Abstract
Background:
Current recommendations for echocardiographic assessment of diastolic function (2016 guidelines of the American Society of Echocardiography (ASE)/European Association of Cardiovascular Imaging (EACVI) in patients with metabolic syndrome and overweight/obesity result in a significant number of patients with indeterminate diastolic dysfunction (LVDD). The aim of this article is to study whether the use of the left atrial strain criterion (LALS) reduces the number of indeterminate patients.
Methods:
229 patients were studied with a complete echocardiographic study that included left ventricular longitudinal strain (LVLS) analysis, LALS and a maximal ergospirometry test with assessment of oxygen uptake (VO2max).
Results:
The mean age was 65±5 years, 153 (67%) males, with a mean EF of 60±5%. The mean LVLS was −19.4±2% and the LALS Reservoir was 23.8±7%. There were 140 patients who did not meet LVDD criteria and 82 who did meet the indeterminate LVDD criterion. When the left atrial volume index (LAVI) >34 ml/m2 criterion was replaced in the 2016 ASE/EACVI algorithm by LALS Reservoir ≤20%, the number of indeterminate patients was reduced from 36% to 23% (p<0.001) at the expense of increasing normal studies (61% and 74%). Adding the LALS Reservoir criterion ≤23% in the 82 patients of the indeterminate group resulted in two groups with a different VO2max (11.6±3 and 18±5 ml/kg/min, p:0.081).
Conclusions:
This study confirms the low prevalence of diastolic dysfunction in overweight/obese patients with metabolic syndrome. Adding left atrial strain criterion to the current recommendations significantly reduces the number of indeterminate patients by reclassifying them as normal.
Keywords: Diastolic dysfunction, Doppler echocardiography, Exercise capacity, Left atrial strain, Metabolic syndrome
1. Introduction
The presence of alterations in left ventricular diastolic function is well established in patients with cardiovascular risk factors who are obese and/or overweight1,2 and in clinical practice this finding is detected using Doppler echocardiography. Current recommendations for the assessment of diastolic function by echocardiography are based on the use of different echocardiographic criteria3, and although there is evidence that this strategy has increased specificity in the diagnosis of diastolic dysfunction (DDVI), these algorithms applied to the individual result in a high proportion of indeterminate diagnoses4–5. In order to improve the echocardiographic classification of LVDD, other echocardiographic parameters have been proposed, mainly those assessing left atrial function and specifically left atrial strain6,7. Abnormalities of diastolic function have been shown to be associated with reduced aerobic functional capacity (FAC) in people with diabetes, hypertension and obesity5,8. The main objective of this study is to assess whether adding the LALS criterion to the current classification can reduce the number of patients diagnosed with indeterminate LVDD.
2. Materials and methods
2.1. Patient population and design
Prospective cohort study of Predimed Plus trial participants in the Vitoria node at study entry. Predimed Plus is a multicentre, randomised, parallel-group, primary cardiovascular prevention study that compares the effect on cardiovascular morbidity and mortality of an intensive lifestyle intervention based on a low-calorie Mediterranean diet, increased physical activity and behaviour therapy (intervention group) with a non-intensive Mediterranean diet intervention without calorie restriction (control group)9. Inclusion criteria for the study were male volunteers aged 55–75 years or women aged 60–75 years, overweight or obese (BMI ≥ 27 and < 40 kg/m2) and at least 3 of the 5 components of the metabolic syndrome with no evidence of cardiovascular disease. The study is registered in the International Standard Randomized Controlled Trial (ISRCT;http://www.irsctn.com/ISRCTN89898870) under the number: 89898870.
2.2. Echocardiographic study
All studies were performed in left lateral decubitus position with a Vivid E9 BT 11 (GE Vingmed Ulatrasound AS, Horten, Norway), using an M5S-D [1.5–4.5 MHz] sectorial cardiac probe and acquired by a highly experienced technician, following a defined acquisition protocol and evaluated by a single echocardiographer. The study included a comprehensive conventional assessment following current recommendations10, including mitral flow velocity, tissue Doppler measurement of the lateral mitral annulus and septal annulus, detection of the tricuspid velocity gradient from various echocardiographic planes and biplane LA measurement (Supplementary material).
2.3. Ergospirometry
A symptom-limited maximal treadmill stress test (General Electric model T2100) was performed according to the ramped Bruce protocol with continuous electrocardiographic monitoring. Exhaled gas analysis was performed using a metabolic test system (MetaSoft CPX testing from GE Medical Systems Information Technologies. Freiburg. Germany) with gas analyser (MetaLyzer 3B, Firmware Version 2.0 from Cortex, Leipzig. Germany). Maximal oxygen uptake (VO2max) was considered to be the maximum value reached at peak effort. The external work performed, expressed in METs, was generated by the device software as well as the VO2 predicted, based on the Wasserman equations. (Supplementary material).
