Abstract
The association between fruit and vegetable consumption before and during pregnancy and offspring’s physical growth has been well reported, but no study has focused on offspring’s neurological development. We aimed to explore the association between maternal fruit and vegetable consumption before and during pregnancy and developmental delays in their offspring aged 2 years. Between July 2013 and March 2017, 23 406 women were recruited for the Tohoku Medical Megabank Project Birth and Three-Generation Cohort Study. Fruit and vegetable consumption was calculated using FFQ, and offspring’s developmental delays were evaluated by the Ages & Stages Questionnaires, Third Edition (ASQ-3) for infants aged 2 years. Finally, 10 420 women and 10 543 infants were included in the analysis. Totally, 14·9 % of children had developmental delay when screened using the ASQ-3. Women in the highest quartile of vegetable consumption from pre-pregnancy to early pregnancy and from early to mid-pregnancy had lower odds of offspring’s developmental delays (OR 0·74; 95 % CI 0·63, 0·89 and OR 0·70; 95 % CI 0·59, 0·84, respectively) than women in the lowest quartile. Women in the highest quartile of fruit consumption from early to mid-pregnancy had lower odds of offspring’s developmental delays (OR 0·78; 95 % CI 0·66, 0·92) than women in the lowest quartile. In conclusion, high fruit and vegetable consumption before and during pregnancy was associated with a lower risk of developmental delays in offspring aged 2 years.
Key words: Vegetables, Fruit, Child Development, Fetal Development, Pregnancy
Developmental delays in early life including speech and language development, motor development, social-emotional development and cognitive development(1) can affect individuals throughout their life. Generally, developmental delays are determined when a child does not achieve milestones compared with peers of the same age range; the estimated prevalence is approximately 8·4 % in infants under 5 years of age(2). Infants with developmental delays have a higher risk of learning disabilities, behaviour problems and difficulty building friendships later in life(3). They are also at higher risk of educational attainment and well-being compared with children without such disabilities(4) and require lifelong treatment and medical care, resulting in a greater social and economic burden.
Diet during pregnancy has a close relationship with the development of the child. It has been reported that fish, PUFA, folic acid and multivitamin intake before and during pregnancy have a positive effect on the offspring’s neurological development(5–9). Fruit and vegetables are major sources of vitamins and minerals, but the association between fruit and vegetable consumption before and during pregnancy and offspring’s neurological development has not been investigated. Because fruit and vegetable consumption has been reported to be beneficial for children’s physical growth and the prevention of other diseases(10–13), it may also be valuable for children’s neurodevelopment.
Modifying environmental factors before and during pregnancy can prevent developmental disorders and is necessary especially because developmental disorders require long-term treatment and specialised education once it occurs. Identifying the association between dietary habits before and during pregnancy, which is one of the environmental factors within one’s control, and offspring’s developmental delays may provide valuable clues to aid in its prevention. Therefore, this study aimed to investigate the association between fruit and vegetable consumption before and during pregnancy and offspring’s development at 2 years of age, when they are at the early stages of development. In addition, we investigated the association between changes in fruit and vegetable consumption before and during pregnancy and offspring’s development at 2 years of age to evaluate the appropriate period for these consumption.
Methods
Study design and population
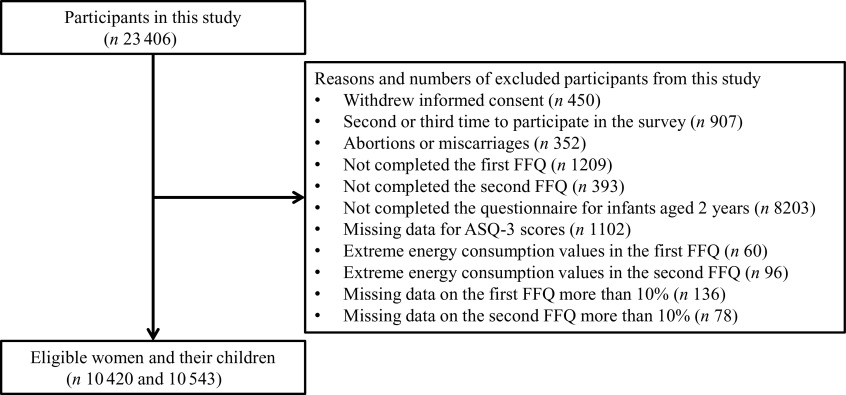
This study was based on data obtained in the Tohoku Medical Megabank Project Birth and Three-Generation Cohort Study (TMM BirThree Cohort Study). The TMM BirThree Cohort Study was a prospective cohort study undertaken in Miyagi Prefecture, Japan, and the design and methods have been reported previously(14,15). Between July 2013 and March 2017, 23 406 pregnant women, including women who participated in the study multiple times for different pregnancies, were recruited. Written informed consent was obtained from all participants. We used the data set as of 1 April 2020. The flow chart of participant exclusion criteria is shown in Fig. 1. Among women who participated in the study multiple times for different pregnancies, data from the second and subsequent participation were excluded to eliminate the effect of repeated measurements on a single participant. This study was conducted according to the guidelines stipulated in the Declaration of Helsinki, and all procedures involving human participants were approved by the institutional review board of the Tohoku University Tohoku Medical Megabank Organization (2019-4-057) and KAGOME CO., LTD. (2018-R10).
Fig. 1.
