Abstract
The use of human immunodeficiency virus (HIV) protease inhibitors in children has lagged behind that in adults because of the lack of suitable pediatric formulations and information on safe and effective dosing regimens. This study was designed to obtain pharmacokinetic information on indinavir, administered to HIV-infected children also receiving therapy with two nucleoside agents, and to explore relationships between pharmacokinetic parameters and anti-HIV effect. Indinavir was initiated at a dose of 500 mg/m2 every 8 h. Plasma indinavir concentrations were measured every 4 weeks; the dose or dosing interval was adjusted to maintain trough concentrations of ≥0.1 mg/liter. All children were evaluated clinically at baseline and every 4 weeks. Plasma HIV RNA was quantitated at baseline and at weeks 4, 12, and 24. Eighteen children participated in this study. The average daily dose of indinavir was 2,043 mg/m2; nine children received indinavir at 6-h intervals. Pharmacokinetic characteristics of indinavir (mean ± standard deviation) were the following: oral clearance, 1.4 ± 0.5 liters/h/kg; half-life, 1.1 ± 0.43 h; and trough concentration, 0.29 ± 0.32 mg/liter. In nine children that completed 24 weeks of therapy, the baseline-to-week-24 change in HIV RNA level was related to indinavir trough concentration and didanosine area under the curve. This study illustrates the ability to obtain pharmacokinetic information from children during routine clinic visits and to use this information to provide a safeguard against underdosing. The incorporation of pharmacologic knowledge with virologic, immunologic, and behavioral considerations should result in improved clinical outcomes for children infected with HIV.
Combination therapy with an inhibitor of human immunodeficiency virus (HIV) protease and two nucleoside HIV reverse transcriptase inhibitors has become the standard of care for many HIV-infected adults. The use of protease inhibitors in children has lagged behind that in adults because of the lack of suitable pediatric drug formulations and information on safe and effective dosing regimens. Even so, combination therapy including protease inhibitors is recommended as initial therapy for HIV-infected children (5). Indinavir is one of five protease inhibitors currently available to adults. This study was designed to obtain pharmacokinetic information on indinavir, administered to HIV-infected children receiving concomitant therapy with didanosine and stavudine, and to explore relationships between pharmacokinetic parameters and antiviral effect.
MATERIALS AND METHODS
Patients and study design.
This pharmacologic study was conducted with children receiving indinavir. Twelve of the children participated in an open trial of combination therapy with indinavir, didanosine, and stavudine. Virologic, immunologic, and safety information for all 12 and first-dose pharmacokinetic data for 5 of these children have been previously reported (9). Briefly, the patients enrolled in this pilot study included HIV-infected children who were able to swallow capsules consistently and who had a history of good compliance with prescribed medication regimens and scheduled clinic visits. The presence of symptomatic HIV disease (Centers for Disease Control and Prevention [CDC] clinical category A, B, or C) or immunosuppression (CDC immunologic category 2 or 3) and a history of at least 1 year of nucleoside antiretroviral therapy were required (4). The following baseline laboratory values were required: a hemoglobin concentration of 7 g/dl or greater; a polymorphonuclear leukocyte count of at least 400/μl; a platelet count of at least 50,000/μl; aspartate aminotransferase, alanine aminotransferase, and bilirubin less than 10 times the upper limit of normal; and a normal serum creatinine concentration. Additional children were eligible to participate in this pharmacologic study if they were receiving indinavir in combination with nucleoside antiretroviral drugs. There was no requirement for prior antiretroviral therapy or CDC clinical or immunologic category in these children.
In all children, indinavir therapy was initiated at a dose of 500 mg/m2 every 8 h; standard pediatric doses were used for other concomitantly prescribed antiretroviral agents. Standard approved formulations of all the drugs were employed. All patients received prophylaxis for Pneumocystis carinii pneumonia, and nutritional support and antibiotic therapy were prescribed as needed. The use of immunomodulators, antiretroviral agents other than the study drugs, and agents known to interact with indinavir (e.g., rifampin, rifabutin, and ketoconazole) was prohibited. The study was approved by the Review Board for Human Subject Research at Baylor College of Medicine. Informed consent was obtained from each subject's parent or legal guardian. In the case of children 7 years of age or older, the assent of the minor subject also was obtained.
Clinical and laboratory monitoring.
All children were evaluated clinically at baseline and every 4 weeks thereafter. The complete blood count, routine blood chemistries (including creatinine, aspartate aminotransferase, alanine aminotransferase, bilirubin, amylase, and creatine phosphokinase), and urinalysis were monitored at baseline and every 4 weeks thereafter. Immunologic monitoring included lymphocyte monoclonal antibody phenotyping at baseline and at weeks 4, 12, and 24. Plasma HIV RNA concentrations were measured by a PCR (Roche Molecular Systems, Inc., Branchburg, N.J.) at baseline and at weeks 4, 12, and 24. Four children with undetectable plasma HIV RNA levels underwent lumbar puncture after study week 12 for measurement of cerebrospinal fluid (CSF) HIV RNA and antiretroviral drug concentrations.
