Abstract
Fluoxetine (FLX) is an antidepressant that is increasingly being detected in aquatic environments. However, this contaminated FLX can affect aquatic organisms. Therefore, the aim of this study was to evaluate the genotoxic, mutagenic, and cytotoxic potential of FLX on erythrocytes in Nile tilapia (Oreochromis niloticus) after acute exposure. Fish were exposed to different concentrations of FLX (10, 100 and 1000 µg/L) for 96 h. Then, the condition factor (K value) was used to assess the general fish condition. The genotoxicity was investigated using a comet assay, and the mutagenicity was examined using micronucleus (MN) and erythrocytic nuclear abnormalities (ENAs) assays. In addition, the cytotoxicity was analyzed by erythrocyte morphometry and erythrocyte maturity index (EMI). The results showed that FLX did not affect the fish’s health. Nevertheless, 100 and 1000 µg/L FLX significantly increased DNA damage. Furthermore, a higher concentration of FLX presented a significantly increased frequency of MNs and ENAs, also leading to changes in some erythrocyte morphometric indices and significantly decreased mature erythrocytes. In conclusion, our results indicate that FLX induces genotoxic, mutagenic, and cytotoxic effects in erythrocytes of O. niloticus.
Keywords: Fluoxetine, Genotoxicity, Mutagenicity, Cytotoxicity, Erythrocyte, Oreochromis niloticus
Graphical Abstract
1. Introduction
Currently, pharmaceutical drugs are continuously being released into the aquatic environment through sewage from hospitals and wastewater treatment plants, including pharmaceutical manufacturing, to the point that the natural sources of water are toxic [1]. Most of these drugs are detected in effluents at various concentrations that range from ng/L to µg/L [2]. Concentrations greater than mg/L have also been detected in surface water [3]. Recently, antidepressants have been detected in various environments, such as ground surfaces and surrounding wastewater treatment plants, potentially including residues of these drugs in water resources [4]. One of the antidepressants found in water bodies is fluoxetine (FLX), an antidepressant in the class of selective serotonin reuptake inhibitors (SSRIs) [5]. FLX is considered a widespread drug used to treat patients with psychiatric disorders such as depression, obsessive compulsive disorder, and panic disorder [6]. In the past few years, a wastewater treatment plant near the Great Lakes, Canada, has reportedly detected FLX at concentrations of 0.013–0.099 µg/L in sewage [7]. In addition, FLX concentrations ranging from 0.012 to 0.929 µg/L were found in surface waters of the United States of America [8]. The distribution or residue of FLX in the aquatic environment can possibly be caused by humans who continue to use FLX [9]. In addition, ineffective wastewater treatment can lead to the continuous release of low concentrations of fluoxetine in the environment, resulting in high levels of FLX residues in the aquatic environment and potentially affecting aquatic organisms [10].
The environmental risks of FLX contaminants are now reported to be found in many nontarget aquatic organisms, such as invertebrates and vertebrates. In zebrafish (Danio rerio), FLX deleteriously affected embryo development and can interfere with egg production, concomitantly with changes in antioxidant activity of superoxide dismutase (SOD) and catalase (CAT) [8], [11]. Some studies have reported that FLX significantly reduces the aggressive behavior of Siamese fighting fish (Betta splendens) and Arabian killifish (Aphanius dispar) [12], [13]. Chen et al. [14] reported that topmouth gudgeon (Pseudorasbora parva) after FLX exposure increased acetylcholinesterase (AChE) activity. FLX caused hepatic biotransformation enzymes in common goby (Pomatoschistus microps), but no significant oxidative stress responses were observed [4]. In addition, FLX affected mRNA expression of genes involved in neurotransmitter in European bass (Dicentrarchus labrax) [15]. In a recent investigation, FLX significantly accumulated in tissues of Japanese medaka (Oryzias latipes) [16]. Moreover, FLX has been resulted in decreased food intake in goldfish (Carassius auratus) [17].
To assess the impact of waterborne contaminants, fish are considered a good choice for use in biomonitoring because they are susceptible to environmental changes [18], [19]. Currently, the ability of fish is not only used for the assessment of the impacts of water contaminants but can also be used as a biological indicator for the investigation of genotoxic, mutagenic, and carcinogenic effects in aquatic organisms, as well as to prevent these effects from entering the food chain [20]. Nile tilapia (Oreochromis niloticus) is one of the fish commonly used in toxicity testing because of its ability to adapt and tolerate environmental pollution very well [21]. Therefore, O. niloticus is considered a good biomarker for assessing toxic substances in aquatic habitats [22]. In addition, it is noted that O. niloticus is a widely distributed freshwater fish and is economically important in terms of consumption. For these reasons, we chose to conduct our study on this species to determine the possible deleterious effects of FLX exposure on fish health [23].
The toxicological studies in this study focused mainly on erythrocytes because they can respond to environmental pollution and reflect on the overall pathophysiological condition of the organism [24], [25]. However, studies on the genotoxic, mutagenic, and cytotoxic potential of FLX on fish erythrocytes are limited. For example, DNA damage has previously been assessed in the meager (Argyrosomus regius) [3] and Javanese medaka (Oryzias javanicus) [26] exposed to fluoxetine. Thus, we conducted this research to highlight the damage of FLX to erythrocytes. There are several methods for assessing the toxicity of FLX on erythrocytes in fish. One method is the comet assay, which is a suitable method for evaluating genotoxic effects and detecting DNA strand breaks in individual cells [27]. This assay can determine the DNA strand breaks before the occurrence of DNA repair mechanisms [28]. In addition, micronucleus (MN) and erythrocyte nuclear abnormalities (ENAs) assays can indicate chromosome damage and mutagenic effects [29]. MNs and ENAs may occur from either mitotic spindle dysfunction or chromosomal breakage [30]. Importantly, the comet assay and MN and ENA assays can be used to assess environmental toxicity [31]. This information may not be sufficient to determine environmental toxicity, so we decided to use erythrocyte morphometry and erythrocyte maturity determination to assess the cytotoxicity of pollution-sensing organisms that are sensitive enough to detect environmental pollution [32], [33], [34]. Several studies have suggested that these techniques are considered effective for the assessment of genotoxicity, mutagenicity and cytotoxicity, particularly in aquatic organisms [35], [36].
Given this background, the objectives of the present study were to verify the genotoxic and mutagenic effects of FLX on erythrocytes of O. niloticus using the comet assay, MN induction and other nuclear alterations, as well as to examine the cytotoxicity of O. niloticus after FLX exposure by measuring erythrocyte morphometry and considering the erythrocyte maturity index (EMI).
2. Materials and methods
2.1. Chemicals
Fluoxetine (FLX, CAS 56296-78-7) and colchicine (CAS 64-86-8) were purchased from Tokyo Chemical Industry Co., Ltd. (TCI), Japan. dimethyl sulfoxide (DMSO, CAS 67-68-5) was purchased from Loba Chemie Pvt. Ltd.
2.2. Animals and experimental design
Nile tilapia (Oreochromis niloticus) were obtained from Suphanburi Inland Fisheries Research and Development Center. Two weeks before the experiment, the fish were acclimated under The Central Laboratory for Aquaculture Research, Department of Zoology, Faculty of Science, Kasetsart University. In addition, fish were fed commercial fish food pellets twice a day, and three-fourths of the water was renewed every 2 days. After acclimation for 2 weeks, the fish were randomly divided into 6 groups (10 fish per group) and placed in glass aquariums (50 L) for the experiment. During the experiment, they were controlled in oxygenated water under a natural photoperiod (12:12 h light-dark cycle). In addition, the physical and chemical parameters of water were measured every day by a Multi-Probe System (YSI 556MPS), such as dissolved oxygen (7.06 ± 0.37 mg/L), pH (7.51 ± 0.17), conductivity (455.40 ± 17.86 µS/cm), total dissolved solids (0.28 ± 0.01 mg/L) and temperature (27.89 ± 0.26 °C).
Before exposure, FLX was dissolved in DMSO so that the final concentration of DMSO did not exceed 0.02%. The experimental design consisted of a negative control group (dechlorinated tap water); a solvent control group (DMSO, 0.02%); a positive control group (colchicine, 1 mg/kg, i.p.); and three groups of FLX (10, 100 and 1000 µg/L). The concentrations of fluoxetine used in this study were chosen from other previously published studies [26], [37], [38]. All these fish were exposed for 96 h. After 96 h exposure, the fish were anaesthetized with tricaine methanesulfonate (MS-222), and tissue papers were used to mop the water and slime of the fish's body. Then, a heparinized syringe was inserted in the cardiac region of the fish for blood drawing and blood samples were collected from all treatment groups for assessment of erythrocyte integrity, such as genetic damage, maturity stage and morphometry of the erythrocyte. In addition, the somatic condition of the fish was also examined.
The animal use experiment followed the Organization for Economic Co-operation and Development (OECD) [39] for the guidelines for the acute toxicity test of fish. This research was approved by the Institutional Animal Care and Use Committee, Faculty of Science, Kasetsart University, Thailand under permit number ACKU64-SCI-001.
