This economic analysis compares robotic-assisted radical prostatectomy with open and laparoscopic radical prostatectomies using contemporary data on clinical outcomes, costs, and surgical volumes among patients with localized prostate cancer in the UK.
Key Points
Question
Is robotic-assisted radical prostatectomy (RARP) cost-effective compared with open (ORP) and laparoscopic-assisted (LRP) radical prostatectomies to treat localized prostate cancer in the UK?
Findings
This economic evaluation using a Markov model found that RARP was associated with less cost than LRP and increased quality-adjusted life-years (QALYs). Compared with ORP, RARP was associated with £526 (US $693) higher cost but 0.12 more QALYs gained, driven by RARP’s lower reported biochemical recurrence rate and resulting in an incremental cost-effectiveness ratio of £4293 (US $5653)/QALY.
Meaning
These findings suggest that RARP is more cost-effective than ORP and LRP to treat localized prostate cancer in the UK.
Abstract
Importance
The cost-effectiveness of different surgical techniques for radical prostatectomy remains a subject of debate. Emergence of recent critical clinical data and changes in surgical equipment costs due to their shared use by different clinical specialties necessitate an updated cost-effectiveness analysis in a centralized, largely government-funded health care system such as the UK National Health Service (NHS).
Objective
To compare robotic-assisted radical prostatectomy (RARP) with open radical prostatectomy (ORP) and laparoscopic-assisted radical prostatectomy (LRP) using contemporary data on clinical outcomes, costs, and surgical volumes in the UK.
Design, Setting, and Participants
This economic analysis used a Markov model developed to compare the cost-effectiveness of RARP, LRP, and ORP to treat localized prostate cancer. The model was constructed from the perspective of the UK NHS. The model simulated 65-year-old men who underwent radical prostatectomy for localized prostate cancer and were followed up for a 10-year period. Data were analyzed from May 1, 2020, to July 31, 2021.
Exposures
Robotic-assisted radical prostatectomy, LRP, and ORP.
Main Outcomes and Measures
Quality-adjusted life-years (QALYs), costs (direct medical costs and costs outside the NHS), and incremental cost-effectiveness ratios (ICERs).
Results
Compared with LRP, RARP cost £1785 (US $2350) less and had 0.24 more QALYs gained; thus, RARP was a dominant option compared with LRP. Compared with ORP, RARP had 0.12 more QALYs gained but cost £526 (US $693) more during the 10-year time frame, resulting in an ICER of £4293 (US $5653)/QALY. Because the ICER was below the £30 000 (US $39 503) willingness-to-pay threshold, RARP was more cost-effective than ORP in the UK. The most sensitive variable influencing the cost-effectiveness of RARP was the lower risk of biochemical recurrence (BCR). Scenario analysis indicated RARP would remain more cost-effective than ORP as long as the BCR hazard ratios comparing RARP vs ORP were less than 0.99.
Conclusions and Relevance
These findings suggest that in the UK, RARP has an ICER lower than the willingness-to-pay threshold and thus is likely a cost-effective surgical treatment option for patients with localized prostate cancer compared with ORP and LRP. The results were mainly driven by the lower risk of BCR for RARP. These findings may differ in other health care settings where different thresholds and costs may apply.
Introduction
Prostate cancer is the second most frequent malignant neoplasm after lung cancer among men worldwide1 and is the most common cancer among men in the UK.2 Radical prostatectomy is the criterion standard for definitive therapy for localized prostate cancer. Radical prostatectomy can be performed via an open technique (ORP) or using minimally invasive techniques, including laparoscopic-assisted radical prostatectomy (LRP) and robotic-assisted radical prostatectomy (RARP).
Perioperative clinical effectiveness of RARP has been well documented in the literature. Compared with ORP, RARP has been shown to reduce postoperative complications and hospital length of stay (LOS) and to enable faster recovery.3,4 Compared with conventional laparoscopy, RARP conveys additional degrees of wrist movements and 3-dimensional visualization of the operative field.5
Compared with other urological surgical procedures, the diffusion of robotic surgery was highest for prostatectomy, representing most of the surgical volume in 2014 among National Health Service (NHS) hospitals.6 However, one of the remaining concerns about RARP is its cost-effectiveness. The cost-effectiveness of RARP in a centralized, largely government-funded health care system such as the NHS may be attributed to the decreased conversion to open surgery, reduction in rates of complications and positive surgical margins, and improved functional outcomes to counterpoise the higher cost of the surgical equipment. A UK economic assessment by the National Institute for Health Research in 2012 showed that RARP was more cost-effective than LRP for 10 years when the annual volume per system exceeded 150 cases.7 Although other studies have examined the cost-effectiveness of RARP,7,8,9,10,11,12,13,14 conclusive evidence is still lacking and warrants further research. Emergence of recent clinical data provides an opportunity to develop a more robust cost-effectiveness model. Since 2012, several meta-analyses on RARP15,16,17 reported important long-term outcomes, including biochemical recurrence (BCR) and recurrence-free survival. Moreover, previous studies have not addressed the multispecialty use of the robot, which is important when calculating surgical volume per system and surgical cost per procedure. In addition, ORP was not included as a comparison arm in the 2012 National Institute for Health Research assessment. Against this backdrop, we sought to perform a cost-effectiveness analysis from the NHS perspective by comparing RARP with ORP and LRP using more contemporary data on clinical outcomes, costs, and surgical volumes.
