NR, Martin M, Morgan M, Espitia S, Saldana HR, Bjenning C, Whelan KT, Grottick AJ, Menzaghi F, Thomsen WJ, 2008. Discovery and structure-activity relationship of (1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-benzazepine (Lorcaserin), a selective serotonin 5-HT2C receptor agonist for the treatment of obesity. J. Med. Chem. 51, 305–313. [DOI] [PubMed] [Google Scholar]
- Soto CA, Shashack MJ, Fox RG, Bubar MJ, Rice KC, Watson CS, Cunningham KA, Gilbertson SR, Anastasio NC, 2018. Novel bivalent 5-HT2A receptor antagonists exhibit high affinity and potency in vitro and efficacy in vivo. ACS Chem. Neurosci. 9, 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubrié P, 1986. Reconciling the Role of Central Serotonin Neurons in Human and Animal Behavior. Behavioral and Brain Sciences, pp. 319–364. [Google Scholar]
- Stromberg C, 1988. Interactions of antidepressants and ethanol on spontaneous locomotor activity and rotarod performance in NMRI and C57BL/6 mice. J. Psychopharmacol. 2, 61–66. [DOI] [PubMed] [Google Scholar]
- Swinford-Jackson SE, Anastasio NC, Fox RG, Stutz SJ, Cunningham KA, 2016. Incubation of cocaine cue reactivity associates with neuroadaptations in the cortical serotonin (5-HT) 5-HT2C receptor (5-HT2CR) system. Neuroscience 324, 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, Al-Shamma H, Smith B, Chalmers D, Behan D, 2008. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J. Pharmacol. Exp. Therapeut. 325, 577–587. [DOI] [PubMed] [Google Scholar]
- Vanover KE, Betz AJ, Weber SM, Bibbiani F, Kielaite A, Weiner DM, Davis RE, Chase TN, Salamone JD, 2008. A 5-HT2A receptor inverse agonist, ACP-103, reduces tremor in a rat model and levodopa-induced dyskinesias in a monkey model. Pharmacol. Biochem. Behav. 90, 540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanover KE, Robbins-Weilert D, Wilbraham DG, Mant TG, Van Kammen DP, Davis RE, Weiner DM, 2007. Pharmacokinetics, tolerability, and safety of ACP103 following single or multiple oral dose administration in healthy volunteers. J. Clin. Pharmacol. 47, 704–714. [DOI] [PubMed] [Google Scholar]
- Vanover KE, Weiner DM, Makhay M, Veinbergs I, Gardell LR, Lameh J, Del Tredici AL, Piu F, Schiffer HH, Ott TR, Burstein ES, Uldam AK, Thygesen MB, Schlienger N, Andersson CM, Son TY, Harvey SC, Powell SB, Geyer MA, Tolf BR, Brann MR, Davis RE, 2006. Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N'-(4-(2-methylpropylo xy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inverse agonist. J. Pharmacol. Exp. Therapeut. 317, 910–918. [DOI] [PubMed] [Google Scholar]