2.3. Condition factor
Individual fish from each group were measured for weight (g) and total length (cm) after 96 h of exposure. Then, Futon's condition factor (K) was calculated according to Omar et al. [40], as shown in this formula:
| K = W/L3 |
where W and L denote weight (g) and total length (cm), respectively.
2.4. Genotoxicity
The comet assay was performed according to the procedure of Botelho et al. [41] with modification. First, blood samples were collected from cardiac puncture, and 3 µL of blood samples were diluted in phosphate buffered saline (1000 µL). Second, 5 µL of diluted blood samples were mixed with 15 µL of phosphate buffered saline, 0.7% low melting point agarose was added at 37 °C, and the samples were embedded on 1% normal melting point agarose-coated slides. A coverslip was added to the slide, and the slide was placed on an ice pack for 10 min. After softly removing the coverslips, the slides were added to low melting point agarose (85 µL) and covered with coverslips again. Third, the slide was removed and immersed in lysis buffer (2.5 M NaCl, 100 mM NaEDTA, 10 mM Tris, 10% DMSO, 1% Triton X-100 and pH 10) for 24 h. After lysis, the slides were transformed in electrophoresis buffer (1 mM Na EDTA, 300 mM NaOH and pH 13) at 25 V and 270 mV for 1 h, and the slides were kept in the dark and neutralized in neutralization buffer (0.4 M Tris and pH 13) for 10 min. Finally, the slides were stained with propidium iodide (PI) and analyzed with a fluorescence microscope (Olympus BX51) under 20 × magnification. One hundred cells were randomly analyzed per blood sample for each fish, and the CaspLab software program was used to measure the percentage of DNA damage in the tail (%DNA damage), Olive tail moment (OTM) and tail length (TL), as described by Guimarães et al. [34]. Moreover, the percentage of DNA damage in the tail (%) was classified into five levels according to Trigueiro et al. [42]: minimal damage (0–10%), low damage (10–25%), mid damage (25–50%), high damage (50–75%) and extreme damage (75–100%).
2.5. Mutagenicity
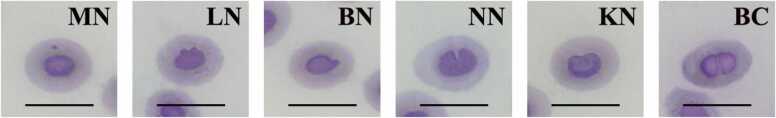
Micronucleus (MN) and erythrocytic nuclear abnormalities (ENAs) assays were performed according to the methods of Kosai et al. [43] and Pradhan et al. [44] with modification. Blood samples were obtained from cardiac puncture. Next, blood was smeared on a slide and air-dried. The smear was fixed with methanol for 1 min. Then, the slide was stained with Wright-Giemsa for 5 min. Then, 2000 erythrocytes were randomly analyzed per fish under a light microscope (Olympus BX51) with immersion lenses (100 × magnification). The ENAs were classified as follows: MN, lobed nucleus (LN), blebbed nucleus (BN), notched nucleus (NN), binucleated cell (BC) and kidney-shaped nucleus (KN) as described by Ossa-López et al. [45] and Roda et al. [46] with adaptations. Finally, the total ENA frequencies (MN, LN, BN, NN, BC and KN) were calculated from the formula of Barreto et al. [31] as follows:
2.6. Cytotoxicity
The cytotoxicity of erythrocytes was evaluated using both erythrocyte morphometry and erythrocyte maturity index determination through the same slides used in the MN and ENA assays.
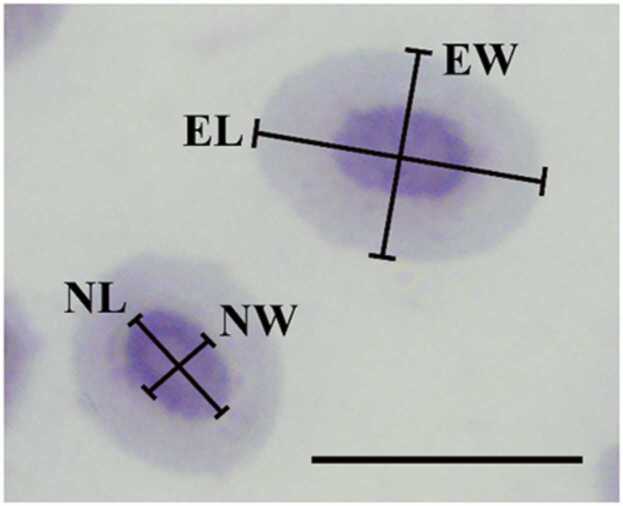
The erythrocyte morphometry was performed according to Ahmed and Sheikh [47] with modification. One hundred erythrocyte measurements were randomly processed using ImageJ 1.52av software [34] per fish under a light microscope at a magnification of 100 × magnification (Olympus BX51). The morphometric indices of erythrocytes in the fish were examined using indices as described by Mofizur Rahman and Baek [33], such as the erythrocyte major axis (EL), erythrocyte minor axis (EW), nucleus major axis (NL) and nucleus minor axis (NW), as shown in Fig. 1. Erythrocyte size (ES) and nucleus size (NS) were also analyzed using the following formulas: [(EL × EW × π)/4] and [(NL × NW × π)/4], respectively [33], [48]. In addition, erythrocyte shapes and their nuclear shapes were evaluated with EL/EW and NL/NW ratios, and the ratio between nuclear and erythrocyte area was assessed with the NS/ES ratio [49].
Fig. 1.
The major axis and minor axis determination of erythrocytes and nucleus in Oreochromis niloticus. erythrocyte major axis (EL), erythrocyte minor axis (EW), nucleus major axis (NL), nucleus minor axis (NW). Scale bars = 10 µm.
The erythrocytic maturity index (EMI) determination was performed according to Castro et al. [32] with modifications. From the same microscope fields used to screen erythrocyte morphometry, the EMI value was calculated for 25 erythrocytes by dividing NW by EL values. The EMI values were divided into one of the 10 maturity classes as described by Marques et al. [50]. Finally, each maturity class was calculated as the frequency (%) of cells.
2.7. Statistical analysis
All data were checked using the StatPlus program for Windows version 2017 (AnalystSoft Inc., Canada) by one-way analysis of variance (ANOVA) and a post hoc test followed by the least significant difference (LSD) test. All results are presented as the means ± standard deviations. The differences in the data were significant when the p value was less than 0.05.
3. Results
3.1. Condition factor
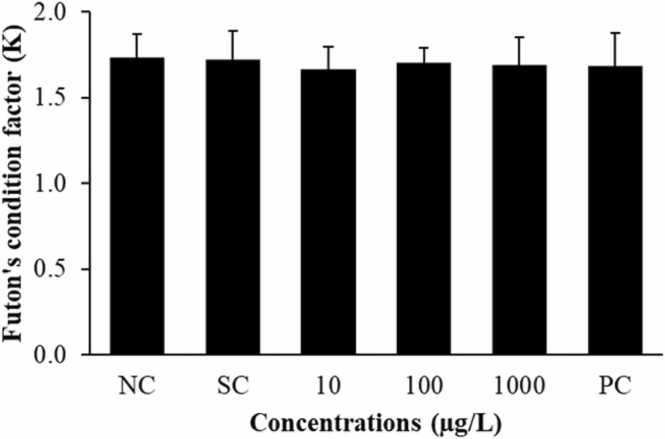
The condition factor of O. niloticus was evaluated from the K value. In this study, the K value of the fish exposed to 0.02% DMSO (solvent control group) and different concentrations of FLX for 96 h, as well as 1 mg/kg colchicine (positive control group), was not significantly different from that of the negative control group (Fig. 2), revealing that the fish exposed to DMSO, FLX and colchicine were in somatic conditions similar to those of the negative control group and had no effect on fish health.
Fig. 2.
Futon's condition factor (K) of Oreochromis niloticus after exposure to fluoxetine for 96 h. The results are expressed as the mean ± standard deviation. NC: negative control group; SC: solvent control group; PC: positive control group; 10, 100, 1000: treatment groups exposed to 10 µg/L, 100 µg/L and 1000 µg/L of FLX, respectively.