Methods
Study Overview
We developed a Markov model to simulate and compare the costs and cost-effectiveness of RARP with those of LRP and ORP to treat localized prostate cancer. The base case analysis was simulated for 65-year-old men who underwent radical prostatectomy for localized prostate cancer and were followed up for a 10-year period. Medical costs were calculated from sources provided by the UK NHS, and instrument costs were extracted from publicly available price information. Costs and outcomes beyond 1 year were discounted at an annual rate of 3.5% according to the UK National Institute for Health and Care Excellence (NICE) guideline.18 The incremental cost-effectiveness ratio (ICER) was computed as the ratio of the difference in costs divided by the difference in quality-adjusted life-years (QALYs) between the 2 procedures. Results were presented as QALYs, costs, and ICERs of different surgical approaches. This study followed the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline.
Model Structure
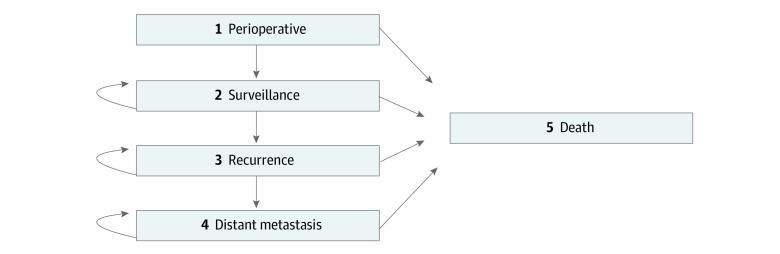
The Markov model was constructed with 5 health states: perioperative surgery, surveillance, BCR, metastasis, and death (Figure 1). Patients cycled through the model until death or the end of the model’s time frame (10 years postoperatively).
Figure 1. Markov State Transition Diagram.
Includes 5 health states. Arrows indicate transitions.
After radical prostatectomy, patients may have perioperative complications or mortality. Only patients alive at 1 year postoperatively would progress to the surveillance state. Patients remain in the surveillance state until BCR (2 consecutive serum prostate-specific antigen levels >0.2 ng/mL [to convert to micrograms per liter, multiply by 1.0]) occurs. Patients with BCR may undergo radiotherapy, hormone therapy, or a combination of both. Once BCR occurs, patients may develop metastasis and are assumed to receive systemic therapy and palliative care until death.
Model Inputs
Clinical Parameters and Use of Health Care Resources
The base case values of clinical inputs were extracted from the literature according to evidence-based medicine hierarchy: systematic literature reviews or meta-analyses were preferred to individual randomized clinical trials (RCTs) and observational studies; recent literature was preferred to older publications; studies with 3-arm comparisons (RARP, LRP, and ORP) were preferred to 2-arm comparisons; and observational studies must have adjusted for covariates. Moreover, the hospital LOS was based on a UK database study19 to reflect local practice. All parameters are presented in eTable 1 in the Supplement.
Transitional Probability
The probabilities for patients to remain in the same health state or transition to a different one were based on the available literature (eTable 1 in the Supplement). The probability of perioperative death was extracted from a meta-analysis.20 At 1 year after surgery, all surviving patients transition to the surveillance state. The probability of developing BCR after RARP was also derived from a meta-analysis.21 Hazard ratios and risk ratios of BCR comparing RARP, LRP, and ORP were used to calculate the probabilities of developing BCR from the surveillance state in the ORP and LRP arms. Patients in the surveillance, BCR, and metastasis states were at risk of all-cause mortality based on age- and sex-specific mortality estimates in the UK.22
Utility Inputs
A utility value was assigned for each health state based on the literature (eTable 2 in the Supplement). The total QALY represented the sum of each estimated health state–specific QALY across the time horizon of the model.
Costs
Direct medical costs were calculated in the base case, and non-NHS costs were further included in the sensitivity analysis (eTables 3-6 in the Supplement). Direct medical costs included costs of surgical equipment and instruments, operating room, and hospitalization as well as treatments for urinary and sexual dysfunctions, surveillance, salvage therapy, and distant metastasis.
The da Vinci robotic capital cost included the cost to purchase a single console da Vinci X system (Intuitive Surgical, Inc) with its necessary add-ons as well as the annual maintenance service fees. The up-front capital investment was assumed to last for 7 years, and we conservatively assumed no residual value at the end of the service life. Given that the robotic system is also used in other specialties, the capital cost per RARP was calculated by dividing the annual capital cost by the annual number of procedures, estimated at 250 cases based on the utilization data of the da Vinci surgical systems in the UK. All costs were adjusted to the 2019 pound sterling using inflation and the price index for medical services from the UK Office of National Statistics.23
Data Analysis
The mean total direct cost per patient and the QALYs were calculated as well as several intermediate outcomes such as life-years, probability of BCR, and BCR-free years. The ICERs and incremental net monetary benefit were computed as the cost of gaining an additional QALY for RARP vs ORP and RARP vs LRP. One intervention was considered dominant over another if the intervention was associated with higher effectiveness and lower cost. If an intervention was more costly but had higher effectiveness, the ICER was calculated and compared with the willingness-to-pay (WTP) threshold suggested by the NICE (£30 000 [US $39 503]/QALY). The intervention was deemed cost-effective if the ICER was less than the WTP.