2.4. Echocardiographic criteria for diastolic dysfunction
To define the presence of LVDD by echocardiography, the diagnostic algorithm for diastolic dysfunction proposed by the latest ASE/EACVI-2016 guidelines was applied in patients with preserved ejection fraction3. The presence of the following criteria is considered: 1. Mitral septal annulus velocity e’ <7 cm/s or mitral lateral annulus velocity <10 cm/s. 2. Mitral E-wave velocity ratio and mean value of lateral e’ and septal e’ velocities of the mitral annulus >14. 3. Peak tricuspid regurgitation velocity (PTRV) >2.8 m/s. 4. Left atrial volume index (LAVI) >34 ml/m2. Patients with 3 or 4 criteria meet diagnostic criteria for LVDD and those with only one criterion are considered to have normal diastolic function. Those with two criteria are classified as indeterminate. These guidelines3 also include another classification of diastolic dysfunction into grades (Table IV of the guidelines) which is based on quantitative values of classical echographic parameters of LVD: Diastolic dysfunction is considered grade I if the mitral E/A ratio≤0.8, E/e’ ratio <10 and Peak Tricuspid Regurgitation Velocity (PTRV) is <2.8 m/s, grade II if E/A is >0.8 and <2, E/e’ 10–14 and PTRV is >2.8 m/s and grade III if E/A>2, E/e’ >14 and PTRV >2.8 m/s.
2.5. Assessment of left ventricular and left atrial strain.
Myocardial deformation parameters were measured by two-dimensional speckle-tracking echocardiography (2DSTE) using commercially available software (EchoPac version 110.1.1 BT 11; GE Vingmed Ultrasound AS). LV global longitudinal strain (LVGLS) is calculated as the average of the strain obtained in the three apical planes (Supplementary Material).
Left atrial strain (LAS) analysis was performed in the apical four-chamber plane using the apical four-chamber LVS calculation tool applied to the left atrium (EchoPAc version 202.34.0; GE Vingmed Ultrasound AS). For each patient, strain during the reservoir phase (LALSRes), during the conduction phase (LALSCond) and during the atrial contraction phase (LALSPump) was calculated11. A representative patient case is illustrated in supplementary figure 1. All measurements were carried out by a single experienced observer (Supplementary material). Although the threshold of LALS Reservoir that predicts the presence of LVDD is not established, we explored the value of 23% because it was the mean value in our population and 20% because that threshold predicted good association with filling pressures obtained by cardiac catheterisation12.
2.6. Statistical analysis
Quantitative variables are presented as mean ± standard deviation or median [interquartile range] as appropriate and qualitative variables are expressed as n (%). Differences between groups were compared with χ2 and Student’s t-tests or analysis of variance where appropriate. To assess the association between variables and the LALS, Pearson correlations were used. ROC curves were used to determine whether there was an association between LALS and aerobic functional capacity, defined by VO2 max, with a threshold of 15 ml/kg/min. Both LALS reservoir and LALS pump were assessed. This analysis was also performed with the variable left atrial volume index.
The usefulness of LALS to improve echocardiographic classification of LVDD was explored in two ways: 1. By replacing in the algorithm ASE/EACVI-2016 the echocardiographic criterion LAVI>34 ml/m2 with LALS (≤ 23% or ≤ 20%) and grouping into three groups; normal, indeterminate LVDD and definite LVDD. 2. By assessing whether the group of patients with indeterminate DD and who had a reduced LALS (≤ 23% or ≤ 20%) had a lower FAC than the rest. To measure the variability of the LALS, it was recalculated in a sample of 15 randomly selected patients, with a second measurement more than three months later. Reproducibility was assessed with the intraclass correlation coefficient (ICC) and the Bland-Altman analysis providing the disagreement or average of the differences between two sets of measurements (supplementary material). For all analyses, a value of p < 0.05 was considered significant. IBM SPSS Statistics version 23 and R2.5 were used for statistical analysis.
3. Results
3.1. Demographics and clinical characteristic of population
Of the 274 patients who met the entry criteria for the Predimed Plus study, 235 were selected who were in sinus rhythm, had an ejection fraction greater than 50%, had a complete echocardiographic study including 2DSTE of the left ventricle and a valid ergospirometry. In 229 it was possible to obtain measures of the LALS in its three components. The mean age was 65±5 years and 76 (33%) were women. Table 1 shows the demographic, analytical and echocardiographic data of the group, which shows a population with overt obesity, very high prevalence of hypertension (84%), hyperlipidaemia (69%) and 22% of diabetics. The mean value of the echocardiographic criteria for diastolic dysfunction reached the threshold values for lateral and septal e’ wave and LAVI. PTRV was obtained in 103 (45%) patients of the total group.