Flow chart of participant exclusion criteria in this study. This flow chart describes the exclusion criteria and the number of participants, excluded participants, and eligible participants in total. ASQ-3, Ages & Stages Questionnaires, Third Edition.
Dietary assessment
Two semi-quantitative FFQ were used to evaluate dietary consumption during two different periods. The first FFQ that was administered in early pregnancy enquired about the frequency and amount of food and beverages consumed in the past year to evaluate dietary consumption from pre- to early pregnancy. The second FFQ was administered at mid-pregnancy and enquired about the frequency and amount of food and beverages consumed since the first FFQ was completed to evaluate dietary consumption from early to mid-pregnancy. Mean response periods of the first and second FFQ in the participants included in the analysis were 19·5 (sd 7·2) weeks and 27·7 (sd 5·5) weeks of gestation, respectively. Frequency and amount of 130 food items and beverage consumption were evaluated through each FFQ. Frequency and amount of consumption of food items and beverages were converted into average daily consumption by multiplying the frequency and quantity. These items included eighteen fruits, twenty-six vegetables, eighteen meats, nineteen fish, nine grains, four potatoes, seven beans and seven dairy products. The total consumption of each dietary group was identified by summing the responses to the consumption query of each dietary group. Total energy and nutrient consumption such as carotene, vitamin C, vitamin K and folate were calculated using the Standard Tables of Food Composition in Japan (Fifth Revised and Enlarged Edition 2005)(16). The consumption of each dietary and nutrient was energy-adjusted using the residual method(17), and the values were included in the analysis as continuous or categorical values, which were divided into quartiles. Spearman’s correlation coefficient between fruit consumption from pre- to early pregnancy and that from early to mid-pregnancy was 0·559 (online Supplementary Table S1). Similarly, Spearman’s correlation coefficient between vegetable consumption from pre- to early pregnancy and that from early to mid-pregnancy was 0·600. Additionally, we defined four categories based on changes from pre- to mid-pregnancy in the total fruit, vegetable, meat, fish, grain, potato, bean, dairy product, carotene, vitamin C, vitamin K and folate consumption. The definitions of the four categories were described as follows: women whose answers to the first and second FFQ were in the first or second quartile (low-low group), women whose answers to the first FFQ were in the first or second quartile and whose answers to the second FFQ were in the third or fourth quartile (low-high group), women whose answers to the first FFQ were in the third or fourth quartile and whose answers to the second FFQ were in the first or second quartile (high-low group), and women whose answers to the first and second FFQ were in both the third or fourth quartile (high-high group). More detailed methods are described elsewhere(10).
Outcome variables
The Ages & Stages Questionnaires, Third Edition (ASQ-3) was used to evaluate offspring’s developmental delays. The ASQ-3 is a broadband questionnaire to screen offspring’s developmental delays, and it consists of twenty-one age-specific questionnaires for ages 1–66 months. In the present study, we used the validated Japanese translation of the ASQ-3(18) and obtained information using the ASQ-3 for infants aged 2 years. Each questionnaire contains thirty questions divided into five developmental domains: ‘communication’, ‘fine motor’, ‘gross motor’, ‘problem solving’ and ‘personal-social’. Each domain has a set of six items, and each item is scored as 10, 5 and 0 corresponding to ‘yes’, ‘sometimes’ and ‘not yet’, respectively. The total score ranges from 0 to 60 for each domain. Each domain was classified as a failed domain when the score was <2 sd below the mean(19). Infants were identified as having developmental delays if they failed in one or more of the domains(19).
Confounders
Variables related to birth dates of mothers, fathers and their infants were obtained from medical records and the informed consent form. Maternal age (years) and paternal age at birth of their infant (years) were calculated by subtracting the infants’ birth dates from each of their parents’ birth dates. Infant birth season was based on the birth date of infants categorised under any one of the four seasons, ‘spring (March–May)’, ‘summer (June–August)’, ‘autumn (September–November)’ and ‘winter (December–February)’. Variables related to maternal height (cm), pre-pregnancy weight (kg), parity (never, one or more) and infant sex (male and female) were obtained from medical records. Maternal age was divided into four categories (<25, 25–29, 30–34 and ≥35 years), and paternal age was divided into six categories (<25, 25–29, 30–34, 35–39, 40–44 and ≥45 years). Pre-pregnancy BMI (kg/m2) was calculated by dividing the pre-pregnancy weight (kg) by the square of maternal height (m2) and was classified into three categories (<18·5, 18·5–24·9 and ≥25·0 kg/m2). Variables related to cigarette smoking (never, stopped before pregnancy, stopped after pregnancy and current), alcohol consumption (never, former and current), folic acid supplementation during early pregnancy (yes and no) and fertility treatment (yes and no) were obtained from the questionnaire during early pregnancy. Data on variables related to household income (<4 000 000, 4 000 000–5 999 999 and ≥6 000 000 Japanese Yen/year) were obtained from the questionnaire during mid-pregnancy. Maternal educational attainment data (high school graduate or less, junior college or vocational college graduate, university graduate or above, others), maternal personal pervasive developmental disorders history (yes and no) and paternal personal pervasive developmental disorders history (yes and no) were gathered a year after birth. Breast-feeding duration data were obtained from the questionnaire for infants aged 6 months and 1 year and were classified into two categories (<6 and ≥6 months). Frequencies of fruit, green vegetable, red and yellow vegetables, and light-coloured vegetable consumption in the offspring at age 2 years in the past week (never, once or twice a week, three or four times a week, five or six times a week, once a day, twice a day, three or more times a day) were obtained from the questionnaire for infants aged 2 years. Total meat, fish, grain, potato, bean and dairy product consumption and their changes from pre- to mid-pregnancy were also included in the analyses as confounders.