Sensitivity Analysis
One-way deterministic sensitivity analyses were conducted to address uncertainties associated with the parameter values. Published sources were used to obtain ranges for parameters whenever possible. If they were unavailable, parameter uncertainty was assessed by increasing and decreasing the base case values by 20%. The ranges and distributions of parameter values are listed in eTables 1-3 in the Supplement.
Probabilistic sensitivity analysis was conducted using 10 000 iterations of Monte Carlo simulations by sampling each parameter simultaneously from their distributions. The probability of cost-effectiveness for each intervention was calculated at the WTP threshold of £30 000 (US $39 503)/QALY, and the cost-effectiveness acceptability curve for different WTP thresholds was plotted accordingly.
Scenario Analysis
The first set of scenarios considers different RARP surgical equipment or instrument costs: when new da Vinci instrument pricing is applied (estimated to decrease £277 (US $365) per RARP24); when the RARP surgical system is from charity donations; at minimum volume requirement from the NICE (150 cases per system); or when different generations of da Vinci systems were purchased (the least expensive [Si] and most expensive [Xi] systems). Second, the cost-effectiveness from the societal perspective was tested by including non-NHS costs such as work and productivity loss, informal care cost, and patient out-of-pocket cost (eTable 3 in the Supplement). Furthermore, the time horizon was extended to lifetime. Last, given the central importance of oncological outcomes on cost-effectiveness analyses, scenario analysis was performed under a conservative assumption that RARP and ORP have no difference in BCR rates.
The model was programmed in TreeAge Pro Healthcare 2020 (TreeAge Software). The analysis was performed from May 1, 2020, to July 31, 2021, and the statistical analysis data can be found in the eMethods in the Supplement.
Results
Base Case Analysis
During the 10-year time horizon, the BCR rate for RARP was estimated to be 28%; for LRP, 37%; and for ORP, 32%. Robotic-assisted radical prostatectomy had the longest recurrence-free survival at 8.02 years (compared with 7.33 years for LRP and 7.75 years for ORP) and the highest QALYs gained at 7.93 (compared with 7.69 for LRP and 7.81 for OPR) (Table 1).
Table 1. Base Case Results of Cost-effectiveness Analysis.
| Procedure | BCR rate, % | Recurrence-free duration, y | Life-years | QALY | Direct costs, £ (US $) | ICER, £ (US $) | INMB, £/US $a | ||
|---|---|---|---|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | ||||||
| RARP | 28 | 8.02 | 9.22 | 7.93 | NA | 13 247 (17 443) | NA | RARP dominant | 8993/ (11 842) |
| LRP | 37 | 7.33 | 9.09 | 7.69 | 0.24 | 15 032 (19 794) | −1785 (−2350) | ||
| RARP | 28 | 8.02 | 9.22 | 7.93 | NA | 13 247 (17 443) | NA | 4293 (5653) | 3149 (4146) |
| ORP | 32 | 7.75 | 9.17 | 7.81 | 0.12 | 12 721 (16 751) | 526 (693) | ||
Abbreviations: BCR, biochemical recurrence; ICER, incremental cost-effectiveness ratio; INMB, incremental net monetary benefit; LRP, laparoscopic radical prostatectomy; NA, not applicable; ORP, open radical prostatectomy; QALY, quality-adjusted life-year; RARP, robotic-assisted radical prostatectomy.
Calculated at the willingness-to-pay threshold of less than £30 000 (US $39 503)/QALY gained.
The total direct 10-year costs of RARP were estimated at £13 247 (US $17 443); those of LRP, at £15 032 (US $19 794); and those of ORP, at £12 721 (US $16 751). Robotic-assisted radical prostatectomy had the highest surgical equipment cost at £2775 (US $3654), followed by LRP at £1360 (US $1791) and ORP at £638 (US $840) (Table 2). Compared with ORP, RARP had a £2137 (US $2814) higher operating room cost, but lower costs attributed to shorter hospital LOS, a lower complication rate, and lower rates of urinary and sexual dysfunctions and BCR. As such, RARP costs were £526 (US $693) more than ORP costs during the 10-year period. Compared with LRP, RARP had a £1414 (US $1862) higher surgical equipment cost, but lower cost attributed to shorter hospital LOS, lower operating room cost, and reduced rates of complications and BCR. Robotic-assisted radical prostatectomy was associated with £1785 (US $2350) saved during the 10-year period (Table 2).
