Abstract
This study was carried out to assess the distribution of trace metals in soil samples from selected automobile mechanic workshops (AMWs) in Benin City, spatially map the concentrations and estimate the health risk indices for the exposed populace. Topsoil samples were collected from twenty-one (21) AMWs in Benin City in 3 composites for three months at each station. Soil samples were analyzed for heavy metals (Pb, Cd, Fe, Cu, Ni, Cr, and Zn) using Atomic Absorption Spectrophotometer. The non-carcinogenic risks caused by exposure to the metals were insignificant, characterized by in mean values of hazard quotient (HQ) and hazard index (HI) below one (1) in adults and children. Carcinogenic risk (CR) occurred only in the children exposed to nickel through ingestion; attributable to ingestion. In adults and children, the risks associated with the uptake routes were in the order of ingestion > dermal contact > inhalation. The hazard index (HI) values of heavy metals for children and adults decreased in the order of Pb > Cr > Cd > Cu > Zn > Ni and were all lower than one (1), which indicated that the children and adults were not at non-carcinogenic risk. The contamination factors (CF) of all metals analyzed were lower than one (1), suggesting low contamination. The average CF decreased in the order of Pb (0.3715) > Zn (0.14) > Cu (0.087) > Cr (0.013) > Ni (0.01) > Fe (0.0007). Potential ecological risks of the trace metals in soils of these workshops revealed low pollution of the soils by the metals. Results indicated that the three routes of uptake in adults and children decreased in the order of ingestion > dermal > inhalation. The non-carcinogenic risks posed by metals to the children and adults were insignificant. Ingested nickel however posed potential carcinogenic risk to only the children. The toxicodynamics of heavy metals in the soil profile demonstrated in this study could be a vital information for future studies and decisions on the management of the health and environment of the study area.
Keywords: Trace metals, Health risk, Ecological risk, Automobile, Geoaccumulation index, Pollution load index
Graphical Abstract
Highlights
-
•
The exposure pathways of metals in adults and children decreased in the order of ingestion > dermal > inhalation.
-
•
The average of contamination factor decreased in the order of Pb > Zn > Cu > Cr > Ni> Fe.
-
•
The non-carcinogenic risk potentials for metal intake in children were higher than that of adults.
1. Introduction
Recently in Benin City, Nigeria, there has been a rise in emigration rates to European countries. This has allegedly increased the rate of importations of used cars, commonly called “Tokunbo” car into the country, particularly in Benin City. Following the increase in demand for maintenance of these cars, there has been a commensurate sharp rise in the number of automobile mechanic workshops (AMWs) within the Benin metropolis. A survey of Benin City indicates that there are about 507 automobile departmentalized workshops scattered all over the city rendering car services which involve a change of the used engine oil and parts which are indiscriminately discarded in the environment may be a great source of soil contamination [14], [38]. These workshops procure spare parts from two major automobile spare part markets within the city: Uwelu and Evbareke spare part markets [37].
Cadmium is released from tires' wear and tear and vehicular emissions, aided by lubricant oil. Cadmium is also used an automobile coating which offers good corrosion resistance. When exposed to cadmium, zinc (a vital trace element) is usually displaced by cadmium, thus may disrupt metabolic processes [42]. In developed countries, vehicular emissions are monitored by various regulations, and policies compared to developing countries which are the destination of automobiles that fail vehicular emission tests. Lead is released by the exhaust of such vehicles and from their worn metal alloys in the engine [2], [84]. Lead might adversely affect mental health, causing cardiovascular and neurological diseases in humans, especially children. Exposure to lead may cause kidney and brain damage and ultimately death. Impairment of cognitive performance by lead has also been reported [10]. Lead and cadmium are carcinogenic and can cause bone fractures and malformation, nephrological, neurological, hematological dysfunctions; hypertension, and immunosuppression [9].
Zinc is sourced mainly from the wear of galvanized parts such as fuel tanks. Brake wear is the primary source of copper and lead [84]. When an automobile engine is running, the engine oil, transmission oil, and hydraulic fluid pick up worn metal debris which contains Pb, Cd, Zn, Fe, Cu [25]. The incidence of engine wear and tear depends on the automobile's age, condition, and the transmission systems [1], [87]. Sadly, used vehicles and spare part reuse are predominant in the metropolis of Benin City [3], [16], [56]. Contamination of soils with trace metals from AMWs has been widely reported in Nigeria [39], [38], [56], [7], [37]. For example, David and Sunday [23] reported a strong correlation between vehicular emissions and trace metal accumulation in the soil samples from roadsides within Ota metropolis, Ogun State, Nigeria. Elsewhere, Pam et al. [69] discovered the concentration levels of Pb, Cd, Ni, Cu, Mn, and Zn in soils around two major AMWs in Benue State were above background levels and permissible limits recommended for soils. Recently, Anegbe et al. [15] observed higher concentrations of trace metals in soil samples collected within the metropolis of Benin City were higher than the average concentration in other neighboring states. Ibrahim et al. [36] linked the contamination of soil samples from Borno State, Nigeria, to servicing practices of AMWs using pollution load indices.
Furthermore, health hazards associated with heavy metals in soil have also been reported over the years in Nigeria. Odukoya et al. [61] reported notable health risk indices associated with exposure to concentrations of Mo, Ag, Cr, Ni, Cu, Pb, Zn, As, Sb, Bi, Cr, Tl, Se, Hg and Cd in soil samples within industrial estates of Southwestern Nigeria. Later on, Adedeji et al. [4] reported significant health risks due to exposure to Cd, Cr, Cu, Mn, Ni, Pb, and Zn the soil samples collected from Ijebu-Ode, Ogun State, Nigeria. Recently, Isibor et al. [43] reported notable health risk impacts of selected trace metals detected in the water and soil samples of a tropical rainforest river in Benin City, Nigeria. Soil is however an essential resource to agricultural and global food security. Because it plays a crucial role in maintaining the proper functioning and sustenance of the earth’s ecosystems [12]), its contamination is thus a fundamental environmental problem in areas densely populated with AMWs [43].
The mechanics and residents within the catchment area are at risk of exposure to contaminated soil through dermal, ingestion, and inhalation. It has been reported that automobiles introduce several toxic metals into the Nigerian environment [50], [60] through the wear and tear of tires and engine parts, grease and oil leaks, metal seepages from automobile catalysts, and panel beating [5]. These mechanic-associated anthropogenic activities increase the likelihood of exposure of the populace in the metropolis of Benin City, Nigeria [41], [56], [57], from which used engine oils and other motor oils containing trace metals are indiscriminately discharged by artisans in the business of automobile repairs and servicing [15]. Even essential metals, when in excess is injurious. Excess zinc in the human body can also disrupt the immune system [10]. Excess copper intake can elicit liver damage and initiate gastrointestinal complications [10]. Nickel is considered one of the leading human carcinogens. The study aimed to assess the spatial distribution of trace metals in soil samples within the catchment areas of automobile mechanic workshops and estimate the associated health risk. The study seeks to employ Geo-accumulation indices (Igeo) and the potential ecological risk index (PER) methods to evaluate and assess the pollution severity.
2. Materials and method
2.1. 2.1 The study area
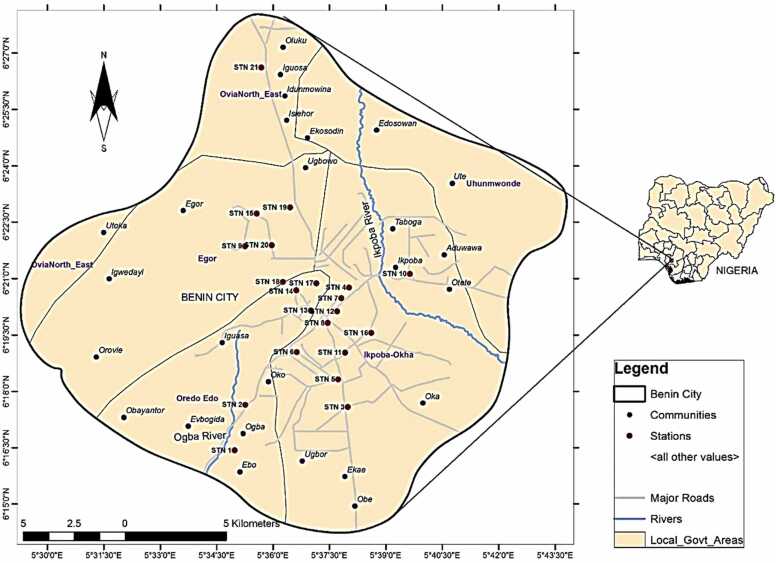
The sampling locations were 21 AMWs, located in selected parts of Benin City, Edo State, Nigeria (Fig. 1).
Fig. 1.
Map showing sampled locations.
Map The map was designed using QGIS software version 3.10.1 'A Coruña' [70].
URL: https://qgis.org/en/site/forusers/download.html#.
The longitudes and latitudes of the stations’ decimal degrees were 6.27368, 5.58302 (Station 1); 6.29393, 5.58775 (Station 2); 6.29288, 5.63317 (Station 3); 6.34599, 5.63379 (Station 4); 6.30521, 5.6289 (Station 5); 6.31736, 5.61061 (Station 6); 6.3412, 5.63032 (Station 7); 6.33023, 5.62429 (Station 8); 6.36433, 5.58767 (Station 9); 6.35201, 5.66074 (Station 10); 6.31709, 5.63196 (Station 11); 6.33538, 5.62848 (Station 12); 6.33575, 5.61679 (Station 13); 6.3447, 5.61034 (Station 14); 6.3788, 5.59276 (Station 15); 6.32582, 5.64361(Station 16); 6.34782, 5.61927 (Station 17); 6.34853, 5.60439 (Station 18); 6.38149, 5.6076 (Station 19); 6.36474, 5.59944 (Station 20); 6.44357, 5.59486 (Station 21).