Statistical analysis
Continuous variables were expressed as mean and standard deviation, and categorical variables were indicated as frequencies and percentages. The association between dietary and nutrient consumption and offspring’s developmental delays was evaluated by multivariate logistic regression analysis to calculate the OR, 95 % CI and P for trend. The association between changes in dietary and nutrient consumption and offspring’s developmental delays was evaluated by the same method used to calculate the OR, 95 % CI. Missing data for confounders were imputed using multivariate imputation methods by chained equations assuming missing data at random(20). For the present study, multivariate imputation methods by chained equations imputed each incomplete confounder by generating plausible synthetic values based on exposure, outcome and the other confounders in the data. We independently analysed twenty copies of the data, each with missing values imputed in the multivariate analyses. The first quartile and low-low group were used as the reference group. In a sub-analysis, OR, 95 % CI and P for trend were estimated using multivariate logistic regression with generalised estimating equations, specifying an exchangeable correlation structure. Generalised estimating equations were used to account for the correlation of repeated observations (multiple births, multiple participation) by a single participant. Spearman’s correlation analysis was used to assess correlations among mothers and their offspring’s fruit and vegetable consumption. P < 0·05 was considered statistically significant. Statistical analyses were performed with R, version 4.0.2.
Results
Characteristics of the study population
In total, 10 420 women and 10 543 infants (including twins and triplets) were included in the analysis (Fig. 1). Among the women included in the analysis, the mean age was 32·1 (sd 4·8) years old, the mean maternal height was 158·5 (sd 5·4) cm and the mean pre-pregnancy weight was 53·9 (sd 9·1) kg (Table 1). A small number of mothers and fathers had personal pervasive developmental disorders history, and approximately 10 % parents underwent fertility treatment. Totally, 1572 infants (14·9 %) had developmental delay when screened using the ASQ-3. Comparison of carotene, vitamin C and folate consumption between fruit and vegetable quartiles showed that the fourth quartile of vegetable consumption had higher carotene and folate consumption than the fourth quartile of fruit consumption (online Supplementary Table S2–S5). Vitamin C consumption was slightly higher in the fourth quartile of fruit consumption than the fourth quartile of vegetable consumption. No strong correlation of fruit and vegetable consumption before and during pregnancy with the frequency of fruit and vegetable consumption by the offspring at age 2 years was observed (online Supplementary Table S1). The information on the frequency of dietary consumption in offspring aged 2 years is shown in Supplementary Tables S6–S9.
Table 1.
Characteristics of the study population
(Numbers and percentages; mean values and standard deviations)
| n | % | |
|---|---|---|
| n | 10 543 | |
| Maternal characteristics | ||
| Age (years) | ||
| Mean | 32·1 | |
| sd | 4·8 | |
| Height (cm) | ||
| Mean | 158·5 | |
| sd | 5·4 | |
| Pre-pregnancy weight (kg) | ||
| Mean | 53·9 | |
| sd | 9·1 | |
| Pre-pregnancy BMI* | ||
| <18·5 (kg/m2) | 1453 | 13·8 |
| 18·5–24·9 (kg/m2) | 7710 | 73·1 |
| ≥25·0 (kg/m2) | 1224 | 11·6 |
| Missing | 156 | 1·5 |
| Parity | ||
| Never | 4802 | 45·5 |
| One or more | 5722 | 54·3 |
| Missing | 19 | 0·2 |
| Educational attainment | ||
| High school graduate or less | 2901 | 27·5 |
| Junior college or vocational college graduate | 3703 | 35·1 |
| University graduate or above | 2809 | 26·6 |
| Others | 22 | 0·2 |
| Missing | 1108 | 10·5 |
| Household income | ||
| <4 000 000 (Japanese Yen/year) | 3443 | 32·7 |
| 4 000 000–5 999 999 (Japanese Yen/year) | 3409 | 32·3 |
| ≥6 000 000 (Japanese Yen/year) | 3287 | 31·2 |
| Missing | 404 | 3·8 |
| Cigarette smoking | ||
| Never | 6690 | 63·5 |
| Stopped before pregnancy | 2496 | 23·7 |
| Stopped after pregnancy | 1167 | 11·1 |
| Current | 162 | 1·5 |
| Missing | 28 | 0·3 |
| Alcohol consumption | ||
| Never | 4774 | 45·3 |
| Former | 3592 | 34·1 |
| Current | 2151 | 20·4 |
| Missing | 26 | 0·2 |
| Folic acid supplementation during pregnancy | ||