Table 2. Cost Components for ORP, LRP, and RARP.
| Cost component | Direct cost, £ (US $) | Incremental cost, £ (US $) | |||
|---|---|---|---|---|---|
| ORP | LRP | RARP | RARP vs ORP | RARP vs LRP | |
| Surgical equipment | 638 (840) | 1360 (1791) | 2775 (3654) | 2137 (2814) | 1414 (1862) |
| Cost for operation room | 3457 (4552) | 4826 (6355) | 3829 (5042) | 372 (490) | −997 (−1313) |
| Cost for hospital stay | 2087 (2748) | 1300 (1712) | 1115 (1468) | −972 (−1280) | −185 (−244) |
| Cost for complication | 11 (14) | 27 (36) | 3 (4) | −8 (−11) | −24 (−32) |
| Bladder neck construction | 88 (116) | 42 (55) | 16 (21) | −72 (−95) | −26 (−34) |
| Sexual dysfunction treatment | 380 (500) | 311 (410) | 218 (287) | −162 (−213) | −93 (−122) |
| Urinary incontinence treatment | 93 (122) | 85 (112) | 76 (100) | −17 (−22) | −8 (−11) |
| Surveillance | 729 (960) | 690 (909) | 754 (993) | 25 (33) | 64 (84) |
| Recurrence treatment | 3591 (4728) | 4323 (5692) | 3071 (4044) | −520 (−685) | −1252 (−1649) |
| Distant metastasis treatment | 983 (1294) | 1230 (1620) | 830 (1093) | −153 (−201) | −401 (−528) |
| Palliative care | 664 (874) | 836 (1101) | 560 (737) | −105 (−138) | −277 (−365) |
| All | 12 721 (16 751) | 15 032 (19 794) | 13 247 (17 443) | 526 (693) | −1785 (−2350) |
Abbreviations: LRP, laparoscopic radical prostatectomy; ORP, open radical prostatectomy; RARP, robotic-assisted radical prostatectomy.
Robotic-assisted radical prostatectomy had 0.12 more QALYs gained than ORP, resulting in an estimated ICER of £4293 (US $5653)/QALY. Because the ICER was lower than the WTP threshold, RARP was a dominant option compared with LRP: RARP cost £1785 (US $2350) less and gained 0.24 more QALYs.
Sensitivity Analysis
RARP vs ORP
In deterministic sensitivity analysis of RARP vs ORP (eFigure 1A in the Supplement), the hazard ratio of BCR was associated with the most variability in ICER (−£1993 [US −$2624]/QALY to £53 451 [US $70 382]/QALY gained). In addition, RARP’s annual case volume and operative time and ORP’s hospital LOS and operative time each heavily impacted the ICER. For instance, when the case volume was 100 cases per year the ICER was £18 000 (US $23 702)/QALY, but when the case volume increased to 400 cases per year the ICER dropped to £3000 (US $3950)/QALY.
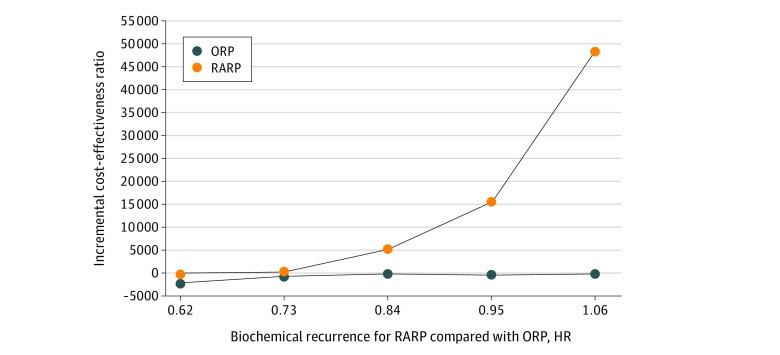
Given the variability in the ICER caused by BCR, 1-way sensitivity analysis was further performed (Figure 2). When BCR hazard ratios (RARP vs ORP) varied from 1.06 to 0.70, ICERs ranged between £50 705 (US $66 767)/QALY and £0 (US $0)/QALY, respectively. In addition, when the BCR hazard ratios were lower than 0.99, RARP remained more cost-effective than ORP. Furthermore, when the BCR hazard ratios decreased to less than 0.70, RARP became dominant over ORP.
Figure 2. One-Way Sensitivity Analysis of Hazard Ratio (HR) for Biochemical Recurrence .
Robotic-assisted radical prostatectomy (RARP) is compared with open radical prostatectomy (ORP). Data are from 1 to 5 years of the time horizon.
The probabilistic sensitivity analysis showed RARP had a higher percentage of cost-effective iterations vs ORP if the WTP threshold was greater than £6250 (US $8230)/QALY gained. At a WTP threshold of £30 000 (US $39 503)/QALY gained, the probability of RARP being cost-effective or dominant over ORP was 99.0% (23.5% dominant and 75.5% cost-effective) (eFigures 2 and 3A in the Supplement).
RARP vs LRP
In the deterministic sensitivity analysis of RARP vs LRP (eFigure 1B in the Supplement), RARP was dominant over LRP for all the ranges tested. In probabilistic sensitivity analysis of RARP vs LRP, RARP had a higher percentage of cost-effective iterations at any threshold (eFigure 2 in the Supplement). At a WTP threshold of £30 000 (US $39 503)/QALY, RARP was more cost-effective or dominant over LRP in 99.9% of the iterations (98.4% dominant and 1.6% cost-effective) (eFigure 3B in the Supplement).
Scenario Analysis
From a societal perspective, RARP was dominant over LRP and more cost-effective than ORP in a lifetime scenario. Once non-NHS costs were included in the model, RARP became dominant over ORP and increased its dominance over LRP (Table 3).