Table 1:
Clinical characteristics of the total population
| Number | 229 |
| Age (years) | 65±5 |
| Males | 153 (67%) |
| Weight (kg) | 87±12 |
| Size (cm) | 166±8 |
| Body surface, m2 | 1.94±0.17 |
| Body mass index (kg/m2) | 31.5±3.4 |
| Smoking (last 5 years) | 108 (47%) |
| High blood pressure | 192 (84%) |
| Diabetes | 51 (22%) |
| Hyperlipemia | 158 (69%) |
| Glucose (mg/dL) | 113±24 |
| Total cholesterol (mg/dL) | 215±34 |
| HDL-Cholesterol (mg/dL) | 46±10 |
| LDL-Cholesterol (mg/dL) | 131±29 |
| Triglycerides (mg/dL) | 187±79 |
| LVEDD (mm) | 47,7±5 |
| LVESD (mm) | 31±4 |
| Septum (mm) | 11±1,6 |
| Posterior wall (mm) | 9.7±1.5 |
| Mass (g) | 160±43 |
| Mass index (g/m2) | 82±19 |
| LA area (cm2) | 21.4±4,8 |
| LA volume (ml) | 69±18 |
| LA volume index (ml/m2) | 35.3±8.5 |
| LVEDV (ml) | 102±25 |
| LVESV (ml) | 41±12 |
| Ejection fraction (%) | 60.3±4 |
| E-wave mitral flow (m/s) | 0.67±0.14 |
| A-wave mitral flow (m/s) | 0.81±0.14 |
| E/A ratio | 0.85±0.2 |
| Deceleration time (ms) | 225±51 |
| Lateral e’ wave (cm/s) | 8.4±2 |
| Septal e’ wave (cm/s) | 6.7±1.5 |
| Average E/e’ ratio | 9.1±2.3 |
| PTRV (m/s) | 2.4±0.2 |
LA: Left atrium. LVEDD: LV end-diastolic diameter. LVESD: LV end-systolic diameter. LALS: Left atrial longitudinal strain. LVGLS: LV global longitudinal strain. LVEDV: LV end-diastolic volume. LVESV: LV end-systolic volume. PTRV: Peak tricuspid regurgitation velocity.
3.2. Echocardiographic criteria for diastolic dysfunction among the population.
Supplementary figure 2 shows the number of patients with 1, 2, 3 or 4 criteria. The most frequent isolated criterion was a reduction in lateral mitral annulus velocity, which was met by 166 (72%) patients, followed by an increase in LAVI, which was met by 106 patients (46%). Two criteria were met by 36% of the population. The prevalence of LVDD according to the guidelines is also shown, which corresponds to 140 normal patients, 82 with indeterminate diagnosis and 7 with definite LVDD. When the LVDD grade classification3 was considered, the distribution of the population was as follows: Grade I LVDD showed 72 (31%) patients, Grade II LVDD 3 (1.3%) and none Grade III.
3.3. Parameters of functional aerobic capacity
The mean duration of the stress test was 9.3±2.4 minutes, representing an external work of 10±2.4 mets. The mean VO2max was 19.6±4.8 ml/kg/min representing 94±18% of the theoretical maximal exercise capacity. Of the total, 166 (72%) of the patients exceeded 85% of the theoretical FAC and 63 (28%) did not. The mean value of maximum HR was 140±17 (90±10% of theoretical maximum HR) and 74% of patients reached ≥85% of theoretical maximum HR.
3.4. Left ventricular and left atrial strain.
The mean LVGLS value of the population was −19.4±2.4% obtained from the LVLS in apical planes 3 (−19.4±3.2%), 4 (−18.9±2.85) and 2 chambers (−19.9±2.9%).
The LA deformation study showed a mean LALSRes value of 23.8±6.7%, LALSCond of 10±3.8% and LALSPump of 13.8±4.6%. LALS could be obtained in 97% of patients in whom LVLS could be studied. Supplementary table 1 shows the correlation between LALSRes and total group parameters. There was only correlation with age and LVGLS, and no correlation was found with echocardiographic variables defining diastolic dysfunction or with FAC indexes. Reproducibility assessment showed an excellent ICC (±IC) for LALSRes of 0.942 (0.836–0.980), p<0.005, for LALSCond of 0.900 (0.728–0.965), p<0.005 and for LALSPump of 0.852 (0.613–0.947), p<0.005.
3.5. Left atrial strain and diastolic dysfunction
Table 2 shows the age and echocardiographic parameters of diastolic dysfunction and strain distributed according to the algorithm and the ASE/EACVI-2016 grade classification. A significant reduction in the value of isolated echocardiographic criteria was observed in the definite LVDD group compared to the normal and indeterminate groups. However, the LVGLS and LALS values, although lower, do not reach statistical significance. Similar findings were found when comparing patients without LVDD and those classified as group I and II. Supplementary table 2 shows the percentage of patients with threshold LALSRes≤23% or LALSRes≤20%. All patients with LVDD showed a strain ≤20% and 70% of the normal and indeterminate group >20%. Figure 1 shows the classification of the ASE/EACVI-2016 algorithm into the three groups according to whether the LAVI was used as a criterion or whether it was replaced by the two threshold values of the LALSRes. For LALSRes≤20%, there is a significant reduction in indeterminate cases (23% and 36%, p<0.001) at the expense of defining a higher number of normal patients (74% and 61%).