Pharmacokinetic analyses.
A single timed blood sample was obtained from each child during a routine clinic visit within a window of 3 to 8 h after an indinavir dose at least every 4 weeks throughout the study. Five children (as previously described) received the simultaneous administration of indinavir, didanosine, and stavudine on study day 2, and serial blood samples were collected. No dosing recommendation or published experience with indinavir in children was available at the time this study was initiated. Therefore, indinavir concentrations in plasma were determined within 2 weeks of the samples being obtained, and doses were adjusted in each child to achieve a trough concentration of ≥0.1 mg/liter. This value was selected because it is approximately the average trough observed in adults receiving the standard dose of 800 mg every 8 h recommended by the manufacturer (Crixivan [indinavir sulfate] package insert; Merck and Co., Inc., West Point, Pa.). A proportional adjustment of the dose or a change in the dosing interval was done as necessary to achieve the target trough concentration. Trough concentrations were either directly measured or extrapolated from the measured concentration by using an elimination half-life determined for the individual child. A population half-life of 1 h was used if sufficient data had not been obtained from which to determine an individualized half-life.
A reverse-phase high-pressure liquid chromatography (HPLC) method was developed to quantitate indinavir concentrations. The mobile phase consisted of a 35:65 (vol/vol) solution of acetonitrile in a 50 mM phosphoric acid o-phosphoric acid buffer adjusted to a pH of 3.10 with triethylamine. A liquid-liquid extraction procedure was used to separate compounds of interest from the plasma fractions of samples, standards, and quality controls. Twenty microliters of the appropriate standard solutions was added to 250 μl of plasma. Plasma samples and blank plasma (250 μl) were mixed with 20 μl of the internal standard solution (30 μg of L-738,804 per ml) and extracted with 2 ml of tert-butyl ether. After mixing and centrifugation, the aqueous plasma layer was flash-frozen in a dry ice–2-propanol bath for 5 min. The organic layer was decanted and evaporated under N2 with an N-Evap dryer at 40°C. The residue was reconstituted with 200 μl of HPLC mobile phase. A 50-μl aliquot of the reconstituted extract was injected onto the column. Indinavir and the internal standard were separated on a 250- by 4.6-mm, 5-μm Spherisorb C8 cartridge column. The flow rate was set at 1.25 ml/min on a Shimadzu LC-600 pump, and the column temperature was maintained at 40°C with a column heater. The compounds were detected at 210 nm on a Shimadzu SPD-6AV variable-wavelength UV detector. A 7-point standard curve (run in triplicate) was constructed over the range of 0.02 to 16 mg/liter (0.03 to 13 μM). Quality control samples were prepared at concentrations of 0.1, 0.5, and 5 mg/liter. The lower limit of quantitation was 0.02 mg/liter with a coefficient of variation (CV) of <10% at all concentrations. Interday and intraday CVs were less than 7 and 5%, respectively, for each of the three quality control samples.
Didanosine and stavudine were quantitated by a validated simultaneous HPLC procedure. The mobile phase consisted of 5% acetonitrile in 50 mM ammonium phosphate buffer adjusted to pH 6.75; the flow rate was 1.0 ml/min. A Shimadzu LC-600 HPLC system was used with a Shimadzu SIL-9A automatic injector, and detection was via a Shimadzu UV detector set at 266 nm. A solid-phase extraction procedure (3M Empore C18 extraction disc cartridges [7 mm and 3 ml]) was used to extract 200 μl of plasma, standards, quality controls, and unknown samples. All plasma samples were spiked with 25 μl of β-hydroxyethyl-theophylline as the internal standard. A 50-μl injection volume was delivered to a Waters Spherisorb C8 column (250- by 4.6-mm [inside diameter]; 5-μm particle size) for separation at 40°C. The retention times for stavudine, didanosine, and β-hydroxethyl-theophylline were approximately 5, 6, and 7.7 min, respectively. An eight-point standard curve (run in triplicate) was determined for stavudine and didanosine. The standards ranged from 0.02 to 4.48 mg/liter (0.10 to 20 μM) for stavudine and 0.01 to 2.36 mg/liter (0.05 to 10 μM) for didanosine. Validation criteria were determined using quality control samples at three levels run in triplicate. For stavudine, the within- and between-day variabilities ranged from 3.1 to 6.7% and 4.2 to 8.2%, respectively. The within- and between-day variabilities for didanosine ranged from 3.8 to 10.3% and 4.2 to 12.0%, respectively. Quantification of indinavir, didanosine, and stavudine in CSF was accomplished by modifying the assays described above to use a simulated CSF for the standard and quality control matrix. The simulated CSF consisted of 0.9% NaCl, 0.24 mM phosphoric acid, and 0.5% EDTA-derived human plasma; the final pH was adjusted to 7.4.