Table 3. Scenario Analysis Result of Cost-effectiveness Analysis.
| RARP vs ORP | RARP vs LRP | |||||
|---|---|---|---|---|---|---|
| Incremental | ICER | Incremental | ICER | |||
| Cost, £ (US $) | QALY | Cost, £ (US $) | QALY | |||
| Base case | 526 (693) | 0.12 | 4293 | −1785 (−2350) | 0.24 | RARP dominant |
| Scenarios | ||||||
| Societal perspective | ||||||
| Include non-NHS costs | −7797 (−10 267) | 0.12 | RARP dominant | −22 392 (−29 485) | 0.24 | RARP dominant |
| Lifetime time horizon | 309 (407) | 0.26 | 1190 | −2133 (−2809) | 0.54 | RARP dominant |
| RARP surgical cost change | ||||||
| RARP instruments using new pricea | 248 (327) | 0.12 | 2025 | −2062 (−2715) | 0.24 | RARP dominant |
| RARP surgical system via charity donations | −137 (−180) | 0.12 | RARP dominant | −2447 (−3222) | 0.24 | RARP dominant |
| Annual volume 150 per systemb | 1242 (1635) | 0.12 | 10 140 | −1068 (−1406) | 0.24 | RARP dominant |
| RARP using da Vinci Xi system | 916 (1206) | 0.12 | 7633 | −1395 (−1837) | 0.24 | RARP dominant |
| RARP using da Vinci Si system | 378 (498) | 0.12 | 3150 | −1933 (−2545) | 0.24 | RARP dominant |
| RARP does not reduce risk of BCR (BCR rate same as ORP) | 1444 (1901) | 0.03 | 42 689 | NA | NA | NA |
| New assumptions on LOS: ORP, 3 d; LRP, 2 d; RARP, 2 d | 1056 (1390) | 0.12 | 8619 | −1604 (−2112) | 0.24 | RARP dominant |
Abbreviations: BCR, biochemical recurrence; ICER, incremental cost-effectiveness ratio; LOS, length of stay; LRP, laparoscopic radical prostatectomy; NA, not applicable; NHS, National Health Service; NICE, National Institute for Health and Care Excellence; ORP, open radical prostatectomy; QALY, quality-adjusted life-year; RARP, robotic-assisted radical prostatectomy.
New price list for da Vinci surgical instruments is found at Extended Use Program for da Vinci X/Xi Instruments, Intuitive Surgical Inc.24
NICE guideline recommends RARP at the centers with annual volume exceeding 150 cases per system.
If we consider the new robotic instrument prices, RARP remained dominant over LRP and became more cost-effective than ORP. If the capital cost is excluded (ie, acquired through donation), RARP became dominant over ORP and increased its dominance over LRP. The results remained unchanged when prices from different generations of da Vinci systems were used. If the annual volume is 150 cases for each system as per the NICE guideline, RARP would still be more cost-effective than ORP (ICER = £11 561 [US $15 223]/QALY) and dominant over LRP (£975 [US $1284] less cost and 0.24 more QALYs). However, if we were to assume that RARP and ORP have the same BCR rate, RARP would not be cost-effective (ICER = £42 689 [US $56 211]/QALY).
Discussion
From the UK NHS perspective, our model found that RARP is more cost-effective than LRP and ORP to treat localized prostate cancer. During a 10-year follow-up period after surgery, RARP was associated with lower BCR rates, longer BCR-free survival, and more QALYs gained compared with LRP or ORP.
Despite its higher equipment costs, RARP’s subsequent medical costs were lower than those of LRP and ORP. This decrease is attributed to RARP’s lower perioperative complication rate, reduced rates of urinary and sexual dysfunctions, and lower rate of cancer recurrence.6,16,17 Our results corroborate the findings of a real-world evidence study based on the UK Hospital Episode Statistics data,25 which showed that RARP was associated with reduced long-term use of health resources and downstream cost savings compared with LRP and ORP. Observational studies conducted in other countries26,27,28 also showed similar results, although they might not be directly comparable with our results given the differences in study designs, data sources, and/or settings. For instance, a previous claims data analysis from the US27 showed that the reduction in health care services attributed to RARP may offset the higher cost during the index hospitalization.
In our analysis, the cost-effectiveness of RARP was driven by the lower risk of BCR associated with RARP. Specifically, the results of our sensitivity analyses suggest that RARP would remain more cost-effective than ORP at a WTP threshold of £30 000 (US $39 503)/QALY as long as RARP had a lower BCR rate than ORP (any hazard ratio <0.99). However, RARP would not be cost-effective if it had the same or a higher BCR rate than ORP. The BCR data used herein are based on a meta-analysis, which showed a reduction in 5-year BCR risk for RARP; however, both RCTs and observational studies were included in the meta-analysis.15 The only RCT to compare RARP with ORP3 showed that RARP had a lower BCR than ORP. Hence, further rigorous investigations comparing long-term outcomes by different surgical approaches of radical prostatectomy are warranted.
Although a previous cost-effectiveness analysis accounted for adjuvant radiotherapy,12 recent clinical trials and guidelines recommend against it. Similar to more recent cost-effectiveness reports, we have accounted for salvage instead of adjuvant radiotherapy.