Table 2:
Echocardiographic parameters according to the 2016 ASE/EACVI algorithm and classification into grades.
| 2016 ASE-EACVI Algorithm | 2016 ASE-EACVI Grade Classification | |||||||
|---|---|---|---|---|---|---|---|---|
| Normal | Indeterm. | LVDD | p | Normal | Grade I | Grade II | p | |
| No. of patients | 140 | 82 | 7 | 154 | 72 | 3 | ||
| Age | 64±5 | 66±5 | 68±4 | 0.023 | 64±5 | 66±4 | 68±3 | 0.002 |
| lateral e’ | 8.8±2 | 7.9±1 | 7±1 | <0.001 | 8.5±2 | 8.3±1 | 8±0.5 | 0.641 |
| septal e’ | 7±1 | 6.3±1 | 6±1 | 0.002 | 6.8±2 | 6.4±1 | 6.3±1 | 0.209 |
| E/e’ Ratio | 8.6±1.7 | 9.6±2.3 | 14.8±1.6 | <0.001 | 9.7±2 | 7.8±1 | 14.5±2 | <0.001 |
| LAVI (ml/m2) | 31.6±7 | 40.8±7 | 45.8±7 | 0.001 | 35.6±8 | 34±8 | 49.7±7 | 0.005 |
| PTRV (m/s) | 2.4±0.2 | 2.3±0.2 | 2.7±0.2 | 0.003 | 2.4±0.2 | 2.4±0.2 | 2.8±0.1 | 0.001 |
| LVGLS | 19.4±2 | 19.5±2 | 18.7±2 | 0.681 | 19.4±2 | 19.5±2 | 20±4 | 0.681 |
| LALSRes | 23.9±6.7 | 23.8±6.7 | 20.8±5.2 | 0.502 | 23.6±6.7 | 24.1±6.7 | 23.5±4.1 | 0.910 |
| LALSCond | 10.4±4 | 9.5±3.6 | 10.1±2.9 | 0.256 | 10.3±3.7 | 9.4±4 | 12±2.7 | 0.188 |
| LALSPump | 13.6±4.3 | 14.3±5 | 10.7±2.9 | 0.113 | 13.4±4.6 | 14.6±4.6 | 11.6±1.3 | 0.121 |
LVDD: LV diastolic dysfunction. Indeterm.: Indeterminate. LAVI: Left atrial volume index. LALSRes: Left atrial longitudinal reservoir strain. LALSCond: Left atrial longitudinal conduction strain. LALSPump: Left atrial longitudinal pump strain. LVGLS: LV global longitudinal strain. PTRV: Peak tricuspid regurgitation velocity.
Figure 1:
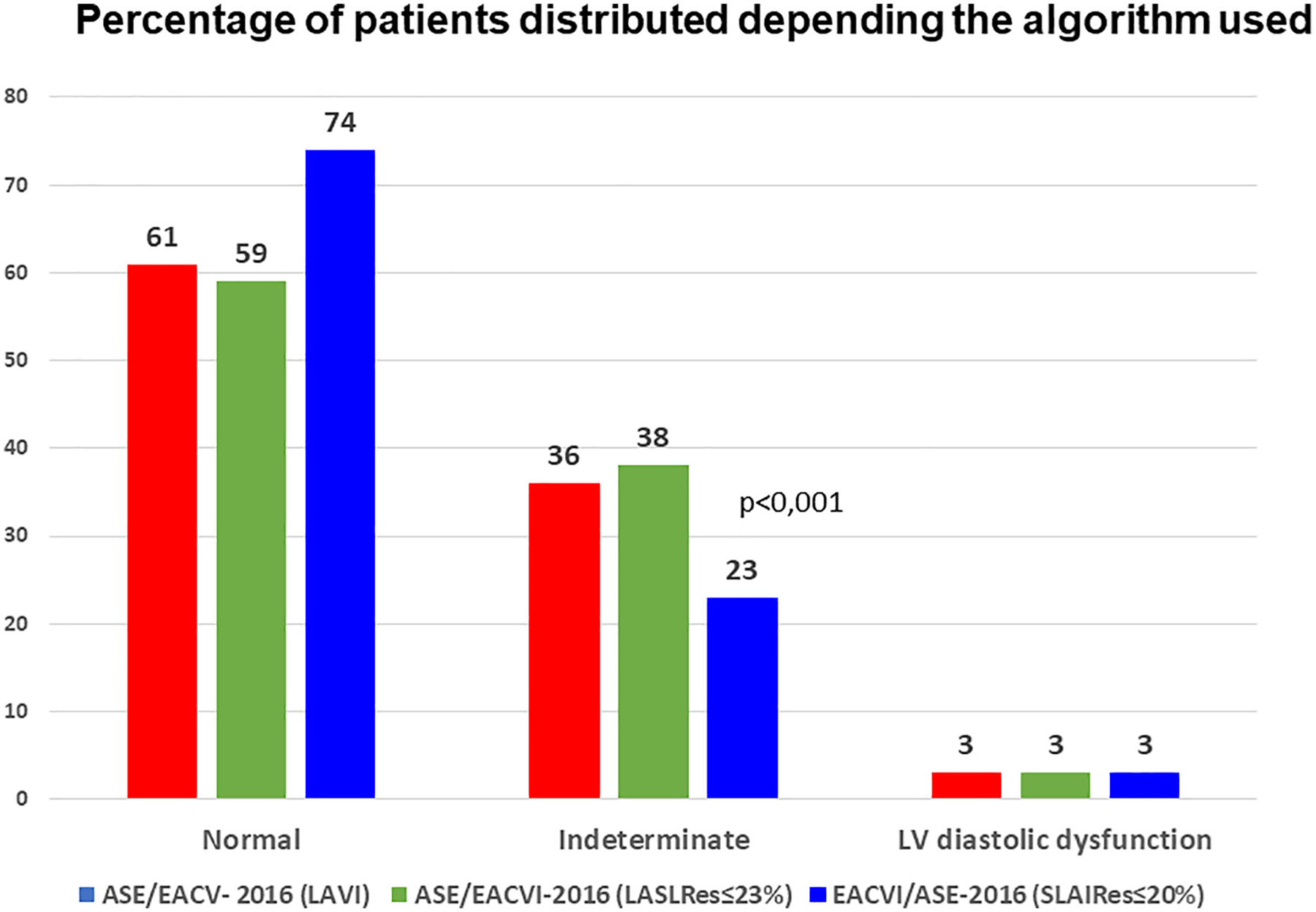
Percentage of patients distributed as normal, indeterminate or LVDD depending on whether the algorithm used the LA volume or different threshold values of the LALSRes.
3.6. Association of exercise capacity and diastolic dysfunction criteria
Supplementary table 3 shows the findings of exercise capacity, defined as VO2max, duration in minutes or number of METs achieved, distributed in three subgroups, depending on whether the ASE/EACVI-2016 algorithm or its grading was considered. In the first case, the subgroup with LVDD reached lower values for all FAC parameters than the rest. In the group classification, both normal and grade I patients achieved similar values. Table 3 shows the FAC distributed in the normal, indeterminate or LVDD groups, in the reclassification made when the