Indinavir, didanosine, and stavudine pharmacokinetic parameters were determined with the nonlinear mixed-effects model program NONMEM (version IV, level 2.1) (13). The pharmacokinetic parameters of each drug were determined independently from those of the other two drugs; however, all concentrations available for each drug from all children were simultaneously analyzed. A one-compartment oral absorption model was assumed for each drug. Several models of drug input were explored, including zero- and first-order absorption processes and absorption lag times. During modeling of the zero-order absorption process, the duration of a constant-rate input required to deliver the nominal dose was estimated. The variances of the log of the pharmacokinetic parameters were modeled to be constant, thus providing estimates of variability in terms of a CV. Off-diagonal covariances were not estimated. Residual variability was estimated using a proportional error model. The first-order conditional estimation procedure was used in the final NONMEM analysis for each drug. This method provides Bayesian estimates of individualized parameter values for each subject. Relationships between changes in HIV RNA levels and indinavir, didanosine, and stavudine concentrations in plasma were explored with regression analysis in only the 12 children that participated in the open trial. If relationships were found, Monte Carlo simulation studies were then performed to determine the proportion of children that might be expected to maintain systemic exposure at or above the estimated pharmacodynamic target level. Each simulation study used the population pharmacokinetic parameters determined under the final pharmacostatistical model. Simulations of 500 children per drug with demographics consistent with those of the children enrolled in this study were obtained.
RESULTS
Eighteen children participated in the pharmacokinetic evaluation of indinavir; 12 of these children were enrolled in the original study of indinavir, stavudine, and didanosine. The other 6 children were receiving indinavir and dual nucleoside therapy; 6 were receiving stavudine, 4 were receiving didanoine, and 2 were receiving lamivudine. All children were protease inhibitor treatment naïve at the time indinavir therapy was initiated. The 18 children in this pharmacokinetic study fall into the following categories: female, 10; male, 8; white, 4; black, 11; Hispanic, 3. Their distribution among CDC clinical and immunologic categories at the time this study began was as follows: N2, 1; A2, 1; B2, 2; A3, 7; B3, 1; and C3, 6. The mean age of these children was 9.2 years (range, 4 to 17 years). The average dose of indinavir in these children at week 12 of therapy (after any pharmacokinetic-based dose or dosing interval adjustment) was 2,043 mg/m2/day; 9 children received indinavir at 6-h intervals, and 9 at 8-h intervals. For didanosine, the average dose was 171 mg/m2/day; the average stavudine dose was 1.8 mg/kg of body weight/day. Didanosine and stavudine were both administered twice daily.
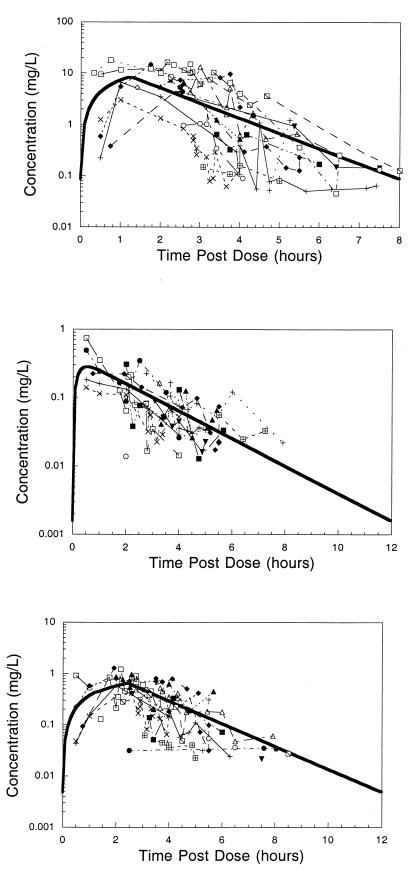
Differences in pharmacokinetic behavior and analytical sensitivity among the three drugs resulted in a varying number of concentrations and patients available for analysis. In the final analysis, 123 indinavir concentrations were available from 18 patients. For didanosine, 14 patients provided 84 concentrations, and for stavudine, 100 concentrations were available from 17 patients. Table 1 gives the population pharmacokinetic parameter point estimates and approximate 95% confidence intervals from the NONMEM analyses. The absorption of didanosine was rapid, and few data were available to characterize the absorption process. Hence, the absorption was assumed to be first order, and the rate constant was fixed at 5.0 h−1. Indinavir and stavudine both conformed better to a zero-order absorption process, and the duration of absorption was estimated in NONMEM. Incorporating an absorption lag time did not improve any of the models. Figure 1 shows composite plots of concentration-time data for indinavir, didanosine, and stavudine. Data points are connected by patient in time order, but that is not to imply that the data result from a single intensively sampled profile. With the exception of the 5 patients with areas under the curve (AUCs) obtained on day 2, all data points were obtained as single timed postdose samples. Table 2 presents summary statistics of the individual Bayesian post hoc parameter values.
TABLE 1.