2.2. Sample collection
Topsoil samples were collected at 10 cm depth from 21 AMWs in Benin City by digging 10 cm beneath the soil with the aid of sterilized trowel. This exercise was conducted monthly between May and July, 2019. At each station, three composites of soil samples were collected with the aid of a soil ager and then rigorously stirred with the aid of a rotator at to get a representative sample. The collected soil samples were preserved in labeled aluminum and transported to the laboratory. The sampling duration was spread over three months to capture the impacts of rain on the soil samples which might occur through surface runoff.
2.3. Sample preparation and analysis
The soil samples were air-dried for 48 h, sieved, and ground to smoothness using ceramic mortar and pestle. Soil samples were digested according to the modified method of Enuneku et al. [28]. Afterward, 1 g of the sample was digested in 10 mL freshly prepared aqua regia (3:1, HNO3:HCl) in a hot sand bath for 45 min and allowed to cool afterward Twenty (20) mL of distilled water was added [28]. The sample was filtered using a Whatman filter paper (110 mm) into a 100 mL standard flask [28]. Thereafter the sample was made up to 100 mL mark with distilled water. Samples were then analyzed for the concentrations of lead, copper, zinc, iron, nickel, cadmium, and chromium using atomic absorption spectrophotometer (Buck Scientific, 210 VGP).
2.3.1. Quality control and quality assurance
The precision of the AAS was validated by repeating the experimental procedure three times. Certified reference materials (CRM) and standard reference materials (SRM) provided by the Federal Environmental Protection Agency (2003) were adopted as a guide. The recovery rates ranged from 89% to 96%.
The relative standard deviation (SD) was < 6% suggested data reliability [43]. The curves were derived from the reference solutions prepared from analyte grade stock solutions containing 1000 g/kg of lead, copper, zinc, iron, nickel, cadmium, and chromium. The concentrations were stated in mg/kg [43].
The limits of detection (LOD) and the limits of quantification (LOQ) were estimated from the standard deviation of 10 readings from the analytical blanks and the slopes of the analytical curves (LOD = 3σ/ slope and LOQ = 10σ/ slope) [43]. The values were 0.05–0.07 µg/kg for Fe, 0.07–0.123 µg/kg for Pb, 0.06–0.121 µg/kg for Cd, 0.043–0.127 µg/kg for Cr, 0.023–0.132 µg/kg for Cu, and 0.013–0.117 µg/kg for Ni.
2.4. Health risk estimations
Human risk assessment was used to estimate the possible risk level inflicted by the metals on exposed individuals [47]. Identification of hazards, assessment of exposure, dose-response assessment, and risk characterization [59]. Health risk assessment was determined using the non-carcinogenic and carcinogenic risks for both adults and children. The health risks posed by metals were expressed as chronic daily intake (CDI) (mg/kg/day). The risks associated with uptake of metals through incidental ingestion (CDIing) and dermal contact (CDIderm) were calculated thus;
CS= metal concentration in the soil (mg/kg); IRs= ingestion rate (mg/day); CF= conversion factor (kg/mg); FI= fraction ingested from the contaminated source (unitless); ED= exposure duration (years); EF of the CDIing= exposure frequency (days/year); BW= average body weight (kg); AT= average time (days); ABS= dermal absorption factor (unitless); SA= exposed surface area of skin (cm2/event); AF= skin adherence factor (mg/cm2); EF of CDIderm= exposure frequency (events/year); PEF= the particle emission factor.
2.4.1. Non-carcinogenic risk assessment
The hazard quotient (HQ) was employed to assess the non-carcinogenic health risk of each metal on the individuals in the study area. It was expressed as the ratio of the average daily dose that each individual receives through the three routes of uptake to a reference value RfD [85]. The Hazard Index (HI) was expressed as the sum of the HQ values for the metals; expressed as the non-carcinogenic risk posed collectively by the heavy metals.
The equations were calculated thus:
2.4.2. Carcinogenic risk
Carcinogenic risk (CR) was expressed as the product of the chronic daily intake (CDI) and the cancer slope factor (CSF) over a lifetime of an individual.
CR was calculated thus:
2.5. Data analysis
Results in this study were presented as mean ± standard deviation. Before conducting parametric tests, data characteristics were tested for homogeneity of variance and normality. Shapiro-Wilk’s test was conducted, including a visual inspection of histograms, standard Q-Q plots, which showed that data were normally distributed as the null hypothesis was accepted at p = 0.001. One-way analysis of variance was carried out to test for significant differences in heavy metal concentrations (spatial and temporal), and subsequently, post-hoc tests were done to locate the differences using SPSS (version 20).
2.5.1. Spatial mapping of metal distribution
Spatial mapping of heavy metal distribution in the soil of the catchment area of the AMWs was done using ArcGIS (version 10.4) software. The interpolation method adopted was inverse distance weighting (IDW) in the Geostatistical Analyst of ArcToolbox in the software [18]. IDW is a commonly used tools in the interpolation of pollution data [20].
2.5.2. Pollution load, geo-accumulation and potential ecological risk indices
The pollution load index (PLI) was calculated thus;.
| PLI= (CF1 X CF2 X CF3 X CF4 X……CFn)1/n |
| Contamination factor (CF) = [Cmetal]/ [Cbackground] |
The contamination was graded on a scale range of 1–6 (0 = not polluted, 1 = none to moderately polluted, 2 = moderately polluted, 3 = moderately to strongly polluted, 4 = strongly polluted, 5 = strongly to very strongly polluted, 6 = very strongly polluted [53].
The geo-accumulation index was computed to analyze the concentrations of the metals in the soil in comparison to the natural background level [49]. This gives a clear picture as to the progress of the soil contamination in time and space.
Geo-accumulation index (Igeo) was calculated thus;.
| Igeo= Log2 (B6/ B8 x B9) |
| Igeo= log2 Cs |
2.6. 1.5 X Gb
Cs= concentration of metal in soil, the B8 = conversion factor (1.5), and Gb is the geochemical background value of the metal.
The potential ecological risk index (PER) of the trace metals in soil samples was estimated to determine the possibility of the metals causing ecological hazards that might impair the ecological services such as ground water purification and support for plants.
PER was calculated thus;
= pollution coefficient of each metal.
= measured concentration of each soil sample.
= background concentration of metal in soil.
= toxicity factors of Pb (5), Cd (5), Fe (1), Cu (5), Ni (10), Cr (2), and Zn (1) were adopted from Xu et al. [86].
= potential ecological risk factor of each metal.
3. Results
3.1. Concentrations of trace metals in soil samples
The concentrations of Pb, Fe, Zn, and Cu in the soil samples at all the stations exceeded the established regulatory limits set by FEPA (Table 1). Conversely, the concentrations of chromium were below the established limit of FEPA [30]. The lead concentration at Stations 2 and 18 were significantly higher than the concentration at Station 14, which was in turn higher than other stations (p < 0.05).
Table 1.