LAVI was replaced by the LALS. There was a continuing tendency for lower VO2max in all three groups, although there was no significant difference between the normal and indeterminate groups. Supplementary table 4 shows again the FAC parameters in the normal, indeterminate and indeterminate group according to their LALSRes≤23%. The VO2max achieved in indeterminate patients with reduced strain was lower than in patients with LALS above 23% (11.6±3 and 18±5, p:0.081). However, this subgroup did not differ significantly from the normal group or the indeterminate group without reclassification. The ROC curve analysis that studied the association between LALS and FAC, defined by a threshold of ≤15 ml/kg/min of VO2max reached an area under the curve value in the total group of 0.530 (0.431–0. 630); but when the subgroup of 106 patients aged less than 65 years was analysed, areas under the curve of 0.719 (0.556–0.882) for LALSPump and 0.661 (0.490–0.832) for LALSReservoir were reached (Supplementary Material).
Table 3:
Exercise capacity according to the 2016 ASE/EACVI algorithm, when the LA volume criterion was replaced by LA strain (<23% and <20%).
| 2016 ASE/EACVI (SLAI <23%) | 2016 ASE/EACVI (SLAI <20%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-LVDD | Indeterm. | LVDD | p | NON-LVDD | Indeterm. | LVDD | p | |
| No. of patients | 135 | 88 | 6 | 171 | 52 | 6 | ||
| VO2max ml/kg | 20.3±5 | 18.6±4 | 16±3 | 0.007 | 20±4 | 18.3±4 | 16±2 | 0.015 |
| ST duration (min) | 9.6±2 | 8.9±2 | 7±2 | 0.008 | 9.4±2 | 8.7±2 | 7±1 | 0.012 |
| METS number | 10.3±2 | 9.7±2 | 7±2 | 0.014 | 10.2±2 | 9.6±2 | 7.7±1 | 0.018 |
LVDD: LV diastolic dysfunction. ST: Stress test. VO2: Oxygen uptake.
4. Discussion
The main results of this study, in which we have assessed the echocardiographic diagnosis of diastolic dysfunction in a population with metabolic syndrome and overweight/obesity, were: 1) to find a higher prevalence of diastolic dysfunction with a higher percentage of inconclusive studies than in the normal population. 2) to document the value of LALS to reduce the percentage of patients with indeterminate studies. We have used the ASE/EACVI-2016 recommendations because their accuracy has been confirmed in studies that have compared echocardiographic findings with LV filling pressures obtained by cardiac catheterisation12,13.