NONMEM pharmacokinetic parametersa
| Parameter | Result (approximate 95% confidence interval)
|
||
|---|---|---|---|
| Indinavir | Didanosine | Stavudine | |
| CL/F (liters/h/kg) | 1.3 (0.8, 1.8) | 4.64 (3.72, 5.56) | 0.54 (0.35, 0.73) |
| VD/F (liters/h/kg) | 1.92 (1.12, 2.72) | 10.1 (5.57, 14.6) | 1.05 (0.57, 1.53) |
| Half-life (h) | 1.0 | 1.5 | 1.4 |
| Ka (h−1) or Tdur (h) | Tdur, 1.26 (0.72, 1.8) | Ka (fixed), 5.0 | Tdur, 2.43 (1.81, 3.06) |
| Variability CL/F (% CV) | 41 | 17 | 49 |
| Variability VD/F (% CV) | NE | 35 | 45 |
| Residual variability (% CV) | 70 | 48 | 49 |
Abbreviations: CL/F, apparent oral clearance; VD/F, apparent volume of distribution; Ka, first-order absorption rate constant; Tdur, duration of zero-order absorption; NE, not estimated.
FIG. 1.
Composite plots of concentration-versus-time postdose data for indinavir (top), didanosine (middle) and stavudine (bottom). Data points are connected by patient in time order and do not imply that the data result from a single intensively sampled profile. The bold line represents the profile for the standard-size individual receiving approximately 500 mg of indinavir/m2 every 8 h, 90 mg of didanosine/m2 every 12 h, and 1 mg of stavudine/kg every 12 h in this study.
TABLE 2.
Pharmacokinetic characteristicsa
| Drug (n) | Doseb
|
AUC0–τ (mg · h/liter) | CL/F
|
t1/2 (h) | Cmax (mg/liter) | Cmin (mg/liter) | ||
|---|---|---|---|---|---|---|---|---|
| mg/kg/d | mg/m2/d | liters/h/kg | liters/h/m2 | |||||
| Indinavir (18) | ||||||||
| Mean | 75 | 2,043 | 17.7 | 1.41 | 39 | 1.1 | 7.3 | 0.29 |
| SD | 22 | 490 | 10.1 | 0.50 | 13.2 | 0.43 | 2.8 | 0.32 |
| CV | 29 | 24 | 57 | 36 | 34 | 40 | 38 | 110 |
| Min | 39 | 954 | 7.8 | 0.63 | 15 | 0.6 | 2.6 | 0.01 |
| Max | 109 | 2,673 | 40 | 2.37 | 68 | 2.1 | 13.5 | 1.28 |
| Didanosine (16) | ||||||||
| Mean | 6.48 | 171 | 0.73 | 3.9 | 120.5 | 1.52 | 0.48 | ND |
| SD | 1.26 | 27.2 | 0.16 | 1.4 | 23.1 | 0.41 | 0.43 | ND |
| CV | 19 | 16 | 22 | 36 | 19 | 27 | 89 | ND |
| Min | 4.4 | 120 | 0.47 | 1.5 | 93 | 0.73 | 0.16 | ND |
| Max | 8.6 | 222 | 0.96 | 6 | 182.2 | 2.4 | 1.56 | ND |
| Stavudine (18) | ||||||||
| Mean | 1.8 | 49 | 1.83 | 0.59 | 15.9 | 1.41 | 0.51 | ND |
| SD | 0.24 | 7.8 | 0.86 | 0.26 | 7 | 0.62 | 0.19 | ND |
| CV | 13 | 16 | 47 | 44 | 44 | 44 | 37 | ND |
| Min | 1.36 | 31.8 | 0.77 | 0.27 | 7.49 | 0.6 | 0.29 | ND |
| Max | 2.2 | 63.2 | 3.26 | 1.17 | 33.2 | 2.9 | 0.89 | ND |
n, number of patients; AUC0–τ, AUC curve within a dosing interval; CL/F, apparent oral clearance; t1/2, elimination half life; Cmax, maximum concentration in plasma; Cmin, trough concentration in plasma; ND, not determined, as average trough concentrations were ≤0.01 mg/liter; d, day.
Dose at week 24.
CSF samples were obtained from 4 children receiving indinavir, didanosine, and stavudine between 2.5 and 7.5 h following a dose of all three agents administered simultaneously; plasma was obtained within 10 min of CSF collection. The indinavir concentrations in CSF have been previously reported (9) and ranged from 0.15 to 0.98 mg/liter. The ratio of CSF-to-plasma concentrations ranged from 0.03 at 2.5 h postdose to 0.94 at 7.5 h postdose. Didanosine was measurable in the CSF of only 1 child, with a concentration of 0.01 mg/liter and a CSF-to-plasma concentration ratio of 0.17 at 2.5 h postdose. Stavudine was measurable in all CSF samples, with concentrations ranging from 0.13 to 0.17 (average ± standard deviation, 0.15 ± 0.02) mg/liter; the CSF-to-plasma concentration ratios ranged from 0.19 at 2.5 h postdose to 1.27 at 7.5 h postdose.