3.2. Genotoxicity
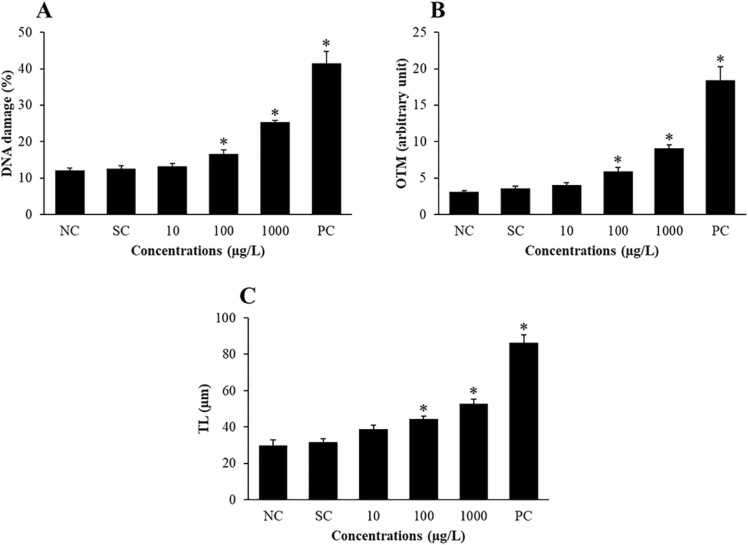
DNA damage to erythrocytes in O. niloticus was evaluated by using the comet assay, and the results are summarized in Fig. 3. This experiment presented three parameters: the percentage of DNA damage in the tail (%DNA damage; Fig. 3A), Olive tail moment (OTM; Fig. 3B) and tail length (TL; Fig. 3C). The solvent control group showed no difference in %DNA damage, OTM or TL compared with the negative control group. %DNA damage and OTM of the group exposed to 10 µg/L FLX did not differ from the negative control group. However, the TL of erythrocytes in the group exposed to FLX at concentrations of 10 µg/L was significantly higher than that in the negative control group. In addition, the fish exposed to 100, 1000 µg/L FLX and injected with 1 mg/kg colchicine demonstrated that all three DNA damage parameters were significantly different from those of the negative control group (p < 0.05).
Fig. 3.
DNA damage parameters of Oreochromis niloticus exposed to fluoxetine for 96 h: A) Percentage of DNA damage in the tail (DNA damage), B) Olive tail moment (OTM) and C) Tail length (TL). The results are expressed as the mean ± standard deviation. * Statistically significant differences compared to the negative control group (p < 0.05). NC: negative control group; SC: solvent control group; PC: positive control group; 10, 100, 1000: treatment groups exposed to 10 µg/L, 100 µg/L and 1000 µg/L of FLX, respectively.
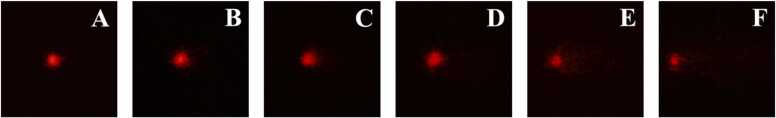
Some of the frequencies of DNA damage levels in the erythrocytes of the fish are depicted in Table 1. DNA damage images after exposure to FLX are shown in Fig. 4. Mostly, the negative control group, the solvent control group and the group obtained 10 µg/L FLX, demonstrating minimal and low DNA damage levels. In contrast, the group exposed to FLX at 100 µg/L showed a significantly higher mid DNA damage level than the negative control group. Moreover, at 1000 µg/L FLX, the positive control group also displayed a significant increase in the level of mid- and high DNA damage compared to the negative control group. However, the level of extreme DNA damage after exposure to FLX and colchicine was not detected during the experimental period, and thus, the results are not presented in the table.
Table 1.
Frequency (%) of DNA damage in erythrocytes of Oreochromis niloticus at each level during 96 h of treatment with fluoxetine.
| Level of DNA damages | Treatment groups |
|||||
|---|---|---|---|---|---|---|
| NC | SC | 10 µg/L | 100 µg/L | 1000 µg/L | PC | |
| Minimal | 45.20 ± 3.49 | 45.90 ± 2.81 | 42.30 ± 4.35 | 26.20 ± 3.77* | 11.30 ± 1.42* | 1.00 ± 0.53* |
| Low | 46.80 ± 5.81 | 44.10 ± 3.03 | 47.70 ± 4.00 | 59.50 ± 4.50* | 35.70 ± 2.87* | 11.50 ± 4.24* |
| Mid | 7.90 ± 3.25 | 9.90 ± 0.88 | 9.80 ± 1.62 | 13.20 ± 1.81* | 47.60 ± 3.72* | 56.25 ± 4.77* |
| High | 0.10 ± 0.32 | 0.10 ± 0.32 | 0.20 ± 0.42 | 1.10 ± 0.74 | 5.40 ± 1.65* | 30.63 ± 5.85* |
The results expressed as mean ± standard deviation. * Statistically significant differences to the negative control group (p < 0.05). NC: negative control group; SC: solvent control group; PC: positive control group; 10, 100, 1000: treatment groups exposed to 10 µg/L, 100 µg/L and 1000 µg/L of FLX, respectively.
Fig. 4.
Representative images of DNA damage in erythrocytes of Oreochromis niloticus exposed to fluoxetine for 96 h: A) negative control group, B) solvent control group, C) group exposed to 10 µg/L fluoxetine, D) group exposed to 100 µg/L fluoxetine, E) group exposed to 1000 µg/L fluoxetine, F) positive control group.
3.3. Mutagenicity
The results of the analysis of MNs and ENAs (LN, BN, NN, KN and BC) in erythrocytes of O. niloticus induced by FLX are presented in Fig. 5 and Table 2. There was a significant increase in the total erythrocytic nuclear abnormalities frequency (%ENAs) in the fish exposed to 1000 µg/L FLX and 1 mg/kg colchicine compared with the negative control group. The frequency of MNs in erythrocytes of fish exposed to FLX and colchicine showed a significant increase in MN frequency when compared to the negative control group, except for the solvent control group and the 10 µg/L exposure group of FLX. Furthermore, this study showed that the frequencies of LN, NN and KN of the fish exposed to 1000 µg/L FLX and the positive control group were significantly higher than those of the negative control group. In the positive control group, the frequency of BN was significantly different from that in the negative control group. However, the BC frequency of all groups did not differ significantly when compared to the negative control group.
Fig. 5.
Representative photomicrographs of erythrocytic nuclear abnormalities in Oreochromis niloticus after exposure to fluoxetine for 96 h: micronucleus (MN), lobed nucleus (LN), blebbed nucleus (BN), notched nucleus (NN), kidney-shaped nucleus (KN) and binucleated cell (BC). Scale bars = 10 µm.
Table 2.
Frequency (%) of erythrocytic nuclear abnormalities (ENAs) of Oreochromis niloticus exposed to fluoxetine for 96 h.
| Frequency (%) of ENAs | Treatment groups |
|||||
|---|---|---|---|---|---|---|
| NC | SC | 10 µg/L | 100 µg/L | 1000 µg/L | PC | |
| MN | 0.04 ± 0.04 | 0.06 ± 0.03 | 0.07 ± 0.04 | 0.24 ± 0.11* | 0.26 ± 0.11* | 0.56 ± 0.32* |
| LN | 0.05 ± 0.04 | 0.05 ± 0.04 | 0.06 ± 0.05 | 0.07 ± 0.04 | 0.14 ± 0.08* | 0.17 ± 0.08* |
| BN | 0.15 ± 0.06 | 0.15 ± 0.06 | 0.16 ± 0.06 | 0.20 ± 0.07 | 0.25 ± 0.14 | 0.72 ± 0.29* |
| NN | 0.20 ± 0.07 | 0.20 ± 0.06 | 0.21 ± 0.07 | 0.26 ± 0.07 | 0.37 ± 0.14* | 0.84 ± 0.35* |
| KN | 0.33 ± 0.15 | 0.34 ± 0.14 | 0.36 ± 0.16 | 0.41 ± 0.15 | 0.60 ± 0.16* | 0.95 ± 0.24* |
| BC | 0.01 ± 0.02 | 0.01 ± 0.02 | 0.01 ± 0.02 | 0.01 ± 0.02 | 0.01 ± 0.02 | 0.03 ± 0.05 |
| Total ENAs | 0.76 ± 0.20 | 0.79 ± 0.23 | 0.86 ± 0.27 | 1.18 ± 0.20* | 1.62 ± 0.48* | 3.26 ± 0.64* |
The results expressed as mean ± standard deviation. * Statistically significant differences to the negative control group (p < 0.05). NC: negative control group; SC: solvent control group; PC: positive control group; 10, 100, 1000: treatment groups exposed to 10 µg/L, 100 µg/L and 1000 µg/L of FLX, respectively; MN: micronucleus; LN: lobed nucleus; BN: blebbed nucleus; NN: notched nucleus; BC: binucleated cell; KN: kidney-shaped nucleus.
3.4. Cytotoxicity
The morphological indices of erythrocytes and their nuclei in O. niloticus after FLX exposure are presented in Table 3. The solvent control group showed no difference in morphological indices compared with the negative control group. The fish exposed to 100 and 1000 µg/L FLX and the positive control group presented a significant decrease in EL, EL/EW, NL/NW and ES compared to the negative control group. In addition, there were significant differences in NL and NS/ES in the groups exposed to 1000 µg/L FLX and the positive control group when compared with the negative control group. Furthermore, only EW was significantly lower in the positive control group than in the negative control group. Nevertheless, no significant differences were found in the NW and NS results of all groups when compared to the negative control group.
Table 3.