4.1. Echocardiographic criteria for diastolic dysfunction
A key finding in our study was to confirm the high prevalence of isolated echocardiographic criteria validated by the 2016 guidelines (supplementary figure 2). Three studies have evaluated this algorithm in large populations without heart disease and preserved ejection fraction, finding a very high proportion of isolated echocardiographic criteria for LVDD. The group of indeterminate patients was 13.7%14, 15.2%4 and 20%15, values lower than those found by us, which should not be surprising, as it has been proven that this diagnosis increases with the increase in age when using the ASE/EACVI-2016 algorithm15. We have also been able to document that the presence of LVDD, defined according to the algorithm, reaches significantly lower VO2max and exercise capacity values than the normal and indeterminate groups (supplementary table 3). When the patients were distributed according to grades, it was also confirmed that those with more advanced LVDD had a lower exercise capacity (supplementary table 3). These findings are consistent with the approach of some authors that, in patients with preserved ejection fraction, a reduction in VO2max can be considered a surrogate for diastolic function16. Our data did not allow us to find a linear association between LALS and exercise capacity, although we were able to confirm the ability of the LALS≤−20% threshold to predict reduced FAC in the subgroup of participants aged less than 65 years. Interestingly, we found that the LA volume index did not have this predictive ability (Supplementary material).
4.2. Left atrial longitudinal strain and diastolic dysfunction
Numerous studies have confirmed that the progressive effect of LVDD leads to an increase in LA size and a deterioration of its functionality that can be documented by analysis of systolic function and/or mechanical properties17,18. The quantification of LA strain by 2DSTE echocardiography has been postulated as a robust, easily assessed and reproducible index19. Although three components are considered in LALS (supplementary figure 1), the most commonly used parameter in clinical studies is the LALS reservoir. The pathophysiological mechanism of impaired reservoir function in patients with LVDD involves elevated LA pressures, increased LA wall stress, reduced pulmonary venous emptying and, consequently, reduced LA filling and reduced LALS Reservoir20. The LALS values obtained in our population, with respect to those found in a systematic review in 2542 healthy individuals21, are lower in both LALS Reservoir (23.8% and 39%), LALS Conduction (10% and 23%) and LALS Pump (13.8% and 17%). Our values are also lower than those found in a reference population that used the same equipment and software to calculate the LALS22. In healthy subjects the physiological determinants of LA strain are age, LVGLS, LV volume and some parameters of diastolic function23, but in our population we only found an association with age and LVGLS, with no correlation with any of the isolated echocardiographic criteria studied or LA dimensions (supplementary table 1).
The echocardiographic criterion LAVI is included in the ASE/EACVI-2016 guidelines, as there is very strong evidence that LVDD leads to an increase in LA volume and its quantification and reproducibility is superior to other parameters. Although it reflects the chronic effect of increased filling pressures, it cannot be used as a marker for improvement of diastolic dysfunction due to slow and often incomplete LA remodeling and provides limited information in event prediction19. The LALS, unlike traditional parameters, changes progressively with the severity of LVDD, and its feasibility is excellent, so we have explored its usefulness in improving the profitability of the diagnostic algorithm.
When we substitute the parameter LAVI>34 ml/m2 for LALS ≤20%, we get a significant decrease in indeterminate studies at the expense of an increase in normal studies (Figure 1). Two recent studies have confirmed our results: Morris et al. in 517 patients at risk of developing LVDD, proposed the strategy of considering the presence of LAVI>34 ml/m2 or LALS<23% as positive criteria, obtaining a reduction of indeterminate studies at the expense of a higher proportion of patients with a diagnosis of LVDD25. Potter et al. in 758 asymptomatic patients studied the value of replacing the criterion LAVI>34 ml/m2 with LALS <24%, reducing the number of indeterminate cases, finding that cases defined as normal had the lowest proportion of incident heart failure at two-year follow-up26. The accuracy of our classification (replacing LAVI with LALS) is supported by the finding that there is a progressive reduction in VO2max with increasing progression of diastolic dysfunction (table 3).
The other approach to improve the classification was to study whether adding the LALS criterion to the indeterminate group delimited a group of patients who could present LVDD, using VO2max as a surrogate for LVDD. We were able to confirm that patients with LALS≤23% achieved a lower value than the rest within the indeterminate group (supplementary table 4). This finding is consistent with that previously found in patients with preserved EF, where using the <23% threshold in the indeterminate group detected a higher proportion of patients who developed heart failure25.
4.3. Limitations
The sample size is limited because the data are from a single centre, but our findings are applicable to the population with metabolic syndrome and overweight/obesity. The echocardiographic LVDD assessment did not include echocardiographic parameters other than the criteria proposed by the guidelines, as we decided to use a strategy that can be implemented in the general population. Although in the obese population the VO2/kg peak can be corrected (as body fat, which may represent a significant proportion of total body weight, does not consume oxygen during exercise) we have not done so since it is controversial which correction should be applied. We have also assumed the normality values for the LAVI that are established for the normal population, even though we know that in the overweight population the normality of the volume may reach higher values. The LALS threshold values we have used have been exploratory for this population and certainly do not reflect the diagnostic cut-off point of the increase in filling pressures in other populations, which may be lower27. We have not considered other parameters of left atrial mechanics, such as strain rate, which have been shown to be useful in this field. LALS threshold values must be considered in the context of having been conducted with a particular platform and software and may not be applicable to studies conducted with equipment from different vendors.