Trace metal concentration (mg/kg) in soil samples of AMWs.
| Stations |
Lead |
Iron |
Zinc |
Copper |
Nickel |
Cadmium |
Chromium |
|---|---|---|---|---|---|---|---|
| Mean±SD(Min – Max) | Mean±SD(Min – Max) | Mean±SD(Min – Max) | Mean±SD(Min – Max) | Mean±SD(Min – Max) | Mean±SD(Min – Max) | Mean±SD(Min – Max) | |
| STN 1 | 2.07 ± 0.93(1.30 – 3.10) | 34.80 ± 2.96(31.40 – 36.80) | 7.90 ± 8.32(3.05 – 17.50) | 1.23 ± 0.84(0.41 – 2.08) | 0.33 ± 0.22(0.19 – 0.59) | 0.00 ± 0.00(0.00 – 0.00) | 1.13 ± 0.27(0.81 – 1.29) |
| STN 2 | 7.43 ± 6.20 A(1.20 – 13.60) | 35.97 ± 3.79(31.80 – 39.20) | 5.60 ± 5.31(2.01 – 11.70) | 0.89 ± 0.65(0.31 – 1.60) | 0.28 ± 0.21(0.13 – 0.52) | 0.01 ± 0.02(0.00 – 0.03) | 1.03 ± 0.39(0.68 – 1.45) |
| STN 3 | 3.00 ± 2.09(1.60 – 5.40) | 33.77 ± 0.38(33.50 – 34.20) | 2.57 ± 0.91(1.83 – 3.59) | 0.62 ± 0.81(0.00 – 1.54) | 0.28 ± 0.34(0.06 – 0.67) | 0.00 ± 0.01(0.00 – 0.01) | 0.23 ± 0.14(0.13 – 0.39) |
| STN 4 | 1.70 ± 0.26(1.40 – 1.90) | 30.30 ± 5.24(24.60 – 34.90) | 6.22 ± 8.42(0.00 – 15.80) | 1.04 ± 0.64(0.50 – 1.75) | 0.32 ± 0.05(0.26 – 0.36) | 0.01 ± 0.01(0.00 – 0.02) | 1.11 ± 0.25(0.85 – 1.35) |
| STN 5 | 3.48 ± 2.43(1.50 – 6.20) | 34.07 ± 1.93(31.90 – 35.60) | 5.57 ± 5.69(1.74 – 12.10) | 0.49 ± 0.32(0.31 – 0.86) | 0.35 ± 0.15(0.18 – 0.45) | 0.01 ± 0.02(0.00 – 0.03) | 0.61 ± 0.13(0.46 – 0.71) |
| STN 6 | 1.77 ± 1.36(0.70 – 3.30) | 33.10 ± 2.63(31.20 – 36.10) | 8.90 ± 12.26(0.73 – 23.00) | 0.64 ± 0.53(0.25 – 1.24) | 0.34 ± 0.16(0.16 – 0.46) | 0.03 ± 0.02(0.01 – 0.05) | 0.66 ± 0.23(0.40 – 0.84) |
| STN 7 | 2.88 ± 1.29(1.40 – 3.70) | 35.40 ± 3.94(32.20 – 39.80) | 10.16 ± 11.64(3.23 – 23.60) | 1.29 ± 0.34(0.96 – 1.64) | 0.28 ± 0.25(0.03 – 0.52) | 0.04 ± 0.02(0.02 – 0.05) | 0.88 ± 0.51(0.40 – 1.42) |
| STN 8 | 0.72 ± 0.68(0.00 – 1.35) | 33.67 ± 0.72(33.20 – 34.50) | 9.92 ± 13.40(2.13 – 25.40) | 0.55 ± 0.32(0.31 – 0.91) | 0.18 ± 0.24(0.03 – 0.46) | 0.01 ± 0.02(0.00 – 0.03) | 1.09 ± 0.37(0.71 – 1.45) |
| STN 9 | 1.62 ± 0.84(0.65 – 2.20) | 34.50 ± 2.86(32.20 – 37.70) | 11.72 ± 15.40(2.31 – 29.50) | 3.54 ± 5.60 A(0.17 – 10.00) | 0.06 ± 0.05(0.01 – 0.09) | 0.01 ± 0.01(0.00 – 0.02) | 0.94 ± 0.69(0.40 – 1.72) |
| STN 10 | 2.80 ± 2.07(1.10 – 5.10) | 34.43 ± 2.04(32.20 – 36.20) | 6.24 ± 6.20(2.53 – 13.40) | 0.79 ± 0.15(0.62 – 0.88) | 0.68 ± 0.71(0.12 – 1.48) | 0.00 ± 0.01(0.00 – 0.010) | 0.75 ± 0.43(0.45 – 1.25) |
| STN 11 | 1.10 ± 0.44(0.60 – 1.40) | 35.83 ± 3.69(31.60 – 38.40) | 2.72 ± 0.31 A(2.36 – 2.91) | 0.55 ± 0.22(0.30 – 0.71) | 0.15 ± 0.13(0.02 – 0.28) | 0.01 ± 0.02(0.00 – 0.03) | 0.37 ± 0.06(0.31 – 0.42) |
| STN 12 | 1.94 ± 0.58(1.52 – 2.60) | 35.00 ± 1.01(34.10 – 36.10) | 9.31 ± 12.21(2.00 – 23.40) | 1.19 ± 0.68(0.41 – 1.64) | 0.65 ± 0.75(0.11 – 1.51) | 0.01 ± 0.02(0.00 – 0.04) | 0.79 ± 0.93(0.00 – 1.82) |
| STN 13 | 1.37 ± 0.06(1.30 – 1.40) | 33.76 ± 2.36(31.07 – 35.50) | 2.12 ± 0.78 A(1.36 – 2.92) | 2.54 ± 2.00 A(0.60 – 4.60) | 0.11 ± 0.10(0.00 – 0.19) | 0.01 ± 0.00(0.01 – 0.01) | 0.11 ± 0.06(0.06 – 0.17) |
| STN 14 | 4.17 ± 3.26B(1.50 – 7.80) | 32.90 ± 5.21(27.10 – 37.20) | 13.28 ± 9.25(3.45 – 21.80) | 1.27 ± 1.1(0.33 – 2.56) | 0.33 ± 0.09(0.26 – 0.43) | 0.01 ± 0.02(0.00 – 0.03) | 0.33 ± 0.40(0.00 – 0.78) |
| STN 15 | 1.21 ± 0.21(1.00 – 1.42) | 33.47 ± 1.07(32.30 – 34.40) | 4.55 ± 3.08 A(2.62 – 8.10) | 3.93 ± 5.11 A(0.65 – 9.81) | 0.20 ± 0.17(0.06 – 0.39) | 0.00 ± 0.00(0.00 – 0.00) | 0.55 ± 0.23(0.39 – 0.81) |
| STN 16 | 1.90 ± 0.44(1.40 – 2.20) | 34.20 ± 2.81(31.30 – 36.90) | 1.47 ± 1.29 A(0.00 – 2.39) | 2.98 ± 0.49 A(2.45 – 3.41) | 0.29 ± 0.11(0.17 – 0.39) | 0.01 ± 0.01(0.00 – 0.02) | 0.74 ± 0.17(0.57 – 0.90) |
| STN 17 | 2.20 ± 1.37(0.70 – 3.40) | 32.53 ± 1.58(30.80 – 33.90) | 2.42 ± 0.37 A(2.09 – 2.82) | 0.61 ± 0.02(0.59 – 0.62) | 0.08 ± 0.01(0.07 – 0.09) | 0.00 ± 0.01(0.00 – 0.01) | 0.64 ± 0.19(0.47 – 0.84) |
| STN 18 | 7.42 ± 9.28 A(0.65 – 18.00) | 34.53 ± 1.33(33.00 – 35.40) | 5.87 ± 6.58(1.22 – 13.40) | 1.56 ± 1.19(0.19 – 2.40) | 0.09 ± 0.12(0.00 – 0.23) | 0.01 ± 0.01(0.00 – 0.02) | 0.65 ± 0.29(0.34 – 0.91) |
| STN 19 | 0.93 ± 0.21(0.70 – 1.10) | 34.10 ± 2.10(32.00 – 36.20) | 2.04 ± 0.66 A(1.42 – 2.73) | 0.32 ± 0.14(0.19 – 0.46) | 0.29 ± 0.11(0.18 – 0.40) | 0.00 ± 0.01(0.00 – 0.010) | 0.69 ± 0.63(0.20 – 1.40) |
| STN 20 | 1.12 ± 0.28(0.85 – 1.40) | 34.17 ± 2.39(31.50 – 36.10) | 2.48 ± 0.33 A(2.22 – 2.85) | 0.65 ± 0.12(0.51 – 0.73) | 0.24 ± 0.12(0.11 – 0.34) | 0.01 ± 0.01(0.00 – 0.02) | 0.97 ± 0.53(0.38 – 1.41) |
| STN 21 | 1.58 ± 0.36(1.35 – 2.00) | 34.70 ± 1.76(32.70 – 36.00) | 2.19 ± 0.75 A(1.65 – 3.05) | 0.71 ± 0.42(0.44 – 1.19) | 0.15 ± 0.16(0.00 – 0.31) | 0.02 ± 0.03(0.00 – 0.05) | 0.76 ± 0.12(0.63 – 0.85) |
| P value[30]) | p< 0.050.05 | p> 0.05< 1 | p< 0.051.5 | p< 0.050.3 | p> 0.05̶ | p> 0.050.05 | p> 0.050.05 |
Iron concentrations in soil samples at Stations 11, 13, 15, 16, 17, 19, 20, and 21 were significantly lower than the concentrations at other stations. The concentrations of copper at Stations 9, 13, 15, and 16 were significantly higher than the concentrations detected in soil samples at other stations (p < 0.05). No significant difference occurred in nickel and chromium concentrations in the soil samples across all stations (p > 0.05).
Numbers with the same superscript are significantly different (p < 0.05). Sample size (N) = 21.
3.2. Human health risk assessment
The components for computations of health risks posed to adults and children (Table 2) were adopted were adopted from USEPA [80], USEPA [81] and [78]). Hazard quotients (HQ) or hazard index (HI) > /= 1, and cancer risk (CR) from 10−6 – 10−4 were considered significant [80]. The non-carcinogenic risks of Pb, Ni, Cu, Cd, Zn, and Cr in soil samples at AMWs in Benin City through the three exposure routes showed that the risks posed to adult and children were insignificant; with all HQ mean values < 1 (Table 3, Table 4). Results indicated that the three routes of uptake in adults and children were in the decreasing order of ingestion > dermal > inhalation (Table 3, Table 4).
Table 2.
Computed chronic reference doses for dermal, inhalation and ingestion of metals by adults and children.
| Metals | CM (mg/kg) | RfDderm (mg/kg/day) | RfDinh (mg/kg/day) | RfDing (mg/kg/day) | CDIderm (mg/kg/day) | CDIinh (mg/kg/day) | CDIing (mg/kg/day) | SFinh | SFing | SFderm |
|---|---|---|---|---|---|---|---|---|---|---|
| Pb | 2.50 | 0.000525 | 0.0035 | 0.0035 | 1.591 × 10−7 | 1.203 × 10−9 | 3.989 × 10−6 | _ | 0.0085 | _ |
| Ni | 0.27 | 0.00540 | 0.0206 | 0.020 | 1.725 × 10−8 | 1.304 × 10−10 | 4.323 × 10−7 | 0.81 | 0.84 | _ |
| Fe | 34.06 | _ | _ | _ | 2.172 × 10−6 | 1.641 × 10−8 | 5.443 × 10−5 | _ | _ | _ |
| Cu | 1.30 | 0.012 | 0.0402 | 0.040 | 8.317 × 10−8 | 6.286 × 10−10 | 2.084 × 10−6 | _ | _ | _ |
| Cd | 0.01 | 0.001 | 0.001 | 0.001 | 6.377 × 10−10 | 4.820 × 10−12 | 1.598 × 10−8 | 6.30 | 5.01 × 10−1 | 2.00 × 101 |
| Zn | 5.87 | 0.060 | 0.300 | 0.300 | 3.743 × 10−7 | 2.829 × 10−9 | 9.380 × 10−6 | _ | _ | _ |
| Cr | 0.72 | 0.000006 | 0.0000286 | 0.003 | 4.56 × 10−8 | 3.449 × 10−10 | 1.144 × 10−6 | 42 | _ | _ |
Legends: CM= concentration of metals in soil, RfDderm= reference dermal dose, RfDinh= reference inhalation dose, RfDing= reference ingestion dose, CDIderm= dermal chronic daily intake, CDIinh= inhalation chronic daily intake, CDIing= ingestion chronic daily intake, SFinh= inhalation slope factor, SFderm= dermal slope factor [78], [80], [81]
Table 3.