Morphometric indices of erythrocytes and nuclei of Oreochromis niloticus exposed to fluoxetine for 96 h.
| Morphometric indices | Treatment groups |
|||||
|---|---|---|---|---|---|---|
| NC | SC | 10 µg/L | 100 µg/L | 1000 µg/L | PC | |
| EL (µm) | 10.59 ± 0.38 | 10.54 ± 0.27 | 10.52 ± 0.32 | 9.83 ± 0.30* | 9.33 ± 0.59* | 9.06 ± 0.23* |
| EW (µm) | 7.32 ± 0.34 | 7.47 ± 0.20 | 7.35 ± 0.20 | 7.33 ± 0.22 | 7.18 ± 0.24 | 6.81 ± 0.21* |
| EL/EW | 1.46 ± 0.09 | 1.42 ± 0.06 | 1.44 ± 0.06 | 1.35 ± 0.06* | 1.31 ± 0.06* | 1.34 ± 0.06* |
| NL (µm) | 4.77 ± 0.29 | 4.96 ± 0.11 | 4.97 ± 0.09 | 4.63 ± 0.12 | 4.51 ± 0.31* | 4.27 ± 0.30* |
| NW (µm) | 3.03 ± 0.11 | 3.04 ± 0.04 | 3.08 ± 0.05 | 3.12 ± 0.10 | 3.15 ± 0.27 | 3.11 ± 0.20 |
| NL/NW | 1.60 ± 0.10 | 1.64 ± 0.04 | 1.62 ± 0.05 | 1.50 ± 0.04* | 1.45 ± 0.09* | 1.38 ± 0.07* |
| ES (µm2) | 60.92 ± 3.66 | 61.74 ± 2.12 | 60.69 ± 2.16 | 56.63 ± 2.50* | 52.69 ± 4.67* | 48.49 ± 2.04* |
| NS (µm2) | 11.39 ± 0.93 | 11.88 ± 0.37 | 12.03 ± 0.29 | 11.36 ± 0.57 | 11.21 ± 1.56 | 10.50 ± 1.34 |
| NS/ES | 0.19 ± 0.01 | 0.19 ± 0.01 | 0.20 ± 0.01 | 0.20 ± 0.01 | 0.21 ± 0.02* | 0.22 ± 0.02* |
The results expressed as mean ± standard deviation. * Statistically significant differences to the negative control group (p < 0.05). NC: negative control group; SC: solvent control group; PC: positive control group; 10, 100, 1000: treatment groups exposed to 10 µg/L, 100 µg/L and 1000 µg/L of FLX, respectively; EL: erythrocyte major axis; EW: erythrocyte minor axis; NL: nucleus major axis; NW: nucleus minor axis; ES: erythrocyte size; NS: nucleus size.
The maturity stage of erythrocytes after FLX exposure was evaluated using EMI determination. The EMI results in this study showed only class 1–6 erythrocytes, as shown in Table 4. The frequencies of erythrocytes in classes 1, 2 and 6 were detected in all experimental groups, but these classes were very rarely detected. The solvent control group showed no difference in EMI value compared with the negative control group. The erythrocytes in class 3 were noticed most frequently in the negative control group, the solvent control group and the FLX 10 µg/L exposure group. In addition, the frequency of class 4 was abundantly observed in the groups exposed to 100 and 1000 µg/L FLX and the positive control group, also showing a significant decrease in erythrocytes of class 3 when compared to the negative control group. Moreover, the FLX 1000 µg/L exposure group and the positive control group demonstrated a significantly increased frequency of class 5 erythrocytes compared to the negative control group.
Table 4.
Frequency (%) of erythrocytic maturity index (EMI) classes of Oreochromis niloticus exposed to fluoxetine for 96 h.
| Frequency of EMI classes | Treatment groups |
|||||
|---|---|---|---|---|---|---|
| NC | SC | 10 µg/L | 100 µg/L | 1000 µg/L | PC | |
| Class 1 | 0.40 ± 1.26 | 0.40 ± 1.26 | 0.40 ± 1.26 | 0.57 ± 1.51 | 0.40 ± 1.26 | 0.50 ± 1.41 |
| Class 2 | 1.60 ± 2.80 | 2.00 ± 2.83 | 0.40 ± 1.26 | 0.57 ± 1.51 | 0.40 ± 1.26 | 0.50 ± 1.41 |
| Class 3 | 67.60 ± 3.50 | 67.60 ± 2.95 | 66.40 ± 3.37 | 37.14 ± 3.02* | 26.80 ± 2.70* | 21.50 ± 2.07* |
| Class 4 | 28.80 ± 3.16 | 28.40 ± 2.95 | 30.80 ± 2.70 | 59.43 ± 2.76* | 66.40 ± 3.37* | 71.00 ± 2.83* |
| Class 5 | 1.20 ± 1.93 | 1.20 ± 2.70 | 1.60 ± 2.80 | 1.71 ± 2.14 | 5.20 ± 3.29* | 6.00 ± 3.02* |
| Class 6 | 0.40 ± 1.26 | 0.40 ± 1.26 | 0.40 ± 1.26 | 0.57 ± 1.51 | 0.80 ± 1.69 | 0.50 ± 1.41 |
The results expressed as mean ± standard deviation. * Statistically significant differences to the negative control group (p < 0.05). NC: negative control group; SC: solvent control group; PC: positive control group; 10, 100, 1000: treatment groups exposed to 10 µg/L, 100 µg/L and 1000 µg/L of FLX, respectively.
4. Discussion
At the present time, pharmaceuticals and personal care products (PPCPs) are found to be more widespread in aquatic ecosystems [38]. FLX, an antidepressant (selective serotonin reuptake inhibitors; SSRIs), is most often detected as a residue on surface water and wastewater [51]. Moreover, it is possible that FLX affects aquatic animals such as microalgae, water fleas, plankton, and midges, including some fishes [52]. Fish are often used as bioindicators to determine the genotoxic and mutagenic effects of contaminants present in the aquatic environment [53]. In the past decade, there have been limited reports of FLX genotoxicity in fish. Therefore, this research demonstrates the genotoxic potential of FLX in O. niloticus, focusing mainly on its toxic effect on erythrocytes. Erythrocytes are often used as a diagnostic tool for the response of fish exposed to aquatic toxicants [54]. Furthermore, erythrocytes also provide reliable, fast, simple, and accurate information [32]. Importantly, they can indicate the physiological stress of organisms caused by environmental factors [55].
In the present study, fish health was estimated by the condition factor (K) value. The condition factor is an important tool for assessing the condition or well-being of fish [56], which can indicate the animal's ability to tolerate stressful environments and can investigate the harmful effects of exposure to toxic substances [35], [40]. In this work, FLX had no effect on K values in fish that were exposed to FLX. Consistent with previous reports, Argyrosomus regius exposed to FLX, even at the highest concentration (3 µg/L), did not have a statistically significant effect on changes in the K value [3]. Therefore, O. niloticus exposed to FLX may not have any harmful effects on general health.
According to Cotelle and Férard [57], the MN assay detects the breakage of unrepaired DNA strands, whereas the comet assay detects DNA strand breaks that occur before the DNA repair process. Therefore, the present study used a comet assay, including MN and ENA assays that should be evaluated together, to assess genotoxic substances in fish erythrocytes, as suggested by Barreto et al. [31] The comet assay is always used in biological monitoring or genotoxicity testing with a focus on organisms, and this assay has been proven to be a method of rapidity and sensitivity [57]. The comet assay was mostly used to examine various types of damage caused by DNA strand breaks in individual cells, such as double strand breaks, single strand breaks, alkali labile sites, DNA cross-linking and incomplete excision repair sites [58]. In this work, the comet assay was used to detect DNA damage after O. niloticus were exposed to FLX for 96 h. FLX concentrations above 100 µg/L can induce DNA damage in the erythrocytes of O. niloticus. Although few studies have evaluated the genotoxic mechanism of fluoxetine, some research has indicated that fluoxetine can affect DNA damage through direct or indirect mechanisms. Normally, antidepressants have mutagenic and carcinogenic potential that can cause DNA damage through direct and indirect mechanisms. The direct mechanism is probably due to the direct binding of antidepressants to DNA, leading to conformational or base pair changes in DNA [59]. According to Kashanian et al. [60], FLX demonstrates a slight change in the binding of base pairs of DNA and DNA grooves, but FLX does not affect DNA conformational changes. However, a higher concentration of FLX may also affect the conformational changes of DNA. In addition, some components in the chemical structure of antidepressants (aromatic rings, fluorobenzene groups and nitro groups) may also cause genetic damage. The nitro groups might be transformed into nitroso compounds and form alkylating molecules, leading to lesions such as DNA adducts, DNA strand breaks and DNA cross-links [59]. In terms of indirect mechanisms, antidepressants might influence organelles, such as mitochondria [20]. It is mainly associated with oxidative stress caused by reactive oxygen species (ROS), leading to DNA damage, such as base modification and single- and double-strand breaks [61], as well as alterations in the expression of genes associated with the DNA damage response, DNA repair, cell proliferation and cell cycle control [20]. For example, intracellular ROS production in haemocytes of Mytilus edulis was increased after exposure to FLX at concentrations of 10 and 50 mg/L, thus affecting haemocyte DNA damage [62]. Additionally, ROS can disrupt DNA replication and transcription [46], as well as inhibit DNA repair [53]. A previous study found that Carassius auratus had increased malondialdehyde (MDA) content after exposure to FLX [63]. MDA is a byproduct of lipid peroxidation (LPO) and affects DNA damage via DNA adducts formed by the binding of MDA to DNA bases [64]. Importantly, with a high MDA content, it can be inferred that the ROS level is also high, and some antioxidant enzymes in fish, such as superoxide dismutase (SOD), may not be sufficient to defend against the oxidative stress of FLX [63], leading to DNA damage. In addition, Duarte et al. [3] reported that Pomatoschistus microps exposed to 3 µg/L FLX, the highest concentration at that time, demonstrated an increase in LPO levels and DNA damage in their liver. Therefore, in this study, it is possible that DNA damage in erythrocytes of O. niloticus after FLX exposure can be caused by both direct and indirect mechanisms.