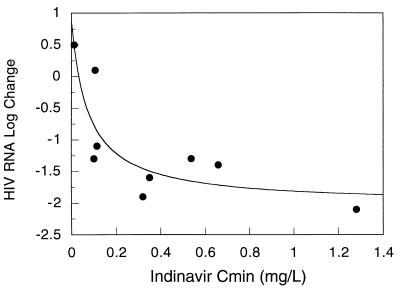
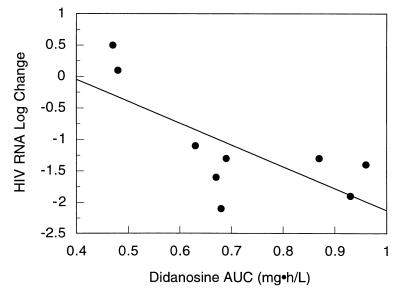
Pharmacodynamic relationships were explored in 9 children. These children were enrolled in the original pilot trial of indinavir, didanosine, and stavudine, and all completed 24 weeks of therapy. Of 3 children in the original study who did not complete 24 weeks of therapy, 2 were withdrawn at the request of the parent or guardian and 1 was withdrawn because of noncompliance. In the 9 children, the log10 change in plasma HIV RNA levels from baseline to week 24 of therapy was related in an inverse manner to the AUC and trough concentration of indinavir and the AUC of didanosine. There was no statistically significant relationship between the stavudine AUC and change in HIV RNA levels (r2 = 0.3; P = 0.12). For indinavir, the relationship was strongest for trough concentration (r2 = 0.73, versus 0.43 for AUC) and was best described with a maximal inhibitory effect model (Fig. 2). Using this model, the estimated value for maximal suppression of plasma HIV RNA was 2 log; an indinavir trough concentration of 0.08 mg/liter was associated with a 50% effect, or a 0.58 log10 drop in HIV RNA from baseline to week 24. The relationship between the didanosine AUC and changes in plasma HIV RNA concentrations is shown in Fig. 3. In a stepwise regression analysis of indinavir trough, didanosine and stavudine AUC, and the baseline-to-week-24 change in HIV RNA level, the final model included indinavir trough concentration and didanosine AUC (r2 = 0.65; P = 0.018). Seven of the 9 children in the pharmacodynamic analysis had a >1-log10 drop in plasma HIV RNA levels after 24 weeks of therapy. All 7 had indinavir trough concentrations of ≥0.1 mg/liter (range, 0.1 to 1.28 mg/liter) and didanosine AUCs of >0.6 mg · h/liter (range, 0.63 to 0.96 mg · h/liter). The remaining 2 children had an average increase of 0.3 log in plasma HIV RNA. These two children had indinavir troughs of 0.1 and <0.02 mg/liter and didanosine AUCs of 0.48 and 0.47 mg · h/liter.
FIG. 2.
Trough concentrations (Cmin) of indinavir versus baseline-to-week-24 change in plasma HIV RNA levels. The solid line represents the line of best fit as determined with a maximal inhibitory effect model. The estimated parameters of this line are the following: baseline effect, 0.87; maximal inhibitory effect, 2.89; and concentration at 50% effect, 0.077 mg/liter.
FIG. 3.
Didanosine AUC versus baseline-to-week-24 changes in plasma HIV RNA levels. The solid line represents the line of best fit as determined with linear regression; the equation for this line is y = 1.337 − (3.47 × AUC). r2 = 0.51; P = 0.03.
Monte Carlo simulations were performed to determine the percentage of children that might be expected to maintain indinavir trough concentrations above 0.1 mg/liter and didanosine AUCs above 0.6 mg · h/liter. When given an indinavir regimen of 500 mg/m2 every 8 h (doses were rounded to 400, 600, or 800 mg), only 28% of the 500 children in the simulation achieved a trough concentration at steady state of >0.1 mg/liter. An indinavir regimen of 500 mg/m2 every 6 h was predicted to result in 52% of children attaining trough concentrations of >0.1 mg/liter. The simulation study with a didanosine regimen of 90 mg/m2 every 12 h found that 57% of subjects would have an AUC of >0.6 mg · h/liter. A didanosine regimen of 120 mg/m2 every 12 h increased the percentage to 88.
DISCUSSION
The pharmacokinetic characteristics of indinavir determined in these 18 children are generally consistent with the limited data reported in the literature (11). The pharmacokinetic characteristics determined for didanosine and stavudine are similarly comparable with data from pediatric investigations (2, 7, 8, 10). All concentration data from this study arise from subjects with a range of weights, oral clearances, and dosing regimens. In the case of indinavir, these dosing regimens vary not only across subjects but also for each subject. The composite plots of concentration versus time-postdose data (Fig. 1) illustrate that the curves generated from typical pharmacokinetic parameters are reasonably characteristic of the observed data.