| Yes | 6265 | 59·4 |
| No | 4265 | 40·5 |
| Missing | 13 | 0·1 |
| Personal PDD history | ||
| Yes | 4 | 0·0 |
| No | 9492 | 90·0 |
| Missing | 1047 | 9·9 |
| Fertility treatment | ||
| Yes | 1238 | 11·7 |
| No | 9280 | 88·0 |
| Missing | 25 | 0·2 |
| HG from conception to early pregnancy | ||
| No | 1483 | 14·1 |
| Just nausea | 4793 | 45·5 |
| Vomiting, but was able to eat | 3197 | 30·3 |
| Vomiting, and was unable to eat | 1055 | 10·0 |
| Missing | 15 | 0·1 |
| HG from early to mid-pregnancy | ||
| No | 7039 | 66·8 |
| Just nausea | 2414 | 22·9 |
| Vomiting, but was able to eat | 985 | 9·3 |
| Vomiting, and was unable to eat | 85 | 0·8 |
| Missing | 20 | 0·2 |
| Paternal characteristics | ||
| Personal PDD history | ||
| Yes | 5 | 0·0 |
| No | 9491 | 90·0 |
| Missing | 1047 | 9·9 |
| Infant characteristics | ||
| Infant sex | ||
| Male | 5468 | 51·9 |
| Female | 5065 | 48·0 |
| Missing | 10 | 0·1 |
| Birth season | ||
| Spring (March–May) | 2655 | 25·2 |
| Summer (June–August) | 2516 | 23·9 |
| Autumn (September–November) | 2776 | 26·3 |
| Winter (December–February) | 2596 | 24·6 |
| Breast-feeding duration | ||
| <6 (months) | 1864 | 17·7 |
| ≥6 (months) | 7056 | 66·9 |
| Missing | 1623 | 15·4 |
| ASQ-3 | ||
| Failed one domain | 1004 | 9·5 |
| Failed two domains | 284 | 2·7 |
| Failed three domains | 154 | 1·5 |
| Failed four domains | 62 | 0·6 |
| Failed five domains | 68 | 0·6 |
| Failed one or more of the domains | 1572 | 14·9 |
ASQ-3, Ages & Stages Questionnaires, Third Edition; HG, hyperemesis gravidarum; PDD, pervasive developmental disorder.
Pre-pregnancy BMI was calculated by dividing the pre-pregnancy weight (kg) by the square of maternal height (m2).
Cut-off scores of each Ages & Stages Questionnaires, Third Edition domain and number of infants below the cut-off score
Cut-off scores of each ASQ-3 domain and number of infants below the cut-off score are shown in Table 2. The cut-off score of communication was lowest and that of fine motor was the highest. The percentage of infants with problem-solving delays was the lowest (4·2 %), while gross motor delays were the highest (5·8 %).
Table 2.
Cut-off scores of each Ages & Stages Questionnaires, Third Edition (ASQ-3) domain and the number of infants below the cut-off score
(Numbers and percentages)
| Cut-off scores* | Number of infants below the cut-off score | ||
|---|---|---|---|
| n | % | ||
| Communication | 18·1 | 548 | 5·2 |
| Gross motor | 35·8 | 612 | 5·8 |
| Fine motor | 36·5 | 455 | 4·3 |
| Problem solving | 28·6 | 438 | 4·2 |
| Personal social | 30·9 | 569 | 5·4 |
Each domain was classified as a failed domain when the score was 2 sd below the mean.
Energy-adjusted fruit and vegetable consumption from pre- to mid-pregnancy and offspring’s developmental delays
In the multivariable models, women in the highest quartile of fruit consumption from early to mid-pregnancy had lower odds of offspring’s developmental delays (OR 0·78; 95 % CI 0·66, 0·92) than women in the lowest quartile (Table 3). In addition, high fruit consumption from early to mid-pregnancy was negatively associated with odds of offspring’s developmental delays (P = 0·004). Women in the highest quartile of vegetable consumption from pre-pregnancy to early pregnancy and from early to mid-pregnancy had lower odds of offspring’s developmental delays (OR 0·74; 95 % CI 0·63, 0·89 and OR 0·70; 95 % CI 0·59, 0·84, respectively) than women in the lowest quartile. High vegetable consumption from pre-pregnancy to early pregnancy and from early to mid-pregnancy was negatively associated with odds of offspring’s developmental delays (P = 0·004 and P < 0·001, respectively). The same results were also obtained in the analysis using GEE (online Supplementary Table S10). In addition, by analysing both of these periods of vegetable consumption in the same model, only vegetable consumption from early to mid-pregnancy was negatively associated with odds of offspring’s developmental delays (P = 0·014) (online Supplementary Table S11). The effect size of vegetable consumption on ASQ-3 score was greater than that of fruit consumption in both periods. Moreover, higher carotene, vitamin C and folate consumption from pre-pregnancy to early pregnancy and these from early to mid-pregnancy were negatively associated with odds of offspring’s developmental delays (online Supplementary Table S12).
Table 3.