5. Conclusions
This study confirms the low prevalence of diastolic dysfunction in overweight/obese metabolic syndrome and the limitations of the recommended classification for its echocardiographic diagnosis. Adding left atrial strain criterion significantly reduces the number of indeterminate studies by reclassifying them as normal. The values obtained for maximal oxygen uptake, considered as a surrogate for diastolic dysfunction, support the usefulness of the left atrial strain as a main criterion for echocardiographic diagnosis.
Supplementary Material
Supplementary figure 1: Longitudinal left atrial strain of a representative patient showing the calculated parameters that correspond to the reservoir, conduction and pump phases. LALS: Left atrial longitudinal strain.
Supplementary figure 2: Number of patients who met echocardiographic criteria for LVDD and prevalence according to ASE/EACVI 2016 guidelines.
Acknowledgements
Jordi Salas-Salvadó, co-lead author, gratefully acknowledges the financial support of ICREA in the framework of the ICREA Academy programme.
Source of Funding
This work has been funded: 1.- Carlos III Health Institute (Ministry of Economy, Industry and Competitiveness) through a health research fund (FIS) project coordinated by Dr. Jordi Salas-Salvadó (PI13/01056), by the CIBEROBN (Biomedical Research Networking Center for Physiopathology of Obesity and Nutrition). 2.- The European Research Council (Advanced Research Grant 2014-2019; 340918) awarded to Dr. Miguel A. González-Martínez. 3.- Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Numbers R01HL137338 and K24HL148521. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
Alvaro Alonso: Reports NIH grants during the conduct of the study.
Jordi Salas-Salvadó: Declares having received grants from CIBEROBN, ISCIII, Spain, during the study; non-financial support from the Nut and Dried Fruit Foundation, personal fees from the Danone Institute Spain, grants from the Nut and Dried Fruit Foundation, personal fees from the Nut and Dried Fruit Foundation, non-financial support from Danone Institute International, others from the Patronato Español Olivarero, others from the Almond Board of California, others from American Pistachio Growers, outside the submitted work.
Abbreviations
- ASE/EACVI 2016
2016 Guidelines for the evaluation of left ventricular diastolic function by echocardiography of the American Society of Echocardiography/European Association of Cardiovascular Imaging
- LAS
Left atrial strain
- FAC
Functional aerobic capacity
- LAVI
Left atrial volume index
- LVDD
Left ventricular diastolic dysfunction
BIBLIOGRAPHY
- 1.Russo C, Jin Z, Homma S, et al. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol. 2011; 57:1368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park SK, Ryoo JH, Oh CM, et al. Effect of Overweight and Obesity (Defined by Asian-Specific Cutoff Criteria) on Left Ventricular Diastolic Function and Structure in a General Korean Population. Circ J. 2016; 80:2489–2495 [DOI] [PubMed] [Google Scholar]
- 3.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016; 29:277–314. [DOI] [PubMed] [Google Scholar]
- 4.Almeida JG, Fontes-Carvalho R, Sampaio F, et al. Impact of the 2016 ASE/EACVI recommendations on the prevalence of diastolic dysfunction in the general population. Eur Heart J Cardiovasc Imaging. 2018; 19:380–386. [DOI] [PubMed] [Google Scholar]
- 5.Alonso-Gómez AM, Tojal Sierra L, Fortuny Frau E, et al. Diastolic dysfunction and exercise capacity in patients with metabolic syndrome and overweight/obesity. Int J Cardiol Heart Vasc. 2019; 22:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh A, Addetia K, Maffessanti F, Mor-Avi V, Lang RM. LA Strain for Categorization of LV Diastolic Dysfunction. JACC Cardiovasc Imaging. 2017; 10:735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh JK, Miranda WR, Bird JG, Kane GC, Nagueh SF. The 2016 Diastolic Function Guideline: Is it Already Time to Revisit or Revise Them? JACC Cardiovasc Imaging. 2020; 13:327–335. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, Kim MG, Kang S, et al. Obesity and Hypertension in Association with Diastolic Dysfunction Could Reduce Exercise Capacity. Korean Circ J. 2016; 46:394–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sayón-Orea C, Razquin C, Bulló M, et al. Effect of a Nutritional and Behavioral Intervention on Energy-Reduced Mediterranean Diet Adherence Among Patients With Metabolic Syndrome: Interim Analysis of the PREDIMED-Plus Randomized Clinical Trial. JAMA. 2019; 322:1486–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015; 16:233–70. [DOI] [PubMed] [Google Scholar]