The hazard quotient for dermal, inhalation,l ingestion, and hazard index for adults.
| Metals | HQing |
HQderm |
HQinh |
HI |
CR |
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | CRing | CRinh | ||
| Pb | 1.14 × 10−3 | 8.27 × 10−4 | 303 × 10−4 | 2.20 × 10−4 | 3.44 × 10−7 | 2.49 × 10−7 | 1.44 × 10−3 | 3.39 × 10−8 | _ |
| Ni | 2.16 × 10−5 | 1.25 × 10−5 | 3.20 × 10−6 | 1.85 × 10−6 | 6.32 × 10−7 | 3.67 × 10−9 | 3.81 × 10−6 | 3.63 × 10−7 | 1.06 × 10−10 |
| Cu | 5.21 × 10−5 | 4.07 × 10−5 | 6.93 × 10−6 | 5.41 × 10−6 | 1.56 × 10−8 | 1.22 × 10−8 | 5.91 × 10−5 | _ | _ |
| Cd | 1.60 × 10−5 | 1.56 × 10−5 | 6.38 × 10−5 | 6.22 × 10−5 | 4.82 × 10−9 | 4.70 × 10−9 | 7.97 × 10−5 | 7.99 × 10−9 | 1.45 × 10−12 |
| Zn | 3.13 × 10−5 | 1.86 × 10−5 | 6.24 × 10−6 | 3.72 × 10−6 | 9.43 × 10−9 | 5.62 × 10−9 | 3.75 × 10−5 | _ | _ |
| Cr | 3.81 × 10−4 | 1.49 × 10−4 | 7.61 × 10−4 | 2.97 × 10−4 | 1.21 × 10−5 | 4.71 × 10−6 | 9.29 × 10−4 | _ | 1.45 × 10−8 |
| ΣHQ/CR/HI | 1.64 × 10−3 | 1.06 × 10−3 | 1.14 × 10−3 | 5.90 × 10−4 | 1.32 × 10−5 | 4.99 × 10−6 | 2.54 × 10−3 | 4.05 × 10−7 | 1.46 × 10−8 |
Legends: HQ= hazard quotient, HI= hazard index, CR= cancer risk, HQing= hazard quotient of ingestion, HQderm= hazard quotient of dermal, HQinh= hazard quotient of inhalation. CRing= carcinogenic risk of ingestion, CRinh= carcinogenic risk of inhalation
Table 4.
The mean Hazard quotient for dermal, inhalation, ingestion, and hazard Index for children.
| Metal | HQing |
HQdermal |
HQinh |
HI |
CR |
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | CRing | CRinh | ||
| Pb | 9.121 × 10−3 | 6.622 × 10−3 | 1.70 × 10−3 | 1.24 × 10−3 | 2.75 × 10−6 | 2.00 × 10−6 | 1.08 × 10−2 | 2.71 × 10−7 | _ |
| Ni | 1.73 × 10−4 | 1.00 × 10−4 | 1.79 × 10−5 | 1.04 × 10−5 | 5.06 × 10−8 | 2.93 × 10−8 | 1.91 × 10−4 | 2.9 0 × 10−6 | 8.45 × 10−10 |
| Cu | 4.17 × 10−4 | 3.25 × 10−4 | 3.89 × 10−5 | 3.05 × 10−5 | 1.25 × 10−7 | 9.76 × 10−8 | 4.56 × 10−4 | _ | _ |
| Cd | 1.28 × 10−4 | 1.25 × 10−4 | 3.58 × 10−4 | 3.49 × 10−4 | 3.86 × 10−8 | 3.76 × 10−8 | 4.86 × 10−4 | 6.39 × 10−8 | 2.43 × 10−10 |
| Zn | 2.50 × 10−4 | 1.49 × 10−4 | 3.50 × 10−5 | 2.09 × 10−5 | 7.54 × 10−8 | 4.50 × 10−8 | 2.85 × 10−4 | _ | _ |
| Cr | 3.05 × 10−3 | 1.19 × 10−3 | 4.27 × 10−3 | 1.67 × 10−3 | 9.65 × 10−5 | 3.77 × 10−5 | 7.42 × 10−3 | _ | 1.45 × 10−8 |
| ΣHQ/CR/HI | 1.31 × 10−2 | 8.51 × 10−3 | 6.42 × 10−3 | 3.32 × 10−3 | 9.95 × 10−5 | 3.99 × 10−5 | 1.96 × 10−2 | 3.23 × 10−6 | 1.56 × 10−8 |
The contribution of HQing to HI was the highest, 67% for children and 55% for adults, suggesting that ingestion was the main exposure pathway associated with significant health risks tendencies. The HI values of heavy metals for adult and children was in the order of Pb > Cr > Cd > Cu > Zn > Ni (Table 3, Table 4). The HI values for all the metals were < 1, indicating no non-carcinogenic risk for adults (Table 3). Only ingested nickel posed CR to the children, while other elements posed no significant risk (Table 4).
Legends: HQ= hazard quotient, HI= hazard index, CR= cancer risk, HQing= hazard quotient of ingestion, HQderm= hazard quotient of dermal, HQinh= hazard quotient of inhalation. CRing= carcinogenic risk of ingestion, CRinh= carcinogenic risk of inhalation. Emboldened figure is significant.
3.3. Pollution load index (PLI) for trace metals
The result of the contamination factors () and pollution load index (PLI) for Pb, Fe, Zn, Cu, Ni,Cd and Cr at the stations were all < 1, which is an indication of low contamination. Analysis of the results showed that the average was in the order of Pb (0.3715) > Zn (0.14) > Cu (0.087) > Cr (0.013) > Ni (0.01) > Fe (0.0007). The pollution load index (PLI) values show that the highest value (0.0157) was both in stations 2 and 12, while the lowest (0.0070) was in station 13 (Table 5).
Table 5.
Pollution load index of the metals in the soil samples.
| Stations | Pb | Fe | Zn | Cu | Ni | Cd | Cr | PLI |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.1035 | 0.0007 | 0.0832 | 0.0273 | 0.0049 | 0.0000 | 0.0126 | 0.0147 |
| 2 | 0.3715 | 0.0008 | 0.0589 | 0.0198 | 0.0041 | 0.0333 | 0.0114 | 0.0157 |
| 3 | 0.1500 | 0.0007 | 0.0271 | 0.0138 | 0.0041 | 0.0000 | 0.0026 | 0.0086 |
| 4 | 0.0850 | 0.0006 | 0.0655 | 0.0231 | 0.0047 | 0.0333 | 0.0123 | 0.0129 |
| 5 | 0.1740 | 0.0007 | 0.0586 | 0.0109 | 0.0051 | 0.0333 | 0.0068 | 0.0118 |
| 6 | 0.0885 | 0.0007 | 0.0937 | 0.0142 | 0.0050 | 0.1000 | 0.0073 | 0.0119 |
| 7 | 0.1440 | 0.0008 | 0.1069 | 0.0287 | 0.0041 | 0.1333 | 0.0098 | 0.0153 |
| 8 | 0.0360 | 0.0007 | 0.1044 | 0.0122 | 0.0026 | 0.0333 | 0.0121 | 0.0100 |
| 9 | 0.0810 | 0.0007 | 0.1234 | 0.0787 | 0.0009 | 0.0333 | 0.0104 | 0.0131 |
| 10 | 0.1400 | 0.0007 | 0.0657 | 0.0176 | 0.0100 | 0.0000 | 0.0083 | 0.0145 |
| 11 | 0.0550 | 0.0008 | 0.0286 | 0.0122 | 0.0022 | 0.0333 | 0.0041 | 0.0071 |
| 12 | 0.0970 | 0.0007 | 0.0980 | 0.0264 | 0.0096 | 0.0333 | 0.0088 | 0.0157 |
| 13 | 0.0685 | 0.0007 | 0.0223 | 0.0564 | 0.0016 | 0.0333 | 0.0012 | 0.0070 |
| 14 | 0.2085 | 0.0007 | 0.1398 | 0.0282 | 0.0049 | 0.0333 | 0.0037 | 0.0146 |
| 15 | 0.0605 | 0.0007 | 0.0479 | 0.0873 | 0.0029 | 0.0000 | 0.0061 | 0.0120 |
| 16 | 0.0950 | 0.0007 | 0.0155 | 0.0662 | 0.0043 | 0.0333 | 0.0082 | 0.0115 |
| 17 | 0.1100 | 0.0007 | 0.0255 | 0.0136 | 0.0012 | 0.0000 | 0.0071 | 0.0077 |
| 18 | 0.3710 | 0.0007 | 0.0618 | 0.0347 | 0.0013 | 0.0333 | 0.0072 | 0.0132 |
| 19 | 0.0465 | 0.0007 | 0.0215 | 0.0071 | 0.0043 | 0.0000 | 0.0077 | 0.0074 |
| 20 | 0.0560 | 0.0007 | 0.0261 | 0.0144 | 0.0035 | 0.0333 | 0.0108 | 0.0091 |
| 21 | 0.0790 | 0.0007 | 0.0231 | 0.0158 | 0.0022 | 0.0667 | 0.0084 | 0.0085 |
3.4. Geoaccumulation index (Igeo) of trace metals in soils of the AMWs
The geoaccumulation indices () values for the seven metals were: − 5.38 to − 2.01 for Pb, − 11.19 to − 10.94 for Fe, − 6.60 to − 3.42 for Zn, − 7.72 to – 4.10 for Cu, − 10.73–7.23 for Ni, 5.49–3.49 for Cd and 10.26–6.90 for Cr. The average values of were negative in all stations and decreased in the following order: Pb (−3.87) > Zn (−4.89) > Cd (−5.18) > Cu (−6.05) > Cr (−7.73) > Ni (8.80). > Fe (−11.02) with all the metals showing no sign of contamination. The calculations for Indicate that the metals in the soil samples presented no ecological risk (Table 6).
Table 6.