Evidence suggests that hospital and surgeon volumes correlate strongly with oncological outcomes for a complex procedure such as radical prostatectomy.29,30,31,32,33 Given the importance of oncological outcomes to the overall cost-effectiveness equation, our findings highlight the need for centralized RARP.34 Similarly, the impact of case volume on cost-effectiveness was also demonstrated.7,9 The NICE recommends the use of RARP at centers with more than 150 cases per system per year to achieve superior cost-effectiveness over LRP. Our study showed that RARP is more cost-effective than ORP and LRP at an annual volume of 250 cases per system, which reflects the current use of the robotic system across different specialties in the UK,35 as well as at an annual volume of 150 cases as recommended by the NICE. Although we only assessed the association of surgical volume with cost by sharing the capital cost across specialties, maintaining a certain volume of RARPs might also improve treatment efficacy and patient outcomes as surgeons become more skilled.32
Several peer-reviewed studies and heath technology assessment reports13 have assessed the cost-effectiveness of RARP, but all have presented some weaknesses, which the current model aims to address. First, both short- and long-term outcomes from the most recent literature have been considered. Second, we replicated a real-world scenario where the robotic system is shared among different surgical specialties. Last, in contrast to previous cost-effectiveness studies, we compared the 3 most common surgical techniques of radical prostatectomy and conducted thorough sensitivity and scenario analyses.
We have addressed several medical device-specific factors that could influence cost-effectiveness analysis, including organizational impact, learning curve, incremental innovation, and dynamic pricing.36,37 We have accounted for the capital cost of acquiring the robotic system and its use by different specialties, an aspect of organizational impact. We have also examined the effect of different surgical volumes as a proxy for surgeons’ learning curves.38,39 To account for incremental product innovation, scenario analysis was conducted using the latest prices for the various generations of da Vinci systems; however, differential effectiveness was not included owing to lack of relevant clinical data.
Limitations
Multiple assumptions were made in this simulation-based model. Despite the efforts to perform multiple probabilistic and deterministic sensitivity analyses to better assess uncertainties associated with data selection, it is not possible to reflect all complexities surgeons encounter in real-world practice. Although we did not account for readmissions, we accounted for major perioperative complications that lead to readmissions. In addition, we have accounted for the association of perioperative complications with cost and patient quality of life. Second, to ensure the highest possible level of evidence in this economic analysis, we prioritized data based on the hierarchy of level of evidence for clinical outcomes regardless of their origin. However, nonrandomized studies were used to inform our model when RCTs were lacking. The driving parameter in our model was BCR risk, which was derived from a published meta-analysis of RCTs and observational studies.12 Although sensitivity analyses were conducted to examine cost-effectiveness with different BCR risks, the model should be updated when more rigorous data become available. In addition, it is possible to adapt the current Markov model to other health care settings.
Cost-effectiveness analysis has been widely used to inform stakeholders in health care; however, several additional aspects are worthwhile to consider.40 These may include affordability and budgetary impact on the health system; a wider variety of nonhealth benefits such as surgeon ergonomics, clinician competition, and patients’ preferences; and equitable access to new technology.
Conclusions
This economic analysis found that in the UK, RARP has an ICER lower than the WTP threshold (ICER <£30 000 [US $39 503]), suggesting that it may be a cost-effective surgical treatment option compared with ORP and LRP in patients with localized prostate cancer, in part driven by the lower risk of BCR. Our findings may differ in other health care settings where different thresholds and costs may apply.
eTable 1. Clinical and Resource Utilization Parameters
eTable 2. Utility Parameters
eTable 3. Cost Parameters in the Model
eTable 4. Surgical Equipment Cost
eTable 5. Micro-costing for the Treatment Cost Associated With Dysfunctional Outcome
eTable 6. Micro-costing for the Treatment Cost Associated With Radiation Therapy Adverse Event
eTable 7. Micro-costing for the Cost Associated With Distant Metastasis Treatment
eFigure 1. Deterministic Sensitivity Analysis
eFigure 2. Cost-effectiveness Acceptability Curve at Willingness-to-Pay
eFigure 3. The ICER Plane of RARP vs ORP and RARP vs LRP From Monte Carlo Simulation
eMethods. Statistical Analysis Plan
References
- 1.Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10(2):63-89. doi: 10.14740/wjon1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Research UK . Prostate cancer statistics. 2021. Accessed July 29, 2021. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer#heading-Zero