In three children that participated in a phase I study of indinavir, the 8-h AUC at a dose of 500 mg/m2 ranged between 14.4 and 18.3 mg · h/liter, and the estimated apparent oral clearance was between 27.3 and 34.7 liters/h/m2; indinavir maximum concentrations ranged from 2.8 mg/liter to 8.8 mg/liter. In this same study, the estimated apparent oral clearance in eight children receiving a dose of 350 mg/m2 was 32 liters/h/m2. The terminal half-life of indinavir averaged 0.9 h (11). The mean AUC for indinavir in this study was 17.7 mg · h/liter, the apparent oral clearance was 39 liters/h/m2, the mean maximum concentration in plasma was 7.3 mg/liter, and the half-life was 1.1 h. In this study, we attempted to administer an indinavir dose to children by using commercially available dosage forms, which would maintain trough concentrations of ≥0.1 mg/liter. We found that 50% of the children in this study required a more frequent dosing interval. After indinavir dose adjustments, the mean indinavir trough concentration was 0.29 mg/liter, and concentrations ranged from 0.01 to 1.28 mg/liter. Even with a change in dosing interval, five children still had trough concentrations of <0.1 mg/liter.
Sixteen children received concomitant therapy with didanosine, and 14 took some indinavir and didanosine doses simultaneously. This was allowed based upon our earlier finding with five children that coadministration of didanosine with indinavir did not reduce the concentrations of indinavir, in contrast with the substantial reduction reported in adults (9; Crixivan package insert [Merck and Co., Inc.]). The consistency of the indinavir pharmacokinetic characteristics in these 18 children with those described in the literature (not receiving coadministration with didanosine) is further support that indinavir pharmacokinetic behavior was not substantially altered. The reasons for this discrepant finding are unknown. We would recommend that clinicians not coadminister indinavir with didanosine unless they can document with measured indinavir concentrations that pharmacokinetic disposition is unaltered by simultaneous administration.
In the present study, the average AUC and apparent oral clearance for didanosine were 0.73 mg · h/liter and 132 liters/h/m2. In a study of children receiving a didanosine dose of 90 mg/m2, the AUC was reported to average between 0.41 and 0.78 mg · h/liter (2, 10). From these data, the apparent oral clearance of didanosine is estimated to be 116 to 220 liters/h/m2. In a phase I study of stavudine monotherapy in children, the average apparent oral clearance at a dose of 1 mg/kg was estimated at 16 to 19 liters/h/m2 (7). The elimination half-life averaged 1.13 h, and the mean stavudine AUC was 1.63 mg · h/liter. In the 18 children in our study, the average apparent oral clearance was 15.9 liter/h/m2, and the average AUC was 1.83 mg · h/liter.
Appreciable concentrations of indinavir were found in the CSF of 4 children, with CSF-to-plasma ratios of 0.03 to 0.94. Indinavir has also been measured in the CSF of adults (S. L. Letendre, E. Capparelli, R. J. Ellis, D. Dur, and J. A. McCutchan, Abstr. 6th Conf. Retrovir. Opportunistic Infect. abstr. 407, 1999). In 22 individuals at steady state on an indinavir-containing regimen and free of opportunistic infections, the median indinavir concentration in CSF was 0.055 mg/liter (range, 0.016 to 0.18), and the median CSF-to-serum concentration ratio was 0.14 (range, 0 to 2.28). Both didanosine and stavudine have been previously shown to reach detectable concentrations in the CSF of children. CSF samples were obtained 2 h after an oral dose of didanosine in 20 children; concentrations in CSF were measurable in only three children (2). Two children receiving a didanosine dose of 180 mg/m2 had concentrations in CSF of 0.095 and 0.083 mg/liter; one child receiving a dose of 90 mg/m2 had a concentration in CSF of 0.035 mg/liter (2). Concentrations in CSF were obtained 2 to 3 h postdose in seven children receiving either 0.25, 1, or 2 mg of oral stavudine per kg per day (7). Stavudine concentrations in the CSF ranged from 0.008 to 0.12 mg/liter; CSF penetration ranged from 16 to 97% of concomitant concentrations in plasma. At the dose of 1 mg/kg twice daily, the concentrations of stavudine in the CSF of two children were 0.063 and 0.12 mg/liter.