Geoaccumulation index (Igeo) of heavy metals in soils of AMWs.
| Stations | Pb | Fe | Zn | Cu | Ni | Cd | Cr |
|---|---|---|---|---|---|---|---|
| 1 | -3.8573 | -10.9904 | -4.1730 | -5.7782 | -8.2719 | -6.9005 | |
| 2 | -2.0135 | -10.9427 | -4.6694 | -6.2449 | -8.5089 | -5.4919 | -7.0342 |
| 3 | -3.3219 | -11.0338 | -5.7931 | -6.7665 | -8.5089 | -9.1971 | |
| 4 | -4.1414 | -11.1902 | -4.5179 | -6.0202 | -8.3163 | -5.4919 | -6.9263 |
| 5 | -3.1078 | -11.0210 | -4.6771 | -7.1060 | -8.1870 | -5.4919 | -7.7899 |
| 6 | -4.0831 | -11.0627 | -4.0010 | -6.7207 | -8.2288 | -3.9069 | -7.6763 |
| 7 | -3.3808 | -10.9658 | -3.8100 | -5.7094 | -8.5089 | -3.4919 | -7.2612 |
| 8 | -5.3808 | -11.0381 | -3.8445 | -6.9393 | -9.1464 | -5.4919 | -6.9525 |
| 9 | -4.2109 | -11.0029 | -3.6039 | -4.2531 | -10.7313 | -5.4919 | -7.1661 |
| 10 | -3.4215 | -11.0059 | -4.5133 | -6.4169 | -7.2288 | -7.4919 | |
| 11 | -4.7694 | -10.9484 | -5.7112 | -6.9393 | -9.4094 | -5.4919 | -8.5112 |
| 12 | -3.9508 | -10.9822 | -3.9360 | -5.8259 | -7.2939 | -5.4919 | -7.4169 |
| 13 | -4.4527 | -11.0342 | -6.0708 | -4.7320 | -9.8569 | -5.4919 | -10.2612 |
| 14 | -2.8468 | -11.0714 | -3.4236 | -5.7320 | -8.2719 | -5.4919 | -8.6763 |
| 15 | -4.6319 | -11.0467 | -4.9690 | -4.1023 | -8.9944 | -7.9393 | |
| 16 | -3.9809 | -11.0155 | -6.5990 | -4.5015 | -8.4583 | -5.4919 | -7.5112 |
| 17 | -3.7694 | -11.0878 | -5.8798 | -6.7899 | -10.3163 | -7.7207 | |
| 18 | -2.0155 | -11.0017 | -4.6015 | -5.4353 | -10.1464 | -5.4919 | -7.6983 |
| 19 | -5.0116 | -11.0198 | -6.1263 | -7.7207 | -8.4583 | -7.6122 | |
| 20 | -4.7434 | -11.0168 | -5.8445 | -6.6983 | -8.7313 | -5.4919 | -7.1208 |
| 21 | -4.2470 | -10.9946 | -6.0239 | -6.5709 | -9.4094 | -4.4919 | -7.4727 |
3.5. Potential ecological risk index (PER) of trace metals in soils of AMWs
The range of the potential ecological risk assessment (PER) of the metals were Pb= 0.18–1.86, Zn= 0.01–0.12, Cu= 0.03–0.44, Ni= 0.00–0.03, Cr= 0.00–0.03 and Cd= 0.00–4.00. The PER of the metals was in the order of Cd > Pb > Cu > Zn > Ni > Cr.
3.6. Spatial distribution of trace metals in sampled locations
Mapping the distribution of trace metals across the 21 sampling stations around the AMWs in Benin City was conducted using inverse distance weighing in ArcGIS 10.4.
The hotspots where Cr concentration was dominant were Stations 1, 2, 4, 7, 8, 9, and 20, which were the significant sources of spread; from where it tapered to the minimum at Stations 3 and 11; between which a slight intermediate rise occurred at Station 5 (Fig. 2), while an abrupt rise occurred at Station 13; from range 0.12 mg/kg - 0.37 mg/kg, through 0.69 mg/kg - 0.74 mg/kg to the peak (0.96 mg/kg - 1.13 mg/kg) at Stations 7 and 8. Other peaks occurred at Stations 1, 2, 4, 9, and 20 instead of linked in constant ascending gradients.
Fig. 2.
Spread of Cr across the study area.
The spread of nickel contamination was fareasonablyow compared to that of chromium. Tnickel peakkel was only reached at Stations 8 and 10, while the minimum concentrations occurred at Stations 9, 13, 17, 18, and 21 (Fig. 3). A smooth transition from lowest to highest concentrations occurred between Stations 21 and 10, respectively. Station 10 was the primary source of distribution of Ni to other stations.
Fig. 3.
Spread of Ni across the study area.
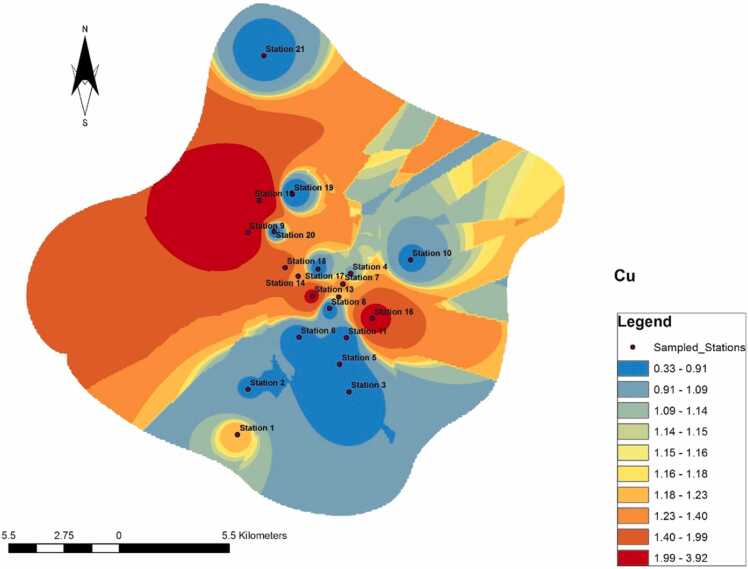
A notable and dramatic spread occurred in copper across the sampled stations, dominated by highest and lowest concentrations, with minimal intermediate concentrations (Fig. 4). The lowest concentrations occurred at Stations 2, 3, 5, 6, 8, 10, 11, 18, 19, 20, and 21. Intermediate stations were scanty, and the low concentrations were abruptly interjected by the highest concentrations, which occurred only at Stations 9, 13, 15, and 16. A spread occurred from Stations 9 and 16 to Station 21.
Fig. 4.
Spread of Cu across the study area.
The peak concentrations of zinc occurred at Stations 6, 8, 9, 14, which were dominated by the lowest concentrations, which occurred at Stations 3, 11, 13, 16, 17, 19, 20, and 21 (Fig. 5). The intermediate boundaries between the clusters of the highest and lowest Stations were locations of gradient transitions.
Fig. 5.
Spread of Zn across the study area.
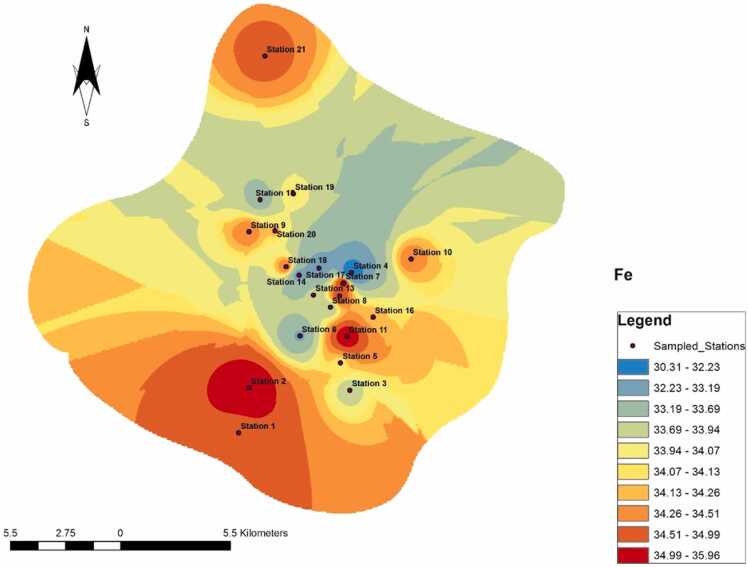
The study area was dominated by peak concentrations of Fe, which occurred at Stations 1, 2, 7, 9, 10, and 11, while the lowest concentrations occurred only at Station 4 (Fig. 6). The border zones between the highest and lowest points were dominated by interspersed mixtures of abrupt and gradient fluctuations. A free and uninterrupted spread occurred from Station 11 to Station 6; and, and Station 21 to Station 16.
Fig. 6.
Spread of Fe across the study area.
Conversely, high lowest concentrations of Pb dominated the study area, occurring at Stations 8, 11, 15, and 19. The highest concentrations of Pb occurred at Stations 2 and 18, spreading at a constantly decreasing gradient towards Stations 6, 8, 9, 11, 13, 16, 19, 20, and 21 (Fig. 7).
Fig. 7.
Spread of Pb across the study area.
The distribution for cadmium was not interpolated due to very low concentrations.
4. Discussion
Assessments of heavy metals contamination from soils in AMWs offer some insight into the levels of contamination of the soils in automobile shops [6]. The heavy metal levels in soils of AMWs in Benin City were assessed in this study, and their human health risks and potential ecological risk were determined.
4.1. Heavy metal concentration in soil samples
The lead concentrations in all the soil samples greater than 1.0 mg/kg indicates that the AMWs may be the source of pollution [28]. The lead concentration in this study ranged between 0.70 and 7.43 mg/kg across the stations. This consistent result indicates that the pollution of the soils samples with lead is attributable to the automobile activities or spill of automobile fluids at AMWs [[40], [45], [46], [54], [65]]. This is of concern as AMWs are almost evenly distributed around residential areas inhabited by middle-class citizens. The implication of this is that children in such areas are often left to play on the streets with peer groups. Some of the children's activities that may elicit lead-associated health risks include playing with sands and scavenging discarded auto parts. Although the mean concentration of Pb in the current study may be lower than 15.1 mg.kg−1 reported by [7], 28.1 mg/kg reported by Anegbe et al. [15] as well as 14.13 mg/ kg reported by Babatunde et al. [17], 76.92 mg/ kg reported by Okunola et al. [64], 47.8 mg/ kg reported by Sitkol et al. [76], and the set limit of Department of Petroleum Resources [24]- 85 mg/ kg, the current study area being a residential environment is however of a great concern. Details of the current study and previous reports of works done in Nigeria consistently show that most of the Nigerian soil samples analyzed were contaminated with lead. Hence, stringent measures may be required to place restrictions and sanctions on the use of lead-containing products. Elevated lead concentrations may be linked to lead deposition in soils within the AMWs and, hence, the topsoil's retention [15]. Studies have shown that the application of tetraethyl lead as an anti-knocking agent in gasoline releases lead from fossil fuel combustion through emissions from automobiles [11], [66], [68], [74].