The MN assay is widely used in biomonitoring to assess the impact of pollutants on aquatic organisms [65], and this assay is proposed as a technique for the detection of mutagenicity (chromosomal damage) [58]. In the current study, O. niloticus in the group exposed to 100 and 1000 µg/L FLX for 96 h showed an increased frequency of MN. MNs are small and round nuclei that can occur due to several factors, such as age and metabolism, including various environmental factors [66]. The possible origins of MN can cause a variety of reasons, such as the breakage of acentric chromosome fragments or whole chromosomes during anaphase of dividing cells, and cannot be included in the main nucleus during the telophase of the cell division process [67], including defects in kinetochore proteins, mitotic spindles and anaphase checkpoints, as described by Luzhna et al. [68]. Previous reports have shown that the ENA assay can be a tool to investigate mutagenicity in fish erythrocytes [18]. For example, guppy (Poecilia reticulata) [29], grass carp (Ctenopharyngodon idella) [34], climbing perch (Anabas testudineus) [27], streaked prochilod (Prochilodus lineatus) [46], Gilthead seabream (Sparus aurata) [69], etc. The formation of nuclear buds (lobed, blebbed and notched nucleus) originated from the dysfunction of tubulin, chromosome segregation and chromosomal instability via the breakage-fusion-bridge cycle [31], [70]. Furthermore, nuclear buds can contain genetic information with respect to oncogenes that may lead to cancer cell formation [45]. The presence of binucleated cells is possibly due to abnormalities in cell division during the M phase of the cell cycle [70]. With respect to kidney-shaped nuclei, these may occur from invagination of the nucleus [31], whereas some studies have identified that they are caused by the same phenomena as micronuclei or binucleated cells [71]. Moreover, Al-Obaidi and Al-Shawi [72] reported findings similar to our results; they reported that FLX has genotoxic potential and can induce MN in polychromatic erythrocytes in the bone marrow of male rats. However, the origins of these morphological alterations of the nucleus have been found to arise from genotoxic events caused by exposure to xenobiotic agents [73].
To support the toxicological response information to the cytotoxicity of erythrocytes in O. niloticus, this research uses morphometric analysis of erythrocytes and their nuclei along with EMI determination. Both morphometric indices of erythrocytes and EMI assays have been recognized as suitable techniques for the early detection of early warning signs of environmental stress in fish, and these techniques are also sensitive to environmental pollution [32], [35]. Mofizur Rahman et al. [33] previously explained that assessment of morphometric indices of erythrocytes and their nucleus in fish can measure stress and be useful in monitoring fish health and has also reported that changes in the structure of erythrocytes and their nucleus may be due to disruption of lipid solubility in erythrocyte membranes, uneven hemoglobin distribution and erythrocytic necrosis. Importantly, stress is one of the factors in the welfare of fish that can affect morphological indices of erythrocytes [74]. For these reasons, abnormal or distorted erythrocytes can interfere with erythrocyte function and lead to tissue hypoxia, resulting from reduced oxygen-carrying capacities of hemoglobin in red blood cells [75]. This is consistent with the description of Zabotkina et al. [76] that values of the nucleus/cytoplasmic ratio can indicate the relative elevation of the share of the hemoglobin-containing cytoplasm. In this study, after exposure to FLX above 100 µg/L, O. niloticus showed changes in the morphological indices of erythrocytes and their nuclei.
In addition, using the EMI determination in this work, we observed that the stage of erythrocyte maturation decreased with increased exposure to FLX concentrations. This may be due to the change in erythrocyte shape from elongated to round resulting from the effects of a decrease in erythrocyte major axis (EL) and nucleus minor axis (NW) enlargement [33]. Interestingly, the EMI assay indicates the balance between the production and elimination of erythrocytes, including an elevated presence of degenerate erythrocytes and immature erythrocytes in the peripheral blood, which are associated with pollution in the environment where the organisms live [50], [77]. Therefore, it can detect aquatic environments that may be contaminated with pollutants [32]. Consistent with the research of Osman et al. [78], pollution might have stimulated an increase in the number of immature red blood cells in the fish circulation. Furthermore, stress can also affect the release of immature red blood cells [79]. Importantly, immature erythrocytes and erythrocyte deformability can affect their inefficient functions, such as inadequate blood flow and reduced oxygen transport to the tissues of organs within the organism [75]. Ultimately, if the morphometric parameters and maturation of erythrocytes continue to be abnormal, it leads to complete degeneration of red blood cells and destruction [80], depending on fish species, size, age, health and environmental conditions [33].
Furthermore, colchicine was used as a positive control group in this study because colchicine is known as an aneugenic agent that can affect tubulin inhibition and cause cell cycle arrest. Moreover, colchicine also affects mitotic spindle damage and leads to the formation of micronuclei within living cells [81], [82]. Previous studies by Gustavino et al. [83] and Melo et al. [84] showed that Cyprinus carpio and Oreochromis niloticus demonstrated that the number of erythrocytes with micronuclei was higher after colchicine exposure. In addition, colchicine induces DNA damage and leads to cell death by apoptosis [85]. Our results indicated that O. niloticus treated with 1 mg/kg colchicine showed effects on erythrocytes in terms of DNA damage, nuclear alterations, morphological changes and abnormal maturation, and our results are consistent with previous research. Therefore, researchers chose to use colchicine as a positive control in toxicity studies.
Overall, this research showed that the combined assessment of comet, MN and ENA assays as well as erythrocyte morphometry and EMI assays can be used as tools to support the evaluation of erythrocyte toxicity in fish. In addition, the present study increases the knowledge concerning the harmful effects of FLX on fish, but further investigation is needed to provide a comprehensive assessment of FLX toxicity in fish, such as enzyme assays and other organ pathological studies. Our results showed that FLX had no effects on fish health. On the other hand, our study demonstrated that FLX induced erythrocyte toxicity in fish in a concentration-dependent manner. However, if FLX contamination persists in the aquatic environment, aquatic organisms may accumulate these substances from the food or the water they ingest and can be transferred in these substances between trophic levels through the food chain [86], resulting in the higher trophic level organisms in the food chain are at greater risk of toxic buildup in their body [87]. For this reason, people who consume aquatic organisms (e.g. fishes) contaminated with FLX can unconsciously get FLX. Ultimately, if these substances accumulate in the body for a long time that can be harmful to human health and increase the risk of severe illness. Thus, existing FLX (including other pharmaceuticals) in the aquatic environment must be managed to avoid exposure of fish and aquatic organisms to contaminated environments.
5. Conclusion
The findings of the present study indicated that after 96 h of exposure to FLX at concentrations greater than 100 µg/L, Oreochromis niloticus were able to induce erythrocyte damage, such as genotoxicity (DNA damage), mutagenicity (micronucleus formation and nuclear alterations) and cytotoxicity (morphological changes and abnormal maturation). Therefore, we should be aware of the treatment of wastewater that may be contaminated with FLX before discharge into aquatic environments to avoid harming the livelihoods of aquatic organisms.
CRedit authorship contribution statement
V. B., M. K. and T. T. contributed to the study conception and experimental design in this research. All authors performed material preparation, sample collection and data analysis. The manuscript was drafted by V. B. and P.V. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research is funded by Kasetsart University through the Graduate School Fellowship Program and Department of Zoology, Faculty of Science, Kasetsart University. VB would like to thank you Kasetsart University Reseach and Development Institute for supporting fund for page charge fee.
Handling Editor: Lawrence Lash
Contributor Information
Mesayamas Kongsema, Email: fscimmk@ku.ac.th.
Vasakorn Bullangpoti, Email: fscivkb@ku.ac.th.