Energy-adjusted fruit and vegetable consumption from pre- to mid-pregnancy and offspring’s developmental delays*
(Odd ratios and 95 % confidence intervals)
| From pre- to early pregnancy | From early to mid-pregnancy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quartiles | Daily consumption | Crude | Multivariable model† | Daily consumption | Crude | Multivariable model† | ||||
| OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | |||
| lower, upper | lower, upper | lower, upper | lower, upper | |||||||
| Fruit | ||||||||||
| First quartile | < 76·2 g/d | Reference | Reference | < 73·8 g/d | Reference | Reference | ||||
| Second quartile | 76·3–130·4 g/d | 0·94 | 0·81, 1·10 | 0·90 | 0·77, 1·06 | 73·8– 124·5 g/d | 1·10 | 0·95, 1·28 | 1·09 | 0·93, 1·27 |
| Third quartile | 130·4–199·9 g/d | 1·00 | 0·86, 1·16 | 1·01 | 0·86, 1·19 | 124·6–191·8 g/d | 0·99 | 0·85, 1·15 | 1·01 | 0·86, 1·18 |
| Fourth quartile | ≥ 199·9 g/d | 0·84 | 0·72, 0·98 | 0·86 | 0·73, 1·01 | ≥ 191·8 g/d | 0·75 | 0·64, 0·88 | 0·78 | 0·66, 0·92 |
| P for trend | 0·07 | 0·28 | < 0·001 | 0·004 | ||||||
| Vegetables | ||||||||||
| First quartile | < 100·1 g/d | Reference | Reference | < 95·6 g/d | Reference | Reference | ||||
| Second quartile | 100·1–139·3 g/d | 0·77 | 0·67, 0·89 | 0·75 | 0·64, 0·87 | 95·7–131·4 g/d | 0·91 | 0·79, 1·05 | 0·88 | 0·76, 1·03 |
| Third quartile | 139·4–190·2 g/d | 0·75 | 0·65, 0·88 | 0·77 | 0·65, 0·91 | 131·4–179·4 g/d | 0·83 | 0·72, 0·96 | 0·83 | 0·71, 0·98 |
| Fourth quartile | ≥ 190·3 g/d | 0·70 | 0·61, 0·82 | 0·74 | 0·63, 0·89 | ≥ 179·4 g/d | 0·67 | 0·58, 0·79 | 0·70 | 0·59, 0·84 |
| P for trend | < 0·001 | 0·004 | < 0·001 | < 0·001 | ||||||
HG, hyperemesis gravidarum; PDD, pervasive developmental disorder.
The odds of offspring developmental delays were evaluated using ASQ-3.
Adjusted for maternal age (<25, 25–29, 30–34 and ≥35 years), pre-pregnancy BMI (<18·5; 18·5–24·9 and ≥25·0 kg/m2), parity (never; one or more), educational attainment (high school graduate or less; junior college or vocational college graduate; university graduate or above; others), household income (<4 000 000; 4 000 000–5 999 999; ≥6 000 000 Japanese Yen/year), cigarette smoking (never; stopped before pregnancy; stopped after pregnancy; current), alcohol drinking (never; former; current), folic acid supplementation during pregnancy (yes; no), maternal PDD history (yes; no), fertility treatment (yes; no), HG from conception to early pregnancy or HG from early to mid-pregnancy (no; just nausea; vomiting, but was able to eat; vomiting, and was unable to eat), paternal PDD history (yes; no), infant sex (male; female), birth season (spring; summer; autumn; winter), breast-feeding duration (<6, ≥6 months), frequency of fruit, green vegetable, red and yellow vegetable and light-coloured vegetable consumption in the offspring at age 2 years (never; once or twice a week; three or four times a week; five or six a week; once a day; twice a day; three or more times a day) and total fruit, vegetable, meat, fish, grain, potato, bean, and daily product consumption (in quartiles) except for exposures.
Changes in fruit and vegetable consumption from pre- to mid-pregnancy and offspring’s developmental delays
No significant differences were identified between changes in fruit consumption from pre- to mid-pregnancy and offspring’s developmental delays (Table 4). Compared with women with low vegetable consumption before and during pregnancy (low-low group), women with high consumption only from early to mid-pregnancy (low-high group) and women with high consumption before and during pregnancy (high-high group) had offspring with lower odds of developmental delays (OR 0·84; 95 % CI 0·70, 1·00 and OR 0·82; 95 % CI 0·71, 0·95, respectively). No association was identified in women with high consumption only from pre- to early pregnancy (high-low group) (OR 0·92; 95 % CI 0·77, 1·10). Similar results were also observed in the analysis using GEE (online Supplementary Table S13). Moreover, women with high carotene, vitamin C and folate consumption before and during pregnancy (high-high group) had offspring with lower odds of developmental delays than women with low consumption before and during pregnancy (low-low group) (online Supplementary Table S14).
Table 4.
Changes in fruit and vegetable consumption from pre- to mid-pregnancy and offspring developmental delays*
(Odd ratios and 95 % confidence intervals)
| Quartiles | Crude | Multivariable model† | |||
|---|---|---|---|---|---|
| OR | 95 % CI | OR | 95 % CI | ||
| n | Lower, upper | Lower, upper | |||
| Fruit | |||||
| Low-low‡ | 3732 | Reference | Reference | ||
| Low-high§ | 1539 | 0·86 | 0·73, 1·02 | 0·87 | 0·73, 1·04 |
| High-low|| | 1539 | 1·08 | 0·92, 1·26 | 1·10 | 0·93, 1·30 |
| High-high¶ | 3733 | 0·84 | 0·74, 0·96 | 0·88 | 0·77, 1·01 |
| Vegetables | |||||
| Low-low‡ | 3845 | Reference | Reference | ||
| Low-high§ | 1426 | 0·81 | 0·68, 0·96 | 0·84 | 0·70, 1·00 |
| High-low|| | 1426 | 0·88 | 0·75, 1·04 | 0·92 | 0·77, 1·10 |
| High-high¶ | 3846 | 0·74 | 0·66, 0·84 | 0·82 | 0·71, 0·95 |
ASQ-3, Ages & Stages Questionnaires, Third Edition; HG, hyperemesis gravidarum; PDD, pervasive developmental disorder.