Although chromium is an essential element, it may become toxic at high levels. Elevated levels of chromium can pose health risks to humans because chromium can be accumulated in the skin, lungs, muscles, and liver, where it can be linked to various health conditions [71]. Chromium concentrations in the current study ranged between 0.11 and 1.13 mg/kg. The mean chromium levels observed in the soil samples analyzed were lower than the standard regulatory limits of CCME (1999) whose assessment criteria is 20.00 mg/ kg and the DPR target value of 100 mg/ kg. The concentrations of chromium in the soil samples of the AMWs were also lower than those reported by Idugboe et al. [37] and Zakir et al. [88] but higher than values reported by Adebayo et al. [6].
Cadmium has no biological purpose in the human body [48] but it is rather of notable ecological significance [6]. Cadmium was below detectable limits (0.00 mg/kg) in station 1 and 15 throughout the months of sampling. According to Ebong et al. [27], the presence of cadmium could be due to the disposal of polyvinyl chloride plastics, nickel-cadmium batteries, motor oil and disposal sludge in the AMWs. The mean cadmium concentration in the soils of the current study was lower than values reported by Anegbe et al. [15] in some auto AMWs in Benin City. The discrepancy in the concentrations of cadmium may be due to the age difference of the AMWs. The current study area being a new residential environment, and the AMWs also being less than a decade of operation, may explain the relatively concentrations of most heavy metals detected in the soil samples.
Plants take up nickel from nickel-rich soils such as soils of lands designated for tea, beans, and vegetables cultivation. The risks of cancers of the lung, nose, larynx, prostate, respiratory failures, congenital disabilities, and heart diseases are associated with nickel exposure [15], [26]. The nickel concentrations in the current study area ranged between 0.06 mg/kg - 0.68 mg/kg in the stations. The highest concentration of nickel obtained in this study is lower than values reported by Oguntimehin and Ipinmoroti [63] and Nwachukwu et al., [58]. The highest mean concentration of nickel obtained in this study is however higher than that reported by Pam et al. [69] and Adebayo et al. [6].
The mean iron concentration ranged between 30.30 mg/kg- 35.97 mg/kg in the soils across the stations, thus making iron the metal with the highest concentration in the soils. The high concentration of iron in soils has been alluded to by several authors [13], [22], [63], [8]. The mean values of iron in this study are lower than those reported by Osakwe [67], Oguntimehin and Ipinmoroti [63], Nwachukwu et al. [57], Idugboe et al. [37] and Anegbe et al. [15]. High concentrations of iron are attributable to rust of old vehicles bodies, welding and vehicular panel beating activities at the site. Acute exposure of Fe may elicit cardiac depression and life-threatening metabolic acidosis [6].
The mean zinc concentration ranged between 1.47 mg/kg - 13.28 mg/kg across the stations. The range of values in this study was higher than the values reported by Nwachukwu et al. [58], Ubwa et al. [77] and Anegbe et al. [15]. Oguntimehin and Ipinmoroti, [63], Zakir et al. [88] and Osakwe [67] all reported higher values than in the current study. Elevated zinc concentration in the soils is attributable to the prolonged period the site has been existing on the roadside. Moreover, contaminated roadside soil is attributable to the wear and tear of vehicle parts [88]. The heat conductivity of zinc is applied in brake linings of vehicles, engine oil and vehicle tyres [11], [51], [52]. In the event of spills of automobile oil, coolants, and other fluids, zinc contamination of soil occurs [55], [67].
4.2. Human health risk assessment
The results presented in this study indicate that the order of HQ in the three exposure pathways to metals in adults and children was ingestion > dermal contact > inhalation. Nickel is the metal that requires strict monitoring to avoid the synergy AMW source with other anthropogenic sources [[9], [11], [21]]. Since most of the automobile chemicals used are in the liquid form, the inhalation might not be as impactful as the dermal route of exposure. This conforms to the results showing inhalation route ranked the last in the order of impacts. As observed in the results presented, the children are expected to be more susceptible than adults as playing with sand is predominant among children in the sub-urban areas of the country. The situation might be worse if their playground is located near any of these mechanic workshops. This may explain why the non-carcinogenic risk was higher in the children that in the adults. The findings of the current study conform to the observations of Wei and Yang [82], and Xiao et al. [85]. The highest route of exposure was ingestion because risk in children is attributable to the frequency of hand-to-mouth habit [82]. Notably, although the concentration of nickel in the soil was below the regulatory limit, the CR posedto the children was however significant. This shows that only studying background concentrations of contaminants in the environment is not sufficient, health risk analyses are effective in giving early warning signals. Nickel is a ubiquitous element in the environment. The metal has no determinate physiological function in animals and human beings [32]. Exposure to nickel can cause allergy, cardiovascular and kidney dysfunctions, lung fibrosis. Most importantly, the children in the current study area could be afflicted with cancer of the respiratory system [32]. Due to the molecular mechanisms of nickel-induced toxicity, mitochondrial dysfunctions and oxidative stress have a primary and crucial role. Nickel induces cancer through epigenetic alterations which perturb the genome [[31], [33], [34]]. This metal can cause an allergy that manifests as contact dermatitis, headaches, gastrointestinal and respiratory manifestations [54], [65], [73], [75], [86], [87]. The molecular mechanisms of Ni induced neurotoxicity are initiated through mitochondrial damage which precedes mitochondrial membrane potential damage, followed by reduced mitochondrial ATP concentration and ultimately mitochondrial DNA damage. The damage exerted on mitochondrial functions interferes with the mitochondrial transport chain, amplifies ROS and enhances oxidative stress. The current study indicates that the inadvertent hand-to-mouth habit in children may put them at higher health risk than adults as characterized by the high risks recorded in the ingestion pathway.
The sensitivity of biological communities to heavy metals was presented as potential ecological risk (PER) as proposed by Islam et al. [44]. As proposed by Hakanson [35], the elemental abundance and release capacity was represented as potential ecological risk index (PER) in this study. PRI was thus employed as a tool to assess the degree of heavy metal pollution in the soil samples based on the toxicity of heavy metals and the corresponding response of the environment [19], [83]. The mean PER of the analyzed metals was in the order of Cd > Pb > Cu > Zn > Ni > Cr. The results of the current study indicate that Cd may have posed the highest ecological risk. Similarly, Enuneku et al. [28] reported high potential ecological risk posed by Cd among other heavy metals investigated. In the current study, the ecological risk of other heavy metals in the sampled soils were relatively low across all the study stations.The concentrations of metals in the soil may rise to notable levels in the future due to the indices obtained from the geoaccumulation and bioaccumulation analyses [19], [29], [33], [62].
4.3. Recommendations and conclusion
Potential ecological risks of the trace metals in soils of these workshops revealed low pollution of the soil. Although the concentrations of metals in the soil were within safe limits, the children are however at risk of nickel-initiated cancer through hand-to-mouth habits. Parents are thus advised to prevent their children from contact with the soil and enforce regular hand washing as recommended by World Health Organization. Although naturally, surface runoff and percolations in the ground may reduce the concentrations of metals in the soil overtime but these will cause pollution problems in the receiving destinations which are surface water bodies and underground water respectively. The soil in the study area can however be remediated by cost-effective and sustainable techniques such as phytoaccumulation or phytoextraction in which the plants uptake the metals into their tissues through absorption and accumulation [72], [73], [75], [79], [89]. The toxicodynamics of heavy metals in the soil profile demonstrated in this study could be a vital information for future studies and decisions on the management of the health and environment of the study area. Further studies for proper validation of data obtained for ingestion routes using consumable environmental media have however been suggested.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2022.03.021.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Abdulsalam S., Adefila S.S., Bugaje I.M., Ibrahim S. Bioremediation of soil contaminated with used motor oil in a closed system. J. Bioremed. Biodegrad. 2012;3(12):172–179. [Google Scholar]
- 2.Abdulrashid L., Yaro A., Isah A. Heavy metals contamination in urban soils of Nigerian: a review. Int. J. Innov. Biosci. Res. 2017;5(3):1–12. [Google Scholar]
- 3.Abechi E.S., Okunola O.J., Zubairu S.M.J., Usman A.A., Apene E. Evaluation of metals in roadside soils of major streets in Jos Metropolis, Nigeria. J. Environ. Chem. Ecotoxicol. 2010;2(7):98–102. [Google Scholar]
- 4.Adedeji, O.H., Olayinka, O.O., and Tope-Ajayi, O.O. (2019). Spatial Distribution and Health Risk Assessment of Soil Pollution by Heavy Metals in Ijebu-Ode, Nigeria. Journal of health & pollution, 9(22), 190601. https://doi.org/10.5696/2156–9614-9.22.190601. [DOI] [PMC free article] [PubMed]
- 5.Achuba F.I., Peretiemo-Clarke B.O. Effect of spent engine oil on soil catalase and dehydrogenase activities. Int. Agrophys. 2008;22:1–4. [Google Scholar]
- 6.Adebayo A.J., Jayoye J.T., Ilemobayo I.O., Labunmi L. Delineation of heavy metals in soils from auto-mechanic workshops within Okitipupa, Ondo State, Nigeria. Int. Res. J. Public Environ. Health. 2017;4(7):136–147. [Google Scholar]
- 7.Adelekan B.A., Abegunde K.D. Heavy metal contamination of soil and ground water at automobile mechanic villages in Ibadan, Nigeria. Int. J. Phys. Sci. 2011;6(5):1045–1058. [Google Scholar]
- 8.Ademoroti C.M.A. Environmental chemistry and toxicology. Foludex Press Ltd Ibadan. 1996:150–188. [Google Scholar]
- 9.Agency for Toxic Substances and Disease Registry (ATSDR). (2005). Public Health Statement for Zinc: Public Service. Department of Health and Human Services. Pp 42.