- 11.Badano LP, Kolias TJ, Muraru D, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. Eur Heart J Cardiovasc Imaging. 2018; 19:591–600. [DOI] [PubMed] [Google Scholar]
- 12.Singh A, Medvedofsky D, Mediratta A et al. Peak left atrial strain as a single measure for the non-invasive assessment of left ventricular filling pressures. Int J Cardiovasc Imaging 2019; 35:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lancellotti P, Galderisi M, Edvardsen T, et al. Echo-Doppler estimation of left ventricular filling pressure: results of the multicentre EACVI Euro-Filling study. Eur Heart J Cardiovasc Imaging. 2017; 18:961–968. [DOI] [PubMed] [Google Scholar]
- 14.Balaney B, Medvedofsky D, Mediratta A, et al. Invasive Validation of the Echocardiographic Assessment of Left Ventricular Filling Pressures Using the 2016 Diastolic Guidelines: Head-to-Head Comparison with the 2009 Guidelines. J Am Soc Echocardiogr. 2018; 31:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorrentino R, Esposito R, Santoro C, et al. Practical Impact of New Diastolic Recommendations on Noninvasive Estimation of Left Ventricular Diastolic Function and Filling Pressures. J Am Soc Echocardiogr. 2020; 33:171–181. [DOI] [PubMed] [Google Scholar]
- 16.Yeung DF, Jiang R, Behnami D, et al. Impact of the updated diastolic function guidelines in the real world. Int J Cardiol. 2021; 326:124–130. [DOI] [PubMed] [Google Scholar]
- 17.Guazzi M, Bandera F, Ozemek C et al. Cardiopulmonary Exercise Testing: What Is its Value? J Am Coll Cardiol. 2017; 70:1618–1636. [DOI] [PubMed] [Google Scholar]
- 18.Nagueh SF. Left Ventricular Diastolic Function: Understanding Pathophysiology, Diagnosis, and Prognosis With Echocardiography. JACC Cardiovasc Imaging. 2020; 13:228–244. [DOI] [PubMed] [Google Scholar]
- 19.Thomas L, Muraru D, Popescu BA, et al. Evaluation of Left Atrial Size and Function: Relevance for Clinical Practice. J Am Soc Echocardiogr. 2020; 33:934–952. [DOI] [PubMed] [Google Scholar]
- 20.Cho GY, Hwang IC. Left Atrial Strain Measurement: A New Normal for Diastolic Assessment? JACC Cardiovasc Imaging. 2020; 13:2327–2329. [DOI] [PubMed] [Google Scholar]
- 21.Braunauer K, Düngen HD, Belyavskiy E, et al. Potential usefulness and clinical relevance of a novel left atrial filling index to estimate left ventricular filling pressures in patients with preserved left ventricular ejection fraction. Eur Heart J Cardiovasc Imaging. 2020; 21:260–269. [DOI] [PubMed] [Google Scholar]
- 22.Pathan F, D’Elia N, Nolan MT, Marwick TH, Negishi K. Normal ranges of left atrial strain by speckle-tracking echocardiography: a systematic review and meta-analysis. J Am Soc Echocardiogr 2017; 30:59–70. [DOI] [PubMed] [Google Scholar]
- 23.Sugimoto T, Robinet S, Dulgheru R, et al. NORRE Study. Echocardiographic reference ranges for normal left atrial function parameters: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. 2018; 19:630–638. [DOI] [PubMed] [Google Scholar]
- 24.Miglioranza MH, Badano LP, Mihăilă S, et al. Physiologic Determinants of Left Atrial Longitudinal Strain: A Two-Dimensional Speckle-Tracking and Three-Dimensional Echocardiographic Study in Healthy Volunteers. J Am Soc Echocardiogr. 2016; 29:1023–1034. [DOI] [PubMed] [Google Scholar]
- 25.Morris DA, Belyavskiy E, Aravind-Kumar R, et al. Potential Usefulness and Clinical Relevance of Adding Left Atrial Strain to Left Atrial Volume Index in the Detection of Left Ventricular Diastolic Dysfunction. JACC Cardiovasc Imaging. 2018; 11:1405–1415. [DOI] [PubMed] [Google Scholar]
- 26.Potter EL, Ramkumar S, Kawakami H et al. Association of Asymptomatic Diastolic Dysfunction Assessed by Left Atrial Strain With Incident Heart Failure. JACC Cardiovasc Imaging. 2020; 13:2316–2326. [DOI] [PubMed] [Google Scholar]
- 27.Inoue K, Khan FH, Remme EW et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur Heart J Cardiovasc Imaging. 2021. Jan 26: jeaa415. doi: 10.1093/ehjci/jeaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1: Longitudinal left atrial strain of a representative patient showing the calculated parameters that correspond to the reservoir, conduction and pump phases. LALS: Left atrial longitudinal strain.
Supplementary figure 2: Number of patients who met echocardiographic criteria for LVDD and prevalence according to ASE/EACVI 2016 guidelines.