- 3.Coughlin GD, Yaxley JW, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomised controlled study. Lancet Oncol. 2018;19(8):1051-1060. doi: 10.1016/S1470-2045(18)30357-7 [DOI] [PubMed] [Google Scholar]
- 4.Yaxley JW, Coughlin GD, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet. 2016;388(10 049):1057-1066. doi: 10.1016/S0140-6736(16)30592-X [DOI] [PubMed] [Google Scholar]
- 5.Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Hu JC. Use, costs and comparative effectiveness of robotic assisted, laparoscopic and open urological surgery. J Urol. 2012;187(4):1392-1398. doi: 10.1016/j.juro.2011.11.089 [DOI] [PubMed] [Google Scholar]
- 6.Marcus HJ, Hughes-Hallett A, Payne CJ, et al. Trends in the diffusion of robotic surgery: a retrospective observational study. Int J Med Robot. 2017;13(4):e1870. doi: 10.1002/rcs.1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsay C, Pickard R, Robertson C, et al. Systematic review and economic modelling of the relative clinical benefit and cost-effectiveness of laparoscopic surgery and robotic surgery for removal of the prostate in men with localised prostate cancer. Health Technol Assess. 2012;16(41):1-313. doi: 10.3310/hta16410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooperberg MR, Ramakrishna NR, Duff SB, et al. Primary treatments for clinically localised prostate cancer: a comprehensive lifetime cost-utility analysis. BJU Int. 2013;111(3):437-450. doi: 10.1111/j.1464-410X.2012.11597.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parackal A, Tarride JE, Xie F, et al. Economic evaluation of robot-assisted radical prostatectomy compared to open radical prostatectomy for prostate cancer treatment in Ontario, Canada. Can Urol Assoc J. 2020;14(8):E350-E357. doi: 10.5489/cuaj.6376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratchanon S, Apiwattanasawee P, Prasopsanti K. A cost-utility analysis of laparoscopic radical prostatectomy and robotic-assisted laparoscopic radical prostatectomy in men with localized prostate cancer in Thailand. J Med Assoc Thai. 2015;98(suppl 1):S14-S20. [PubMed] [Google Scholar]
- 11.Health Quality Ontario . Robotic surgical system for radical prostatectomy: a health technology assessment. Ont Health Technol Assess Ser. 2017;17(11):1-172. [PMC free article] [PubMed] [Google Scholar]
- 12.Close A, Robertson C, Rushton S, et al. Comparative cost-effectiveness of robot-assisted and standard laparoscopic prostatectomy as alternatives to open radical prostatectomy for treatment of men with localised prostate cancer: a health technology assessment from the perspective of the UK National Health Service. Eur Urol. 2013;64(3):361-369. doi: 10.1016/j.eururo.2013.02.040 [DOI] [PubMed] [Google Scholar]
- 13.Song C, Kreaden U, Cheng L, Yankovsky A, Li Y. PCN188 systematic literature review on cost-effectiveness analysis of robotic-assisted radical prostatectomy for prostate cancer. Value Health. 2021;24(suppl 1):S54. doi: 10.1016/j.jval.2021.04.279 [DOI] [Google Scholar]
- 14.Hohwü L, Borre M, Ehlers L, Venborg Pedersen K. A short-term cost-effectiveness study comparing robot-assisted laparoscopic and open retropubic radical prostatectomy. J Med Econ. 2011;14(4):403-409. doi: 10.3111/13696998.2011.586621 [DOI] [PubMed] [Google Scholar]
- 15.Lee SH, Seo HJ, Lee NR, Son SK, Kim DK, Rha KH. Robot-assisted radical prostatectomy has lower biochemical recurrence than laparoscopic radical prostatectomy: systematic review and meta-analysis. Investig Clin Urol. 2017;58(3):152-163. doi: 10.4111/icu.2017.58.3.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo HJ, Lee NR, Son SK, Kim DK, Rha KH, Lee SH. Comparison of robot-assisted radical prostatectomy and open radical prostatectomy outcomes: a systematic review and meta-analysis. Yonsei Med J. 2016;57(5):1165-1177. doi: 10.3349/ymj.2016.57.5.1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Wang B, Ai Q, et al. Long-term cancer control outcomes of robot-assisted radical prostatectomy for prostate cancer treatment: a meta-analysis. Int Urol Nephrol. 2017;49(6):995-1005. doi: 10.1007/s11255-017-1552-8 [DOI] [PubMed] [Google Scholar]
- 18.National Institute for Health and Care Excellence . Developing NICE guidelines: the manual. October 31, 2014. Accessed July 29, 2021. https://www.nice.org.uk/process/pmg20/chapter/incorporating-economic-evaluation [PubMed]
- 19.Laird A, Fowler S, Good DW, et al. ; British Association of Urological Surgeons (BAUS) . Contemporary practice and technique-related outcomes for radical prostatectomy in the UK: a report of national outcomes. BJU Int. 2015;115(5):753-763. doi: 10.1111/bju.12866 [DOI] [PubMed] [Google Scholar]
- 20.Tewari A, Sooriakumaran P, Bloch DA, Seshadri-Kreaden U, Hebert AE, Wiklund P. Positive surgical margin and perioperative complication rates of primary surgical treatments for prostate cancer: a systematic review and meta-analysis comparing retropubic, laparoscopic, and robotic prostatectomy. Eur Urol. 2012;62(1):1-15. doi: 10.1016/j.eururo.2012.02.029 [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Zhao X, Song Y, Cai A, Xi H, Chen L. A systematic review and meta-analysis of robot-assisted versus laparoscopically assisted gastrectomy for gastric cancer. Medicine (Baltimore). 2017;96(48):e8797. doi: 10.1097/MD.0000000000008797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Office for National Statistics . Monthly mortality analysis, England and Wales: January 2021. February 25, 2021. Accessed October 9, 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/monthlymortalityanalysisenglandandwales/january2021