- 10.Agency for Toxic Substances and Disease Registry (ATSDR). (2019). Toxicological Profile for Lead. U.S. Department of Health and Human Services. Pp 561.
- 11.Akbar N.F., Hale W.H.G., Headley A.D., Athar M. Heavy metal contamination of roadside soils. Soil Water Res. 2000;1(4):158–163. [Google Scholar]
- 12.Akinsanya B., Isibor P.O., Onadeko B., Abe-Alimi Impacts of trace metals on African common toad, Amietophrynus regularis (Reuss, 1833) and depuration effects of the toad’s emteric parasite, Amplicaecum africanum (Taylor, 1924) sampled within Lagos metropolis, Nigeria. Heliyon. 6(2020) 2020;e03570(1–12) doi: 10.1016/j.heliyon.2020.e03570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aluko O.O., Oluwande P.A. Characterization of leachates from a municipal solid waste landfill site in Ibadan, Nigeria. J. Environ. Health Res. 2003;2:83–84. [PubMed] [Google Scholar]
- 14.Amukali O.1, Bariweni P., Imaitor-Uku E.E. Spatial distribution of heavy metal contamination indexes in soils around auto-mechanic workshop clusters in Yenagoa metropolis, Bayelsa State, Nigeria. Global J. Earth Environ. Sci. 2018;3(4):23–33. [Google Scholar]
- 15.Anegbe B., Okuo J.M., Okieimen F.E., Ugbune U.E., Emina R.A. Levels of heavy metals in soil sample from active automobile workshops in Benin City. Int. J. Environ. Chem. 2019;3(1):7–17. [Google Scholar]
- 16.Baba A.A., Adekola F.A., Lawal A. Trace metal concentration in road side dust of Ilorin Town, Nigeria. Acad. J. Sci. Eng. 2014;1(1):88–96. [Google Scholar]
- 17.Babatunde O.A., Oyewale O.A., Steve P.I. Bioavailability of trace element in soils around Nnpc Oil Depot Jos Nigeria. J. Environ. Sci. Toxicol. Food Technol. 2014;8:47–56. [Google Scholar]
- 18.Bai J., Cui B., Wang Q., Ga H., Ding Q. Assess-ment of heavy metal contamination of roadside soils in Southwest China. Stoch. Environ. Res. Risk Assess. 2009;23:341–347. [Google Scholar]
- 19.Chen H.Y., Teng Y.G., Lu S.J., Wang Y.Y., Wang J.S. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015:512–513. doi: 10.1016/j.scitotenv.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y., Jiang X., Wang Y., Zhuang D. Spatial characteristics of heavy metal pollution and the potential ecological risk of a typical mining area: a case study in China. Process Saf. Environ. Protect. 2018;113:204–219. [Google Scholar]
- 21.Chirenje T., Ma L.Q., Clark C., Reeves M. Cu, Cr and As distribution in soils adjacent to pressure-treated decks, fences and poles. J. Environ. Pollut. 2003;124:407–417. doi: 10.1016/s0269-7491(03)00046-0. [DOI] [PubMed] [Google Scholar]
- 22.Dara S.S. S. Chand and Company Ltd; Ram Naga, New Delhi: 1993. A textbook of environmental and pollution control. [Google Scholar]
- 23.David O.O., Sunday A.A. Assessment of vehicular pollution of roadside soil in Ota Metropolis, Ogun State, Nigeria International. J. Civil Environ. Eng. 2012;12(4):12–17. [Google Scholar]
- 24.Department of Petroleum Resources (DPR). (2002) Environmental guidelines and Standards for the petroleum industries in Nigeria Depaertment of Petroleum Resources, Ministry of Petroleum and Mineral Resources, Abuja, Nigeria.
- 25.Dike B.U., Okoro B.C., Nwakwasi N.N., Agbo K.C. Remediation of used motor engine oil contaminated soil: a soil washing treatment. J. Civil Environ. Eng. 2013;3(1):129–131. [Google Scholar]
- 26.Duda-Chodak A., Blaszczyk U. The impact of nickel on human health. J. Elementol. 2008;13(4):685–696. [Google Scholar]
- 27.Ebong G.A., Akpan M.M., Mkpene V.N. Heavy metal content of municipal and rural dumpsite soils and rate of accumulation by Carica papaya and Talinum triangulare in Uyo Nigeria. E-J. Chem. 2008;5(2):281–290. [Google Scholar]
- 28.Enuneku, A.A., Abhulimen, P.I., Isibor, P.O., Asemota, C.O., Okpara, B., Imoobe, T.O. T., & Ezemonye, L.I. (2020). Interactions of trace metals with bacteria and fungi in selected agricultural soils of Egbema Kingdom, Warri North, Delta State, Nigeria. Heliyon. 6(2020) e04477.[. [DOI] [PMC free article] [PubMed]
- 29.Fagbote E.O., Olanipekun E.O. Evaluation of the status of heavy metal pollution of soil and plant (Chromolaena odorata) of Agbabu bitumen deposit area, Nigeria. American-Eurosian. J. Sci. Res. 2010;5(4):241–248. [Google Scholar]
- 30.Federal Environmental Protection Agency (FEPA). (2003).Guidelines and Standards for Environmental pollution control in Nigeria. Pp 420.
- 31.Ferreira-Baptista L., De Miguel E. Vol. 39. A tropical urban environment; Angola: 2005. Geochemistry and risk assessment of street dust in Luanda; pp. 4501–4512. (Atmos. Environ.). [Google Scholar]
- 32.Giuseppe G., Alessia C., Graziantonio L., Maria S.S., Alessia F. Nickel: human health and environmental toxicology. Int. J. Environ. Res. Public Health. 2019;2020(17):679. doi: 10.3390/ijerph17030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong C., Ma L., Cheng H., Liu Y., Xu D., Li B., Liu F., Ren Y., Liu Z., Zhao C., Yang Y., Nie H., Lang C. Characterization of the particle size fraction associated heavy metals in tropical arable soils from Hainan Island. China. J Geochem. Explor. 2014;139 109–14. [Google Scholar]
- 34.Haifeng Gao Junhong Bai, Rong Xiao Peipei Liu, Wei Jiang Junjing Wang. Levels, sources and risk assessment of trace elements in wetland soils of a typical shallow freshwater lake, China. Stochastic Environ. Res. Risk Assess. 2012:2012. [Google Scholar]
- 35.Hakanson L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980;14:975–1001. [Google Scholar]
- 36.Ibrahim D., Abdullahii S.U., Adamu I.U., Dazi L.L., Salihu A.I., Simon I.A. Heavy metal contamination of soil and ground water at automobile mechanic workshops in Borno State, Nigeria Nigerian Research. J. Chem. Sci. 2019;7:197–213. [Google Scholar]
- 37.Idugboe S.O., Tawari-Fufeyin P., Midonu A.A. Soil pollution in two auto mechanic villages in Benin city, Nigeria. IOSR J. Environ. Sci. Toxicol. Food Technol. 2014;8(I):9–14. [Google Scholar]
- 38.Ilemobayo O., Kolade I. Profile of heavy metals from Automobile workshop in Akure, Nigeria. J. Environ. Sci. Technol. 2008;1(1):19–26. [Google Scholar]
- 39.Ipeaiyeda A.R., Dawodu M. Heavy metals contamination of topsoil and dispersion in the vicinities of reclaimed auto-repair workshops in Iwo, Nigeria. Bull. Chem. Soc. Ethiopia. 2008;22(3):339–348. [Google Scholar]
- 40.Ilemobayo O., Kolade I. Profile of heavy metals from Automobile workshop in Akure, Nigeria. J. Environ. Sci. Technol. 2008;1(1):19–26. [Google Scholar]
- 41.Inam E., Edet J.B., Offiong N.O. Levels and occupational health risk assessment of trace metals in soils from automobile repair workshop village and environs in Uyo metropolis, Nigeria. Afr. J. Environ. Sci. Technol. 2015;9(7):584–591. [Google Scholar]
- 42.Isibor P.O., Imoobe T.O.T. Comparative analysis of contaminability between Clariasgariepinusand tilapia mariae. Annu. Res. Rev. Biol. 2017;16(5):1–14. doi: 10.9734/ARRB/2017/34920. [DOI] [Google Scholar]
- 43.Isibor P.O., Imoobe T.O.T., Enuneku A.A., Akinduti P.A., Dedeke G., Adagunodo T.A., Obafemi Y.D. Principal components and hierarchical cluster analyses of trace metals and total hydrocarbons in gills, intestines and muscles of clarias gariepinus (Burchell, 1822) Scientific Rep. 2020;10:5180. doi: 10.1038/s41598-020-62024-9. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Islam M.S., Ahmed M.K., Habibullah-Al-Mamun M., Masunaga S. Potentialecological risk of hazardous elements in different land-use urban soils of Bangladesh. Sci. Total Environ. 2015;512–513:94–102. doi: 10.1016/j.scitotenv.2014.12.100. [DOI] [PubMed] [Google Scholar]
- 45.Iwegbue C.M.A., Isirimah N.O., Igwe C., William E.S. Characteristic levels of heavymetals in soil profiles of automobile mechanic waste dumps in Nigeria. J. Environ. 2006;26(2):123–128. [Google Scholar]
- 46.Kakulu S.E. Trace metal concentration in roadside surface soil and tree barks. A measurement of local atmosphere pollution in Abuja, Nigeria. Environ. Monit. Assess. 2003;89:233–242. doi: 10.1023/a:1026149914428. [DOI] [PubMed] [Google Scholar]
- 47.Kolluru, R.V., Bartell, S.M., Pitblado, R.M. and Stricoff, R.S. (1996) Risk Assessment and Management Handbook; McGrow-Hill: New York, NY, USA.