Pharmacodynamic relationships were explored only with the children that participated in the open pilot study of combination therapy with indinavir, didanosine, and stavudine. Only these children met standardized entry criteria of prior antiretroviral therapy and CDC clinical and immunologic category. Nine of these twelve children completed 24 weeks of therapy with indinavir, didanosine, and stavudine. There was a strong relationship between the baseline-to-week-24 fall in plasma HIV RNA and trough indinavir concentrations and didanosine AUC. The finding in this study of a relationship between indinavir trough concentrations and suppression of HIV RNA supports the use of trough concentrations as a relevant determinant of effect for this drug. The optimal trough concentration of indinavir, however, cannot be determined from this study with children. Our decision to target trough concentrations of more than 0.1 mg/liter is supported by the relationship found in these children predicting that at a trough concentration of <0.1 mg/liter, the suppression of plasma HIV RNA at 24 weeks would be <1 log10. Five children completed 48 weeks of therapy with indinavir, didanosine, and stavudine. Four of the five maintained a >1-log10 suppression in plasma HIV RNA from baseline, while one had a 0.9-log10 increase from baseline at week 48. All four children that maintained viral suppression had indinavir trough concentrations of >0.1 mg/liter and didanosine AUCs of >0.6 mg · h/liter. The child that had a rebound in viral load had an indinavir trough concentration of <0.02 mg/liter (model estimated concentration was 0.01 mg/liter) and a didanosine AUC of 0.47 mg · h/liter.
The observation of a relationship between indinavir trough concentration and antiviral effect has been reported by other investigators (1, 6, 14). For example, in 23 protease inhibitor-naïve adults that received an indinavir-containing antiretroviral regimen, those that had suppression of plasma HIV RNA to undetectable levels had higher trough indinavir concentrations than did those with plasma HIV RNA that remained detectable (1). The average trough concentration in the group with undetectable levels of plasma HIV RNA was 0.15 mg/liter versus 0.03 mg/liter in the detectable group (P = 0.007). The finding of a relationship between the trough concentrations of indinavir and change in plasma HIV RNA levels is consistent also with observations for children receiving the protease inhibitor ritonavir. In a study of 41 children, trough ritonavir concentrations were found to be an important predictor of anti-HIV response (12). Although the active moiety of didanosine is an intracellular triphosphate, relationships between plasma didanosine concentrations and anti-HIV effect have been observed (2, 3). For example, an evaluation of didanosine monotherapy in children found a relationship between didanosine AUC and response. Those children who responded with a decrease in HIV antigen and an improvement in IQ score had a higher didanosine AUC than did the nonresponders (0.46 mg · h/liter versus 0.19 mg · h/liter) (2). No pharmacodynamic relationship between stavudine and a drop in viral load was detected in this study. There was a trend of greater suppression of plasma HIV RNA with a higher stavudine AUC, but there was no statistically significant relationship (r2 = 0.3; P = 0.12). This of course does not mean that stavudine is a dispensable component of the therapeutic regimen. Rather, it is most likely that in these children, stavudine was contributing its maximal pharmacodynamic effect at the doses used.
An indinavir regimen of 500 mg/m2 every 8 h is currently being used in clinical trials evaluating the safety and anti-HIV effect in children. If the pharmacokinetic and pharmacodynamic characteristics of the simulated population of 500 subjects approximate those of real-world children, this regimen may not be adequate to maintain a sustained anti-HIV response. The regimen of 500 mg/m2 every 8 h produced trough concentrations of >0.1 mg/liter in only 28% of the simulated population; an indinavir regimen of 500 mg/m2 every 6 h was predicted to yield trough concentrations of >0.1 mg/liter in 52% of subjects in this simulation. The usual didanosine dosing regimen is 90 mg/m2 every 12 h, although a range of 90 to 150 mg/m2 every 12 h has been recommended (5). At a dose of 90 mg/m2, 57% of the simulated subjects would have a didanosine AUC of >0.6 mg · h/liter. An increase in the standard dose to 120 mg/m2 every 12 h would be expected to increase the percentage of subjects with AUCs of >0.6 mg · h/liter to 88. These simulation studies suggest that alternative dosing strategies should be evaluated clinically to determine if therapeutic benefit could be safely optimized.
Contemporary pharmacotherapy of HIV infection is a challenging undertaking. This is particularly true with HIV-infected children, where therapeutic options are limited by available and palatable dosage forms and lack of pediatric-specific pharmacokinetic and pharmacodynamic information. This study illustrates the ability to obtain pharmacokinetic information for children from concentration data collected during routine clinic visits. Furthermore, we have described an approach for using this pharmacokinetic information to adjust dosing regimens in an attempt to achieve a pharmacologic objective and provide a safeguard against underdosing. Lastly, the relationships found between systemic drug concentrations and changes in plasma HIV RNA levels contribute to our understanding of the pharmacologic characteristics associated with therapeutic failure and success. We believe the incorporation of pharmacologic knowledge with virologic and immunologic data and behavioral considerations will result in improved clinical outcomes for children infected with HIV.
ACKNOWLEDGMENTS
This work was supported by grants RO1-AI33835 and UO1-AI27551 from the National Institute of Allergy and Infectious Diseases and grant MO1-RR00188 from the General Clinical Research Centers Program.
We thank Shao-Mei Han for his technical assistance and Bao-Chau Huynh and Sagar P. Kawle for their assistance with the development of the indinavir and combined didanosine and stavudine assays, respectively.