References
- 1.Kovács R., Csenki Z., Bakos K., Urbányi B., Horváth Á., Garaj-Vrhovac V., Gajski G., Gerić M., Negreira N., López de Alda M., Barceló D., Heath E., Kosjek T., Žegura B., Novak M., Zajc I., Baebler Š., Rotter A., Ramšak Ž., Filipič M. Assessment of toxicity and genotoxicity of low doses of 5-fluorouracil in zebrafish (Danio rerio) two-generation study. Water. Res. 2015;77:201–212. doi: 10.1016/j.watres.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Melchor-Martínez E.M., Jiménez-Rodríguez M.G., Martínez-Ruiz M., Peña-Benavides S.A., Iqbal H.M.N., Parra-Saldívar R., Sosa- Hernández J.E. Antidepressants surveillance in wastewater: overview extraction and detection. Case Stud. Chem. Environ. Eng. 2021;3 doi: 10.1016/j.cscee.2020.100074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duarte I.A., Reis-Santos P., Novais S.C., Rato L.D., Lemos M.F.L., Freitas A., Pouca A.S.V., Barbosa J., Cabral H.N., Fonseca V.F. Depressed, hypertense and sore: long-term effects of fluoxetine, propranolol and diclofenac exposure in a top predator fish. Sci. Total. Environ. 2020;712 doi: 10.1016/j.scitotenv.2020.136564. [DOI] [PubMed] [Google Scholar]
- 4.Duarte I.A., Pais M.P., Reis-Santos P., Cabral H.N., Fonseca V.F. Biomarker and behavioural responses of an estuarine fish following acute exposure to fluoxetine. Mar. Environ. Res. 2019;147:24–31. doi: 10.1016/j.marenvres.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Nałęcz-Jawecki G., Wawryniuk M., Giebułtowicz J., Olkowski A., Drobniewska A. Influence of selected antidepressants on the ciliated protozoan Spirostomum ambiguum: toxicity, bioaccumulation, and biotransformation products. Molecules. 2020;25:1476. doi: 10.3390/molecules25071476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrés-Costa M.J., Proctor K., Sabatini M.T., Gee A.P., Lewis S.E., Pico Y., Kasprzyk-Hordern B. Enantioselective transformation of fluoxetine in water and its ecotoxicological relevance. Sci. Rep. 2017;7:15777. doi: 10.1038/s41598-017-15585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon J.W., Armbrust K.L. Laboratory persistence and fate of fluoxetine in aquatic environments. Environ. Toxicol. Chem. 2006;25:2561–2568. doi: 10.1897/05-613r.1. [DOI] [PubMed] [Google Scholar]
- 8.Cunha V., Rodrigues P., Santos M.M., Moradas-Ferreira P., Ferreira M. Danio rerio embryos on Prozac – effects on the detoxification mechanism and embryo development. Aquat. Toxicol. 2016;178:182–189. doi: 10.1016/j.aquatox.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Henry T.B., Black M.C. Acute and chronic toxicity of fluoxetine (selective serotonin reuptake inhibitor) in western mosquitofish. Arch. Environ. Contam. Toxicol. 2008;54:325–330. doi: 10.1007/s00244-007-9018-0. [DOI] [PubMed] [Google Scholar]
- 10.Santos L.H., Araújo A.N., Fachini A., Pena A., Delerue-Matos C., Montenegro M.C. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard Mater. 2010;175:45–95. doi: 10.1016/j.jhazmat.2009.10.100. [DOI] [PubMed] [Google Scholar]
- 11.Lister A., Regan C., Van Zwol J., Van Der Kraak G. Inhibition of egg production in zebrafish by fluoxetine and municipal effluents: a mechanistic evaluation. Aquat. Toxicol. 2009;95:320–329. doi: 10.1016/j.aquatox.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Lynn S.E., Egar J.M., Walker B.G., Sperry T.S., Ramenofsky M. Fish on Prozac: a simple, noninvasive physiology laboratory investigating the mechanisms of aggressive behavior in Betta splendens. Adv. Physiol. Educ. 2007;31:358–363. doi: 10.1152/advan.00024.2007. [DOI] [PubMed] [Google Scholar]
- 13.Barry M.J. Effects of fluoxetine on the swimming and behavioural responses of the Arabian killifish. Ecotoxicology. 2013;22:425–432. doi: 10.1007/s10646-012-1036-7. [DOI] [PubMed] [Google Scholar]
- 14.Chen H., Zeng X., Mu L., Hou L., Yang B., Zhao J., Schlenk D., Dong W., Xie L., Zhang Q. Effects of acute and chronic exposures of fluoxetine on the Chinese fish, topmouth gudgeon Pseudorasbora parva. Ecotoxicol. Environ. Saf. 2018;160:104–113. doi: 10.1016/j.ecoenv.2018.04.061. [DOI] [PubMed] [Google Scholar]
- 15.Costa C., Semedo M., Machado S.P., Cunha V., Ferreira M., Urbatzka R. Transcriptional analyses reveal different mechanism of toxicity for a chronic exposure to fluoxetine and venlafaxine on the brain of the marine fish Dicentrarchrus labrax. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021;250 doi: 10.1016/j.cbpc.2021.109170. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura Y., Yamamoto H., Sekizawa J., Kondo T., Hirai N., Tatarazako N. The effects of pH on fluoxetine in Japanese medaka (Oryzias latipes): acute toxicity in fish larvae and bioaccumulation in juvenile fish. Chemosphere. 2008;70:865–873. doi: 10.1016/j.chemosphere.2007.06.089. [DOI] [PubMed] [Google Scholar]
- 17.Mennigen J.A., Sassine J., Trudeau V.L., Moon T.W. Waterborne fluoxetine disrupts feeding and energy metabolism in the goldfish Carassius auratus. Aquat. Toxicol. 2010;100:128–137. doi: 10.1016/j.aquatox.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz de Arcaute C., Soloneski S., Larramendy M.L. Toxic and genotoxic effects of the 2,4-dichlorophenoxyacetic acid (2,4-D)-based herbicide on the Neotropical fish Cnesterodon decemmaculatus. Ecotoxicol. Environ. Saf. 2016;128:222–229. doi: 10.1016/j.ecoenv.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 19.Kim J.H., Choi H., Sung G.H., Seo S.A., Kim K.I., Kang Y.J., Kang J.C. Toxic effects on hematological parameters and oxidative stress in juvenile olive flounder, Paralichthys olivaceus exposed to waterborne zinc. Aquac. Rep. 2019;15 [Google Scholar]
- 20.Silva A.M., Barbosa I.A., Seabra C., Beltrão N., Santos R., Vega-Naredo I., Oliveira P.J., Cunha-Oliveira T. Involvement of mitochondrial dysfunction in nefazodone-induced hepatotoxicity. Food. Chem. Toxicol. 2016;94:148–158. doi: 10.1016/j.fct.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Sayed A.E.-D.H., Mahmoud U.M., Mekkawy I.A. Erythrocytes alterations of monosex tilapia (Oreochromis niloticus, Linnaeus, 1758) produced using methyltestosterone, Egypt. J. Aquat. Res. 2016;42:83–90. [Google Scholar]
- 22.Bücker A., Conceição M.B.d. Genotoxicity evaluation of tilapia (Oreochromis niloticus) exposed to waters from two sites of Itajai-Acu River (SC, Brazil) J. Braz. Soc. Ecotoxicol. 2012;7:51–56. [Google Scholar]
- 23.Firidin G. Oxidative stress parameters, induction of lipid peroxidation, and ATPase activity in the liver and kidney of Oreochromis niloticus exposed to lead and mixtures of lead and zinc. Bull. Environ. Contam. Toxicol. 2018;100:477–484. doi: 10.1007/s00128-018-2281-0. [DOI] [PubMed] [Google Scholar]
- 24.Khatun M.M., Mostakim G.M., Moniruzzaman M., Rahman U.O., Islam M.S. Distortion of micronuclei and other peripheral erythrocytes caused by fenitrothion and their recovery assemblage in zebrafish. Toxicol. Rep. 2021;8:415–421. doi: 10.1016/j.toxrep.2021.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehra S., Chadha P. Naphthalene-2-sulfonate induced toxicity in blood cells of freshwater fish Channa punctatus using comet assay, micronucleus assay and ATIR-FTIR approach. Chemosphere. 2021;265 doi: 10.1016/j.chemosphere.2020.129147. [DOI] [PubMed] [Google Scholar]
- 26.Yamindago A., Lee N., Lee N., Jo Y., Woo S., Yum S. Fluoxetine in the environment may interfere with the neurotransmission or endocrine systems of aquatic animals. Ecotoxicol. Environ. Saf. 2021;227 doi: 10.1016/j.ecoenv.2021.112931. [DOI] [PubMed] [Google Scholar]
- 27.Sumi N., Chitra K.C. Cytogenotoxic effects of fullerene C60 in the freshwater teleostean fish, Anabas testudineus (Bloch, 1792) Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019;847 doi: 10.1016/j.mrgentox.2019.503104. [DOI] [PubMed] [Google Scholar]
- 28.Abdel-Khalek A.A., Dajem S.B., Morsy K. The potential use of rice husk for reducing the genotoxic effects of iron and aluminum oxides nanoparticles in Oreochromis niloticus. Water Air Soil Pollut. 2020;231:139. [Google Scholar]