The odds of offspring developmental delays were evaluated using ASQ-3.
Adjusted for maternal age (<25; 25–29; 30–34; ≥35 years), pre-pregnancy BMI (<18·5; 18·5–24·9; ≥25·0 kg/m2), parity (never; one or more), educational attainment (high school graduate or less; junior college or vocational college graduate; university graduate or above; others), household income (<4 000 000; 4 000 000–5 999 999; ≥6 000 000 Japanese Yen/year), cigarette smoking (never; stop before pregnancy; stop after pregnancy; current), alcohol drinking (never; former; current), folic acid supplementation during pregnancy (yes; no), maternal PDD history (yes; no), fertility treatment (yes; no), HG from conception to early pregnancy or HG from early to mid-pregnancy (no; just nausea; vomiting, but was able to eat; vomiting, and was unable to eat), paternal PDD history (yes; no), infant sex (male; female), birth season (spring; summer; autumn; winter), breast-feeding duration (<6, ≥6 months), frequency of fruit, green vegetable, red and yellow vegetable and light-coloured vegetable consumption in the offspring at age 2 years (never; once or twice a week; three or four times a week; five or six a week; once a day; twice a day; three or more times a day) and changes in fruit, vegetable, meat, fish, grain, potato, bean, and daily product consumption (low-low; low-high; high-low; high-high) except for exposures.
Women whose answers to the first and second FFQs were in the first or second quartile.
Women whose answers to the first FFQ were in the first or second quartile and whose answers to the second FFQ were in the third or fourth quartile.
Women whose answers to the first FFQ were in the third or fourth quartile and whose answers to the second FFQ were in the first or second quartile.
Women whose answers to the first and second FFQ were in both the third or fourth quartile.
The associations between fruit and vegetable consumption and each domain of ASQ-3 are presented in online Supplementary Tables S15 and S16.
Discussion
This study investigated the association between fruit and vegetable consumption before and during pregnancy and development of offspring aged 2 years. The results showed that high fruit and vegetable consumption before and during pregnancy was associated with a lower risk of developmental delays in offspring, aged 2 years.
Fruit and vegetables are rich sources of various vitamins, minerals, carotenoids and phenolics(21). Previous studies have shown that folic acid and/or multivitamin intake before and during pregnancy were beneficial for offspring’s development(7–9). Fruit and vegetables abundantly contain pro-vitamin A, vitamin C, vitamin K and folate(21,22), and these three components are most abundantly consumed from vegetables in Japan(23). In the results of the present study, an inverse association between higher consumption of carotene (precursor to vitamin A), vitamin C and folate and offspring’s developmental delays was observed (online Supplementary Table S12). Vitamin A supplementation during pregnancy has been reported to be potentially beneficial in reducing maternal anaemia and maternal infections in vitamin A-deficient areas(24). Another previous study reported that vitamin C may benefit pregnancies with intra-uterine growth retardation and preeclampsia(25). Vitamin C is also involved in the absorption of Fe in the body and therefore may have a useful effect during pregnancy by preventing anaemia. Folate is a methyl donor necessary for DNA synthesis and cell division, and the value of folate for the prevention of neural tube defects is well known(26,27). In fact, anaemia, infections, intra-uterine growth retardation and preeclampsia during pregnancy were reported to be risk factors for offspring’s development(28–32). Therefore, carotene, vitamin C and folate consumption could partly explain the association between fruit and vegetable consumption and offspring’s developmental delays.
Our results showed that vegetable consumption may have a greater influence on offspring’s development than fruit consumption. Vegetables accounted for 53·1 %, 40·0 % and 38·0 % of the dietary vitamin A, vitamin C and folic acid intake, respectively, in Japan(23). Fruit accounted for 5·4, 31·9 and 5·9 % of the dietary vitamin A, vitamin C and folic acid intake, respectively, in Japan(23). In our sub-analyses, the fourth quartile of vegetable consumption had higher carotene and folate consumption than the fourth quartile of fruit consumption in both first and second FFQ (online Supplementary Tables S4 and S5). The fact that vegetable consumption had a greater influence on dietary vitamin intake than fruit consumption may be one reason for vegetable consumption having a greater effect on offspring’s development than fruit consumption.
This study is the first to investigate the relationship between fruit and vegetable consumption, the timing of this consumption and the offspring’s developmental delays. Several previous studies reported that folic acid intake before and during pregnancy reduces offspring’s developmental delays(7,8). Pre-pregnancy folic acid is essential for the development of the central nervous system which begins immediately after conception; therefore, folic acid intake prior to pregnancy is critical(33). However, our results showed that offspring’s developmental delays were lower not only with higher fruit and vegetable consumption before and throughout pregnancy but also with higher consumption from early pregnancy. These results may suggest that fruit and vegetable consumption not only before and during early pregnancy but also throughout pregnancy may play an important role in the development of the central nervous system. The knowledge on the timing of certain food and nutritional component consumption is still limited. Therefore, further research is necessary to provide guidance on appropriate dietary timing and to elucidate the underlying mechanisms.
In addition to fruit, vegetables and the vitamins contained in them, several previous studies reported that fish and PUFA such as DHA and EPA were beneficial for neurodevelopment in offspring(5,6). Comparing fish and unsaturated fatty acid consumption by quartiles of fruit and vegetable consumption, the higher the quartile of vegetable consumption, the higher the fish and unsaturated fatty acid consumption (online Supplementary Tables S2–S5). The difference in fish consumption among the quartiles of vegetable consumption was a maximum of 13·7 g/d (online Supplementary Table S4). However, this difference in consumption is not expected to influence the results of the present analysis because we adjusted for fish consumption as a confounder.