REFERENCES
- 1.Acosta E P, Henry K, Baken L, Page L M, Fletcher C V. Indinavir concentrations and antiviral effect. Pharmacotherapy. 1999;19:708–712. doi: 10.1592/phco.19.9.708.31544. [DOI] [PubMed] [Google Scholar]
- 2.Balis F M, Pizzo P A, Butler K M, Hawkins M E, Brouwers P, Husson R N, Jacobsen F, Blaney S M, Gress J, Jarosinski P, Poplack D G. Clinical pharmacology of 2′-3′-dideoxyinosine in human immunodeficiency virus-infected children. J Infect Dis. 1992;165:99–104. doi: 10.1093/infdis/165.1.99. [DOI] [PubMed] [Google Scholar]
- 3.Butler K M, Husson R N, Balis F M, Brouwers P, Eddy J, El-Amin D, Gress J, Hawkins M, Jarosinski P, Moss H, Poplack D, Santacroce S, Venzon D, Weiner L, Wolters P, Pizzo P A. Dideoxyinosine in children with symptomatic human immunodeficiency virus infection. N Eng J Med. 1991;324:137–144. doi: 10.1056/NEJM199101173240301. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1994 revised classification system for human immunodeficiency virus infection in children less than 13 years of age. Morbid Mortal Weekly Rep. 1994;43(RR-12):1–10. [Google Scholar]
- 5.Centers for Disease Control and Prevention. Guidelines for the use of antiretroviral agents in pediatric HIV infection. Morbid Mortal Weekly Rep. 1998;47(RR-4):1–43. [PubMed] [Google Scholar]
- 6.Harris M, Durakovic C, Rae S, Raboud J, Fransen S, Shillington A, Conway B, Montaner J S G. A pilot study of nevirapine, indinavir, and lamivudine among patients with advanced human immunodeficiency virus disease who have had failure of combination nucleoside therapy. J Infect Dis. 1998;177:1514–1520. doi: 10.1086/515317. [DOI] [PubMed] [Google Scholar]
- 7.Kline M W, Dunkle L M, Church J A, Goldsmith J C, Harris A T, Federici M E, Schultze M E, Woods L, Loewen D F, Kaul S, Cross A, Rutkiewicz V L, Rosenblatt H M, Hanson I C, Shearer W T. A phase I/II evaluation of stavudine (d4T) in children with human immunodeficiency virus infection. Pediatrics. 1995;96:247–252. [PubMed] [Google Scholar]
- 8.Kline M W, Fletcher C V, Federici M E, Harris A T, Evans K D, Rutkiewicz V L, Shearer W T, Dunkle L M. Combination therapy with stavudine and didanosine in children with advanced human immunodeficiency virus infection: pharmacokinetic properties, safety, and immunologic and virologic effects. Pediatrics. 1996;97:886–890. [PubMed] [Google Scholar]
- 9.Kline M W, Fletcher C V, Harris A T, Evans K D, Brundage R C, Remmel R P, Calles N R, Kirkpatrick S B, Simon C. A pilot study of combination therapy with indinavir, stavudine (d4T), and didanosine (ddI) in children infected with the human immunodeficiency virus. J Pediatr. 1998;132:543–546. doi: 10.1016/s0022-3476(98)70039-3. [DOI] [PubMed] [Google Scholar]
- 10.Mueller B U, Butler K M, Stocker V L, Balis F M, Brouwers P, Jarosinski P, Husson R N, Lewis L L, Venzon D, Pizzo P A. Clinical and pharmacokinetic evaluation of long-term therapy with didanosine in children with HIV infection. Pediatrics. 1994;94:724–731. [PubMed] [Google Scholar]
- 11.Mueller B U, Sleasman J, Nelson R P, Smith S, Deutsch P J, Ju W, Steinberg S M, Balis F M, Jarosinski P F, Brouwers P, Mistry G, Winchell G, Zwerski S, Sei S, Wood L V, Zeichner S, Pizzo P A. A phase I/II study of the protease inhibitor indinavir in children with HIV infection. Pediatrics. 1998;102:101–109. doi: 10.1542/peds.102.1.101. [DOI] [PubMed] [Google Scholar]
- 12.Mueller B U, Zeichner S L, Kuznetsov V A, Heath-Chiozzi M, Pizzo P A, Dimitrov D S. Individual prognoses of long-term responses to antiretroviral treatment based on virological, immunological and pharmacological parameters measured during the first week under therapy. AIDS. 1998;12:F191–F196. doi: 10.1097/00002030-199815000-00004. [DOI] [PubMed] [Google Scholar]
- 13.NONMEM Project Group. NONMEM user's guide, parts I-VI. San Francisco: Division of Clinical Pharmacology, University of California at San Francisco; 1991. [Google Scholar]
- 14.Stein D S, Fish D G, Bilello J A, Preston S L, Martineau G L, Drusano G L. A 24 week open label phase I/II evaluation of the HIV protease inhibitor MK-639 (indinavir) AIDS. 1996;10:485–492. doi: 10.1097/00002030-199605000-00006. [DOI] [PubMed] [Google Scholar]