- 29.Qualhato G., Rocha T.L., de Oliveira Lima E.C., Silva D.M.E., Cardoso J.R., Koppe Grisolia C., de Sabóia-Morais S.M.T. Genotoxic and mutagenic assessment of iron oxide (maghemite-γ-Fe2O3) nanoparticle in the guppy Poecilia reticulata. Chemosphere. 2017;183:305–314. doi: 10.1016/j.chemosphere.2017.05.061. [DOI] [PubMed] [Google Scholar]
- 30.Monteiro V., Cavalcante D.G., Viléla M.B., Sofia S.H., Martinez C.B. In vivo and in vitro exposures for the evaluation of the genotoxic effects of lead on the Neotropical freshwater fish Prochilodus lineatus. Aquat. Toxicol. 2011;104:291–298. doi: 10.1016/j.aquatox.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Barreto A., Luis L.G., Soares A., Paíga P., Santos L., Delerue-Matos C., Hylland K., Loureiro S., Oliveira M. Genotoxicity of gemfibrozil in the gilthead seabream (Sparus aurata) Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2017;821:36–42. doi: 10.1016/j.mrgentox.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Castro D., Mieiro C.L., Coelho J.P., Guilherme S., Marques A., Santos M.A., Duarte A.C., Pereira E., Pacheco M. Addressing the impact of mercury estuarine contamination in the European eel (Anguilla anguilla L., 1758) - An early diagnosis in glass eel stage based on erythrocytic nuclear morphology. Mar. Pollut. Bull. 2018;127:733–742. doi: 10.1016/j.marpolbul.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 33.Mofizur Rahman M., Baek H.J. Evaluation of erythrocyte morphometric indices in juvenile red spotted grouper, Epinephelus akaara under elevated water temperature. Dev. Reprod. 2019;23:345–353. doi: 10.12717/DR.2019.23.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guimarães A.T.B., Estrela F.N., Pereira P.S., de Andrade Vieira J.E., de Lima Rodrigues A.S., Silva F.G., Malafaia G. Toxicity of polystyrene nanoplastics in Ctenopharyngodon idella juveniles: a genotoxic, mutagenic and cytotoxic perspective. Sci. Total. Environ. 2021;752 doi: 10.1016/j.scitotenv.2020.141937. [DOI] [PubMed] [Google Scholar]
- 35.Almeida S., Rocha T.L., Qualhato G., Oliveira L., Amaral C., Conceição E., Sabóia-Morais S., Bailão E. Acute exposure to environmentally relevant concentrations of benzophenone-3 induced genotoxicity in Poecilia reticulata. Aquat. Toxicol. 2019;216 doi: 10.1016/j.aquatox.2019.105293. [DOI] [PubMed] [Google Scholar]
- 36.Witeska M. Erythrocytes in teleost fishes: a review. Zool. Ecol. 2013;23:275–281. [Google Scholar]
- 37.Blahova J., Doubkova V., Plhalova L., Lakdawala P., Medkova D., Vecerek V., Svobodova Z., Faggio C. Embryotoxicity of selective serotonin reuptake inhibitors–comparative sensitivity of zebrafish (Danio rerio) and African clawed frog (Xenopus laevis) embryos. Appl. Sci. 2021;11:10015. [Google Scholar]
- 38.Weinberger J., 2nd, Klaper R. Environmental concentrations of the selective serotonin reuptake inhibitor fluoxetine impact specific behaviors involved in reproduction, feeding and predator avoidance in the fish Pimephales promelas (fathead minnow) Aquat. Toxicol. 2014;151:77–83. doi: 10.1016/j.aquatox.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.OECD, Test No. 203: Fish, Acute Toxicity Test, 2019.
- 40.Omar W.A., Zaghloul K.H., Abdel-Khalek A.A., Abo-Hegab S. Genotoxic effects of metal pollution in two fish species, Oreochromis niloticus and Mugil cephalus, from highly degraded aquatic habitats. Mutat. Res. 2012;746:7–14. doi: 10.1016/j.mrgentox.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Botelho R.G., Christofoletti C.A., Correia J.E., Ansoar Y., Olinda R.A., Tornisielo V.L. Genotoxic responses of juvenile tilapia (Oreochromis niloticus) exposed to florfenicol and oxytetracycline. Chemosphere. 2015;132:206–212. doi: 10.1016/j.chemosphere.2015.02.053. [DOI] [PubMed] [Google Scholar]
- 42.Trigueiro N., Gonçalves B.B., Dias F.C., de Oliveira Lima E.C., Rocha T.L., Sabóia-Morais S. Co-exposure of iron oxide nanoparticles and glyphosate-based herbicide induces DNA damage and mutagenic effects in the guppy (Poecilia reticulata) Environ. Toxicol. Pharmacol. 2021;81 doi: 10.1016/j.etap.2020.103521. [DOI] [PubMed] [Google Scholar]
- 43.Kosai P., Jiraungkoo W., Synsatayak A., Jiraungkoo K. Efficacy of calcium reducing lead toxicity in hematology of Oreochromis niloticus. J. Fish. Aquat. Sci. 2011;6:346–355. [Google Scholar]
- 44.Pradhan D., Singh R.K., Verma S.K. Genotoxic potential assessment of the herbicide bispyribac-sodium in a fresh water fish Clarias batrachus (Linn.) Bull. Environ. Contam. Toxicol. 2020;105:715–720. doi: 10.1007/s00128-020-03003-8. [DOI] [PubMed] [Google Scholar]
- 45.Ossa-López P.A., Castaño-Villa G.J., Rivera-Páez F.A. Genotoxic effects and gene expression in Danio rerio (Hamilton 1822) (Cypriniformes: Cyprinidae) exposed to mining-impacted tributaries in Manizales, Colombia. Environ. Monit. Assess. 2017;189:520. doi: 10.1007/s10661-017-6231-9. [DOI] [PubMed] [Google Scholar]
- 46.Roda J.F.B., Lauer M.M., Risso W.E., Dos Reis Martinez C. Bueno. Microplastics and copper effects on the neotropical teleost Prochilodus lineatus: is there any interaction? Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020;242 doi: 10.1016/j.cbpa.2020.110659. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed I., Sheikh Z.A. Comparative study of hematological parameters of snow trout Schizopyge plagiostomus and Schizopyge niger inhabiting two different habitats. Eur. Zool. J. 2020;87:12–19. [Google Scholar]
- 48.Najiah M., Nadirah M., Marina H., Lee S.W., Nazaha W.H. Quantitative comparisons of erythrocyte morphology in healthy freshwater fish species from Malaysia. Res. J. Fish. Hydrobiol. 2008;3:32–35. [Google Scholar]
- 49.Zhelev Z.M., Arnaudova D.N., Popgeorgiev G.S., Tsonev S.V. Determinations of erythrocyte sizes in adult Pelophylax ridibundus (Amphibia: Anura: Ranidae) inhabiting industrial area in Southern Bulgaria. Water Air Soil Pollut. 2021;232:125. [Google Scholar]
- 50.Marques A., Marçal R., Pereira V., Pereira P., Mieiro C., Guilherme S., Marques C., Santos M.A., Pereira R., Abreu H., Gaivão I., Pacheco M. Macroalgae-enriched diet protects gilthead seabream (Sparus aurata) against erythrocyte population instability and chromosomal damage induced by aqua-medicines. J. Appl. Phycol. 2019;32:1477–1493. [Google Scholar]
- 51.Corcoran J., Winter M.J., Tyler C.R. Pharmaceuticals in the aquatic environment: a critical review of the evidence for health effects in fish. Crit. Rev. Toxicol. 2010;40:287–304. doi: 10.3109/10408440903373590. [DOI] [PubMed] [Google Scholar]
- 52.Brooks B.W., Foran C.M., Richards S.M., Weston J., Turner P.K., Stanley J.K., Solomon K.R., Slattery M., La Point T.W. Aquatic ecotoxicology of fluoxetine. Toxicol. Lett. 2003;142:169–183. doi: 10.1016/s0378-4274(03)00066-3. [DOI] [PubMed] [Google Scholar]
- 53.Hemalatha D., Nataraj B., Rangasamy B., Maharajan K., Ramesh M. Exploring the sublethal genotoxic effects of class II organophosphorus insecticide quinalphos on freshwater fish Cyprinus carpio. J. Oceanol. Limnol. 2020;39:661–670. [Google Scholar]
- 54.Islam S.M.M., Rohani M.F., Zabed S.A., Islam M.T., Jannat R., Akter Y., Shahjahan M. Acute effects of chromium on hemato-biochemical parameters and morphology of erythrocytes in striped catfish Pangasianodon hypophthalmus. Toxicol. Rep. 2020;7:664–670. doi: 10.1016/j.toxrep.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simide R., Richard S., Prévot-D′Alvise N., Miard T., Gaillard S. Assessment of the accuracy of physiological blood indicators for the evaluation of stress, health status and welfare in Siberian sturgeon (Acipenser baerii) subject to chronic heat stress and dietary supplementation. Int. Aquat. Res. 2016;8:121–135. [Google Scholar]
- 56.Datta S.N., Kaur V.I., Dhawan A., Jassal G. Estimation of length-weight relationship and condition factor of spotted snakehead Channa punctata (Bloch) under different feeding regimes. Springerplus. 2013;2:436. doi: 10.1186/2193-1801-2-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cotelle S., Férard J.F. Comet assay in genetic ecotoxicology: a review. Environ. Mol. Mutagen. 1999;34:246–255. [PubMed] [Google Scholar]