As the result confirming the correlation of the fruit and vegetable consumption before and during pregnancy with the frequency of fruit and vegetable consumption by the offspring aged 2 years showed, no strong correlation was found (online Supplementary Table S1). This suggests that the offspring of mothers who had higher fruit and vegetables did not necessarily consume fruit and vegetables. In other words, the results of this study do not suggest that the amount of fruit and vegetables consumed by the offspring themselves influenced their developmental delay.
This study has several limitations. First, ASQ-3 is a screening tool, not a diagnostic tool, for developmental delays. However, ASQ-3 is considered to have high reliability because it has been validated in many countries around the world and used in a variety of studies(18,34,35). Second, in the present study, the results of ASQ-3 were used only at 2 years of age and it is necessary to study the offspring at later ages as the follow-up study progresses. Third, the first and second FFQ were made by modifying the FFQ used in the Japan Public Health Centre-Based Prospective study(36,37) and were added to the response option ‘constitutionally unable to eat it’ to identify genetic factors. The FFQ used in the Japan Public Health Centre-Based Prospective study was validated in the general Japanese population(36–39), but not in pregnant women. Fourth, it was not possible to clarify the causal relationship between exposures and outcomes because this was an observational study. Fifth, although Fe intake during pregnancy is known to be associated with cognitive function in the offspring, Fe intake during pregnancy could not be taken into account in this study.
The present study had several strengths. The TMM BirThree Cohort Study is a prospective birth cohort design with a large sample size. It was possible to evaluate changes in fruit and vegetable consumption before and during pregnancy and find the association with offspring’s development. Moreover, we could research the long-term effect of fruit and vegetable consumption before and during pregnancy using two kinds of FFQ given at different periods.
In conclusion, fruit and vegetable consumption before and during pregnancy was associated with a lower risk of developmental delays in offspring aged 2 years. Dietary habits before and during pregnancy might be one of the key factors that influence the growth of offspring.
Acknowledgements
We are sincerely grateful to the participants of the TMM BirThree Cohort Study and the staff of the Tohoku Medical Megabank Organization, Tohoku University. The full list of members is available at https://www.megabank.tohoku.ac.jp/english/a201201/. We would also like to thank Editage (www.editage.com) for English language editing.
This work was supported by the Japan Agency for Medical Research and Development (AMED), Japan (grant number, JP20km0105001). KAGOME CO., LTD. provided support in the form of salaries for authors Y. Y., T. Y., S. S. and H. S.
Y. Y. designed the study, analysed the data, wrote the manuscript and was primarily responsible for the final content. T. Obara and S. K. reviewed the drafts. T. Obara, M. I., J. S. and S. K. contributed to data collection. All authors provided critical feedback and approved the final manuscript.
Y. Y., T. Y., S. S., and H. S. are employees of KAGOME CO., LTD. Y. Y. and T. Y. are collaborative researchers of Tohoku University and KAGOME CO., LTD. None of the other authors have reported a conflict of interest related to the study.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0007114521002154.
click here to view supplementary material
References
- 1. Oberklaid F & Efron D (2005) Developmental delay: identification and management. Aust Fam Physician 34, 739–742. [PubMed] [Google Scholar]
- 2. Global Research on Developmental Disabilities Collaborators (2018) Developmental disabilities among children younger than 5 years in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Glob Health 6, e1100–e1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Keogh BK, Bernheimer LP & Guthrie D (2004) Children with developmental delays twenty years later : where are they? how are they? Am J Ment Retard 109, 219–230. [DOI] [PubMed] [Google Scholar]
- 4. Villagomez AN, Muñoz FM, Peterson RL, et al. (2019) Neurodevelopmental delay: case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 37, 7623–7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hamazaki K, Matsumura K, Tsuchida A, et al. (2020) Maternal dietary intake of fish and PUFAs and child neurodevelopment at 6 months and 1 year of age: a nationwide birth cohort-the Japan Environment and Children’s Study (JECS). Am J Clin Nutr 112, 1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Starling P, Charlton K, McMahon AT, et al. (2015) Fish intake during pregnancy and foetal neurodevelopment: a systematic review of the evidence. Nutrients 7, 2001–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmidt RJ, Tancredi DJ, Ozonoff S, et al. (2012) Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr 96, 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levine SZ, Kodesh A, Viktorin A, et al. (2018) Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatr 75, 176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmidt RJ, Hansen RL, Hartiala J, et al. (2011) Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology 22, 476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yonezawa Y, Obara T, Yamashita T, et al. (2020) Fruit and vegetable consumption before and during pregnancy and birth weight of new-borns in Japan: the Tohoku medical megabank project birth and three-generation cohort study. Nutr J 19, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jang W, Kim H, Lee BE, et al. (2018) Maternal fruit and vegetable or vitamin C consumption during pregnancy is associated with fetal growth and infant growth up to 6 months: results from the Korean Mothers and Children’s Environmental Health (MOCEH) cohort study. Nutr J 17, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Willers SM, Devereux G, Craig LC, et al. (2007) Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax 62, 773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miyake Y, Sasaki S, Tanaka K, et al. (2010) Consumption of vegetables, fruit, and antioxidants during pregnancy and wheeze and eczema in infants. Allergy 65, 758–765. [DOI] [PubMed] [Google Scholar]
- 14. Kuriyama S, Metoki H, Kikuya M, et al. (2019) Cohort profile: tohoku medical megabank project birth and three-generation cohort study (TMM BirThree Cohort Study): rationale, progress and perspective. Int J Epidemiol 49, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuriyama S, Yaegashi N, Nagami F, et al. (2016) The tohoku medical megabank project: design and mission. J Epidemiol 26, 493–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ministry of Education, Culture, Sports, Science and Technology (2005) Standard tables of food composition in japan fifth revised and enlarged edition -2005- (Japanese). https://www.mext.go.jp/b_menu/shingi/gijyutu/gijyutu3/toushin/05031802.htm (accessed November 2020).