- 48.Lenntech, W.T. (2009) Chemical Properties, Health and Environmental Effects of Copper. Lenntech WaterTreatment and Purification Holding B. V. www. Lenntech. comeriodic/element/cu.htm.
- 49.Li, P., wu, J., Qian, H., Zhou, W. (2016). Distribution, enrichment, and sources of trace metals in the topsoil in the vicinity of a steel wire plant along the Silk Road economic belt, northwest China, environ Health Science 75(10):909.
- 50.Lonati G., Giugliano M., Cernuschi S. The role of traffic emissions from weekends and weekday fine PM data in Milan. Atmos. Environ. 2006;40:5998–6011. [Google Scholar]
- 51.Manno E., Varrica d, Dongarra G. Metal Distribution in Road dust samples collected in an urban area close to a petrochemical plant at Gela, Sicily. Atmos. Environ. 2006;40:5929–5941. [Google Scholar]
- 52.Matthews –Amune O.C., Kakulu S. Investigation of heavy metal levels in road-side agricultural soil and plant samples in Adogo, Nigeria. Acad. J. Environ. Sci. 2013;1(2):31–35. [Google Scholar]
- 53.Muller G. Index of geoaccumulation in sediments of the Rhine River. Geojournal. 1969;2:108–118. [Google Scholar]
- 54.National Environment Protection Council of Australia, NEPCA (2010) Limits of Heavy Metals in Soils. Available online at www.newzealand.govt.nz.
- 55.Nnabo P.N., Orazulike D.M., Offor O.C. The preliminary assessment of the levels of heavy element contaminations in stream bed sediments of Enyigba and Environs, Southeastern Nigeria. J. Basic Phys. Res. 2011;2:43–52. [Google Scholar]
- 56.Nwachukwu M.A., Feng H., Alinnor J. Assessment of heavy metals in soil and their implications within and around mechanic villages. Int. J. Environ. Sci. Technol. 2010;7(2):347–358. [Google Scholar]
- 57.Nwachukwu M.A., Feng H., Alinnor J. Trace metal dispersion in soil from auto mechanica village to urban residential areas in Owerri, Nigeria. Proc. Environ. Sci. 2011;4:310–322. [Google Scholar]
- 58.Nwachukwu, M.A., Ntesat, B., and Mbaneme, F.C. (2013). Assessment of direct soil pollution in automobile junk market. Journal of Environ. Chem. Ecotoxicol.
- 59.NRC (National Research Council) (1983) NAS (National Academy of Sciences). Risk Assessment in the Federal Government: Managing the Process; National Academy Press: Washington, DC, USA. [PubMed]
- 60.Obafemi Y.D., Isibor P.O., Adeyemi A.O., Taiwo O.S. Plasmid profiling of crude petroleum degrading bacterial strains isolated from polluted soils in Ota, Nigeria. Annu. Res. Rev. Biol. 2018;25(5):1–11. [Google Scholar]
- 61.Odukoya A.M., Olobaniyi S.B., Abdussalam M. Metal pollution and health risk assessement of soil within an urban idustrial estate, South West Nigeria. lfe J. Sci. 2016;18(2):573–583. [Google Scholar]
- 62.Ogunkunle C.O., Fatoba P.O. Pollution loads and the ecological risk assessment of soil heavy metals around a mega cement factory in southwest Nigeria. Polish J. Environ. Stud. 2013;22:487–493. [Google Scholar]
- 63.Oguntimehin I., Ipinmoroti K. Profile of heavy metals from automobile workshops in Akure Nigeria. J. Environ. Sci. Technol. 2008;1(1):19–26. [Google Scholar]
- 64.Okunola O.J., Uzairu A., Ndukwe G. Levels of trace metals in soiland vegetation along major and minor roads in metropolitan city of Kaduna, Nigeria. Afr. J. Biotechnol. 2007;6(14):1703–1709. [Google Scholar]
- 65.Olajire A.A., Ayodele E.T. Contamination of roadside soil and grass with heavy metals. Environ. Int. 1997;23(2):91–101. [Google Scholar]
- 66.Onder S., Dursun S., Gezgin S., Demirbas A. Determination of heavy metal pollution in grass and soil of city centre green areas Konya, Turkey. Polish J. Environ. Study. 2007;16(1):145–154. [Google Scholar]
- 67.Osakwe S.A. Heavy metal contamination and physicochemical characteristics of soils from automobile workshops in Abraka, Delta State, Nigeria. Int. J. Nat. Sci. Res. 2014;2(4):48–58. [Google Scholar]
- 68.Oztas T., Sibel A.S. Distribution patterns of lead accumulation in roadside soils: a case study from Erzurum. Turkey. Int. J. Environ. Pollut. 2002;18(2):190–196. [Google Scholar]
- 69.Pam A.A., Sha’Ato R., Offem O.J. Contributions of automobile mechanic sites to heavy metals in soil: a case study of north bank mechanic Village Makurdi, Benue State, Central Nigeria. J. Chem. Biol. Phys. Sci. 2013;3(3):2337–2347. [Google Scholar]
- 70.QGIS Development Team. (2019). QGIS Geographic Information System. Open Source Geospatial Foundation Project. URL: https://qgis.org/en/site/forusers/download.html#.
- 71.Reyes-Gutiérrez L.R., Romero-Guzmán E.T., Cabral-Prieto A., Rodríguez- Castillo R. Characterization of chromium in contaminated soil studied by SEM, EDS, XRD and mössbauer spectroscopy. J. Miner. Mater. Charact. Eng. 2007;7(1):59–70. [Google Scholar]
- 72.Sakai Y., Ma Y., Xu C., Wu H., Zhu W., Yang J. Phytodesalination of a salt-affected soil with four halophytes in China. J. Arid Land Stud. 2012;2022:17–20. [Google Scholar]
- 73.Saeedi M., Hosseinzadeh M., Jamshidi A., Pajooheshfar S. Assessment of heavy metals contamination and leaching characteristics in highway sideway side soils, Iran. Environ. Monit, Assess. 2014;15(9):431–441. doi: 10.1007/s10661-008-0264-z. [DOI] [PubMed] [Google Scholar]
- 74.Sharma S., Parasade F.M. Accumulation of lead and cadmium in soil and vegetable crops along major highways in Agra, India. J. Chem. 2010;7(4):1174–1183. [Google Scholar]
- 75.Sherene T. Mobility and transport of heavy metals in polluted soil environment. Biol. forum Int. J. 2010;2(2):112–121. [Google Scholar]
- 76.Sitkol L.R., Zawisza B., Jurczyk J., Buhl F., Zielonka U. Determination of high Zn and Pb concentrations in polluted soils using energy-dispersive X-ray Fluorescence Spectrometry. Polish J. Environ. Stud. 2004;13(1):91–96. [Google Scholar]
- 77.Ubwa S.T., Atoo G.H., Offem J.O., Abah J., Asemave K. Effect of activities at gboko abattoir on some physical properties and heavy metals levels of surrounding soil. Int. J. Chem. 2013;5(1):49–57. [Google Scholar]
- 78.USDOE (United State Department of Energy). (2011). U.S. Department of Energy’s Oak Ridge Operations Office (ORO). The Risk Assessment Information System (RAIS).
- 79.US EPA. (2008) Integrated Risk Information System of the US Environmental Protection Agency; Office of Emergency and Remedial Response: Washington, DC, USA.
- 80.US EPA (US Environmental Protection Agency). (2010). Risk-Based Concentration Table. United States Environmental Protection Agency. Available at: http://www.epa.gov/reg3hwmd/risk/human/index.htm.
- 81.US EPA (US Environmental Protection Agency). (2011). Recommended Use of BW3/4 as the Default Method in Derivation of the Oral Reference Dose. EPA/100/R11/001. Office of the Science Advisor. http://www.epa.gov/raf/publications/pdfs/recommended-use-of-bw34.pdf.
- 82.Wei B.G., Yang L.S. A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem. J. 2010;94(2):99–107. [Google Scholar]
- 83.Wu S., Peng S., Zhang X., Wu D., Luo W., Zhang T., Zhou S., Yang G., Wan H., Wu L. Levels and health risk assessments of heavy metals in urban soils in Dongguan, China. J. Geochem. Explor. 2015;148:71–78. [Google Scholar]
- 84.Yan Xuedong, Zhang Fan, Zeng Chen, Zhang Man, Devkota Lochan Prasad, Yao Tandong. “Relationship between Heavy Metal Concentrations in Soils and Grasses of Roadside Farmland in Nepal”. Int. J. Environ. Res. Public Health. 2012 doi: 10.3390/ijerph9093209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiao Q., Zong Y., Lu S. Assessment of heavy metal pollution and human health risk in urban soils of steel industrial city (Anshan), Liaoning, Northeast China. Ecotoxicol. Environ. Saf. 2015;120:377–385. doi: 10.1016/j.ecoenv.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 86.Xu Z., Ni S., Tuo X., Zhang C. Calculation of heavy metals’ toxicity coefficient in the evaluation of potential ecological risk index. Environ. Sci. Technol. 2008;31(2):112–115. [Google Scholar]
- 87.Zaja C.G., Szyszlak-Bargłowicz J., Słowik T., Kuranc A., Kaminska A. Designation of chosen heavy metals in used engine oils using the XRF method. Polish J. Environ. Stud. 2015;24(5):23–34. [Google Scholar]
- 88.Zakir H.M., Sultana N., Akter M. Heavy metal contamination in roadside soils and grasses: a case study from Ahaka City, Bangladest. J. Chem. Biol. Physical Sci. 2014;4(2):1661–1673. [Google Scholar]
- 89.Zhang F., Yan X., Zeng C., Zhang M., Shrestha S., Devkota L.P., Yao T. Influence of traffic activity on heavy metal concentrations of roadside farmland soil in mountainous areas. Int. J. Environ. Res. Public Health. 2012;9(5):1715–1731. doi: 10.3390/ijerph9051715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material