- 58.Kang S.H., Kwon J.Y., Lee J.K., Seo Y.R. Recent advances in in vivo genotoxicity testing: prediction of carcinogenic potential using comet and micronucleus assay in animal models. J. Cancer Prev. 2013;18:277–288. doi: 10.15430/JCP.2013.18.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahmadimanesh M., Balarastaghi S., Rashedinia M., Yazdian-Robati R. A systematic review on the genotoxic effect of serotonin and norepinephrine reuptake inhibitors (SNRIs) antidepressants. Psychopharmacology. 2020;237:1909–1915. doi: 10.1007/s00213-020-05550-8. [DOI] [PubMed] [Google Scholar]
- 60.Kashanian S., Javanmardi S., Chitsazan A., Omidfar K., Paknejad M. DNA-binding studies of fluoxetine antidepressant. DNA Cell Biol. 2012;31:1349–1355. doi: 10.1089/dna.2012.1657. [DOI] [PubMed] [Google Scholar]
- 61.Kohen R., Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002;30:620–650. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- 62.Lacaze E., Pédelucq J., Fortier M., Brousseau P., Auffret M., Budzinski H., Fournier M. Genotoxic and immunotoxic potential effects of selected psychotropic drugs and antibiotics on blue mussel (Mytilus edulis) hemocytes. Environ. Pollut. 2015;202:177–186. doi: 10.1016/j.envpol.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 63.Ding J., Lu G., Li Y. Interactive effects of selected pharmaceutical mixtures on bioaccumulation and biochemical status in crucian carp (Carassius auratus) Chemosphere. 2016;148:21–31. doi: 10.1016/j.chemosphere.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 64.Ren X., Wang Z., Gao B., Liu P., Li J. Toxic responses of swimming crab (Portunus trituberculatus) larvae exposed to environmentally realistic concentrations of oxytetracycline. Chemosphere. 2017;173:563–571. doi: 10.1016/j.chemosphere.2017.01.078. [DOI] [PubMed] [Google Scholar]
- 65.Jindal R., Verma S. In vivo genotoxicity and cytotoxicity assessment of cadmium chloride in peripheral erythrocytes of Labeo rohita (Hamilton) Ecotoxicol. Environ. Saf. 2015;118:1–10. doi: 10.1016/j.ecoenv.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 66.Sommer S., Buraczewska I., Kruszewski M. Micronucleus assay: the state of art, and future directions. Int. J. Mol. Sci. 2020;21:1534. doi: 10.3390/ijms21041534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Terradas M., Martín M., Tusell L., Genescà A. Genetic activities in micronuclei: is the DNA entrapped in micronuclei lost for the cell? Mutat. Res. 2010;705:60–67. doi: 10.1016/j.mrrev.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 68.Luzhna L., Kathiria P., Kovalchuk O. Micronuclei in genotoxicity assessment: from genetics to epigenetics and beyond. Front. Genet. 2013;4:131. doi: 10.3389/fgene.2013.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodrigues S., Antunes S.C., Correia A.T., Nunes B. Ecotoxicological evaluation of gilthead seabream (Sparus aurata) exposed to the antibiotic oxytetracycline using a multibiomarker approach. Mar. Environ. Res. 2018;141:233–246. doi: 10.1016/j.marenvres.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 70.Anifowoshe A., Oyebanji J., Oladipo O., Oyeyemi F., Abdulrahim M., Abdulkareem S., Mustapha M. Histological changes, micronuclei induction and nuclear abnormalities in the peripheral erythrocytes of Clarias gariepinus (Burchell 1822) exposed to water sample from Apodu reservoir. J. Life Sci. Res. 2020;1:01–07. [Google Scholar]
- 71.Alkaladi A., El-Deen N.A., Afifi M., Zinadah O.A. Hematological and biochemical investigations on the effect of vitamin E and C on Oreochromis niloticus exposed to zinc oxide nanoparticles. Saudi J. Biol. Sci. 2015;22:556–563. doi: 10.1016/j.sjbs.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Al-Obaidi I.A., Al-Shawi N.N. Assessment the genotoxic potential of fluoxetine and amitriptyline at maximum therapeutic doses for four-week treatment in experimental male rats. Iraqi J. Pharmaceut. Sci. 2020;30:81–90. [Google Scholar]
- 73.Sadiqul I.M., Kabir S.M., Ferdous Z., Mansura K.M., Khalilur R.M. Chronic exposure to quinalphos shows biochemical changes and genotoxicty in erythrocytes of silver barb, Barbonymus gonionotus. Interdiscip. Toxicol. 2017;10:99–106. doi: 10.1515/intox-2017-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berillis P., Mente E., Nikouli E., Makridis P., Grundvig H., Bergheim A., Gausen M. Improving aeration for efficient oxygenation in sea bass sea cages. Blood, brain and gill histology. Open Life Sci. 2016;11:270–279. [Google Scholar]
- 75.Aliko V., Korriku R.S., Pagano M., Faggio C. Double-edged sword: fluoxetine and ibuprofen as development jeopardizers and apoptosis' inducers in common toad, Bufo bufo, tadpoles. Sci. Total. Environ. 2021;776 doi: 10.1016/j.scitotenv.2021.145945. [DOI] [PubMed] [Google Scholar]
- 76.Zabotkina E.A., Golovanov V.K., Golovanova I.L. Effects of roundup herbicide and increase in water temperature on the parameters of peripheral blood cells in Amur sleeper Perccottus glenii Dybowski. Inland Water Biol. 2018;11:207–213. [Google Scholar]
- 77.Maceda-Veiga A., Monroy M., Viscor G., De Sostoa A. Changes in non-specific biomarkers in the Mediterranean barbel (Barbus meridionalis) exposed to sewage effluents in a Mediterranean stream (Catalonia, NE Spain) Aquat. Toxicol. 2010;100:229–237. doi: 10.1016/j.aquatox.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 78.Osman A.G.M., AbouelFadl K.Y., Abd El Reheem A.E.B.M., Mahmoud U.M., Kloas W., Moustafa M.A. Blood biomarkers in Nile tilapia Oreochromis niloticus niloticus and African catfish Clarias gariepinus to evaluate water quality of the river Nile. J. Fishscicom. 2018;12:1–15. [Google Scholar]
- 79.Kori R.S., Aladkatti R.H., Desai S.D., Das K.K. Effect of anti-stress activity of fluoxetine on restrained stress induced male albino rats in hematological parameters and whole brain histopathology. J. Young Pharm. 2017;9:246–250. [Google Scholar]
- 80.Sharma J., Dar S.A., Sayani A.N., Langer S. Effect of stressors on haematological and hormonal parameters of Garra gotyla gotyla. Int. J. Curr. Microbiol. App. Sci. 2017;6:357–369. [Google Scholar]
- 81.Attia S.M. Molecular cytogenetic evaluation of the aneugenic effects of teniposide in somatic and germinal cells of male mice. Mutagenesis. 2012;27:31–39. doi: 10.1093/mutage/ger051. [DOI] [PubMed] [Google Scholar]
- 82.Ergul M., Bakar-Ates F. Investigation of molecular mechanisms underlying the antiproliferative effects of colchicine against PC3 prostate cancer cells. Toxicol. In Vitro. 2021;73 doi: 10.1016/j.tiv.2021.105138. [DOI] [PubMed] [Google Scholar]
- 83.Gustavino B., Scornajenghi K.A., Minissi S., Ciccotti E. Micronuclei induced in erythrocytes of Cyprinus carpio (teleostei, pisces) by X-rays and colchicine. Mutat. Res. 2001;494:151–159. doi: 10.1016/s1383-5718(01)00191-7. [DOI] [PubMed] [Google Scholar]
- 84.Melo K.M., Grisolia C.K., Pieczarka J.C., de Souza L.R., Filho Jde S., Nagamachi C.Y. FISH in micronucleus test demonstrates aneugenic action of rotenone in a common freshwater fish species, Nile tilapia (Oreochromis niloticus) Mutagenesis. 2014;29:215–219. doi: 10.1093/mutage/geu005. [DOI] [PubMed] [Google Scholar]
- 85.Cho J.H., Joo Y.H., Shin E.Y., Park E.J., Kim M.S. Anticancer effects of colchicine on hypopharyngeal cancer. Anticancer Res. 2017;37:6269–6280. doi: 10.21873/anticanres.12078. [DOI] [PubMed] [Google Scholar]
- 86.Zenker A., Cicero M.R., Prestinaci F., Bottoni P., Carere M. Bioaccumulation and biomagnification potential of pharmaceuticals with a focus to the aquatic environment. J. Environ. Manag. 2014;133:378–387. doi: 10.1016/j.jenvman.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 87.Du B., Haddad S.P., Luek A., Scott W.C., Saari G.N., Kristofco L.A., Connors K.A., Rash C., Rasmussen J.B., Chambliss C.K., Brooks B.W. Bioaccumulation and trophic dilution of human pharmaceuticals across trophic positions of an effluent-dependent wadeable stream. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369 doi: 10.1098/rstb.2014.0058. 20140058. [DOI] [PMC free article] [PubMed] [Google Scholar]