- 23.Office for National Statistics . CPI INDEX: Medical services (S) 2015=100. Accessed February 16, 2022. https://www.ons.gov.uk/economy/inflationandpriceindices/timeseries/dkc3/mm23
- 24.Intuitive Surgical, Inc. Instruments Program Notice: Extended Use Program for da Vinci X/Xi Instruments. Date. Accessed February 16, 2022. https://www.intuitive.com/en-us/about-us/company/instruments-program-notice
- 25.Hughes D, Camp C, O’Hara J, Adshead J. Health resource use after robot-assisted surgery vs open and conventional laparoscopic techniques in oncology: analysis of English secondary care data for radical prostatectomy and partial nephrectomy. BJU Int. 2016;117(6):940-947. doi: 10.1111/bju.13401 [DOI] [PubMed] [Google Scholar]
- 26.Wu SY, Chang SC, Chen CI, Huang CC. Latest comprehensive medical resource consumption in robot-assisted versus laparoscopic and traditional open radical prostatectomy: a nationwide population-based cohort study. Cancers (Basel). 2021;13(7):1564. doi: 10.3390/cancers13071564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okhawere KE, Shih IF, Lee SH, Li Y, Wong JA, Badani KK. Comparison of 1-year health care costs and use associated with open vs robotic-assisted radical prostatectomy. JAMA Netw Open. 2021;4(3):e212265. doi: 10.1001/jamanetworkopen.2021.2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Oliveira RAR, Guimarães GC, Mourão TC, et al. Cost-effectiveness analysis of robotic-assisted versus retropubic radical prostatectomy: a single cancer center experience. J Robot Surg. 2021;15(6):859-868. doi: 10.1007/s11701-020-01179-z [DOI] [PubMed] [Google Scholar]
- 29.Barocas DA, Mitchell R, Chang SS, Cookson MS. Impact of surgeon and hospital volume on outcomes of radical prostatectomy. Urol Oncol. 2010;28(3):243-250. doi: 10.1016/j.urolonc.2009.03.001 [DOI] [PubMed] [Google Scholar]
- 30.Williams SB, Ray-Zack MD, Hudgins HK, et al. Impact of centralizing care for genitourinary malignancies to high-volume providers: a systematic review. Eur Urol Oncol. 2019;2(3):265-273. doi: 10.1016/j.euo.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sammon JD, Karakiewicz PI, Sun M, et al. Robot-assisted versus open radical prostatectomy: the differential effect of regionalization, procedure volume and operative approach. J Urol. 2013;189(4):1289-1294. doi: 10.1016/j.juro.2012.10.028 [DOI] [PubMed] [Google Scholar]
- 32.Trinh QD, Bjartell A, Freedland SJ, et al. A systematic review of the volume-outcome relationship for radical prostatectomy. Eur Urol. 2013;64(5):786-798. doi: 10.1016/j.eururo.2013.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leow JJ, Chang SL, Meyer CP, et al. Robot-assisted versus open radical prostatectomy: a contemporary analysis of an all-payer discharge database. Eur Urol. 2016;70(5):837-845. doi: 10.1016/j.eururo.2016.01.044 [DOI] [PubMed] [Google Scholar]
- 34.Cole AP, Leow JJ, Chang SL, et al. Surgeon and hospital level variation in the costs of robot-assisted radical prostatectomy. J Urol. 2016;196(4):1090-1095. doi: 10.1016/j.juro.2016.04.087 [DOI] [PubMed] [Google Scholar]
- 35.Lam K, Clarke J, Purkayastha S, Kinross JM. Uptake and accessibility of surgical robotics in England. Int J Med Robot. 2021;17(1):1-7. doi: 10.1002/rcs.2174 [DOI] [PubMed] [Google Scholar]
- 36.Drummond M, Griffin A, Tarricone R. Economic evaluation for devices and drugs—same or different? Value Health. 2009;12(4):402-404. doi: 10.1111/j.1524-4733.2008.00476_1.x [DOI] [PubMed] [Google Scholar]
- 37.Taylor RS, Iglesias CP. Assessing the clinical and cost-effectiveness of medical devices and drugs: are they that different? Value Health. 2009;12(4):404-406. doi: 10.1111/j.1524-4733.2008.00476_2.x [DOI] [PubMed] [Google Scholar]
- 38.Tarricone R, Torbica A, Drummond M. Challenges in the assessment of medical devices: the MedtecHTA Project. Health Econ. 2017;26(suppl 1):5-12. doi: 10.1002/hec.3469 [DOI] [PubMed] [Google Scholar]
- 39.Tarricone R, Torbica A, Drummond M; MedtecHTA Project Group . Key recommendations from the MedtecHTA Project. Health Econ. 2017;26(suppl 1):145-152. doi: 10.1002/hec.3468 [DOI] [PubMed] [Google Scholar]
- 40.Thokala P, Devlin N, Marsh K, et al. Multiple criteria decision analysis for health care decision making—an introduction: report 1 of the ISPOR MCDA Emerging Good Practices Task Force. Value Health. 2016;19(1):1-13. doi: 10.1016/j.jval.2015.12.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Clinical and Resource Utilization Parameters
eTable 2. Utility Parameters
eTable 3. Cost Parameters in the Model
eTable 4. Surgical Equipment Cost
eTable 5. Micro-costing for the Treatment Cost Associated With Dysfunctional Outcome
eTable 6. Micro-costing for the Treatment Cost Associated With Radiation Therapy Adverse Event
eTable 7. Micro-costing for the Cost Associated With Distant Metastasis Treatment
eFigure 1. Deterministic Sensitivity Analysis
eFigure 2. Cost-effectiveness Acceptability Curve at Willingness-to-Pay
eFigure 3. The ICER Plane of RARP vs ORP and RARP vs LRP From Monte Carlo Simulation
eMethods. Statistical Analysis Plan