- 17. Willett W & Stampfer MJ (1986) Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 124, 17–27. [DOI] [PubMed] [Google Scholar]
- 18. Mezawa H, Aoki S, Nakayama SF, et al. (2019) Psychometric profile of the ages and stages questionnaires, Japanese translation. Pediatr Int 61, 1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Squires J, Twombly E, Bricker D, et al. (2009) ASQ-3 User’s Guide. Baltimore: Brookes Publishing. [Google Scholar]
- 20. Buuren SV (2020) Package ‘mice’. https://cran.r-project.org/web/packages/mice/mice.pdf. (accessed November 2020).
- 21. Liu RH (2013) Health-promoting components of fruits and vegetables in the diet. Adv Nutr 4, 384S–392S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Slavin JL & Lloyd B (2012) Health benefits of fruits and vegetables. Adv Nutr 3, 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ministry of Education, Culture, Sports, Science and Technology (2020) The National Health and Nutrition Survey -2018- (Japanese). https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/eiyou/h30-houkoku_00001.html (accessed November 2020).
- 24. van den Broek N, Dou L, Othman M, et al. (2010) Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database Syst Rev 11, CD008666. [DOI] [PubMed] [Google Scholar]
- 25. Myatt L & Cui X (2004) Oxidative stress in the placenta. Histochem Cell Biol 122, 369–382. [DOI] [PubMed] [Google Scholar]
- 26. Crider KS, Bailey LB & Berry RJ (2011) Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients 3, 370–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jägerstad M (2012) Folic acid fortification prevents neural tube defects and may also reduce cancer risks. Acta Paediatr 101, 1007–1012. [DOI] [PubMed] [Google Scholar]
- 28. Wiegersma AM, Dalman C, Lee BK, et al. (2019) Association of prenatal maternal anemia with neurodevelopmental disorders. JAMA Psychiatr 76, 1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zerbo O, Qian Y, Yoshida C, et al. (2017) Association between influenza infection and vaccination during pregnancy and risk of autism spectrum disorder. JAMA Pediatr 171, e163609. [DOI] [PubMed] [Google Scholar]
- 30. Croen LA, Qian Y, Ashwood P, et al. (2019) Infection and fever in pregnancy and autism spectrum disorders: findings from the study to explore early development. Autism Res 12, 1551–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leonard H, Nassar N, Bourke J, et al. (2008) Relation between intrauterine growth and subsequent intellectual disability in a ten-year population cohort of children in Western Australia. Am J Epidemiol 167, 103–111. [DOI] [PubMed] [Google Scholar]
- 32. Walker CK, Krakowiak P, Baker A, et al. (2015) Preeclampsia, placental insufficiency, and autism spectrum disorder or developmental delay. JAMA Pediatr 169, 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roffman JL (2018) Neuroprotective effects of prenatal folic acid supplementation: why timing matters. JAMA Psychiatr 75, 747–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chong KC, Zhou VL, Tarazona D, et al. (2017) ASQ-3 scores are sensitive to small differences in age in a Peruvian infant population. Child Care Health Dev 43, 556–565. [DOI] [PubMed] [Google Scholar]
- 35. Agarwal PK, Xie H, Sathyapalan Rema AS, et al. (2020) Evaluation of the Ages and Stages Questionnaire (ASQ 3) as a developmental screener at 9, 18, and 24 months. Early Hum Dev 147, 105081. [DOI] [PubMed] [Google Scholar]
- 36. Sasaki S, Kobayashi M, Tsugane S, et al. (2003) Validity of a self-administered food frequency questionnaire used in the 5-year follow-up survey of the JPHC Study Cohort I: comparison with dietary records for food groups. J Epidemiol 13, S57–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsugane S, Kobayashi M, Sasaki S, et al. (2003) Validity of the self-administered food frequency questionnaire used in the 5-year follow-up survey of the JPHC Study Cohort I: comparison with dietary records for main nutrients. J Epidemiol 13, S51–S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Iso H, Moriyama Y, Yoshino K, et al. (2003) Validity of the self-administered food frequency questionnaire used in the 5-year follow-up survey for the JPHC Study to assess folate, vitamin B6 and B12 intake: comparison with dietary records and blood level. J Epidemiol 13, S98–S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kobayashi M, Sasaki S, Tsugane S, et al. (2003) Validity of a self-administered food frequency questionnaire used in the 5-year follow-up survey of the JPHC Study Cohort I to assess carotenoids and vitamin C intake: comparison with dietary records and blood level. J Epidemiol 13, S82–S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0007114521002154.
click here to view supplementary material